ABSTRACT
Mycobacterium abscessus is the etiological agent of severe pulmonary infections in vulnerable patients, such as those with cystic fibrosis (CF), where it represents a relevant cause of morbidity and mortality. Treatment of pulmonary infections caused by M. abscessus remains extremely difficult, as this species is resistant to most classes of antibiotics, including macrolides, aminoglycosides, rifamycins, tetracyclines, and β-lactams. Here, we show that apoptotic body like liposomes loaded with phosphatidylinositol 5-phosphate (ABL/PI5P) enhance the antimycobacterial response, both in macrophages from healthy donors exposed to pharmacological inhibition of cystic fibrosis transmembrane conductance regulator (CFTR) and in macrophages from CF patients, by enhancing phagosome acidification and reactive oxygen species (ROS) production. The treatment with liposomes of wild-type as well as CF mice, intratracheally infected with M. abscessus, resulted in about a 2-log reduction of pulmonary mycobacterial burden and a significant reduction of macrophages and neutrophils in bronchoalveolar lavage fluid (BALF). Finally, the combination treatment with ABL/PI5P and amikacin, to specifically target intracellular and extracellular bacilli, resulted in a further significant reduction of both pulmonary mycobacterial burden and inflammatory response in comparison with the single treatments. These results offer the conceptual basis for a novel therapeutic regimen based on antibiotic and bioactive liposomes, used as a combined host- and pathogen-directed therapeutic strategy, aimed at the control of M. abscessus infection, and of related immunopathogenic responses, for which therapeutic options are still limited.
IMPORTANCE Mycobacterium abscessus is an opportunistic pathogen intrinsically resistant to many antibiotics, frequently linked to chronic pulmonary infections, and representing a relevant cause of morbidity and mortality, especially in immunocompromised patients, such as those affected by cystic fibrosis. M. abscessus-caused pulmonary infection treatment is extremely difficult due to its high toxicity and long-lasting regimen with life-impairing side effects and the scarce availability of new antibiotics approved for human use. In this context, there is an urgent need for the development of an alternative therapeutic strategy that aims at improving the current management of patients affected by chronic M. abscessus infections. Our data support the therapeutic value of a combined host- and pathogen-directed therapy as a promising approach, as an alternative to single treatments, to simultaneously target intracellular and extracellular pathogens and improve the clinical management of patients infected with multidrug-resistant pathogens such as M. abscessus.
KEYWORDS: chronic infection, cystic fibrosis, host-pathogen interactions, infectious disease, innate immunity, liposomes, nontuberculous mycobacteria, pulmonary infection
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive genetic disease caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) channel, resulting in a systemic disease which affects different organs and apparatuses, such as the pancreas, liver, reproductive tract, and mainly, the lungs (1). Here, the loss of function of CFTR causes a defective mucociliary clearance, a dramatic production of sticky mucus, and dysfunction in the phagocytosis process (2, 3), leading to chronic bacterial infection and colonization by opportunistic pathogens, such as Mycobacterium abscessus (4). M. abscessus represents a relevant cause of morbidity and mortality in CF patients, where it causes severe pulmonary infections, which are very difficult to treat due to its trend to form biofilms and its natural resistance to a wide range of antibiotics (5). On these grounds, the identification of novel therapeutic strategies capable of supporting or complementing the few currently available antibiotic options represents an urgent issue to be addressed.
Phagocytosis is a key effector mechanism of the antimicrobial innate immune response and is mediated by a timely coordinated and a topologically distributed expression of second lipid messengers, which recruit signal proteins on the nascent or maturing phagosome through recognition of specific lipid-binding domains (6, 7). We have previously demonstrated that selected second lipid messengers, involved in phagolysosome biogenesis and delivered by apoptotic body-like liposomes (ABLs), enhance innate (myco)bactericidal responses in different in vitro, ex vivo, and in vivo experimental models of pathogen-inhibited and host-impaired phagosome maturation (8, 9), as well as simultaneously reducing potentially damaging tissue inflammatory responses (9, 10). In particular, the treatment with ABLs loaded with phosphatidic acid (PA) of Mycobacterium tuberculosis-infected mice resulted in a 100-fold reduction of pulmonary mycobacterial loads, with a concomitant 10-fold reduction of serum tumor necrosis factor alpha (TNF-α), interleukin-1beta (IL-1β), and gamma interferon (IFN-γ) (9). Furthermore, ABLs loaded with phosphatidylinositol 5-phosphate (PI5P) improve the antimicrobial response to multidrug-resistant (MDR) Pseudomonas aeruginosa in impaired macrophages from CF patients and limit in vivo airway inflammatory response (10). Therefore, we evaluated ABLs loaded with selected bioactive lipids, alone or in combination with amikacin, as a novel formulation of a combined host- and pathogen-directed therapeutic approach against in vitro and in vivo M. abscessus infection.
RESULTS
ABLs loaded with PA, PI3P, and PI5P improve M. abscessus uptake and intracellular killing both in dTHP-1 cells and monocyte-derived macrophages (MDM) treated or not treated with INH172.
The bioactive lipids PA, PI5P, phosphatidylinositol 3-phosphate (PI3P), lysobisphosphatidic acid (LBPA), sphingosine 1-phosphate (S1P), and arachidonic acid (AA), chosen because of their capability to drive the phagocytosis process (6, 7), were individually included in the inner layer of ABLs (see Fig. S1A in the supplemental material) to test their in vitro capability to enhance intracellular mycobacterial killing. These liposome formulations were preliminarily tested in flow cytometry for size distribution by comparing their forward scatter parameters (FS) with the FS of commercially available beads of known diameter. Results show a size distribution of the different liposome formulations between 0.8 μm and 6 μm in diameter (Fig. S1B to G), which indicates their suitability to be used for inhalation against pulmonary infections (11). The different liposome formulations were then used to stimulate differentiated THP1 (dTHP-1) cells infected with M. abscessus and were evaluated in terms of mycobacterial phagocytosis and replication index. Results show that liposomes loaded with PA (ABL/PA), PI3P (ABL/PI3P), and PI5P (ABL/PI5P) enhanced internalization of M. abscessus in dTHP-1 cells treated (Fig. 1A) or not treated (Fig. S2A) with the pharmacological inhibitor of CFTR, INH172. After mycobacterial internalization, phagosome acidification and reactive oxygen species (ROS) generation are sequential steps responsible for the killing of intracellular pathogens (12). Macrophages exposed to the same three liposome formulations promote phagosome acidification (Fig. S3A) and ROS generation (Fig. S3B). To measure the functional consequences of phagosome acidification and of ROS generation, we measured intracellular M. abscessus viability following stimulation with all liposome formulations. The analysis confirmed that ABL/PA, ABL/PI3P, and ABL/PI5P, among six liposome formulations, significantly reduced the mycobacterial replication index in dTHP-1 (Fig. S2B) and in dTHP-1 following pharmacological inhibition of CFTR (Fig. 1B). These results were further confirmed in primary macrophages from healthy donors (Fig. 1C).
FIG 1.
