Abstract
Background:
Pregnancy, infancy, and childhood are sensitive windows for environmental exposures. Yet the health effects of exposure to nano- and microplastics (NMPs) remain largely uninvestigated or unknown. Although plastic chemicals are a well-established research topic, the impacts of plastic particles are unexplored, especially with regard to early life exposures.
Objectives:
This commentary aims to summarize the knowns and unknowns around child- and pregnancy-relevant exposures to NMPs via inhalation, placental transfer, ingestion and breastmilk, and dermal absorption.
Methods:
A comprehensive literature search to map the state of the science on NMPs found 37 primary research articles on the health relevance of NMPs during early life and revealed major knowledge gaps in the field. We discuss opportunities and challenges for quantifying child-specific exposures (e.g., NMPs in breastmilk or infant formula) and health effects, in light of global inequalities in baby bottle use, consumption of packaged foods, air pollution, hazardous plastic disposal, and regulatory safeguards. We also summarize research needs for linking child health and NMP exposures and address the unknowns in the context of public health action.
Discussion:
Few studies have addressed child-specific sources of exposure, and exposure estimates currently rely on generic assumptions rather than empirical measurements. Furthermore, toxicological research on NMPs has not specifically focused on child health, yet children’s immature defense mechanisms make them particularly vulnerable. Apart from few studies investigating the placental transfer of NMPs, the physicochemical properties (e.g., polymer, size, shape, charge) driving the absorption, biodistribution, and elimination in early life have yet to be benchmarked. Accordingly, the evidence base regarding the potential health impacts of NMPs in early life remains sparse. Based on the evidence to date, we provide recommendations to fill research gaps, stimulate policymakers and industry to address the safety of NMPs, and point to opportunities for families to reduce early life exposures to plastic. https://doi.org/10.1289/EHP9086
Introduction
Globally, humans are in close and frequent contact with plastics and their degradation products (Vianello et al. 2019), particularly nano- and microplastics (NMPs). Because of their complex physical and chemical properties (e.g., polymer, size, shape, charge) (Figure 1), NMPs may negatively affect human health, yet these exposures and their health implications remain either largely uninvestigated or unknown (Koelmans et al. 2019; WHO 2019). In this commentary, we describe why the special vulnerability of children and pregnant women to these exposures must be considered when framing research questions and designing methods for plastics research, as well as when developing policy around plastics.
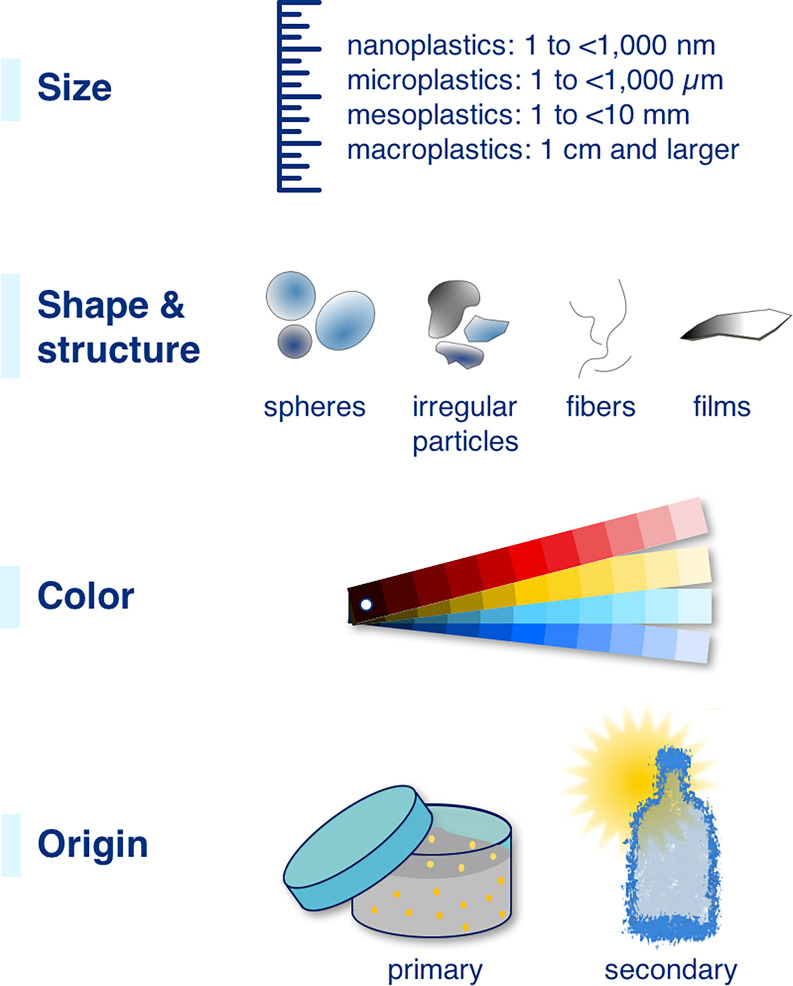
Figure 1.
Major characteristics of nano- and microplastic particles. Figure and definitions of size categories (nanoplastics: 1 to ; microplastics: 1 to ) adapted with permission from Hartmann et al. (2019) © 2019 American Chemical Society.
Emerging evidence indicates that humans are ubiquitously exposed to NMPs (Koelmans et al. 2019). Plastic products have also been found to contain a cocktail of more than 40,000 chemicals (Zimmermann et al. 2020), and certain plastics have been shown to leach over 80% of their chemicals into water, highlighting the potential for human exposure (Zimmermann et al. 2021). As plastics degrade in the environment, they generate potentially toxic modified products. Thus, plastics are a source of exposure to particles and chemicals alike, and their health effects will likely be driven by both physical and chemical toxicity.
Although the chemicals present in plastics have become a major research topic, their presence as particles is critically unexplored, in part due to significant methodological barriers. Yet these microscopic (and smaller) plastic particles may exert potential health effects in multiple ways. Mechanisms could include the ability to a) deposit and cause inflammatory reactions if present in large numbers in sensitive areas (e.g., the alveoli region with its limited removal processes) (Hinds 1999); b) translocate through biological barriers owing to their small size (with different sizes relevant for different barriers/organs) (Amato-Lourenço et al. 2020; Riediker et al. 2019; Stone et al. 2017); and c) act as carriers of chemical mixtures, thereby contributing to chemical exposures (Campanale et al. 2020; Eriksson et al. 2020; Rochman et al. 2019).
Recently, microplastics were discovered in human placenta (Ragusa et al. 2021), meconium, and infant stool (Zhang et al. 2021; Schwabl et al. 2019; Braun et al. 2021). Yet the impacts of exposure to plastic particles during early windows of vulnerability are almost entirely unknown. Our lack of knowledge on the health impacts of NMPs and the chemicals they carry prevents evidence-based assessment and effective management of the potential health risks arising from plastic exposures (Senathirajah et al. 2021). Early exposures to NMPs—alongside other environmental contaminants—should, we believe, be researched as contributors to the developmental origins of health and disease. Fundamental questions remain wide open: To what extent are humans exposed to NMPs, especially during pregnancy and the sensitive early years? And what are the effects on human health over the long term?
Plastics are chemically complex and release both NMPs and chemicals during production, use, and degradation (Hahladakis et al. 2018). The majority of NMPs come from fragmentation of larger plastic items (usually litter), but there is also significant direct release to the environment of microplastics (e.g., loss of preproduction pellets). To date, just a handful of studies have measured these exposures at human scale. One 2019 study estimated daily microplastic intake at 203 particles for girls and 223 particles for boys based on consumption of specific foodstuffs and drinking water in the United States (Cox et al. 2019). Another study modeled the lifetime accumulation of microplastics using a physiologically based pharmacokinetic model and estimated daily intake rates of 553 particles for children (Mohamed Nor et al. 2021). Yet these estimates might be “drastic underestimates” (Cox et al. 2019); more child-focused research estimated a single infant’s intake of microplastics from feeding bottles to be in the range of 14,600–4,550,000 particles/d, with the lowest levels seen in Africa and Asia (Li et al. 2020). This enormous range highlights the large uncertainty around human exposure to NMPs, particularly in early life, and the considerable analytical challenges of quantifying NMPs.
Another major knowledge gap is around the potential human health effects of NMPs. In 2019, a systematic review by the Norwegian Food Safety Authority found only three studies relevant for human health and concluded that it was currently impossible to assess the health risks of NMPs (VKM 2019). This mirrors earlier assessments by the European Food Safety Authority (EFSA 2016), United Nations Food and Agriculture Organization (Lusher et al. 2017), and Science Advice for Policy by European Academies (Koelmans et al. 2019).
Pregnancy and childhood are windows of vulnerability to environmental toxicants, as research over the last several decades has established (Landrigan and Etzel 2013). Early life exposures to hazardous chemicals, even in small quantities, can impact human health over the entire life span (Vizcaino et al. 2014). During infancy and childhood, child-specific behaviors, such as crawling and hand-to-mouth activity, mean that children are exposed to the environment in different ways than adults (Moya et al. 2004). Moreover, children eat, drink, and breathe more per unit body weight than adults. Consequently, pollutants in the environment are disproportionately taken up by the youngest, and we believe the same is true for NMPs (Figure 2). These higher exposures occur at the same time as critical development of the neurobehavioral, immune, metabolic, cardiovascular, and other important body systems. Children also have more years of life ahead of them than adults, making the early years a critical window to prevent long-lasting damage to health. Nevertheless, very little research on NMPs has taken an early life exposures approach (Street and Bernasconi 2021). Measurements of early life exposures to NMPs, their toxicity, and long-term outcomes are therefore scarce.
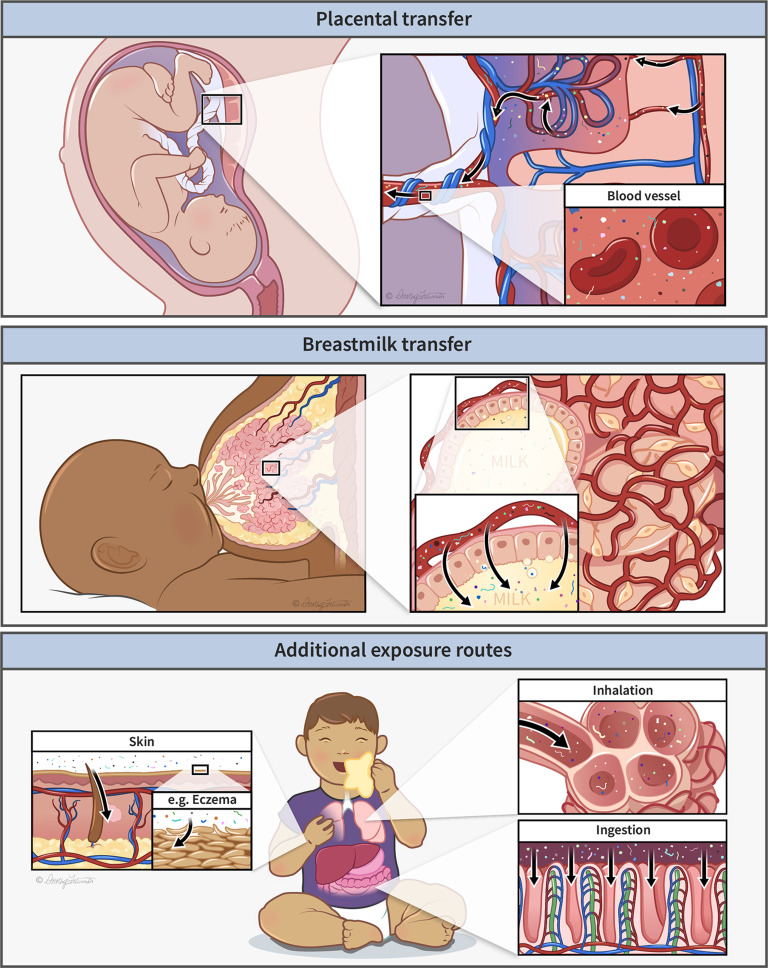
Figure 2.
Early life exposure pathways to plastic particles, showing potential routes of exposure via (top) placenta, (middle) breastmilk, and (bottom) dermal, respiratory, and gastrointestinal systems. Illustration printed with permission, © Dorothy Fatunmbi (https://elementusillustrations.com).
The aim of this commentary is to summarize the available evidence on child- and pregnancy-related exposures to NMPs via inhalation, placental transfer, ingestion and breastmilk, and dermal absorption. By providing an overview of the topic for the public health and pediatrics communities, we aim to engage them more actively in NMP research. We a) briefly review the state of the science on early life exposures to NMPs and identify knowledge needs; b) demonstrate how new methodologies may be able to fill these knowledge gaps; c) guide study design of future studies; d) put these issues in the context of policy and global health adaptations; and e) provide recommendations for research and the relevant stakeholders.
