Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) is a highly complex, costly procedure for patients with oncologic, hematologic, genetic, and immunologic diseases. Demographics and socioeconomic status as well as donor availability and type of health care system are important factors that influence access to and outcomes following allo-HCT. The last decade has seen an increase in the numbers of allo-HCTs and teams all over the world, with no signs of saturation. More than 80 000 procedures are being performed annually, with 1 million allo-HCTs estimated to take place by the end of 2024. Many factors have contributed to this, including increased numbers of eligible patients (older adults with or without comorbidities) and available donors (unrelated and haploidentical), improved supportive care, and decreased early and late post-HCT mortalities. This increase is also directly linked to macro- and microeconomic indicators that affect health care both regionally and globally. Despite this global increase in the number of allo-HCTs and transplant centers, there is an enormous need for increased access to and improved outcomes following allo-HCT in resource-constrained countries. The reduction of poverty, global economic changes, greater access to information, exchange of technologies, and use of artificial intelligence, mobile health, and telehealth are certainly creating unprecedented opportunities to establish collaborations and share experiences and thus increase patient access to allo-HCT. A specific research agenda to address issues of allo-HCT in resource-constrained settings is urgently warranted.
Learning Objectives
Understand the panorama of allogeneic hematopoietic cell transplantation (allo-HCT) activity worldwide
Review factors affecting the access to allo-HCT across the globe
Discuss possible strategies for expanding allo-HCT activity in resource-constrained areas
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a highly specialized, technologically sophisticated, resource- intense, and expensive procedure for patients with otherwise incurable hematologic diseases. Numerous factors influence whether a patient eligible for allo-HCT actually goes on to receive it: donor availability, social issues, economic status, and health care system.1 The degree to which these factors, particularly socioeconomic factors, might influence access to allo-HCT is a question of debate.1
In recent decades a worldwide increase in the use of allo-HCT has been seen due to (1) better donor availability, (2) optimization of indications and rapid evolution of molecular diagnostic/prognostic techniques, (3) use of novel reduced-intensity conditioning regimens for older adult patients and/or patients with comorbidities, and (4) improvements in early and late outcomes due to better supportive care. However, allo-HCT is still associated with significant morbidity and mortality, requiring advanced care consisting of significant infrastructure and a network of specialists from all fields of medicine.
The World Health Organization (WHO) has recognized the broad idea of the provision of medical products of human origin as an important global medical task and a governmental responsibility at the national level.2,3 Data collection and data analysis are also recognized as integral parts of the treatment to achieve an efficient and cost-effective use of resources. The Worldwide Network for Blood and Marrow Transplantation (WBMT), as a nongovernmental organization in official relations with the WHO, has taken up the challenge of collecting global HCT activity data. Information on HCT trends over time provides a sound basis for scientific societies, politicians, and health care agencies to develop or optimize national HCT programs.
This article presents a global overview of the numbers and types of allo-HCT in different world regions collected by members of the WBMT (the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the Asia-Pacific Blood and Marrow Transplantation Group) or directly from transplant centers (where no regional registry is in place) using a uniform reporting sheet.4,5 As many patient, disease, donor, and transplant-related factors may improve outcomes, including those affecting access to allo-HCT, this article also describes these aspects.
Worldwide allo-HCT activity and overall trends
Worldwide, 1 298 897 HCTs (42.9% allo-HCT) procedures have been recorded from the first HCT in 1957 to 2016.5 The annual activity increased continuously from 46 563 in 2006 (the foundation of WBMT and the first global report) to 82 718 in 2016 (the latest complete global survey, Table 1),4-6 amounting to a global increase of 77.6% since 2006, which was somewhat higher in allo-HCT (89.0%) than in auto-HCT (68.9%).5
Table 1.
Increase in HCT numbers from 2006 to 2016 according to transplant type (allogeneic and autologous), donor type, and source4-6
| Year | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allogeneic HCT | 20,333 | 21,904 | 23,989 | 25,539 | 27,189 | 29,815 | 31,926 | 33,572 | 35,333 | 35,897 | 38,425 |
| Related | 11,002 | 11,611 | 12,362 | 12,641 | 12,945 | 14,303 | 15,493 | 16,305 | 17,705 | 18,515 | 20,594 |
| Identical | n.a. | 10,107 | 10,599 | 10,624 | 10,912 | 11,751 | 12,322 | 12,475 | 12,579 | 12,511 | 12,463 |
| Nonidentical | n.a. | 1,504 | 1,763 | 2,017 | 2,033 | 2,552 | 3,171 | 3,830 | 5,126 | 6,004 | 8,131 |
| CB | 0 | 102 | 92 | 131 | 149 | 143 | 251 | 239 | 298 | 358 | 117 |
| Unrelated | 9,331 | 10,293 | 11,627 | 12,898 | 14,244 | 15,512 | 16,433 | 17,267 | 17,628 | 17,382 | 17,831 |
| CB | 1,722 | 2,031 | 2,266 | 2,508 | 2,804 | 2,927 | 2,859 | 2,787 | 2,568 | 2,474 | 2,477 |
| Autologous HCT | 26,230 | 26,805 | 27,547 | 30,081 | 31,730 | 33,756 | 36,220 | 37,464 | 38,188 | 41,698 | 44,293 |
| Total | 46,563 | 48,709 | 51,536 | 55,620 | 58,919 | 63,571 | 68,146 | 71,036 | 73,521 | 77,595 | 82,718 |
n.a.
