Abstract
Obstructive sleep apnea (OSA) is regarded as an independent risk factor for hypertension. The possible mechanism includes oxidative stress, endothelial injury, sympathetic excitement, renin–angiotensin–aldosterone system activation, etc. Clinical studies have found that there is a high coexistence of OSA and primary aldosteronism in patients with hypertension and that elevated aldosterone levels are independently associated with OSA severity in resistant hypertension. The underlying mechanism is that aldosterone excess can exacerbate OSA through increasing overnight fluid shift and affecting the mass and function of upper airway muscles during the sleep period. Thus, a bidirectional influence between OSA and aldosterone exists and contributes to hypertension in OSA patients, especially resistant hypertension.
Keywords: obstructive sleep apnea, aldosterone, hypertension, continuous positive airway pressure (CPAP), mineralocorticoid receptor antagonists
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive overnight hypoxic episodes and subsequent sleep fragmentation due to a complete or partial collapse of the upper airway. It was estimated that 936 million adults aged 30–69 years have OSA globally, including 425 million moderate to severe ones (1). OSA is related to not only endothelial injury and sympathetic excitement but also endocrine dysfunction such as elevated secretion of catecholamine and renin–angiotensin–aldosterone system activation (2–5). Those mechanisms make OSA a well-established independent risk factor of HTN (6). About 25% hypertensive patients had OSA (7). The incidence of OSA could reach 83%~92.3% among patients with resistant hypertension (RHTN) (8, 9). Untreated moderate to severe OSA increased the risk of RHTN (OR = 2) (10). Recently, it has been noticed that OSA is highly present in primary aldosteronism (PA) and vice versa. Meanwhile, elevated aldosterone is associated with OSA severity in RHTN. Herein, we will give a review of the role of aldosterone in OSA and its related HTN.
Coexistence of PA and OSA
OSA in PA
As we know, OSA brings great health burden in the general population. Higher incidence of OSA in PA patients was identified ( Table 1 ). The RESIST-POL study found that OSA occurred more frequently in RHTN patients with PA than those without PA (59.4% vs. 42.4%) (15). In a population consisting of 3,003 consecutive patients with HTN, 321 were diagnosed with PA, among whom 45.8% were OSA patients (14). Then, a cross-sectional multiethnic study that screened for OSA in 207 patients with PA showed that the prevalence was 67.6% and there was no obvious difference of the prevalence between the white and Chinese (64.4% vs. 70%) (13). It was also reported the OSA prevalence of 55% among 71 Japanese patients with PA. Body mass index (BMI) and triglyceride content increased the risk of OSA in PA patients (OR = 1.27 and OR = 1.01, respectively) (11). Besides, an early retrospective study reported that among 3,428 hypertensive patients, patients with hyperaldosteronism had a higher prevalence of sleep apnea than those without hyperaldosteronism (18% vs. 9%), despite a lack of confirmation of PA (16). It should be mentioned that a much lower prevalence of OSA (10%) in a retrospective study including 677 PA patients may be attributed to the fact that polysomnography (PSG) was only conducted in those with snoring, which resulted in the underestimation of the actual prevalence of OSA in PA (12).
Table 1.
The prevalence of OSA in PA patients.
| Author | Study design | Nation | Subjects | Assessment of PA | Assessment of OSA | Prevalence of OSA |
|---|---|---|---|---|---|---|
| Nakamura (11) | Cross-sectional | Japan | 71 PA | Screening test (ARR > 20 ng/dl: ng/ml/h) and confirmatory test including CCT (ARR ≥ 20), FUT (PRA < 2.0 ng/ml/h), and SIT (aldosterone level ≥ 6.0 ng/dl). PA was diagnosed if ≥1 of the 3 tests were positive. | Smart Watch PMP-300E (REI ≥ 15 or REI ≥ 5 together with symptoms) | 55% |
| Li (12) | Retrospective | China | 677 PA | Screening test (ARR > 24 ng/dl: ng/ml/h) and confirmatory test (SIT: aldosterone level ≥ 6.0 ng/dl) | PSG (used only in snoring patients with PA) (AHI ≥ 5) | 10% |
| Buffolo (13) | Cross-sectional, multiethnic, multicenter | Europe | 104 PA | PA was diagnosed according to the 2016 ES guideline. Different centers have different standards for the screening test and confirmatory test. | Cardiorespiratory polygraphy (AHI ≥ 5) | 64.40% |
| China | 100 PA | Same as above | Same as above | 70% | ||
| Wang (14) | Cross-sectional study | China | 321 PA | A suppressed PRA (<1.0 μg/L/h) and an elevated aldosterone level (>12 ng/L) or an elevated ARR (>20 ng/dl: ng/ml/h) and a confirmation test by saline infusion test (aldosterone level was >5 ng/dl) | All patients suspicious of OSA underwent full-night PSG (AHI ≥5) | 45.80% |
| Prejbisz (15) | Cross-sectional | Poland | 32 PA and 172 non-PA amid 204 RHTN | screening test (ARR > 30 ng/dl: ng/ml/h, PAC > 15 ng/dl) and confirming test (CCT: aldosterone level fails to decrease by more than 30%) | All patients irrespective of the symptoms of OSA were evaluated by PSG (AHI ≥ 15) | 59.4% in PA; 42.4% in non-PA, P = 0.058 |
| Sim (16) a | Retrospective | USA | 3,428 HTN (575 had HA | HA was defined as ARR >30 (ng/dl: ng/ml/h) and PAC >20 ng/dl or ARR >50 | Sleep apnea was defined by ICD-9 coding or procedural coding for dispensation of positive airway devices. | 18% in HA vs. 9% in non-HA (P<0.001) |
OSA, obstructive sleep apnea; HTN, hypertension; RHTN, resistant hypertension; PA, primary aldosteronism; HA, hyperaldosteronism; ESS, Epworth Sleepiness Scale; PSG, polysomnography; AHI, apnea–hypopnea index; PAC, plasma aldosterone concentration; PRA, plasma renin activity; ARR, aldosterone-to-renin ratio; UAldo, urine aldosterone level; CCT, the captopril challenge test; FUT, the furosemide upright test; SIT, the saline infusion test; ICD-9, International Classification of Disease, Ninth Revision.
