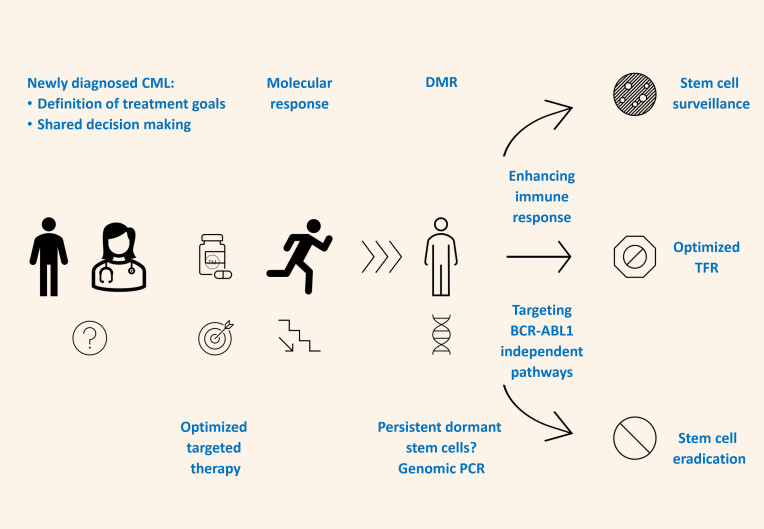
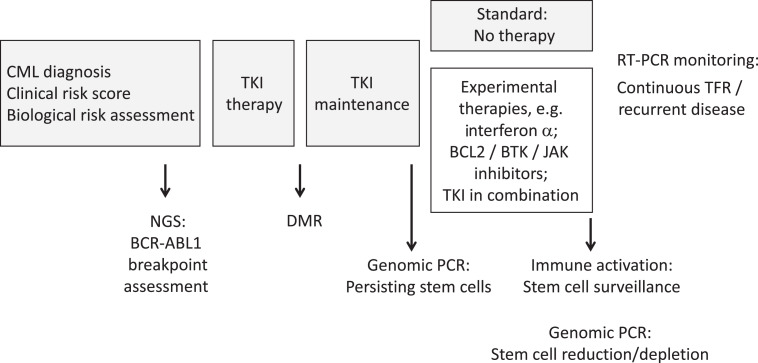
Visual Abstract
Abstract
Treatment-free remission (TFR) is a new and significant goal of chronic myeloid leukemia management. TFR should be considered for patients in stable deep molecular response (DMR) after careful discussion in the shared decision-making process. Second-generation tyrosine kinase inhibitors (TKIs) improve the speed of response and the incidence of DMR. Treatment may be changed to a more active TKI to improve the depth of response in selected patients who have not reached DMR. Stem cell persistence is associated with active immune surveillance and activation of BCR-ABL1-independent pathways, eg, STAT3, JAK1/2, and BCL2. Ongoing studies aim to prove the efficacy of maintenance therapies targeting these pathways after TKI discontinuation.
Learning Objectives
Review the options for achieving deep molecular remission in CML patients, with the possibility of discontinuing therapy and staying in long-term treatment-free remission, which is equivalent to an operational cure
Discuss the biology of persisting leukemic stem cells as the origin of relapse
Define the mechanism of action of experimental therapies to eradicate or silence residual leukemia
Introduction
A significant proportion of chronic myeloid leukemia (CML) patients achieve a deep molecular response (DMR) with tyrosine kinase inhibitor (TKI) therapy defined as residual BCR-ABL1 transcript levels of at least MR4 on the international scale (IS). The speed of response is faster, and the incidence of DMR at specific time points is higher under treatment with second-generation inhibitors (2G-TKIs) than with imatinib.1
An attempt to discontinue therapy can be considered after the achievement and long-term maintenance of DMR. The concept was established with the Stop Imatinib 1 (STIM1) trial. After a median follow-up of 77 months after stopping TKI therapy, 38% of patients maintained a molecular remission. In this trial, patients were eligible for treatment discontinuation with negative results of a sensitive quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) sustained for 2 years before stopping.2,3 After this innovative pilot study, multiple trials were conducted, each with different entry criteria and definitions of relapse, prompting a restart of TKIs. Concurrently, PCR methodology and definitions of PCR sensitivity were standardized in order to harmonize recommendations for treatment discontinuation and increase the safety of stopping procedures.4,5
As a multicenter academic approach, the European Stop Kinase Inhibitor (EURO-SKI, NCT01596114) study analyzed 755 patients with similar overall results. The minimum requirements for stopping in most studies were a duration of TKI therapy of at least 3 years and a sustained DMR of at least 1 year.6
In general, about 50% of patients will relapse, regardless of the TKI used. Most molecular recurrences occur within the first 6 months after TKI discontinuation. However, treatment discontinuation is safe if eligible patients are carefully selected, and high-quality standardized qRT-PCR is employed. The available data and procedures required should be discussed with the patient. Some patients eligible for treatment-free remission (TFR) may prefer to remain on their current treatment. A loss of major molecular response (MMR; BCR-ABL1 > 0.1% IS) has been established as the trigger for restarting therapy.5,7 However, considering that the rate of DMR in a newly diagnosed patient on current treatment options is about 40% and the chance for stable TFR is about 50%, the overall chance of long-term TFR is only 20% for a CML patient newly diagnosed in 2021. In addition, the cost of TKI therapy is a significant problem for patients and the health system in general. Hence, it is crucial to increase the rate of durable DMR and to convert DMR into functional cures, defined as nonrecurrence of active CML after stopping TKIs.8
CLINICAL CASE
Our patient is a 27-year-old woman, a teacher, diagnosed with chronic-phase CML in May 2017. Calculation of the EUTOS long-term survival score revealed low-risk disease. Cytogenetics demonstrated the involvement of 3 chromosomes, 46,XX,t(1;9;22)(q25;q34;q11), but multiplex PCR revealed a typical e14a2 BCR-ABL1 transcript. Using next-generation sequencing, no BCR-ABL1-independent mutations were detected.
