Abstract
Objective
To investigate whether vitamin D and marine derived long chain omega 3 fatty acids reduce autoimmune disease risk.
Design
Vitamin D and omega 3 trial (VITAL), a nationwide, randomized, double blind, placebo controlled trial with a two-by-two factorial design.
Setting
Nationwide in the United States.
Participants
25 871 participants, consisting of 12 786 men ≥50 years and 13 085 women ≥55 years at enrollment.
Interventions
Vitamin D (2000 IU/day) or matched placebo, and omega 3 fatty acids (1000 mg/day) or matched placebo. Participants self-reported all incident autoimmune diseases from baseline to a median of 5.3 years of follow-up; these diseases were confirmed by extensive medical record review. Cox proportional hazard models were used to test the effects of vitamin D and omega 3 fatty acids on autoimmune disease incidence.
Main outcome measures
The primary endpoint was all incident autoimmune diseases confirmed by medical record review: rheumatoid arthritis, polymyalgia rheumatica, autoimmune thyroid disease, psoriasis, and all others.
Results
25 871 participants were enrolled and followed for a median of 5.3 years. 18 046 self-identified as non-Hispanic white, 5106 as black, and 2152 as other racial and ethnic groups. The mean age was 67.1 years. For the vitamin D arm, 123 participants in the treatment group and 155 in the placebo group had a confirmed autoimmune disease (hazard ratio 0.78, 95% confidence interval 0.61 to 0.99, P=0.05). In the omega 3 fatty acids arm, 130 participants in the treatment group and 148 in the placebo group had a confirmed autoimmune disease (0.85, 0.67 to 1.08, P=0.19). Compared with the reference arm (vitamin D placebo and omega 3 fatty acid placebo; 88 with confirmed autoimmune disease), 63 participants who received vitamin D and omega 3 fatty acids (0.69, 0.49 to 0.96), 60 who received only vitamin D (0.68, 0.48 to 0.94), and 67 who received only omega 3 fatty acids (0.74, 0.54 to 1.03) had confirmed autoimmune disease.
Conclusions
Vitamin D supplementation for five years, with or without omega 3 fatty acids, reduced autoimmune disease by 22%, while omega 3 fatty acid supplementation with or without vitamin D reduced the autoimmune disease rate by 15% (not statistically significant). Both treatment arms showed larger effects than the reference arm (vitamin D placebo and omega 3 fatty acid placebo).
Study registration
ClinicalTrials.gov NCT01351805 and NCT01169259
Introduction
Autoimmune diseases, characterized by an inflammatory autoimmune response to self-tissues, are the third leading cause of morbidity in the industrialized world and a leading cause of mortality among women.1 2 Autoimmune diseases are chronic conditions with increasing prevalence with age and major societal and economic burdens due to a lack of effective treatments.3 4
Vitamin D and marine derived, long chain omega 3 fatty acids are two nutritional supplements investigated as potential autoimmune disease treatments. In vitro, the lipid soluble active form of vitamin D (1,25-hydroxyvitamin D) regulates genes involved in inflammation and acquired and innate immune responses.5 Animal models of autoimmune disease have reported vitamin D to be beneficial because it inhibits the development or progression of disease,5 6 7 8 but observational studies have found conflicting results9 10 11 12; small trials of vitamin D supplementation in people with established autoimmune disease have mainly reported disappointing results.13 14 Whether vitamin D supplementation can prevent autoimmune disease onset is still unknown and has not been tested in clinical trials. Randomized controlled trials of people with prevalent rheumatoid arthritis, systemic lupus erythematosus,15 and psoriasis16 have also shown improvements in outcomes with omega 3 fatty acids, but few studies have examined omega 3 fatty acids in autoimmune disease prevention. A Danish observational study found a 49% reduction in rheumatoid arthritis risk for each 30 g increase in daily fatty fish intake (≥8 g fat/100 g fish).17 However, randomized controlled trials examining omega 3 fatty acid intake and autoimmune disease risk are lacking.
We report the effects of vitamin D and omega 3 fatty acid supplementation on autoimmune disease incidence (including rheumatoid arthritis, polymyalgia rheumatica, autoimmune thyroid disease, and psoriasis) within the large scale vitamin D and omega 3 trial (VITAL) over approximately five years of randomized follow-up. We assessed whether the effects differed by age, sex, race, body mass index, and by baseline concentrations of vitamin D, or by eicosapentaenoic acid plus docosahexaenoic acid or dietary fish intake.
Methods
Trial design and oversight
This randomized, double blind, placebo controlled, two-by-two factorial design trial was conducted to examine the benefits and risks of vitamin D (cholecalciferol; 2000 IU/day) and marine omega 3 fatty acids (1 g/day as a fish oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) in the prevention of cancer and cardiovascular disease among 25 871 participants (men aged ≥50 years; women aged ≥55 years; NCT 01169259). Aggregate incident autoimmune disease was a prespecified endpoint of a funded ancillary study started before trial recruitment (NCT01351805). Trial protocol, oversight, and CONSORT diagram (consolidated standards of reporting trials; supplementary fig 1), as adhered to in this study, have been previously published.18 This trial did not intend to examine vitamin D supplementation in a population that was vitamin D deficient, but in participants representative of vitamin D levels in other large trials and in the general older adult population in the United States.
Eligible participants, recruited throughout the US, were required to limit vitamin D use from outside sources to no more than 800 IU/day, and to forego the use of fish oil supplements. At trial entry, those with a history of renal failure or dialysis, cirrhosis, hypercalcemia, cancer (except non-melanoma skin cancer), cardiovascular disease, or other serious illness were ineligible. A total of 25 871 people consented to enrollment; 5106 were black and 2152 were other racial and ethnic groups (non-white). These participants successfully completed a three month placebo run-in period and were randomized to treatment (vitamin D, n=12 927; omega 3 fatty acids, n=12 933) or placebo arms (vitamin D placebo, n=12 944; omega 3 fatty acid placebo, n=12 938) within sex, race, and five year age groups in blocks of eight. Randomization occurred between November 2011 and March 2014, and the intervention was completed as prespecified after five years of randomized assignment in December 2017.19
Baseline questionnaires collected data on clinical and lifestyle risk factors, and queried vitamin D supplement use and fish and dairy intake (supplement 1). Blood samples, obtained at baseline from all willing participants (n=16 956), were assayed for 25-hydroxyvitamin D and plasma omega 3 index (eicosapentaenoic acid plus docosahexaenoic acid as percentage of total fatty acids; Quest Diagnostics, liquid chromatography tandem mass spectrometry). Questionnaires were completed six months and one year after randomization, and then annually. These questionnaires asked about trial supplement adherence, new doctor diagnosed diseases, potential side effects of trial agents, and new cancer or cardiovascular disease risk factors. Calendar packs containing trial capsules (similar in appearance) were mailed to participants with the questionnaires. The vitamin D pill and its matching placebo, which contained soybean oil, were prepared by Pharmavite LLC (Northridge, California, USA). The omega 3 fatty acid pill and its matching placebo, which contained olive oil, were prepared by Pronova BioPharma (Norway).
