Abstract
The ability of cholesterol to uncoil (i.e., condense) the acyl chains of phospholipids has been known for a century. Despite extensive studies of the interactions between cholesterol and phospholipids, a molecular-level understanding of this uncoiling phenomenon has remained elusive. Equally unclear has been whether cholesterol’s two different faces (i.e., its relatively smooth α face and its relatively rough β face) contribute to its condensing power. Because cholesterol’s condensing effect is believed to play a major role in controlling the fluidity, structure, and functioning of all animal cell membranes, its biological importance cannot be overstated. This Perspective focuses on experimental evidence that addresses (i) the credibility of a popular “umbrella” mechanism that has been used to account for cholesterol’s condensing effect, (ii) the credibility of an alternate “template” mechanism, (iii) the importance of cholesterol two-faced character with respect to its condensing power, and (iv) the viability of a surface occupancy model.
Keywords: Cholesterol, condensing, phospholipid, monolayer, umbrella, alpha and beta face, nearest-neighbor
Introduction
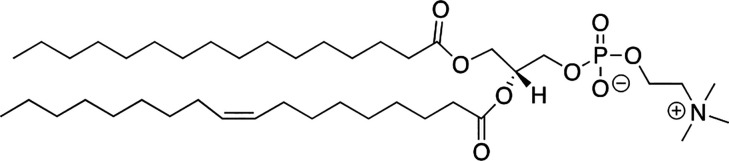
The main building blocks of all animal cell membranes consist of high-melting lipids, low-melting lipids, cholesterol, and an assortment of proteins. By use of the term, high-melting lipids, what I’m referring to are naturally occurring lipids that, by themselves, will form relatively incompressible and solid-like monolayers at 37 °C. Such monolayers are commonly described as being in the solid phase (So), where the hydrocarbon chains favor a fully extended anti conformation and the average chain occupies a surface area of ca. 20 Å2.1 The term, “low-melting lipids” refers to naturally occurring lipids that, by themselves, will form compressible, fluid-like monolayers at 37 °C that are commonly described as being in the liquid-disordered (Ld) phase. Here, the presence of permanent “kinks” (i.e., one or more cis-double bonds) causes the average hydrocarbon chain to occupy a surface area that is greater than ca. 40 Å2.2 Cholesterol is distinct by being a rigid lipid molecule that has negligible compressibility with a surface area of 36 Å2/molecule.3
While cholesterol exhibits a fluidizing effect on high-melting lipids, this same sterol has a condensing effect on low-melting lipids.4 The lipid raft hypothesis—a very popular hypothesis that has emerged in cell biology—is based on the assumption that cholesterol combines with high-melting lipids in animal cell membranes to form transient domains (“lipid rafts”), which play a major role in fundamental processes such as signal transduction and membrane protein trafficking.5,6
Model systems that are used to examine the lipid raft hypothesis are usually derived from cholesterol and high-melting lipids or saturated phospholipids that serve as mimics of high-melting lipids. These model systems are then described as being in the liquid-ordered (Lo) phase, which has structural and physical properties that are intermediate between those found in the Ld and So phases.
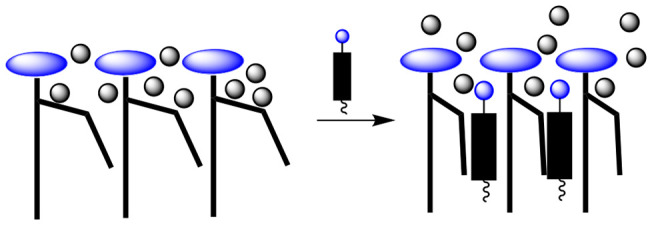
In contrast to the lipid raft hypothesis, which continues to be the subject of debate, cholesterol’s condensing effect (i.e., its ability to uncoil low-melting lipids) was firmly established in 1925.1 Despite numerous studies of this effect, a detailed molecular-level understanding of how cholesterol is able to uncoil the acyl chains of low-melting lipids has remained a mystery. A stylized illustration of cholesterol’s condensing effect is shown in Figure 1. Here a kink in one of the hydrocarbon chains reflects the presence of one or more cis-double bonds (or one or more gauche conformations) in the chain.
Figure 1.
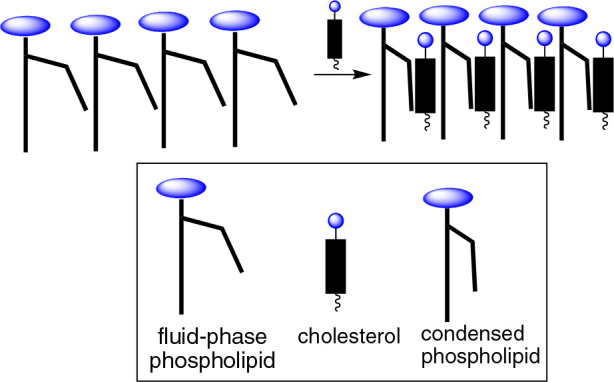
Stylized illustration of cholesterol’s condensing effect on phospholipids in the Ld phase.
In a recent Perspective, I have described how the “nearest-neighbor recognition” method allows one to quantify cholesterol’s preference for associating with mimics of high-melting lipids over mimics of low-melting lipids.7 In that Perspective, I also explained how these nearest-neighbor interactions can serve as a driving force for lipid raft formation and that they are best thought of as “push” and “pull” forces that maximize the number of hydrocarbon contacts and attractive van der Waals interactions within the membrane.7
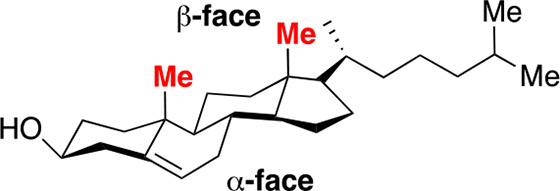
In this Perspective, I will focus, sharply, on cholesterol’s condensing effect. To date, the most popular model that has emerged to account for this effect is the “umbrella model”.8−15 In essence, it has been proposed that the headgroups of phospholipids act like umbrellas in minimizing the contact that their lipophilic acyl chains make with water. It has also been proposed that because there is limited space beneath the headgroups, these acyl chains are forced to become more compact (i.e., condensed) to make room for cholesterol as it becomes part of the lipophilic assembly. A related issue that has attracted considerable attention is whether the fact that cholesterol has two different faces (a smooth α face and a relatively rough β face) contributes to its condensing power (Chart 1).16−20
Chart 1. Molecular Structure of Cholesterol Showing Its Smooth α and Rough β Face.