Spleen size was normal, and no medication or concomitant diseases were reported. There was a strong desire to establish a family in the coming years. We thoroughly discussed with the patient the diagnosis, treatment options, goals of therapy, and risks and benefits of each treatment option. Shared decision-making led to the conclusion to start nilotinib 300 mg twice daily in June 2017. Treatment was well tolerated; the only side effect was a grade 1 facial exanthema. Blood counts normalized within 4 weeks. BCR-ABL1 transcript levels in peripheral blood were measured in a specialized lab standardized for sensitive quantification according to the IS:
September 2017: 3.2%
December 2017: 0.41%
March 2018: 0.23%
June 2018: 0.072%
December 2018: 0.015%
June 2019: 0.0030%
September 2019: 0.0015%
December 2019: 0.0023%
March 2020: 0.0010%
June 2020: 0% with 96 000 ABL1 transcripts (MR4.5).
At this stage, 3 years after the start of therapy, the patient requested that therapy be discontinued.
Clinical experience
The prospective, nonrandomized EURO-SKI was designed to define optimal conditions for treatment discontinuation. Sixty-one sites in 11 European countries participated. Eligibility criteria included chronic-phase CML, TKI treatment for at least 3 years (without treatment failure according to European LeukemiaNet recommendations), and confirmed MR4 or better for at least 1 year. The primary end point was molecular relapse-free survival, defined as survival without a loss of MMR. Secondary end points were the analysis of prognostic factors affecting MMR maintenance and the economic impact of stopping therapy. Molecular relapse-free survival was 61% and 50% at 6 and 24 months, respectively. In the prognostic analysis of patients who received first-line imatinib therapy, longer treatment duration and longer DMR duration were advantageous for maintenance of MMR over a period of six months. Treatment discontinuation did not result in serious adverse events. For study participants, the overall cost savings were estimated at about €22 million.6
Confirmation of loss of MMR with a second PCR is not recommended and could delay restarting therapy. Almost all patients with molecular recurrence regain their initial MR level after a TKI restart. The choice of TKI for the restart depends on the previous tolerability, comorbidities, risk of long-term toxicity, and expectations toward a second TFR attempt. So far, very few of the thousands of patients in TFR trials have had unfavorable outcomes. There are occasional reports of progression to blast phase, but it is uncertain whether TKI discontinuation contributed to the underlying biology of progression. Irrespective of higher eligibility rates for TFR studies with second-generation TKI, the probability of maintaining TFR has been about 50% after nilotinib or dasatinib therapies, similar to the results after stopping imatinib.9-13
The French RE-STIM trial investigated the outcome of second or subsequent TFR attempts. Seventy patients who regained DMR after molecular recurrence after the first TKI discontinuation stopped treatment for a second time. The proportion of patients without molecular recurrence was 35% at 36 months after the second TKI discontinuation. RE-STIM data actually suggest that patients in TFR losing MMR have a lower chance for a successful second TFR attempt than patients with molecular recurrence according to the original STIM definition (positive PCR).14
Patients' perspective
In general, recommendations for safe discontinuation and treatment restart are well established and published. However, the experience of patients with regard to their decision about TFR and their needs during TFR are widely unknown. Using a survey, international patient advocates sought to check the patient perspective, to identify areas of unmet needs, and to create recommendations for improvements. Fear or anxiety during treatment discontinuation were reported by 56% of patients, but only 7% of patients were asked about their needs for psychological support. After treatment restart, 59% of patients felt scared or anxious, and 56% felt depressed. Twenty-six percent of reinitiated patients received psychological and/or emotional support. Sixty percent of patients experienced withdrawal symptoms after treatment discontinuation. However, 40% of patients with withdrawal symptoms reported a lack of support from their physician in symptom management. Hence, the monitoring of psychological consequences before, during, and after TFR should be considered.15