The questionnaire response rate averaged 93.1%, and follow-up about mortality was greater than 98%.20 Adherence to the trial regimen (percentage of participants who took at least two thirds of trial capsules) averaged 81%. Blood samples from a subgroup at one year found mean 25-hydroxyvitamin D levels (n=1644) increased by 40% (from 29.8 ng/ml at baseline to 41.8 ng/ml at one year) in the vitamin D group and changed minimally in the placebo group; the mean omega 3 index (n=1583) increased 54.7% (to 4.1% at one year in the omega 3 group) and changed by less than 2% in the placebo group. The trial was approved by the institutional review board of Partners’ HealthCare and was monitored by an external data and safety monitoring board.
Autoimmune disease endpoints
The primary endpoint was total confirmed autoimmune disease incidence. Annual questionnaires inquired about new onset doctor diagnosed rheumatoid arthritis, polymyalgia rheumatica, autoimmune thyroid disease, psoriasis, and inflammatory bowel disease, with space to write in all other new onset autoimmune diseases. Participants who reported a new incident autoimmune disease were asked to sign a release for medical records. Two trained physicians (including a board certified rheumatologist, endocrinologist, and gastroenterologist), blinded to treatment assignment, reviewed each record and confirmed or disconfirmed the autoimmune disease according to classification criteria when available. For autoimmune thyroid disease in particular, insufficient medical record documentation, often consisting of a doctor’s diagnosis of Hashimoto’s thyroiditis or Graves’ disease and abnormal thyroid function tests without confirmatory studies, led to an inability to classify these participants as having confirmed disease according to our rigorous criteria. We classified participants with evidence of incident autoimmune disease, but insufficient documentation for certainty, as having probable autoimmune disease; these participants were added to those with confirmed autoimmune disease for secondary endpoints.
Date of first symptoms attributed to the autoimmune disease and date of doctor’s diagnosis were recorded from the medical records. New onset autoimmune disease was not confirmed if the disease was diagnosed or onset of its first symptoms occurred before randomization. Deaths were confirmed by review of medical records and death certificates, as previously reported.18
Statistical analyses
Analyses were based on the intention-to-treat principle. In a priori power calculations based on the log rank test, we calculated that the trial sample size would have at least 80% power to detect a 30% rate reduction using the projected incidence of validated composite autoimmune disease over five years. We conducted t tests or χ2 tests to compare baseline characteristics of participants randomized to supplementation or placebo. For our primary analyses, we compared the separate main effects of vitamin D or omega 3 fatty acid supplement assignment on autoimmune disease incidence by using Cox regression models. To account for randomization stratification and study design,21 we additionally adjusted for age, sex, self-reported race, and randomization to the other supplement. Person time was counted until diagnosis of a new confirmed autoimmune disease, death, or the end of the trial. Because autoimmune diseases develop slowly over time,22 we examined our a priori interest in whether effects varied over time by using cumulative incidence plots; we also ran models including linear and quadratic interactions with time and conducted analyses of the primary outcomes excluding events that occurred during the first two years. Additionally, we assessed hazard ratio by year of study (supplementary table 1).
To assess for synergistic effects of supplementation with omega 3 fatty acids and vitamin D, specified a priori, we examined four group cumulative incidence curves, added an interaction term for treatment with vitamin D and omega 3 fatty acids to models, and repeated Cox models with the vitamin D placebo/omega 3 fatty acid placebo group as the reference arm compared with the three intervention arms.
We assessed the effects of treatment on individual disease endpoints (rheumatoid arthritis, polymyalgia rheumatica, autoimmune thyroid disease, and psoriasis) and grouped all other autoimmune diseases as an additional endpoint of other autoimmune diseases (supplementary table 2). Because people with an existing autoimmune disease are at high genetic risk of developing a new autoimmune disease, for each autoimmune disease endpoint we included validated reports of diagnoses of another autoimmune disease (eg, those with autoimmune thyroid disease at baseline were followed for other incident autoimmune disease). We ran models including interaction terms between treatment and the variable of interest to test whether the effect of treatment on incident autoimmune disease varied by age, sex, race, randomization to the other arm of the trial, baseline body mass index, family history of autoimmune disease, baseline blood levels of 25-hydroxyvitamin D or vitamin D intake (for the vitamin D arm), or baseline blood levels of eicosapentaenoic acid plus docosahexaenoic acid (omega 3 fatty acid arm). In addition to prespecified dichotomized subgroup analyses, for the continuous variables, such as age and body mass index, we ran models including linear and quadratic interaction terms between treatment and the variable of interest. To test the sensitivity of results to our strict definition of autoimmune disease, we ran models with all probable and definite autoimmune disease as an endpoint. In other preplanned sensitivity analyses, we ran models in which we excluded all participants who reported any autoimmune disease at baseline. Data analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina, USA).
Patient and public involvement
This was a randomized controlled trial inspired by physicians’ awareness of limited choices for treating patients with autoimmune disease, and desire by patients for effective treatments. Patients and the public were not further involved in the design or conduct of this double blind trial.
Results
Baseline characteristics of the 25 871 participants were balanced between treatment and placebo groups (table 1; details of the cohort given by Manson and colleagues20). Fifty one per cent were women; mean age was 67.1 years. The racially diverse cohort consisted of 71% who self-identified as non-Hispanic white, 20% black, and 9% other racial or ethnic groups. A total of 4555 participants (18%) reported at least one autoimmune disease before randomization. Numbers of deaths and participants who reported side effects were low, as previously reported.19
Table 1.