Methods: Literature Review
To identify studies that investigated health effects of NMP particles in the context of pregnancy or child health and explore knowledge gaps in the field, we carried out a literature search on 9 April 2021 in Web of Science and Scopus. The following search strings were used:
-
Web of Science:
((microplast* OR nanoplast* OR micro-plast* OR nano-plast* OR “plastic particle*” OR polystyrene OR microbead*) AND (infant OR child* OR baby OR pregnan* OR neonatal OR prenatal OR utero OR gestation* OR transgenerational OR intergenerational OR placenta* OR breastmilk OR breastfeed*))
-
Scopus:
TITLE-ABS-KEY ((microplast* OR nanoplast* OR micro-plast* OR nano-plast* OR “plastic particle*” OR polystyrene OR microbead*) AND (infant OR child* OR baby OR pregnan* OR neonatal OR prenatal OR utero OR gestation* OR transgenerational OR intergenerational OR placenta* OR breastmilk OR breastfeed*))
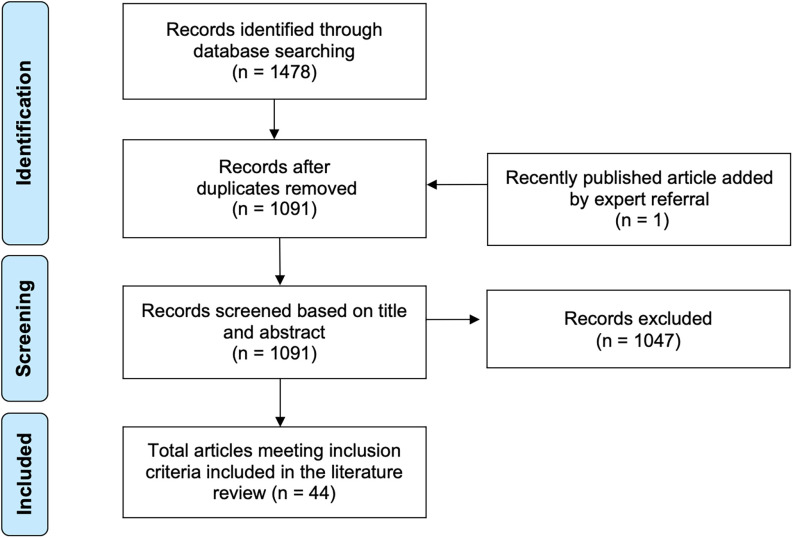
The search resulted in 1,091 unique studies after removal of duplicates using EndNote 20. Two authors (K.S. and M.W.) independently screened titles and abstracts for inclusion using Rayyan (Ouzzani et al. 2016); discrepancies were resolved jointly. A total of articles were included (Figure 3), of which were primary research articles (Table 1). The full list of included articles is provided in “Literature search bibliography” in the Supplemental Material.
Figure 3.
PRISMA flow chart for literature review. Note: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Studies on health effects of nano- and microplastics relevant for pregnancy and childhood identified via literature search.
| Type of study | Study focus | Reference |
|---|---|---|
| Epidemiological studies | Exposure and irritation in schools | Malmberg et al. 2000 |
| Exposure studies | MP in indoor or outdoor air or in dust | Abbasi et al. 2017, 2019 |
| Akhbarizadeh et al. 2021 | ||
| Dehghani et al. 2017 | ||
| Dris et al. 2017 | ||
| Liu et al. 2019 | ||
| Zhang et al. 2020 | ||
| MP in fish and seafood for human consumption | Akhbarizadeh et al. 2018, 2019, 2020 | |
| Barboza et al. 2020 | ||
| Martinez-Tavera et al. 2021 | ||
| MP in bottled water | Makhdoumi et al. 2021 | |
| Zuccarello et al. 2019 | ||
| MP in fruits and vegetables | Conti et al. 2020 | |
| MP release by infant feeding bottles | Li et al. 2020 | |
| Aerosol deposition in the nose | Zhou et al. 2013 | |
| Meta-analyses with exposure estimates | Cox et al. 2019 | |
| Mohamed Nor et al. 2021 | ||
| Experimental studies | Toxicokinetics—inhalation | Becquemin et al. 1991a, 1991b |
| Fournier et al. 2020 | ||
| Han et al. 2021 | ||
| Toxicokinetics—ingestion | Smyth et al. 2005 | |
| Toxicokinetics—placental/embryonic transfer | Grafmueller et al. 2015a, 2015b | |
| Gruber et al. 2020 | ||
| Huang et al. 2015 | ||
| Ragusa et al. 2021 | ||
| Wick et al. 2010 | ||
| Fournier et al. 2020 | ||
| Tian et al. 2009 | ||
| Toxicodynamics—allergy, asthma, inflammation | Alberg et al. 2014 | |
| Inocencio et al. 2017 | ||
| Toxicodynamics—embryo development | Bosman et al. 2005 | |
| Toxicodynamics—reproduction | Han et al. 2021 | |
| Toxicodynamics—metabolism | Luo et al. 2019a, 2019b | |
| Toxicodynamics—systemic toxicity | Han et al. 2021 |
Note: MP, microplastic.
Inclusion criteria were primary studies of humans or other mammals, as well as review articles and meta-analyses that included NMPs in terms of toxicity, hazard, or exposure. The literature search was date and language unrestricted. Studies were excluded if they used in vitro or nonmammalian models or did not have clear relevance for pregnancy or child health. The large majority of excluded studies used NMPs as consumables (e.g., antibody-coated microbeads or polystyrene plates) rather than as the research topic, or reported on medical procedures, drugs, or diagnostics that used NMPs. A small number of search results were excluded when the abstract did not have sufficient detail and full text was unavailable.
Results: Literature Review
Included studies were published between 1991 and 2021, with more than half published in 2019 or later. We also found seven review articles (listed in “Review articles on health effects of nano- and microplastics relevant for pregnancy and childhood” in the Supplemental Material), thus nearly 16% of the total included search results were review articles. In the research articles, the most common topic ( studies) was exposure to microplastics, predominantly quantified in air or seafood. Interestingly, only one study focused on a child-specific source of exposure, namely plastic feeding bottles (Li et al. 2020). Exposure estimates for children (Cox et al. 2019; Mohamed Nor et al. 2021) were based on generic assumptions (e.g., food consumption) rather than empirical information on the levels of NMP in matrices relevant for child exposure (e.g., toys, indoor air in schools, playground soils, infant foods, baby personal care products).
With regard to the child-specific hazards of NMPs, 12 studies investigated toxicokinetic aspects and 7 studies focused on the toxicodynamics, both in mammalian models. Only 1 study took an epidemiological approach. Most toxicokinetics work has addressed the placental transfer of plastic particles (), followed by studies on the biodistribution of NMP after inhalation (). We identified 6 rodent studies and 1 study with lambs that investigated the effects of NMPs in the context of in utero, neonatal, or early life exposures. Based on these few studies, no specific trend in the toxicological research on NMP is apparent. It is noteworthy that all but 1 study (Huang et al. 2015) used commercially available polystyrene beads, which are of limited relevance for human exposure given their spherical shape. Real-world NMPs have a wide variety of physical and chemical properties (Figure 1).
In general, the literature search revealed large gaps remaining in our knowledge about early life exposures to NMPs; pregnancy- and child-specific absorption, distribution, metabolism, and elimination of NMPs; and the toxicodynamic profiles of NMPs. The overall size of the evidence base for NMPs was substantially smaller than for plastic chemicals. We tested this by running the same search replacing NMP-related terms with “bisphenol*” ( studies) or “phthalate*” (), compared with the studies found for NMPs. Although this comparison has its limitations (e.g., chemicals vs. particles, more time spent on research on chemicals), it highlights the nascent nature of NMP research in general and the lack of focus on child health in particular.
In addition to studies captured by this literature search, some lessons can be drawn from the environmental health and safety research on engineered nanomaterials, for which more information is available (Schüepp 2010; Machado et al. 2010). For instance, some engineered nanomaterials in food and the domestic environment can be ingested or inhaled by children (Tang et al. 2015; Tulve et al. 2015) or transferred via the placenta to the fetus (Bongaerts et al. 2020). Infants’ immature respiratory systems may make them more vulnerable to nanomaterials (Semmler-Behnke et al. 2012). However, there are also certain crucial differences between engineered nanomaterials and nanoplastics: the former are intentionally manufactured, and the latter are often unintentionally generated during or after the use of plastic products. Accordingly, NMPs are more heterogeneous in shape, size, chemical composition, and other properties and will fragment further and faster in the environment (Gigault et al. 2021). Thus, insights from engineered nanomaterials cannot be simply translated to NMPs. However, as with the intersection of research on ambient ultrafine particles (Stone et al. 2017), it is worth exploring common lessons to be learned. The following sections are based on evidence from the emerging literature base around NMPs, as well as on insights drawn from other nanomaterials and classically monitored chemicals.
Discussion
Exposure Routes
Plastics are unavoidable for children, infants, and pregnant women around the world. The presence of microplastics is predominantly studied in air, foodstuffs, and beverages (Table 1) (Kannan and Vimalkumar 2021; Lehner et al. 2019) although early life exposures can also occur via the placenta and breastmilk (Figure 2), as well as from food contact materials. The relative importance of these exposure routes has not yet been established.
Microplastics have been discovered in human placenta (Ragusa et al. 2021), meconium, and infant stool (Zhang et al. 2021; Schwabl et al. 2019), but the sources of these plastics are difficult to trace. Microplastics have been detected in a variety of foods (Mortensen et al. 2021), such as fish (Akhbarizadeh et al. 2020; Barboza et al. 2020; Martinez-Tavera et al. 2021), seafood (Danopoulos et al. 2020; Van Cauwenberghe and Janssen 2014), and salt (Yang et al. 2015), as well as in food contact materials (Li et al. 2020), drinking water (Belz et al. 2021; WHO 2019), and in both indoor and ambient air (Abbasi et al. 2017; Allen et al. 2019; Cai et al. 2017; Liu et al. 2019; Zhang et al. 2019). Absorption of NMPs from these sources in utero, infancy, and childhood has yet to be benchmarked.
In the following sections, we outline key findings and knowledge gaps for child-relevant NMP exposure routes. Variation in size and other properties of NMPs (Figure 1) will influence their behavior and relevance for the different exposure routes.
Inhalation.
Plastic degradation products, depending on their size (), may become airborne and available for inhalation (Amato-Lourenço et al. 2020; Prata 2018). Microplastics have been detected in ambient air in environments ranging from urban (Abbasi et al. 2019; Akhbarizadeh et al. 2021; Amato-Lourenço et al. 2020; Cai et al. 2017) to remote mountain areas (Allen et al. 2019). The presence of microplastics in both indoor air (Soltani et al. 2021; Dris et al. 2017) and settled dust (Liu et al. 2019) has also been reported. Compared with outdoors, much higher concentrations of synthetic fibers including microplastics have been reported indoors, predominantly composed of polypropylene and polyethylene terephthalate (PET) polymers. Given the instrumental limitations of the prior research, only fibers have been analyzed. This size fraction () is not respirable, that is, it will not reach the tracheobronchial and alveolar region during inhalation (Hinds 1999; ICRP 1994). However, once settled on surfaces, such particles can be ingested by infants during their typical mouthing behaviors (Xue et al. 2007). Particles between 10 and typically do not reach the thoracic and alveolar region but are deposited in the nose and mouth (head airways region), which, despite effective clearance mechanisms (Hinds 1999; Thomas 2013), can be the site of allergic reactions. The respirable fraction of particles () can reach the thoracic and alveolar region (Hinds 1999; ICRP 1994). The presence of such small synthetic fibers and NMPs in indoor environments, relevant for deposition in the lower respiratory system, has not yet been studied. Considering that people in high-income countries spend about 90% of their time indoors (Brasche and Bischof 2005), assessing exposures indoors via inhalation is central to understanding potential health effects.