This retrospective study did not have PA confirmed.
Management of PA improved OSA symptoms. Wolley et al. performed repeated PSG on 20 patients with PA pre- and post-treatment. Seven patients underwent adrenalectomy and 13 patients received treatment with spironolactone, amiloride, or both. The mean apnea–hypopnea index (AHI) was reduced from 22.5 to 12.3 events/h (P = 0.02) after an 8-month follow-up (17). A retrospective study including 83 PA patients (48 surgically managed and 35 medically managed) also showed a significant reduced OSA probability assessed by the Berlin questionnaire after PA treatment (from 64% to 35%, mean Berlin score 1.64 to 1.35, P < 0.001) with the follow-up duration up to 2.6 years. It seemed to be independent of BMI, for BMI remains unchanged before and after treatment for PA. Both obese and non-obese patients showed significant decreases in OSA probability (18).
PA in OSA
PA was present in 2.6%–12.7% of the general HTN patients (19). Studies showed that the incidence of PA in OSA patients was at least 5% in OSA-HTN patients and up to 36% in RHTN patients with probable OSA (13, 14, 20–23) ( Table 2 ). PA is likely to be more frequent in patients with OSA and HTN than in the general hypertensive population. Among 114 subjects with RHTN, 72 subjects had a high risk for sleep apnea based on the Berlin questionnaire who were more likely to have a diagnosis of PA than those with low risk of sleep apnea (36% vs. 19%) (20). Furthermore, the study of Di Murro et al., in which both PA and OSA were definitely confirmed, found that OSA patients (n = 53) had a higher prevalence of PA compared with non-OSA patients (n = 272) (34% vs. 9.2%) (21).
Table 2.
The prevalence of PA among OSA.
| Author | Study design | Nation | Subjects | Assessment of PA | Assessment of OSA | Prevalence of PA |
|---|---|---|---|---|---|---|
| Dobrowolski (23) | Prospective | Poland | 94 moderate/severe OSA and HTN | Confirmed by SIT (aldosterone level > 10 ng/dl) plus low baseline PRA | PSG (AHI ≥ 15) | 21.30% |
| Chee (22) a | Prospective | Australia | 40 OSA and HTN | Only screening test performed [ARR > 70 pmol/mU (approximate 20 ng/dl: ng/ml/h)] | PSG (AHI ≥ 5) | 5% had likely PA and 22.5% had possible PA |
| Buffolo (13) | Cross-sectional, multiethnic, multicenter | Europe | 102 patients with OSA and HTN | PA was diagnosed according to the 2016 ES guideline. Different centers have different standards for the screening test and confirmatory test | Cardiorespiratory polygraphy (AHI ≥ 5) | 11.80% |
| China | 101 patients with OSA and HTN | Same as above | Same as above | 5.90% | ||
| Wang (14) | Cross-sectional study | China | 888 patients with OSA and HTN | A suppressed PRA (<1.0 μg/L/h) and an elevated aldosterone level (>12 ng/L) or an elevated ARR (>20 ng/dl: ng/ml/h) and a confirmation test by SIT (aldosterone level was >5 ng/dl) | PSG (AHI ≥ 5) | 16.55% |
| Di Murro (21) | Prospective | Italy | 53 OSA and HTN | ARR >40 (ng/dl: ng/ml/h) in the presence of PAC >15 ng/dl and suppressed PRA and confirmatory test [SIT (PAC >5 ng/dl)] | Only those have features of OSA and ESS ≥10 underwent PSG (AHI ≥ 5) | 34% |
| Calhoun (20) | Prospective | USA | 114 RHTN (72 subjects had a high probability and 42 subjects had a low probability of having sleep apnea) | PA was defined as a suppressed PRA (<1.0 ng/mL/h) and elevated 24-h UAldo >12 μg in the setting of high dietary sodium ingestion (>200 mEq/24 h) | Berlin questionnaire | Subjects at high risk for sleep apnea were almost two times more likely to have PA diagnosed (36% vs. 19%, P < 0.05) |
OSA, obstructive sleep apnea; HTN, hypertension; RHTN, resistant hypertension; PA, primary aldosteronism; PSG, polysomnography; ESS, Epworth Sleepiness Scale; AHI, apnea–hypopnea index; PAC, plasma aldosterone concentration; PRA, plasma renin activity; ARR, aldosterone-to-renin ratio; UAldo, urine aldosterone level; SIT, the saline infusion test.
This study inferred that the likelihood and possibility of PA in hypertensive OSA patients were 5% and 22.5% mainly based on the results of screening test and usage of antihypertensive agents, because no one completed the confirmation test.
There was a high coexistence of PA and OSA, discrepancies in the results notwithstanding. The possible reason for the variation in prevalence should be considered such as sample sizes, differences in the criteria for the selection of patients, and different methodologies and criteria for the diagnosis of PA and OSA ( Tables 1 and 2 ).
Aldosterone Level in OSA
Higher Aldosterone Levels in OSA
Several studies showed that aldosterone excess was present in OSA and suggested its involvement in the pathogenesis of OSA-related HTN. A meta-analysis showed that OSA patients tended to have a higher aldosterone level compared with the control group. A subgroup analysis further found that this effect was solely significant in OSA patients with HTN rather than in OSA patients without HTN (24). Later, a cross-sectional study with 534 primary RHTN patients (493 OSA and 41 non-OSA) demonstrated that the severe OSA group had elevated plasma aldosterone concentration (PAC) compared with those in the non-OSA group or mild/moderate OSA group. PAC and 24-h urine aldosterone level (24-h UAldo) were positively associated with AHI (β = 0.32 and β = 0.35, respectively, P < 0.05), after adjustment of age, gender, BMI, smoking status, plasma renin activity (PRA), diuretic and angiotensin-converting enzyme inhibitors (ACEI), and/or angiotensin receptor blocker (ARB) usage (9). Similarly, de Souza and colleagues found that higher PAC and 24-h UAldo were associated with severe OSA after adjustment for age, sex, BMI, 24 h BPs, and dipping status (25).