Characteristics of VITAL trial participants at baseline according to randomized assignment to active supplementation (vitamin D or omega 3 fatty acids) or placebo. Data are numbers (%) unless indicated otherwise
| Characteristic | Total (n=25 871) | Vitamin D | Omega 3 fatty acids | |||
|---|---|---|---|---|---|---|
| Treatment group (n=12 927) | Placebo group (n=12 944) | Treatment group (n=12 933) | Placebo group (n=12 938) | |||
| Female sex | 13 085 (50.6) | 6547 (50.7) | 6538 (50.5) | 6547 (50.7) | 6538 (50.5) | |
| Age (years), mean (SD) | 67.1 (7.1) | 67.1 (7.1) | 67.1 (7.1) | 67.2 (7.1) | 67.1 (7.1) | |
| Race or ethnic group* | ||||||
| Non-Hispanic white | 18 046 (71.3) | 9013 (71.3) | 9033 (71.4) | 9044 (71.5) | 9002 (71.2) | |
| Black | 5106 (20.2) | 2553 (20.2) | 2553 (20.2) | 2549 (20.2) | 2557 (20.2) | |
| Other | 2152 (8.5) | 1081 (8.6) | 1071 (8.5) | 1060 (8.4) | 1092 (8.6) | |
| Geographical region of residence | ||||||
| North east | 7161 (27.7) | 3603 (27.9) | 3558 (27.5) | 3544 (27.4) | 3617 (28) | |
| Midwest | 5541 (21.4) | 2774 (21.5) | 2767 (21.4) | 2790 (21.6) | 2751 (21.3) | |
| West | 5926 (22.9) | 2935 (22.7) | 2991 (23.1) | 2993 (23.1) | 2933 (22.7) | |
| South east | 7242 (28.0) | 3615 (28.0) | 3627 (28.0) | 3605 (27.9) | 3637 (28.1) | |
| Highest level of education | ||||||
| ≤High school | 3304 (12.8) | 1650 (12.8) | 1654 (12.8) | 1683 (13) | 1621 (12.6) | |
| ≥High school | 22 514 (87.2) | 11 252 (87.2) | 11 262 (87.2) | 11226 (87) | 11288 (87.4) | |
| Self-reported annual income ($)† | ||||||
| <50 000 | 8523 (36.6) | 4293 (36.9) | 4230 (36.4) | 4238 (36.4) | 4285 (36.9) | |
| ≥50 000 | 14 750 (63.4) | 7344 (63.1) | 7406 (63.6) | 7405 (63.6) | 7345 (63.2) | |
| Current supplemental vitamin D use ≤800 IU/day | 11 030 (42.6) | 5497 (42.5) | 5533 (42.7) | 5498 (42.5) | 5533 (42.7) | |
| Serum 25-hydroxyvitamin D, mean (SD) | 30.7 (10) | 30.7 (10) | 30.7 (10) | 30.7 (10) | 30.7 (10) | |
| Serum 25-hydroxyvitamin D <20 ng/mL | 2161 (12.9) | 1049 (12.5) | 1112 (13.3) | 1072 (12.8) | 1089 (13.0) | |
| Serum 25-hydroxyvitamin D <30 ng/mL | 7646 (45.6) | 3803 (45.4) | 3843 (45.9) | 3823 (45.6) | 3823 (45.7) | |
| Omega index,‡ mean (SD) | 2.6 (0.9) | 2.6 (0.9) | 2.6 (0.9) | 2.6 (0.9) | 2.6 (0.9) | |
| Body mass index,§ mean (SD) | 28.1 (5.7) | 28.1 (5.7) | 28.1 (5.8) | 28.1 (5.7) | 28.1 (5.8) | |
| Physical activity total MET hours/week, median (IQR) | 15.4 (4.6-31.6) | 15.2 (4.5-31.5) | 15.5 (4.7-32.0) | 15.5 (4.6-31.5) | 15.4 (4.5-31.7) | |
| Current smoker | 1836 (7.2) | 921 (7.2) | 915 (7.2) | 920 (7.2) | 916 (7.2) | |
| Family history of autoimmune disease | 8168 (34.3) | 4092 (34.4) | 4076 (34.3) | 4125 (34.6) | 4043 (34.1) | |
| Randomized to active group, other arm of trial | 12 933 (50.0) | 6463 (50.0) | 6470 (50.0) | 6463 (50) | 6464 (50) | |
IQR=interquartile range; MET=metabolic equivalent; SD=standard deviation.
Percentages might not add up to 100 because of rounding. No significant differences were found in baseline characteristics between groups. Race or ethnic group: n=25 304; serum vitamin D: n=16 757; omega index: n=16 478; body mass index: n=25 254; physical activity: n=25 619; smoking: n=25 485; family history: n=23 779. Number of participants with autoimmune disease self-reported at baseline: rheumatoid arthritis, 1279; polymyalgia rheumatica, 162; psoriasis, 846; autoimmune thyroid disease, 2075; other autoimmune disease, 851.
Race and ethnic group were reported by participants.
Income data missing for 10% of participants.
Serum eicosapentaenoic acid plus docosahexaenoic acid/total lipids.
Body mass index is weight in kilograms divided by square of height in meters.