As they grow, infants and children undergo changes in lung architecture and breathing rates and patterns. In rodents, the translocation of inhaled nanoparticles from the alveolar air space to secondary organs was over one order of magnitude greater in neonatal rodents than in adults (Semmler-Behnke et al. 2012); to what extent this applies to human inhalation of NMPs—and its potential for nanotoxicity (Donaldson et al. 2004)—is unresolved. Studies on inhalation of NMPs come mainly from adults, and primarily from occupational settings, such as synthetic textile production sites (Kremer et al. 1994). Both cellulosic and plastic microfibers have been observed in nonneoplastic and malignant lung tissue taken from patients with different types of lung cancer (Pauly et al. 1998) where fibers were present in 87% of 114 lung human lung tissue specimens examined and showed little deterioration, indicating their biopersistence. Observations in the study by Pauly et al. (1998) confirmed that some fibers are capable of reaching the deep lung and avoiding clearance mechanisms. Because they persist, these foreign bodies may induce acute or chronic inflammation.
Placental transfer.
Maternal exposures can become determinants of a child’s health. Toxicants a mother is exposed to while pregnant—or those stored in her body from years earlier—can be released to her child during pregnancy and breastfeeding. A range of experimental studies is available on the placental transfer of polystyrene NMPs within a nanomaterial health and safety context (Fournier et al. 2020; Grafmueller et al. 2015a, 2015b; Gruber et al. 2020; Tian et al. 2009; Wick et al. 2010). Recently, Ragusa et al. (2021) discovered microplastics in the human placenta in the size range of using Raman microspectroscopy. Ragusa et al. (2021) located pigmented microplastic fragments in four of the six human placenta samples studied, on both the fetal and maternal sides, as well as on chorioamniotic membranes. However, the route of NMP transfer into the body, into the bloodstream, and then into the placenta is unknown. Braun et al. (2021) detected microplastics in the placenta and meconium after cesarean, while emphasizing the need for researchers to carefully evaluate the potential for contamination of samples from air fallout. Research suggests that the degree of particle transfer across the placenta changes across the trimesters with increasing gestational age (Vähäkangas and Myllynen 2009). It is unclear what mechanisms may be triggered by the transport of NMPs to the embryo or fetus. In a mouse model, carboxylate-modified polystyrene nanoparticles ( in size) have been reported to cross the placenta, induce trophoblast apoptosis, and show uptake by fetal organs (Huang et al. 2015). Research has demonstrated NMP transfer in human cells using placental perfusion ex vivo in the size range of (Grafmueller et al. 2015a; Poulsen et al. 2015; Wick et al. 2010) and in vitro (Aengenheister et al. 2018; Cartwright et al. 2012; Hesler et al. 2019; Kloet et al. 2015). Yet, limited research has explored the placental kinetics of nanoparticles and engineered nanomaterials in humans (Bové et al. 2019; Muoth et al. 2016); we encourage further research on this topic.
Breastfeeding and ingestion.
Chemical contaminants can be transferred from mother to child by breastfeeding (Mogensen et al. 2015). Depending on the duration of breastfeeding, the child’s body burden reflects the mother’s exposure. The degree to which NMPs can be transferred by lactation is not known. As a toddler, a child can inhale and digest NMP-containing dust in their home environments as well. Tasting, licking, and chewing plastic toys and textiles are potential exposure routes for NMPs and associated contaminants. In one study, infant stool was found to have significantly higher concentrations of PET microplastics compared with adult stool, a finding that the authors attributed primarily to ingestion, whereas the stool concentration of polycarbonate microplastics did not differ significantly between infants and adults (Zhang et al. 2021). In addition, plastic packaging of child food items and baby bottles must be considered potential sources of NMPs and plastic chemicals. Li et al. (2020) showed that when shaken with warm water, plastic baby bottles released up to microplastics per liter and that sterilization and high-temperature water significantly increased microplastics release. Overall, they concluded that children fed via plastic baby bottles will be exposed to 14,600–4,550,000 particles/d in the size range captured by pore sized filters. The analysis by Li et al. (2020) also found trillions of nano-sized plastic particles per liter, with a mean particle diameter of . We call for future research to collect data on plastic use during pregnancy and in early childhood.
Ongoing and future cohort studies should gather information on the type of plastic packaging used during pregnancy and childhood. Such an approach is already being implemented in the sixth Faroese Birth Cohort, which was established in June 2020 and has received support from the U.S. National Institutes of Health and the Danish Environmental Protection Agency. To date, 550 mother–child pairs are participating. Parents provide information on plastic-avoiding behavior via written questionnaire, and children are examined at birth, 2 wk, 3 months, and 12 months postpartum. The generated data may be used to estimate changes in ingestion-related exposure hazards.
Dermal absorption.
Although research has suggested a low probability for dermal exposure to NMPs given the surface properties of plastic particles and the excellent barrier properties of adult skin (Lehner et al. 2019), it is unknown how vulnerable children’s skin may be. The adult skin barrier is different from those of infants and children. Preterm-born infants in particular have a thinner and less efficient stratum corneum, which is the most superficial layer of skin, compared with adults (Mancini 2004). Skin barrier maturation has been reported to continue into a child’s fourth year of life (Mack et al. 2016). Little evidence exists for transfer of nanoplastics (Bouwstra et al. 2001); for smaller NMPs, ports of entry could be hair follicles, sweat glands (Alvarez-Román et al. 2004), or damaged skin, such as related to atopic dermatitis (eczema), which is a common childhood disease (Biagini Myers and Khurana Hershey 2010). Frequent use of plastic packaging, emollients, and other baby personal care products could also be sources of exposure to NMPs, but research on relevant sources is lacking.
Toxicology
Similar to our limited understanding of human exposures, the health impacts of plastic particles remain poorly understood. Recent assessments point toward large knowledge gaps regarding the toxicokinetics and -dynamics of NMPs (WHO 2019). Our literature search identified just five studies in this area that investigated inhalation ( studies) or ingestion ( study) using animal models (Table 1). The major line of thinking is that smaller plastic particles have a higher probability of crossing biological barriers and, thus, entering tissues or becoming systematically distributed. Limited evidence suggests that NMPs in size can be transported actively across epithelia (e.g., by M cells in the small intestines), whereas larger microplastics up to can translocate passively via persorption (Wright and Kelly 2017). However, the proportion of NMPs physically entering the human body in the sense of a tissue translocation remains largely unknown. The same is true for elimination and excretion (Schwabl et al. 2019; Zhang et al. 2021). Moreover, the physicochemical properties of NMPs (e.g., polymer, size, shape, surface charge) driving the uptake by cells (including in placental tissue and mammary glands), cytotoxicity, and pro-inflammatory responses have yet to be benchmarked. In addition, nearly no studies of NMPs and plastics take into account that plastic may pose distinct human health risks at every stage of its lifecycle (Eibner 2007), including hazards from incinerating plastic waste.
Thus far, toxicological research has not specifically focused on the effects of NMPs on child health. This is problematic because the body’s defense mechanisms—the lungs and immune system—are not fully developed at birth, leading to, for example, much higher rates of respiratory infections and asthma among children than adults (Landrigan and Etzel 2013). This means that environmental exposures tolerable for adults may have adverse health effects when they occur early in life. Humans are equipped with a blunted immune system at birth, with decreased neutrophil and monocyte cell counts, decreased natural killer cell cytolytic function, and a T-cell population skewed toward immunotolerance, instead relying on maternally derived immunoglobulins and other immune factors to combat infections (Pollard and Bijker 2021; Simon et al. 2015). Little, if anything, is known about how exposure to NMPs affects the developing human immune system. However, mouse dams fed NMPs in drinking water displayed a dose-dependent increase in immunoglobulin A levels, fewer mature dendritic cells, and an increased ratio of helper/cytotoxic T-cells (Park et al. 2020). The dendritic cell phenotype was recapitulated in male, but not female, pups, whereas the T-cell population in pups of both sexes was skewed toward helper T-cells. Although suggestive of NMP immunomodulation, the impact this has on infection and autoimmunity is unknown. It is important to close these knowledge gaps to assess how NMP exposure affects the developing immune response and the implications for immune responses later in life.
Recent studies seem to confirm that polystyrene particles, frequently considered an inert material, can, in the nanosize range, exert toxicological effects. Polystyrene nanoparticles (25 and ) have been reported to exert toxicological effects on human alveolar epithelial cells and to affect cell viability, cause cell cycle S phase arrest, oxidative stress, activated inflammatory gene transcription, and change the expression of proteins associated with the cell cycle and pro-apoptosis (Dong et al. 2020; Xu et al. 2019b). However, it remains open if these effects are caused by the polystyrene itself, a stabilizer present on the particle surface, or a degradation product of polystyrene forming in vivo. Research is needed to find out the extent of exposure and characteristics of particles that determine where and at what dose the particles deposit in the respiratory tract, as well as their associated toxicological effects. Insoluble particles , which can reach and deposit in large numbers in the alveolar region, may cause inflammation and systemic changes even if the material they are composed of is not potentially toxic (Garcés et al. 2021). This is due to the site of deposition and limited clearance mechanisms, which in the case of alveoli entails engulfment by macrophages only, with a clearance process of insoluble particles occurring over months to years (Hinds 1999; Nagre et al. 2019). Plastic particles may serve as carriers of mixtures of chemicals ad- and absorbed on them. These chemicals may be delivered with particles to the sensitive lower parts of the lungs, where they may dissolve upon particle deposition and enter the bloodstream. There is emerging evidence confirming translocation of ambient insoluble particles (called ultrafine particles in air pollution studies) to the blood circulation through the pulmonary alveoli (Saenen et al. 2017). An understanding of the toxicity of particles beyond the effects of incorporated or adsorbed low-molecular weight substances is critically needed.
A small number of animal studies relevant to early life exposures points toward toxicological effects of NMPs on metabolism, maternal–fetal immune balance, and the microbiome, as well as an induction of oxidative stress and inflammation (Alberg et al. 2014; Bosman et al. 2005; Han et al. 2021; Hu et al. 2021; Inocencio et al. 2017; Luo et al. 2019a, 2019b) (Table 1). As an example, exposure to inhaled polystyrene nanoplastics exacerbated lung injury and inflammation in preterm-born lambs (Inocencio et al. 2017). In a study with pregnant mice, an exposure to polyethylene microplastics via drinking water did not affect reproduction but, rather, resulted in higher body and organ weight in dams and neonates (Han et al. 2021). Another study with pregnant mice exposed during gestation and lactation to polystyrene microplastics via drinking water reported metabolic effects in F1 and F2 animals (Luo et al. 2019a). The translocation to tissues, the cellular uptake of NMPs, a range of cytotoxic and apoptotic effects, as well as inflammation, are potential mechanisms of toxicological action from NMP exposure. However, given the early stage of research, these studies have certain limitations, such as the use of very high doses of spherical NMPs predominantly consisting of polystyrene (WHO 2019) and the focus on high-dose, acute dose-limiting toxicity, often neglecting long-term effects of NMP exposure. Potential NMP toxicity on other systems and the developing brain is also critically unexplored.
Our literature search identified only one epidemiological study of health effects following exposure to NMPs in children. Malmberg et al. (2000) studied an acrylate–styrene copolymer floor polish in a school environment that began powdering after layers of polish peeled off and turned to dust (including particles in diameter). Teenagers at the school reported irritation in their eyes and lower airways. Chemical analysis confirmed the presence of acrylate monomers, which could be transported on respirable floor dust particles.
In contrast to the dearth of experimental and epidemiological data on NMPs, occupational health data from workers in plastics industries and orthopedic research on plastic implants is more abundant. Occupational exposure to plastic fibers and dust caused respiratory symptoms and impaired pulmonary function (Koelmans et al. 2019), and abrasion of plastic implants induced inflammation in an in vitro study using human cells (Wooley et al. 1996). In the 1980s, researchers investigated spontaneous abortions in women employed in the plastics industry (Lindbohm et al. 1985; McDonald et al. 1988). Although these examples represent very specific exposure scenarios, they may serve as indicators of potential health effects caused by a chronic exposure to NMPs in the general population. Addressing this through the lens of child health is particularly pertinent because we know that children are widely affected by ambient air pollution (WHO 2018, 2021), which includes NMPs.
Interactions between NMPs and other chemicals make it impossible to assess the health impacts of plastic exposures by investigating NMPs alone. Plastic particles can act as carriers for a diverse group of chemicals (Hartmann et al. 2017; Rochman et al. 2019; Wiesinger et al. 2021). These include chemicals used in plastic production [such as phthalates (Bølling et al. 2020), bisphenols (Asimakopoulos et al. 2016), and brominated flame retardants (Sindiku et al. 2015; Tsydenova and Bengtsson 2011)], as well as chemicals [such as polycyclic aromatic hydrocarbons (PAHs), organochlorine pesticides, and polychlorinated biphenyls (PCBs)] sorbed to plastics from the environment (Gasperi et al. 2018). Plastic particles can also contain or accumulate metals such as cadmium, zinc, nickel, and lead, added as colorants, biocides, or stabilizers (Campanale et al. 2020). A number of chemicals used in plastics and others that NMPs carry are classified as endocrine-disrupting chemicals (Li et al. 2010). Older plastic toys with unsafe levels of lead, cadmium, and bromine are still widely available in secondhand shops and among hand-me-downs and donated items (Turner 2018). Altogether, this results in an exposure to a cocktail of chemicals and particles that remains largely unquantified and uncharacterized (Zimmermann et al. 2019). Accordingly, toxicity of NMPs may be related to the particles themselves or the “Trojan Horse” effects via the release of sorbed pollutants and/or the leaching of plastic chemicals (Bouwmeester et al. 2015; Vethaak and Legler 2021). Attempting to disentangle the physical toxicity (i.e., presence of particles) of NMPs from their chemical impacts experimentally is, we believe, at the same time difficult and too simplistic.