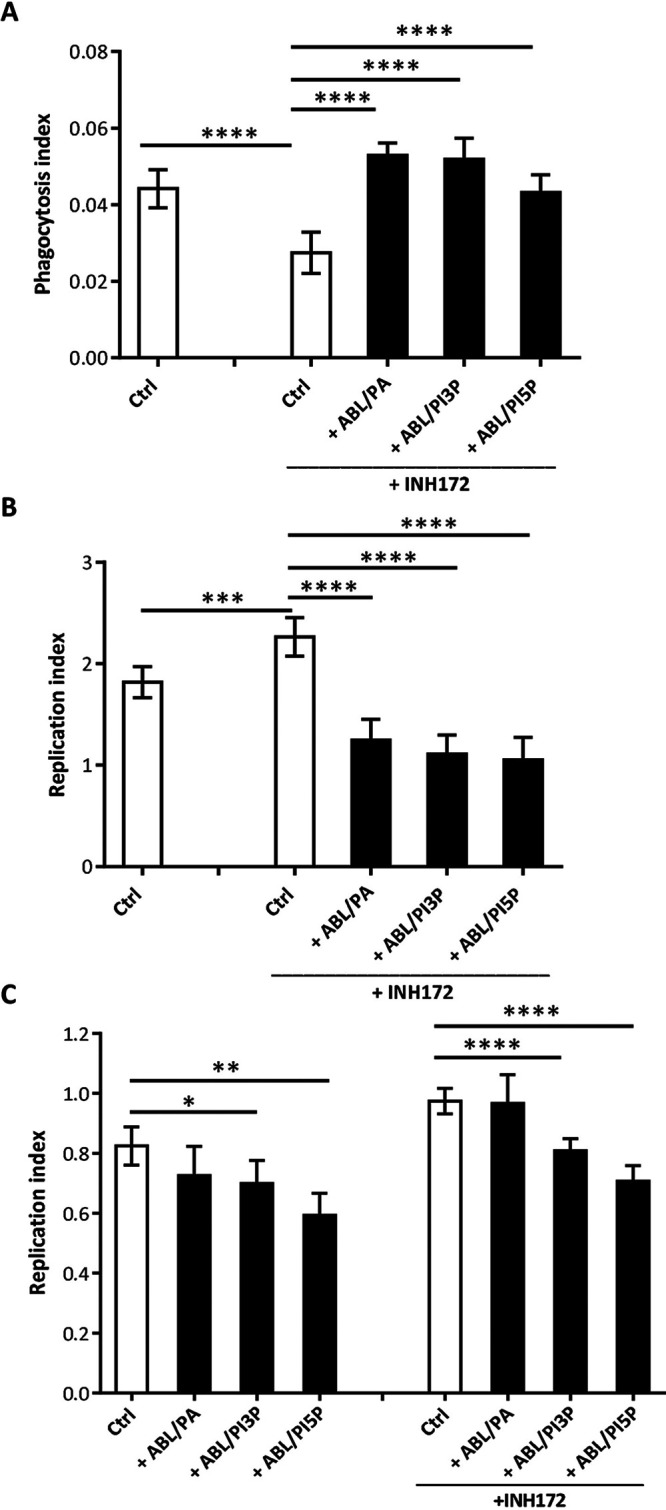
ABL/PA, ABL/PI3P, and ABL/PI5P enhance both internalization and intracellular killing of M. abscessus in dTHP-1 cells and monocyte-derived macrophages (MDM) from healthy donors (HD) with pharmacologically inhibited CFTR. (A to C) dTHP-1 cells (A and B) and primary MDM from healthy donors (C), treated or not treated with INH172, were cultured at 5 × 105 cells/well in 24-well plates and 2 × 105 cells/well in 96-well plates. (A) Cells were stimulated with selected ABL formulations before infection for 30 min and then infected with the M. abscessus reference strain (ATCC 19977). Bacterial uptake was quantified by CFU assay and indicated as phagocytosis index, calculated as the ratio between the CFU obtained immediately after the infection and those from the inoculum. (B and C) Cells, dTHP-1 cells (B) and MDM from healthy donors (C), were exposed or not exposed to INH172, infected with M. Abscessus, and treated for 18 h with the selected ABL formulations. Bacterial growth was assessed by CFU assay. The replication index was calculated as the ratio between the CFU obtained 18 h after infection, in the presence or absence of ABL formulations, and the CFU obtained before the addition of liposomes. The results are shown as the mean ± standard deviation of the values obtained from triplicates of each condition, and panel C is representative of experiments with cells from four different donors. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by Student’s t test.
ABL/PI5P promotes intracellular M. abscessus killing dependent on ROS production and phagosome acidification in CF macrophages.
Previous results induced us to initially select ABL/PA, ABL/PI3P, and ABL/PI5P for subsequent analyses on primary monocyte-derived macrophages isolated from CF patients. Macrophages from CF patients were infected in vitro with M. abscessus, and bacterial viability was measured by CFU assay and shown as replication index. Results show that no significant effect was observed following stimulation with ABL/PA (Fig. S4A), whereas ABL/PI3P and, most significantly, ABL/PI5P induced a significant improvement of antimycobacterial activity compared to unstimulated cells (Fig. S4B and Fig. 2A), indicating that the effect was specific and dependent upon the second lipid messenger used. Given that ABL/PI5P resulted in the most significant effect, we next investigated its antimicrobial activity mechanism, observing that intracellular mycobacterial killing was dependent on phagosome acidification and ROS generation. In particular, CF macrophages were exposed to concanamycin A, an inhibitor of V-ATPase (13), or to pegylated catalase (PEG-Cat), which converts hydrogen peroxide to water and oxygen and thus reduces ROS activity. Results indicate that concanamycin A (Fig. 2B) and PEG-Cat (Fig. 2C) almost completely abolish the ABL-induced intracellular M. abscessus killing, demonstrating that the main mechanism of ABL/PI5P action relies on phagosome acidification and ROS generation.
FIG 2.
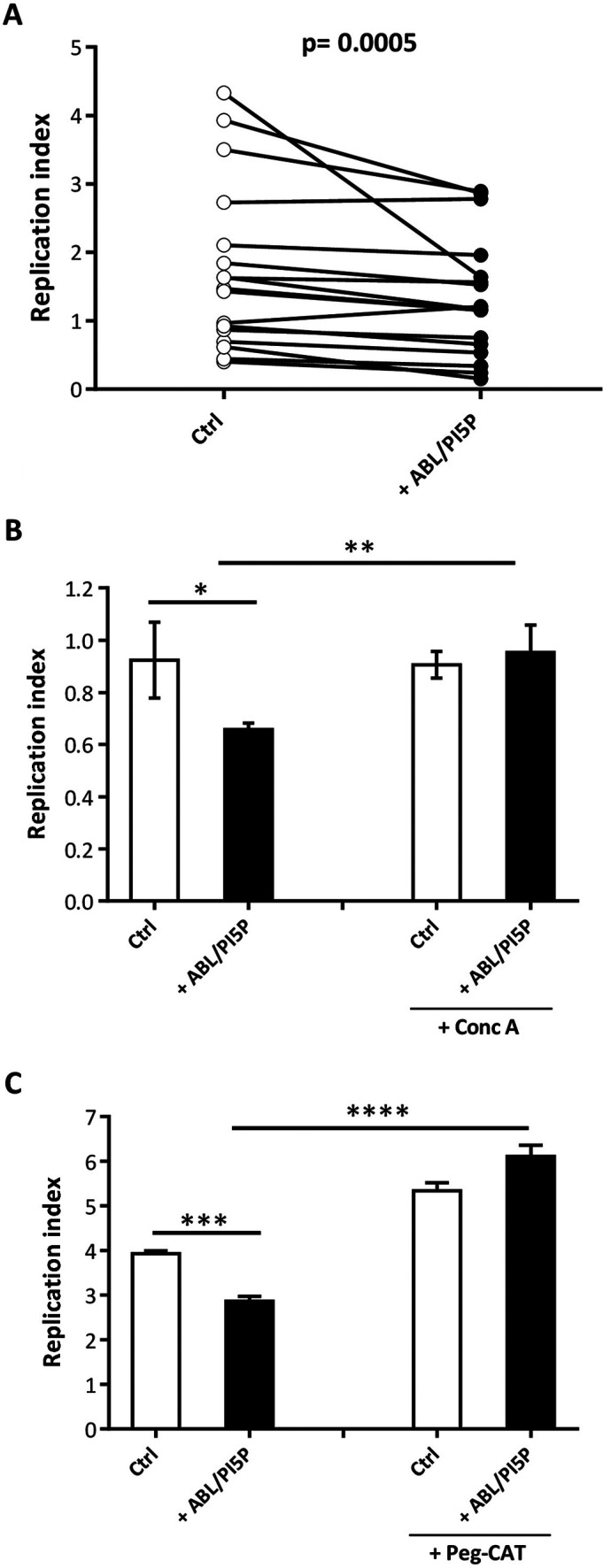
ABL/PI5P promotes both ROS- and phagolysosome acidification-dependent intracellular M. abscessus killing in CF MDM. (A to C) MDM isolated from CF patients (n = 17) were plated at the concentration of 1 × 106 cells/mL, infected with the M. abscessus reference strain (ATCC 19977), and then stimulated for 18 h with ABL carrying PI5P (A) (n = 17) in the presence or absence of catalase (PEG-Cat) (B) (representative of n = 4) or concanamycin A (Conc A), a specific inhibitor of vacuolar type H+-ATPase activity (C) (representative of n = 4) at the concentration of 100 U/mL or 1 nM, respectively. Bacterial growth was assessed by CFU assay, and the replication index was calculated as the ratio between the CFU obtained 18 h after infection, in the presence or absence of ABL formulations, and the CFU obtained before the addition of liposomes. (A) Statistical analysis was performed using the two-sided Wilcoxon matched-pair signed rank test (P = 0.0005). (B and C) The results are shown as the mean ± standard deviation of the values obtained from each condition and are representative of experiments with cells from four different CF patients. *, P < 0.05; **, P < 0.01, ***, P < 0.001; ****, P < 0.0001 by Student’s t test.