Primary endpoint
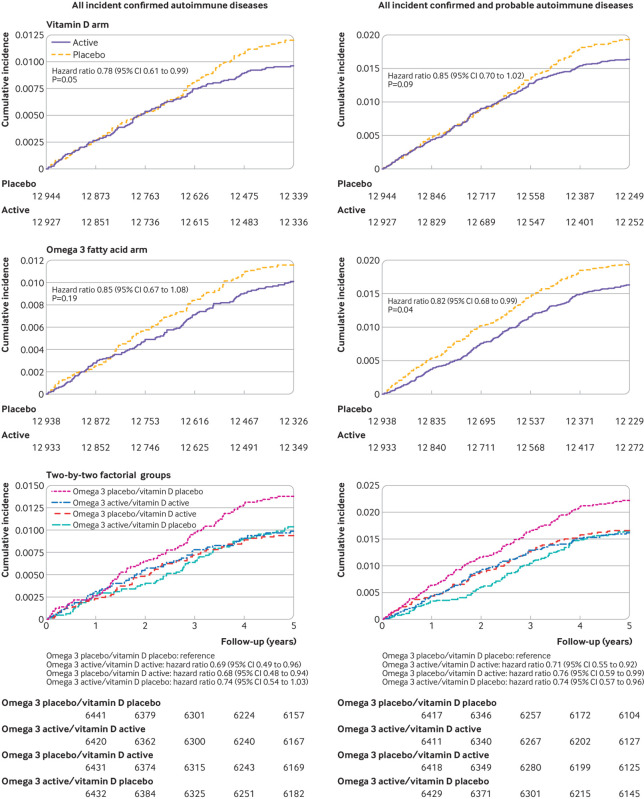
For the vitamin D arm, 123 participants in the treatment group and 155 in the placebo group had confirmed autoimmune disease (adjusted hazard ratio 0.78, 95% confidence interval 0.61 to 0.99, P=0.05; table 2). For the omega 3 fatty acid group, confirmed autoimmune disease occurred in 130 participants in the treatment group and 148 in the placebo group (0.85, 0.67 to 1.08, P=0.19; table 3). Cumulative incidence of autoimmune disease over the five years of the trial was lower for the treatment group than the placebo group in both arms of the trial (fig 1).
Table 2.
Hazard ratios and 95% confidence intervals for primary and secondary endpoints according to randomized assignment to vitamin D or placebo
| Endpoint | Vitamin D group (n=12 927) | Placebo group (n=12 944) | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|
| Primary endpoint | ||||
| Confirmed autoimmune diseases | 123 | 155 | 0.78 (0.61 to 0.99) | 0.05 |
| Secondary endpoints | ||||
| Confirmed+probable autoimmune diseases | 210 | 247 | 0.85 (0.70 to 1.02) | 0.09 |
| Analyses excluding all prerandomization autoimmune diseases | ||||
| Confirmed autoimmune diseases | 102 | 128 | 0.79 (0.61 to 1.03) | 0.08 |
| Confirmed+probable autoimmune diseases | 170 | 209 | 0.81 (0.66 to 1.00) | 0.05 |
| Analyses excluding first two years of follow-up | ||||
| Confirmed autoimmune diseases | 54 | 87 | 0.61 (0.43 to 0.86) | 0.005 |
| Confirmed+probable autoimmune diseases | 94 | 133 | 0.69 (0.53 to 0.90) | 0.007 |
| Individual autoimmune diseases | ||||
| Confirmed rheumatoid arthritis | 15 | 24 | 0.58 (0.30 to 1.13) | 0.11 |
| Confirmed+probable rheumatoid arthritis | 18 | 27 | 0.63 (0.34 to 1.15) | 0.13 |
| Confirmed polymyalgia rheumatic* | 31 | 43 | 0.70 (0.44 to 1.12) | 0.14 |
| Confirmed+probable polymyalgia rheumatica | 32 | 43 | 0.72 (0.46 to 1.15) | 0.17 |
| Confirmed autoimmune thyroid disease | 21 | 11 | 1.63 (0.77 to 3.45) | 0.20 |
| Confirmed+probable autoimmune thyroid disease | 99 | 94 | 1.05 (0.78 to 1.41) | 0.74 |
| Confirmed psoriasis† | 15 | 23 | 0.72 (0.37 to 1.39) | 0.32 |
| Confirmed+probable psoriasis | 17 | 25 | 0.70 (0.37 to 1.32) | 0.27 |
| Confirmed other autoimmune disease | 40 | 56 | 0.74 (0.49 to 1.11) | 0.15 |
| Confirmed+probable other autoimmune disease | 45 | 63 | 0.73 (0.50 to 1.08) | 0.12 |
Analyses were from Cox regression models controlled for age, sex, race, and omega 3 fatty acid randomization group.
Fourteen participants had confirmed polymyalgia rheumatica without giant cell arteritis, 18 had confirmed giant cell arteritis without polymyalgia rheumatica, and two were confirmed with both.
No participants had psoriatic arthritis.
Table 3.
Hazard ratios and 95% confidence intervals for primary and secondary endpoints according to randomized assignment to omega 3 fatty acids or placebo within VITAL trial in intention-to-treat analyses
| Endpoint | Omega 3 group (n=12 933) | Placebo group (n=12 938) | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|
| Primary endpoint | ||||
| Confirmed autoimmune disease | 130 | 148 | 0.85 (0.67 to 1.08) | 0.19 |
| Secondary endpoints | ||||
| Confirmed+probable autoimmune disease | 208 | 249 | 0.82 (0.68 to 0.99) | 0.04 |
| Analyses excluding all prerandomization autoimmune disease | ||||
| Confirmed autoimmune disease | 111 | 119 | 0.91 (0.70 to 1.18) | 0.48 |
| Confirmed+probable autoimmune disease | 180 | 199 | 0.90 (0.73 to 1.10) | 0.30 |
| Analyses excluding first two years of follow-up | ||||
| Confirmed autoimmune disease | 67 | 74 | 0.90 (0.64 to 1.26) | 0.54 |
| Confirmed+probable autoimmune disease | 110 | 117 | 0.94 (0.72 to 1.23) | 0.66 |
| Individual autoimmune diseases | ||||
| Confirmed rheumatoid arthritis | 15 | 24 | 0.58 (0.30 to 1.13) | 0.11 |
| Confirmed+probable rheumatoid arthritis | 17 | 28 | 0.57 (0.31 to 1.05) | 0.07 |
| Confirmed polymyalgia rheumatica | 34 | 40 | 0.87 (0.55 to 1.38) | 0.55 |
| Confirmed+probable polymyalgia rheumatica | 34 | 41 | 0.85 (0.54 to 1.34) | 0.48 |
| Confirmed autoimmune thyroid disease | 12 | 20 | 0.53 (0.25 to 1.14) | 0.10 |
| Confirmed+probable autoimmune thyroid disease | 85 | 108 | 0.80 (0.59 to 1.07) | 0.13 |
| Confirmed psoriasis | 23 | 15 | 1.57 (0.80 to 3.07) | 0.19 |
| Confirmed+probable psoriasis | 25 | 17 | 1.44 (0.76 to 2.72) | 0.26 |
| Confirmed other autoimmune disease | 45 | 51 | 0.84 (0.56 to 1.26) | 0.40 |
| Confirmed+probable other autoimmune disease | 48 | 60 | 0.76 (0.52 to 1.12) | 0.17 |
VITAL=vitamin D and omega 3 trial.