Allo-HCT team activity in 2016
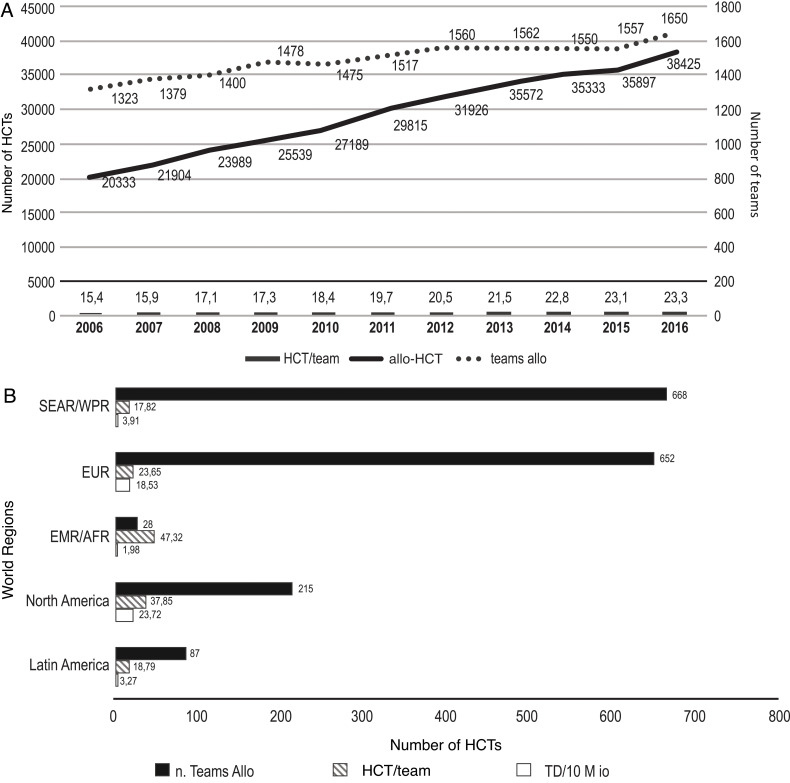
In 2016, 38 425 first allo-HCTs, in comparison to 20 333 in 2006, were performed worldwide (Figure 1). The increase took place in all regions of the world, and in contrast to 2006, more related (53.6%) than unrelated HCTs were reported.5 Increases of 20% and 50% were noted in the number of allo-HCT teams and allo-HCTs, respectively, while the allo-HCT/team ratio varied from 15.4 in 2006 to 23.3 in 2016 (Figure 2A). Team density (TD, the number of teams/10 million persons) was highest in North America (NA; 23.72), followed by Europe (EUR; 18.53), the South-East Asian/Western Pacific Region (SEAR/WPR; 3.91), Latin America (LA; 3.27), and the Eastern Mediterranean/African Region (EMR/AFR; 1.98; Figure 2B). Accordingly, allo-HCT/team ratios were higher in EMR/AFR and NA, demonstrating a particularly high concentration of procedures in few transplant centers in EMR/AFR (Figure 2B).5
Figure 1.
Allo-HCT activity according to donor type (related and unrelated), irrespective of source and matching (PBSC, BM, or CB; matched or mismatched), and region comparing 2006 to 2016. BM, bone marrow; CB, cord blood; PBSC, peripheral blood stem cell.
Figure 2.
(A) Number of allo-HCTs, number of allo-HCT teams, and allo-HCTs per team. (B) Number of allo-HCT teams, number of allo-HCTs per team, and TD according to region in 2016.
Trends in indications, donor type, and stem cell source
The number of transplants for all indications using all type of donors (except for plasma cell disorders and lymphomas in related allo-HCT; data not shown) increased.5 Leukemia was the most frequent indication, of which acute myeloid leukemia (AML) amounted 14 334 HCTs. HCT for myelodysplastic syndromes (MDS) and, to a lower extent, myeloproliferative disorders increased steadily over the observation period. Increased allo-HCT activities were observed for almost all indications in all regions except for solid tumors in almost all regions but SEAR/WPR and lymphoproliferative disorders in NA (Figure 3). The highest increments were observed for severe aplastic anemia and hemoglobinopathies (Δ2007-2016 > 1000%) and for leukemia (Δ2007-2016 > 550%; data not shown).5
Figure 3.
Change in % of allo-HCT activity from 2006 and 2016 activity according to diagnosis and region. Adapted with permission of Haematologica.5
Allo-HCTs (range, 0-7850) were reported from 76 countries, including grafts from unrelated donors (UDs) and from cord blood (CB) in 55 and in 41 countries, respectively. Absolute UD-HCT numbers ranged from 0 to 4311, and those of CB ranged from 0 to 1233 in individual countries. Overall, related-HCT has become more frequent than UD-HCT, starting in 2014, due to the increased use of related haploidentical (haplo) donors (39.5% of related HCT). Haplo-HCTs (n = 8131) were evenly distributed in 62 countries, with absolute numbers ranging from 0 to 2554. It is not surprising that more haplo-HCTs were performed in regions without or with few UD registries (LA, EMR, AFR, and SEAR/WPR).