Studies have explored the impact of continuous positive airway pressure (CPAP), the gold-standard therapy for moderate to severe OSA, on aldosterone levels in OSA patients with a follow-up varying from 1 to 14 months (25–34) ( Table 3 ). Concerning about normotensive population, two observational studies were conducted by Nicholl and colleagues to evaluate the effect of 1-month CPAP on renin–angiotensin–aldosterone system (RAAS) activity in OSA patients without HTN and diabetes in 2014 and 2021. Not only PAC was significantly reduced but also BP and renal function were improved after CPAP treatment (26, 28), especially in those with severe hypoxemia (26). Of note, patients in both studies were asked to keep a high-salt diet 3 days before the first visit until the second visit to avoid the impact of salt status on the aldosterone level. However, Møller and coworkers found that no laboratory data including PAC were changed in 13 symptomatic OSA patients without HTN after a successful 14-month CPAP treatment (29). A parallel randomized controlled trial (RCT) of 1-month CPAP enrolling 101 males with OSA was conducted, in which both the placebo and active treatment groups have similar use of CPAP. Unexpectedly, PAC rose by 30% in both groups and the sham/active difference was not significant (33). The result should be interpreted with caution due to lack of demographic information about coexisting diseases and drug usages.
Table 3.
Effect of CPAP on aldosterone levels.
| Author | Nation | Study design | Number (male) | With HTN? | Follow-up (months) | Compliance (h/night) | Outcome |
|---|---|---|---|---|---|---|---|
| Nicholl (26) | Canada | Observational | 30 (20) | Normotensive | 1 | >4 | PAC ↓ |
| Nicholl (28) | Canada | Observational | 20 (15) | Normotensive | 1 | >4 | PAC ↓ |
| Møller (29) | Denmark | Observational | 13 (12) | Normotensive | 14 | Sufficient compliance | PAC, PRA, Ang II → |
| Meston (33) | Britain | RCT | 101 (101) | No data | 1 | Placebo: 4.6 ± 2.4; active: 5.4 ± 1.6 | PAC ↑ in both groups and sham/active differences → |
| Joyeux-Faure (34) | Spain | RCT | 37 (32) | RHTN | 3 | CPAP: 3.90; sham CPAP: 1.86 | Increase of PAC was significant in the sham CPAP group compared with active CPAP; renin → |
| De Souza (25) | Brazil | RCT | 117 (47) | RHTN | 6 | >4 (45 patients in the CPAP group meet good compliance) | 24-h UAldo |
| ↓ solely in patients with true RHTN, but not in those with whitecoat RHTN | |||||||
| Sánchez-de-la-Torre (27) | Spain | Observational | 37 (37) | RHTN | 3 | >4 | PAC → |
| Decrease of ARR was significantly greater in the responder group (n = 18) | |||||||
| Lloberes (32) | Spain | RCT | 78 (59) | RHTN | 3 | 5.6 ± 1.5 | PAC ↓ was found in nine patients with whitecoat RHTN, but not in the 27 patients with true RHTN |
| Pedrosa (31) | Brazil | Randomized | 35 (27) | RHTN | 6 | 6.01 ± 0.20 | PAC → |
| Saarelainen (30) | Finland | Observational | 11 (11) | HTN | 3 | >4 | PAC ↓, renin → |
OSA, obstructive sleep apnea; HTN, hypertension; RHTN, resistant hypertension; AHI, apnea–hypopnea index; BP, blood pressure; PAC, plasma aldosterone concentration; PRA, plasma renin activity; CPAP, continuous positive airway pressure; RCT, randomized controlled trial; ARR, aldosterone-to-renin ratio; UAldo, urine aldosterone; Ang Ⅱ, angiotensin Ⅱ; ↑, significantly increase; ↓, significantly decrease; →, insignificant change.
The change of aldosterone level by CPAP treatment was also tested in OSA patients with HTN or RHTN. PAC in 11 hypertensive OSA subjects without any medications was found to be significantly decreased after 3 months of CPAP treatment (P = 0.046) (30). Improvement in nighttime mean BP was associated with changes of aldosterone. Lloberes et al. conducted one RCT, which showed that a 3-month CPAP treatment significantly decreased PAC and this change was independently associated with changes in office DBP (32). Intriguingly, Joyeaux-Faure and colleagues reported a significant increase in PAC during a 3-month sham CPAP compared with the active CPAP (P = 0.01) (34). The 24-h UAldo tended to decrease after 6 months of CPAP treatment in another RCT (P = 0.074) (25). This reduction was more noticeable in those with the non-dipping pattern, not using spironolactone, less obese, and with lowest sleep SaO2 levels (25). Although some studies found unchanged PAC by CPAP (27, 31), a significant decrease of aldosterone-to-renin ratio (ARR) was observed in the responder group whose mean blood pressure (BP) was reduced by at least 4.5 mmHg after CPAP treatment (27).
In summary, a higher level of aldosterone was present in hypertensive OSA patients, and most studies indicated a decline of aldosterone level after CPAP treatment. Several factors blamed for some controversy in the results may belong to methodological concerns such as different demographic characteristics (OSA severity, BMI) and inconsistent control of variables (antihypertensive drugs, salt status, body position when sampling, etc.) known to influence aldosterone production.