Analyses were from Cox regression models controlled for age, sex, race, and vitamin D randomization group.
Fig 1.
Cumulative incidence rates of total autoimmune diseases in the VITAL (vitamin D and omega 3) trial. Hazard ratios are from Cox models controlled for age, sex, race, and randomization group in the opposite arm of the trial
Preplanned analyses excluding the first two years of follow-up (n=25 499) to test the latency of treatment effects revealed a significantly lower incidence of confirmed autoimmune disease in the vitamin D group compared with the placebo group (0.61, 0.43 to 0.86, P=0.005; table 2); this was not observed in the omega 3 group (table 3). When hazard ratios were calculated for each year of the trial (supplementary table 1), although the numbers of participants with confirmed autoimmune disease in a given year were small, hazard ratios for vitamin D treatment were consistently lower in the last three years than in the first two years of the trial. However, when modeled as a linear association over the five years of the study, there was no clear statistical evidence that treatment effects varied by time for vitamin D (P for interaction=0.14) or omega 3 fatty acids (P for interaction=0.57). The nonlinear effects of time were similarly non-significant (P for interaction=0.34 for vitamin D, 0.59 for omega 3 fatty acids).
Secondary analyses
We investigated the effects across the four subgroups of this trial’s two-by-two factorial design. The cumulative incidence of confirmed autoimmune disease over the five years of the trial (fig 1) was lower in all three of the groups receiving supplementation (vitamin D and omega 3 fatty acid; vitamin D and omega 3 fatty acid placebo; vitamin D placebo and omega 3 fatty acid) than in the group receiving vitamin D placebo and omega 3 fatty acid placebo (log rank P=0.08). In a Cox model adjusted for age, sex, and race, with a separate term for each group (vitamin D placebo and omega 3 fatty acid placebo as the reference group), the incidence of confirmed autoimmune disease was lower among those randomized to vitamin D with omega 3 fatty acids (hazard ratio 0.69, 95% confidence interval 0.49 to 0.96) or without omega 3 fatty acids (0.68, 0.48 to 0.94) compared with those who received only placebo (table 4). For omega 3 fatty acids alone, the benefit was marginally significant (0.74, 0.54 to 1.03). A test of multiplicative interaction between the two treatments was not statistically significant (P=0.20).
Table 4.
Hazard ratios and 95% confidence intervals for primary and secondary endpoints according to two-by-two randomized assignment to vitamin D, omega 3 fatty acids, and placebo
| Endpoint | Hazard ratio (95% CI)* | P value |
|---|---|---|
| Primary endpoint: definite autoimmune diseases | ||
| Vitamin D active/omega 3 active | 0.69 (0.49 to 0.96) | 0.03 |
| Vitamin D active/omega 3 placebo | 0.68 (0.48 to 0.94) | 0.02 |
| Vitamin D placebo/omega 3 active | 0.74 (0.54 to 1.03) | 0.07 |
| All probable autoimmune diseases | ||
| Vitamin D active/omega 3 active | 0.71 (0.55 to 0.92) | 0.01 |
| Vitamin D active/omega 3 placebo | 0.76 (0.59 to 0.99) | 0.04 |
| Vitamin D placebo/omega 3 active | 0.74 (0.57 to 0.96) | 0.02 |
| Definite rheumatoid arthritis | ||
| Vitamin D active/omega 3 active | 0.23 (0.07 to 0.81) | 0.02 |
| Vitamin D active/omega 3 placebo | 0.85 (0.38 to 1.89) | 0.69 |
| Vitamin D placebo/omega 3 active | 0.85 (0.38 to 1.89) | 0.69 |
| All probable rheumatoid arthritis | ||
| Vitamin D active/omega 3 active | 0.27 (0.09 to 0.80) | 0.02 |
| Vitamin D active/omega 3 placebo | 0.87 (0.41 to 1.83) | 0.71 |
| Vitamin D placebo/omega 3 active | 0.80 (0.38 to 1.71) | 0.57 |
| Definite polymyalgia rheumatica | ||
| Vitamin D active/omega 3 active | 0.67 (0.37 to 1.21) | 0.19 |
| Vitamin D active/omega 3 placebo | 0.44 (0.23 to 0.88) | 0.02 |
| Vitamin D placebo/omega 3 active | 0.59 (0.32 to 1.09) | 0.09 |
| All probable polymyalgia rheumatica | ||
| Vitamin D active/omega 3 active | 0.67 (0.37 to 1.21) | 0.18 |
| Vitamin D active/omega 3 placebo | 0.48 (0.25 to 0.93) | 0.03 |
| Vitamin D placebo/omega 3 active | 0.59 (0.32 to 1.09) | 0.09 |
| Definite autoimmune thyroid disease | ||
| Vitamin D active/omega 3 active | 0.89 (0.34 to 2.31) | 0.81 |
| Vitamin D active/omega 3 placebo | 1.11 (0.45 to 2.73) | 0.82 |
| Vitamin D placebo/omega 3 active | 0.23 (0.05 to 1.05) | 0.06 |
| All probable autoimmune thyroid disease | ||
| Vitamin D active/omega 3 active | 0.84 (0.56 to 1.27) | 0.41 |
| Vitamin D active/omega 3 placebo | 1.00 (0.67 to 1.48) | 0.99 |
| Vitamin D placebo/omega 3 active | 0.75 (0.49 to 1.14) | 0.18 |
| Definite psoriasis | ||
| Vitamin D active/omega 3 active | 1.13 (0.44 to 2.93) | 0.80 |
| Vitamin D active/omega 3 placebo | 0.76 (0.26 to 2.18) | 0.61 |
| Vitamin D placebo/omega 3 active | 1.63 (0.68 to 3.94) | 0.28 |
| All probable psoriasis | ||
| Vitamin D active/omega 3 active | 1.00 (0.42 to 2.41) | 0.99 |
| Vitamin D active/omega 3 placebo | 0.61 (0.22 to 1.67) | 0.33 |
| Vitamin D placebo/omega 3 active | 1.31 (0.57 to 2.98) | 0.53 |
| Definite other autoimmune disease | ||
| Vitamin D active/omega 3 active | 0.64 (0.37 to 1.13) | 0.13 |
| Vitamin D active/omega 3 placebo | 0.61 (0.35 to 1.09) | 0.09 |
| Vitamin D placebo/omega 3 active | 0.71 (0.41 to 1.22) | 0.22 |
| All probable other autoimmune disease | ||
| Vitamin D active/omega 3 active | 0.58 (0.34 to 1.00) | 0.05 |
| Vitamin D active/omega 3 placebo | 0.64 (0.38 to 1.08) | 0.09 |
| Vitamin D placebo/omega 3 active | 0.67 (0.40 to 1.11) | 0.12 |
Comparison group: vitamin D placebo/omega 3 fatty acids placebo. Data from Cox models adjusted for age, sex, and race.