Of the 38 425 allo-HCTs, 20% were derived from bone marrow (BM), 73% from peripheral blood (PB), and 7% from CB.5
Factors affecting access to allo-HCT
Donor availability
UD registries: impact of ethnicity and economic issues
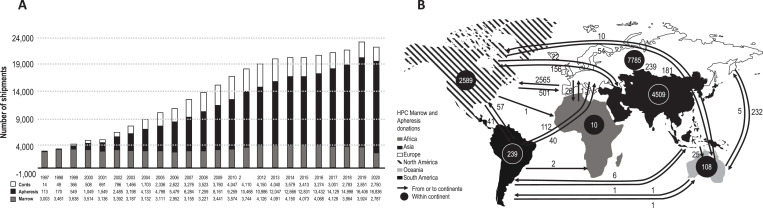
In the absence of an HLA-identical related donor, hematopoietic cells from HLA-matched or mismatched UD, haplo donors, or CB cells can be used (Figure 4). Countries with UD registries increased from 2 in 1987 to 57 in 2012 and 119 in 2021. Accordingly, the donor pool has increased in the last 40 years to >40 million UD, including CB (May 2021; https://wmda.info/). Rates of UD per 10 000 inhabitants vary per continent (2.98 in Africa, 16.5 in Asia, 61.64 in Oceania, 179.45 in South America, 198.28 in NA, and 247.33 in Europe; Joris M., personal communication, May 2021). The numbers of unrelated grafts (BM, PB, and CB cells) expanded >7-fold from 1997 to 2020, with increased use of PB (except for the COVID-19 pandemic period in 2020) and decreased use of BM and CB cells (Figure 5A). Figure 5B shows the global exchange of products (BM + PB) in 2020. In a National Marrow Donor Program study, the probability of finding a UD for black Americans is <17%.7 In a recent study of the Brazilian UD registry (REDOME), this probability was <10% for sickle cell disease patients.8 In addition, access to searching for and using UD grafts is limited by financial issues even in different areas of high-income countries (HICs). In the US, Paulson et al. found that patients with leukemia from poorer areas were less likely to undergo UD allo-HCT compared to patients from wealthier countries.9 Similar findings were also reported in Italy.10 Despite many retrospective studies and some prospective trials reporting similar outcomes after UD, CB, or haplo-HCT, the use of UD over haplo or CB depends on patient/donor ethnicity, availability of a UD registry, and financial issues—graft-related costs being an important factor in low- and middle-income countries (LICs, LMICs).
Figure 4.
Factors associated with access to allo-HCT: an international perspective.
Figure 5.
(A) Unrelated BM, mobilized PB, and CB grafts shipped from 1997 to 2020. (B) Global exchange of hematopoietic cell products (BM + PB) in 2020.
Impact of haplo donors on access to allo-HCT
The use of haplo donors is increasing worldwide, but interestingly, this is more evident in LICs and LMICs, probably due to the unavailability of HLA-matched UD, HCT-associated costs, infrastructure of centers, lack of access to drugs, and other factors. According to the last WBMT report, the haplo-HCT transplant rate (TR; HCT/10 million population) represented 32% and 26% of all allo-HCTs in SEA/WPR and LA, respectively, but only 14% in Europe and EMR/AFR and 4.9% in NA.5
Patient demographics: age, gender, and ethnicity
Studies investigating age as a predictor of access to HCT indicate that younger patients are more likely to receive a transplant compared to older patients, even in HICs.11,12 Only 5.5% of older adult patients with AML (n = 17 555) underwent HCT in the US between 2003 and 2012. Other factors associated with a lower likelihood of receiving HCT included care at a nonacademic hospital, race other than White, Charlson comorbidity score of ≥1, uninsured status, and lower educational status.12 No significant gender effect has been reported.13 The role of race in turn must be interpreted with caution because race is a complex social, cultural, and political construct and not only a biological concept. The availability of a donor for non-Caucasians and ethnic minorities has improved over the last decades with the use of haplo donors and CB units.14 However, many studies have linked economic issues with race and ethnicity to address access to allo-HCT.9,15
Social factors
Geographic distance
The distance between a patient and an HCT facility appears to be an important factor to HCT access.16 To ensure proper follow-up care, allo-HCT patients with limited geographic access must decide whether to make numerous long trips or relocate near the HCT facility, either of which can be a significant financial burden. There are conflicting results from published data on the role of distance from the transplant center or the impact of urban/rural areas on outcomes after allo-HCT.9,17 However, in a recent study, poor access to care, defined as low socioeconomic status and a far distance to the transplant center, was associated with increased 1-year mortality.18
Patient/family attitudes and availability of caregivers
Family and patient psychological factors may be important for HCT access and outcome. To our knowledge no study has evaluated how psychological factors affect access, yet diagnoses of depression, anxiety, or posttraumatic stress disorder following HCT were associated with suboptimal health outcomes and increased mortality.19-21 Caregiver availability may also be a barrier to patients considering and proceeding to HCT.22 Parents/guardians are often the natural caregivers for their children, but for adult patients, the quantity and quality of relationships and the caregiver's income loss may affect the availability of such a person.23,24
Economics
Macro- and microeconomic factors: impact on access to transplantation
From a global perspective, access to and rates of allo- HCT are closely related to the socioeconomic status of countries and regions of the globe. International findings show striking differences in absolute transplant numbers, TD, and the spread of allo-HCT, which are affected mainly by a country's or region's macro- or microeconomic factors related to resources and infrastructure.4 For instance, a recent study showed that adults with AML treated in São Paulo (Brazil) had inferior 5-year overall survival (OS) and higher early mortality primarily due to multiresistant gram-negative bacterial and fungal infections compared to patients from Oxford (UK). Importantly, Brazilian patients were less likely to undergo allo-HCT (28% vs 75%; P < .001) and waited longer for HCT (median, 23.8 vs 7.2 months; P < .001).25
The following economic indicators have been associated with TR (HCTs/10 million), other transplant indicators,4 and outcomes26-28: (1) human development index (HDI), a composite variable containing information about life expectancy, education, and gross national income (GNI); (2) health care expenditure (HCE) per capita; (3) GNI per capita; (4) World Bank categories: HICs, HMICs, LMICs, and LICs.