Analytical Tools to Characterize NMPs
Knowledge-based policy development on a global scale critically depends on the ability to identify and characterize the health hazards of different classes of NMPs. Yet we believe that the greatest current barrier to this knowledge is the absence of methods to readily locate, identify, and quantify NMPs in the human body. This challenge increases for plastic particles in the submicron size range (e.g., nanoplastics). Compared with small molecules NMPs can be considered massive, and their size makes them challenging to ionize for conventional mass spectrometric analysis. However, by employing field flow fractionation and pyrolysis gas chromatography mass spectrometry, researchers can determine the size of the particles and the type of polymer the plastic is made of (Mintenig et al. 2018). Another method to determine the type of plastic material is to take advantage of the interaction of light with the chemical functional groups present in the particle using Fourier transform infrared spectroscopy (FTIR) or Raman spectroscopy (Cabernard et al. 2018; Xu et al. 2019a).
The current gold standard for detection and identification of NMPs is the use of vibrational spectroscopic imaging or mapping (Chen et al. 2020; Renner et al. 2018; Xu et al. 2019a) although these methods are not high-throughput. Mass spectrometric methods also provide important information but are often not sufficient to identify few very small particles. Vibrational spectroscopy can be deployed to identify NMPs in typical infant foods, such as breastmilk or infant formula. Raman mapping has previously been used to study plastics (Xu et al. 2020) and is, in principle, capable of identifying particles in the submicron range (Schwaferts et al. 2019). Studying infant-specific food samples would be a logical first step toward approaching more challenging sample matrices relevant for children. The NMP community may benefit from specific protocols, such as for preconcentration, developed specifically for infant-specific food samples. A combination of vibrational spectroscopic imaging with statistical learning methods would likely be the way forward to identify the presence of NMP particles (Renner et al. 2019); commercial software packages for analytical chemistry are available, and many important functions are available as packages in R or Python. With such methods, detection in more challenging matrices, such as breastmilk or even tissue, may also become feasible. Detection of specific toxic chemicals dissolved in NMPs, or from NMP degradation products, would be a further challenge.
Sampling and analysis of NMPs in different matrices.
Improved sampling of NMPs in air, food, packaging, and other child- and pregnancy-relevant matrices is an important steppingstone toward estimating early life exposure. Yet there is no “magic box” available for obtaining such data on NMPs. Based on our experience, it is difficult to analyze very small microplastics, and it is currently impossible to analyze nanoplastics in complex matrices, such as breastmilk.
Take the example of measuring airborne NMPs. Few relevant studies have analyzed atmospheric fallout samples (Dris et al. 2015), filter samples (Dris et al. 2017), and settled dust (Dris et al. 2017; Liu et al. 2019). Such studies typically rely on microscopic analysis (counting and size determination) and offline chemical analysis (e.g., FTIR coupled with attenuated total reflectance, micro-FTIR, micro-Raman spectroscopy). One promising technique that so far has not been used for NMP detection is real-time aerosol mass spectrometry, which allows determination of the chemical composition of particles and their size in the range that is relevant for possible lung deposition (). Aerosol mass spectrometry was recently used to study phthalates in the particle phase (Eriksson et al. 2020) and may, therefore, be applied to detect airborne nanoplastics. Simpler detection techniques could be developed for airborne particles of using gravimetric analysis combined with scanning electron microscopy and energy dispersive X-ray for chemical composition analysis and automated counting and size determination through image analysis.
We encourage prioritizing sampling for NMPs in child-relevant environments, such as hospital nurseries and neonatal intensive care units, homes, playgrounds, school buses, and schools and kindergartens, as has been done with plasticizers (Bekö et al. 2013). We urge that foods, personal care products, toys, textiles (e.g., clothing, carpets, bedding), and other items that pregnant women, infants, and children are disproportionately exposed to also be prioritized for sampling.
Plastic chemicals.
Chemical additives for plastics manufacturing, nonintentionally added substances, and chemicals accrued onto these particles add to the complexity surrounding NMP exposures. Mass spectrometry allows for the characterization of these chemicals. As an example, a recent study used a suspect screening approach with industrial chemicals and identified previously unknown plasticizers in paired maternal and cord serum samples (Wang et al. 2021a). Nontargeted chemical analysis of mother–child biosamples, supported by extensive targeted screening of chemicals, is an important, although resource-intensive, step toward identifying plastic-associated chemicals. We believe that identifying and characterizing chemical biomarkers that reflect our total plastic exposure—from chemicals, NMPs, and their degradation products—is likely the most promising approach. Validated methods to interpret the huge amounts of information generated from these analyses are, in our experience, critical. Moreover, targeted and nontargeted screenings can identify substances associated with plastics, but they must be combined with toxicological data (Zimmermann et al. 2020, 2021). We recommend that bioanalytical tools, health data (ideally from prospective longitudinal cohort studies), cheminformatics, and holistic human health risk analyses should be integrated to obtain meaningful information about potential relationships between chemical signals and their toxicity for child health. However, even if analytical methods advance significantly in the mid-term, we believe that achieving the capability to monitor NMPs in human populations on a scale needed to compile convincing epidemiological evidence is unrealistic. We therefore argue foremost for a precautionary approach to NMPs and child health, even while data collection and risk analysis is still underway (see the “Recommendations” section).
Data science.
Understanding the relationships between exposure and risks is extremely complex because of the high volume of heterogeneous data from different cohorts and different exposure routes. Once more data become available on the health impacts of NMPs, advanced information technologies can be applied to facilitate acquiring, storing, and managing knowledge and big data for the development of evidence-based toxicology (Escher et al. 2020). Although the risks of hard-to-decipher results are clear, big data approaches also provide an opportunity to reexamine existing data with a new perspective. For example, existing mother–child cohort studies can provide valuable data resources. A combination of genetics analysis, mathematical modeling, and machine learning tools, we believe, will be valuable to evaluate the associations between potential exposure, potential risk factors on child development, and health hazards from early life exposure to NMPs and plastics-associated chemicals.
Global Context, Policy, and Health Inequalities
NMPs are contaminants of emerging concern that are not yet subject to extensive risk assessment or regulation at the international level. By contrast, the harmful effects of contaminants, such as methylmercury, lead, and persistent organic pollutants (Storelli 2008), have been studied for decades, with well-documented adverse impacts on the environment and humans, in part because of the easier methodology compared with NMPs. Research on these traditional contaminants has led to specific global policies that have boosted human health, such as the banning of leaded gasoline internationally and the Basel, Rotterdam, Stockholm, and Minamata Conventions (Wang et al. 2021b).
An estimated 79% of all plastic waste ever produced has accumulated in landfills, dumps, or the natural environment while 12% has been incinerated and only 9% recycled (United Nations 2021). Regulatory policies for plastics differ drastically between countries. This introduces variability in children’s exposure to plastic particles and chemicals depending on where in the world they were born (Danopoulos et al. 2020; Sripada 2017). Geography and climatic conditions in a number of coastal communities in developing countries also make them likely receivers of marine and air NMP pollution that is generated elsewhere (Chassignet et al. 2021; Nel et al. 2021; Premti 2018). Children in low- and middle-income countries face substantial changes in the plastic landscape without corresponding regulatory safeguards.
Hazardous waste—such as discarded electrical and electronic equipment (e-waste)—exported from high-income countries to developing countries for recycling or waste processing is a large source of NMPs and a major public health threat for children (Hale et al. 2020; Lebbie et al. 2021). E-waste often contains plastic (e.g., cables, casings, electronic chips) and, when shredded and burned, releases plastic particles along with highly toxic fumes and runoff (Heacock et al. 2016; Labunska et al. 2013; Lebbie et al. 2021; Sindiku et al. 2015). Children and adolescents who collect, process, shred, and burn plastic waste or who live near dump sites face risks related to NMPs on top of numerous other social and environmental hazards (U.S. Department of Labor 2020; Fonbuena 2019; Lebbie et al. 2021; Heacock et al. 2016; Perkins et al. 2014).
Consumption of highly processed foods continues to rise globally. Children’s exposure is, therefore, likely also rising to plastic food contact materials used throughout the manufacturing process, including food processing equipment, food packaging, and single-use containers (Muncke et al. 2020). The global market for baby bottles, along with the use of infant formula, is also expanding. When a country’s gross domestic product per capita doubles, breastfeeding prevalence at 12 months of age decreases by 10% (Victora et al. 2016). Although glass and stainless steel bottles are common alternatives, plastic bottles still dominate this growing market and are a known source of NMPs (Li et al. 2020). Indeed, 63% of infants months of age are not exclusively breastfed in low- and middle-income countries; this figure is higher in high-income countries (Victora et al. 2016) and among high-income groups within countries. Plastic baby bottles have even been inappropriately marketed as hygiene measures during the Coronavirus disease (COVID-19) pandemic, in violation of national and international codes (van Tulleken et al. 2020).
Early life exposures to NMPs and plastics reflect numerous commercial and social determinants of health and are likely linked to health inequalities. Global inequalities in pregnant women’s body burden of plastic chemicals has, for example, been reported by income (Wenzel et al. 2018), by race/ethnic group (Bloom et al. 2019; James-Todd et al. 2017), by neighborhood (Bustamante-Montes et al. 2021), and by education (Zhu et al. 2016), among other socioeconomic factors. Some of this variation may reflect differences in proximity to pollution sources, dietary behaviors, occupational exposures, use of personal care products (Helm et al. 2018; Zota and Shamasunder 2017), and intersections of these factors. Inequalities in exposure to NMPs and health impacts may fall along similar lines.
On the international stage, the amendment of the Basel Convention in 2019 (Basel Convention 2019) makes global trade in plastic waste more transparent while ensuring that its management is safer for the environment and human health. In 2020, the Basel Convention Plastic Waste Partnership working group specifically included microplastics in its portfolio. Yet despite the global efforts to encourage a culture of recycling, the capacity for collection, sorting, and recycling continues to underperform. This is attributed to the increased material diversity and complexity of plastics compared with other materials (Crippa et al. 2019). Stricter policy measures in certain regions have limited or banned the use of some microplastics, such as microbeads (Burton 2015). However, largely untested replacements will likely be used in their place. As plastics production continues to rise globally, new mixtures are constantly emerging (Ryan 2015; Sackmann et al. 2018). It is therefore critical that these knowledge gaps are filled with high-quality human-relevant data so that the Basel Convention secretariat and other policy entities have the information needed to both promote a circular economy and protect public health.
Recommendations
Policymakers.
Children’s right to a healthy environment is enshrined in the United Nations Convention on the Rights of the Child. According to this treaty—to which 196 countries are party—states have the duty to secure children’s enjoyment of the highest attainable standard of health, which includes states’ taking action to prevent and reduce exposure to harmful substances or environmental conditions that directly or indirectly affect children’s health (UNHCR 2020). Given the unknowns surrounding the risk of NMP exposures and effects in pregnancy and childhood, we advocate for using the precautionary principle to guide policymakers’ approach to NMPs and child health (Leslie and Depledge 2020; Harremoës et al. 2002). We argue that appropriate and proportionate measures to reduce the exposure of children to NMPs should be taken even without comprehensive knowledge of the scale of the risk. At the same time, it is necessary that policymakers promote research that enables us to better understand and quantify the risks of NMPs. Along those lines, we call for greater surveillance of NMPs in children’s environments that is guided by the latest research. The process the government of California, USA, takes to monitor microplastics in drinking water and, in parallel, to assess the associated health risks can be a model for this (Coffin et al. 2021). We emphasize that humans will always be exposed to NMP particles along with chemicals released by all the plastic products we use, and therefore plastics cannot be studied or regulated as a single entity (Lambert et al. 2017). Youth involved in plastic waste collection and e-waste burning—especially those exploited or forced into hazardous child labor—must be prioritized for prevention and health promotion (Lebbie et al. 2021; U.S. Department of Labor 2020).