This Perspective addresses four key issues that involve cholesterol’s condensing effect: (i) the credibility of the popular “umbrella” mechanism, (ii) the credibility of an alternate “template” mechanism, (iii) the importance of cholesterol two-faced character with respect to its condensing power, and (iv) the viability of a surface occupancy model. Before discussing each of these issues, I want to briefly mention two classic studies because of their historical significance and also because they have inspired many researchers over many decades to investigate cholesterol’s condensing effect, i.e., Leathe’s pioneering discovery of this effect and a subsequent investigation by Demel, Van Deenen, and Pethica that provided important clarification.1,21
Cholesterol’s Condensing Effect
The discovery of cholesterol’s condensing effect on low-melting lipids was first reported by J. B. Leathes in 1925.1 In that seminal paper, surface pressure–area isotherms were measured for cholesterol, egg phosphatidylcholine (egg PC, a relatively compressible mixture of phosphocholines), and a 1/1 mixture of egg PC and cholesterol. What Leathes discovered was that when he subtracted the area that was occupied by cholesterol from the total area that was occupied by the 1/1 mixture of cholesterol/egg PC at various surface pressures, the area that was occupied by the average hydrocarbon chain of the phospholipid was significantly reduced, relative to the area that it occupied in the absence of cholesterol (Figure 2A). In other words, the presence of cholesterol had a condensing effect on the average acyl chain of egg PC.
Figure 2.
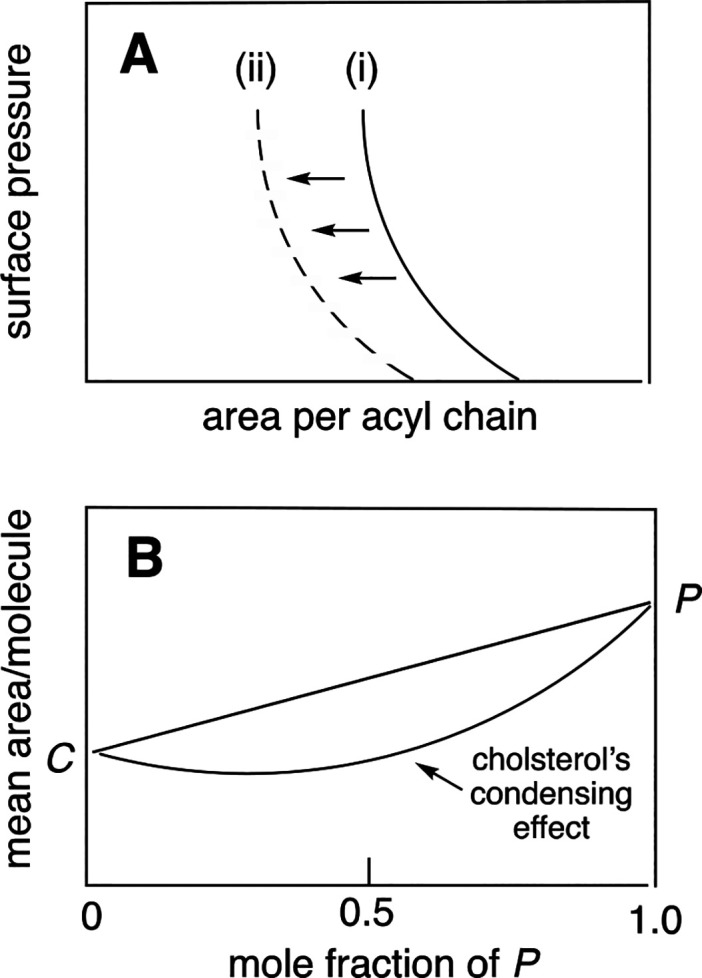
Methods used to detect cholesterol’s condensing effect on phospholipids. Schematic showing (A) the area occupied per acyl chain in (i) egg PC and (ii) egg PC/cholesterol (1/1, mol/mol) as a function of surface pressure and (B) a plot of the mean area per molecule as a function of the mole fraction of phospholipid present in a mixture of cholesterol (C) and phospholipid (P) at constant surface pressure.
Because egg PC is composed of a mixture of phosphocholines of which 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine is dominant, Demel, Van Deenen, and Pethica investigated the condensing properties of cholesterol on pure synthetic phospholipids (Chart 2).21 What they discovered was that cholesterol’s condensing effect was significantly dependent on the specific structure and composition of the phospholipid’s acyl chains. Also noteworthy was that cholesterol’s condensing effect was detected in a different way, i.e., as a negative deviation from linearity when the mean molecular area of cholesterol and the phospholipid was plotted as a function of the mole fraction of the phospholipid at constant surface pressure (Figure 2B).21 Thus, when cholesterol was combined with a condensable phospholipid, the surface area that was occupied by the two lipids was less than what would have been predicted if one assumes that the surface area contribution by each lipid was the same as that measured alone. From a thermodynamic standpoint, this negative deviation is considered to be nonideal mixing of the two lipids. If the mixing were ideal, the observed surface areas would be linearly weighted by the composition of the lipid mixture. Thus, a negative deviation from linearity reflects the formation of a nonideal solution.
Chart 2. Molecular Structure of 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC).
The Umbrella Model
To account for cholesterol’s condensing effect, an “umbrella model” was proposed by Huang and Feigenson in 1999.8 According to this model, “in a bilayer, nonpolar cholesterol relies on polar phospholipid headgroup coverage to avoid the unfavorable free energy of cholesterol contact with water. Thus, at high cholesterol mole fraction, this unfavorable free energy, not any favorable cholesterol–phospholipid interaction, dominates the mixing behavior. This physical origin also explains the “cholesterol condensing effect” and the increase in acyl chain order parameter in cholesterol–phospholipid mixtures.”8 In other words: “Acyl chains and cholesterols become more tightly packed as cholesterol content increases because they share limited space under phospholipid headgroups. The hydrophobic nature of cholesterol thus forces cholesterol and acyl chains together.”8
The Template Mechanism
An alternative hypothesis that we, ourselves, proposed to account for cholesterol’s condensing effect is based on a template mechanism.22 Specifically, we proposed that the attractive hydrophobic interaction between the rigid sterol nucleus and the acyl chains of the phospholipids (which are flexible and able to complement, perfectly, the shape of cholesterol such that the number of hydrophobic contacts is maximized and the packing is tight) is the dominant factor that leads to the uncoiling of the phospholipid’s acyl chains. In other words, no umbrella action by the phospholipid headgroups is required.
Distinguishing between the Umbrella Model and the Template Mechanism
Experimental Design
To distinguish between the umbrella model and the template mechanism, we compared the condensing power of cholesterol with that of dihydrocholesterol and coprostanol (Chart 3).23
Chart 3. Comparative Structures of Cholesterol, Dihydrocholesterol, and Coprostanol.
