Visual Abstract
Abstract
Pregnancy and childbirth pose an important hemostatic challenge for women with von Willebrand disease (VWD) and can be associated with an increased risk of maternal and neonatal bleeding complications. VWD is a genetically and clinically heterogeneous bleeding disorder caused by a deficiency or an abnormality in the function of von Willebrand factor. Understanding inheritance pattern, hemostatic response to pregnancy, and response to treatment is essential for provision of individualized obstetric care and optimal outcome. A multidisciplinary approach to management with a close liaison between the obstetric team and the hemophilia treatment center is required for continuity of care from preconception counseling through to antenatal, peripartum, and postpartum care. Delivery plan must be coordinated by the multidisciplinary team and include decisions on place and mode of delivery, implementation of safe analgesia/anesthesia, and peripartum hemostasis. In this clinical case-based review, we aim to deliver evidence-based practical guidance for challenges encountered during pregnancy and management of childbirth and puerperium.
Learning Objectives
Evaluate the clinical challenges in the management of pregnancy and childbirth in VWD
Recognize heterogeneity in the maternal and fetal risks in different types of VWD
Discuss multidisciplinary management and best practice for optimal maternal and neonatal outcome
CLINICAL CASE
A 20-year-old woman with severe type 2M von Willebrand disease (VWD) is pregnant at 10 weeks' gestation. This is her first pregnancy, and she is seen in the multidisciplinary clinic for women with bleeding disorders at the hemophilia treatment center (HTC) to discuss and plan management of pregnancy and delivery.
Preconceptional care and prenatal diagnosis
Understanding different types of inherited bleeding disorders (IBDs), their inheritance pattern, and maternal and fetal/neonatal bleeding risks is important for appropriate counseling in relation to prenatal diagnosis (PND) and planning management of pregnancy and childbirth. Ideally, women with IBDs should be seen preconceptionally, to review their historic diagnosis, perform genetic testing when applicable, and assess their bleeding risk and response to treatment.
The inheritance of most cases of type 1, 2A, 2B, and 2M VWD is autosomal dominant, with a 50% chance of an affected fetus in each pregnancy. Types 2N and 3 are inherited in an autosomal recessive manner,1 and there is a 25% risk of having an affected child in each pregnancy when both parents are carriers of the genetic mutation. Genetic testing and PND are not routinely performed in VWD but are considered in families with type 3 VWD.2 PND in late pregnancy (usually at 34 weeks' gestation) is also considered in women with severe types of VWD if the mutation is known to assist management of delivery3,4; for example, if the baby is affected, delivery can be planned in a tertiary center, or delivery can be managed without any restrictions and possibly in the local maternity unit if the baby is unaffected.
CLINICAL CASE (continued)
The patient was seen for preconception counseling in the previous year. Her diagnosis was confirmed to be type 2M. Her baseline factor VIII (FVIII) level was 0.58 IU/mL, von Willebrand factor (VWF):antigen (Ag) was 0.28 IU/mL, VWF:Ristocetin cofactor (RCo) was <0.4 IU/mL, and VWF:collagen binding was 0.18 IU/mL, with normal multimeric analysis. Genetic analysis had confirmed deletion on allele 4222_4224delAAG. She was counseled by a multidisciplinary obstetric/hematology team about inheritance, bleeding risks for her and her baby, and management of pregnancy.
Changes in coagulation factors in pregnancy
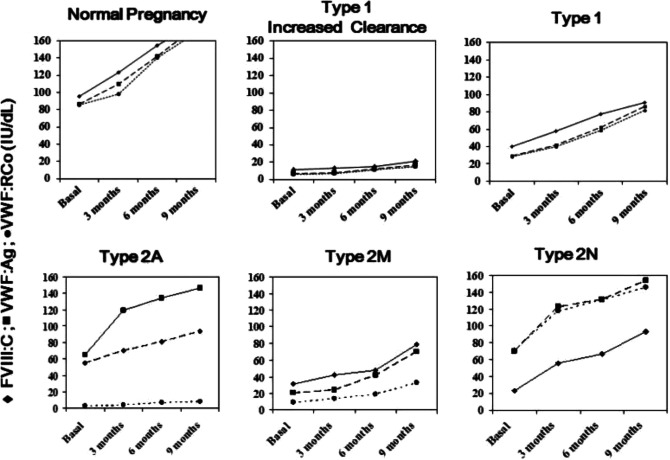
There are several physiological adaptations from early stages of pregnancy in preparation for labor and delivery. Hypercoagulability is one of the physiologic changes that involves a progressive increase in levels of VWF and FVIII throughout pregnancy, with a peak at the time of delivery.5 This pregnancy-induced rise in VWF and FVIII is also seen in most pregnant women with VWD but not to the same extent that is seen in healthy pregnant controls.6 In addition, due to the heterogeneity of genotypic and phenotypic features of VWD, each type exhibits a different response to pregnancy.