Gratwohl et al4 and Niederwieser et al5 demonstrated that global numbers of allo-HCT are still increasing. By the end of 2024, almost 1 million allo-HCT will be performed in 76 of the 194 WHO member states, yet no HCT was performed in countries with a population of <300 000 inhabitants, surface area <700 km2, and GNI < US$1260/person. TR was higher in countries with greater gross domestic product, GNI, and HCE per person, higher HDI, more donors, and larger CB banks.4 The association between transplant and macroeconomic factors varied substantially between donor types, WHO regions, and World Bank categories. Macroeconomic resources generally showed higher associations with auto-HCT than allo-HCT, with no substantial differences for related- or UD-HCT. Ease of access to HCT as determined by TD generally showed a higher association for auto- than allo-HCT. Generally, associations between macroeconomic factors and TR were higher in the Americas and SEAR/WPR Regions. Considering the World Bank categories, associations were generally low, with a stronger impact of financial resources on the LMIC category and TD for auto-HCT in the HMIC category.
Little data are available on whether economic factors may also explain the TR differences found among regions of a particular country. This microlevel scenario generally involves the health care system and economics, while clinical differences should not be expected to cause different TR among regions within a country with a public health care system. An interesting Spanish study showed that auto-HCT TR was associated with only TD, whereas macroeconomic determinants exerted a strong influence in the case of allo-TR.29 In particular, an increase of €1000 in gross domestic product per capita would cause an average increase of 1.4 transplants per 1 million inhabitants.29 Therefore, there may be inequality in access to HCT by region due to per capita income, TD, and public hospital expenditure. These findings may be useful to health authorities in making decisions both at the macroeconomic and local levels.
Macro- and microeconomic factors: impact on outcomes of allo-HCT
Studies analyzing socioeconomic factors and outcomes after allo-HCT are restricted to patients with sufficient resources to proceed to HCT and might not be representative of all transplant candidates. Hence, although research on outcomes following allo-HCT in disadvantaged groups is important, it is equally relevant to ensure that patients in these groups actually have access to transplantation.
In 2010 we evaluated the association of HDI with rates and outcomes of allo-HCT for acute leukemia in European countries. Allo-HCTs performed in countries belonging to the upper HDI category were associated with higher leukemia-free survival compared to other categories.27 In another European study, lower current HCE and HDI were associated with a decreased probability of OS in allo-HCT recipients with acute lymphoblastic leukemia.26 An important Center for International Blood and Marrow Transplant Research study analyzing 11 261 allo-HCT recipients with acute lymphoblastic leukemia across 38 countries showed that transplants performed in countries within the lowest HDI quartile were associated with an OS inferior to countries in the highest HDI quartile.30
The association between country-level macroeconomic indicators and outcomes after allo-HCT needs further evaluation. HCT in lower-income settings may be associated with decreased available resources, especially early post HCT.31 In fact, prior work has documented extensive variability in the provision of supportive practices across countries.30 It is possible that care delivery-related factors may have contributed to the high proportion of deaths from infections seen in HMIC/LMIC/LIC settings (eg, lack of adequate intensive care support).25,30,32 Alternatively, worse macroeconomic indicators may reflect deficiencies in training, experience, or staffing models in centers in resource-constrained settings. On a patient level, lower socioeconomic status may be associated with adverse outcomes owing to chronic stress, low educational level, unaffordability of medications, and difficulties in reaching the transplant center.
Health care systems
Health care is a sector where governments have a leading role. Access to and outcomes following allo-HCT depend on how much a government invests in general health and tertiary care. To our knowledge, there are no data comparing the type of health system (public vs private or universal vs nonuniversal) and access to allo-HCT.
HCT is costly and may drain considerable resources from primary care, maternal and childhood care, or countrywide vaccination programs. On the other hand, caring for patients with endemic diseases (eg, hemoglobinopathies in most emerging countries) may be costly over time, and HCT may be more cost-effective in the long run.33 Thus, when it comes to strategic priorities, countries should tailor the implementation of tertiary care facilities according to socioeconomic conditions and country-specific diseases. It is also critical to evaluate the clinical/economic effectiveness and sustainability of such an endeavor.34 Unfortunately, there are scarce data on what type of donor or graft-versus- host disease (GVHD) prophylaxis could be most cost-effective in developing economies, an issue that urgently warrants further research.
Referral of patients to transplant and posttransplant care
The complex interplay of patients, referring physicians, payors, and transplant center-related factors have been previously described.35 These factors need to be comprehensively studied to deliver optimal care. Both payors and accreditation agencies should also attempt to elevate the standards of care affecting transplant outcomes after discharge from the transplant center.35
National and international regulations
In many health care settings, benchmarking for complex procedures has become a mandatory requirement by stakeholders to assure clinical performance, cost-effectiveness, and patient safety.36 Benchmarking has also been integrated into the Foundation for the Accreditation of Cellular Therapy-Joint Accreditation Committee of the International Society for Cellular Therapy and the European Society for Blood and Marrow Transplantation quality management standards and has been established mainly for developed countries but not for resource-constrained nations.36 Developing meaningful benchmarking systems for countries with similar macroeconomic factors may help address and correct underperformance.
Establishing new HCT programs
Table 2 summarizes important steps in establishing new or optimizing established HCT programs. Close cooperation with health authorities, politicians, and physicians is needed to ensure equality of treatment around the world. It is essential to allocate funds for this treatment and have support from regional and global scientific societies in association with the WHO. Furthermore, HCT-specific minimum requirements are needed. The Transplant Center and Recipient Issues Standing Committee of the WBMT organized a structured review and analyzed, described, and scored (by independent transplant physicians) minimum requirements to establish an HCT program.37 This structured set of recommendations guides the prioritization to establish a transplant program and set the path for expansion and further development.37
Table 2.