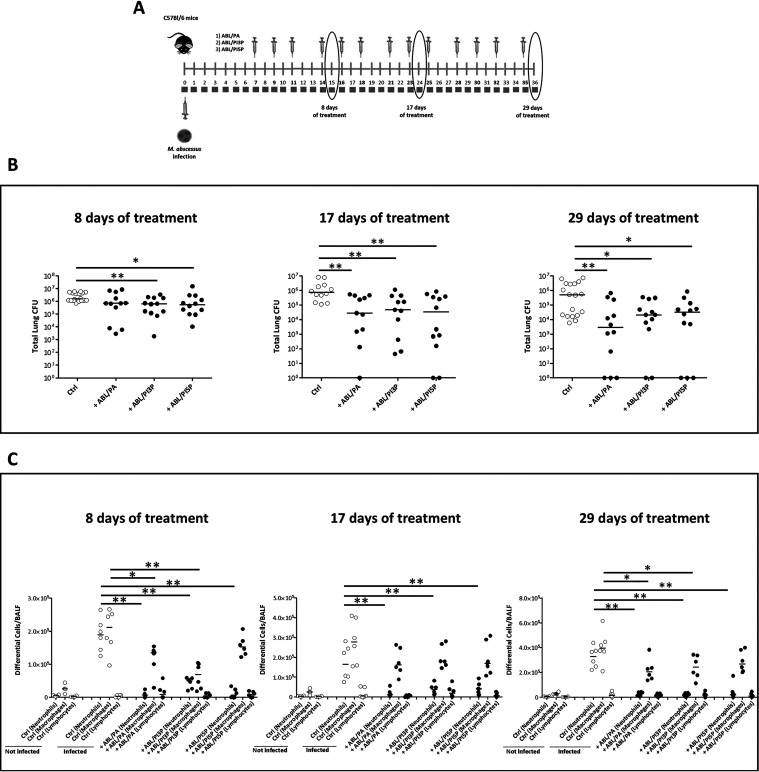
ABL/PI5P induces M. abscessus clearance and leukocyte recruitment in wild-type (WT) and CF mice.
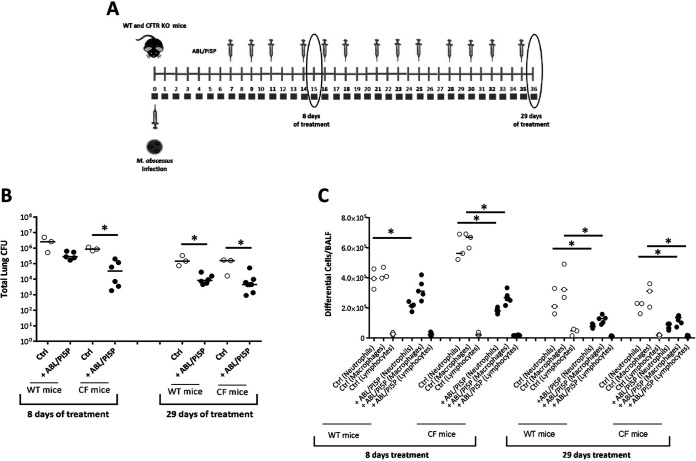
Next, we tested the therapeutic value of the treatment with ABL/PA, ABL/PI3P, and ABL/PI5P in an in vivo model of M. abscessus infection. To this aim, immunocompetent C57BL/6 mice were intratracheally (i.t.) infected with M. abscessus (reference strain ATCC 19977), and a chronic lung infection was established by a previously described agar bead method (14). After 1 week from M. abscessus infection, mice were treated 3 times per week for 4 consecutive weeks by intranasal inoculation of 105 ABLs loaded with PA, PI3P, or PI5P. After 8, 17, and 29 days of treatment, mice were sacrificed, bronchoalveolar lavage (BAL) was performed, and the total lung was processed for microbiological analysis and for the assessment of inflammatory parameters (Fig. 3A). Results showed that all ABL formulations induced a progressive reduction of pulmonary mycobacterial burden over time, reaching about a 2-log reduction of mean pulmonary CFU after 29 days of treatment. Notably, after 29 days of treatment with ABL/PA and ABL/PI5P, in 3 out of 12 mice the total lung CFU was not detectable by the viable count assay (Fig. 3B). The analysis of the inflammatory infiltrate revealed a strongly significant reduction of neutrophils in bronchoalveolar lavage fluid (BALF) of mice treated with all ABL formulations at all time points analyzed and a less pronounced, but still significant, reduction of macrophages in BALF of mice treated with ABL/PA and ABL/PI3P for 8 and 29 days (Fig. 3C). Such a reduction of inflammatory cells in the lungs of treated mice was associated, only in the case of ABL/PI5P treatment, with a progressive decrease of keratinocyte-derived chemokine (KC) (albeit not significant) and a significant decrease of both IL-1β and IFN-γ after 8 and 29 days of treatment, whereas TNF-α and IL-6 did not show any significant modulation over time (Fig. S5). Based on the above-described in vitro and in vivo results, and considering the crucial role played by the inflammatory response in the pathogenesis of CF (15), we selected ABL/PI5P for subsequent in vivo analysis on CF mice. In particular, CFTR knockout (KO) and wild-type mice were infected with M. abscessus and, starting from day 8 after infection, treated 3 times per week for 4 consecutive weeks by intranasal inoculation of 105 ABLs carrying PI5P. After 8 and 29 days of treatment, mice were sacrificed, BALF was recovered, and total the lung was processed for cell counts and CFU analysis, respectively (Fig. 4A). Results showed an about 2-log reduction of pulmonary bacterial burden following liposome administration both in wild-type and CFTR KO mice treated for 8 and 29 days (Fig. 4B), which was associated with a significant reduction of total cell counts in BALF (Fig. 4C).
FIG 3.
ABL/PI5P reduces both bacterial burden and leukocyte recruitment in WT mice infected with M. abscessus. (A) C57BL/6N mice were chronically infected with 105 CFU of the M. abscessus reference strain (ATCC 19977) by intratracheal (i.t.) injection and treated after 1 week of infection with ABL loaded with PA, PI3P, or PI5P three times a week for 8, 17, and 29 days. (A and B) After these time points mice were sacrificed, and BALF and lungs were processed for microbiological analysis (B) and evaluation of neutrophil, macrophage, and lymphocyte BALF recruitment (C). Data from two independent experiments were pooled. The results are shown as the median of the values, and statistical significance by Mann-Whitney test was determined (*, P < 0.05; **, P < 0.01).
FIG 4.
ABL/PI5P reduces both bacterial burden and leukocyte recruitment in CF mice infected with M. abscessus. (A) WT and CF mice were chronically infected with 105 CFU of the M. abscessus reference strain (ATCC 19977) by i.t. injection and treated with ABL/PI5P three times a week for 8 and 29 days. (B and C) Mice were then sacrificed, and BALF and lungs were processed for (B) microbiological analysis and (C) evaluation of neutrophil, macrophage, and lymphocyte BALF recruitment to establish the therapeutic efficacy of liposomes. The results are shown as the median of the values, and statistical significance by Mann-Whitney test was determined (*, P < 0.05; **, P < 0.01).
ABL/PI5P-amikacin combined treatment induces both clearance and anti-inflammatory responses in CF MDM and in an in vivo model of M. abscessus infection.