Women with type 1 VWD with a prepregnancy baseline VWF and FVIII level of >0.3 IU/mL are likely to achieve factor levels within the normal range by the end of pregnancy.6 In type 2 VWD, WVF and FVIII levels increase significantly, but lack of high molecular weight multimers does not change and the VWF:RCo remains reduced.7 Pregnancy may also exacerbate preexisting thrombocytopenia in type 2B VWD.8 Both VWF and FVIII levels go up in type 2N VWD, but FVIII is usually lower in comparison to VWF in most cases.9 Women with type 3 VWD typically do not show any increase in FVIII and VWF during pregnancy10 (Figure 1).
Figure 1.
Modifications of FVIII and VWF levels during normal pregnancy and in women with the more frequent types of VWD.10
CLINICAL CASE (continued)
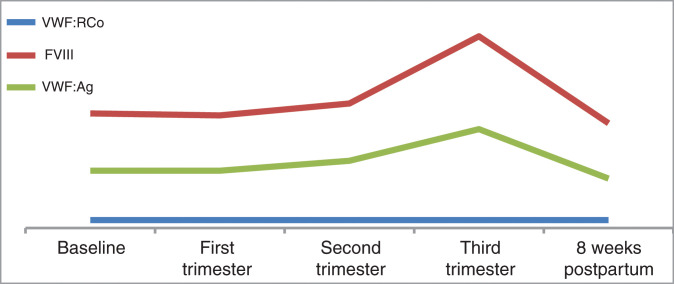
In our patient, changes in her factor levels were congruent with changes described in type 2 VWD; VWF:Ag and FVIII levels increased, whereas VWF:RCo remained unchanged.
First trimester: FVIII: 0.57 IU/mL, VWF:RCo: <0.4 IU/mL, and VWF:Ag: 0.29 IU/mL
Second trimester: FVIII: 0.64 IU/mL, VWF:RCo: <0.4 IU/mL, and VWF:Ag: 0.34 IU/mL
Third trimester: FVIII: 0.97 IU/mL, VWF:RCo: <0.4 IU/mL, and VWF:Ag: 0.50 IU/mL
Antenatal management
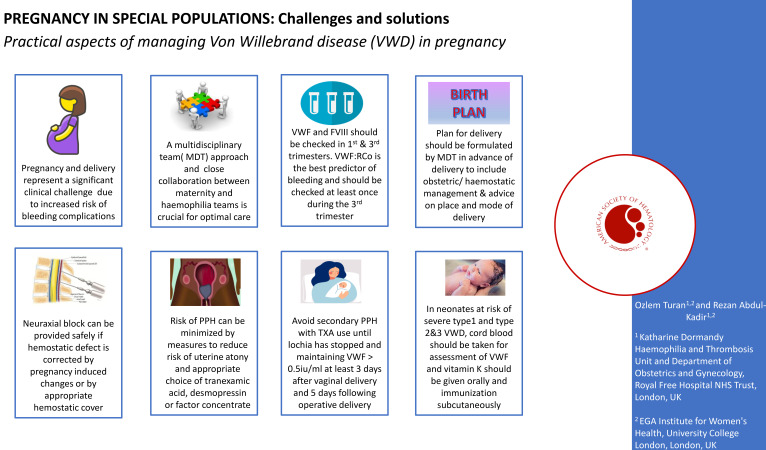
Women with all types of IBDs require a multidisciplinary team (MDT) approach for management of pregnancy and delivery, as well as a close collaboration between the obstetric team and HTC.11,12 Women with VWD do not appear to have an increased risk of miscarriage or antepartum hemorrhage7,13-15 and do not usually require prophylactic treatment in pregnancy; thus, antenatal care can be provided in their local obstetric units in collaboration with the HTC, except those with severe type 2 and 3 VWD, who require care in a tertiary center.16
There are a number of common early pregnancy complications associated with high risk of hemorrhagic sequalae. Miscarriage is the most prevalent one that affects up to 15% of all pregnancies before 12 weeks' gestation.17 In women with VWD, the pregnancy-induced rise in factor levels is not achieved in the first half of pregnancy, and they remain at an increased risk of bleeding after a miscarriage or invasive PND procedures. In these circumstances, it is vital to assess their factor levels and consider provision of hemostatic support.16
Antenatal monitoring should include checking VWF:Ag and FVIII:procoagulant activity at least during their first visit and the third trimester of pregnancy (32-34 weeks), unless first-trimester levels are already within the normal range.10,15,18 VWF:RCo is the best predictor of bleeding risk and should be checked at least once during the third trimester, especially in women with type 2 VWD. Women with type 2B VWD should also have monitoring of their platelet count.
Women with VWD are not at an increased risk of obstetric complications.19 A national database from the United States, including 4067 deliveries, found no increase in fetal growth restriction, placental abruption, or preterm birth in women with VWD.19 Therefore, they do not require additional obstetric assessment or monitoring.
Iron deficiency and iron-deficiency anemia, secondary to heavy menstrual bleeding, is a common diagnosis among women with VWD. There is an increased iron requirement, especially at late stages of pregnancy. Anemia is associated with adverse fetal and maternal outcome, including risk of primary postpartum hemorrhage (PPH).20-22 In a cohort study of 506 women with VWD, 28% of those who experienced PPH were anemic antenatally.23 Therefore, regular monitoring and correction of anemia and iron deficiency are a very important part of antenatal care.