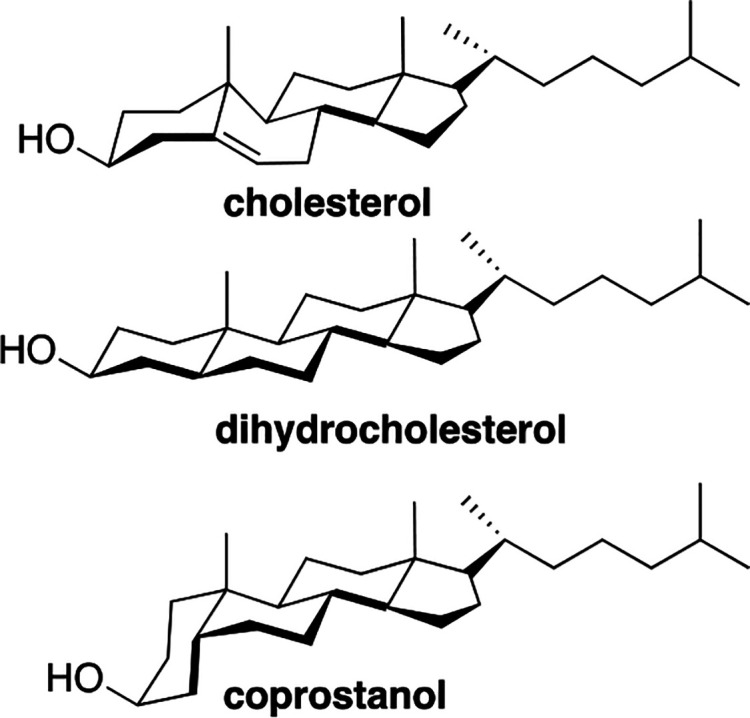
Because cholesterol and dihydrocholesterol are similar in structure and shape, they have virtually identical cross-sectional areas of 36 Å2.3 Also, due to their similarity in shape, these sterols are expected to produce a similar number of hydrophobic contacts with the acyl chains of a neighboring phospholipid and occupy a similar amount of space beneath the phospholipid’s headgroup. Thus, based on both the template mechanism and the umbrella model, both sterols are expected to have a similar condensing power.23 However, in the case of coprostanol, the umbrella model and the template mechanism lead to predictions that are diametrically opposed to one another. Specifically, if an umbrella mechanism was operating, due to the cis-fusion of its A and B ring and the corresponding larger cross-sectional area (41 Å2), coprostanol’s condensing effect is expected to be stronger than that of cholesterol and dihydrocholesterol due to increased crowding beneath the phospholipid’s headgroup (Figure 3). In other words, more uncoiling of the acyl chains would be needed to prevent the sterol nucleus and neighboring acyl chains from making contact with water. However, if a template mechanism was operating, then coprostanol is expected to have a weaker condensing effect since the acyl chains of a neighboring phospholipid would not be able to complement, perfectly, the shape of the coprostanol molecule.
Figure 3.
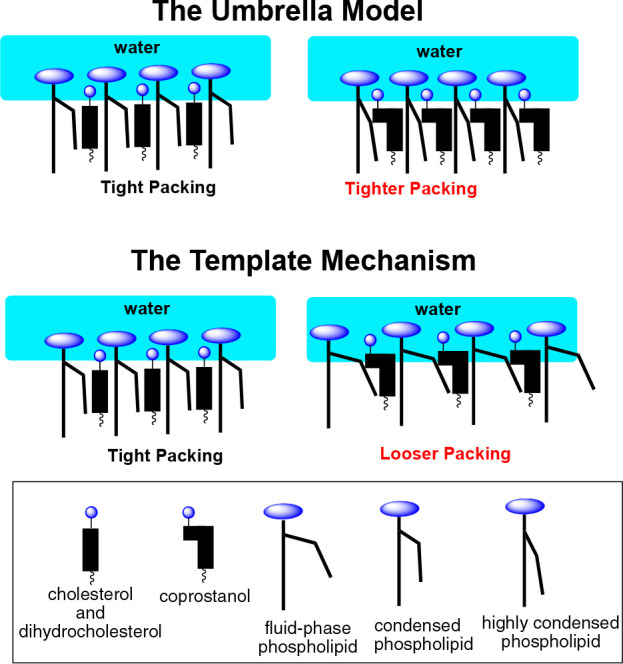
Experimental design for distinguishing between the umbrella model and the template mechanism.
To judge the relative condensing power of cholesterol, dihydrocholesterol, and coprostanol, we measured their ability to increase the compactness of fluid bilayers derived from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) using the nearest-neighbor recognition (NNR) method and also a fluorescence assay that uses the phase-sensitive probe, Laurdan.23 Because the So to Ld phase transition temperature of DPPC is 41 °C, a higher temperature (45 °C) was used in the NNR experiments to ensure the existence of the Ld phase.
Nearest-Neighbor Recognition Measurements
The nearest-neighbor recognition (NNR) technique is a chemical method that allows one to quantify nearest-neighbor interactions by measuring equilibrium mixtures of dimers.7 In a typical NNR experiment, a heterodimer that is composed of two lipids of interest, A and B, is partially reduced within a host membrane. Monomer exchange with the heterodimer and with the resulting homodimers that are formed via thiolate-disulfide interchange then yields an equilibrium mixture of dimers that reflects nearest-neighbor preferences (Figure 4). An equilibrium constant, K, is then given by (AB)2/(AA)(BB).
Figure 4.
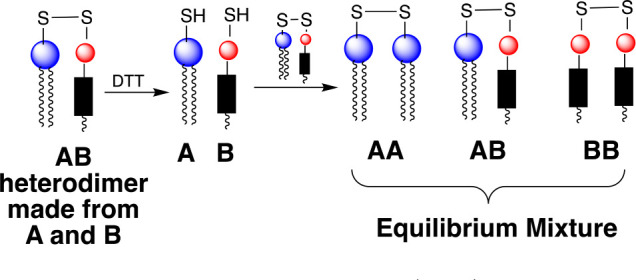
Nearest-neighbor recognition (NNR) method.