Musculoskeletal and/or joint pain starting a few weeks or months after TKI discontinuation has been observed in about 25% of patients. This TKI withdrawal syndrome is likely based on a release of off-target effects of the TKI. In most cases symptoms are mild and transient, but some patients may require temporary anti-inflammatory treatment.16 Surveillance and symptomatic treatment of withdrawal symptoms should be a priority during treatment discontinuation.15
Predictors of success
Various prognostic factors for a successful TFR have been reported. Longer duration of TKI therapy and DMR and prior treatment with interferon alpha (IFNA) were identified as important predictors for successful TFR in EURO-SKI; of these, duration of DMR was shown to be the most important factor, corroborating data from a study in Japan.6,17 A Canadian TFR study revealed 6 years of imatinib therapy and 4.5 years MR4 duration as optimal time frames for a successful TFR.18
Age and sex have been reported to be associated with TFR in early studies.6 The Sokal score was reported to predict TFR outcome, eg, in the STIM study.2 However, the EURO-SKI study failed to demonstrate the predictive value of age, sex, and Sokal score for TFR in patients after first-line imatinib. A selection bias of patients on IFNA therapy prior to imatinib might play a role. Data published recently indicate that the BCR-ABL1 transcript type might influence TFR outcome, with a lower incidence of relapse in patients and e14a2 compared to e13a2.19
So far, the duration of first-line nilotinib or dasatinib therapy has not been found to affect the probability of TFR success. The duration of DMR on second-line nilotinib was associated with successful TFR in ENESTop (Table 1).9,10,19
Table 1.
| Parameter | Impact on eligibility for TFR | Impact on stability of TFR | Study examples |
|---|---|---|---|
| Prognostic score | DMR more frequent in low-risk patients | TFR more stable in low-risk patients; risk of relapse high in high-risk patients | STIM, STIM2, ENESTfreedom, TWISTER |
| TKI type | DMR more frequent with 2G-TKIs vs imatinib | No major difference | |
| Initial slope of BCR-ABL1 transcript decline or “halving time” | Probability of DMR better in patients with EMR | No major difference | |
| MMR at 12 months | Probability of DMR better in patients with MMR | No major difference | |
| Type of BCR-ABL1 transcript | DMR more likely in patients with e14a2 vs e13a2 (inconsistent) | TFR more stable with e14a2 (inconsistent) | |
| Mutations outside BCR-ABL1 | MMR and DMR more likely in patients lacking BCR-ABL1- independent mutations | Data still immature | |
| Duration of DMR | Longer DMR predictive | EURO-SKI | |
| Level of MR before stop | Probably yes; undetectable BCR-ABL1 predictive | EURO-SKI, ENESTfreedom | |
| Duration of TKI therapy | Inconsistent, overlapping with duration of DMR | STIM | |
| Proportion of natural killer cells | Reduced risk of relapse after imatinib and dasatinib but not after nilotinib |
EMR, early molecular response.
Differences in trial designs must be considered when results from various studies are compared: the proportion of successful TFR attempts is higher in studies that define disease relapse as loss of MMR than in those that specify loss of undetectable BCR-ABL1 transcripts as the criterion. In addition, a deeper MR prior to treatment discontinuation may be associated with a better chance of successful TFR.17
CLINICAL CASE (continued)
In June 2020, 3 years after starting therapy and 1 year in MR4.5, nilotinib therapy was discontinued. Grade 2 muscle and joint pain, responsive to acetaminophen and lasting about 4 weeks, were signs of a mild TKI withdrawal syndrome.
During follow-up, qRT-PCR revealed an increase of BCR-ABL1 transcripts:
September 2020: 0.06% (IS)
December 2020: 0.15%.