Women with IBDs should be reviewed by the MDT team in the third trimester of pregnancy (32-34 weeks) ahead of the expected date of delivery for a delivery plan. The plan should include details of place and mode of delivery (MOD), hemostatic cover for delivery and postpartum period, pain relief and anesthesia, and management of mother and baby during labor and after delivery. The plan is agreed on with the mother and communicated to all involved in her care.
Management of labor and delivery
Women with type 1 VWD who have a VWF:RCo level >0.5 IU/mL at 32 to 34 weeks' gestation and a mild bleeding phenotype can plan delivery in their local maternity unit unless they have additional bleeding risk such as previous PPH.16 However, for women with a severe IBD, including type 2 and 3 VWD, or those carrying a baby potentially at risk of a severe disorder, it is recommended that they deliver in a maternity unit with an affiliated HTC, where the necessary expertise in management and resources for laboratory testing and clotting factor replacement are readily available.16,24
The aim of planning the MOD is to achieve the least trauma to the mother and infant. VWD is usually not an indication for a cesarean section, and the decision for MOD should be guided by obstetric indications.18,25 Unlike hemophilia, there is a lack of data on MOD and risk of cranial bleeding for fetuses at risk of severe VWD (types 2 and 3). Labor and delivery are associated with an increase in VWF and FVIII in neonates,26 and thus neonates with mild type 1 VWD have a negligible bleeding risk.4 Neonates with type 2 and 3 and severe type 1 VWD still have reduced levels of VWF and FVIII at birth and have a theoretical risk of increased bleeding.27 Despite this theoretical risk, bleeding risks in neonates with VWD, including type 3 VWD, are uncommon, and life-threatening intracranial hemorrhage is very rare. Small case series, including 6 from Canada and 2 from the United Kingdom, show that there were no neonatal cases of intracranial hemorrhage in infants with VWD.27,28 However, cephalohematoma has been reported.29,30 In a cohort of 113 pediatric patients (aged 0-16 years) with VWD, cephalohematoma at birth was reported in 10 of 113 (9%) children29; 50% of them were born by a ventouse delivery. In the same study, bleeding from invasive monitoring using a fetal scalp electrode was reported in 2 cases. In another study, 5 of 7 (71%) children had a cephalohematoma after being born by a ventouse delivery.30 Therefore, precautions to reduce hemorrhagic risk in the newborns need to be implemented. These measures are presented in Table 1, based on risk stratification and recommendations by the Royal College of Obstetricians and Gynaecologists guidelines on management of IBDs in pregnancy.16
Table 1.
Risk stratification of bleeding risk for the fetus/neonate in women with VWD16
| Risk level | Possible or confirmed fetal diagnosis | Suggested management of delivery |
|---|---|---|
| High | Type 3 VWD | • Discuss MOD, taking maternal and fetal factors into consideration, but avoid midcavity forceps, ventouse delivery; FBS, FSE, rotational forceps, and external cephalic version |
| Medium | Type 2 VWD | • Avoid midcavity forceps, ventouse, rotational forceps, and external cephalic version • Judicious use of FBS and FSE to facilitate vaginal delivery |
| Mild | Clinically moderate or severe type 1 VWD in family | • Consider avoidance of ventouse and external cephalic version • Judicious use of rotational forceps, FBS, and FSE |
| Unlikely to be at risk | Clinically mild type 1 VWD in family | • No special precautions |
FBS, fetal blood sampling; FSE, fetal scalp electrode.
Neuraxial analgesia/anesthesia
Neuraxial analgesia provides effective pain control in labor, but there is a concern of a theoretical increased risk of spinal canal hematoma in women with bleeding disorders due to their coagulation defect. In total, 161 550 epidurals were sited for obstetric analgesia during a 2-week national audit period in the United Kingdom, with a reported incidence of 5 cases of spinal canal hematoma. There were zero cases of spinal canal hematoma out of a total of 133 525 obstetric spinal anesthesia sited during the same audit period.31 On the other hand, in the event of an operative delivery, the alternative to neuraxial anesthesia would be general anesthesia, which is associated with an 8 times higher risk of uterine atony and PPH, a 50 times higher risk of failed intubation, and low neonatal Apgar scores.32 In women with IBDs, the data on the risk of spinal canal hematoma are scarce and limited to case reports and small case series, all showing a safe administration of neuraxial analgesia/anesthesia when the coagulation factors are normalized due to physiologic changes of pregnancy or with appropriate hemostatic cover. Two of the largest series included 15 and 33 cases of VWD,33,34 with no hemostatic treatment or only tranexamic acid (TXA) for those with mild VWD but factor concentrate administration for moderate and severe cases.
The UK guideline on the management of women with IBDs recommends avoidance of neuraxial block in women with VWD unless VWF activity is more than 0.5 IU/mL and the hemostatic defect has been corrected.16 A target VWF activity level of 0.50 to 1.50 IU/mL is recommended to allow neuraxial block, and VWF activity should be maintained above 0.50 IU/mL while the neuraxial catheter is in place and for at least 6 hours after removal.35 In cases with a mild risk of bleeding (ie, type 1 VWD with corrected VWF activity >0.5 IU/mL), hemostatic support with TXA is sufficient.16 In women with type 2 and 3 VWD, adequate hemostasis may not be achieved despite factor replacement and normal laboratory VWF activity level; such cases should be individually assessed by an MDT with expertise in VWD.