When A and B mix ideally, this is reflected by a value of K = 4.0. Favored homoassociations are indicated by values of K that are less than 4.0, and favored heteroassociations are revealed when K is greater than 4.0. Taking statistical considerations into account, it can be shown that the nearest- neighbor interaction free energy between A and B, ωAB, is given by ωAB= −1/2RT ln K/4.24
Connecting the Dots
Before discussing some of our key findings that relate to the umbrella model versus the template mechanism, you may be asking yourself where did this NNR method come from? Let me briefly explain how it came about. In the early 1980s, shortly after introducing the concept of polymerized vesicles, we began exploring a variety of methods for forming covalent bonds between neighboring lipids in vesicle form.25 One of these methods involved the reversible formation of disulfide bonds from thiol-bearing phospholipids.26,27 Around that time, a friend of mine (the late Francois Kezdy, University of Chicago) showed me a paper that he thought was interesting. This paper described some highly unusual differential scanning calorimetry (DSC) results that were interpreted as evidence for the existence of discrete domains of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and DPPC in the Ld phase.28 These results intrigued me and made me think (as an organic chemist) of how one might be able to quantify such mixing at the molecular level. It did not take me long to “connect the dots” between lipid mixing, reversible bond formation via thiol–disulfide interchange, chemical equilibrium, phospholipid synthesis, and HPLC chromatography to devise the NNR method. With only this concept in hand (i.e., without a shred of preliminary results), I submitted a proposal to the National Insitutes of Health for support for testing its feasibility. Based on its novelty, the proposal was met with enthusiasm and was funded. Then, with the aid of a truly outstanding graduate student (Sharon Krisovitch), we published a series of papers that not only demonstrated the feasibility of the NNR method but also provided compelling evidence that our mimics of DMPC and DPPC did not form domains in the Ld phase; instead, they mixed randomly.29−32 In one of those papers, we also presented a kinetic argument that cast serious doubt on key interpretations that were made in that DSC study.30
The Fidelity of The NNR Method
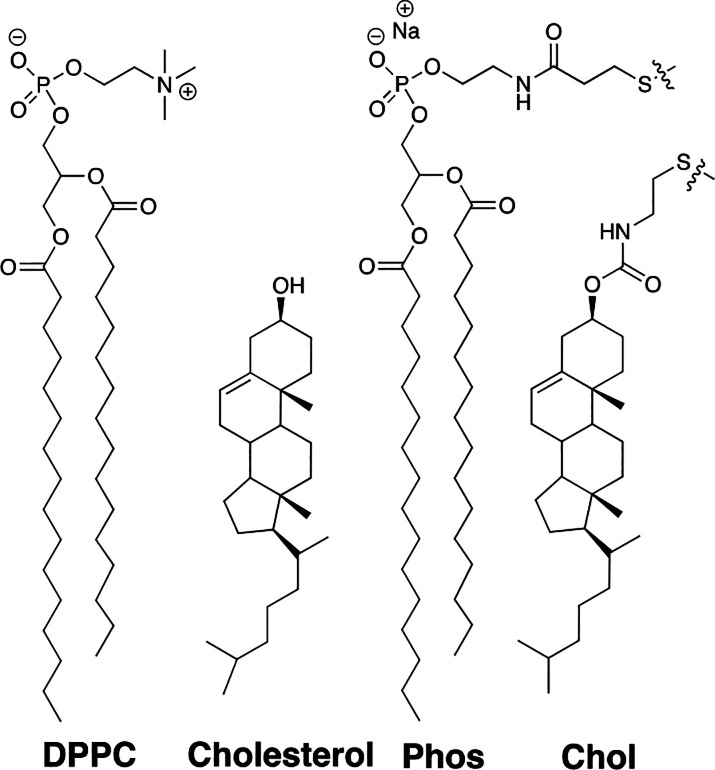
In Chart 4 are shown exchangeable lipids Phos and Chol, which we have used as mimics of DPPC and cholesterol.
Chart 4. Structures of DPPC, Cholesterol, and Exchangeable Mimics Phos and Chol.
In previous work, we demonstrated that Phos and Chol are, in fact, excellent mimics of these natural lipids through monolayer measurements at the air/water interface and DSC measurements of bilayers.22,30 We also showed that the equilibrium constant, K, which defines the mixing of Phos with Chol, can be used to monitor the compactness of a bilayer where higher values of K reflect increased compactness.33 It was also noteworthy that the mixing of Phos with Chol closely matched the transition from the Ld to the Lo state for mixtures of DPPC and cholesterol.33,34 Finally, that such exchangeable mimics closely reflect the energetics of the interactions between naturally occurring analogs was demonstrated by a comparison of the ωAB value for cholesterol/POPC in model membranes with the ωAB value that was determined via the NNR method for our exchangeable mimics. Specifically, based on a combination of fluorescence resonance energy transfer measurements (25–37 °C) and Monte Carlo simulations, the ωAB value for POPC/cholesterol was estimated to be +200 cal/mol.35 The ωAB value that we determined for the exchangeable mimics of POPC/cholesterol at 45 °C was +160 ± 30 cal/mol.7
The Condensing Power of Cholesterol, Dihydrocholesterol, and Coprostanol
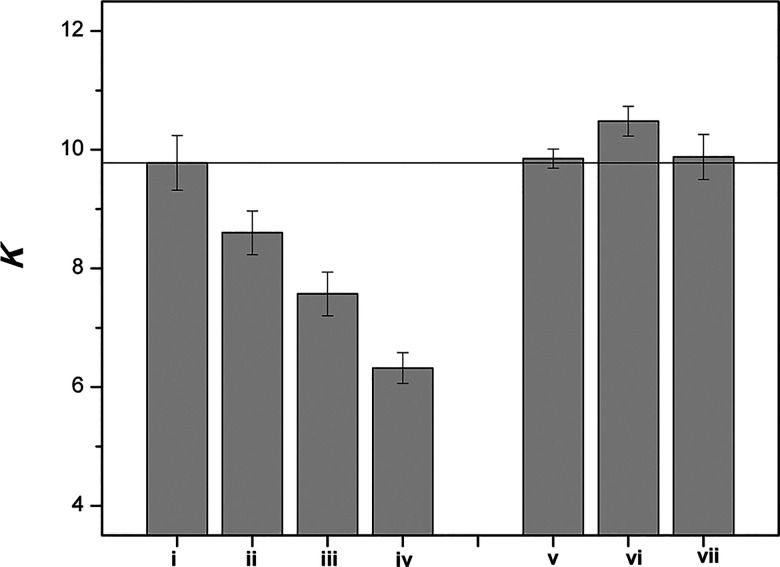
When 2.5 mol % of Phos and Chol were included in bilayers made from DPPC, a K value of ca. 4 was obtained at 45 °C. However, in the presence of 40 mol % cholesterol (i.e., when the membrane was converted from the Ld phase to the Lo phase), K was found to increase to a value of ca. 9.5.23 As shown in Figure 5, incremental replacement of cholesterol with coprostanol resulted in a steady decrease in K, reflecting reduced compactness of the bilayer. In sharp contrast, incremental replacement of cholesterol with dihydrocholesterol had a negligible effect on the value of K.
Figure 5.
Bar graph showing K in liposomes containing the following mole percentages of cholesterol/coprostanol: (i) 40/0, (ii) 30/10, (iii) 20/20, (iv) 2.5/37.5, and cholesterol/dihydrocholesterol: (v) 30/10, (vi) 20/20 and (vii) 2.5/37.5. Here, Chol was included in the cholesterol count. Error bars represent one standard deviation. All thiolate-disulfide exchange reactions were carried out at 45 °C. (Reprinted from ref (23). Copyright 2011 American Chemical Society.)