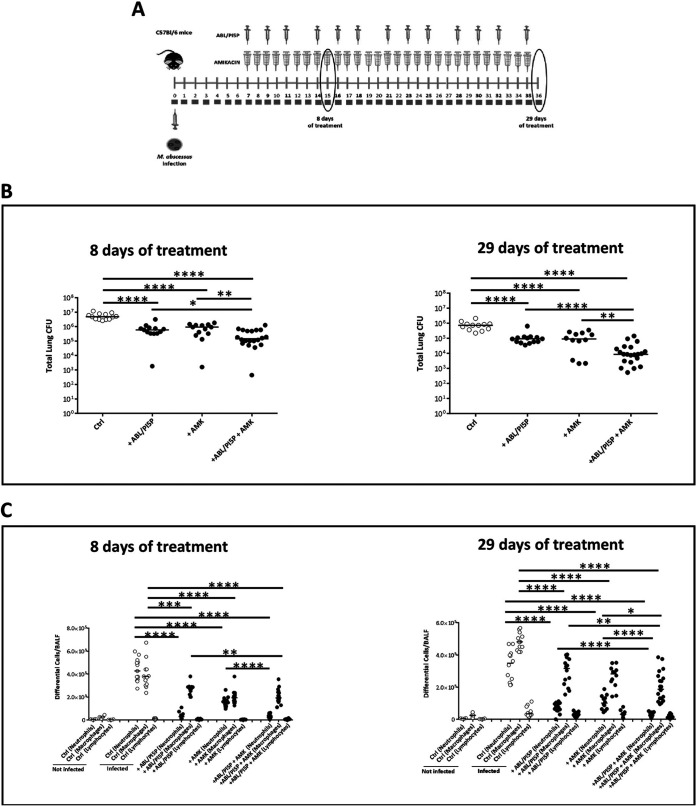
As a combination therapy may represent a valuable strategy to differentially target extracellular and intracellular pathogens, we combined ABL/PI5P with amikacin antibiotic treatment, chosen for its clinical use in both intensive and continuation therapeutic regimens for M. abscessus infection (16) and for its low penetration capability in macrophages (17). Initially the combined treatment was tested in vitro on M. abscessus-infected dTHP-1 cells treated or not treated with CFTR inhibitor (INH172) as well as on macrophages from CF patients. Next, we evaluated, in an in vivo model of chronic M. abscessus infection, the efficacy of the combined strategy with administration of intranasal (i.n.) ABL/PI5P and intraperitoneal (i.p.) amikacin.
Results show that ABL/PI5P reduces the intracellular M. abscessus replication in dTHP-1 cells irrespective of CFTR inhibition (Fig. S6B and D), without any direct effect on the extracellular pathogen (Fig. S6A and C). The combination treatment with ABL/PI5P and AMK (Fig. S6B and D) induced a significantly higher reduction of M. abscessus replication index than the single treatments. Similar analysis was then performed on monocyte-derived macrophages from CF patients, and results confirmed the increased therapeutic value of the in vitro combined treatment, compared to the single treatments, on extracellular and intracellular pathogens (Fig. 5A and B). Such a combination of host-directed (ABL/PI5P) and pathogen-directed (amikacin) therapy, was finally tested on wild-type C57BL/6 mice intratracheally infected with M. abscessus. Mice were intratracheally infected with M. abscessus (reference strain ATCC 19977) and, starting on day 7 after infection, were inoculated daily, via the i.p. route, with 100 mg/kg body weight of amikacin, whose dose was chosen in preliminary in vivo experiments (Fig. S7) and/or treated 3 times per week for 4 consecutive weeks by intranasal inoculation of 105 liposomes. After 8 and 29 days of treatment mice were sacrificed, BALF was obtained, and the total lung was processed for microbiological analysis and assessment of inflammatory parameters (Fig. 6A). Results show that the combination therapy induced a highly significant decrease in pulmonary mycobacterial burden compared to the single therapies (Fig. 6B) along with a significant reduction of neutrophils and macrophages in BALF (Fig. 6C), in the absence of any signs of kidney and liver toxicity (Fig. S8). The reduction of inflammatory response was also evaluated in terms of cytokines in BALF and in total lung. Results obtained after 29 days of treatment show that the combination therapy induced a more significant reduction of IL-1β, TNF-α, KC, IL-17a, and IFN-γ in total lung than the single treatments (Fig. S9).
FIG 5.
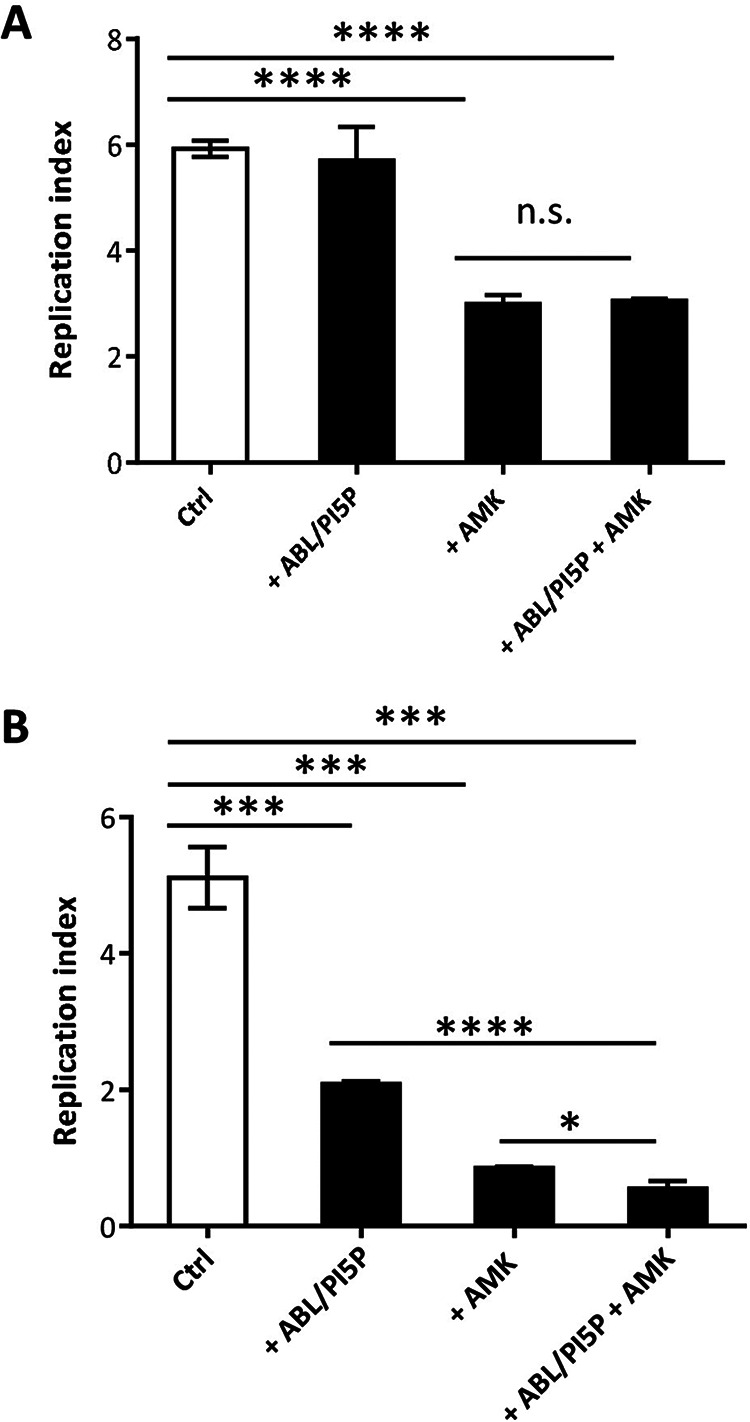
ABL/PI5P-amikacin combined treatment promotes higher reduction of M. abscessus intracellular growth than single treatments in CF MDM. CF MDM were cultured and infected as described in Materials and Methods. (A and B) Finally, supernatant was collected, cells were lysed, and both were analyzed for extracellular (A) and intracellular (B) bacterial growth. The replication index was calculated as the ratio between the CFU obtained 18 h after infection in the presence or absence of ABL/PI5P and/or amikacin (AMK) and those obtained immediately after infection, before the addition of the stimuli. Results are shown as the mean ± standard deviation of the values obtained from triplicates of each condition and are representative of four CF patients. n.s., not significant; *, P < 0.05; ***, P < 0.001; ****, P < 0.0001 by Student’s t test.
FIG 6.