Management of obstetric analgesia and anesthesia in women with VWD requires a prior individualized risk assessment and planning by the MDT. Risks and benefits of neuraxial block and its alternatives should be considered to help the mother make an informed decision. Table 2 summarizes conditions for the use of neuraxial block in women with IBDs.
Table 2.
Conditions for the use of regional block in women with IBDs during labor and delivery36
| Multidisciplinary management involving hematologists, anesthetists, obstetricians, and the mother |
| Detailed counseling on the benefits and risks of regional block and its alternatives to help the mother make an informed decision |
| Careful assessment of coagulation status, including assessment of clotting factor during the third trimester, and bleeding phenotype, including personal and family bleeding history |
| Availability of therapeutic products and laboratory facilities to ensure adequate response to treatment |
| Plan of management made antenatally during the third trimester, clearly documented, and readily available to professionals attending the woman in labor |
| Normalization of coagulation defect by either a pregnancy-induced rise in coagulation factors or the use of appropriate prophylactic treatment prior to regional block procedures |
| Meticulous technical skills in the administration of regional block by an experienced anesthetist |
| Where an epidural catheter is placed, adequate hemostasis should be maintained prior to catheter removal, as the risk of bleeding is no less than with insertion |
| Awareness and surveillance for symptoms and signs of potential complications |
Postpartum hemorrhage
PPH is defined as blood loss of 500 mL or more within 24 hours of childbirth and complicates on average 3% to 6% of all deliveries.35,37 Lack of consistency in the definition used for PPH in the VWD literature was recognized by a multidisciplinary panel working on the recent VWD guideline38; the panel has proposed a PPH definition as “blood loss of 1000 mL or more within 24 hours of birth or any blood loss with the potential to produce hemodynamic instability” to be used in future VWD research.38 Women with VWD have a greater risk of experiencing PPH,39 and a recent systematic review reported an incidence of primary PPH of 34% among 811 deliveries in women with VWD.40 In a large national cohort study including 4067 women with VWD,19 the risk of PPH was 6% in women with VWD compared with 4% in those without VWD, and women with VWD were 5 times more likely to receive a blood transfusion after childbirth.19
Although the etiology for PPH is often multifactorial, uterine atony is the commonest obstetric cause.41 Other common obstetric causes are retained placental tissue and genital tract trauma. In women with IBDs, coagulation defect can be the primary or contributing factor to PPH. Prevention of PPH should include obstetric measures to minimize risk of uterine atony and birth trauma as well as hemostatic measures to correct their coagulation defect. Active management of the third stage of labor, which incorporates prophylactic use of uterotonic (oxytocin), early clamping, and controlled cord traction to deliver the placenta, reduces the risk of severe primary PPH greater than 1000 mL42 and should be implemented for all women with IBDs. Additional uterotonic agents such as prostaglandin E2 (misoprostol) have been recommended for prophylactic use in women with moderate and severe IBDs.25 Obstetric management should also include avoidance of factors that increase the risk of PPH, such as prolonged labor, maternal birth tract injury during vaginal delivery, and assurance of adequate surgical hemostasis during repair or during cesarean delivery.
Hemostatic management includes prior assessment of PPH risk and planning an appropriate level of hemostatic cover. In women with VWD, risk assessment should be individualized, taking into consideration the VWF level during the third trimester, bleeding phenotype, previous response to treatment, and obstetric risk factors for PPH (eg, multiple pregnancy, placenta previa). Several studies have shown a high rate of PPH in women with VWD, with a third-trimester VWF of <0.5 IU/dL despite prophylactic treatment with VWF concentrates (plasma derived or recombinant).6,43-45 However, these studies provide no data on treatment regimes, target VWF activity and factor-level monitoring during treatment, cause of PPH, and the use of other treatments such as uterotonics and TXA.
There is an increased systemic and local fibrinolysis after childbirth, and TXA inhibits fibrinolysis and reduces blood loss and mortality with PPH.46 Prophylactic use of TXA is recommended for all women with VWD: alone for those with a VWF level >0.5 IU/mL and in conjunction with desmopressin or VWF concentrate if VWF:RCo is <0.5 IU/mL.16,35 This can be started at the onset of established labor and given 6 hourly and continued for several weeks until lochia is light. The intravenous route may be preferred due to slower gastric emptying and increased risk of vomiting in labor.
