Abstract
SARS-COV 2 is recognized to be responsible for a multi-organ syndrome. In most patients, symptoms are mild. However, in certain subjects, COVID-19 tends to progress more severely.
Most of the patients infected with SARS-COV2 fully recovered within some weeks. In a considerable number of patients, like many other viral infections, various long-lasting symptoms have been described, now defined as “long COVID-19 syndrome”.
Given the high number of contagious over the world, it is necessary to understand and comprehend this emerging pathology to enable early diagnosis and improve patents outcomes.
In this scenario, AI-based models can be applied in long-COVID-19 patients to assist clinicians and at the same time, to reduce the considerable impact on the care and rehabilitation unit.
The purpose of this manuscript is to review different aspects of long-COVID-19 syndrome from clinical presentation to diagnosis, highlighting the considerable impact that AI can have.
Keywords: AI, COVID-19, SARS-COV2, Long-COVID
Abbreviations: COVID-19, coronavirus disease 2019; MERS, Middle East respiratory syndrome; ICU, Intensive care unit; CT, computed tomography; CMR, cardiac magnetic resonance; CTA, computed tomography angiography; DM, diabetes mellitus; AI, Artificial intelligence; DL, Deep Learning; ML, Machine Learning
1. Introduction
As of October 12th, 2021, global casualties due to coronavirus disease 2019 (COVID-19) infections approach 200 million, several patients are asymptomatic or have mild symptoms, while few others show an aggressive and life-threatening disease [1], [2], [3].
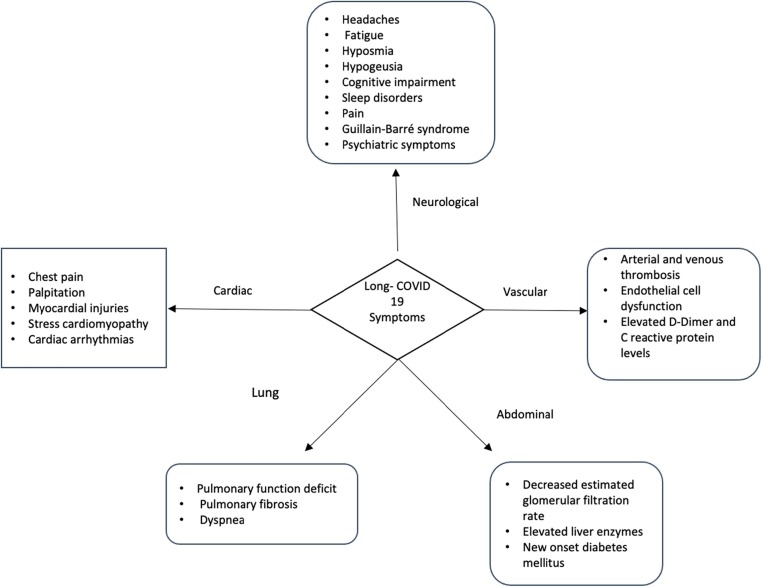
Through a large proportion of subjects infected with SARS-COV-2 present a restitutio ad integrum within a few weeks, an emerging aspect of COVID-19 infection is the long-term effect [4], [5]. Among those surviving the acute infection, a fraction of the COVID-19 patients reports persistent symptoms for 12 weeks or longer beyond the initial period of acute infection and illness [5]. The symptoms are diverse and related to multiorgan involvement, the most described symptoms are fatigue, headache, attention disorder, hair loss, and dyspnea (Fig. 1 ) [6]. Dennis et al. investigated the medium-term organ impairment in 201 symptomatic patients after initial COVID-19 symptoms reporting that 70 % of patients have impairment in one or more organs after 4 months following COVID-19 infection [4]. Similar findings were reported from other studies. An Italian case series reported persistent COVID-19 symptoms in 87,4 % of patients who had recovered from COVID-19, particularly fatigue and dyspnea [7]. Thus, the authors reported a worsening of the quality of life in 44,1 % of patients [7].
Fig. 1.
Long COVID-19 manifestations in different organs.
In view of several reports, the definition of a new syndrome has been proposed, namely long COVID-19 syndrome. In the UK NICE guidelines, a distinction of long-COVID has been made in relation to the duration after the acute onset of the syndrome. Especially, post-acute COVID-19 is defined as ongoing symptomatic COVID-19 for patients who still have symptoms between 4 and 12 weeks after the start of acute symptoms, on the other hand, post-COVID-19 syndrome for people who still have symptoms for more than 12 weeks after the start of acute symptoms [8].
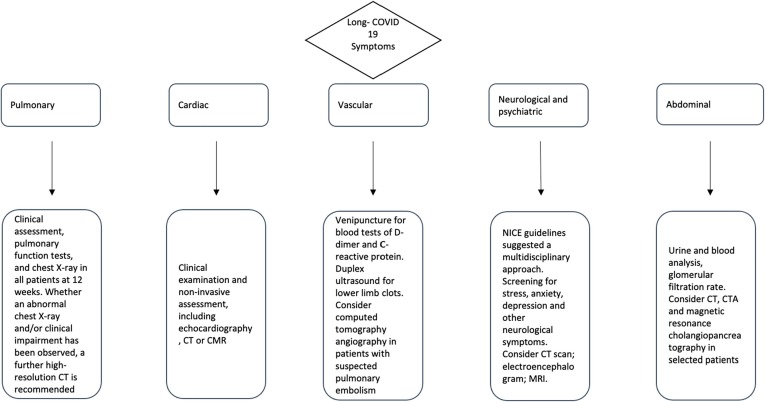
Care and management for COVID-19 patients do not conclude after acute infection but continue in the outpatient setting. Consequently, a large amount of time and resources will be needed to help clinicians understand and manage long-term COVID-19 sequelae. In view of the multi-organ involvement during post-COVID-19 syndrome, an inter-disciplinary approach is mandatory, where clinical and laboratory data must be embedded with the radiological data (Fig. 2 ).
Fig. 2.
Proposed management for patients with long COVID-19.
This scenario is a new future frontier for artificial intelligence (AI) such as its ability to join office-based, laboratory-based, and image-based data. We provide a review of the current literature on post-COVID-19 syndrome, its clinical presentation, and imaging-based diagnosis. Finally, we discuss the potential role of the multi-disciplinary approach with the support of AI.
2. Respiratory system
2.1. Clinical manifestations
The lung is a predominant organ involvement during COVID-19 infection [1], but data on residual lung damage are still scarce. Among COVID-19 survivors several pulmonary manifestations, ranging from cough to dyspnea, have been described [9]. Previous studies evaluating SARS 2002/2003 outbreak showed that 27,8 % of patients presented persistent lung functional deficit at 1-year follow-up [10]. Similarly, a decreased lung function and sign of pulmonary fibrosis have been demonstrated during the Middle East respiratory syndrome (MERS) in up to 33 % of patients [11].
Similar findings were described following SARS-COV 2 infection. A multicenter European study showed an impaired performance status in 41 % of survivors and persisting symptoms such as dyspnea in 36 % of survivors 100 days after COVID-19 onset [12]. In detail, the authors reported a reduction in forced vital capacity and/or forced expiratory volume in 1 s in 22%, a reduced total lung capacity in 11%, and an impaired diffusing capacity of the lung for carbon monoxide in 21% of all survivors [12]. Huang et al investigated the long-term health consequences in 1733 discharged patients with COVID-19, reporting a lower median 6-min walking distance than normal reference values in 24 % patients at 6 months [13]. Further analysis showed the characteristic of patients with persistent lung injuries, including male, overweight, comorbidity, oxygen therapy, intensive care unit (ICU) admission, and invasive mechanical ventilation [14].