ABL/PI5P-amikacin combined treatment induces both M. abscessus clearance and anti-inflammatory responses in WT mice. (A) WT mice were chronically infected with 105 CFU of the M. abscessus reference strain (ATCC 19977) and treated 1 week after infection with AMK and/or ABL/PI5P as described in Materials and Methods. (B and C) After 8 and 29 days of treatment, mice were sacrificed, and BALF and lungs were processed for microbiological analysis (B) and evaluation of neutrophil, macrophage, and lymphocyte BALF recruitment (C). The results are shown as the median of the values, and statistical significance by Mann-Whitney test was determined (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
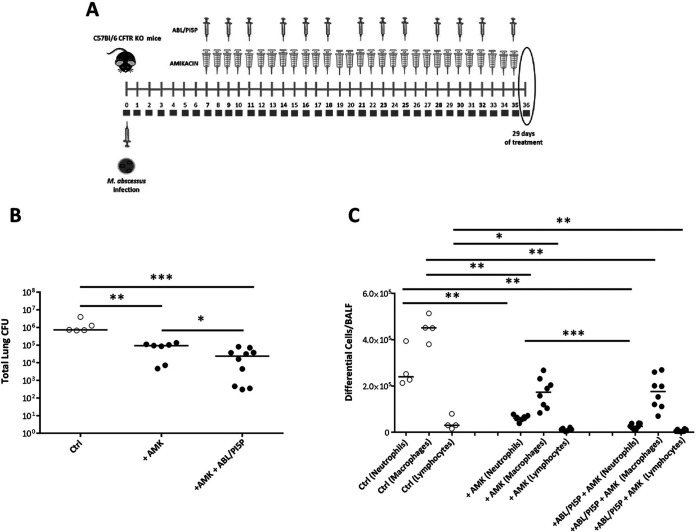
The combination of host- and pathogen-directed therapy was finally tested in CFTR KO mice, a relevant experimental model of CF, after M. abscessus infection (Fig. 7A). Results show that a significant reduction of pulmonary mycobacterial burden (Fig. 7B) and inflammatory response (Fig. 7C) was observed following combination therapy.
FIG 7.
ABL/PI5P-amikacin combined treatment induces both M. abscessus clearance and anti-inflammatory responses in CF mice. (A) CF mice were chronically infected with 105 CFU of the M. abscessus reference strain (ATCC 19977) and treated 1 week after infection with AMK and/or ABL/PI5P as described in Materials and Methods. (B and C) After 29 days of treatment mice were sacrificed, and BALF and lungs were processed for microbiological analysis (B) and evaluation of neutrophil, macrophage, and lymphocyte BALF recruitment (C). The results are shown as the median of the values, and statistical significance by Mann-Whitney test was determined (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
DISCUSSION
The therapeutic management of M. abscessus infection remains extremely difficult, as this pathogen exhibits resistance to many different antibiotics (18). In addition to mutations in drug targets, resistance mechanisms may involve (i) intrinsic drug resistance, (ii) low permeability of the cell wall, (iii) induction of drug efflux pumps, (iv) mutations in mycobacterial enzymes which do not convert prodrugs into active metabolites, and/or (v) the expression of numerous enzymes that can either neutralize drugs or modify their specific targets (5). The standard therapy of pulmonary M. abscessus infection in CF patients requires an intensive phase and a continuation phase based on different therapeutic regimens, whose duration depends upon the severity of infection and upon the response to treatment (19). In particular, the intensive phase consists of a daily oral administration of a macrolide together with the intravenous inoculation of amikacin and one or more among tigecycline, imipenem, or cefoxitin. The continuation phase is based on daily oral administration of a macrolide in association with inhaled amikacin together with 2 or 3 among minocycline, clofazimine, moxifloxacin, and linezolid (16). Such a complex therapeutic regimen is worsened by drug intolerance and drug-related toxicity, frequently occurring in CF patients, often requiring changes in antibiotic therapy (16). Thus, the discovery of additional and more effective anti-M. abscessus drugs and/or the identification of novel therapeutic strategies are urgently needed for the clinical management of this pathogen.
CF macrophages show signs of impaired phagolysosome maturation, due to the CFTR mutation-dependent block of phagosomal acidification, which leads to a reduced antimicrobial response (3, 10). In addition, M. abscessus has been reported to further inhibit phagosome maturation and to escape from the phagosome microenvironment by the ESX-4 system (20), which may further facilitate colonization of vulnerable CF patients. Thus, an efficient host-directed therapy aimed to enhance intracellular mycobacterial killing should rescue both the pathogen-inhibited and host-impaired phagosome maturation process. In the present study, we show in vitro and in vivo results supporting the therapeutic value of the combined treatment with ABL/PI5P and amikacin, used as host- and pathogen-directed therapy, aimed at specifically targeting the intracellular and extracellular pathogen, respectively.
The role of PI5P in the activation of innate antimicrobial responses has been reported in different kinds of infection. In particular, we have recently reported that ABL loaded with PI5P could enhance intracellular killing of P. aeruginosa in impaired macrophages from CF patients (10) and of in vivo acquired Klebsiella pneumoniae, Klebsiella oxytoca, Acinetobacter baumannii, and methicillin-resistant Staphylococcus aureus in BALF cells from patients with pneumonia caused by the respective pathogens (8). Also, the role of phosphoinositide 5-kinase (PIKfive), which catalyzes the formation of PI3,5P2 and PI5P, has been recently described to play an important role in the early phases of phagosome maturation and the intracellular restriction of Legionella pneumophila infection (21). The results reported here further extend such evidence, indicating a very efficient capability of ABL/PI5P liposome formulation to restrict intracellular M. abscessus growth, by a phagosome acidification-dependent and ROS-mediated mechanism, in macrophages from healthy donors as well as CF patients. Although the exact molecular pathway activated by PI5P and leading to intracellular mycobacterial killing is still unknown, a role of PI5P in the activation of VPS34-independent noncanonical autophagy has been reported (22), which may be responsible for the observed phagosome acidification, ROS production, and intracellular mycobacterial killing.
Airway inflammation is a hallmark of CF disease characterized by elevated levels of proinflammatory cytokines and chemokines (15), resulting in chronic inflammation, neutrophil recruitment, and progressive airway destruction leading to the decline in lung function. Thus, the concomitant reduction of in vivo inflammatory responses may represent a further important goal for the treatment of chronic M. abscessus infection. The functional distribution of PS at the outer surface of cell membranes represents an “eat me” signal (23) through which apoptotic bodies are specifically eliminated by macrophages through an anti-inflammatory phagocytosis (24, 25). These features have highlighted the possibility of using apoptotic bodies and/or PS liposome formulations to manipulate the immune response for therapeutic gain by reducing immunopathologic responses (26–28). Moreover, we recently reported that the treatment with ABL loaded with PI5P reduces NF-κB activation and the downstream proinflammatory cytokine production in in vitro P. aeruginosa-infected macrophages and limits airway inflammation in in vivo-infected mice (10). Coherently with this already published data, the results reported here show a significant reduction of airway inflammation in M. abscessus-infected mice, which is more significant following amikacin-ABL/PI5P combination therapy, possibly as an additional effect of the antibiotic-mediated reduction of extracellular bacilli.
The main limitation of our study is based on the absence of a formal pharmacokinetic, pharmacodynamic, or toxicity assessment following the combined treatment with ABL/PI5P and amikacin. Despite the demonstration of efficacy against in vivo infection with M. abscessus and the absence of liver and renal toxicity in mice undergoing the treatment, this study is also limited by the demonstration of efficacy only in the murine models of infection. Thus, the clinical potential cannot be fully established until safety and efficacy trials of combined treatment are undertaken in humans.
In conclusion, our results show that the host- and pathogen-directed combination therapy reported here may offer the unique advantage to simultaneously target extracellular and intracellular mycobacteria. In this context, the intracellular hiding of bacterial pathogens in mammalian cells, such as phagocytes, can prevent antibiotics from killing pathogens and plays an underappreciated role in the recurrence of bacterial infections (29). The intracellular localization of a bacterial pathogen may hinder the antibiotic efficacy (i) by reducing the local active concentration of the drug due to either a poor permeability of several antibiotics to the cell membrane and/or a higher efflux/influx ratio, (ii) by activating the expression of genes associated with latency and by reducing the bacterial metabolism, both necessary to survive inside a hostile intracellular microenvironment (30), and (iii) by colonizing intracellular compartments that are difficult for the drug to reach (31). These persisting intracellular mycobacteria that are difficult for the drug to reach are crucial for the establishment of chronic infection and granuloma formation (5). Thus, the higher efficiency of host- and pathogen-directed combination therapy in treating in vivo infections by targeting intracellular and extracellular mycobacteria may translate to a reduction of the time of therapy and to minimizing the risk of emergence of additional drug resistances.