Fluorescence Measurements
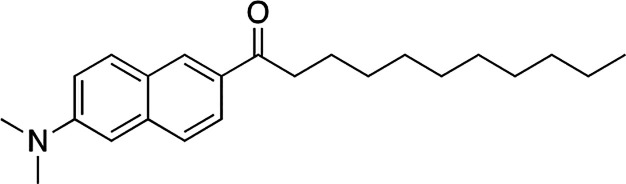
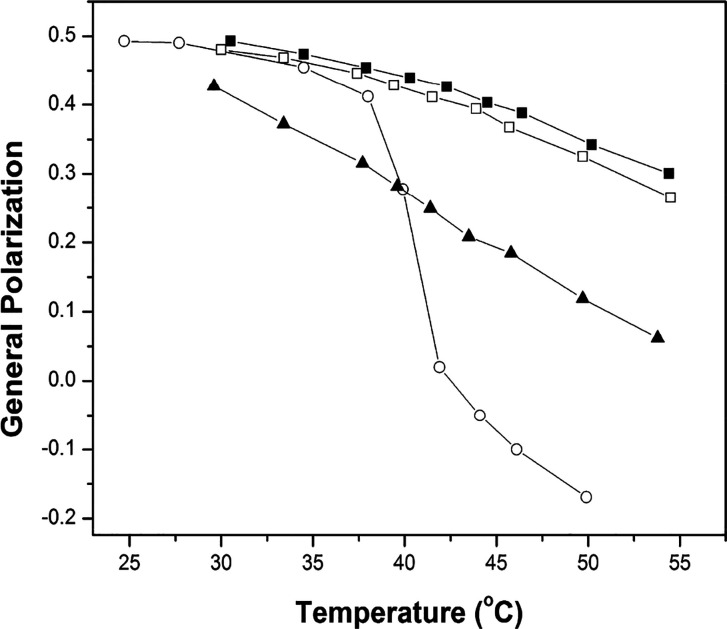
Related fluorescence measurements that were carried out using the phase-sensitive probe, Laurdan, and the empirical general polarization method confirmed, qualitatively, the results that were obtained from the NNR measurements (Chart 5 and Figure 6). This fluorescence method relies on the fact that as a membrane becomes looser, the polarity surrounding membrane-bound Laurdan increases. This change in polarity can then be followed by measuring the fluorescence intensities at 490 and 440 nm and determining general polarization values, where GP = (I440 – I490)/(I440 + I490). In Figure 6 are shown GP values for lipid bilayers made from DPPC in the presence of cholesterol, dihydrocholesterol, and coprostanol as a function of temperature. When only 2.5 mol % cholesterol is present, (i.e., the membrane is made almost entirely from DPPC), the GP value decreases, precipitously, with increasing temperatures as the membrane is converted from the So to the Ld phase. In sharp contast, only a modest decrease in the GP values are seen over this same temperature range when cholesterol and dihydrocholesterol are included in the membrane as the membrane is maintained in the Lo phase. In contrast, when coprostanol is included in the membrane, all of the GP values are intermediate in magnitude reflecting a bilayer that is intermediate in compactness.23 Taken together, these NNR and fluorescence results are inconsistent with the umbrella model but are fully consistent with the template mechanism for cholesterol’s condensing effect.
Chart 5. Molecular Structure of Laurdan.
Figure 6.
Plot of general polarization versus temperature for liposomes made from the following molar percentages of lipids: (○) DPPC/DPPG/cholesterol 95/2.5/2.5; (■) DPPC/DPPG/cholesterol, 57.5/2.5/40; (□) DPPC/DPPG/cholesterol/dihydrocholesterol, 57.5/2.5/2.5/37.5; (▲)DPPC/DPPG/cholesterol/coprostanol, 57.5/2.5/2.5/37.5. (Reprinted from ref (23). Copyright 2011 American Chemical Society.)
Cholesterol’s Two-Faced Character
Previous molecular dynamics simulations suggest that the interactions of saturated phospholipid chains with the smooth face of cholesterol are stronger than with the sterol’s rough face.17,18 Atomic-scale molecular dynamics simulations that have compared membranes made from cholesterol and DPPC with ones made from 18,19-dinorcholesterol (DChol) and DPPC suggest that cholesterol’s two different faces may add to its condensing power by lowering its degree of tilt, thereby allowing it to lie in a more upright position and parallel to the acyl chains of neighboring phospholipids.19,20
To examine how the two different faces of cholesterol influence its condensing power, experimentally, we relied on a heroic synthetic effort by our collaborators, Prof. Covey and co-workers at Washington University, who converted 19-nortestosterone into 18,19-dinorcholesterol (Dchol) in 18 steps (Scheme 1).36
Scheme 1. Conversion of 19-Nortestosterone into 18,19-Dinorcholesterol (DChol).
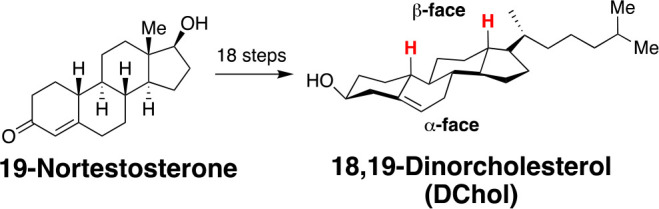
Nearest-Neighbor Recognition, Fluorescence, and Monolayer Measurements
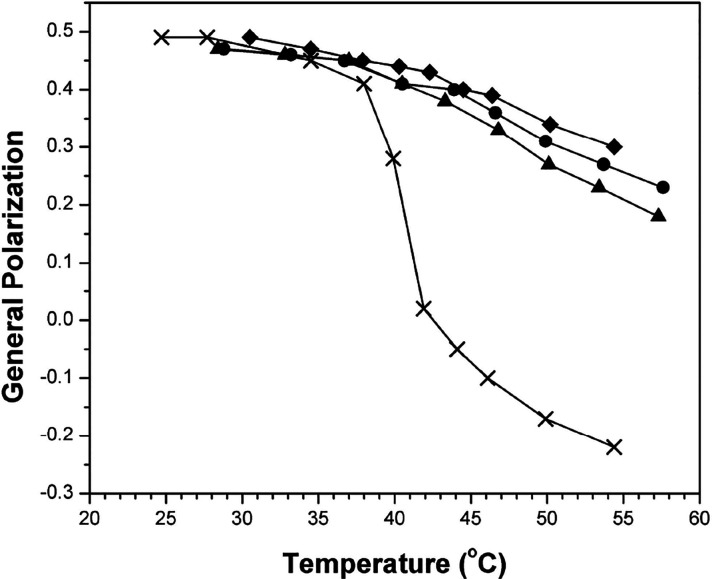
Based on a combination of nearest-neighbor recognition and fluorescence measurements using fluid bilayers of DPPC, as well as monolayer measurements using DMPC, we found that cholesterol has a stronger condensing effect than DChol but only slightly so-- on the order of tens of calories per mole (Table 1 and Figures 7 and 8).37 For these monolayer experiments, DMPC was chosen instead of DPPC because of its lower Tm value of 23 °C that made it easier to maintain the fluid phase at the air/water interface especially at low sterol concentrations (Figure 8).