2.2. Imaging features
Computed tomography (CT) imaging revealed pulmonary alteration in 63 % of survivors, including ground-glass opacities, consolidation, and reticulation with bilateral involvement in 75 % of survivors under analysis [12].
The document from the European Society of Thoracic Imaging and the European Society of Radiology discussed the CT features in COVID-19 survivors at discharge and during the follow-up[15]. The authors proposed a glossary of appropriate definitions to describe the lung abnormalities post-COVID-19 pneumonia, which include, for example, the term “fibrotic-like changes” representing potential precursors of fibrosis, but with a high probability of resolving over time[15].
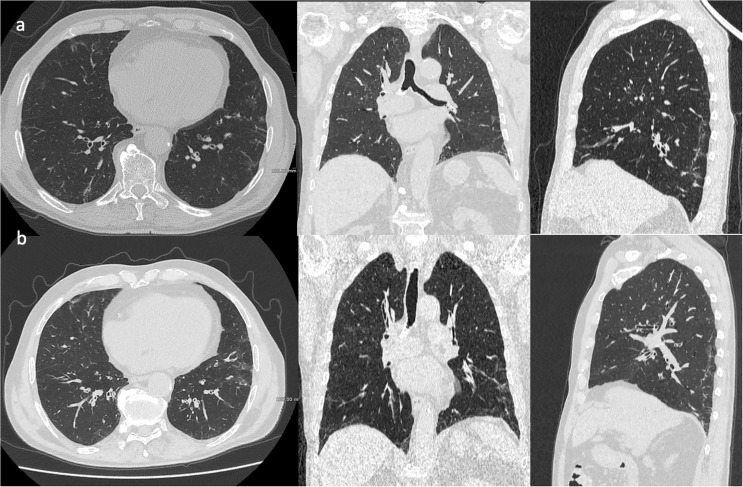
Fig. 3 showed an example of pulmonary fibrosis in Long-COVID patients.
Fig. 3.
A 68-years old male patient presented with fever, dyspnea and cough diagnosed as positive for COVID-19 by PCR. HRCT chest three month after the diagnosis (Fig. 3a) showing bilateral interlobular thickening with scattered reticular and ground glass infiltration. Follow-up HRCT was done 6 months from start of symptoms revealing a worsening with bilateral fibrotic-like changes, represents by parenchimal bands and peri-bronchial thickening (Fig. 3b).
Given the high number of infected patients with SARS-COV2, persistent functional deficits described are likely to represent a significant disease burden. Therefore, prompt therapy may prevent potentially permanent fibrosis and functional impairment.
2.3. Diagnostic work-up
The British Thoracic Society provided guidance for the respiratory follow-up for patients with COVID-19 pneumonia in two separate algorithms based on the severity of acute COVID-19 infection and ICU admission required [16]. Both algorithms recommended clinical assessment, pulmonary function tests, and chest X-rays in all patients at 12 weeks. Subsequently, whether an abnormal chest X-ray and/or clinical impairment has been observed, a further high-resolution CT is recommended [16]. Also, NICE guidelines make recommendations for clinical investigation of patients with long-COVID, including chest X-rays in patients with persistent pulmonary symptoms [8].
3. Cardiac involvement
3.1. Clinical manifestations
A review of evidence performed by the NICE described the presence of chest pain and palpitation in 10–44% survivors [17]. In an Italian case series, Carfi et al. reported the presence of chest pain in up to 20% of patients 60 days after acute infection [7]. Myocardial injuries have been described after SARS-COV 2 infection[18]. The psychological, social, and economic stress related to the COVID-19 was associated with an increased incidence of stress cardiomyopathy in comparison with pre-pandemic periods (7,8% vs 1,5–1,8%, respectively) [19]. Also, cardiac arrhythmias in Long-COVID patients were reported, including atrial fibrillation, supraventricular tachycardia, complete heart block, and ventricular tachycardia [20].
3.2. Imaging features
Puntmann et al in a study of 100 patients, who recovered from COVID-19, described cardiac involvement in 78 % of patients and ongoing myocardial inflammation in 60 % of patients by cardiac magnetic resonance (CMR) independents of preexisting comorbidities, the severity of the acute illness, and time from the original diagnosis [18]. Native T1 and T2 showed the best discriminatory power to detect COVID-19 related myocardial damage (AUC of 0,83 and 0,82) [18].
Another CMR imaging study among 26 competitive college athletes with SARS-COV 2 infection revealed that 46% of enrolled patients had evidence of myocarditis or prior myocardial injury [21].
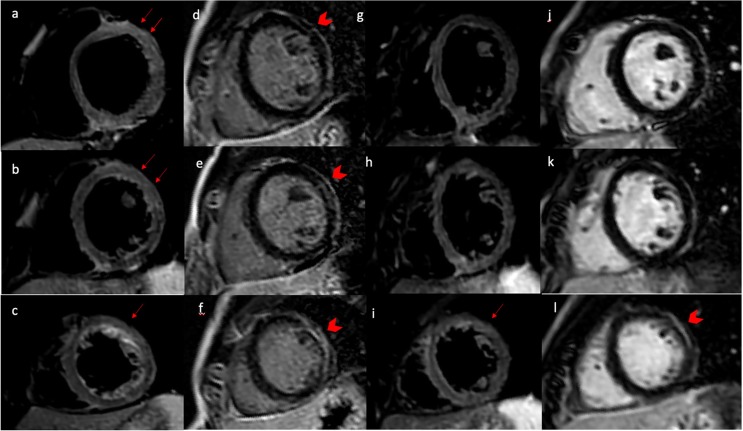
Similarly, Clark et al. investigated the presence of cardiac damage by CMR among soldiers with cardiopulmonary symptoms in the late convalescent phase of recovery from SARS-CoV-2, reporting the prevalence of myocarditis -LGE pattern in 8 % of patients enrolled [22]. See Fig. 4 .
Fig. 4.
A 21-year-old, normal weight and non-smoker man presented to the emergency department with new onset of acute chest pain, ten days after RT-PCR-confirmed SARS-COV 2 infection. T2-short tau inversion recovery CMR short axis (Panel a-c) view revealing edema in the antero-lateral segments (red arrow). Late gadolinium enhancement imaging in a short-axis view demonstrating an intramyocardial scar in the same segment (arrowhead in panel d-f). Follow-up HRCT was done 3 months from start of symptoms showing a persistence edema (arrow in panel i) and intramyocardial scar (arrowhead in panel i) in lateral apical segment (arrow in panel l).
3.3. Diagnostic work-up
The Canadian Cardiovascular Society Rapid Response Team provide guidance to health care providers for patients who still have symptoms 4 weeks after the start of acute symptoms and (1) persistent or new unexplained chest pain, (2) shortness of breath, (3) frequent palpitations, and (4) postural lightheadedness. This guideline suggested clinical examination and non-invasive assessment, including echocardiography, CT, or CMR to rule out myopericarditis, coronary artery disease, congestive heart failure, and pulmonary hypertension [17].
4. Vascular complications
4.1. Clinical and imaging features
Recently, elevated D-Dimer and C reactive protein levels have been reported in 30% and 9,5 % of survivors, respectively [23]. A retrospective observational cohort study of 163 patients with confirmed COVID-19 not receiving anticoagulation reported an incidence of thrombosis, including arterial and venous events, of 2,5 % (95% CI, 0,8% to 7,6%) at day 30 after discharge. The same study revealed hemorrhagic events during the follow-up period (6 of 163; 3,7%) [24]. Despite recovery from COVID-19 infection, survivors may still live thrombosis due to endothelial dysfunction with a high prevalence of pulmonary vascular dysfunction[16].