Due to loss of MMR, treatment reintroduction was discussed. Good response and good tolerability of nilotinib was balanced against a second chance with an alternative TKI. The decision was made to commence dasatinib 100 mg/d, leading to a rapid decline of BCR-ABL1 transcripts:
March 2021: 0.0020%.
June 2021: 0% with 290 000 ABL1 transcripts (MR5).
A bone marrow aspiration was performed to quantify persisting stem cells on a genomic level and to characterize their environment. The optimal duration of TKI treatment for the second attempt of TFR is unknown. The inhibition of BCR-ABL1-independent pathways responsible for stem cell persistence and for activation of immune surveillance is a leading topic in current research.
Analyzing persistent stem cells
In several studies, highly sensitive DNA-based BCR-ABL1 PCR demonstrated persistence of the CML clone in most patients with negative qRT-PCR, indicating low BCR-ABL1 expression in persisting CML stem cells. The detection of the patient-specific genomic BCR-ABL1 break point requires a DNA sample collected at diagnosis.20-22 The analysis of bone marrow vs peripheral blood for this purpose is ongoing.23
In a European study, DNA and mRNA BCR-ABL1 levels by quantitative PCR and by digital PCR were compared. A good correlation was found at BCR-ABL1 transcript levels above MR4; the correlation was poor for samples taken in DMR. A combination of genomic and RT-PCR was able to better predict molecular relapse-free survival after TKI stop.24
Leukemic stem cells are CD34+/CD38−, but this phenotype is not exclusive to CML. Dipeptidyl peptidase-4 (CD26) may represent a specific marker for CML stem cells.25 Using the CD34+CD38−CD26+ phenotype, a study identified CML stem cells circulating in the peripheral blood in the majority of patients in TFR after TKI discontinuation,26 which is, however, still conflicting. Another study identified persisting leukemic stem cells in the bone marrow of patients in DMR, in contrast to the peripheral blood.23 Further, CD93 expression has been consistently shown in a lin-CD34+CD38−CD90+ population with stem cell characteristics.27
Using fluorescence-activated cell sorting and genomic PCR, residual disease in TFR differed according to the cell lineage. BCR-ABL1 was identified predominantly in the lymphoid compartment and never in granulocytes. B cells were more often BCR-ABL1+ than T cells. Lineage-specific assessment of persisting disease prior to treatment discontinuation could improve the prediction of successful TFR.28
Innovative approaches
JAK, BTK
In TKI-resistant CML, STAT3 inhibition was able to reduce survival of CML cells. Kuepper et al identified Jak1 but not Jak2 as the STAT3-activating kinase. The combination of BCR-ABL1 and JAK1 inhibitors further reduced colony-forming units from murine CML bone marrow and human CML mononuclear cells as well as CD34+ CML cells. Combination therapy induced apoptosis even in quiescent leukemic stem cells, suggesting JAK1 as a potential therapeutic target for curative CML therapies.29 Ruxolitinib, a dual JAK1/2 inhibitor, combined with nilotinib may selectively decrease CML stem cells.25 A few early-phase studies testing the combination of BCR-ABL1 targeting TKIs with ruxolitinib were prematurely closed due to toxicity; others are still recruiting (Table 2). Fc gamma receptor IIb (CD32b) was shown to be critical in stem cell persistence. Hence, targeting FcγRIIb downstream signaling, eg, with a BTK inhibitor, provides a promising therapeutic approach.30
Table 2.
Clinical trials to maintain TFR in addition to TKI
| TIGER nilotinib ± peg-IFNA2b (phase 3) | NCT01657604 |
| PETALs nilotinib ± peg-IFNα2a (phase 3) | NCT02201459 |
| IFNA maintenance therapy | |
| TIGER peg-IFNA2b (phase 3) | NCT01657604 |
| ENDURE pegylated-proline-IFNA2b (phase 3) | NCT03117816 |
| Checkpoint inhibitors | |
| ACTIW pioglitazone (phase 1), avelumab (phase 2) | NCT02767063 |
| Dasatinib nivolumab (phase 1B) | NCT02011945 |
| JAK inhibition | |
| Preclinical data | |
| Nilotinib + ruxolitinib phase 1/2 | NCT01914484 |
| Nilotinib + ruxolitinib phase 1 | NCT02253277 |
| Nilotinib + ruxolitinib phase 1 | NCT01702064 |
| ABL-TKI + ruxolitinib phase 2 | NCT03610971 |
| ABL-TKI + ruxolitinib phase 2 | NCT03654768 |
| BCL-2 inhibition | |
| Preclinical data | |
| Dasatinib ± venetoclax phase 3 | NCT02689440 |
| BTK inhibition | |
| Preclinical data | |
BCL2
BCL2-related antiapoptotic proteins are expressed at higher levels in CML stem cells compared to normal stem cells. Inhibiting BCL2 increases killing of CML stem cells by TKIs in models. Venetoclax combined with a TKI reduces the serial re-engraftment capacity of CML stem cells in mice better than a TKI alone. Thus, BCL2 inhibition may be another option for targeting persisting CML stem cells unresponsive to BCR-ABL1 TKIs.31,32 A randomized first-line study testing the impact of the addition of venetoclax to dasatinib in newly diagnosed CML patients is ongoing (NCT02689440). However, the impact of venetoclax on persistent stem cells should be tested in a maintenance format after achievement of DMR.