Table 1. Nearest-Neighbor Interactions between Chol and Phos in Fluid Bilayers of DPPCa.
| sterol | mol % | K | ωAB (cal/mol) |
|---|---|---|---|
| Chol | 0 | 3.9 ± 0.3 | 8 ± 21 |
| Chol | 40 | 9.2 ± 0.2 | –260 ± 7 |
| Chol/DChol | 20/20 | 8.1 ± 0.7 | –220 ± 27 |
| DChol | 40 | 8.3 ± 0.7 | –230 ± 28 |
Measurements were made at 45 °C in host membranes derived from DPPC and the indicated sterol(s). In each case, 2.5 mol % Phos and 2.5 mol % Chol were present.37
Figure 7.
Plot of general polarization versus temperature in liposomes made from DPPC/DPPG/cholesterol/DChol with the following molar percentages: (◆) 57.5/2.5/40/0; (●) 57.5/2.5/20/20; (▲) 57.5/2.5/0/40; (×) 95/2.5/2.5/0. Error values lie within the data points themselves. (Reprinted ref (37). Copyright 2014 American Chemical Society.)
Figure 8.
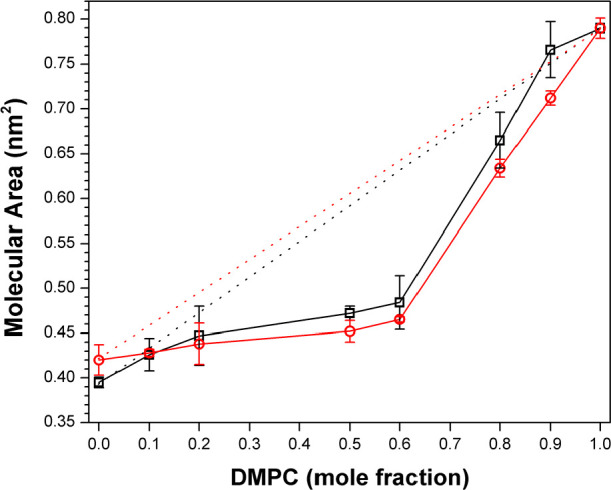
Molecular area–-additivity curves for (□) DMPC/DChol and (○) DMPC/Chol with a surface pressure of 10 mN/m. Ideal additivities are shown by dotted lines. Error bars that are not visible lie within the symbols themselves. (Reprinted ref (37). Copyright 2014 American Chemical Society.)
Condensed Complex Formation and Thermodynamics of Phos–Chol Association
The nearest-neighbor recognition measurements that are described in this Perspective have provided unique insight into the interactions between individual Phos molecules with individual Chol molecules in host membranes. When the host membrane consists mainly of DPPC and the bilayers are in the Ld phase Phos and Chol exhibit K values that are close to the statistical value of 4.0. In sharp contrast, when a sufficient amount of cholesterol is present and the membrane has been converted into the Lo phase, the K value is now greater than 4.0, which clearly reflects an affinity between the two lipids. These findings imply that cholesterol’s condensing effect requires more than just one cholesterol molecule interacting with one neighboring phospholipid. In this regard, several reports have provided evidence for the formation of discrete complexes of varying stoichiometries. Some of these reports were based on epifluorescence microscopy of monolayers derived from dihydrocholesterol and a variety of phospholipids where coexisting liquid phases were observed.38−41 Other studies have used X-ray lamellar diffraction to measure the phosphate-to-phosphate distances across lipid bilayers.42 Kinetic measurements of the lability of cholesterol toward cholesterol oxidase in liposomes containing biological phospholipids and varying concentrations of cholesterol have also provided evidence for cholesterol-phospholipid complexes of varying stoichiometry43 While more studies are needed to understand the formation of condensed complexes of phospholipids and cholesterol, and how such complexes interact with neighboring complexes and uncomplexed lipids, the template mechanism is likely to play a key role in cholesterol’s condensing effect.22,23
To gain insight into the thermodynamics of cholesterol’s condensing effect, we first measured the temperature dependence of K for Chol and Phos in bilayers made from DPPC and 10 mol % cholesterol. In the Ld phase, K was found to be ca. 4 and independent of temperature from 45 to 65 °C, reflecting random mixing.44 However, the situation was quite different when the cholesterol content was increased to 40 mol %, which placed the membrane in the Lo phase. In this case, K steadily increased on going from 60 to 45 °C, reflecting an increased affinity between Chol and Phos.44 Based on the observed temperature dependence of K, this affinity was characterized by ΔHo = −2.06 ± 0.14 kcal/mol of phospholipid and ΔSo = −4.48 ± 0.44 cal/K mol of phospholipid. It is noteworthy that this enthalpy driven affinity is consistent with explicit solvent molecular dynamics simulations that have predicted that water in a hydrophobic cavity is more disorganized than bulk water, and that hydrophobic association can be enthalpy driven.45 For extensive discussions of the thermodynamics of cholesterol–phosphocholine interactions in lipid membranes, the reader is referred to two recent papers by Almeida.46,47
Surface Occupancy Model
Our finding that the association between Phos and Chol is enthalpy-driven supports a surface occupancy model that we proposed for cholesterol’s condensing effect.48,49 If one considers the fact that phospholipid headgroups occupy only about half of the surface area of fluid-phase phospholipid bilayers, then the remaining surface area must be occupied by partially hydrated CH2 groups of the acyl chains. By replacing the space that is occupied by these “wet” CH2 groups with rigid hydrophobic sterols, disorganized water can then be released from hydrophobic cavities, resulting in a partial straightening of the acyl chains, strong chain–chain interactions, and tighter packing (Figure 9).
Figure 9.
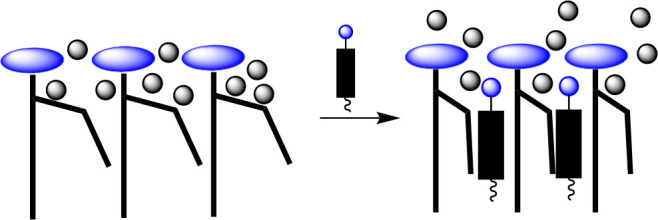
Stylized illustration of cholesterol’s condensing effect showing the release of disorganized water from hydrophobic cavities.
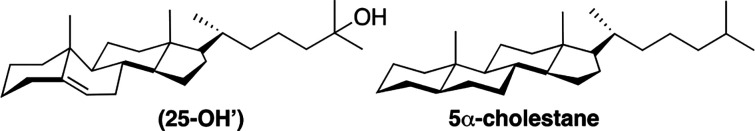
Experimental evidence in support of this surface occupancy model was obtained by comparing the condensing power of cholesterol with that of a derivative of 25-hydroxycholesterol (i.e., 25-OH′) and 5α-cholestane (Chart 6).48,49 Here, the relocation of cholesterol’s hydroxyl group from the C-3 to the C-25 position 5-OH′ should allow only the pendant alkyl chain to be favored at the membrane surface. With 5α-cholestane, which is completely devoid of polar groups, the entire molecule is expected to favor the hydrocarbon interior. Thus, if cholesterol’s condensing power relies on having its sterol nucleus occupying space at the surface of the bilayer, then both 25-OH′ and 5α-cholestane are expected to have a much weaker condensing power.