MATERIALS AND METHODS
Liposome preparation.
ABLs were produced as previously described (9). Briefly, the inner monolayer lipids (0.05 mg/mL) were suspended in anhydrous dodecane (Sigma). The following bioactive lipids were used for the inner monolayer: l-α-phosphatidic acid (PA), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol-3′-phosphate) (PI3P), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol-5′-phosphate) (PI5P), d-erythro-sphingosine-1-phosphate (S1P), lysobisphosphatidic acid (LBPA), (all from Avanti Polar Lipids), and arachidonic acid (AA) (Sigma). l-α-phosphatidylserine (PS) (Avanti Polar Lipids) was used as the outer monolayer lipid and was added to a 99:1 dodecane:silicone solution to obtain a final concentration of 0.05 mg/mL. Asymmetric liposomes were prepared by adding 2 mL of outer monolayer lipid suspension over 3 mL of complete medium. Finally, 100 μL of the inner monolayer lipid suspensions were added over 2 mL of lipid phase, and the samples were centrifuged at 120 × g for 10 min. After the centrifugation, ABLs were collected in an aqueous phase using a 5-mL syringe with a 16-gauge stainless steel needle. The following six ABL formulations were produced: PS outside/PA inside (ABL/PA), PS outside/PI3P inside (ABL/PI3P), PS outside/PI5P inside (ABL/PI5P), PS outside/S1P inside (ABL/S1P), PS outside/LBPA inside (ABL/LBPA), and PS outside/AA inside (ABL/AA). Liposomes were then quantified and analyzed in terms of dimension by two different flow cytometers, FACSCalibur (Becton, Dickinson) and FACSCelesta (Becton, Dickinson).
Cell culture.
The human promonocytic THP-1 leukemia cell line was supplied by the European Collection of Cell Culture, grown in RPMI 1640 containing fetal bovine serum (10%), gentamicin (5 μg/mL), l-glutamine (2 mM), nonessential amino acids (1 mM), and sodium pyruvate (1 mM) and cultured in 75-cm2 polystyrene flasks. Before the experiments, cells (5 × 105 or 1 × 106 per well) were seeded in 24-well plates, and the cells were induced to differentiate by stimulation for 72 h with phorbol 12-myristate 13-acetate (PMA) (20 ng/mL) and used as a model of human macrophages (dTHP-1).
Primary monocyte-derived macrophages (MDM) were prepared as previously described (10). Briefly, peripheral blood mononuclear cells (PBMC) from healthy donors and CF patients were isolated by Ficoll density gradient, and the monocytes were then positively sorted using anti-CD14 monoclonal antibodies conjugated to magnetic microbeads (Miltenyi Biotec) according to manufacturer’s instructions. The monocytes were then suspended in complete medium and incubated for a further 5 days in 96-well plates at the concentration of 106 cells/mL in the presence of macrophage colony-stimulating factor (M-CSF) (50 ng/mL) (Miltenyi Biotec) to obtain differentiated macrophages.
Bacteria.
Mycobacterium abscessus (ATCC 19977) was used in in vitro experiments and in the in vivo mouse model of chronic M. abscessus infection (14). The M. abscessus single colonies were collected by streaking on Middlebrook 7H10 medium (7H10; BD Difco) supplemented with oleic acid, albumin, dextrose, and catalase (OADC), then suspended in 15 mL of Middlebrook 7H9 broth (7H9; BD Difco) supplemented with albumin, dextrose, and catalase (ADC), and grown in an Erlenmeyer flask at 37°C with stirring for 48 h. The growth of bacterial cultures was monitored by measuring the optical density at the wavelength of 600 nm by a spectrophotometer (Varioskan LUX multimode microplate reader; Thermo Fisher Scientific). Bacilli were stored at −80°C until use after suspension in the microorganism preservation system Protect (Technical Service Consultants Ltd.).
For in vivo experiments, M. abscessus (ATCC 19977) was grown for 2 days (to reach the exponential phase) in 20 mL of Middlebrook 7H9 broth. Then bacteria were embedded in agar beads, as previously described (14).
Patients.
CF patients (n = 22) were enrolled at “Bambino Gesù” Children’s Hospital in Rome, Italy. All of the CF patients were clinically stable at the time of blood donation (5 mL). Controls were represented by buffy coats from healthy blood donors (n = 4) attending at Blood Transfusion Unit of Policlinico “Tor Vergata” in Rome, Italy. Clinical and demographic features of CF patients are summarized in Table S1.
Mouse strains.
Immunocompetent C57BL/6NCrlBR male mice (8 to 10 weeks of age) from Charles River, gut-corrected CF transmembrane conductance regulator (CFTR)-deficient male and female C57BL/6 Cftrtm1UNCTgN (FABPCFTR)#Jaw mice (CF mice), and the corresponding congenic WT mice, 10 to 17 weeks old, (originally obtained from Case Western Reserve University) were tested in the experiments. Since CF mice had limited utility because most mice die from intestinal obstruction during the first month of life, such lethal intestinal abnormalities have been corrected by expressing the human CFTR (hCFTR) in CFTR−/− mice under the control of the rat intestinal fatty acid-binding protein (FABP) gene promoter to direct expression of the wild-type hCFTR complementary DNA (cDNA) to the intestinal epithelial cells of these mice. Such mice survived and showed functional correction of ileal goblet cell and crypt cell hyperplasia and cyclic AMP-stimulated chloride secretion (32). All mice were maintained in specific-pathogen-free conditions at San Raffaele Scientific Institute, Milan, Italy.
Evaluation of in vitro bacterial uptake and extracellular/intracellular growth.
To assess bacterial uptake, dTHP-1 cells were distributed in 24-well plates at the concentration of 5 × 105 cells/well and were stimulated with ABL carrying PA, PI3P, PI5P, S1P, LBPA, or AA used at the ratio of 1:1 (ABL:MDM) for 30 min before infection in the presence or absence of the CFTR inhibitor INH172 (Sigma), used at the concentration of 10 μM. INH172 binds to the nucleotide binding domain-1 (NBD-1) present on CFTR, leading to a rapid, reversible, and voltage-independent inhibition of the channel (33). Cells were then washed once and infected with M. abscessus for 3 h at 37°C at a multiplicity of infection (MOI) of 10 in the presence or absence of INH172. Thereafter, extracellular bacilli were killed by a 1-h incubation with 250 μg/mL amikacin. Finally, cells were lysed with 1% deoxycholate (Sigma), and samples were diluted in phosphate-buffered saline (PBS)-Tween 80 and CFU-quantified by plating bacilli in triplicate on 7H10.
To assess intracellular bacterial growth, dTHP-1 cells and MDM from healthy donors or from CF patients were distributed in 24- or 96-well plates at the concentration of 5 × 105 cells/mL or 1 × 106 cells/mL, respectively. Cells were infected with M. abscessus, for 3 h at 37°C at an MOI of 10 in the presence or absence of 10 μM INH172. Thereafter, extracellular bacilli were killed by a 1-h incubation with 250 μg/mL amikacin. Cells were then washed and incubated with ABL loaded with PA, PI3P, PI5P, S1P, LBPA, or AA used at the ratio of 1:1 (ABL:MDM) for a further 18 h in the presence or absence of INH172. Finally, cells were lysed with 1% deoxycholate (Sigma), and samples were diluted in PBS-Tween 80 and CFU-quantified by plating bacilli in triplicate on 7H10. To evaluate the role of ROS and of phagosome acidification in intracellular bacterial killing, M. abscessus-infected cells were treated simultaneously with ABL/PI5P with either polyethylene glycol (PEG)-catalase (100 U/mL) or concanamycin A (1 nM). In order to evaluate the in vitro efficacy of a combined therapy on extracellular and intracellular mycobacterial viability, dTHP-1 cells or MDM from CF patients were infected with M. abscessus at an MOI of 10 for 3 h at 37°C. Cells were then stimulated, in presence or absence of INH172, with ABL/PI5P and/or 4 μg/mL amikacin (AMK) for 18 h. Both extracellular and intracellular bacterial growth were assessed by CFU assay.