4.2. Diagnostic work-up
In view of this emerging signal, the British Thoracic Society suggests a computed tomography angiography (CTA) in patients with suspected pulmonary embolism [16]. Another follow-up algorithm for the investigation of patients after COVID-19 is proposed by Dwahan et al. with perfusion imaging as a key tool in the triage tree, including VQ scintigraphy and dual-energy CT [25]. Also, high-performance low field MRI may provide a morphology and function assessment in a single examination without the need to apply intravenous contrast agents or a radiation exposure [26].
5. Neurological sequelae
5.1. Clinical manifestations
Persistent neurological symptoms have been reported in COVID-19 survivors [27] similar to chronic post-SARS and MERS syndrome [28]. The symptoms include headaches, fatigue, hyposmia, hypogeusia, cognitive impairment, sleep disorders, pain, and Guillain-Barré syndrome [28], [29], [27].
Graham et al. investigated the neurological manifestation in 100 non-hospitalized COVID-19 survivors, reporting brain fog (81%), headache (68%), numbness (60%), dysgeusia (59%), anosmia (55%), and myalgias (55%) [30]. See Fig. 5 . In a prospective study of 6-months outcomes of COVID-19 patients, abnormalities in cognitive and functional outcomes were reported in >90 % of survivors. The outcomes were assessed with different scales, such as the modified Ranking scale, Barthel index (for activities of daily living), and Quality of Life in Neurological Disorder (a measurement of anxiety, depression, fatigue, and sleep disorders). In a multivariate analysis, persistent neurological manifestations were independent predictors of impaired activities of daily living and were inversely associated with return to work [31].
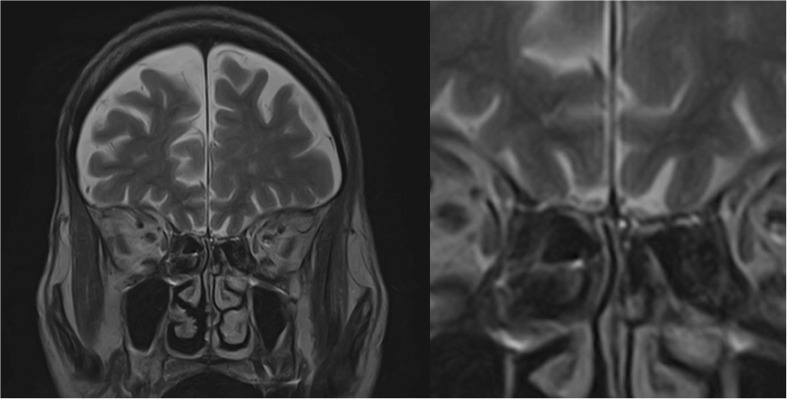
Fig. 5.
Coronal T2 fast spin-echo of the olfactory bulb in patient suffering from post-infection anosmia.
COVID-19 survivors have reported a spectrum of persistent or presenting psychiatric symptoms after initial infection, with a high prevalence in the initial phase after discharge[32]. In a cohort of 126 COVID-19 survivors, 31%, 22,2%, and 38,1% of them suffered stress, anxiety, and depression, respectively [32]. Another study by Mazza et al. screened 402 COVID-19 survivors one month after hospital, reporting approximately 56 % of patients that suffered from psychiatric sequelae [33]. A study published in Lancet Psychiatry evaluated a cohort of 236,379 COVID-19 survivors and a control group among patients with another viral influenza, reporting an incidence of neurological and psychiatric disorders of 33 % in COVID-19 group, with higher values in comparison with a control group [34].
5.2. Imaging features
A recent prospective study on brain MRI findings indicated that COVID-19 survivors with no specific neurological symptoms exhibited brain microstructure abnormalities and a decrease in cerebral blood flow after a 3-months follow-up [35]. The MRI changes were more extensive in patients with severe COVID-19 and highly correlated with the level of inflammatory markers, suggesting an inflammatory storm induced by the immune response as the main underlying mechanism[35].
Similarly, Huang et al. investigated the long-term white matter change in recovered COVID-19 patients at the one-year follow-up by using conventional diffusion tensor imaging, diffusion kurtosis imaging, and neurite orientation dispersion and density models[36]. The authors showed lower axonal density with no significant decline in cognitive function in COVID-19 survivors in comparison with healthy subjects. In addition, patients admitted to intensive care unit and/or required longer hospital stays had more white matter alterations[36].
5.3. Diagnostic work-up
Various guidelines treated long-Covid-19 management or have included a recommendation for long-COVID in their guidelines for COVID-19, NICE guidelines suggested a multidisciplinary approach to identify, refer, and treat these patients [37]. In this scenario, also imaging should play a crucial role. Nuzzo et al. proposed an MRI control of the brain in the management of long-COVID-19 patients with suspected neurological sequelae, in order to assess the potentially neuronal degeneration due to microvascular disorders [38], [39].
6. Abdominal involvement
Various abdominal manifestations were reported in COVID-19 survivors, including renal, digestive, and metabolic sequelae. In a cohort of 287 survivors from COVID-19, approximately 1,4 % of patients experienced renal failure in long-term follow-up [40]. At 6 months after acute infection, 35% of COVID-19 survivors demonstrated a decreased estimated glomerular filtration rate[13]. More importantly, the study reported that 13 % of patients developed a new-onset reduction of estimated glomerular filtration rate after acute infection [13].
The prospective COVERSCAN study evaluated the medium-term organ impact of the long-COVID syndrome in different organs, including kidneys (4%), liver (28%), pancreas (40%), and spleen (4%), with single-organ and multiorgan impairment in 70% and 29%, respectively [4]. A Chinese study evaluated a 2-month follow-up in 253 discharged COVID-19 patients, showing that liver enzymes, such as ALT, AST, GGT, and ALP remained elevated two weeks after acute infection [41]. Similarly, pancreatic abnormalities were reported in COVID-19 survivors. In fact, patients with long-COVID may also experience endocrinopathy, such as diabetes mellitus (DM) in subjects without known DM prior to the acute infection [42]. A possible mechanism explaining the occurrence of DM may be related to the cytolytic effect of SARS-COV 2 on pancreatic β‐cells [43]. In a retrospective cohort study of three data sources from a large United States health plan, among 193 113 discharged COVID‐19 patients, new-onset DM was one of the most common clinical sequelae a median follow‐up of 3 months [44].
7. Role of the AI
In the era of modern medicine, artificial intelligence (AI) is a growing field of interest in diagnostic imaging due to technological innovations that have led to constant development [45], [46].