Chart 6. Molecular Structures of 25-OH′ and 5α-Cholestane.
Using a combination of nearest-neighbor recognition, fluorescence and monolayer measurements, we found that both 25-OH′ and 5α-cholestane do, indeed, exhibit a much weaker condensing effect than cholesterol (not shown), which provide experimental support this surface occupancy model.48,49
Conclusions
The relative condensing power that cholesterol, dehydrocholesterol, and coprostanol have on fluid bilayers of DPPC is inconsistent with the popular umbrella model for cholesterol’s condensing effect but is fully consistent with a template mechanism. Comparison of the condensing action of cholesterol with an analog bearing two smooth faces (i.e., DChol) has further revealed that cholesterol’s two-faced character does contribute to its condensing power but this contribution is exceedingly small—on the order of tens of calories per mole.
A thermodynamic analysis of the association of an exchangeable mimic of cholesterol (i.e., Chol) with an exchangeable mimic of DPPC (i.e., Phos) has revealed that this association in the liquid-condensed phase is enthalpy driven—a fact that supports a surface occupany model where disorganized water molecules at the surface of the membrane are released into the bulk water phase as the rigid sterol nucleus replaces “wet” CH2 groups of the phospholipid’s acyl chains. Experimental evidence that supports this surface occupancy model has come from the fact that cholesterol has a much stronger condensing power than 25-OH′ and also 5α-cholestane.
Additional studies are needed to understand the formation of condensed complexes of cholesterol and phospholipids and how these complexes interact with neighboring complexes and uncomplexed lipids to produce the condensed Lo phase. While cholesterol’s two-faced character appears to make a very week contribution to its condensing power, it may be more important in other ways, e.g., in controlling the structure, lateral organization and functioning of membrane proteins. This is an intriguing question but one that remains to be answered.
Acknowledgments
I am grateful to my co-workers who are listed in our publications that are cited in this Perspective and also to the late Professor Ferenc J. Kezdy for many valuable discussions. I am also grateful to the National Institutes of Health (PHS GM56149) and the National Science Foundation (CHE-1145500), for their financial support, which has made much of our research in this area and this Perspective possible.
The author declares no competing financial interest.
References
- Pearson R. H.; Pascher I. The molecular structure of lecithin dihydrate. Nature 1979, 281, 499–501. 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Leathes J. B. Croonian lectures on the role of fats in vital phenomena. Lancet 1925, 205, 853–856. 10.1016/S0140-6736(01)22310-1. [DOI] [Google Scholar]
- Stottrup B. L.; Keller S. L. Phase behavior of lipid monolayers containing dppc and cholesterol analogs. Biophys. J. 2006, 90, 3176–3183. 10.1529/biophysj.105.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L. R.; Smith B. A.; McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc. Natl. Acad. Sci. U. S. A. 1979, 76, 15–18. 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K.; Ikonen E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Brown D. A.; London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes?. Biochem. Biophys. Res. Commun. 1997, 240, 1–7. 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- Regen S. L. The origin of lipid rafts. Biochemistry 2020, 59, 4617–4621. 10.1021/acs.biochem.0c00851. [DOI] [PubMed] [Google Scholar]
- Huang J.; Feigenson G. W. A macroscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 1999, 76, 2142–2157. 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. Exploration of molecular interactions in cholesterol superlattices: effect of multibody interactions. Biophys. J. 2002, 83, 1014–1025. 10.1016/S0006-3495(02)75227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills T. T.; Toombes G. E. S.; Tristram-Nagle S.; Smilgies D.-M.; Feigenson G. W.; Nagle J. F. Order parameters and areas in fluid-phase oriented membranes using wide angle x-ray scattering. Biophys. J. 2008, 95, 669–681. 10.1529/biophysj.107.127845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer F.; Smit B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 3654–3658. 10.1073/pnas.0809959106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J.; Alwarawrah M.; Huang J. Instability of cholesterol clusters in lipid bilayers and the cholesterol’s umbrella effect. J. Phys. Chem. B 2010, 114, 840–848. 10.1021/jp909061h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett M. A.; Zheng S.; Toppozini L. A.; Alsop R. J.; Dies H.; Wang A.; Jago N.; Moore M.; Rheinstadter M. C. Solubility of cholesterol in lipid membranes and the formation of immiscible cholesterol plaques at high cholesterol concentrations. Soft Matter 2013, 9, 9342–9351. 10.1039/c3sm50700a. [DOI] [Google Scholar]
- Kurniawan J.; Yin N.-N.; Liu G-y.; Kuhl T. L. Interaction forces between ternary lipid bilayers containing cholesterol. Langmuir 2014, 30, 4997–5004. 10.1021/la500341c. [DOI] [PubMed] [Google Scholar]
- Leeb F.; Maibaum L. Spatially resolving the condensing effect of cholesterol in lipid bilayers. Biophys. J. 2018, 115, 2179–2188. 10.1016/j.bpj.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannock D. A.; Lewis R. N. A. H.; McElhaney R. N. Comparative calorimetric and spectroscopic studies of the effects of lanosterol and cholesterol on the thermotropic phase behavior and organization of dipalmitoylphosphatidylcholine bilayer membranes. Biophys. J. 2006, 91, 3327–3340. 10.1529/biophysj.106.084368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit S. A.; Jakobsson E.; Scott H. L. Simulation of the early stages of nano-domain formation in mixed bilayers of sphingomyelin, cholesterol, and dioleylphosphatidylcholine. Biophys. J. 2004, 87, 3312–3322. 10.1529/biophysj.104.046078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog T.; Pasenkiewicz-Gierula M. Non-polar interactions between cholesterol and phospholipids: a molecular dynamics simulation study. Biophys. Chem. 2004, 107, 151–164. 10.1016/j.bpc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Rog T.; Pasenkiewicz-Gierula M.; Vattulainen I.; Karttunen M. What happens if cholesterol is made smoother: importance of methyl substituents in cholesterol ring structure on phosphatidylcho- line-sterol interaction. Biophys. J. 2007, 92, 3346–3357. 10.1529/biophysj.106.095497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyry S.; Rog T.; Karttunen M.; Vattulainen I. Significance of cholesterol methyl groups. J. Phys. Chem. B 2008, 112, 2922–2929. 10.1021/jp7100495. [DOI] [PubMed] [Google Scholar]