Desmopressin increases circulatory levels of VWF and FVIII due to release of an endothelial store of VWF. It is a valid treatment option for VWD, primarily type 1 VWD and selected type 2 cases. It is effective in patients with baseline VWF and FVIII levels higher than 0.10 IU/mL, but due to variability in response, a prior test dose is recommended.10,35 Desmopressin is generally contraindicated in type 2B VWD as it can transiently exacerbate thrombocytopenia.47 It is not used in type 3 VWD because the clinical response is very poor or absent.9 A systematic review of 216 pregnancies in 30 studies demonstrated a safe and effective use of desmopressin for the prevention and treatment of bleeding in pregnancy and childbirth in most cases.48 The most common indication for its use was as a prophylactic hemostatic agent for the prevention of PPH in 172 cases with no significant bleeding in 167 deliveries. There were no adverse fetal outcomes. Maternal side effects included well-tolerated facial flashing and headache and only 1 case of water intoxication seizure.48 Thus, fluid intake should be monitored and limited to 1 to 1.5 L in 24 hours, and electrolytes should be monitored if additional fluid is required. Repeated dosing of more than 2 to 3 doses 12 to 24 hours apart should be avoided. Desmopressin should also be avoided if fluid restriction or monitoring is not feasible and in women with preeclampsia due to its vasoconstrictive effect, exacerbating vascular resistance and hypertension.47
Factor replacement is required when desmopressin is ineffective or contraindicated. Treatment with factor concentrate is given when the mother is in active labor and as near to delivery as possible or prior to insertion of a neuraxial catheter. The peak target VWF activity level is aimed at 1.0 IU/mL, and the level should be maintained above 0.5 IU/mL until hemostasis is secured.16 Monitoring of pre- and posttreatment levels of VWF activity and factor VIII levels is recommended after delivery, if labor is prolonged, or in case of excessive bleeding.16 There are a number of plasma-derived products with different VWF/FVIII ratios or recombinant VWF, which is devoid of FVIII. The choice of factor concentrate will depend on the local availability and FVIII levels in the third trimester.9 An increased level of FVIII is associated with a risk of venous thromboembolism (VTE). Products that limit a patient's exposure to FVIII allows for more frequent dosing of VWF if needed, without the risk of VTE secondary to accumulation of FVIII.49 Plasma-derived concentrates with a low FVIII are advisable for women with a high third-trimester FVIII level, such as women with type 2 VWD. Recombinant VWF concentrates contain no FVIII with a longer half-life, now licensed for use in several countries, and they can also be used in these women.9,44 In women with type 2B VWD, platelet count should be checked when in labor and platelet transfusion is recommended to maintain a platelet count of >50 × 109.18
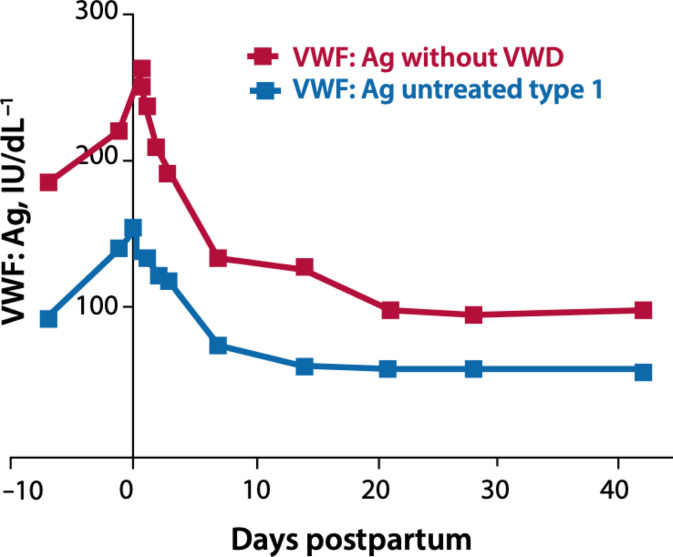
Women with VWD are also at risk of secondary PPH and prolonged and heavy lochia loss during the puerperium.50 A pregnancy-induced rise in VWF and FVIII returns to prepregnancy levels within a few days after delivery6,51 (Figure 2). For women who are receiving factor concentrates, it is important to ensure VWF and FVIII levels are maintained above 0.5 IU/mL for 3 to 5 days after an uncomplicated vaginal delivery and 5 to 7 days following operative deliveries.52 In women with severe VWD, especially type 3 VWD, VWF concentrate may be required for a few weeks after delivery.16 In addition, TXA 1 g 6 hourly during the puerperium should be considered in all women with VWD until the lochia has stopped. All women should be briefed, with a safety net put in place before discharge regarding increased risk of secondary PPH, and they are advised to report any excessive vaginal bleeding.
Figure 2.
Postnatal changes in VWF.6
Pregnancy and the postpartum period are associated with an increased risk of VTE. Women with VWD were no less likely to experience a pulmonary embolism after childbirth than women without VWD in a large cohort study.19 Therefore, women should be carefully assessed for thrombotic risk postdelivery against their risk of bleeding, and appropriate postnatal VTE prophylaxis should be advised. Low-molecular-weight heparin can be used for women with adequate correction of VWF and FVIII levels, but mechanical methods should be employed when factor levels are less than 0.5 IU/mL.16
Management of neonates
VWF level is raised at birth, making diagnosis of mild VWD difficult in the neonatal period and reducing neonatal bleeding risk. However, factor levels in neonates with severe type 1 and types 2 and 3 remain low, and thus these neonates are at risk of bleeding complications. It is recommended that a cord blood sample is obtained at the time of delivery for assessment of FVIII and VWF antigen and activity in these neonates. Cranial imaging and short-term prophylaxis with factor concentrate should be considered in neonates with type 3 VWD, especially if delivery was potentially traumatic.16 Neonates with reduced VWF are also at risk of muscle bleeds following intramuscular injection, and vitamin K should be administered orally in neonates with type 2 and 3 VWD. Bleeding postvaccination has also been reported in these neonates, and it was the second most frequent pediatric bleeding symptom in 1 study.30 Therefore, all immunizations should be given subcutaneously with the smallest gauge needle.53 Review at the HTC with the pediatric team should be planned and arranged before discharge.