Immunotherapy
Numerous studies have revealed an association between cellular immune parameters and TFR, including higher numbers of natural killer cells and lower numbers of both T regulatory cells and CD86+ plasmacytoid dendritic cells. Cumulative results suggest an immunological control of persisting CML stem cells in patients with successful TFR.19,33
According to preclinical and clinical studies, IFNA was able to induce cytogenetic remissions in a minority of CML patients. Moreover, a small percentage of patients treated with IFNA sustained durable remissions after discontinuing therapy. The mechanisms behind this clinical observation are not well understood; activation of leukemia-specific immunity may be a key factor.34 Treatment with IFNA permits the discontinuation of imatinib in most patients after prior imatinib/IFNA combination therapy and may result in improved MR. Induction of a proteinase 3-specific cytotoxic T-lymphocyte response by IFNA may contribute to this effect.35 Studies investigating the combination of IFNA with nilotinib or dasatinib are ongoing.
In a phase 1 study, the combination of imatinib and ropeginterferon α2b was shown to be safe and resulted in a DMR in patients with chronic-phase CML who did not achieve a DMR with imatinib alone.36 Clinical trials exploring the impact of checkpoint inhibition include nivolumab as a programmed cell death 1 protein inhibitor in combination with dasatinib (NCT02011945) and avelumab as a programmed cell death 1 ligand 1 inhibitor combined with TKIs (ACTIW trial, NCT02767063; Table 2, Figure 1).37
Figure 1.
Innovative strategies to improve chances of treatment-free remission.
In any attempt to enhance the stability of TFR, the anticipated effects should be balanced against the risk of additional side effects caused by the immune activation of the block of BCR-ABL1-independent pathways.
Recommendations
The recommendations of the European LeukemiaNet panel, the National Comprehensive Cancer Network, and the French CML Study Group are summarized in Table 3.5,38,39
Table 3.
Recommendations for TFR
| Parameter | European LeukemiaNet 20205 | National Comprehensive Cancer Network 202138 | French Chronic Myeloid Leukemia Study Group 201839 |
|---|---|---|---|
| Mandatory | |||
| Age, phase of the disease, disease history | Adult patients, CML in first chronic phase only. No prior treatment failure. | Age ≥18 years. Chronic-phase CML. No prior history of accelerated or blast-phase CML. | Age ≥18 years. Chronic-phase CML. No prior history of allogeneic stem cell transplantation, progression, resistance, suboptimal response, or warning. |
| Motivation, communication, information | Motivated patient with structured communication. Patient's acceptance of more frequent PCR tests after stopping treatment. |
Consultation with a CML specialist to review eligibility for TKI discontinuation and potential risks and benefits of treatment discontinuation, including TKI withdrawal syndrome. Discontinuation of TKI therapy should only be performed in consenting patients after a thorough discussion of the potential risks and benefits. | Adherence to monitoring and retreatment recommendations. Written information and instructions may be provided. |
| Molecular-monitoring infrastructure | Access to high-quality qRT-PCR using the IS with rapid turnaround of PCR test results | Access to a reliable quantitative PCR test with a sensitivity of detection of at least MR4.5 (BCR-ABL1 ≤ 0.0032% IS) that provides results within 2 weeks. | Results should be available within 2-3 weeks. Molecular biologists should be aware of TKI discontinuations in individual patients. |