- Demel R. A.; Van Deenen L. L. M.; Pethica B. A. Monolayer interactions of phospholipids and cholesterol. Biochim. Biophys. Acta, Biomembr. 1967, 135, 11–19. 10.1016/0005-2736(67)90003-X. [DOI] [Google Scholar]
- Sugahara M.; Uragami M.; Yan X.; Regen S. L. The structural role of cholesterol in biological membranes. J. Am. Chem. Soc. 2001, 123, 7939–7940. 10.1021/ja016199c. [DOI] [PubMed] [Google Scholar]
- Daly T. A.; Wang M.; Regen S. L. The origin of cholesterol’s condensing effect. Langmuir 2011, 27, 2159–2161. 10.1021/la105039q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida P. F. Thermodynamics of lipid interactions in complex bilayers. Biochim. Biophys. Acta, Biomembr. 2009, 1788, 72–85. 10.1016/j.bbamem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Czech B.; Singh A.; Regen S. L. Polymerized vesicles. J. Am. Chem. Soc. 1980, 102, 6638–6640. 10.1021/ja00541a078. [DOI] [Google Scholar]
- Regen S. L.; Yamaguchi K.; Samuel N. K. P.; Singh M. Polymerized-depolymerized vesicles: a reversible phosphatidylcholine-based membrane. J. Am. Chem. Soc. 1983, 105, 6354–6355. 10.1021/ja00358a050. [DOI] [Google Scholar]
- Samuel N. K. P.; Singh M. M.; Yamaguchi K.; Regen S. L. Polymerized-depolymerized vesicles: reversible thiol-disulfide-based phosphatidylcholine membranes. J. Am. Chem. Soc. 1985, 107, 42–47. 10.1021/ja00287a008. [DOI] [Google Scholar]
- Melchior D. L. Lipid domains in fluid membranes: a quick freeze differential scanning calorimetry study. Science 1986, 234, 1577–1580. 10.1126/science.3787264. [DOI] [PubMed] [Google Scholar]
- Krisovitch S. M.; Regen S. L. Nearest-neighbor recognition in phospholipid bilayers: probing lateral organization at the molecular level. J. Am. Chem. Soc. 1991, 113, 8175–8177. 10.1021/ja00021a058. [DOI] [Google Scholar]
- Krisovitch S. M.; Regen S. L. Nearest-neighbor recognition in phospholipid membranes: a molecular-level approach to the study of membrane suprastructure. J. Am. Chem. Soc. 1992, 114, 9828–9835. 10.1021/ja00051a015. [DOI] [Google Scholar]
- Krisovitch S. M.; Regen S. L. Cholesterol-induced nearest-neighbor recognition in a fluid phospholipid membrane. J. Am. Chem. Soc. 1993, 115, 1198–1199. 10.1021/ja00056a083. [DOI] [Google Scholar]
- Davidson S. M. K.; Liu Y.; Regen S. L. The influence of cholesterol on nearest-neighbor recognition in saturated phospholipid membranes. J. Am. Chem. Soc. 1993, 115, 10104–10110. 10.1021/ja00075a028. [DOI] [Google Scholar]
- Cao H.; Zhang J.; Jing B.; Regen S. L. A chemical sensor for the liquid-ordered phase. J. Am. Chem. Soc. 2005, 127, 8813–8816. 10.1021/ja0513988. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B.; Thompson T. E. Cholesterol-induced fluid-phase immiscibility in membranes. Proc. Natl. Acad. Sci. U. S. A. 1991, 88, 8686–8690. 10.1073/pnas.88.19.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier M. L.; Wright J. R.; Pokorny A.; Almeida P. F. F. Investigation of domain formation in sphingomyelin/cholesterol/popc mixtures by fluorescence resonance energy transfer and monte carlo simulations. Biophys. J. 2007, 92, 2422–2433. 10.1529/biophysj.106.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydock-McGrane L.; Rath N. P.; Covey D. F. The synthesis of a “smoothened” cholesterol: 18,19-di-norcholesterol. J. Org. Chem. 2014, 79, 5636–5643. 10.1021/jo500813n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M. R.; Wang M.; Mydock-McGrane L.; Covey D. F.; Tejada E.; Almeida P. F.; Regen S. L. Eliminating the roughness of cholesterol’s β-face: does it matter?. Langmuir 2014, 30, 12114–12118. 10.1021/la503075e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A.; McConnell H. M. Cholesterol-phospholipid complexes in membranes. J. Am. Chem. Soc. 1999, 121, 486–487. 10.1021/ja9835537. [DOI] [Google Scholar]
- Radhakrishnan A.; McConnell H. M. Condensed complexes of cholesterol and phospholipids. Biophys. J. 1999, 77, 1507–1517. 10.1016/S0006-3495(99)76998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. L.; Radhakrishnan A.; McConnell H. M. Saturated phospholipids with high melting temperatures form complexes with cholesterol in monolayers. J. Phys. Chem. B 2000, 104, 7522–7527. 10.1021/jp000958g. [DOI] [Google Scholar]
- McConnell H. M.; Radhakrishnan A. Condensed complexes of cholesterol and phospholipids. Biochim. Biophys. Acta, Biomembr. 2003, 1610, 159–173. 10.1016/S0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- Hung W.-C.; Lee M.-T.; Chen F.-Y.; Huang H. W. The condensing effect of cholesterol in lipid bilayers. Biophys. J. 2007, 92, 3960–3967. 10.1529/biophysj.106.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Y.; Tabei S. M.; Ye J.; Steck T. L. Stability and stoichiometry of bilayer phospholipid-cholesterol complexes: relationship to cellular sterol distribution and homeostasis. Biochemistry 2013, 52, 6950–6959. 10.1021/bi400862q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Cao H.; Jing B.; Almeida P. F.; Regen S. L. Cholesterol-phospholipid association in fluid bilayers: a thermodynamic analysis from nearest-neighbor recognition measurements. Biophys. J. 2006, 91, 1402–1406. 10.1529/biophysj.106.084152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setny P.; Baron R.; McCammon J. A. How can hydrophobic association be enthalpy driven?. J. Chem. Theory Comput. 2010, 6, 2866–2871. 10.1021/ct1003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida P. F.; et al. Heat capacity of dppc/cholesterol mixtures: comparison of single bilayers with multibilayers and simulations. Langmuir 2018, 34, 9798–9809. 10.1021/acs.langmuir.8b01774. [DOI] [PubMed] [Google Scholar]
- Almeida P. F. How to determine lipid interactions in membranes from experiment through the ising model. Langmuir 2019, 35, 21–40. 10.1021/acs.langmuir.8b03054. [DOI] [PubMed] [Google Scholar]
- Janout V.; Turkyilmaz S.; Wang M.; Wang Y.; Manaka Y.; Regen S. L. An upside down view of cholesterol’s condensing effect: does surface occupancy play a role?. Langmuir 2010, 26, 5316–5318. 10.1021/la100878s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M. R.; Turkyilmaz S.; Regen S. L. Surface occupancy plays a major role in cholestserol’s condensing effect. Langmuir 2013, 29, 10303–10306. 10.1021/la402263w. [DOI] [PubMed] [Google Scholar]