Compared with placebo/placebo reference group.
In preplanned secondary analyses of individual autoimmune diseases, for both vitamin D and omega 3 supplementation, hazard ratios were less than 1 (favoring supplementation) for almost all diseases; however, none of the differences was statistically significant for the individual disorders (table 2, table 3). When participants with probable autoimmune disease were also included, 210 in the vitamin D arm and 247 in the vitamin D placebo arm developed definite or probable autoimmune disease (0.85, 0.70 to 1.02, P=0.09); while 208 in the omega 3 fatty acid arm and 249 in the omega 3 fatty acid placebo arm developed confirmed or probable autoimmune disease (0.82, 0.68 to 0.99, P=0.04). When participants with probable autoimmune disease were included, there was a significant interaction of omega 3 fatty acid treatment with time (P for interaction=0.04), with an apparent increase in effect over time as seen in figure 1. When participants who had reported any other autoimmune disease at baseline were excluded, hazard ratios changed only slightly (table 2, table 3).
Results of prespecified subgroup analyses for confirmed autoimmune disease suggested that people with lower body mass index seem to benefit more from vitamin D treatment (P for interaction=0.02). For example, when we modeled body mass index as a continuous linear term because we found no evidence for nonlinear interactions, for vitamin D treatment versus placebo the hazard ratio was 0.47 (95% confidence interval 0.29 to 0.77) for those with a body mass index of 18, 0.69 (0.52 to 0.90) for those with a body mass index of 25, and 0.90 (0.69 to 1.19) for those with a body mass index of 30. When we stratified by categories of body mass index, for vitamin D treatment versus placebo the hazard ratio was 0.62 (0.42 to 0.93) for body mass index <25, 0.92 (0.61 to 1.38) for body mass index 25-30, and 0.88 (0.54 to 1.44) for body mass index ≥30. The beneficial effect of omega 3 fatty acids on autoimmune disease prevention was greater among those with a family history of autoimmune disease (0.66, 0.43 to 0.99) compared with those with no family history (1.14, 0.82 to 1.58; P for interaction 0.03; supplementary fig 3). All other tested interactions were statistically non-significant (supplementary figs 2 and 3; supplementary table 3).
Discussion
Principal findings
In this large primary prevention trial in diverse older Americans, supplementation with vitamin D at a dose of 2000 IU/day for approximately five years, alone or in combination with 1 g/day of omega 3 fatty acids (460 mg eicosapentaenoic acid and 380 mg docosahexaenoic acid) led to a lower incidence of confirmed autoimmune disease than placebo. Supplementation with omega 3 fatty acids alone did not significantly lower incidence of autoimmune disease. However, when participants with probable autoimmune disease were included, omega 3 fatty acid supplementation did reduce the rate by 18% compared with placebo and a significant interaction was found with time, pointing to an increased effect after longer duration of supplementation. When only the last three years of the intervention were considered, the vitamin D group had 39% fewer participants with confirmed autoimmune disease than the placebo group (P=0.005); while the omega 3 fatty acid group had 10% fewer participants with confirmed autoimmune disease than the placebo group (P=0.54). In this two-by-two trial, supplementation with both vitamin D and omega 3 fatty acids decreased autoimmune disease by about 30% versus placebo alone. Numbers of participants with individual autoimmune diseases were generally fewer in the treatment groups than in the placebo groups; autoimmune thyroid disease (the most challenging to confirm using medical records) and psoriasis were exceptions to this pattern. These individual differences were not statistically significant, perhaps because of the small numbers of participants with individual diseases. Rheumatoid arthritis incidence was approximately 40% lower in the supplementation groups than in the placebo groups, although <40 participants were reported to have definite disease. Following trial participants for a longer period of time will clarify whether these rate reductions persist.
Potential mechanisms and comparison with other studies
Preclinical studies provide several plausible mechanisms for how these supplements might reduce autoimmune disease incidence. Binding to the vitamin D receptor, the vitamin D metabolite 1,25-dihydroxyvitamin D regulates an array of genes, many involved in inflammation and acquired and innate immune responses.23 Vitamin D receptors are found at high density on dendritic cells, T and B lymphocytes and macrophages, whose functions are dramatically affected by activated 1,25-dihydroxyvitamin D binding.24 1,25-dihydroxyvitamin D inhibits expression of interleukin 2 (IL-2), an important growth factor for T lymphocytes, and suppresses T helper 1 cytokines IL-12, interferon γ, and tumor necrosis factor (TNF), while increasing IL-4, IL-5, and IL-10.23 The addition of 1,25-dihydroxyvitamin D to CD4+ T cells also inhibits inflammatory IL-6, an important factor stimulating T helper 17 cells, which play a role in autoimmune disease development.25 1,25-dihydroxyvitamin D inhibits B cell autoantibody production and promotes monocyte differentiation into macrophages, suppressing inflammatory cytokines and chemokines, and reducing antigen presentation capacity by decreasing major histocompatibility complex II expression.26 27 1,25-dihydroxyvitamin D might also increase the production of anti-inflammatory regulatory T cells.28
Animal and in vitro studies indicate that increased dietary intake of eicosapentaenoic acid and docosahexaenoic acid inhibit production of C reactive protein and inflammatory cytokines such as TNFα, IL-1β, and IL-615 29; decrease T cell proliferation and activation30; and serve as substrate for specialized pro-resolving lipid mediators, including resolvins, protectins, and maresins, which promote resolution of inflammation.31 32 33 A substudy of 1561 VITAL participants assessing concentration changes in systemic inflammation biomarkers (IL-6, TNF receptor 2, and high sensitivity C reactive protein) from baseline to one year found no evidence of reductions over the first year.34 Results of other human studies of omega 3 fatty acids and inflammatory proteins are mixed.29 35 36
The observation that people with lower body mass index seem to benefit more from vitamin D supplementation has been made before.37 One potential mechanism might be the dilution effects of body fat, in that vitamin D is fat soluble and can be sequestered in fat cells. However, the D2d study38 observed a major interaction of body mass index with treatment, which did not change when treatment was 4000 IU versus 2000 IU. This finding suggests the effect is not purely dilutional. Further study of how body mass index moderates the effect of vitamin D on autoimmune disease is warranted. Our finding in secondary exploratory analyses that those with a family history of autoimmune disease appear to benefit more from omega 3 fatty acid supplementation also warrants further study because this is a higher risk group.