How to improve access to HCT
| Target | Topic | Actions |
|---|---|---|
| Benchmarking activities among countries and regions | Global HCT activity reports | Biannual survey since 20064,6,47-50 |
| Starting new programs | Alerting health authorities and politicians about the need for programs in countries with low HCT activity | Organization of WBMT workshops in cooperation with the WHO51 |
| Essential medication | Published previously52 | |
| Training of physicians, nurses, technicians, and data managers | Scientific societies; accredited transplant centers | |
| Infrastructure | Define essential infrastructure37,53 | |
| Site visit from experienced physicians | Role of scientific societies | |
| Financial aspects | Optimize treatment | |
| Twinning and telemedicine | Supervisory telemedicine42 | |
| Optimizing existing programs | Outcome registries | Establish outcome registries Analysis of different techniques54 |
| Accreditation | Liaise with JACIE/FACT | |
| Utilization of HCT worldwide | Analyzing incidence (tumor registries) and HCT activities for each disease in regions and countries55 | |
| Establishing alternate donor registries | Describe challenges in developing countries56 | |
| Establishing clinical studies | Structures for local registries, noninterventional, interventional studies |
FACT, Foundation for the Accreditation of Cellular Therapy; JACIE, Joint Accreditation Committee of the International Society for Cellular Therapy.
How to improve access to allo-HCT
Access to allo-HCT is dependent on the multiple factors reported above and summarized in Figure 6. Improvements in access can be obtained by evaluating the need for HCT in individual countries and the ways in which to improve. The easiest way would be to improve the TR in existing centers and establish new transplant centers in regions with low TD. This evaluation provides comparisons for benchmarking and some hints on how to create or optimize transplant programs. New technologies may help in this endeavor.
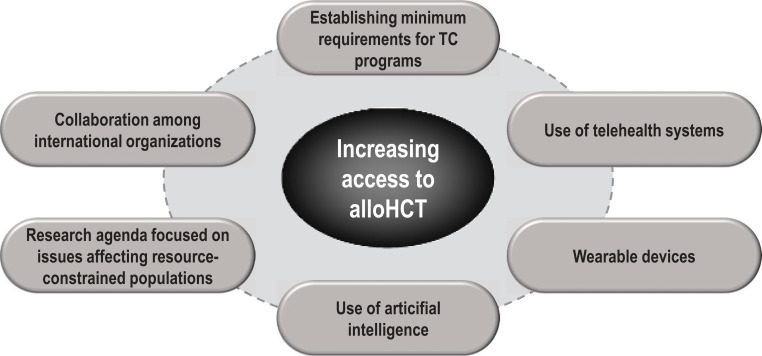
Figure 6.
Increasing access and possibly outcomes to allo-HCT using new technologies: an international perspective.
Role of new technologies
Even in LICs and LMICs, widespread adoption of the mobile phone, investments in electronic medical records, developments in cloud computing, and emergent mobile health (mHealth) applications represent a unique opportunity to narrow the gap of access to HCT between developed and developing nations.
Telehealth has been associated with decreasing costs, gains in productivity, patient adherence, and improved access to health care.38 Since the 2000s there have been a few reports on the use of telehealth in HCT recipients,39,40 but the COVID-19 pandemic really caused the field to gain traction worldwide, including in the developing world. At our public institution in Brazil (V.R., G.F.), a recent survey among 232 HCT recipients during the COVID-19 pandemic showed that one-third of them spent >120 minutes to travel to and from the outpatient clinic, and 42% had a cost >US $10.00 (equivalent to 5% of the minimum wage/month). Approximately half of them had engaged in at least 1 previous interaction via telehealth with our center, and 91% of these patients considered it a good experience.41 Although not fit for all clinical scenarios, telehealth may be efficient and complementary to in-person interactions with HCT patients, may allow a cost reduction for patients, and may be especially useful in the long-term follow-up of HCT survivors,40 which often requires institutions experienced in the management of chronic GVHD and late complications. Telementoring is also a promising approach to implementing allo-HCT programs in that context.42
Wearable devices (eg, the Apple Watch) represent another mHealth modality increasingly assimilated in everyday life. These devices can be continuous or intermittently capture biological data, and recent experiences have been tested in cancer and HCT survivors.43 Wearable devices along with telehealth approaches reduced hospital readmissions following liver transplantation.44 Coupling the massive data generated by these technologies, electronic medical records, and biomarkers with artificial intelligence may potentially predict transplant complications such as febrile neutropenia and GVHD hours or days in advance.45 In fact, artificial intelligence has been used to improve the diagnosis of birth asphyxia and diabetic retinopathy and provide treatment recommendations in cancer in different low-income countries.46 With decreasing costs, these technologies could expand outpatient allo-HCT and allow better utilization of hospital HCT beds in regions where transplant waiting lists are a reality. Prioritization of transplant candidates on waiting lists may also be assisted by intelligent algorithms that take into account personal and disease characteristics, pretransplant workup scheduling, local transplant center performance, and evidence-based data.
Although technological advances offer unprecedented opportunities to enable access to HCT, the validation of effective solutions demands high-quality data sets, internet connectivity, information technology infrastructure, and clinical trials, which are time- and cost-consuming. Hence, stakeholders must ensure that actions are taken to guide sustainable, equitable technology implementation in the HCT realm to fulfill its promises.