| Molecular-monitoring frequency | Monthly molecular monitoring for the first 6 months, bimonthly during months 7-12, and quarterly thereafter (indefinitely) | Monthly during the first half year, every 2 months. In the second half year, quarterly during the second year and then every 3 to 6 months. | |
| Minimal (stop allowed) | |||
| Line of treatment | First-line or second-line therapy if intolerance was the only reason for changing TKI | ||
| BCR-ABL1 transcript type | Typical e13a2 or e14a2 BCR-ABL1 transcripts | Prior evidence of a quantifiable BCR-ABL1 transcript | BCR-ABL1 transcripts e13a2, e14a2, or e13a2/e14a2 |
| Duration of TKI therapy | Duration of TKI therapy >5 years (>4 years for second-generation TKI) |
On approved TKI therapy for at least 3 years | TKI treatment duration ≥5 years. |
| Duration of DMR | DMR (MR4 or better) for >2 years | Stable MR (MR4; BCR-ABL1 ≤ 0.01% IS) for ≥2 years, as documented on at least 4 tests, performed at least 3 mo apart. | DMR at least MR4.5. DMR duration ≥2 years. MR4.5 is confirmed in ≥4 consecutive tests. At most 1 of the 4 assessments showing an MR4 is accepted. |
| Optimal (stop recommended for consideration) | |||
| Duration of TKI therapy >5 years | |||
| Duration of DMR >3 years if MR4 | |||
| Duration of DMR >2 years if MR4.5 | |||
Certain requirements are crucial: the access to a reliable, sensitive, standardized qRT-PCR with rapid turnaround and a motivated patient who accepts more frequent PCR analyses. In general, chronic-phase patients in first-line therapy or in second-line after TKI intolerance should be considered. Data for patients after TKI resistance are still lacking. Standardized qRT-PCR requires specification of the BCR-ABL1 transcript type. For patients with atypical BCR-ABL1 transcripts, the term “individual molecular response” has been coined. Such patients could start TFR if DMR can be reliably quantified for this specific transcript (Table 3).40
CLINICAL CASE (continued)
Our patient decided to continue dasatinib as a monotherapy. The treatment duration and the duration of DMR had been rather short prior to the attempted treatment discontinuation. Current guidelines recommend a treatment duration of at least 4 years with a 2G-TKI and a DMR duration of at least 2 years in MR4.5 (Table 3).5 However, as a low-risk patient with a rapid MR to the first-line treatment, she has a good chance of maintaining DMR and thus discontinuing therapy in about 3 additional treatment years. The variant cytogenetic translocation does not have an impact on prognosis. The predictive impact of mutations outside BCR-ABL1 is the subject of ongoing cooperative studies.
In our clinical practice, we discuss the concept of TFR with chronic-phase CML patients prior to starting treatment. The discussion of the goals of CML treatment and the selection of first-line therapy should include the information that an optimal MR minimizes the risk of progression and maximizes the opportunity for discontinuing therapy. The prospect of TFR can serve as an additional motivator to optimize adherence to TKI therapy.19
Contributor Information
Andreas Hochhaus, Klinik für Innere Medizin II, Universitätsklinikum Jena, Jena, Germany.
Thomas Ernst, Klinik für Innere Medizin II, Universitätsklinikum Jena, Jena, Germany.
Conflict-of-interest disclosure
Andreas Hochhaus: consultancy: Novartis, Pfizer, BMS, Incyte; research funding: Novartis, Pfizer, BMS, Incyte.
Thomas Ernst: honoraria: Novartis, BMS, Incyte; research funding: Novartis.
Off-label drug use
Andreas Hochhaus: experimental design (study proposals): BCL-2, BTK, JAK inhibitors are discussed.
Thomas Ernst: experimental design (study proposals): BCL-2, BTK, JAK inhibitors are discussed.