Possible Mechanism
Aldosterone is a hormone mainly secreted by the adrenal cortex that regulates electrolyte and water balance by increasing the renal retention of sodium and the excretion of potassium. Angiotensin II (Ang II) binds to its receptor (AT1R) on the zona glomerulosa cells of the adrenal cortex, which is one of major regulators of aldosterone synthesis. Chronic intermittent hypoxia (CIH)-induced sympathetic outflow to the kidney stimulates renin release and leads to elevated circulating levels of Ang II (35). It was demonstrated that Ang II was much higher in OSA patients than in healthy controls (24). CIH-treated rats also caused increasing levels of circulating renin activity and Ang II (36). In-vitro studies also confirmed that CIH directly upregulated the expression of renin (37). Besides, obesity, highly concomitant in OSA, has been found to participate in aldosterone excess in RHTN patients (38). Adipocyte-derived factors may be involved in adrenal aldosterone synthesis. Leptin was able to directly activate aldosterone synthase (CYP11B2) of the adrenal gland, resulting in increased production of aldosterone via a Ca2+-dependent mechanism which is independent of RAAS and sympathetic nervous systems (39). However, a subsequent experiment found the effect of leptin on aldosterone synthesis only happened in female mice (40). It was also observed that complement-C1q TNF-related protein-1 (CTRP-1), a secreted protein derived from adipocytes, could also enhance aldosterone production in cells of the human adrenal cortical cell line (41). Additionally, adipose tissue could secrete aldosterone itself (42). Nevertheless, studies are needed to clarify this mechanism in OSA.
Aldosterone Excess Exacerbates OSA
Higher Aldosterone Level Contributes to OSA
In a 109 RHTN population, the severity of OSA was found to be greater in the high PAC (defined as PRA < 1 ng/ml/h and 24-h UAldo ≥ 12 µg) group than in the normal PAC group (AHI, 19.9 vs. 10.0 events/h). PAC and 24-h UAldo were positively and significantly correlated with AHI in the high PAC group, which was not found in the normal PAC group (43). Moreover, hyperaldosteronism [defined as ARR > 30 and PAC > 20 ng/dl or ARR > 50 (ng/dl: ng/ml/h)] was reported to independently increase the risk of OSA after adjustment of age, sex, BMI categories, diabetes mellitus, and chronic heart failure (OR = 1.8, 95% CI: 1.3–2.6) (16).
Two main mineralocorticoid receptor (MR) antagonists, spironolactone and eplerenone, are currently used as secondary agents in hypertensive patients (6). Several studies evaluated their effectiveness on OSA combined with uncontrolled HTN or RHTN (44–48) ( Table 4 ).
Table 4.
Effect of aldosterone inhibition on OSA.
| Author | Nation | Study design | Subjects | Intervention | Follow-up (months) | Outcome |
|---|---|---|---|---|---|---|
| Krasińska (48) | Poland | RCT | 102 RHTN and OSA patients (n = 51 per group) | Therapy group: additional use of eplerenone (50 mg/daily) | 6 | Nighttime BP parameters, left ventricular hypertrophy, AHI, PAC ↓ |
| Control group: standard antihypertensive agents | ||||||
| Yang (44) | China | RCT | 30 RHTN and OSA patients (n = 15 per group) | Therapy group: additional spironolactone 20 mg once daily or 40 mg once their BP remains uncontrolled at 4 weeks | 3 | AHI, BP, and PAC ↓ |
| Control group: usual antihypertensive agents | ||||||
| Krasińska (45) | Poland | Observational | 31 RHTN and OSA patients | Eplerenone at a dose of 50 mg/day with a standard antihypertensive therapy | 3 | AHI, neck circumference, BP, aortic pulse wave, and arterial wall stiffness ↓ |
| Kasai (46) | Canada | Observational | 16 OSA patients with uncontrolled HTN | Intensified diuretic therapy (metolazone 2.5 mg and spironolactone 25 mg daily for 7 days after which the daily dose was doubled for 7 additional days) | 2 weeks | AHI, BP, overnight change in leg fluid volume and overnight change in neck circumference ↓ |
| Gaddam (47) | America | Observational | 12 RHTN and OSA patients | Additional therapy (spironolactone 25 mg once daily and force-titrated to 50 mg once daily at 4 weeks) | 2 | Body weight, BP, AHI ↓ and PRA ↑, a tended but insignificant reduction neck circumference |
OSA, obstructive sleep apnea; HTN, hypertension; RHTN, resistant hypertension; AHI, apnea–hypopnea index; BP, blood pressure; PAC, plasma aldosterone concentration; PRA, plasma renin activity; RCT, randomized controlled trial; ↑, significantly increase; ↓, significantly decrease; →, insignificant change.
Gaddam and coworkers recruited 12 individuals with OSA and RHTN, who administered additional spironolactone. All subjects were asked to remain on their baseline thiazide diuretic, ACEI, and ARB throughout the treatment period. After an 8-week therapy, the reduction in AHI was significant (39.8 ± 19.5 vs. 22.0 ± 6.8 events/h), with a significant decrease of body weight, clinic systolic and diastolic BP, and 24-h ambulatory blood pressure monitoring (AMBP) parameters (24-h systolic BP, for instance, 147 ± 13 vs. 130 ± 19 mmHg) (47). Thirty patients with OSA and RHTN were randomly assigned to a control group who received usual antihypertensive agents and a treatment group who received add-on therapy of spironolactone. After a 12-week follow-up, the reductions of clinic BP, AMBP parameters (mean differences of 24-h systolic BP in the two groups, 16.3 ± 10.0 vs. 5.3 ± 7.0 mmHg), and AHI (mean differences in the two groups, 21.8 ± 15.7 vs. 1.8 ± 12.8 events/h) were significant, as well as PAC (44). Similar results were also observed in 16 OSA patients with uncontrolled HTN. They took metolazone and spironolactone daily for 1 week after which the daily dose was force-titrated to double dose for another week. Then, a significant reduction of AHI, edema scale, body weight, total body fluid and leg fluid volume, and home BP parameters was observed (46).
Krasińska and coworkers led a 3-month observational study and a 6-month RCT in 2014 and 2019, respectively, in which additional use of eplerenone had similar benefits (significant reductions in the AHI, neck circumference, BP), along with improvement in aortic pulse wave, arterial wall stiffness, and left ventricular hypertrophy (45, 48).
The present observational and randomized trials consistently reported obvious improvement of AHI and BP after an add-on therapy of eplerenone or spironolactone in OSA patients with RHTN.