CLINICAL CASE (continued)
The mother opted for a planned cesarean section for fear of labor and to avoid any risk for her baby. After appropriate counseling, a planned cesarean section at 39 weeks' gestation was performed under spinal anesthesia. As her FVIII was high during the third trimester, a plasma-derived concentrate with a low FVIII level was chosen for prophylactic treatment during cesarean section and postnatally. First dose was given prior to insertion of a spinal catheter with 1 g intravenous TXA. Factor concentrate was given 8 hourly for 24 hours, then 12 hourly on day 2, and then daily for 5 days. Tranexamic acid 1 g was given intravenously in the first 24 hours and then orally for 4 weeks or until lochia completely stopped.
She delivered a baby boy weighing 2936 g under spinal anesthesia with no complications. Estimated blood loss at delivery was 450 mL. Cord blood sample indicated that her infant was affected by VWD. Vitamin K was administrated orally. Mother and baby were discharged home on day 3 postnatally with a hemophilia nurse and a community midwife to visit daily for a week.
Conflict-of-interest disclosure
Ozlem Turan: no competing financial interests to declare.
Rezan Abdul Kadir: no competing financial interests to declare.
Off-label drug use
Ozlem Turan: Misoprostol is currently only approved by the Food and Drug Administration (FDA) in the US for the prevention and treatment of gastric ulcers secondary to non-steroidal anti-inflammatory drugs (NSAIDS) use. Misoprostol is also widely used for the prevention and treatment of postpartum haemorrhage.
Rezan Abdul Kadir: Misoprostol is currently only approved by the Food and Drug Administration (FDA) in the US for the prevention and treatment of gastric ulcers secondary to non-steroidal anti- inflammatory drug (NSAID) use. Misoprostol is also widely used for the prevention and treatment of postpartum haemorrhage.
References
- 1.James PD, Goodeve AC. von Willebrand disease. Genet Med. 2011;13(5):365-376. doi: 10.1097/GIM.0b013e3182035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludlam CA, Pasi KJ, Bolton-Maggs P, et al; UK Haemophilia Centre Doctors' Organisation. A framework for genetic service provision for haemophilia and other inherited bleeding disorders. Haemophilia. 2005;11(2): 145-163. doi: 10.1111/j.1365-2516.2005.01070.x. [DOI] [PubMed] [Google Scholar]
- 3.Cutler J, Chappell LC, Kyle P, Madan B. Third trimester amniocentesis for diagnosis of inherited bleeding disorders prior to delivery. Haemophilia. 2013;19(6):904-907. doi: 10.1111/hae.12247. [DOI] [PubMed] [Google Scholar]
- 4.Leebeek FWG, Duvekot J, Kruip MJHA. How I manage pregnancy in carriers of hemophilia and patients with von Willebrand disease. Blood. 2020;136 (19):2143-2150. doi: 10.1182/blood.2019000964. [DOI] [PubMed] [Google Scholar]
- 5.Nowak-Göttl U, Limperger V, Kenet G, et al.. Developmental hemostasis: a lifespan from neonates and pregnancy to the young and elderly adult in a European white population. Blood Cells Mol Dis. 2017;67:2-13. doi: 10.1016/j.bcmd.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 6.James AH, Konkle BA, Kouides P, et al.. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21(1):81-87. doi: 10.1111/hae.12568. [DOI] [PubMed] [Google Scholar]
- 7.Castaman G, Tosetto A, Rodeghiero F. Pregnancy and delivery in women with von Willebrand's disease and different von Willebrand factor mutations. Haematologica. 2010;95(6):963-969. doi: 10.3324/haematol.2009.011239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruse-Jarres R, Johnsen JM. How I treat type 2B von Willebrand disease. Blood. 2018;131(12):1292-1300. doi: 10.1182/blood-2017-06-742692. [DOI] [PubMed] [Google Scholar]
- 9.Castaman G, James PD. Pregnancy and delivery in women with von Willebrand disease. Eur J Haematol. 2019;103(2):73-79. doi: 10.1111/ejh.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castaman G, Goodeve A, Eikenboom J; European Group on von Willebrand Disease. Principles of care for the diagnosis and treatment of von Willebrand disease. Haematologica. 2013;98(5):667-674. doi: 10.3324/haematol.2012.077263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadir RA, Davies J. Hemostatic disorders in women. J Thromb Haemost. 2013;11(suppl 1):170-179. doi: 10.1111/jth.12267. [DOI] [PubMed] [Google Scholar]
- 12.van Galen K, Lavin L, Skouw-Rasmussen N, et al.. European principles of care for women and girls with inherited bleeding disorders. 2021. Accessed June/18/2021. https://eahad.org/eahad-wgbd-poc-webinar-on-9-march/ [DOI] [PubMed]