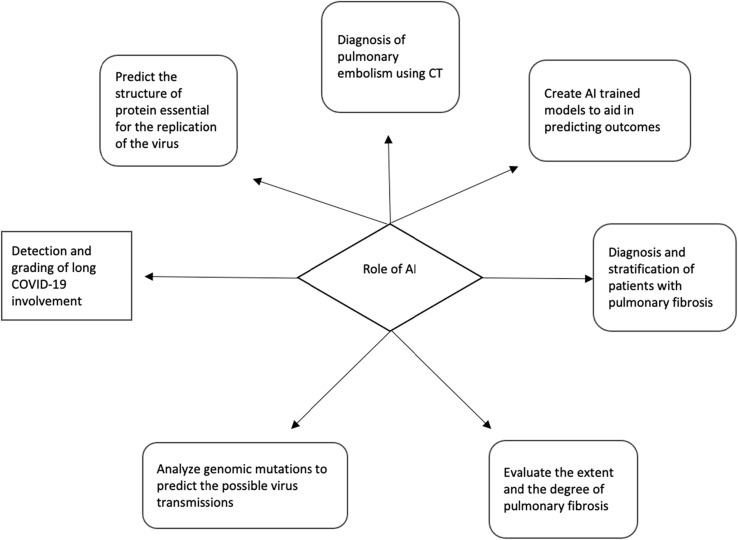
During the pandemic, the number of imaging investigations performed has been increasing dramatically and it will continue to grow due to the persistent symptomatology in Long-COVID patients [47]. AI has numerous potential applications in diagnostic imaging, including image analysis, decision-making, and prognosis prediction [48] (Fig. 6 ), and has been widely used in the fight against the COVID-19 pandemic [49], [50], [51], [52].
Fig. 6.
Potential role of AI in patients with long COVID-19.
7.1. AI for detection and grading
For example, AI has been shown to be able to differentiate COVID-19 pneumonia from community-acquired pneumonia and other lung conditions [53], [54]. A deep learning model achieved high sensitivity (90%) and high specificity (96%)in the detection of COVID-19 using chest CT with an area under the curve of 0.96 and an average time for each CT scan of 4,51 s[53]. In addition, an AI algorithm can identify patients who developed severe COVID-19 symptoms [55], [56]. Quiroz-Juarez has presented a study for the early identification of high-risk patients among those exposed to the SARS-COV-2 virus, using a supervised artificial neural network[55]. The machine learning models were trained using comorbidities, patient demographic data, as well as recent COVID-19-related medical information. The authors reported that the disease outcome can be predicted with specificity greater than 82%, sensitivity greater than 86%, and accuracy greater than 84%[55].
After the COVID-19 pandemic, an increasing number of survivors continue to battle the symptoms of the disease. Various studies have found that a fraction of COVID-19 survivors developed fibrotic abnormalities [12], [57]. Zou et al. evaluated an AI-assisted chest CT technology to quantitively measure the extent and the degree of pulmonary inflammation in 239 patients that developed pulmonary fibrosis after COVID-19 pneumonia at 30, 60, and 90 days after discharge [58]. The authors reported that the AI inflammation score showed a good correlation with the quantitative pulmonary fibrosis score, concluding that AI-assisted chest CT technology may provide qualitative and quantitative data to analyze the long-term evolution of pulmonary fibrosis [58].
In addition, AI has already proven to be an excellent tool in the diagnosis and stratification of patients with pulmonary fibrosis [59], [60]. Christe et al. investigated the performance of AI-based models for the automatic classification of idiopathic interstitial pneumonia into radiological CT patterns, based on chest CT scans and clinical markers in comparison with two expert readers[59]. This model achieved similar accuracy in comparison with human readers: 0,81, 0,70, and 0,81, respectively [59].
Wang et al. evaluated a deep-learning model that combined CT imaging and clinical data in a multi-institutional international cohort of 1051 COVID-19 patients with the purpose to predict future deterioration to critical illness in those patients [61]. The prediction model achieved a C-index of 0,80 with an AUC of 0,82, 0,81, and 0,83 for prediction of progression risk at cutoff values of 3, 5, and 7 days, respectively. This model demonstrated the ability to successfully stratify the patients into risk score group [61]. We can speculate that an AI-trained model combining imaging-based and clinical-based data might predict the persistence of symptoms and lung progression abnormalities in long-COVID patients.
7.2. AI for cardio-vascular complications
It is well known that SARS-COV-2 involved microcirculation, leading to endothelial cell and pericytes damage, micro-thrombosis, and capillary congestion that may contribute to persisting COVID-19 symptoms [62]. In addition, the pro-inflammatory state caused by SARS-COV-2 is considered a trigger in promoting plaque vulnerability [63], [64].
Mohamud et al. described a case series of 6 patients with COVID-19 presenting to the emergency department with acute ischemic infarction due to intraluminal carotid artery thrombus [64]. Similar findings have also been described for coronary artery disease [65], given the relationship between carotid and coronary atherosclerosis [66]. The application of AI algorithms in cardiovascular imaging is an evolving field that has shown to be an efficient tool for the diagnosis of atherosclerotic disease as well as for plaque characterization [45]. Various AI-trained models that combined imaging data and clinical risk factors have been applied to aid in predicting outcomes [67], [68], [69], [70], [71]. For example, Tesche et al. developed a ML-model, incorporating CT plaque features and clinical parameters to predict the presence of major cardiac events, achieving a better risk stratification (AUC 0,96) compared to conventional CT risk score (AUCs 0,79–0,89), plaque features (AUCs 0,72–0,82) and traditional cardiovascular risk score (AUCs 0,52–0,76) [71]. The study by Gupta et al. focused on the identification of patients at risk of heart disease post-COVID-19 illness[72]. In their work, the authors train an ML approach based on stacking ensemble from historical and clinical variables. The results show 93,23% accuracy, 95,74% specificity, 95,24% precision, and 92,05 recall[72].
AI system may also have a significant impact on pulmonary embolism detection [73], a condition that may be present in the clinical progression of COVID-19 patients during acute infection [2], as well as in the follow-up [74]. A recent meta-analysis investigated the diagnostic performance of deep learning algorithms for the diagnosis of pulmonary embolism using CT, demonstrating a sensitivity and specificity of 0,88 and 0,86, respectively [73].
7.3. AI for control measures and genome analysis
AI can be harnessed for forecasting the spread of the virus and developing control measures by analyzing the change in the infectious capacity of virus carriers within a few days after infection [75], predicting the long-term trend of the COVID‐19 outbreak [76].
AI models can also help to predict the structure of protein essential for the replication of the virus. A recent study published in Nature in 2020 evaluated a deep-learning approach to protein structure prediction with the purpose to understand the fold shape, the detailed side-chain configurations in binding regions as well as to target mutations to destabilize the protein, improving the biological insight [77]. DeepTracer, a fully automated software based on a customized deep convolutional neural network, was used to derive macromolecules’ 3D maps at a near-atomic resolution of SARS-COV-2 from electron cryomicroscopy [78]. Deep learning algorithms can also analyze genomic mutations in the coding regions of SARS-CoV-2 and their probable protein secondary structure with the purpose to predict the possible virus transmissions between patients, tracking the evolution, and the worldwide spread of the virus [79]. Similarly, Haimed et al. evaluated an AI approach to discover the patterns and evolution behavior of SARS-CoV-2 with a comparison to other viral families, including Orthomyxoviridae, Retroviridae, Filoviridae, Flaviviridae, and Coronaviridae, reporting an accuracy of 72% to predict the next evolved sequence [80]. An ML algorithm to define the aberrant host immune response in COVID-19 patients was explored by Sahoo et al., reporting a common host immune response in all viral pandemics, namely Vip signature, and a subset of 20-genes classified disease severity (i.e severe Vip signature)[81]. This signature may be a powerful tool to assess disease severity, providing a quantitative and qualitative framework for the assessment of the immune response in viral pandemics[81].
An emerging issue is the rapid spread of COVID-19 variants with serious concerns to global health, it is important to be able to promptly identify and detect these emerging variants. Perez-Romero et al. proposed a deep-learning algorithm that was able to deliver the primer sets for each variant, reporting an accuracy above 95 %, with two exceptions, in particular for the variants P1-2 with an accuracy of 88,99 % and for B.1.1.519 with 60,40% % for the forward primer, and 64,32% for the reverse primer [82].