References
- 1.Kantarjian HM, Hughes TP, Larson RA, et al.. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35(2):440-453. doi: 10.1038/s41375-020-01111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahon FX, Réa D, Guilhot J, et al; Intergroupe Français des Leucémies Myéloïdes Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029-1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 3.Etienne G, Guilhot J, Rea D, et al.. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35(3):298-305. doi: 10.1200/JCO.2016.68.2914. [DOI] [PubMed] [Google Scholar]
- 4.Cross NC, White HE, Colomer D, et al.. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29(5):999-1003. doi: 10.1038/leu.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochhaus A, Baccarani M, Silver RT, et al.. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4): 966-984. doi: 10.1038/s41375-020-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saussele S, Richter J, Guilhot J, et al; EURO-SKI Investigators. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747-757. doi: 10.1016/S1470-2045(18)30192-X. [DOI] [PubMed] [Google Scholar]
- 7.Rousselot P, Charbonnier A, Cony-Makhoul P, et al.. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424-430. doi: 10.1200/JCO.2012.48.5797. [DOI] [PubMed] [Google Scholar]
- 8.Zhao H, Deininger MW. Declaration of Bcr-Abl1 independence. Leukemia. 2020;34(11):2827-2836. doi: 10.1038/s41375-020-01037-9. [DOI] [PubMed] [Google Scholar]
- 9.Radich JP, Hochhaus A, Masszi T, et al.. Treatment-free remission following frontline nilotinib in patients with chronic phase chronic myeloid leukemia: 5-year update of the ENESTfreedom trial. Leukemia. 2021;35(5):1344-1355. doi: 10.1038/s41375-021-01205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes TP, Clementino NCD, Fominykh M, et al.. Long-term treatment-free remission in patients with chronic myeloid leukemia after second-line nilotinib: ENESTop 5-year update. Leukemia. 2021;35(6):1631-1642. doi: 10.1038/s41375-021-01260-y. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi N, Nishiwaki K, Nakaseko C, et al.. Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica. 2018;103(11):1835-1842. doi: 10.3324/haematol.2018.194894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi H, Takezako N, Ohashi K, et al.. Treatment-free remission after first-line dasatinib treatment in patients with chronic myeloid leukemia in the chronic phase: the D-NewS Study of the Kanto CML Study Group. Int J Hematol. 2020;111(3):401-408. doi: 10.1007/s12185-019-02801-z. [DOI] [PubMed] [Google Scholar]
- 13.Imagawa J, Tanaka H, Okada M, et al; DADI Trial Group. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2(12):e528-e535. doi: 10.1016/S2352-3026(15)00196-9. [DOI] [PubMed] [Google Scholar]
- 14.Legros L, Nicolini FE, Etienne G, et al; French Intergroup for Chronic Myeloid Leukemias. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123(22):4403-4410. doi: 10.1002/cncr.30885. [DOI] [PubMed] [Google Scholar]
- 15.Sharf G, Marin C, Bradley JA, et al.. Treatment-free remission in chronic myeloid leukemia: the patient perspective and areas of unmet needs. Leukemia. 2020;34(8):2102-2112. doi: 10.1038/s41375-020-0867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter J, Söderlund S, Lübking A, et al.. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J Clin Oncol. 2014;32(25):2821-2823. doi: 10.1200/JCO.2014.55.6910. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi N, Tauchi T, Kitamura K, et al; Japan Adult Leukemia Study Group. Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: the JALSGSTIM213 study. Int J Hematol. 2018;107(2):185-193. doi: 10.1007/s12185-017-2334-x. [DOI] [PubMed] [Google Scholar]
- 18.Kim DDH, Novitzky-Basso I, Kim TS, et al.. Optimal duration of imatinib treatment/deep molecular response for treatment-free remission after imatinib discontinuation from a Canadian tyrosine kinase inhibitor discontinuation trial. Br J Haematol. 2021;193(4):779-791. doi: 10.1111/bjh.17447. [DOI] [PubMed] [Google Scholar]
- 19.Ross DM, Hughes TP. Treatment-free remission in patients with chronic myeloid leukaemia. Nat Rev Clin Oncol. 2020;17(8):493-503. doi: 10.1038/s41571-020-0367-1. [DOI] [PubMed] [Google Scholar]
- 20.Ross DM, Branford S, Seymour JF, et al.. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515-522. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 21.Ross DM, Branford S, Seymour JF, et al.. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24(10):1719-1724. doi: 10.1038/leu.2010.185. [DOI] [PubMed] [Google Scholar]
- 22.Ross DM, Pagani IS, Shanmuganathan N, et al; Australasian Leukaemia and Lymphoma Group. Long-term treatment-free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia. 2018;32(12):2572-2579. doi: 10.1038/s41375-018-0264-0. [DOI] [PubMed] [Google Scholar]