Industry.
NMPs in the environment are a human-made problem. We are convinced that the manufacturers of plastic products that pregnant women, infants, and children will be in contact with have a duty to reduce NMP exposures. As a first step, producers should investigate how many plastic particles their products shed under real-use conditions. If substantial, the manufacturers should adopt a prevention through design approach (NIOSH 2013) and innovate materials and products that release fewer NMPs. Producers must demonstrate that their products are safe for their youngest users, before bringing them to market.
Research.
We urge the research community to work across disciplines and beyond academia to gain a better understanding of early life exposures to NMPs and plastic chemicals. We believe this is essential because research on other contaminants has shown these to be sensitive periods linked to adverse health outcomes later in life (Heindel et al. 2015). A substantially expanded evidence base is needed to undergird regulatory processes and policymaking. Such understanding can only be generated by significant advances in exposure science, toxicology, epidemiology, and public health sciences that aim at quantifying early life exposures, long-term follow-up characterizing their (latent) effects, and linking both in the relevant human populations (Table 2). For instance, ongoing mother–child cohort studies should include aspects of exposure to NMPs, such as sampling in child-relevant materials and questionnaire data on plastics use and contact. Growing the knowledge base about child health and plastics requires the development of a joint terminology and consensus on appropriate quality standards, as well as the establishment of technical, scientific, and science-policy forums to exchange data, knowledge, and ideas; some of this work has already started (Noventa et al. 2021; Zarus et al. 2021). Importantly, outputs should be co-created and communicated with stakeholders, such as families and children themselves. Including children in the study of their own exposure to plastics and other nanomaterials may be both an exercise in interdisciplinary research, as well as an opportunity for citizen science and awareness-building among those most affected (Kraftl et al. 2021).
Table 2.
Recommendations on research goals, tasks, and requirements to stimulate and guide research linking child health and nano- and microplastics.
| Exposure sciences | Toxicological sciences | Epidemiology and public health sciences | Global health inequalities | |
|---|---|---|---|---|
| Research goals | Assess children’s exposure to NMP comprehensively and quantitatively | Quantify and mechanistically understand hazards NMPs potentially pose to child health | Understand whether real-world exposures to NMPs are associated with health impacts in children | Accelerate research on inequalities in NMP exposures and translate this knowledge into preventive actions |
| Research tasks |
|
|
|
|
| Requirements to meet goals |
|
|
|
|
| Transdisciplinary collaboration |
|
|||
Note: NICU, neonatal intensive care unit; NMP, nano- and microplastic.
Families.
The largest actions, we believe, must be taken by states and industry to prevent children’s exposures to NMPs. Families can also take steps by following the recommendations offered for other child-relevant contaminants, which we believe are likely also effective for reducing NMP exposures. These steps include reducing plastic contact of foods for children (Edwards et al. 2021; Sessa et al. 2021; Trasande et al. 2018), regular wet-cleaning of the home (Rhoads et al. 1999), and careful choice of safer personal care products and building materials (Giovanoulis et al. 2019).
Conclusion
Over the last several decades, pregnant women and children globally have been exposed to an extraordinary diversity of plastics. We believe that children are unique in terms of their exposures and vulnerabilities to NMPs. Yet foundational evidence on children’s exposure to NMPs, as well as child-specific toxicology, is sorely lacking. Our assessment of the fragmented (but growing) evidence base around early life exposures to NMPs provides cause for concern.
These knowledge gaps slow public health action around plastics in general and NMPs in particular. The fragmented evidence base on NMP exposures and toxicity, as well as general lack of epidemiological data, prevents us from fully understanding the health risks of early life exposures to NMPs. Effective risk assessment and risk management will depend on substantial expansion of this evidence base. Closing knowledge gaps on human and environmental health impacts of NMPs will also support the development of safer alternatives, such as materials shedding fewer NMPs and leaching fewer plastic chemicals.
The recommendations we offer here provide research directions to the wider scientific community to fill these knowledge gaps. At the same time, we aim to spur policymakers and industry to implement precautionary approaches toward NMPs (Table 2).
Supplementary Material
Acknowledgments
We thank D. Fatunmbi of Elementus Illustrations for creation of the illustration for Figure 2. We also acknowledge R. Etzel, M.D., Ph.D., for helpful feedback on Figure 2. K.S. acknowledges funding from the Research Council of Norway (project 288638) to the Centre for Global Health Inequalities Research at the Norwegian University of Science and Technology. T.V. acknowledges funding from the Research Council of Norway (project 303369) and SINTEF (strategic institute project on immunotherapy 102020958). M.W. acknowledges funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant (agreement 860720).
References
- Abbasi S, Keshavarzi B, Moore F, Delshab H, Soltani N, Sorooshian A. 2017. Investigation of microrubbers, microplastics and heavy metals in street dust: a study in Bushehr City, Iran. Environ Earth Sci 76(23):798, 10.1007/s12665-017-7137-0. [DOI] [Google Scholar]
- Abbasi S, Keshavarzi B, Moore F, Turner A, Kelly FJ, Dominguez AO, et al. 2019. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ Pollut 244:153–164, PMID: , 10.1016/j.envpol.2018.10.039. [DOI] [PubMed] [Google Scholar]
- Aengenheister L, Keevend K, Muoth C, Schönenberger R, Diener L, Wick P, et al. 2018. An advanced human in vitro co-culture model for translocation studies across the placental barrier. Sci Rep 8(1):5388, PMID: , 10.1038/s41598-018-23410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhbarizadeh R, Dobaradaran S, Amouei Torkmahalleh M, Saeedi R, Aibaghi R, Faraji Ghasemi F. 2021. Suspended fine particulate matter (PM2.5), microplastics (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: their possible relationships and health implications. Environ Res 192:110339, PMID: , 10.1016/j.envres.2020.110339. [DOI] [PubMed] [Google Scholar]
- Akhbarizadeh R, Dobaradaran S, Nabipour I, Tajbakhsh S, Darabi AH, Spitz J. 2020. Abundance, composition, and potential intake of microplastics in canned fish. Mar Pollut Bull 160:111633, PMID: , 10.1016/j.marpolbul.2020.111633. [DOI] [PubMed] [Google Scholar]
- Akhbarizadeh R, Moore F, Keshavarzi B. 2018. Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ Pollut 232:154–163, PMID: , 10.1016/j.envpol.2017.09.028. [DOI] [PubMed] [Google Scholar]
- Akhbarizadeh R, Moore F, Keshavarzi B. 2019. Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: a threat to human health? Food Addit Contam Part A Chem Anal Control Expo Risk Assess 36(11):1696–1708, PMID: , 10.1080/19440049.2019.1649473. [DOI] [PubMed] [Google Scholar]
- Alberg T, Hansen JS, Lovik M, Nygaard UC. 2014. Particles influence allergic responses in mice—role of gender and particle size. J Toxicol Environ Health A 77(5):281–292, PMID: , 10.1080/15287394.2013.863746. [DOI] [PubMed] [Google Scholar]
- Allen S, Allen D, Phoenix VR, Le Roux G, Durántez Jiménez P, Simonneau A, et al. 2019. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat Geosci 12(5):339–344, 10.1038/s41561-019-0335-5. [DOI] [Google Scholar]
- Alvarez-Román R, Naik A, Kalia YN, Guy RH, Fessi H. 2004. Skin penetration and distribution of polymeric nanoparticles. J Control Release 99(1):53–62, PMID: , 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Amato-Lourenço LF, Dos Santos Galvão L, de Weger LA, Hiemstra PS, Vijver MG, Mauad T. 2020. An emerging class of air pollutants: potential effects of microplastics to respiratory human health? Sci Total Environ 749:141676, PMID: , 10.1016/j.scitotenv.2020.141676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimakopoulos AG, Elangovan M, Kannan K. 2016. Migration of parabens, bisphenols, Benzophenone-type UV filters, triclosan, and triclocarban from teethers and its implications for infant exposure. Environ Sci Technol 50(24):13539–13547, PMID: , 10.1021/acs.est.6b04128. [DOI] [PubMed] [Google Scholar]
- Barboza LGA, Cunha SC, Monteiro C, Fernandes JO, Guilhermino L. 2020. Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. Exposure and risk to human consumers. J Hazard Mater 393:1224193, PMID: , 10.1016/j.jhazmat.2020.122419. [DOI] [PubMed] [Google Scholar]
- Basel Convention. 2019. Marine plastic litter and microplastics: overview. http://www.basel.int/Implementation/MarinePlasticLitterandMicroplastics/Overview/tabid/6068/Default.aspx [accessed 29 September 2021].
- Becquemin MH, Swift DL, Bouchikhi A, Roy M, Teillac A. 1991a. Particle deposition and resistance in the noses of adults and children. Eur Respir J 4:694–702. [PubMed] [Google Scholar]
- Becquemin MH, Yu CP, Roy M, Bouchikhi A. 1991b. Total deposition of inhaled particles related to age–comparison with age-dependent model-calculations. Radiat Prot Dosimetry 38:23–28, 10.1093/rpd/38.1-3.23. [DOI] [Google Scholar]
- Bekö G, Weschler CJ, Langer S, Callesen M, Toftum J, Clausen G. 2013. Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PLoS One 8(4):e62442, PMID: , 10.1371/journal.pone.0062442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz S, Bianchi I, Cella C, Emteborg H, Fumagalli FS, Geiss O, et al. 2021. Current Status of the Quantification of Microplastics in Water: Results of a JRC/BAM Interlaboratory Comparison Study on PET in Water. JRC Technical Report. EUR 30799 EN. Luxembourg; Publications Office of the European Union. https://data.europa.eu/doi/10.2760/6228 [accessed 23 December 2021]. [Google Scholar]
- Biagini Myers JM, Khurana Hershey GK. 2010. Eczema in early life: genetics, the skin barrier, and lessons learned from birth cohort studies. J Pediatr 157(5):704–714, PMID: , 10.1016/j.jpeds.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Wenzel AG, Brock JW, Kucklick JR, Wineland RJ, Cruze L, et al. 2019. Racial disparity in maternal phthalates exposure; association with racial disparity in fetal growth and birth outcomes. Environ Int 127:473–486, PMID: , 10.1016/j.envint.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Bølling AK, Sripada K, Becher R, Bekö G. 2020. Phthalate exposure and allergic diseases: review of epidemiological and experimental evidence. Environ Int 139:105706, PMID: , 10.1016/j.envint.2020.105706. [DOI] [PubMed] [Google Scholar]
- Bongaerts E, Nawrot TS, Van Pee T, Ameloot M, Bové H. 2020. Translocation of (ultra)fine particles and nanoparticles across the placenta; a systematic review on the evidence of in vitro, ex vivo, and in vivo studies. Part Fibre Toxicol 17(1):56, PMID: , 10.1186/s12989-020-00386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman SJ, Nieto SP, Patton WC, Jacobson JD, Corselli JU, Chan PJ. 2005. Development of mammalian embryos exposed to mixed-size nanoparticles. Clin Exp Obstet Gynecol 32(4):222–224, PMID: , https://www.imrpress.com/journal/CEOG/32/4/pii/2005063. [PubMed] [Google Scholar]