Strengths and limitations of this study
The strengths of this trial include a large, diverse general population sample; high rates of follow-up and adherence to the trial regimen; validated biomarkers of regimen adherence; and rigorously defined autoimmune disease endpoints. The US population is aging and increased autoantibody and autoimmune disease prevalence is reported.39 Because participants were older adults, the results might not generalize to autoimmune diseases that primarily have their onset in younger people. However, the pathogenesis of many of the specific autoimmune diseases observed (eg, rheumatoid arthritis and psoriasis) is similar in younger adults. The trial tested only one dose and formulation of each supplement. The relatively low number of participants with a confirmed diagnosis of most individual diseases, and the challenge of confirming diagnosis of autoimmune thyroid disease based on medical records, limited statistical power to detect an effect on individual disease outcomes and subgroups of a priori interest. Given the latency of autoimmune disease onset, longer follow-up could be informative, and participants are being followed in an open label extension study.
Clinical implications
This study of more than 25 000 older adults in the US provides evidence that daily supplementation with 2000 IU/day vitamin D or a combination of vitamin D and omega 3 fatty acids for five years reduces autoimmune disease incidence, with more pronounced effects found after two years of supplementation. Autoimmune diseases are a group of heterogeneous conditions with similar underlying pathogenetic mechanisms and together are associated with considerable morbidity and mortality. The clinical importance of these findings is high because these are well tolerated, non-toxic supplements, and other effective treatments to reduce the incidence of autoimmune diseases are lacking. Additionally, we saw consistent results across autoimmune diseases and increasing effects with time. We are continuing to follow participants for two years in an extension study to test the time course of this autoimmune disease reduction effect. Further trials could test these interventions in younger populations, and those with high autoimmune disease risk.
What is already known on this topic
Vitamin D regulates a wide array of genes involved in inflammation and immunity, and has been inconsistently associated with reduced risk of several autoimmune diseases in previous observational studies
Dietary marine derived long chain omega 3 fatty acids decrease systemic inflammation and ameliorate symptoms in some autoimmune diseases
Evidence is needed on whether omega 3 fatty acids lower the risk of developing autoimmune disease
What this study adds
This trial of older adults in the United States found that vitamin D and omega 3 fatty acid supplementation for five years reduced incident autoimmune disease compared with no supplementation
The clinical importance of this trial is high because these are well tolerated, non-toxic supplements, and other effective treatments to reduce the incidence of autoimmune diseases are lacking
Web extra.
Extra material supplied by authors
Web appendix 1: Supplementary tables and figures
Web appendix 2: Supplement 1
Contributors: All authors have contributed substantially to the conception or design of the work, or the acquisition, analysis or interpretation of data; drafting the work or revising it critically for important intellectual content; and final approval of the version published. All authors have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JH is the study guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by the National Institutes of Health grants R01 AR059086, U01 CA138962, R01 CA138962. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Institutes of Health for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Dissemination to participants and related patient and public communities: We disseminate study results to VITAL participants using email and newsletters. We will make great efforts to also disseminate the results to the general public, given that this was a trial in a residential population with results generalizable to older adults. These results were recently presented at the plenary session of the American College of Rheumatology Scientific Convergence 2021 (online annual meeting), and will also be disseminated through press releases.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study protocol was approved by the institutional review board at Partners HealthCare System and monitored by an external data and safety monitoring board.
Data availability statement
Data from this study might be considered for release if the appropriate institutional review board approval and publication clearances have been obtained, and a project is approved by the VITAL oversight committee.