- 23.Ilhan O, Narli Ozdemir Z, Dalva K, et al.. Leukemic stem cells shall be searched in the bone marrow before “tyrosine kinase inhibitor-discontinuation” in chronic myeloid leukemia. Int J Lab Hematol. Published online 9 April 2021. doi: 10.1111/ijlh.13528. [DOI] [PubMed] [Google Scholar]
- 24.Machova Polakova K, Zizkova H, Zuna J, et al.. Analysis of chronic myeloid leukaemia during deep molecular response by genomic PCR: a traffic light stratification model with impact on treatment-free remission. Leukemia. 2020;34(8):2113-2124. doi: 10.1038/s41375-020-0882-1. [DOI] [PubMed] [Google Scholar]
- 25.Houshmand M, Simonetti G, Circosta P, et al.. Chronic myeloid leukemia stem cells. Leukemia. 2019;33(7):1543-1556. doi: 10.1038/s41375-019-0490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bocchia M, Sicuranza A, Abruzzese E, et al.. Residual peripheral blood CD26+ leukemic stem cells in chronic myeloid leukemia patients during TKI therapy and during treatment-free remission. Front Oncol. 2018;8(30 May):194. doi: 10.3389/fonc.2018.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinstrie R, Horne GA, Morrison H, et al.. CD93 is expressed on chronic myeloid leukemia stem cells and identifies a quiescent population which persists after tyrosine kinase inhibitor therapy. Leukemia. 2020;34(6):1613-1625. doi: 10.1038/s41375-019-0684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagani IS, Dang P, Saunders VA, et al.. Lineage of measurable residual disease in patients with chronic myeloid leukemia in treatment-free remission. Leukemia. 2020;34(4):1052-1061. doi: 10.1038/s41375-019-0647-x. [DOI] [PubMed] [Google Scholar]
- 29.Kuepper MK, Bütow M, Herrmann O, et al.. Stem cell persistence in CML is mediated by extrinsically activated JAK1-STAT3 signaling. Leukemia. 2019;33(8):1964-1977. doi: 10.1038/s41375-019-0427-7. [DOI] [PubMed] [Google Scholar]
- 30.Parting O, Langer S, Kuepper MK, et al.. Therapeutic inhibition of FcγRIIb signaling targets leukemic stem cells in chronic myeloid leukemia. Leukemia. 2020;34(10):2635-2647. doi: 10.1038/s41375-020-0977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter BZ, Mak PY, Mu H, et al.. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci Transl Med. 2016;8(355):355ra117. doi: 10.1126/scitranslmed.aag1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houshmand M, Garello F, Stefania R, et al.. Targeting chronic myeloid leukemia stem/progenitor cells using venetoclax-loaded immunoliposome. Cancers (Basel). 2021;13(6):1311. doi: 10.3390/cancers13061311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schütz C, Inselmann S, Saussele S, et al.. Expression of the CTLA-4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia. 2017;31(4):829-836. doi: 10.1038/leu.2017.9. [DOI] [PubMed] [Google Scholar]
- 34.Talpaz M, Hehlmann R, Quintás-Cardama A, Mercer J, Cortes J. Re-emergence of interferon-α in the treatment of chronic myeloid leukemia. Leukemia. 2013;27(4):803-812. doi: 10.1038/leu.2012.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burchert A, Saussele S, Eigendorff E, et al.. Interferon alpha 2 maintenance therapy may enable high rates of treatment discontinuation in chronic myeloid leukemia. Leukemia. 2015;29(6):1331-1335. doi: 10.1038/leu.2015.45. [DOI] [PubMed] [Google Scholar]
- 36.Heibl S, Buxhofer-Ausch V, Schmidt S, et al.. A phase 1 study to evaluate the feasibility and efficacy of the addition of ropeginterferon alpha-2b to imatinib treatment in patients with chronic phase chronic myeloid leukemia (CML) not achieving a deep molecular response (molecular remission 4.5)-AGMT_CML 1. Hematol Oncol. 2020;38(5):792-798. doi: 10.1002/hon.2786. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh YC, Kirschner K, Copland M. Improving outcomes in chronic myeloid leukemia through harnessing the immunological landscape. Leukemia. 2021;35(5):1229-1242. doi: 10.1038/s41375-021-01238-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Comprehensive Cancer Network. Chronic myeloid leukemia. NCCN Clinical Practice Guidelines in Oncology Version 3.2021. J Natl Compr Canc Netw. 2020;18(10):1385-1415. doi: 10.6004/jnccn.2020.0047. [DOI] [PubMed] [Google Scholar]
- 39.Rea D, Ame S, Berger M, et al; French Chronic Myeloid Leukemia Study Group. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: recommendations for clinical practice from the French Chronic Myeloid Leukemia Study Group. Cancer. 2018;124(14):2956-2963. doi: 10.1002/cncr.31411. [DOI] [PubMed] [Google Scholar]
- 40.Schäfer V, White HE, Gerrard G, Möbius S, Saussele S, Franke GN, et al.. Assessment of individual molecular response in chronic myeloid leukemia patients with atypical BCR-ABL1 fusion transcripts: recommendations by the EUTOS cooperative network. J Cancer Res Clin Oncol. 2021;147(3):3081-3089. doi: 10.1007/s00432-021-03569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]