Research agenda for resource-constrained countries
Most of the current evidence in HCT has been generated in HICs and may not be applicable to resource-constrained settings. Many clinical challenges faced by developing countries (ie, the majority of the world's population) are underrepresented in the HCT literature (eg, evidenced-based management of waiting lists, peritransplant multidrug-resistant bacteria, tropical diseases). A research agenda, with a particular focus on clinical trials, directed to and conducted in resource-constrained countries is essential to effectively tackle these issues. Initiatives fostering international cooperation, funding, and local research capacity, such as the American Society of Hematology Global Research Award and Clinical Research Training Institutes, should be multiplied to advance access to HCT and other cellular therapies worldwide.
Conflict-of-interest disclosure
Vanderson Rocha: no competing financial interests to declare.
Giancarlo Fatobene: no competing financial interests to declare.
Dietger Niederwieser: no competing financial interests to declare.
Off-label drug use
Vanderson Rocha: no off-label drug use is discussed.
Giancarlo Fatobene: no off-label drug use is discussed.
Dietger Niederwieser: no off-label drug use is discussed.
References
- 1.Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2010;16(8):1070-1075. doi: 10.1016/j.bbmt.2009.12.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warwick RM, Chapman J, Pruett TL, Wang H. Globally consistent coding systems for medical products of human origin. Bull World Health Organ. 2013;91(5):314-314A. doi: 10.2471/BLT.12.116988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO guiding principles on human cell, tissue and organ transplantation. Accessed 19 October 2021. https://www.who.int/transplantation/Guiding_PrinciplesTransplantation_WHA63.22en.pdf.
- 4.Gratwohl A, Pasquini MC, Aljurf M, et al; Worldwide Network for Blood and Marrow Transplantation. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015;2(3):e91-100. doi: 10.1016/S2352-3026(15)00028-9. [DOI] [PubMed] [Google Scholar]
- 5.Niederwieser D, Baldomero H, Bazuaye N, et al.. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Published online 12 August 2021. Haematologica. doi: 10.3324/haematol.279189. E-publishing ahead of print is increasingly important for the rapid dissemi-nation of science. Haematologica is, therefore, e-publishing PDF files of an early version of manuscripts that have completed a regular peer review and have been accepted for publication. E-publishing of this PDF file has been approved by the authors. After having e-published ahead of print, manuscripts will then undergo technical and English editing, typesetting, proof correction, and be presented for the authors’ final approval; the final version of the manuscript will then appear in print on a regular issue of the journal. All legal disclaimers that apply to the journal also pertain to this production process. [DOI] [Google Scholar]
- 6.Niederwieser D, Baldomero H, Szer J, et al.. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant. 2016;51(6):778-785. doi: 10.1038/bmt.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gragert L, Eapen M, Williams E, et al.. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339-348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunes K, Aguiar VRC, Silva M, et al.. How ancestry influences the chances of finding unrelated donors: an investigation in admixed Brazilians. Front Immunol. 2020;11(6 November):584950. doi: 10.3389/fimmu.2020.584950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulson K, Brazauskas R, Khera N, et al.. Inferior access to allogeneic transplant in disadvantaged populations: a Center for International Blood and Marrow Transplant Research analysis. Biol Blood Marrow Transplant. 2019;25(10): 2086-2090. doi: 10.1016/j.bbmt.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milone G, Sacchi N, Gallina A, et al.. Access to alternative donor hematopoietic search and transplantation for acute leukemia in different macro-regions of Italy: a GITMO/IBMDR study. Bone Marrow Transplant. 2018;53(3):291-299. doi: 10.1038/s41409-017-0026-z. [DOI] [PubMed] [Google Scholar]
- 11.Hershenfeld SA, Matelski J, Ling V, Paterson M, Cheung M, Cram P. Utilisation and outcomes of allogeneic hematopoietic cell transplantation in Ontario, Canada, and New York State, USA: a population-based retrospective cohort study. BMJ Open. 2020;10(10):e039293. doi: 10.1136/bmjopen-2020-039293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt VR, Chen B, Gyawali B, Lee SJ. Socioeconomic and health system factors associated with lower utilization of hematopoietic cell transplantation in older patients with acute myeloid leukemia. Bone Marrow Transplant. 2018;53(10):1288-1294. doi: 10.1038/s41409-018-0164-y. [DOI] [PubMed] [Google Scholar]
- 13.Joshua TV, Rizzo JD, Zhang MJ, et al.. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. 2010;116(14):3469-3476. doi: 10.1002/cncr.25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker JN, Boughan K, Dahi PB, et al.. Racial disparities in access to HLA-matched unrelated donor transplants: a prospective 1312-patient analysis. Blood Adv. 2019;3(7):939-944. doi: 10.1182/bloodadvances.2018028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bona K, Brazauskas R, He N, et al.. Neighborhood poverty and pediatric allogeneic hematopoietic cell transplantation outcomes: a CIBMTR analysis. Blood. 2021;137(4):556-568. doi: 10.1182/blood.2020006252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delamater PL, Uberti JP. Geographic access to hematopoietic cell transplantation services in the United States. Bone Marrow Transplant. 2016;51(2): 241-248. doi: 10.1038/bmt.2015.246. [DOI] [PubMed] [Google Scholar]