References
- 1. Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev 2003;2:119-25. 10.1016/S1568-9972(03)00006-5 [DOI] [PubMed] [Google Scholar]
- 2. Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev 2012;11:754-65. 10.1016/j.autrev.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 3. Roberts MH, Erdei E. Comparative United States autoimmune disease rates for 2010-2016 by sex, geographic region, and race. Autoimmun Rev 2020;19:102423. 10.1016/j.autrev.2019.102423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rose NR. Prediction and prevention of autoimmune disease in the 21st century: a review and preview. Am J Epidemiol 2016;183:403-6. 10.1093/aje/kwv292 [DOI] [PubMed] [Google Scholar]
- 5. Kriegel MA, Manson JE, Costenbader KH, eds. Does vitamin D affect risk of developing autoimmune disease? A systematic review. Seminars in arthritis and rheumatism. Elsevier, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding Y, Liao W, He XJ, Xiang W. Effects of 1,25(OH)2 D3 and vitamin D receptor on peripheral CD4+ /CD8+ double-positive T lymphocytes in a mouse model of systemic lupus erythematosus. J Cell Mol Med 2017;21:975-85. 10.1111/jcmm.13037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallo D, Mortara L, Gariboldi MB, et al. Immunomodulatory effect of vitamin D and its potential role in the prevention and treatment of thyroid autoimmunity: a narrative review. J Endocrinol Invest 2020;43:413-29. 10.1007/s40618-019-01123-5 [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto E, Jørgensen TN. Immunological effects of vitamin D and their relations to autoimmunity. J Autoimmun 2019;100:7-16. 10.1016/j.jaut.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 9. Altieri B, Muscogiuri G, Barrea L, et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev Endocr Metab Disord 2017;18:335-46. 10.1007/s11154-016-9405-9 [DOI] [PubMed] [Google Scholar]
- 10. Costenbader KH, Chang SC, Laden F, Puett R, Karlson EW. Geographic variation in rheumatoid arthritis incidence among women in the United States. Arch Intern Med 2008;168:1664-70. 10.1001/archinte.168.15.1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murdaca G, Tonacci A, Negrini S, et al. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun Rev 2019;18:102350. 10.1016/j.autrev.2019.102350 [DOI] [PubMed] [Google Scholar]
- 12. Skaaby T, Husemoen LLN, Thuesen BH, Linneberg A. Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 2015;50:231-8. 10.1007/s12020-015-0547-4 [DOI] [PubMed] [Google Scholar]
- 13.Giustina A, Adler R, Binkley N, et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Reviews in Endocrine and Metabolic Disorders. 2020:1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scragg R. Limitations of vitamin D supplementation trials: why observational studies will continue to help determine the role of vitamin D in health. J Steroid Biochem Mol Biol 2018;177:6-9. 10.1016/j.jsbmb.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 15. Akbar U, Yang M, Kurian D, Mohan C. Omega-3 fatty acids in rheumatic diseases: a critical review. J Clin Rheumatol 2017;23:330-9. 10.1097/RHU.0000000000000563 [DOI] [PubMed] [Google Scholar]
- 16. Clark CCT, Taghizadeh M, Nahavandi M, Jafarnejad S. Efficacy of ω-3 supplementation in patients with psoriasis: a meta-analysis of randomized controlled trials. Clin Rheumatol 2019;38:977-88. 10.1007/s10067-019-04456-x [DOI] [PubMed] [Google Scholar]
- 17. Pedersen M, Stripp C, Klarlund M, Olsen SF, Tjønneland AM, Frisch M. Diet and risk of rheumatoid arthritis in a prospective cohort. J Rheumatol 2005;32:1249-52. [PubMed] [Google Scholar]
- 18. Bassuk SS, Manson JE, Lee I-M, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials 2016;47:235-43. 10.1016/j.cct.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manson JE, Cook NR, Lee I-M, et al. VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33-44. 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manson JE, Cook NR, Lee I-M, et al. VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23-32. 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Althouse AD, Below JE, Claggett BL, Cox NJ, de Lemos JA, Deo RC, et al. Recommendations for Statistical Reporting in Cardiovascular Medicine: A Special Report From the American Heart Association. Circulation. 2021. [DOI] [PubMed]
- 22. Sparks JA, Costenbader KH. Genetics, environment, and gene-environment interactions in the development of systemic rheumatic diseases. Rheum Dis Clin North Am 2014;40:637-57. 10.1016/j.rdc.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev 2019;40:1109-51. 10.1210/er.2018-00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol 2017;7:697. 10.3389/fimmu.2016.00697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stockinger B. Th17 cells: an orphan with influence. Immunol Cell Biol 2007;85:83-4. 10.1038/sj.icb.7100035 [DOI] [PubMed] [Google Scholar]
- 26. Linker-Israeli M, Elstner E, Klinenberg JR, Wallace DJ, Koeffler HP. Vitamin D(3) and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin Immunol 2001;99:82-93. 10.1006/clim.2000.4998 [DOI] [PubMed] [Google Scholar]
- 27. Korf H, Wenes M, Stijlemans B, et al. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology 2012;217:1292-300. 10.1016/j.imbio.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 28. Fisher SA, Rahimzadeh M, Brierley C, et al. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: a systematic review. PLoS One 2019;14:e0222313. 10.1371/journal.pone.0222313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li K, Huang T, Zheng J, Wu K, Li D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: a meta-analysis. PLoS One 2014;9:e88103. 10.1371/journal.pone.0088103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radzikowska U, Rinaldi AO, Çelebi Sözener Z, et al. The influence of dietary fatty acids on immune responses. Nutrients 2019;11:2990. 10.3390/nu11122990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramirez JL, Gasper WJ, Khetani SA, et al. Fish oil increases specialized pro-resolving lipid mediators in PAD (the omega-pad II trial). J Surg Res 2019;238:164-74. 10.1016/j.jss.2019.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 2018;128:2657-69. 10.1172/JCI97943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015;1851:469-84. 10.1016/j.bbalip.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 34. Costenbader KH, MacFarlane LA, Lee I-M, et al. Effects of one year of vitamin D and marine omega-3 fatty acid supplementation on biomarkers of systemic inflammation in older US adults. Clin Chem 2019;65:1508-21. 10.1373/clinchem.2019.306902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gysemans CA, Cardozo AK, Callewaert H, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology 2005;146:1956-64. 10.1210/en.2004-1322 [DOI] [PubMed] [Google Scholar]
- 36. Grenon SM, Owens CD, Nosova EV, et al. Short-term, high-dose fish oil supplementation increases the production of omega-3 fatty acid-derived mediators in patients with peripheral artery disease (the OMEGA-PAD I Trial). J Am Heart Assoc 2015;4:e002034. 10.1161/JAHA.115.002034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Tan H, Tang J, et al. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: a systematic review and meta-analysis. Diabetes Care 2020;43:1650-8. 10.2337/dc19-1708 [DOI] [PubMed] [Google Scholar]
- 38. Pittas AG, Dawson-Hughes B, Sheehan P, et al. D2d Research Group . Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med 2019;381:520-30. 10.1056/NEJMoa1900906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dillon CF, Weisman MH, Miller FW. Population-based estimates of humoral autoimmunity from the U.S. National Health and Nutrition Examination Surveys, 1960-2014. PLoS One 2020;15:e0226516. 10.1371/journal.pone.0226516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix 1: Supplementary tables and figures
Web appendix 2: Supplement 1
Data Availability Statement
Data from this study might be considered for release if the appropriate institutional review board approval and publication clearances have been obtained, and a project is approved by the VITAL oversight committee.