Possible Mechanisms
Aldosterone promotes sodium–water reabsorption and elevates overnight fluid shifting to the neck in the supine position, resulting in pharyngeal edema and upper airway obstruction. It was proven that OSA severity was strongly correlated with an overnight reduction of leg fluid volume and of calf circumference (49). Clinical studies showed that aldosterone blockade not only reduces PAC and BP but also AHI and neck girth significantly (44, 45, 47, 50). Aldosterone excess could damage the taste sensitivity for sodium chloride (NaCl) and favor more salt intake, further facilitating water–sodium retention (51). Besides, significantly increased secretion of cortisol in patients with PA, independent of PA subtype or adenoma tissue genotypes, could also increase risk of OSA (52, 53).
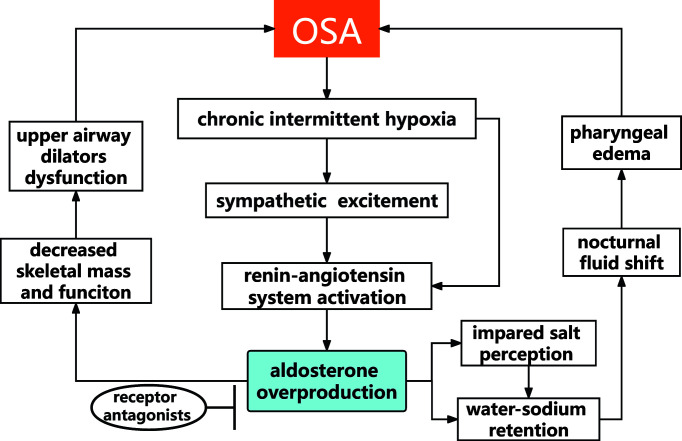
Aldosterone, which was related to sarcopenia (54), might affect the mass and function of upper dilator muscles. Lower skeletal muscle mass was found in female patients with PA than those with non-functional adrenal incidentaloma (55). It was also found that aldosterone impeded myogenesis in vitro and in vivo (56, 57). Both undifferentiated myoblasts and differentiated myotubes express aldosterone receptors (58). In Duchenne muscular dystrophy (DMD) mice model, the non-steroidal mineralocorticoid receptor antagonist (finerenone) brought significant improvements in clinically relevant functional parameters in skeletal muscle (normalized grip strength, lower susceptibility to limb muscle damage, normalized limb muscle force) (59) ( Figure 1 ).
Figure 1.
The possible pathophysiological link between OSA and aldosterone.
Discussion and Future Perspectives
OSA is a long-lasting condition with early repetitive oxidative stress injury and proinflammatory releases, leading to systemic and local inflammation, sympathetic nervous system excitement, renin–angiotensin system activation, and aldosterone overproduction. The consequences are endothelial dysfunction, artery constriction, arterial stiffness, and water–sodium retention, which are potent pathogenic factors for HTN.
In this review, we highlighted the role of aldosterone in OSA-related HTN. Hyperaldosteronism existed in OSA and in turn aggravated OSA, which could set off the onset and development of RHTN. Moreover, with recent studies revealing a proinflammatory, profibrotic, and proinsulin resistance role of MR activation, which could predispose OSA to cardiovascular and metabolic abnormality, it is vital to consider the benefits of PA screening and aldosterone blockade during the course of treatment for patients with OSA and HTN (60).
Although the current recommendation of the Endocrine Society guideline is that all OSA patients should be screened for PA, irrespective of the grade of HTN (61), the screening rate (2.1% in persons with RHTN and 3% in hypertensive OSA inpatients) for PA remained much lower than its prevalence (62, 63). According to the latest decision analysis by weighing the cost of the indiscriminate screening test for PA in all hypertensive OSA patients and of the cardiovascular sequelae from PA when it remains unscreened, Chomsky-Higgins and colleagues indicated that screening per guideline recommendations is cost-saving which had a lower expected cost and higher quality adjusted life-years compared with the current rates of screening (64). Thus, there is great scope to enhance the screening rate of PA. It should be mentioned that aldosterone is a steroid with a low concentration in plasma and is difficult to measure with radioimmunoassay, causing widely varied PAC values across laboratories. Moreover, most studies in this review measured PAC by radioimmunoassay, which might bring some bias for the results. Thus, more accurate and rapid detection methods such as liquid chromatography–tandem mass spectrometry technique and automated chemiluminescent assays are needed to be promoted (65).
Concerning the treatment for hypertensive patients with OSA, most patients would be recommended a CPAP therapy. However, the improvement of BP control by CPAP was relatively modest (2 mmHg for patients with HTN and 5 mmHg for RHTN) (66, 67). What is worse, incompliance to CPAP is high which do affect its effectiveness (68, 69). Moreover, five studies consistently reported that MR antagonists could improve both BP control and OSA severity. Therefore, taking aldosterone blockade as complementary therapy in patients with OSA and RHTN is likely to be a useful strategy. However, there are still some questions that remain to be answered. Should MR antagonists be prioritized to be prescribed if deemed well tolerated in OSA patients with RHTN before considering CPAP? Although its application in the treatment of HTN is still in its infancy, can finerenone with higher tolerance and more safety advantages offer a better alternative? Is there a protective role of the MR antagonists on cardiovascular outcome in general hypertensive patients with OSA?
Conclusion
Current clinical research supports a bidirectional influence between aldosterone level and OSA. More studies are needed to elucidate the underlying pathophysiological link between OSA and aldosterone. Aldosterone blockade is an effective adjunctive therapy for both OSA and OSA-related RHTN.