- 17.Khera N, Gooley T, Flowers MED, et al.. Association of distance from transplantation center and place of residence on outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016; 22(7):1319-1323. doi: 10.1016/j.bbmt.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamy O, Chen A, Battles K, et al.. Impact of access to care on 1-year mortality following allogeneic blood or marrow transplantation. Bone Marrow Transplant. 2021;56(6):1364-1372. doi: 10.1038/s41409-020-01184-8. [DOI] [PubMed] [Google Scholar]
- 19.Amonoo HL, Barclay ME, El-Jawahri A, Traeger LN, Lee SJ, Huffman JC. Positive psychological constructs and health outcomes in hematopoietic stem cell transplantation patients: a systematic review. Biol Blood Marrow Transplant. 2019;25(1):e5-e16. doi: 10.1016/j.bbmt.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 20.El-Jawahri A, Traeger L, Greer JA, et al.. Effect of inpatient palliative care during hematopoietic stem-cell transplant on psychological distress 6 months after transplant: results of a randomized clinical trial. J Clin Oncol. 2017; 35(32):3714-3721. doi: 10.1200/JCO.2017.73.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrlich KB, Miller GE, Scheide T, et al.. Pre-transplant emotional support is associated with longer survival after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(12):1594-1598. doi: 10.1038/bmt.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preussler JM, Mau LW, Majhail NS, et al.. Caregiver availability and patient access to hematopoietic cell transplantation: social worker perspectives inform practice. Support Care Cancer. 2019;27(11):4253-4264. doi: 10.1007/s00520-019-04696-2. [DOI] [PubMed] [Google Scholar]
- 23.Frey P, Stinson T, Siston A, et al.. Lack of caregivers limits use of outpatient hematopoietic stem cell transplant program. Bone Marrow Transplant. 2002;30(11):741-748. doi: 10.1038/sj.bmt.1703676. [DOI] [PubMed] [Google Scholar]
- 24.Sundaramurthi T, Wehrlen L, Friedman E, Thomas S, Bevans M. Hematopoietic stem cell transplantation recipient and caregiver factors affecting length of stay and readmission. Oncol Nurs Forum. 2017;44(5):571-579. doi: 10.1188/17.ONF.571-579. [DOI] [PubMed] [Google Scholar]
- 25.Silveira DRA, Coelho-Silva JL, Silva WF, et al.. A multicenter comparative acute myeloid leukemia study: can we explain the differences in the outcomes in resource-constrained settings? Leuk Lymphoma. 2021;62(1):147-157. doi: 10.1080/10428194.2020.1827252. [DOI] [PubMed] [Google Scholar]
- 26.Giebel S, Labopin M, Ibatici A, et al.. Association of macroeconomic factors with nonrelapse mortality after allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia: an analysis from the Acute Leukemia Working Party of the EBMT. Oncologist. 2016;21(3):377-383. doi: 10.1634/theoncologist.2015-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giebel S, Labopin M, Ehninger G, et al; Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Association of Human Development Index with rates and outcomes of hematopoietic stem cell transplantation for patients with acute leukemia. Blood. 2010;116(1):122-128. doi: 10.1182/blood-2010-01-266478. [DOI] [PubMed] [Google Scholar]
- 28.Frankiewicz A, Peczynski C, Giebel S, et al.. Association of country-specific socioeconomic factors with survival of patients who experience severe classic acute graft-vs.-host disease after allogeneic hematopoietic cell transplantation: an analysis from the Transplant Complications Working Party of the EBMT. Front Immunol. 2020;11(July 23):1537. doi: 10.3389/fimmu.2020.01537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espigado I, Ortega-Ortega M, Montero-Granados R, Rodriguez-Torres N, Márquez-Malaver FJ. Differences in stem cell transplantation activity among regions in Spain: an economic explanation. Bone Marrow Transplant. 2016;51(11):1537-1539. doi: 10.1038/bmt.2016.177. [DOI] [PubMed] [Google Scholar]
- 30.Wood WA, Brazauskas R, Hu ZH, et al.. Country-level macroeconomic indicators predict early post-allogeneic hematopoietic cell transplantation survival in acute lymphoblastic leukemia: a CIBMTR analysis. Biol Blood Marrow Transplant. 2018;24(9):1928-1935. doi: 10.1016/j.bbmt.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong S, Brazauskas R, Hebert KM, et al.. Community health status and outcomes after allogeneic hematopoietic cell transplantation in the United States. Cancer. 2021;127(4):609-618. doi: 10.1002/cncr.33232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva WF, da Rosa LI, Seguro FS, et al.. Salvage treatment for refractory or relapsed acute myeloid leukemia: a 10-year single-center experience. Clinics. 2020;75:1-8. doi: 10.6061/clinics/2020/e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnold SD, Jin Z, Sands S, Bhatia M, Kung AL, Satwani P. Allogeneic hematopoietic cell transplantation for children with sickle cell disease is beneficial and cost-effective: a single-center analysis. Biol Blood Marrow Transplant. 2015;21(7):1258-1265. doi: 10.1016/j.bbmt.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhri NA, Aljurf M, Almohareb FI, et al.. Establishing an autologous versus allogeneic hematopoietic cell transplant program in nations with emerging economies. Hematol Oncol Stem Cell Ther. 2017;10(4):173-177. doi: 10.1016/j.hemonc.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Majhail NS, Jagasia M. Referral to transplant center for hematopoietic cell transplantation. Hematol Oncol Clin North Am. 2014;28(6):1201-1213. doi: 10.1016/j.hoc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Snowden JA, Saccardi R, Orchard K, et al.. Benchmarking of survival outcomes following haematopoietic stem cell transplantation: a review of existing processes and the introduction of an international system from the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Bone Marrow Transplant. 2020;55(4):681-694. doi: 10.1038/s41409-019-0718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasquini MC, Srivastava A, Ahmed SO, et al.. Worldwide Network for Blood and Marrow Transplantation (WBMT) recommendations for establishing a hematopoietic cell transplantation program (part I): minimum requirements and beyond. Hematol Oncol Stem Cell Ther. 2020;13(3):131-142. doi: 10.1016/j.hemonc.