Fluorometric analysis.
Phagosome acidification was assessed using the fluorescent probe LysoSensor green DND 189 (Molecular Probes) (34), which measures the pH of acidic organelles, such as phagolysosomes. Briefly, dTHP-1 cells were pretreated or not treated for 1 h with 10 μM INH172 and then infected with M. abscessus for 3 h at 37°C at an MOI of 10 in the presence or absence of 10 μM INH172. Cells were then washed and incubated for a further 3 h with ABL carrying PA, PI3P, and PI5P, added at the ratio of 1 to 1 (ABL:MDM) in the presence or absence of INH172. Cells were stained for 15 min at 37°C with 1 μM LysoSensor green DND 189. A pH calibration curve was obtained by incubating macrophages in calibration buffers at pH 4.5, 5.5, 6.5, and 7.5 (intracellular pH calibration buffer kit; Molecular Probes) and by labeling cells for 15 min at 37°C with 1 μM LysoSensor green DND 189, according to the manufacturer’s instructions. The intensity of fluorescence was determined at an excitation wavelength of 492 nm and an emission wavelength of 517 nm using a Varioskan LUX multimode microplate reader (Thermo Fisher Scientific).
ROS generation was analyzed by loading dTHP-1 cells with the fluorescent indicator 20,70-dichlorofluorescein diacetate (DCF) (Molecular Probes), used at a concentration of 10 μM, for 40 min at 37°C in the dark. Thereafter, dTHP-1 cells were pretreated or not treated for 1 h with 10 μM INH172 and then infected with M. abscessus for 3 h at 37°C at an MOI of 10 in the presence or absence of 10 μM INH172. Cells were then washed and incubated for a further 3 h with ABL carrying PA, PI3P, and PI5P, added at the ratio of 1 to 1 (ABL:MDM), in the presence or absence of INH172. The production of ROS was evaluated by fluorometry using a Varioskan LUX multimode microplate reader (Thermo Fisher Scientific) and setting the excitation and emission wavelengths at 488 nm and 530 nm, respectively.
Mouse model of chronic infection.
The agar bead method was used to establish a stable infection in mice and to faithfully reproduce a chronic infection. In detail, M. abscessus colonies from 7H10 plates were grown for 2 days (to reach the exponential phase) in 20 mL of Middlebrook 7H9 broth. A bacterial suspension reaching an optical density (OD) of 15 was used for bead preparation. Then 50 mL of white heavy mineral oil and 25 mL of Trypticase soy agar (TSA) were added to the bacterial suspension and mixed at medium speed with a magnet on a stirrer to generate agar beads of the correct size (100 to 200 μm). Agar bead preparations were stored at 4°C for no more than a week, as after this time, the number of viable bacteria included within the agar beads decreases. Fresh preparations were performed every time. Embedding M. abscessus within agar beads and using intratracheal injection physically retained the bacteria in the bronchial airways and provided microanaerobic/anaerobic conditions that allow bacteria to grow in microcolonies (14). For the inoculum, mice were anesthetized, the trachea was exposed and intubated, and 50 μL of bead suspension (1 × 105 CFU) was injected before closing the incision with suture clips. Control mice were intratracheally inoculated with the same volume of empty bead suspension. Agar bead sizes smaller than 100 to 200 μm could be easily cleared by mice, while sizes larger than 100 to 200 μm may not reach the lung during the intratracheal infection. Starting from day 7 after infection and for a further 4 weeks, WT and CF mice were treated with intranasal (i.n.) inoculation of 105 ABL loaded with PA, PI3P, or PI5P suspended in saline solution, given alone or in combination with 100 mg/kg or 200 mg/kg amikacin (AMK), administered by intraperitoneal (i.p.) injection. In particular, mice were treated three times a week for 8, 17, and 29 days and were monitored daily for changes in body weight, appetite, and hair coat; at fixed time points from infection (15, 24, and 36 days) mice were killed by carbon dioxide euthanasia, and total lung CFU were determined by using a viable count assay with an M. abscessus limit of detection of ≥102 CFU.
Evaluation of the in vivo inflammatory response.
BALF was obtained as previously described (35), total cells present in the BALF were counted, and a differential cell count was performed on cytospins stained with Diff Quick. Lungs were recovered and homogenized. BALF and lung serial dilutions were plated on 7H10 plates for CFU counting. The lung supernatants were stored at −80°C for cytokine and chemokine determination. Mouse custom ProcartaPlex 9-plex (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) was used for quantification of lung cytokines and chemokines, and values were normalized at 2,500 μg/mL of quantified proteins in lung supernatants.
Statistics.
Comparison between groups was performed using Student’s t test, as appropriate for normally distributed data. The Wilcoxon rank sum test or Mann-Whitney test was performed for data that were not normally distributed.
Ethics statement.
Buffy coats from anonymized healthy donors, who gave their written informed consent to donate the nonclinically usable components of their blood for scientific research, were obtained from the Blood Transfusion Unit of “Policlinico Tor Vergata” in Rome, Italy (ethics approval no. 16/2020).
Cystic fibrosis patients, giving their (or parental) informed consent to participate in the study, were enrolled at “Bambino Gesù” Children’s Hospital in Rome, Italy, after having received detailed information on the scope and objectives of the study by medical personnel, who explained the patient information leaflet (ethics approval no. 738/2017 of “Bambino Gesù” Children’s Hospital, Rome, Italy).
Animal studies were approved by the Italian Ministry of Health guidelines for the use and care of experimental animals and registered by San Raffaele Scientific Institute Institutional Animal Care and Use Committee (IACUC no. 966, approved in March 2019).
Data availability.
All data are available in the main text or the supplemental material.
Supplementary Material
ACKNOWLEDGMENTS
We thank Carla Viscomi, head nurse of the Cystic Fibrosis Unit, Pediatric Hospital “Bambino Gesù” in Rome, Italy, for her important support in the management and collection of clinical samples from patients and Matteo M. Naldini for English revision of the manuscript.
Conceptualization: M.F., D.M.C., V.L.; methodology: N.P., C.R., T.O., M.R., N.I.L., F.C., F.D.S., M.M.D.A., L.H.D.A.; investigation: N.P., C.R., T.O., M.R., F.D.S., N.I.L.; visualization: N.P., C.R., M.M.D.A., L.H.D.A.; funding acquisition: M.F., D.M.C.; project administration: M.F., D.M.C., V.L.; supervision: M.F., D.M.C., V.L.; resources: F.C., V.L.; writing, original draft: M.F., D.M.C.; writing, review and editing: M.F., D.M.C., N.P., C.R., M.M.D.A.
This study was financially supported by the Italian Cystic Fibrosis Foundation (FFC no. 16/2018 and FFC no. 21/2019), Italian Ministry of Defence (no. a2018.092), and FISM-Fondazione Italiana Sclerosi Multipla (2016/R/22) and financed or cofinanced with “5 per mille” public funding.
Footnotes
Supplemental material is available online only.
Contributor Information
Daniela M. Cirillo, Email: cirillo.daniela@hsr.it.
Maurizio Fraziano, Email: fraziano@bio.uniroma2.it.
Paolo Visca, Università Roma Tre.