Author Contributions
Conception or design of the work: YW, CL, YL, and QL. Drafting the work: YW, CL, YL, and QL. Revising the work critically for important intellectual content: YW, CL, YL, LYZ, SL, LZ, FL, YY, NL, and QL. Final approval of the version submitted for publication: YW, CL, YL, LYZ, SL, LZ, FL, YY, NL, and QL.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82070089, 81770084); Shanghai Municipal Key Clinical Specialty (shslczdzk02202); Shanghai Top-Priority Clinical Key Disciplines Construction Project (2017ZZ02014); Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases (20dz2261100); and Cultivation Project of Shanghai Major Infectious Disease Research Base (20dz2210500).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the Global Prevalence and Burden of Obstructive Sleep Apnoea: A Literature-Based Analysis. Lancet Respir Med (2019) 7(8):687–98. doi: 10.1016/s2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bironneau V, Tamisier R, Trzepizur W, Andriantsitohaina R, Berger M, Goupil F, et al. Sleep Apnoea and Endothelial Dysfunction: An Individual Patient Data Meta-Analysis. Sleep Med Rev (2020) 52:101309. doi: 10.1016/j.smrv.2020.101309 [DOI] [PubMed] [Google Scholar]
- 3. Dissanayake HU, Bin YS, Ucak S, de Chazal P, Sutherland K, Cistulli PA. Association Between Autonomic Function and Obstructive Sleep Apnea: A Systematic Review. Sleep Med Rev (2021) 57:101470. doi: 10.1016/j.smrv.2021.101470 [DOI] [PubMed] [Google Scholar]
- 4. Lavrentaki A, Ali A, Cooper BG, Tahrani AA. MECHANISMS OF ENDOCRINOLOGY: Mechanisms of Disease: The Endocrinology of Obstructive Sleep Apnoea. Eur J Endocrinol (2019) 180(3):R91–125. doi: 10.1530/eje-18-0411 [DOI] [PubMed] [Google Scholar]
- 5. Ceccato F, Bernkopf E, Scaroni C. Sleep Apnea Syndrome in Endocrine Clinics. J Endocrinol Invest (2015) 38(8):827–34. doi: 10.1007/s40618-015-0338-z [DOI] [PubMed] [Google Scholar]
- 6. Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol (2018) 71(19):e127–248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 7. Zhang L, Li J, Li N, Sun N, Xie L, Han Q, et al. Trends in Cause-Related Comorbidities in Hospitalized Patients With Secondary Hypertension in China From 2013 to 2016: A Retrospective Analysis of Hospital Quality Monitoring System Data. J Hypertens (2021) 39(10):2015–21. doi: 10.1097/hjh.0000000000002891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, et al. High Prevalence of Unrecognized Sleep Apnoea in Drug-Resistant Hypertension. J Hypertens (2001) 19(12):2271–7. doi: 10.1097/00004872-200112000-00022 [DOI] [PubMed] [Google Scholar]
- 9. Ke X, Guo W, Peng H, Hu C, Zhang H, Peng C, et al. Association of Aldosterone Excess and Apnea-Hypopnea Index in Patients With Resistant Hypertension. Sci Rep (2017) 7:45241. doi: 10.1038/srep45241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson DA, Thomas SJ, Abdalla M, Guo N, Yano Y, Rueschman M, et al. Association Between Sleep Apnea and Blood Pressure Control Among Blacks. Circulation (2019) 139(10):1275–84. doi: 10.1161/circulationaha.118.036675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakamura Y, Kobayashi H, Tanaka S, Hatanaka Y, Fuke Y, Fukuda N, et al. Primary Aldosteronism and Obstructive Sleep Apnea: A Single-Center Cross-Sectional Study of the Japanese Population. Med (Baltimore) (2021) 100(11):e25049. doi: 10.1097/md.0000000000025049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li M, Ge Q, Sheng CS, Zhang J, Li H, Niu W, et al. Clinical Characteristics of Snoring Patients With Primary Aldosteronism and Obstructive Sleep Apnea-Hypopnea Syndrome. J Hum Hypertens (2019) 33(9):693–700. doi: 10.1038/s41371-019-0208-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buffolo F, Li Q, Monticone S, Heinrich DA, Mattei A, Pieroni J, et al. Primary Aldosteronism and Obstructive Sleep Apnea: A Cross-Sectional Multi-Ethnic Study. Hypertension (2019) 74(6):1532–40. doi: 10.1161/hypertensionaha.119.13833 [DOI] [PubMed] [Google Scholar]
- 14. Wang L, Li N, Yao X, Chang G, Zhang D, Heizhati M, et al. Detection of Secondary Causes and Coexisting Diseases in Hypertensive Patients: OSA and PA Are the Common Causes Associated With Hypertension. BioMed Res Int (2017) 2017:8295010. doi: 10.1155/2017/8295010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prejbisz A, Florczak E, Klisiewicz A, Dobrowolski P, Janaszek-Sitkowska H, Bieleń P, et al. Relationship Between Primary Aldosteronism and Obstructive Sleep Apnoea, Metabolic Abnormalities and Cardiac Structure in Patients With Resistant Hypertension. Endokrynol Pol (2013) 64(5):363–7. doi: 10.5603/ep.2013.0019 [DOI] [PubMed] [Google Scholar]
- 16. Sim JJ, Yan EH, Liu IL, Rasgon SA, Kalantar-Zadeh K, Calhoun DA, et al. Positive Relationship of Sleep Apnea to Hyperaldosteronism in an Ethnically Diverse Population. J Hypertens (2011) 29(8):1553–9. doi: 10.1097/HJH.0b013e3283492219 [DOI] [PubMed] [Google Scholar]
- 17. Wolley MJ, Pimenta E, Calhoun D, Gordon RD, Cowley D, Stowasser M. Treatment of Primary Aldosteronism Is Associated With a Reduction in the Severity of Obstructive Sleep Apnoea. J Hum Hypertens (2017) 31(9):561–7. doi: 10.1038/jhh.2017.28 [DOI] [PubMed] [Google Scholar]