Table 1 summarized previous research regarding the potential role of AI in long-COVID 19 patients.
Table 1.
Previous studies regarding potential role of Artificial Intelligence in long-COVID-19 assessment.
| Authors | Number (patients) | Date published | Research | Results | |
|---|---|---|---|---|---|
| Diagnosis and stratification of patients with pulmonary fibrosis | Zou et al. | 239 | 2021 | Evaluated an AI-assisted chest CT technology to quantitively measure the extent and the degree of pulmonary inflammation | AI inflammation score showed good correlation with the quantitative pulmonary fibrosis score |
| Christe et al. | 104 | 2019 | Investigated the performance of an AI-based models for the automatic classification of idiopathic interstitial pneumonias into radiological CT pattern | AI-based model achieved similar accuracy in comparison with human readers: 0.81, 0.70, and 0.81, respectively | |
| Mäkelä et al | 71 | 2021 | Investigated ad AI model with a deep convolutional neural network to find histological features with a prognostic role in patients with idiopathic pulmonary fibrosis | AI-based model demonstrated median values for false positive, false negative, error, precision, sensitivity, and F1 score were 1.4% (range of 0%–6.7%), 1.0% (range of 0.1%–5.2%), 2.9% (range of 0.6%–9.9%), 54.5% (range of 7.3%–98.2%), 65.2% (range of 7.0%–87.3%), and 55.7% (range of 7.4%–85.5%), respectively. | |
| Grading and stratification of long COVID-19 involvement | Wang et al | 1051 | 2021 | Evaluated a deep-learning model that combined CT imaging and clinical data to predict future deterioration to critical illness in those patients | The prediction model achieved a C-index of 0.80 with an AUC of 0.82, 0.81, and 0.83 for prediction of progression risk at cutoff values of 3, 5, and 7 days, respectively. Therefore, this AI-based model demonstrated the ability to stratify the patients into high-risk and low-risk groups with distinct progression risks (p < 0.0001) |
| Create AI trained models to aid in predicting outcomes | Tesche et al | 361 | 2021 | Investigated the prognostic value of coronary CTA features and clinical parameters on major cardiac adverse events | Implementation of coronary CTA plaque features together with clinical information to a ML model provide better risk stratification (AUC 0,96) compared to conventional CT risk score (AUCs 0,79–0,89), plaque features (AUCs 0,72–0,82) and traditional cardiovascular risk score (AUCs 0,52–0,76) |
| Quiroz-Juarez et al | 475 | 2021 | Investigated a ML models for the early identification of high-risk patients among those exposed to the SARS-COV-2 virus | The authors reported that the disease outcome can be predicted with a specificity greater than 82%, a sensitivity greater than 86%, and an accuracy greater than 84%. | |
| Souza et al. | 13 690 | 2021 | Explored a ML algorithms make a prognosis or early identification of patients at increased risk of developing severe COVID-19 symptoms | Artificial intelligence model showed a ROC area under curve (AUC) of 0.92, a sensitivity of 0.88, and a specificity of 0.82. | |
| Motwani et al | 10,030 | 2017 | ML approach to predict all-cause of mortality in patients with suspected CAD | Machine learning showed an AUC of 0,79 for prediction of 5-year all-cause mortality | |
| Al’ Aref et al | 13,054 | 2020 | ML-model, incorporating clinical factor and the coronary calcium score to predict the presence of obstructive coronary artery disease | Machine learning with coronary calcium score showed an AUC of 0,881 to predict the present of CAD | |
| Lal et al. | 70 | 2020 | AI algorithm to develop a predictive model for adverse neurologic events in patients with carotid atherosclerosis | Artificial intelligence model showed an AUC of 0,86 to predict major adverse neurologic events | |
| Van Rosendal et al. | 8844 | 2018 | ML- based algorithm, including degree of coronary stenosis and plaque composition to improve risk stratification. | Machine learning model demonstrated an AUC of 0.7707 to provide risk stratification. | |
| Gupta et al | 180 | 2021 | Proposed a hybrid ML-model to identify patients at risk of heart disease post-COVID-19 illness. | The results show 93,23% accuracy, 95,74% specificity, 95,24% precision, and 92,05 recall73 | |
| Detection of pulmonary embolism | Soffer et al. | 36,847 | 2021 | Performed a systematic review of applying deep learning for the diagnosis of pulmonary embolism on CT | The application of deep learning achieved pooled sensitivity and specificity for PE detection were 0.88 (95% CI 0.803–0.927) and 0.86 (95% CI 0.756–0.924), respectively. |
| Predict the structure of protein and analyze genomic mutations | Senior et al. | 2020 | Evaluated a deep-learning approach to protein structure prediction | Demonstrated that a deep-learning model can provide accurate prediction of the protein structure. | |
| Pfab et al. | 2021 | Evaluated a fully automated deep learning algorithm to determine the protein structure on a dataset of coronavirus-related cryo-EM maps | The average percentage of matched model residues is 84% for Deep learning model and 49.8% for the previous automatic tool. | ||
| Lopez-Rincon | 2021 | Proposed a deep-learning algorithm that was able to deliver the primer sets for COVID-19 variants | The authors reported an accuracy above 95 %, with two exceptions, in particular for the variants P1-2 with an accuracy of 88.99 % and for B.1.1.519 with 60.40% for the forward primer, and 64.32% for the reverse primer | ||
| Haimed | 2022 | Evaluated an AI approach to discover the patterns and evolution behavior of SARS-CoV-2 | AI models achieved an accuracy of 72% to predict the next evolved sequence |
8. Conclusion
The multi-organ involvement of COVID-19 beyond the acute infection is now recognized in a fraction of the patients, namely “long-COVID”. Given the high number of patients affected by COVID-19 and many patients with symptoms suggestive for long-COVID, early identification and management are vital to improving patient outcomes. In the near future, the adoption of AI may greatly enrich daily clinical practice with the promise to provide solutions to the physician interrogations of risk stratification and the clinical progression of COVID-19 patients.
Disclosure paragraph
All authors agreed with the content and gave consent to submit.
All authors contributed equally as authors to this work.
The authors state that this work is not under consideration elsewhere.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors read and approved the final manuscript.