- 13.Kadir RA, Lee CA, Sabin CA, Pollard D, Economides DL. Pregnancy in women with von Willebrand's disease or factor XI deficiency. Br J Obstet Gynaecol. 1998;105(3):314-321. doi: 10.1111/j.1471-0528.1998.tb10093.x. [DOI] [PubMed] [Google Scholar]
- 14.Lak M, Peyvandi F, Mannucci PM. Clinical manifestations and complications of childbirth and replacement therapy in 385 Iranian patients with type 3 von Willebrand disease. Br J Haematol. 2000;111(4):1236-1239. doi: 10.1046/j.1365-2141.2000.02507.x. [DOI] [PubMed] [Google Scholar]
- 15.Skeith L, Rydz N, O'Beirne M, Goodyear D, Li H, Poon M-C. Pregnancy loss in women with von Willebrand disease: a single-center pilot study. Blood Coagul Fibrinolysis. 2017;28(5):393-397. doi: 10.1097/MBC.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 16.Pavord SR, Rayment R, Madan B, et al.. Management of inherited bleeding disorders in pregnancy. Green-top Guideline No. 71 (joint with UKHCDO). BJOG. 2017;124(8):e193-e263. doi: 10.1111/1471-0528.14592. [DOI] [PubMed] [Google Scholar]
- 17.Quenby S, Gallos ID, Dhillon-Smith RK, et al.. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397(10285):1658-1667. doi: 10.1016/S0140-6736(21)00682-6. [DOI] [PubMed] [Google Scholar]
- 18.Millar CM. Von Willebrand disease. In: Kadir RA, James PD, Lee C, eds. Inherited Bleeding Disorders in Women. 2nd ed. John Wiley; 2019:83-94. [Google Scholar]
- 19.James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5(6):1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x. [DOI] [PubMed] [Google Scholar]
- 20.Kavle JA, Stoltzfus RJ, Witter F, Tielsch JM, Khalfan SS, Caulfield LE. Association between anaemia during pregnancy and blood loss at and after delivery among women with vaginal births in Pemba Island, Zanzibar, Tanzania. J Health Popul Nutr. 2008;26(2):232-240. [PMC free article] [PubMed] [Google Scholar]
- 21.Mirza FG, Abdul-Kadir R, Breymann C, Fraser IS, Taher A. Impact and management of iron deficiency and iron deficiency anemia in women's health. Expert Rev Hematol. 2018;11(9):727-736. doi: 10.1080/17474086.2018.1502081. [DOI] [PubMed] [Google Scholar]
- 22.Nair M, Choudhury MK, Choudhury SS, et al.. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Glob Health. 2016;1(1):e000026. doi: 10.1136/bmjgh-2015-000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malec LM, Moore CG, Yabes J, Li J, Ragni MV. Postpartum haemorrhage in women with von Willebrand disease: an observational study of the Pennsylvania Health Care Cost Containment Council (PHC4) database. Haemophilia. 2015;21(5):e442-e445. doi: 10.1111/hae.12739. [DOI] [PubMed] [Google Scholar]
- 24.Johnsen JM. Current approaches to pregnancy and childbirth in women with von Willebrand disease. Clin Adv Hematol Oncol. 2018;16(4):254-257. [PubMed] [Google Scholar]
- 25.Huq FY, Kadir RA. Management of pregnancy, labour and delivery in women with inherited bleeding disorders. Haemophilia. 2011;17(suppl 1): 20-30. doi: 10.1111/j.1365-2516.2011.02561.x. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni AA, Osmond M, Bapir M, et al.. The effect of labour on the coagulation system in the term neonate. Haemophilia. 2013;19(4):533-538. doi: 10.1111/hae.12115. [DOI] [PubMed] [Google Scholar]
- 27.Labarque V, Stain AM, Blanchette V, Kahr WH, Carcao MD. Intracranial haemorrhage in von Willebrand disease: a report on six cases. Haemophilia. 2013;19(4):602-606. doi: 10.1111/hae.12142. [DOI] [PubMed] [Google Scholar]
- 28.Chalmers E, Alamelu J, Collins P, et al; Paediatric and Rare Disorders Working Parties of UKHCDO. Intracranial haemorrhage in children with inherited bleeding disorders in the UK 2003-2013. J Thromb Haemost. 2018;24(4):641-647. doi: 10.1111/hae.13461. [DOI] [PubMed] [Google Scholar]
- 29.Biss TT, Blanchette VS, Clark DS, et al.. Quantitation of bleeding symptoms in children with von Willebrand disease: use of a standardized pediatric bleeding questionnaire. J Thromb Haemost. 2010;8(5):950-956. doi: 10.1111/j.1538-7836.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 30.Sanders YV, Fijnvandraat K, Boender J, et al; WiN Study Group. Bleeding spectrum in children with moderate or severe von Willebrand disease: relevance of pediatric-specific bleeding. Am J Hematol. 2015;90(12):1142-1148. doi: 10.1002/ajh.24195. [DOI] [PubMed] [Google Scholar]
- 31.Cook TM, Counsell D, Wildsmith JA; Royal College of Anaesthetists Third National Audit Project. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102(2):179-190. doi: 10.1093/bja/aen360. [DOI] [PubMed] [Google Scholar]