- Bouwmeester H, Hollman PCH, Peters RJB. 2015. Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ Sci Technol 49(15):8932–8947, PMID: , 10.1021/acs.est.5b01090. [DOI] [PubMed] [Google Scholar]
- Bouwstra J, Pilgram G, Gooris G, Koerten HK, Ponec M. 2001. New aspects of the skin barrier organization. Skin Pharmacol Appl Skin Physiol 14(suppl 1):52–62, PMID: , 10.1159/000056391. [DOI] [PubMed] [Google Scholar]
- Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, et al. 2019. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun 10(1):3866, PMID: , 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasche S, Bischof W. 2005. Daily time spent indoors in German homes—baseline data for the assessment of indoor exposure of German occupants. Int J Hyg Environ Health 208(4):247–253, PMID: , 10.1016/j.ijheh.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Braun T, Ehrlich L, Henrich W, Koeppel S, Lomako I, Schwabl P, et al. 2021. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics 13(7):921, PMID: , 10.3390/pharmaceutics13070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GA. 2015. Losing sight of science in the regulatory push to ban microbeads from consumer products and industrial use. Integr Environ Assess Manag 11(3):346–347, PMID: , 10.1002/ieam.1645. [DOI] [PubMed] [Google Scholar]
- Bustamante-Montes LP, Borja-Aburto VH, Hernández-Valero MA, García-Fábila MM, Borja-Bustamante P, González-Álvarez R, et al. 2021. Phthalates exposure during pregnancy a study in a Mexican cohort. Toxicol Rep 8:1040–1045, PMID: , 10.1016/j.toxrep.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabernard L, Roscher L, Lorenz C, Gerdts G, Primpke S. 2018. Comparison of Raman and Fourier transform infrared spectroscopy for the quantification of microplastics in the aquatic environment. Environ Sci Technol 52(22):13279–13288, PMID: , 10.1021/acs.est.8b03438. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang J, Peng J, Tan Z, Zhan Z, Tan X, et al. 2017. Characteristic of microplastics in the atmospheric fallout from Dongguan City, China: preliminary research and first evidence. Environ Sci Pollut Res Int 24(32):24928–24935, PMID: , 10.1007/s11356-017-0116-x. [DOI] [PubMed] [Google Scholar]
- Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF. 2020. A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health 17:1212, PMID: , 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright L, Poulsen MS, Nielsen HM, Pojana G, Knudsen LE, Saunders M, et al. 2012. In vitro placental model optimization for nanoparticle transport studies. Int J Nanomedicine 7:497–510, PMID: , 10.2147/IJN.S26601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassignet EP, Xu X, Zavala-Romero O. 2021. Tracking marine litter with a global ocean model: where does it go? Where Does It Come from? Front Mar Sci 8:414, 10.3389/fmars.2021.667591. [DOI] [Google Scholar]
- Chen Y, Wen D, Pei J, Fei Y, Ouyang D, Zhang H, et al. 2020. Identification and quantification of microplastics using Fourier-transform infrared spectroscopy: current status and future prospects. Curr Opin Environ Sci Health 18:14–19, 10.1016/j.coesh.2020.05.004. [DOI] [Google Scholar]
- Coffin S, Wyer H, Leapman JC. 2021. Addressing the environmental and health impacts of microplastics requires open collaboration between diverse sectors. PLoS Biol 19(3):e3000932, PMID: , 10.1371/journal.pbio.3000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti GO, Ferrante M, Banni M, Favara C, Nicolosi I, Cristaldi A, et al. 2020. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environmental Research 187(7):10967, 10.1016/j.envres.2020.109677. [DOI] [PubMed] [Google Scholar]
- Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. 2019. Human consumption of microplastics. Environ Sci Technol 53(12):7068–7074, PMID: , 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- Crippa M, De Wilde B, Koopmans R, Leyssens J, Linder M, Muncke J, et al. 2019. A Circular Economy for Plastics: Insights from Research and Innovation to Inform Policy and Funding Decisions. De Smet M, Linder M, eds. Brussels, Belgium: European Commission. https://op.europa.eu/en/publication-detail/-/publication/33251cf9-3b0b-11e9-8d04-01aa75ed71a1/language-en# [accessed 23 December 2021]. [Google Scholar]
- Dehghani S, Moore F, Akhbarizadeh R. 2017. Microplastic pollution in deposited urban dust, Tehran metropolis, Iran. Environ Sci Pollut Res Int 24:20360–20371, PMID: , 10.1007/s11356-017-9674-1. [DOI] [PubMed] [Google Scholar]
- Danopoulos E, Jenner LC, Twiddy M, Rotchell JM. 2020. Microplastic contamination of seafood intended for human consumption: a systematic review and meta-analysis. Environ Health Perspect 128(12):126002, PMID: , 10.1289/EHP7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJA. 2004. Nanotoxicology. Occup Environ Med 61(9):727–728, PMID: , 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CD, Chen CW, Chen YC, Chen HH, Lee JS, Lin CH. 2020. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J Hazard Mater 385:121575, PMID: , 10.1016/j.jhazmat.2019.121575. [DOI] [PubMed] [Google Scholar]
- Dris R, Gasperi J, Mirande C, Mandin C, Guerrouache M, Langlois V, et al. 2017. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut 221:453–458, PMID: , 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Dris R, Gasperi J, Rocher V, Saad M, Renault N, Tassin B. 2015. Microplastic contamination in an urban area: a case study in greater Paris. Environ Chem 12(5):592–599, 10.1071/EN14167. [DOI] [Google Scholar]
- Edwards L, McCray NL, VanNoy BN, Yau A, Geller RJ, Adamkiewicz G, et al. 2021. Phthalate and novel plasticizer concentrations in food items from U.S. fast food chains: a preliminary analysis. J Expo Sci Environ Epidemiol. Preprint posted online 27 October 2021, PMID: , 10.1038/s41370-021-00392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority). 2016. Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J 14(6):e04501, 10.2903/j.efsa.2016.4501. [DOI] [Google Scholar]
- Eibner W. 2007. International Economic Integration: Selected International Organizations and the European Union. München, Germany: Oldenbourg Verlag. [Google Scholar]
- Eriksson AC, Andersen C, Krais AM, Nøjgaard JK, Clausen PA, Gudmundsson A, et al. 2020. Influence of airborne particles’ chemical composition on SVOC uptake from PVC flooring–time-resolved analysis with aerosol mass spectrometry. Environ Sci Technol 54(1):85–91, PMID: , 10.1021/acs.est.9b04159. [DOI] [PubMed] [Google Scholar]
- Escher BI, Stapleton HM, Schymanski EL. 2020. Tracking complex mixtures of chemicals in our changing environment. Science 367(6476):388–392, PMID: , 10.1126/science.aay6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonbuena C. 2019. “The odour of burning wakes us”: inside the Philippines’ Plastic City. The Guardian, 7 July 2019. https://www.theguardian.com/global-development/2019/jul/08/waste-recycling-smell-pollution-philippines-plastic-city [accessed 23 December 2021].
- Fournier SB, D’Errico JN, Adler DS, Kollontzi S, Goedken MJ, Fabris L, et al. 2020. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part Fibre Toxicol 17(1):55, PMID: , 10.1186/s12989-020-00385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcés M, Cáceres L, Chiappetta D, Magnani N, Evelson P. 2021. Current understanding of nanoparticle toxicity mechanisms and interactions with biological systems. New J Chem 45(32):14328–14344, 10.1039/D1NJ01415C. [DOI] [Google Scholar]
- Gasperi J, Wright SL, Dris R, Collard F, Mandin C, Guerrouache M, et al. 2018. Microplastics in air: are we breathing it in? Curr Opin Environ Sci Health 1:1–5, 10.1016/j.coesh.2017.10.002. [DOI] [Google Scholar]
- Gigault J, El Hadri H, Nguyen B, Grassl B, Rowenczyk L, Tufenkji N, et al. 2021. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat Nanotechnol 16(5):501–507, PMID: , 10.1038/s41565-021-00886-4. [DOI] [PubMed] [Google Scholar]
- Giovanoulis G, Nguyen MA, Arwidsson M, Langer S, Vestergren R, Lagerqvist A. 2019. Reduction of hazardous chemicals in Swedish preschool dust through article substitution actions. Environ Int 130:104921, PMID: , 10.1016/j.envint.2019.104921. [DOI] [PubMed] [Google Scholar]
- Grafmueller S, Manser P, Diener L, Diener PA, Maeder-Althaus X, Maurizi L, et al. 2015a. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ Health Perspect 123(12):1280–1286, PMID: , 10.1289/ehp.1409271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafmueller S, Manser P, Diener L, Maurizi L, Diener PA, Hofmann H, et al. 2015b. Transfer studies of polystyrene nanoparticles in the ex vivo human placenta perfusion model: key sources of artifacts. Sci Technol Adv Mater 16(4):044602, PMID: , 10.1088/1468-6996/16/4/044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber MM, Hirschmugl B, Berger N, Holter M, Radulović S, Leitinger G, et al. 2020. Plasma proteins facilitates placental transfer of polystyrene particles. J Nanobiotechnology 18(1):128, PMID: , 10.1186/s12951-020-00676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. 2018. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 344:179–199, PMID: , 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Hale RC, Seeley ME, Guardia MJL, Mai L, Zeng EY. 2020. A global perspective on microplastics. J Geophys Res Oceans 125(1):e2018JC014719, 10.1029/2018JC014719. [DOI] [Google Scholar]
- Han Y, Song Y, Kim GW, Ha C, Lee J, Kim M, et al. 2021. No prominent toxicity of polyethylene microplastics observed in neonatal mice following intratracheal instillation to dams during gestational and neonatal period. Toxicol Res 37(4):443–450, PMID: , 10.1007/s43188-020-00086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harremoës P, Gee D, MacGarvin M, Stirling A, Keys J, Wynne B, eds. 2002. The Precautionary Principle in the 20th Century: Late Lessons from Early Warnings. 1st ed. Sterling, VA: Earthscan Publications. [Google Scholar]
- Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, et al. 2019. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ Sci Technol 53(3):1039–1047, PMID: , 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- Hartmann NB, Rist S, Bodin J, Jensen LH, Schmidt SN, Mayer P, et al. 2017. Microplastics as vectors for environmental contaminants: exploring sorption, desorption, and transfer to biota. Integr Environ Assess Manag 13(3):488–493, PMID: , 10.1002/ieam.1904. [DOI] [PubMed] [Google Scholar]
- Heacock M, Kelly CB, Asante KA, Birnbaum LS, Bergman ÅL, Bruné MN, et al. 2016. E-waste and harm to vulnerable populations: a growing global problem. Environ Health Perspect 124(5):550–555, PMID: , 10.1289/ehp.1509699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, et al. 2015. Developmental origins of health and disease: integrating environmental influences. Endocrinology 156(10):3416–3421, PMID: , 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm JS, Nishioka M, Brody JG, Rudel RA, Dodson RE. 2018. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res 165:448–458, PMID: , 10.1016/j.envres.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Hesler M, Aengenheister L, Ellinger B, Drexel R, Straskraba S, Jost C, et al. 2019. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol In Vitro 61:104610, PMID: , 10.1016/j.tiv.2019.104610. [DOI] [PubMed] [Google Scholar]