The scientific guarantor of this publication is the corresponding author.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cau R., Falaschi Z., Paschè A., Danna P., Arioli R., Arru C.D., Zagaria D., Tricca S., Suri J.S., Karla M.K., Carriero A., Saba L. CT findings of COVID-19 pneumonia in ICU-patients. J. Public health Res. 2021 doi: 10.4081/jphr.2021.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cau R., Pacielli A., Fatemeh H., Vaudano P., Arru C., Crivelli P., Stranieri G., Suri J.S., Mannelli L., Conti M., Mahammedi A., Kalra M., Saba L. Complications in COVID-19 patients: Characteristics of pulmonary embolism. Clin. Imaging. 2021;77:244–249. doi: 10.1016/j.clinimag.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cau R., Bassareo P.P., Mannelli L., Suri J.S., Saba L. Imaging in COVID-19-related myocardial injury. Int. J. Cardiovasc. Imaging. 2021;37(4):1349–1360. doi: 10.1007/s10554-020-02089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis A., Wamil M., Alberts J., et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11(3) doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carod-Artal F.J. Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev. Neurol. 2021;72(11):384–396. doi: 10.33588/rn.7211.2021230. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfì A., Bernabei R., Landi F. Group GAC-19 P-ACS. persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatesan P. NICE guideline on long COVID. Lancet Respir. Med. 2021;9(2):129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Augustin M., Schommers P., Stecher M., Dewald F., Gieselmann L., Gruell H., Horn C., Vanshylla K., Cristanziano V.D., Osebold L., Roventa M., Riaz T., Tschernoster N., Altmueller J., Rose L., Salomon S., Priesner V., Luers J.C., Albus C., Rosenkranz S., Gathof B., Fätkenheuer G., Hallek M., Klein F., Suárez I., Lehmann C. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg. Heal. – Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui D.S. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das K.M., Lee E.Y., Singh R., Enani M.A., Al Dossari K., Van Gorkom K., Larsson S.G., Langer R.D. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J. Radiol. Imaging. 2017;27(03):342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., Kurz K., Koppelstätter S., Haschka D., Petzer V., Boehm A., Aichner M., Tymoszuk P., Lener D., Theurl M., Lorsbach-Köhler A., Tancevski A., Schapfl A., Schaber M., Hilbe R., Nairz M., Puchner B., Hüttenberger D., Tschurtschenthaler C., Aßhoff M., Peer A., Hartig F., Bellmann R., Joannidis M., Gollmann-Tepeköylü C., Holfeld J., Feuchtner G., Egger A., Hoermann G., Schroll A., Fritsche G., Wildner S., Bellmann-Weiler R., Kirchmair R., Helbok R., Prosch H., Rieder D., Trajanoski Z., Kronenberg F., Wöll E., Weiss G., Widmann G., Löffler-Ragg J., Tancevski I. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur. Respir. J. 2021;57(4):2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L.i., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L.i., Xu D., Li Y., Li C., Peng L.u., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myall K.J., Mukherjee B., Castanheira A.M., Lam J.L., Benedetti G., Mak S.M., Preston R., Thillai M., Dewar A., Molyneaux P.L., West A.G. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martini K., Larici A.R., Revel M.P., et al. COVID - 19 pneumonia imaging follow - up: when and how? A proposition from ESTI and ESR European Society of Radiology. Eur. Radiol. 2021 doi: 10.1007/s00330-021-08317-7. 0123456789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George P.M., Barratt S.L., Condliffe R., Desai S.R., Devaraj A., Forrest I., Gibbons M.A., Hart N., Jenkins R.G., McAuley D.F., Patel B.V., Thwaite E., Spencer L.G. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 17.Paterson I., Ramanathan K., Aurora R., Bewick D., Chow C.-M., Clarke B., Cowan S., Ducharme A., Gin K., Graham M., Gupta A., Jassal D.S., Kazmi M., Krahn A., Lamarche Y., Marelli A., Roifman I., Ruel M., Singh G., Sterns L., Turgeon R., Virani S., Wong K.K., Zieroth S. Long COVID-19: a primer for cardiovascular health professionals, on behalf of the CCS rapid response team. Can. J. Cardiol. 2021;37(8):1260–1262. doi: 10.1016/j.cjca.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., Shchendrygina A., Escher F., Vasa-Nicotera M., Zeiher A.M., Vehreschild M., Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabri A., Kalra A., Kumar A., Alameh A., Adroja S., Bashir H., Nowacki A.S., Shah R., Khubber S., Kanaa’N A., Hedrick D.P., Sleik K.M., Mehta N., Chung M.K., Khot U.N., Kapadia S.R., Puri R., Reed G.W. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw. Open. 2020;3(7):e2014780. doi: 10.1001/jamanetworkopen.2020.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai A.D., Boursiquot B.C., Melki L., Wan E.Y. Management of arrhythmias associated with COVID-19. Curr. Cardiol. Rep. 2020;23(1):2. doi: 10.1007/s11886-020-01434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark D.E., Dendy J.M., Li D.L., Crum K., Dixon D., George-Durrett K., Parikh A.P., Wassenaar J.W., Hughes S.G., Soslow J.H. Cardiovascular magnetic resonance evaluation of soldiers after recovery from symptomatic SARS-CoV-2 infection: a case–control study of cardiovascular post-acute sequelae of SARS-CoV-2 infection (CV PASC) J. Cardiovasc. Magn. Reson. 2021;23(1) doi: 10.1186/s12968-021-00798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., Heightman M., Hillman T.E., Jacob J., Jarvis H.C., Lipman M.C.I., Naidu S.B., Nair A., Porter J.C., Tomlinson G.S., Hurst J.R. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W.C., Bauer K.A., Zwicker J.I. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhawan R.T., Gopalan D., Howard L., Vicente A., Park M., Manalan K., Wallner I., Marsden P., Dave S., Branley H., Russell G., Dharmarajah N., Kon O.M. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir. Med. 2021;9(1):107–116. doi: 10.1016/S2213-2600(20)30407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heiss R., Grodzki D.M., Horger W., Uder M., Nagel A.M., Bickelhaupt S. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn. Reson. Imaging. 2021;76:49–51. doi: 10.1016/j.mri.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neurology T.L. Long COVID: understanding the neurological effects. Lancet Neurol. 2021;20(4):247. doi: 10.1016/S1474-4422(21)00059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordvig A.S., Fong K.T., Willey J.Z., Thakur K.T., Boehme A.K., Vargas W.S., Smith C.J., Elkind M.S.V. Potential neurologic manifestations of COVID-19. Neurol. Clin. Pract. 2021;11(2):e135–e146. doi: 10.1212/CPJ.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., DiBiase R.M., Jia D.T., Balabanov R., Ho S.U., Batra A., Liotta E.M., Koralnik I.J. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frontera J.A., Yang D., Lewis A., Patel P., Medicherla C., Arena V., Fang T., Andino A., Snyder T., Madhavan M., Gratch D., Fuchs B., Dessy A., Canizares M., Jauregui R., Thomas B., Bauman K., Olivera A., Bhagat D., Sonson M., Park G., Stainman R., Sunwoo B., Talmasov D., Tamimi M., Zhu Y., Rosenthal J., Dygert L., Ristic M., Ishii H., Valdes E., Omari M., Gurin L., Huang J., Czeisler B.M., Kahn D.E., Zhou T., Lin J., Lord A.S., Melmed K., Meropol S., Troxel A.B., Petkova E., Wisniewski T., Balcer L., Morrison C., Yaghi S., Galetta S. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021;426:117486. doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai X., Hu X., Ekumi I.O., Wang J., An Y., Li Z., Yuan B.o. Psychological distress and its correlates among COVID-19 survivors during early convalescence across age groups. Am. J. Geriatr. Psychiatry. 2020;28(10):1030–1039. doi: 10.1016/j.jagp.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin Y., Wu J., Chen T., et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J. Clin. Invest. 2021;131(8) doi: 10.1172/JCI147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang S., Zhou Z., Yang D., Zhao W., Zeng M.u., Xie X., Du Y., Jiang Y., Zhou X., Yang W., Guo H.u., Sun H., Liu P., Liu J., Luo H., Liu J. Persistent white matter changes in recovered COVID-19 patients at the 1-year follow-up. Brain. 2021 doi: 10.1093/brain/awab435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19 NICE guideline; c2020. https://www.nice.org.uk/guidance/ng188. No Title. [PubMed]