- 18. Wang E, Chomsky-Higgins K, Chen Y, Nwaogu I, Seib CD, Shen WT, et al. Treatment of Primary Aldosteronism Reduces the Probability of Obstructive Sleep Apnea. J Surg Res (2019) 236:37–43. doi: 10.1016/j.jss.2018.10.040 [DOI] [PubMed] [Google Scholar]
- 19. Yang Y, Reincke M, Williams TA. Prevalence, Diagnosis and Outcomes of Treatment for Primary Aldosteronism. Best Pract Res Clin Endocrinol Metab (2020) 34(2):101365. doi: 10.1016/j.beem.2019.101365 [DOI] [PubMed] [Google Scholar]
- 20. Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone Excretion Among Subjects With Resistant Hypertension and Symptoms of Sleep Apnea. Chest (2004) 125(1):112–7. doi: 10.1378/chest.125.1.112 [DOI] [PubMed] [Google Scholar]
- 21. Di Murro A, Petramala L, Cotesta D, Zinnamosca L, Crescenzi E, Marinelli C, et al. Renin-Angiotensin-Aldosterone System in Patients With Sleep Apnoea: Prevalence of Primary Aldosteronism. J Renin Angiotensin Aldosterone Syst (2010) 11(3):165–72. doi: 10.1177/1470320310366581 [DOI] [PubMed] [Google Scholar]
- 22. Chee MR, Hoo J, Libianto R, Gwini SM, Hamilton G, Narayan O, et al. Prospective Screening for Primary Aldosteronism in Patients With Suspected Obstructive Sleep Apnea. Hypertension (2021) 77(6):2094–103. doi: 10.1161/hypertensionaha.120.16902 [DOI] [PubMed] [Google Scholar]
- 23. Dobrowolski P, Kołodziejczyk-Kruk S, Warchoł-Celińska E, Kabat M, Ambroziak U, Wróbel A, et al. Primary Aldosteronism Is Highly Prevalent in Patients With Hypertension and Moderate to Severe Obstructive Sleep Apnea. J Clin Sleep Med (2021) 17(4):629–37. doi: 10.5664/jcsm.8960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin ZN, Wei YX. Meta-Analysis of Effects of Obstructive Sleep Apnea on the Renin-Angiotensin-Aldosterone System. J Geriatr Cardiol (2016) 13(4):333–43. doi: 10.11909/j.issn.1671-5411.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Souza F, Muxfeldt ES, Margallo V, Cortez AF, Cavalcanti AH, Salles GF. Effects of Continuous Positive Airway Pressure Treatment on Aldosterone Excretion in Patients With Obstructive Sleep Apnoea and Resistant Hypertension: A Randomized Controlled Trial. J Hypertens (2017) 35(4):837–44. doi: 10.1097/hjh.0000000000001254 [DOI] [PubMed] [Google Scholar]
- 26. Nicholl DDM, Hanly PJ, Zalucky AA, Handley GB, Sola DY, Ahmed SB. Nocturnal Hypoxemia Severity Influences the Effect of CPAP Therapy on Renal Renin-Angiotensin-Aldosterone System Activity in Humans With Obstructive Sleep Apnea. Sleep (2021) 44(5):zsaa228. doi: 10.1093/sleep/zsaa228 [DOI] [PubMed] [Google Scholar]
- 27. Sánchez-de-la-Torre M, Khalyfa A, Sánchez-de-la-Torre A, Martinez-Alonso M, Martinez-García M, Barceló A, et al. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. J Am Coll Cardiol (2015) 66(9):1023–32. doi: 10.1016/j.jacc.2015.06.1315 [DOI] [PubMed] [Google Scholar]
- 28. Nicholl DD, Hanly PJ, Poulin MJ, Handley GB, Hemmelgarn BR, Sola DY, et al. Evaluation of Continuous Positive Airway Pressure Therapy on Renin-Angiotensin System Activity in Obstructive Sleep Apnea. Am J Respir Crit Care Med (2014) 190(5):572–80. doi: 10.1164/rccm.201403-0526OC [DOI] [PubMed] [Google Scholar]
- 29. Møller DS, Lind P, Strunge B, Pedersen EB. Abnormal Vasoactive Hormones and 24-Hour Blood Pressure in Obstructive Sleep Apnea. Am J Hypertens (2003) 16(4):274–80. doi: 10.1016/s0895-7061(02)03267-3 [DOI] [PubMed] [Google Scholar]
- 30. Saarelainen S, Hasan J, Siitonen S, Seppälä E. Effect of Nasal CPAP Treatment on Plasma Volume, Aldosterone and 24-H Blood Pressure in Obstructive Sleep Apnoea. J Sleep Res (1996) 5(3):181–5. doi: 10.1046/j.1365-2869.1996.t01-1-00007.x [DOI] [PubMed] [Google Scholar]
- 31. Pedrosa RP, Drager LF, de Paula LKG, Amaro ACS, Bortolotto LA, Lorenzi-Filho G. Effects of OSA Treatment on BP in Patients With Resistant Hypertension: A Randomized Trial. Chest (2013) 144(5):1487–94. doi: 10.1378/chest.13-0085 [DOI] [PubMed] [Google Scholar]
- 32. Lloberes P, Sampol G, Espinel E, Segarra A, Ramon MA, Romero O, et al. A Randomized Controlled Study of CPAP Effect on Plasma Aldosterone Concentration in Patients With Resistant Hypertension and Obstructive Sleep Apnea. J Hypertens (2014) 32(8):1650–7; discussion 7. doi: 10.1097/hjh.0000000000000238 [DOI] [PubMed] [Google Scholar]
- 33. Meston N, Davies RJ, Mullins R, Jenkinson C, Wass JA, Stradling JR. Endocrine Effects of Nasal Continuous Positive Airway Pressure in Male Patients With Obstructive Sleep Apnoea. J Intern Med (2003) 254(5):447–54. doi: 10.1046/j.1365-2796.2003.01212.x [DOI] [PubMed] [Google Scholar]
- 34. Joyeux-Faure M, Baguet JP, Barone-Rochette G, Faure P, Sosner P, Mounier-Vehier C, et al. Continuous Positive Airway Pressure Reduces Night-Time Blood Pressure and Heart Rate in Patients With Obstructive Sleep Apnea and Resistant Hypertension: The RHOOSAS Randomized Controlled Trial. Front Neurol (2018) 9:318. doi: 10.3389/fneur.2018.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of Sleep Apnea. Physiol Rev (2010) 90(1):47–112. doi: 10.1152/physrev.00043.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuan ZM, Chen BY, Wang PX, Li SY, Chen YL, Dong LX. Changes of Angiotensin II and Its Receptor During the Development of Chronic Intermittent Hypoxia-Induced Hypertension in Rats. Zhonghua Jie He He Hu Xi Za Zhi (2004) 27(9):577–80. [PubMed] [Google Scholar]