REFERENCES
- 1.Cutting GR. 2015. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 16:45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly LE, Barnes PJ. 2012. Defective phagocytosis in airways disease. Chest 141:1055–1062. doi: 10.1378/chest.11-2348. [DOI] [PubMed] [Google Scholar]
- 3.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, Palfrey HC, Nelson DJ. 2006. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol 8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 4.Ryan K, Byrd TF. 2018. Mycobacterium abscessus: shapeshifter of the mycobacterial world. Front Microbiol 9:2642. doi: 10.3389/fmicb.2018.02642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen MD, Herrmann JL, Kremer L. 2020. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 18:392–407. doi: 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- 6.Yeung T, Grinstein S. 2007. Lipid signaling and the modulation of surface charge during phagocytosis. Immunol Rev 219:17–36. doi: 10.1111/j.1600-065X.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg BE, Grinstein S. 2008. Pathogen destruction versus intracellular survival: the role of lipids as phagosomal fate determinants. J Clin Invest 118:2002–2011. doi: 10.1172/JCI35433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poerio N, Bugli F, Taus F, Santucci MB, Rodolfo C, Cecconi F, Torelli R, Varone F, Inchingolo R, Majo F, Lucidi V, Mariotti S, Nisini R, Sanguinetti M, Fraziano M. 2017. Liposomes loaded with bioactive lipids enhance antibacterial innate immunity irrespective of drug resistance. Sci Rep 7:45120. doi: 10.1038/srep45120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greco E, Quintiliani G, Santucci MB, Serafino A, Ciccaglione AR, Marcantonio C, Papi M, Maulucci G, Delogu G, Martino A, Goletti D, Sarmati L, Andreoni M, Altieri A, Alma M, Caccamo N, Di Liberto D, De Spirito M, Savage ND, Nisini R, Dieli F, Ottenhoff TH, Fraziano M. 2012. Janus-faced liposomes enhance antimicrobial innate immune response in Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA 109:E1360–E1368. doi: 10.1073/pnas.1200484109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poerio N, De Santis F, Rossi A, Ranucci S, De Fino I, Henriquez A, D’Andrea MM, Ciciriello F, Lucidi V, Nisini R, Bragonzi A, Fraziano M. 2020. Liposomes loaded with phosphatidylinositol 5-phosphate improve the antimicrobial response to Pseudomonas aeruginosa in impaired macrophages from cystic fibrosis patients and limit airway inflammatory response. Front Immunol 11:532225. doi: 10.3389/fimmu.2020.532225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan ZM, Lai GP, Pandey M, Srichana T, Pichika MR, Gorain B, Bhattamishra SK, Choudhury H. 2020. Novel approaches for the treatment of pulmonary tuberculosis. Pharmaceutics 12:1196–1154. doi: 10.3390/pharmaceutics12121196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas A. 2007. The phagosome: compartment with a license to kill. Traffic 8:311–330. doi: 10.1111/j.1600-0854.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 13.Huss M, Ingenhorst G, König S, Gassel M, Dröse S, Zeeck A, Altendorf K, Wieczorek H. 2002. Concanamycin A, the specific inhibitor of V-ATPases, binds to the V(o) subunit c. J Biol Chem 277:40544–40548. doi: 10.1074/jbc.M207345200. [DOI] [PubMed] [Google Scholar]
- 14.Riva C, Tortoli E, Cugnata F, Sanvito F, Esposito A, Rossi M, Colarieti A, Canu T, Cigana C, Bragonzi A, Loré NI, Miotto P, Cirillo DM. 2020. A new model of chronic Mycobacterium abscessus lung infection in immunocompetent mice. Int J Mol Sci 21:6590. doi: 10.3390/ijms21186590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen-Cymberknoh M, Kerem E, Ferkol T, Elizur A. 2013. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax 68:1157–1162. doi: 10.1136/thoraxjnl-2013-203204. [DOI] [PubMed] [Google Scholar]
- 16.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, Van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax 71:88–90. doi: 10.1136/thoraxjnl-2015-207983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rey-Jurado E, Tudó G, Soy D, González-Martín J. 2013. Activity and interactions of levofloxacin, linezolid, ethambutol and amikacin in three-drug combinations against Mycobacterium tuberculosis isolates in a human macrophage model. Int J Antimicrob Agents 42:524–530. doi: 10.1016/j.ijantimicag.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. 2015. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 21:1638–1646. doi: 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degiacomi G, Sammartino JC, Chiarelli LR, Riabova O, Makarov V, Pasca MR. 2019. Mycobacterium abscessus, an emerging and worrisome pathogen among cystic fibrosis patients. Int J Mol Sci 20:5868. doi: 10.3390/ijms20235868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laencina L, Dubois V, Le Moigne V, Viljoen A, Majlessi L, Pritchard J, Bernut A, Piel L, Roux AL, Gaillard JL, Lombard B, Loew D, Rubin EJ, Brosch R, Kremer L, Herrmann JL, Girard-Misguich F. 2018. Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus. Proc Natl Acad Sci USA 115:E1002–E1011. doi: 10.1073/pnas.1713195115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckley CM, Heath VL, Guého A, Bosmani C, Knobloch P, Sikakana P, Personnic N, Dove SK, Michell RH, Meier R, Hilbi H, Soldati T, Insall RH, King JS. 2019. PIKfyve/Fab1 is required for efficient V-ATPase and hydrolase delivery to phagosomes, phagosomal killing, and restriction of Legionella infection. PLoS Pathog 15:e1007551. doi: 10.1371/journal.ppat.1007551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vicinanza M, Korolchuk VI, Ashkenazi A, Puri C, Menzies FM, Clarke JH, Rubinsztein DC. 2015. PI(5)P regulates autophagosome biogenesis. Mol Cell 57:219–234. doi: 10.1016/j.molcel.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segawa K, Nagata S. 2015. An apoptotic “eat me” signal: phosphatidylserine exposure. Trends Cell Biol 25:639–650. doi: 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Birge RB, Ucker DS. 2008. Innate apoptotic immunity: the calming touch of death. Cell Death Differ 15:1096–1102. doi: 10.1038/cdd.2008.58. [DOI] [PubMed] [Google Scholar]
- 25.Greenlee-Wacker MC. 2016. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev 273:357–370. doi: 10.1111/imr.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pujol-Autonell I, Mansilla MJ, Rodriguez-Fernandez S, Cano-Sarabia M, Navarro-Barriuso J, Ampudia RM, Rius A, Garcia-Jimeno S, Perna-Barrull D, Caceres EM, Maspoch D, Vives-Pi M. 2017. Liposome-based immunotherapy against autoimmune diseases: therapeutic effect on multiple sclerosis. Nanomedicine (Lond) 12:1231–1242. doi: 10.2217/nnm-2016-0410. [DOI] [PubMed] [Google Scholar]
- 27.Trahtemberg U, Mevorach D. 2017. Apoptotic cells induced signaling for immune homeostasis in macrophages and dendritic cells. Front Immunol 8:1256. doi: 10.3389/fimmu.2017.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Fernandez S, Pujol-Autonell I, Brianso F, Perna-Barrull D, Cano-Sarabia M, Garcia-Jimeno S, Villalba A, Sanchez A, Aguilera E, Vazquez F, Verdaguer J, Maspoch D, Vives-Pi M. 2018. Phosphatidylserine-liposomes promote tolerogenic features on dendritic cells in human type 1 diabetes by apoptotic mimicry. Front Immunol 9. doi: 10.3389/fimmu.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamaruzzaman NF, Kendall S, Good L. 2017. Targeting the hard to reach: challenges and novel strategies in the treatment of intracellular bacterial infections. Br J Pharmacol 174:2225–2236. doi: 10.1111/bph.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Jia Y, Yang K, Wang Z. 2020. Heterogeneous strategies to eliminate intracellular bacterial pathogens. Front Microbiol 11. doi: 10.3389/fmicb.2020.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McOrist S. 2000. Obligate intracellular bacteria and antibiotic resistance. Trends Microbiol 8:483–486. doi: 10.1016/s0966-842x(00)01854-0. [DOI] [PubMed] [Google Scholar]
- 32.Barinaga M. 1992. Knockout mice offer first animal model for CF. Science 257:1046–1047. doi: 10.1126/science.257.5073.1046. [DOI] [PubMed] [Google Scholar]
- 33.Taddei A, Folli C, Zegarra-Moran O, Fanen P, Verkman AS, Galietta L. 2004. Altered channel gating mechanism for CFTR inhibition by a high-affinity thiazolidinone blocker. FEBS Lett 558:52–56. doi: 10.1016/S0014-5793(04)00011-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Li X, Grassmé H, Döring G, Gulbins E. 2010. Alterations in ceramide concentration and pH determine the release of reactive oxygen species by Cftr-deficient macrophages on infection. J Immunol 184:5104–5111. doi: 10.4049/jimmunol.0902851. [DOI] [PubMed] [Google Scholar]
- 35.Cigana C, Lorè NI, Riva C, De Fino I, Spagnuolo L, Sipione B, Rossi G, Nonis A, Cabrini G, Bragonzi A. 2016. Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections. Sci Rep 6:6:21465. doi: 10.1038/srep21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM02546-21_Supp_1_seq3.pdf, PDF file, 2.3 MB (2.4MB, pdf)
Data Availability Statement
All data are available in the main text or the supplemental material.