- 32.Delgado C, Ring L, Mushambi MC. General anaesthesia in obstetrics. BJA Educ. 2020;20(6):201-207. doi: 10.1016/j.bjae.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd SC, O'Connor AD, Horan MA, et al.. Analgesia, anaesthesia and obstetric outcome in women with inherited bleeding disorders. Eur J Obstet Gynecol Reprod Biol. 2019;239:60-63. doi: 10.1016/j.ejogrb.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 34.Chi C, Lee CA, England A, Hingorani J, Paintsil J, Kadir RA. Obstetric analgesia and anaesthesia in women with inherited bleeding disorders. Thromb Haemost. 2009;101(6):1104-1111. [PubMed] [Google Scholar]
- 35.Connell NT, Flood VH, Brignardello-Petersen R, et al.. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301-325. doi: 10.1182/bloodadvances.2020003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouthors AS, England A, Kadir RA. Analgesia and anaesthesia for pregnant women with inherited bleeding disorders. In: Kadir RA, James PD, Lee C, eds. Inherited Bleeding Disorders in Women. 2nd ed. John Wiley; 2019:191-202. [Google Scholar]
- 37.Knight M, Callaghan WM, Berg C, et al.. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connell NT, James PD, Brignardello-Petersen R, et al.. von Willebrand disease: proposing definitions for future research. Blood Adv. 2021;5(2):565-569. doi: 10.1182/bloodadvances.2020003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James AH. More than menorrhagia: a review of the obstetric and gynaecological manifestations of bleeding disorders. Haemophilia. 2005;11(4):295-307. doi: 10.1111/j.1365-2516.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 40.Punt MC, Waning ML, Mauser-Bunschoten EP, et al.. Maternal and neonatal bleeding complications in relation to peripartum management in women with von Willebrand disease: a systematic review. Blood Rev. 2020;39:100633. doi: 10.1016/j.blre.2019.100633. [DOI] [PubMed] [Google Scholar]
- 41.Chandraharan E, Krishna A.. Diagnosis and management of postpartum haemorrhage. BMJ. 2017;358:j3875. doi: 10.1136/bmj.j3875. [DOI] [PubMed] [Google Scholar]
- 42.Begley CM, Gyte GM, Devane D, McGuire W, Weeks A, Biesty LM. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;2:CD007412. doi: 10.1002/14651858.CD007412.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadir RA, Chi C. Women and von Willebrand disease: controversies in diagnosis and management. Semin Thromb Hemost. 2006;32(6):605-615. doi: 10.1055/s-2006-949665. [DOI] [PubMed] [Google Scholar]
- 44.Machin N, Ragni MV. Recombinant vs plasma-derived von Willebrand factor to prevent postpartum hemorrhage in von Willebrand disease. Blood Adv. 2020;4(14):3234-3238. doi: 10.1182/bloodadvances.2020002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoof SC, van Steenbergen HW, Zwagemaker A, et al.. Primary postpartum haemorrhage in women with von Willebrand disease or carriership of haemophilia despite specialised care: a retrospective survey. Haemophilia. 2015;21(4):505-512. doi: 10.1111/hae.12635. [DOI] [PubMed] [Google Scholar]
- 46.Shakur H, Elbourne D, Gülmezoglu M, et al.. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial. Trials. 2010;11:40. doi: 10.1186/1745-6215-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laffan MA, Lester E, O'Donnell JS, et al.. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol. 2014;167(4):453-465. doi: 10.1111/bjh.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trigg DE, Stergiotou I, Peitsidis P, Kadir RA. A systematic review: the use of desmopressin for treatment and prophylaxis of bleeding disorders in pregnancy. Haemophilia. 2012;18(1):25-33. doi: 10.1111/j.1365-2516.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- 49.Coppola A, Franchini M, Makris M, Santagostino E, Di Minno G, Mannucci PM. Thrombotic adverse events to coagulation factor concentrates for treatment of patients with haemophilia and von Willebrand disease: a systematic review of prospective studies. Haemophilia. 2012;18(3):e173-e187. doi: 10.1111/j.1365-2516.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 50.Chi C, Bapir M, Lee CA, Kadir RA. Puerperal loss (lochia) in women with or without inherited bleeding disorders. Am J Obstet Gynecol. 2010;203(1):56.e1-56.e5. doi: 10.1016/j.ajog.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 51.Huq FY, Kulkarni A, Agbim EC, Riddell A, Tuddenham E, Kadir RA. Changes in the levels of factor VIII and von Willebrand factor in the puerperium. Haemophilia. 2012;18(2):241-245. doi: 10.1111/j.1365-2516.2011.02625.x. [DOI] [PubMed] [Google Scholar]
- 52.National Hemophilia Foundation. MASAC Document 265—MASAC Guidelines for Pregnancy and Perinatal Management of Women with Inherited Bleeding Disorders and Carriers of Hemophilia A or B. National Hemophilia Foundation; 2021. [Google Scholar]
- 53.Carcao M, Bouskill V.. The newborn. In: Kadir RA, James PD, Lee C, eds. Inherited Bleeding Disorders in Women. 2nd ed. John Wiley; 2019:205-221. [Google Scholar]