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snoswell CL, Taylor ML, Comans TA, Smith AC, Gray LC, Caffery LJ. Determining if telehealth can reduce health system costs: scoping review. J Med Internet Res. 2020;22(10):e17298. doi: 10.2196/17298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DuHamel KN, Mosher CE, Winkel G, et al.. Randomized clinical trial of telephone-administered cognitive-behavioral therapy to reduce post- traumatic stress disorder and distress symptoms after hematopoietic stem-cell transplantation. J Clin Oncol. 2010;28(23):3754-3761. doi: 10.1200/JCO.2009.26.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Syrjala KL, Yi JC, Artherholt SB, et al.. An online randomized controlled trial, with or without problem-solving treatment, for long-term cancer survivors after hematopoietic cell transplantation. J Cancer Surviv. 2018;12(4):560-570. doi: 10.1007/s11764-018-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordeiro A, Fatobene G, Mariano LCB, et al.. Telehealth in hematopoietic cell transplantation: perspective from patients at a public hospital in Brazil. Blood. 2020;136(suppl 1):26. doi: 10.1182/blood-2020-143288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frutos C, Enciso ME, von Glasenapp A, Quiroz A, Batista J, Niederwieser D. Bridging the gap using telemedicine: optimizing an existing autologous hematopoietic SCT unit into an allogeneic hematopoietic SCT unit in Paraguay with the help of the WBMT. Blood Adv. 2019;3(suppl 1):45-47. doi: 10.1182/bloodadvances.2019GS121781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mussetti A, Salas MQ, Condom M, et al.. Use of telehealth for domiciliary follow-up after hematopoietic cell transplantation during the COVID-19 pandemic: prospective pilot study. JMIR Form Res. 2021;5(3):e26121. doi: 10.2196/26121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee TC, Kaiser TE, Alloway R, Woodle ES, Edwards MJ, Shah SA. Telemedicine based remote home monitoring after liver transplantation: results of a randomized prospective trial. Ann Surg. 2019;270(3):564-572. doi: 10.1097/SLA.0000000000003425. [DOI] [PubMed] [Google Scholar]
- 45.Abbasi J. Wearable digital thermometer improves fever detection. JAMA. 2017;318(6):510. doi: 10.1001/jama.2017.10248. [DOI] [PubMed] [Google Scholar]
- 46.Owoyemi A, Owoyemi J, Osiyemi A, Boyd A. Artificial intelligence for healthcare in Africa. Front Digit Heal. 2020;2(July):6. doi: 10.3389/fdgth.2020.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gratwohl A, Baldomero H, Gratwohl M, et al; Worldwide Network of Blood and Marrow Transplantation. Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation: a global observational study. Haematologica. 2013;98(8):1282-1290. doi: 10.3324/haematol.2012.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaimovich G, Martinez Rolon J, Baldomero H, et al.. Latin America: the next region for haematopoietic transplant progress. Bone Marrow Transplant. 2017;52(5):671-677. doi: 10.1038/bmt.2016.361. [DOI] [PubMed] [Google Scholar]
- 49.Baldomero H, Aljurf M, Zaidi SZA, et al; Eastern Mediterranean Blood and Marrow Transplantation Group; African Blood and Marrow Transplantation Group; Worldwide Network for Blood and Marrow Transplantation. Narrowing the gap for hematopoietic stem cell transplantation in the East-Mediterranean/African region: comparison with global HSCT indications and trends. Bone Marrow Transplant. 2019;54(3):402-417. doi: 10.1038/s41409-018-0275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gratwohl A, Baldomero H, Aljurf M, et al; Worldwide Network of Blood and Marrow Transplantation. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617-1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harif M, Weisdorf D, Novitzky N, et al.. Special report: summary of the first meeting of African Blood and Marrow Transplantation (AfBMT) group, Casablanca, Morocco, April 19–21, 2018 held under the auspices of the Worldwide Network for Blood and Marrow Transplantation (WBMT). Hematol Oncol Stem Cell Ther. 2020;13(4):202-207. doi: 10.1016/j.hemonc.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 52.El Fakih R, Greinix H, Koh M, et al.. Worldwide Network for Blood and Marrow Transplantation (WBMT) recommendations regarding essential medications required to establish an early stage hematopoietic cell transplantation program. Transplant Cell Ther. 2021;27(3):267.e1-267.e5. doi: 10.1016/j.jtct.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 53.Aljurf M, Weisdorf D, Hashmi SK, et al.. Worldwide Network for Blood and Marrow Transplantation (WBMT) recommendations for establishing a hematopoietic stem cell transplantation program in countries with limited resources (part II): clinical, technical and socio-economic considerations. Hematol Oncol Stem Cell Ther. 2020;13(1):7-16. doi: 10.1016/j.hemonc.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimi A, Baldomero H, Horowitz M, et al; Worldwide Network of Blood and Marrow Transplantation. Global use of peripheral blood vs bone marrow as source of stem cells for allogeneic transplantation in patients with bone marrow failure. JAMA. 2016;315(2):198-200. doi: 10.1001/jama.2015.13706. [DOI] [PubMed] [Google Scholar]
- 55.Cowan AJ, Baldomero H, Atsuta Y, et al.. The global state of hematopoietic cell transplantation for multiple myeloma: an analysis of the Worldwide Network of Blood and Marrow Transplantation database and the global burden of disease study. Biol Blood Marrow Transplant. 2020;26(12):2372-2377. doi: 10.1016/j.bbmt.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aljurf M, Weisdorf D, Alfraih F, et al.. Worldwide Network for Blood and Marrow Transplantation (WBMT) special article, challenges facing emerging alternate donor registries. Bone Marrow Transplant. 2019;54(8):1179-1188. doi: 10.1038/s41409-019-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]