- Hinds WC. 1999. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. 2nd ed. New York, NY: John Wiley & Sons. [Google Scholar]
- Hu J, Qin X, Zhang J, Zhu Y, Zeng W, Lin Y, et al. 2021. Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice. Reprod Toxicol 106:42–50, PMID: , 10.1016/j.reprotox.2021.10.002. [DOI] [PubMed] [Google Scholar]
- Huang JP, Hsieh PCH, Chen CY, Wang TY, Chen PC, Liu CC, et al. 2015. Nanoparticles can cross mouse placenta and induce trophoblast apoptosis. Placenta 36(12):1433–1441, PMID: , 10.1016/j.placenta.2015.10.007. [DOI] [PubMed] [Google Scholar]
- ICRP (International Commission on Radiological Protection). 1994. Human respiratory tract model for radiological protection. A report of a Task Group of the International Commission on Radiological Protection. Ann ICRP 24(1–3):1–482, PMID: . [PubMed] [Google Scholar]
- Inocencio IM, Bischof RJ, Xiang SD, Zahra VA, Nguyen V, Lim T, et al. 2017. Exacerbation of ventilation-induced lung injury and inflammation in preterm lambs by high-dose nanoparticles. Sci Rep 7(1):14704, PMID: , 10.1038/s41598-017-13113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Meeker JD, Huang T, Hauser R, Seely EW, Ferguson KK, et al. 2017. Racial and ethnic variations in phthalate metabolite concentrations across pregnancy. J Expo Sci Environ Epidemiol 27(2):160–166, PMID: , 10.1038/jes.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Vimalkumar K. 2021. A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol (Lausanne) 12:724989, PMID: , 10.3389/fendo.2021.724989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloet SK, Walczak AP, Louisse J, van den Berg HHJ, Bouwmeester H, Tromp P, et al. 2015. Translocation of positively and negatively charged polystyrene nanoparticles in an in vitro placental model. Toxicol In Vitro 29(7):1701–1710, PMID: , 10.1016/j.tiv.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Koelmans B, Pahl S, Backhaus T, Bessa F, van Calster G, Contzen N, et al. 2019. A Scientific Perspective on Microplastics in Nature and Society. Berlin, Germany: SAPEA. [Google Scholar]
- Kraftl P, Lynch I, Jarman P, Menzel A, Walker A, Till R, et al. 2021. So you’re literally taking the piss?! critically analysing and accounting for ethics (and risk) in interdisciplinary research on children and plastics. Child Geogr. Preprint posted online 18 January 2021, 10.1080/14733285.2021.1875124. [DOI] [Google Scholar]
- Kremer AM, Pal TM, Boleij JS, Schouten JP, Rijcken B. 1994. Airway hyper-responsiveness and the prevalence of work-related symptoms in workers exposed to irritants. Am J Ind Med 26(5):655–669, PMID: , 10.1002/ajim.4700260508. [DOI] [PubMed] [Google Scholar]
- Labunska I, Harrad S, Santillo D, Johnston P, Brigden K. 2013. Levels and distribution of polybrominated diphenyl ethers in soil, sediment and dust samples collected from various electronic waste recycling sites within Guiyu town, southern China. Environ Sci Process Impacts 15(2):503–511, PMID: , 10.1039/c2em30785e. [DOI] [PubMed] [Google Scholar]
- Lambert S, Scherer C, Wagner M. 2017. Ecotoxicity testing of microplastics: considering the heterogeneity of physicochemical properties. Integr Environ Assess Manag 13(3):470–475, PMID: , 10.1002/ieam.1901. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Etzel RA. 2013. Textbook of Children’s Environmental Health. Oxford, UK: Oxford University Press. [Google Scholar]
- Lebbie TS, Moyebi OD, Asante KA, Fobil J, Brune-Drisse MN, Suk WA, et al. 2021. E-waste in Africa: a serious threat to the health of children. Int J Environ Res Public Health 18(16):8488, PMID: , 10.3390/ijerph18168488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B. 2019. Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol 53(4):1748–1765, PMID: , 10.1021/acs.est.8b05512. [DOI] [PubMed] [Google Scholar]
- Leslie HA, Depledge MH. 2020. Where is the evidence that human exposure to microplastics is safe? Environ Int 142:105807, PMID: , 10.1016/j.envint.2020.105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbohm ML, Hemminki K, Kyyrönen P. 1985. Spontaneous abortions among women employed in the plastics industry. Am J Ind Med 8(6):579–586, PMID: , 10.1002/ajim.4700080609. [DOI] [PubMed] [Google Scholar]
- Li D, Shi Y, Yang L, Xiao L, Kehoe DK, Gun’ko YK, et al. 2020. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat Food 1(11):746–754, 10.1038/s43016-020-00171-y. [DOI] [PubMed] [Google Scholar]
- Li W, Wang Y, Zhao W. 2010. Application research of TiO2 in plastics. Dev Appl Mater 25:66–68, 10.3969/j.issn.1003-1545.2010.02.015. [DOI] [Google Scholar]
- Liu C, Li J, Zhang Y, Wang L, Deng J, Gao Y, et al. 2019. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ Int 128:116–124, PMID: , 10.1016/j.envint.2019.04.024. [DOI] [PubMed] [Google Scholar]
- Luo T, Wang CY, Pan ZH, Jin CY, Fu ZW, Jin YX. 2019a. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their F1 and F2 offspring. Environ Sci Technol 53(18):10978–10992, PMID: , 10.1021/acs.est.9b03191. [DOI] [PubMed] [Google Scholar]
- Luo T, Zhang Y, Wang C, Wang X, Zhou J, Shen M, et al. 2019b. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ Pollut 255(pt 1):113122, PMID: , 10.1016/j.envpol.2019.113122. [DOI] [PubMed] [Google Scholar]
- Lusher A, Hollman PCH, Mendoza-Hill J. 2017. Microplastics in Fisheries And Aquaculture: Status of Knowledge on their Occurrence and Implications for Aquatic Organisms and Food Safety. Technical Paper 615. Rome, Italy: Food and Agriculture Organization of the United Nations. https://www.fao.org/3/i7677e/i7677e.pdf [accessed 23 December 2021]. [Google Scholar]
- Machado MC, Cheng D, Tarquinio KM, Webster TJ. 2010. Nanotechnology: pediatric applications. Pediatr Res 67(5):500–504, PMID: , 10.1203/PDR.0b013e3181d68e78. [DOI] [PubMed] [Google Scholar]
- Mack MC, Chu MR, Tierney NK, Ruvolo E Jr, Stamatas GN, Kollias N, et al. 2016. Water-holding and transport properties of skin stratum corneum of infants and toddlers are different from those of adults: studies in three geographical regions and four ethnic groups. Pediatr Dermatol 33(3):275–282, PMID: , 10.1111/pde.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhdoumi P, Amin AA, Karimi H, Pirsaheb M, Kim H, Hossini H. 2021. Occurrence of microplastic particles in the most popular iranian bottled mineral water brands and an assessment of human exposure. J Water Process Eng 39:8, 10.1016/j.jwpe.2020.101708. [DOI] [Google Scholar]
- Malmberg B, Leanderson P, Nilsson A, Flodin U. 2000. Powdering floor polish and mucous membrane irritation in secondary school pupils. Int Arch Occup Environ Health 73(7):498–502, PMID: , 10.1007/s004200000176. [DOI] [PubMed] [Google Scholar]
- Mancini AJ. 2004. Skin. Pediatrics 113(suppl 4):1114–1119, PMID: , 10.1542/peds.113.S3.1114. [DOI] [PubMed] [Google Scholar]
- Martinez-Tavera E, Duarte-Moro AM, Sujitha SB, Rodriguez-Espinosa PF, Rosano-Ortega G, Expósito N. 2021. Microplastics and metal burdens in freshwater tilapia (Oreochromis niloticus) of a metropolitan reservoir in Central Mexico: potential threats for human health. Chemosphere 266:128968. Mar, PMID: , 10.1016/j.chemosphere.2020.128968. [DOI] [PubMed] [Google Scholar]
- McDonald AD, Lavoie J, Côté R, McDonald JC. 1988. Spontaneous abortion in women employed in plastics manufacture. Am J Ind Med 14(1):9–14, PMID: , 10.1002/ajim.4700140103. [DOI] [PubMed] [Google Scholar]
- Mintenig SM, Bäuerlein PS, Koelmans AA, Dekker SC, van Wezel AP. 2018. Closing the gap between small and smaller: towards a framework to analyse nano- and microplastics in aqueous environmental samples. Environ Sci Nano 5(7):1640–1649, 10.1039/C8EN00186C. [DOI] [Google Scholar]
- Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E. 2015. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ Sci Technol 49(17):10466–10473, PMID: , 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed Nor NH, Kooi M, Diepens NJ, Koelmans AA. 2021. Lifetime accumulation of microplastic in children and adults. Environ Sci Technol 55(8):5084–5096, PMID: , 10.1021/acs.est.0c07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen NP, Fennell TR, Johnson LM. 2021. Unintended human ingestion of nanoplastics and small microplastics through drinking water, beverages, and food sources. NanoImpact 21:100302, 10.1016/j.impact.2021.100302. [DOI] [PubMed] [Google Scholar]
- Moya J, Bearer CF, Etzel RA. 2004. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics 113(suppl 4):996–1006, PMID: , 10.1542/peds.113.S3.996. [DOI] [PubMed] [Google Scholar]
- Muncke J, Andersson AM, Backhaus T, Boucher JM, Carney Almroth B, Castillo Castillo A, et al. 2020. Impacts of food contact chemicals on human health: a consensus statement. Environ Health 19(1):25, PMID: , 10.1186/s12940-020-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoth C, Aengenheister L, Kucki M, Wick P, Buerki-Thurnherr T. 2016. Nanoparticle transport across the placental barrier: pushing the field forward! Nanomedicine (Lond) 11(8):941–957, PMID: , 10.2217/nnm-2015-0012. [DOI] [PubMed] [Google Scholar]
- Nagre N, Cong X, Pearson AC, Zhao X. 2019. Alveolar macrophage phagocytosis and bacteria clearance in mice. J Vis Exp 145:10.3791/59088, PMID: , 10.3791/59088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel HA, Naidoo T, Akindele EO, Nhiwatiwa T, Fadare OO, Krause S. 2021. Collaboration and infrastructure is needed to develop an African perspective on micro(nano)plastic pollution. Environ Res Lett 16(2):021002, 10.1088/1748-9326/abdaeb. [DOI] [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health). 2013. Prevention through Design. https://www.cdc.gov/niosh/topics/ptd/default.html [accessed 29 September 2021].
- Noventa S, Boyles MSP, Seifert A, Belluco S, Jiménez AS, Johnston HJ, et al. 2021. Paradigms to assess the human health risks of nano- and microplastics. Microplast nanoplast 1 (1):17, PMID: , 10.1186/s43591-021-00011-1.34939039 [DOI] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. 2016. Rayyan—a web and mobile app for systematic reviews. Syst Rev 5(1):210, PMID: , 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Han JS, Park EJ, Seong E, Lee GH, Kim DW, et al. 2020. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol Lett 324:75–85, PMID: , 10.1016/j.toxlet.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Pauly JL, Stegmeier SJ, Allaart HA, Cheney RT, Zhang PJ, Mayer AG, et al. 1998. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol Biomark Prev 7(5):419–428, PMID: . [PubMed] [Google Scholar]
- Perkins DN, Brune Drisse MN, Nxele T, Sly PD. 2014. E-waste: a global hazard. Ann Glob Health 80(4):286–295, PMID: , 10.1016/j.aogh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Pollard AJ, Bijker EM. 2021. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 21(2):83–100, PMID: , 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen MS, Mose T, Maroun LL, Mathiesen L, Knudsen LE, Rytting E. 2015. Kinetics of silica nanoparticles in the human placenta. Nanotoxicology 9(supp1):79–86, PMID: , 10.3109/17435390.2013.812259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata JC. 2018. Airborne microplastics: consequences to human health? Environ Pollut 234:115–126, PMID: , 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Premti A. 2018. Conservation and sustainable use of marine biodiversity of areas beyond national jurisdiction: recent legal developments. UNCTAD Transport and Trade Facilitation Newsletter No. 80. https://unctad.org/news/conservation-and-sustainable-use-marine-biodiversity-areas-beyond-national-jurisdiction-recent [accessed 23 December 2021].
- Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, et al. 2021. Plasticenta: first evidence of microplastics in human placenta. Environ Int 146:106274, PMID: , 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- Renner G, Nellessen A, Schwiers A, Wenzel M, Schmidt TC, Schram J. 2019. Data preprocessing & evaluation used in the microplastics identification process: a critical review & practical guide. TrAC Trends Anal Chem 111:229–238, 10.1016/j.trac.2018.12.004. [DOI] [Google Scholar]
- Renner G, Schmidt TC, Schram J. 2018. Analytical methodologies for monitoring micro(nano)plastics: which are fit for purpose? Curr Opin Environ Sci Health 1:55–61, 10.1016/j.coesh.2017.11.001. [DOI] [Google Scholar]
- Rhoads GG, Ettinger AS, Weisel CP, Buckley TJ, Goldman KD, Adgate J, et al. 1999. The effect of dust lead control on blood lead in toddlers: a randomized trial. Pediatrics 103(3):551–555, PMID: , 10.1542/peds.103.3.551. [DOI] [PubMed] [Google Scholar]
- Riediker M, Zink D, Kreyling W, Oberdörster G, Elder A, Graham U, et al. 2019. Particle toxicology and health—where are we? Part Fibre Toxicol 16(1):19, PMID: , 10.1186/s12989-019-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman CM, Brookson C, Bikker J, Djuric N, Earn A, Bucci K, et al. 2019. Rethinking microplastics as a diverse contaminant suite. Environ Toxicol Chem 38(4):703–711, PMID: , 10.1002/etc.4371. [DOI] [PubMed] [Google Scholar]
- Ryan PG. 2015. A brief history of marine litter research. In: Marine Anthropogenic Litter. Bergmann M, Gutow L, Klages M, eds. Cham, Switzerland: Springer, 1–25. [Google Scholar]
- Sackmann K, Reemtsma T, Rahmberg M, Bunke D. 2018. Impact of European chemicals regulation on the industrial use of plasticizers and patterns of substitution in Scandinavia. Environ Int 119:346–352, PMID: , 10.1016/j.envint.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Saenen ND, Bové H, Steuwe C, Roeffaers MBJ, Provost EB, Lefebvre W, et al. 2017. Children’s urinary environmental carbon load. A novel marker reflecting residential ambient air pollution exposure? Am J Respir Crit Care Med 196(7):873–881, PMID: , 10.1164/rccm.201704-0797OC. [DOI] [PubMed] [Google Scholar]
- Schüepp KG. 2010. The potential harmful and beneficial effects of nanoparticles in children. In: Nanoparticles in Medicine and Environment. Marijnissen JC, Gradon L, eds. Dordrecht, Netherlands: Springer, 211–226. [Google Scholar]
- Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, et al. 2019. Detection of various microplastics in human stool. Ann Intern Med 171(7):453–457, PMID: , 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- Schwaferts C, Niessner R, Elsner M, Ivleva NP. 2019. Methods for the analysis of submicrometer- and nanoplastic particles in the environment. TrAC Trends Anal Chem 112:52–65, 10.1016/j.trac.2018.12.014. [DOI] [Google Scholar]
- Semmler-Behnke M, Kreyling WG, Schulz H, Takenaka S, Butler JP, Henry FS, et al. 2012. Nanoparticle delivery in infant lungs. Proc Natl Acad Sci USA 109(13):5092–5097, PMID: , 10.1073/pnas.1119339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senathirajah K, Attwood S, Bhagwat G, Carbery M, Wilson S, Palanisami T. 2021. Estimation of the mass of microplastics ingested—a pivotal first step towards human health risk assessment. J Hazard Mater 404(pt B):124004, PMID: , 10.1016/j.jhazmat.2020.124004. [DOI] [PubMed] [Google Scholar]
- Sessa F, Polito R, Monda V, Scarinci A, Salerno M, Carotenuto M, et al. 2021. Effects of a plastic-free lifestyle on urinary bisphenol A levels in school-aged children of southern Italy: a pilot study. Front Public Health 9:626070, PMID: , 10.3389/fpubh.2021.626070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AK, Hollander GA, McMichael A. 2015. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282(1821):20143085, PMID: , 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindiku O, Babayemi J, Osibanjo O, Schlummer M, Schluep M, Watson A, et al. 2015. Polybrominated diphenyl ethers listed as Stockholm Convention POPs, other brominated flame retardants and heavy metals in e-waste polymers in Nigeria. Environ Sci Pollut Res Int 22(19):14489–14501, PMID: , 10.1007/s11356-014-3266-0. [DOI] [PubMed] [Google Scholar]
- Smyth SH, Doyle-McCullough M, Cox OT, Carr KE. 2005. Effect of reproductive status on uptake of latex microparticles in rat small intestine. Life Sci 77:3287–3305, PMID: , 10.1016/j.lfs.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Soltani NS, Taylor MP, Wilson SP. 2021. Quantification and exposure assessment of microplastics in Australian indoor house dust. Environ Pollut 283:117064, PMID: , 10.1016/j.envpol.2021.117064. [DOI] [PubMed] [Google Scholar]
- Sripada K. 2017. “Beginning with the smallest intake”: children’s brain development and the role of neuroscience in global environmental health. Neuron 95(6):1242–1245, PMID: , 10.1016/j.neuron.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Stone V, Miller MR, Clift MJD, Elder A, Mills NL, Møller P, et al. 2017. Nanomaterials versus ambient ultrafine particles: an opportunity to exchange toxicology knowledge. Environ Health Perspect 125(10):106002, PMID: , 10.1289/EHP424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storelli MM. 2008. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem Toxicol 46(8):2782–2788, PMID: , 10.1016/j.fct.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Street ME, Bernasconi S. 2021. Microplastics, environment and child health. Ital J Pediatr 47(1):75, PMID: , 10.1186/s13052-021-01034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Wang M, Germ KE, Du HM, Sun WJ, Gao WM, et al. 2015. Health implications of engineered nanoparticles in infants and children. World J Pediatr 11(3):197–206, PMID: , 10.1007/s12519-015-0028-0. [DOI] [PubMed] [Google Scholar]
- Thomas RJ. 2013. Particle size and pathogenicity in the respiratory tract. Virulence 4(8):847–858, PMID: , 10.4161/viru.27172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Razansky D, Estrada GG, Semmler-Behnke M, Beyerle A, Kreyling W, et al. 2009. Surface modification and size dependence in particle translocation during early embryonic development. Inhal Toxicol 21(suppl 1):92–96, PMID: , 10.1080/08958370902942624. [DOI] [PubMed] [Google Scholar]
- Trasande L, Shaffer RM, Sathyanarayana S, Council on Environmental Health, Lowry JA, Ahdoot S, et al. 2018. Food additives and child health. Pediatrics 142(2):e20181410, PMID: , 10.1542/peds.2018-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsydenova O, Bengtsson M. 2011. Chemical hazards associated with treatment of waste electrical and electronic equipment. Waste Manag 31(1):45–58, PMID: , 10.1016/j.wasman.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Tulve NS, Stefaniak AB, Vance ME, Rogers K, Mwilu S, LeBouf RF, et al. 2015. Characterization of silver nanoparticles in selected consumer products and its relevance for predicting children’s potential exposures. Int J Hyg Environ Health 218(3):345–357, PMID: , 10.1016/j.ijheh.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. 2018. Concentrations and migratabilities of hazardous elements in second-hand children’s plastic toys. Environ Sci Technol 52(5):3110–3116, PMID: , 10.1021/acs.est.7b04685. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Labor. 2020. 20 Findings on the Worst Forms of Child Labor. https://www.dol.gov/sites/dolgov/files/ILAB/child_labor_reports/tda2020/2020_TDA_BigBook_Online_optimized.pdf [accessed 23 December 2021].
- UNHCR (United Nations Human Rights Council). 2020. Rights of the child: realizing the rights of the child through a healthy environment. Resolution adopted by the Human Rights Council on 7 October 2020. A/HRC/RES/45/30. Geneva, Switzerland: UNHCR. https://digitallibrary.un.org/record/3888433 [accessed 23 December 2021]. [Google Scholar]
- United Nations. 2021. In images: plastic is forever. https://www.un.org/en/exhibits/exhibit/in-images-plastic-forever [accessed 25 November 2021].
- Vähäkangas K, Myllynen P. 2009. Drug transporters in the human blood-placental barrier. Br J Pharmacol 158(3):665–678, PMID: , 10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberghe L, Janssen CR. 2014. Microplastics in bivalves cultured for human consumption. Environ Pollut 193:65–70, PMID: , 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- van Tulleken CV, Wright C, Brown A, McCoy D, Costello A. 2020. Marketing of breastmilk substitutes during the COVID-19 pandemic. Lancet 396(10259):e58, PMID: , 10.1016/S0140-6736(20)32119-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vethaak AD, Legler J. 2021. Microplastics and human health. Science 371(6530):672–674, PMID: , 10.1126/science.abe5041. [DOI] [PubMed] [Google Scholar]
- Vianello A, Jensen RL, Liu L, Vollertsen J. 2019. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci Rep 9(1):8670, PMID: , 10.1038/s41598-019-45054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, et al. 2016. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387(10017):475–490, PMID: , 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- Vizcaino E, Grimalt JO, Glomstad B, Fernández-Somoano A, Tardón A. 2014. Gestational weight gain and exposure of newborns to persistent organic pollutants. Environ Health Perspect 122(8):873–879, PMID: , 10.1289/ehp.1306758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VKM (Vitenskapskomiteen for mat og miljø). 2019. Microplastics; Occurrence, Levels and Implications for Environment and Human Health Related to Food. Scientific opinion of the Scientific Steering Committee of the Norwegian Scientific Committee for Food and Environment (VKM). VKM report 2019:16. Oslo, Norway: VKM. [Google Scholar]
- Wang A, Abrahamsson DP, Jiang T, Wang M, Morello-Frosch R, Park JS, et al. 2021a. Suspect screening, prioritization, and confirmation of environmental chemicals in maternal-newborn pairs from San Francisco. Environ Sci Technol 55(8):5037–5049, PMID: , 10.1021/acs.est.0c05984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Altenburger R, Backhaus T, Covaci A, Diamond ML, Grimalt JO, et al. 2021b. We need a global science-policy body on chemicals and waste. Science 371(6531):774–776, PMID: , 10.1126/science.abe9090. [DOI] [PubMed] [Google Scholar]
- Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, et al. 2018. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere 193:394–402, PMID: , 10.1016/j.chemosphere.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2018. Air Pollution and Child Health: Prescribing Clean Air. https://apps.who.int/iris/rest/bitstreams/1157950/retrieve [accessed 24 December 2021].
- WHO. 2019. Microplastics in Drinking-Water. https://apps.who.int/iris/rest/bitstreams/1243269/retrieve [accessed 24 December 2021].
- WHO. 2021. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. https://apps.who.int/iris/rest/bitstreams/1371692/retrieve [accessed 24 December 2021]. [PubMed]
- Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, et al. 2010. Barrier capacity of human placenta for nanosized materials. Environ Health Perspect 118(3):432–436, PMID: , 10.1289/ehp.0901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesinger H, Wang Z, Hellweg S. 2021. Deep dive into plastic monomers, additives, and processing aids. Environ Sci Technol 55(13):9339–9351, PMID: , 10.1021/acs.est.1c00976. [DOI] [PubMed] [Google Scholar]
- Wooley PH, Nasser S, Fitzgerald RH Jr.. 1996. The immune response to implant materials in humans. Clin Orthop Relat Res 326:63–70, PMID: , 10.1097/00003086-199605000-00008. [DOI] [PubMed] [Google Scholar]
- Wright SL, Kelly FJ. 2017. Plastic and human health: a micro issue? Environ Sci Technol 51(12):6634–6647, PMID: , 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- Xu JL, Thomas KV, Luo Z, Gowen AA. 2019a. FTIR and Raman imaging for microplastics analysis: state of the art, challenges and prospects. TrAC Trends Anal Chem 119:115629, 10.1016/j.trac.2019.115629. [DOI] [Google Scholar]
- Xu M, Halimu G, Zhang Q, Song Y, Fu X, Li Y, et al. 2019b. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ 694:133794, PMID: , 10.1016/j.scitotenv.2019.133794. [DOI] [PubMed] [Google Scholar]
- Xu XX, Shen XJ, Yang XB, Chen JW, Zhao MD, Liu J, et al. 2020. Rapid analysis of phthalate esters in plastic toys by laser Raman technology. Guang Pu Xue Yu Guang Pu Fen Xi 40(6):1929–1933, 10.3964/j.issn.1000-0593(2020)06-1929-05. [DOI] [Google Scholar]
- Xue J, Zartarian V, Moya J, Freeman N, Beamer P, Black K, et al. 2007. A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal 27(2):411–420, PMID: , 10.1111/j.1539-6924.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Shi H, Li L, Li J, Jabeen K, Kolandhasamy P. 2015. Microplastic pollution in table salts from China. Environ Sci Technol 49(22):13622–13627, PMID: , 10.1021/acs.est.5b03163. [DOI] [PubMed] [Google Scholar]
- Zarus GM, Muianga C, Hunter CM, Pappas RS. 2021. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci Total Environ 756:144010, PMID: , 10.1016/j.scitotenv.2020.144010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Wang L, Kannan K. 2020. Microplastics in house dust from 12 countries and associated human exposure. Environ Int 134:105314, PMID: , 10.1016/j.envint.2019.105314. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang L, Trasande L, Kannan K. 2021. Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ Sci Technol Lett, 10.1021/acs.estlett.1c00559. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao T, Kang S, Sillanpää M. 2019. Importance of atmospheric transport for microplastics deposited in remote areas. Environ Pollut 254 (part A): 112953, 10.1016/j.envpol.2019.07.121. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xi JX, Simpson J, Irshad H, Cheng YS. 2013. Aerosol deposition in a nasopharyngolaryngeal replica of a 5-year-old child. Aerosol Sci and Technol 47:275–282, 10.1080/02786826.2012.749341. [DOI] [Google Scholar]
- Zhu Y, Wssan Y, Li Y, Zhang B, Zhou A, Cai Z, et al. 2016. Free and total urinary phthalate metabolite concentrations among pregnant women from the Healthy Baby Cohort (HBC), China. Environ Int 88:67–73, PMID: , 10.1016/j.envint.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Zimmermann L, Bartosova Z, Braun K, Oehlmann J, Völker C, Wagner M. 2021. Plastic products leach chemicals that induce in vitro toxicity under realistic use conditions. Environ Sci Technol 55(17):11814–11823, PMID: , 10.1021/acs.est.1c01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L, Dierkes G, Ternes TA, Völker C, Wagner M. 2019. Benchmarking the in vitro toxicity and chemical composition of plastic consumer products. Environ Sci Technol 53(19):11467–11477, PMID: , 10.1021/acs.est.9b02293. [DOI] [PubMed] [Google Scholar]
- Zimmermann L, Dombrowski A, Völker C, Wagner M. 2020. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ Int 145:106066, PMID: , 10.1016/j.envint.2020.106066. [DOI] [PubMed] [Google Scholar]
- Zota AR, Shamasunder B. 2017. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol 217(4):418.e1–e6, PMID: , 10.1016/j.ajog.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccarello P, Ferrante M, Cristaldi A, Copat C, Grasso A, Sangregorio D, et al. 2019. Exposure to microplastics (<10 μm) associated to plastic bottles mineral water consumption: The first quantitative study. Water Res 157:365–371, PMID: , 10.1016/j.watres.2019.03.091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.