- 38.Nuzzo D., Cambula G., Bacile I., Rizzo M., Galia M., Mangiapane P., Picone P., Giacomazza D., Scalisi L. Long-term brain disorders in post covid-19 neurological syndrome (PCNS) patient. Brain Sci. 2021;11(4):454. doi: 10.3390/brainsci11040454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee M.-H., Perl D.P., Nair G., Li W., Maric D., Murray H., Dodd S.J., Koretsky A.P., Watts J.A., Cheung V., Masliah E., Horkayne-Szakaly I., Jones R., Stram M.N., Moncur J., Hefti M., Folkerth R.D., Nath A. Microvascular injury in the brains of patients with covid-19. N. Engl. J. Med. 2021;384(5):481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamal M., Abo Omirah M., Hussein A., Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021;75(3) doi: 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.An Y.-W., Song S., Li W.-X., Chen Y.-X., Hu X.-P., Zhao J., Li Z.-W., Jiang G.-Y., Wang C., Wang J.-C., Yuan B.o., Liu H.-Q. Liver function recovery of COVID-19 patients after discharge, a follow-up study. Int J Med Sci. 2021;18(1):176–186. doi: 10.7150/ijms.50691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suwanwongse K., Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: Causality or coincidence? A report of three cases. J. Med. Virol. 2021;93(2):1150–1153. doi: 10.1002/jmv.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sathish T., Anton M.C., Sivakumar T. New-onset diabetes in “long COVID”. J. Diabetes. 2021;13(8):693–694. doi: 10.1111/1753-0407.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daugherty S.E., Guo Y., Heath K., et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cau R., Flanders A., Mannelli L., Politi C., Faa G., Suri J.S., Saba L. Artificial intelligence in computed tomography plaque characterization: a review. Eur. J. Radiol. 2021;140:109767. doi: 10.1016/j.ejrad.2021.109767. [DOI] [PubMed] [Google Scholar]
- 46.Cau R., Cherchi V., Micheletti G., Porcu M., Mannelli L., Bassareo P., Suri J.S., Saba L. Potential role of artificial intelligence in cardiac magnetic resonance imaging. J. Thorac. Imaging. 2021;36(3):142–148. doi: 10.1097/RTI.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal M., Udare A., Patlas M., Ramonas M., Alaref A.A., Rozenberg R., Ly D.L., Golev D.S., Mascola K., van der Pol C.B. Effect of COVID-19 on computed tomography usage and critical test results in the emergency department: an observational study. C open. 2020;8(3):E568–E576. doi: 10.9778/cmajo.20200148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khemasuwan D., Sorensen J.S., Colt H.G. Artificial intelligence in pulmonary medicine: computer vision, predictive model and COVID-19. Eur. Respir. Rev. 2020;29(157):200181. doi: 10.1183/16000617.0181-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suri J.S., Agarwal S., Pathak R., Ketireddy V., Columbu M., Saba L., Gupta S.K., Faa G., Singh I.M., Turk M., Chadha P.S., Johri A.M., Khanna N.N., Viskovic K., Mavrogeni S., Laird J.R., Pareek G., Miner M., Sobel D.W., Balestrieri A., Sfikakis P.P., Tsoulfas G., Protogerou A., Misra D.P., Agarwal V., Kitas G.D., Teji J.S., Al-Maini M., Dhanjil S.K., Nicolaides A., Sharma A., Rathore V., Fatemi M., Alizad A., Krishnan P.R., Frence N., Ruzsa Z., Gupta A., Naidu S., Kalra M. COVLIAS 1.0: lung segmentation in COVID-19 computed tomography scans using hybrid deep learning artificial intelligence models. Diagnostics. 2021;11(8):1405. doi: 10.3390/diagnostics11081405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arora N., Banerjee A.K., Narasu M.L. The role of artificial intelligence in tackling COVID-19. Future Virol. 2020;15(11):717–724. [Google Scholar]
- 51.Summers R.M. Artificial intelligence of COVID-19 imaging: a hammer in search of a nail. Radiology. 2020;298(3):E162–E164. doi: 10.1148/radiol.2020204226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Ginneken B. The potential of artificial intelligence to analyze chest radiographs for signs of COVID-19 pneumonia. Radiology. 2020;299(1):E214–E215. doi: 10.1148/radiol.2020204238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L., Qin L., Xu Z., Yin Y., Wang X., Kong B., Bai J., Lu Y.i., Fang Z., Song Q.i., Cao K., Liu D., Wang G., Xu Q., Fang X., Zhang S., Xia J., Xia J. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296(2):E65–E71. doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harmon S.A., Sanford T.H., Xu S., Turkbey E.B., Roth H., Xu Z., Yang D., Myronenko A., Anderson V., Amalou A., Blain M., Kassin M., Long D., Varble N., Walker S.M., Bagci U., Ierardi A.M., Stellato E., Plensich G.G., Franceschelli G., Girlando C., Irmici G., Labella D., Hammoud D., Malayeri A., Jones E., Summers R.M., Choyke P.L., Xu D., Flores M., Tamura K., Obinata H., Mori H., Patella F., Cariati M., Carrafiello G., An P., Wood B.J., Turkbey B. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-17971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quiroz-Juárez M.A., Torres-Gómez A., Hoyo-Ulloa I., León-Montiel R.d.J., U’Ren A.B., Orzechowski P. Identification of high-risk COVID-19 patients using machine learning. PLoS ONE. 2021;16(9):e0257234. doi: 10.1371/journal.pone.0257234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Souza F.S.H., Hojo-Souza N.S., Dos Santos E.B., Da Silva C.M., Guidoni D.L. Predicting the disease outcome in COVID-19 positive patients through machine learning: a retrospective cohort study with Brazilian data. Front. Artif. Intell. 2021;4:116. doi: 10.3389/frai.2021.579931. https://www.frontiersin.org/article/10.3389/frai.2021.579931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rai D.K., Sharma P., Kumar R. Post covid 19 pulmonary fibrosis. Is it real threat? Indian J. Tuberc. 2021;68(3):330–333. doi: 10.1016/j.ijtb.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zou J.-N., Sun L., Wang B.-R., Zou Y., Xu S., Ding Y.-J., Shen L.-J., Huang W.-C., Jiang X.-J., Chen S.-M., Madeddu G. The characteristics and evolution of pulmonary fibrosis in COVID-19 patients as assessed by AI-assisted chest HRCT. PLoS ONE. 2021;16(3):e0248957. doi: 10.1371/journal.pone.0248957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christe A., Peters A.A., Drakopoulos D., Heverhagen J.T., Geiser T., Stathopoulou T., Christodoulidis S., Anthimopoulos M., Mougiakakou S.G., Ebner L. Computer-aided diagnosis of pulmonary fibrosis using deep learning and CT images. Invest. Radiol. 2019;54(10):627–632. doi: 10.1097/RLI.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mäkelä K., Mäyränpää M.I., Sihvo H.-K., Bergman P., Sutinen E., Ollila H., Kaarteenaho R., Myllärniemi M. Artificial intelligence identifies inflammation and confirms fibroblast foci as prognostic tissue biomarkers in idiopathic pulmonary fibrosis. Hum. Pathol. 2021;107:58–68. doi: 10.1016/j.humpath.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Wang R., Jiao Z., Yang L.i., Choi J.W., Xiong Z., Halsey K., Tran T.M.L., Pan I., Collins S.A., Feng X., Wu J., Chang K., Shi L.-B., Yang S., Yu Q.-Z., Liu J., Fu F.-X., Jiang X.-L., Wang D.-C., Zhu L.-P., Yi X.-P., Healey T.T., Zeng Q.-H., Liu T., Hu P.-F., Huang R.Y., Li Y.-H., Sebro R.A., Zhang P.J.L., Wang J., Atalay M.K., Liao W.-H., Fan Y., Bai H.X. Artificial intelligence for prediction of COVID-19 progression using CT imaging and clinical data. Eur. Radiol. 2022;32(1):205–212. doi: 10.1007/s00330-021-08049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep. 2021;9(3):e14726. doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kataoka Y., Puri R., Nicholls S.J. Inflammation, plaque progression and vulnerability: evidence from intravascular ultrasound imaging. Cardiovasc. Diagn. Ther. 2015;5(4):280–289. doi: 10.3978/j.issn.2223-3652.2015.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohamud A.Y., Griffith B., Rehman M., et al. Intraluminal carotid artery thrombus in COVID-19: another danger of cytokine storm? Am. J. Neuroradiol. 2020;41(9):1677–1682. doi: 10.3174/ajnr.A6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheth A.R., Grewal U.S., Patel H.P., Thakkar S., Garikipati S., Gaddam J., Bawa D. Possible mechanisms responsible for acute coronary events in COVID-19. Med. Hypotheses. 2020;143:110125. doi: 10.1016/j.mehy.2020.110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cademartiri F., Balestrieri A., Cau R., Punzo B., Cavaliere C., Maffei E., Saba L. Insight from imaging on plaque vulnerability: similarities and differences between coronary and carotid arteries—implications for systemic therapies. Cardiovasc. Diagn. Ther. 2020;10(4):1150–1162. doi: 10.21037/cdt-20-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Motwani M., Dey D., Berman D.S., et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur. Heart J. 2017;38(7):500–507. doi: 10.1093/eurheartj/ehw188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al’Aref S.J., Maliakal G., Singh G., et al. Machine learning of clinical variables and coronary artery calcium scoring for the prediction of obstructive coronary artery disease on coronary computed tomography angiography: analysis from the CONFIRM registry. Eur. Heart J. 2020;41(3):359–367. doi: 10.1093/eurheartj/ehz565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Rosendael A.R., Maliakal G., Kolli K.K., Beecy A., Al’Aref S.J., Dwivedi A., Singh G., Panday M., Kumar A., Ma X., Achenbach S., Al-Mallah M.H., Andreini D., Bax J.J., Berman D.S., Budoff M.J., Cademartiri F., Callister T.Q., Chang H.-J., Chinnaiyan K., Chow B.J.W., Cury R.C., DeLago A., Feuchtner G., Hadamitzky M., Hausleiter J., Kaufmann P.A., Kim Y.-J., Leipsic J.A., Maffei E., Marques H., Pontone G., Raff G.L., Rubinshtein R., Shaw L.J., Villines T.C., Gransar H., Lu Y., Jones E.C., Peña J.M., Lin F.Y., Min J.K. Maximization of the usage of coronary CTA derived plaque information using a machine learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J. Cardiovasc. Comput. Tomogr. 2018;12(3):204–209. doi: 10.1016/j.jcct.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 70.Lal B.K., Kashyap V.S., Patel J.B., Gutpa A., Chrencik M.T., King A.H., Khan A.A., Buckler A. Novel application of artificial intelligence algorithms to develop a predictive model for major adverse neurologic events in patients with carotid atherosclerosis. J. Vasc. Surg. 2020;72(1):e176–e177. doi: 10.1016/j.jvs.2020.04.306. [DOI] [Google Scholar]
- 71.Tesche C., Bauer M.J., Baquet M., Hedels B., Straube F., Hartl S., Gray H.N., Jochheim D., Aschauer T., Rogowski S., Schoepf U.J., Massberg S., Hoffmann E., Ebersberger U. Improved long-term prognostic value of coronary CT angiography-derived plaque measures and clinical parameters on adverse cardiac outcome using machine learning. Eur. Radiol. 2021;31(1):486–493. doi: 10.1007/s00330-020-07083-2. [DOI] [PubMed] [Google Scholar]
- 72.Gupta A., Jain V., Singh A. Stacking ensemble-based intelligent machine learning model for predicting post-COVID-19 complications. New Gener. Comput. 2021 doi: 10.1007/s00354-021-00144-0. 0123456789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soffer S., Klang E., Shimon O., Barash Y., Cahan N., Greenspana H., Konen E. Deep learning for pulmonary embolism detection on computed tomography pulmonary angiogram: a systematic review and meta-analysis. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-95249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whyte M.B., Barker R., Kelly P.A., Gonzalez E., Czuprynska J., Patel R.K., Rea C., Perrin F., Waller M., Jolley C., Arya R., Roberts L.N. Three-month follow-up of pulmonary embolism in patients with COVID-19. Thromb. Res. 2021;201:113–115. doi: 10.1016/j.thromres.2021.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng N., Du S., Wang J., Zhang H.e., Cui W., Kang Z., Yang T., Lou B., Chi Y., Long H., Ma M., Yuan Q.i., Zhang S., Zhang D., Ye F., Xin J. Predicting COVID-19 in China using hybrid AI model. IEEE Trans. Cybern. 2020;50(7):2891–2904. doi: 10.1109/TCYB.2020.2990162. [DOI] [PubMed] [Google Scholar]
- 76.Sun J., Chen X.i., Zhang Z., Lai S., Zhao B.o., Liu H., Wang S., Huan W., Zhao R., Ng M.T.A., Zheng Y. Forecasting the long-term trend of COVID-19 epidemic using a dynamic model. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-78084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Senior A.W., Evans R., Jumper J., Kirkpatrick J., Sifre L., Green T., Qin C., Žídek A., Nelson A.W.R., Bridgland A., Penedones H., Petersen S., Simonyan K., Crossan S., Kohli P., Jones D.T., Silver D., Kavukcuoglu K., Hassabis D. Improved protein structure prediction using potentials from deep learning. Nature. 2020;577(7792):706–710. doi: 10.1038/s41586-019-1923-7. [DOI] [PubMed] [Google Scholar]
- 78.Pfab J., Phan N.M., Si D. DeepTracer for fast de novo cryo-EM protein structure modeling and special studies on CoV-related complexes. Proc. Natl. Acad. Sci. 2021;118(2) doi: 10.1073/pnas.2017525118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen T.T., Pathirana P.N., Nguyen T., Nguyen Q.V.H., Bhatti A., Nguyen D.C., Nguyen D.T., Nguyen N.D., Creighton D., Abdelrazek M. Genomic mutations and changes in protein secondary structure and solvent accessibility of SARS-CoV-2 (COVID-19 virus) Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-83105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haimed A.M.A., Saba T., Albasha A., Rehman A., Kolivand M. Viral reverse engineering using Artificial Intelligence and big data COVID-19 infection with Long Short-term Memory (LSTM) Environ. Technol. Innov. 2021;22:101531. doi: 10.1016/j.eti.2021.101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahoo D., Katkar G.D., Khandelwal S., Behroozikhah M., Claire A., Castillo V., Tindle C., Fuller MacKenzie, Taheri S., Rogers T.F., Beutler N., Ramirez S.I., Rawlings S.A., Pretorius V., Smith D.M., Burton D.R., Alexander L.E.C., Duran J., Crotty S., Dan J.M., Das S., Ghosh P. AI-guided discovery of the invariant host response to viral pandemics. EBioMedicine. 2021;68:103390. doi: 10.1016/j.ebiom.2021.103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Romero C.A., Tonda A., Mendoza-Maldonado L., et al. Design of specific primer sets for the detection of SARS-CoV-2 variants of concern B.1.1.7, B.1.351, P.1, B.1.617.2 using artificial intelligence. bioRxiv. 2021 doi: 10.1101/2021.01.20.427043. [DOI] [Google Scholar]