- 37. Takeda Y, Itaya-Hironaka A, Yamauchi A, Makino M, Sakuramoto-Tsuchida S, Ota H, et al. Intermittent Hypoxia Upregulates the Renin and Cd38 mRNAs in Renin-Producing Cells via the Downregulation of miR-203. Int J Mol Sci (2021) 22(18):10127. doi: 10.3390/ijms221810127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dudenbostel T, Ghazi L, Liu M, Li P, Oparil S, Calhoun DA. Body Mass Index Predicts 24-Hour Urinary Aldosterone Levels in Patients With Resistant Hypertension. Hypertension (2016) 68(4):995–1003. doi: 10.1161/hypertensionaha.116.07806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. Adipocyte-Derived Hormone Leptin Is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation (2015) 132(22):2134–45. doi: 10.1161/circulationaha.115.018226 [DOI] [PubMed] [Google Scholar]
- 40. Huby AC, Otvos L, Jr., Belin de Chantemèle EJ. Leptin Induces Hypertension and Endothelial Dysfunction via Aldosterone-Dependent Mechanisms in Obese Female Mice. Hypertension (2016) 67(5):1020–8. doi: 10.1161/hypertensionaha.115.06642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jeon JH, Kim KY, Kim JH, Baek A, Cho H, Lee YH, et al. A Novel Adipokine CTRP1 Stimulates Aldosterone Production. FASEB J (2008) 22(5):1502–11. doi: 10.1096/fj.07-9412com [DOI] [PubMed] [Google Scholar]
- 42. Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, et al. Adipocytes Produce Aldosterone Through Calcineurin-Dependent Signaling Pathways: Implications in Diabetes Mellitus-Associated Obesity and Vascular Dysfunction. Hypertension (2012) 59(5):1069–78. doi: 10.1161/hypertensionaha.111.190223 [DOI] [PubMed] [Google Scholar]
- 43. Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, et al. Severity of Obstructive Sleep Apnea Is Related to Aldosterone Status in Subjects With Resistant Hypertension. J Clin Sleep Med (2010) 6(4):363–8. doi: 10.5664/jcsm.27878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang L, Zhang H, Cai M, Zou Y, Jiang X, Song L, et al. Effect of Spironolactone on Patients With Resistant Hypertension and Obstructive Sleep Apnea. Clin Exp Hypertens (2016) 38(5):464–8. doi: 10.3109/10641963.2015.1131290 [DOI] [PubMed] [Google Scholar]
- 45. Krasińska B, Miazga A, Cofta S, Szczepaniak-Chicheł L, Trafas T, Krasiński Z, et al. Effect of Eplerenone on the Severity of Obstructive Sleep Apnea and Arterial Stiffness in Patients With Resistant Arterial Hypertension. Pol Arch Med Wewn (2016) 126(5):330–9. doi: 10.20452/pamw.3410 [DOI] [PubMed] [Google Scholar]
- 46. Kasai T, Bradley TD, Friedman O, Logan AG. Effect of Intensified Diuretic Therapy on Overnight Rostral Fluid Shift and Obstructive Sleep Apnoea in Patients With Uncontrolled Hypertension. J Hypertension (2014) 32(3):673–80. doi: 10.1097/hjh.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 47. Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, et al. Spironolactone Reduces Severity of Obstructive Sleep Apnoea in Patients With Resistant Hypertension: A Preliminary Report. J Hum Hypertens (2010) 24(8):532–7. doi: 10.1038/jhh.2009.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krasińska B, Cofta S, Szczepaniak-Chicheł L, Rzymski P, Trafas T, Paluszkiewicz L, et al. The Effects of Eplerenone on the Circadian Blood Pressure Pattern and Left Ventricular Hypertrophy in Patients With Obstructive Sleep Apnea and Resistant Hypertension-A Randomized, Controlled Trial. J Clin Med (2019) 8(10):1671. doi: 10.3390/jcm8101671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship Between Overnight Rostral Fluid Shift and Obstructive Sleep Apnea in Drug-Resistant Hypertension. Hypertension (2010) 56(6):1077–82. doi: 10.1161/hypertensionaha.110.154427 [DOI] [PubMed] [Google Scholar]
- 50. Wolf J, Narkiewicz K. Managing Comorbid Cardiovascular Disease and Sleep Apnea With Pharmacotherapy. Expert Opin Pharmacother (2018) 19(9):961–9. doi: 10.1080/14656566.2018.1476489 [DOI] [PubMed] [Google Scholar]
- 51. Adolf C, Görge V, Heinrich DA, Hoster E, Schneider H, Handgriff L, et al. Altered Taste Perception for Sodium Chloride in Patients With Primary Aldosteronism: A Prospective Cohort Study. Hypertension (2021) 77(4):1332–40. doi: 10.1161/hypertensionaha.120.16440 [DOI] [PubMed] [Google Scholar]
- 52. Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, et al. Steroid Metabolome Analysis Reveals Prevalent Glucocorticoid Excess in Primary Aldosteronism. JCI Insight (2017) 2(8):e93136. doi: 10.1172/jci.insight.93136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang LU, Wang TY, Bai YM, Hsu JW, Huang KL, Su TP, et al. Risk of Obstructive Sleep Apnea Among Patients With Cushing’s Syndrome: A Nationwide Longitudinal Study. Sleep Med (2017) 36:44–7. doi: 10.1016/j.sleep.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 54. Burton LA, McMurdo ME, Struthers AD. Mineralocorticoid Antagonism: A Novel Way to Treat Sarcopenia and Physical Impairment in Older People? Clin Endocrinol (Oxf) (2011) 75(6):725–9. doi: 10.1111/j.1365-2265.2011.04148.x [DOI] [PubMed] [Google Scholar]
- 55. Kwak MK, Lee SE, Cho YY, Suh S, Kim BJ, Song KH, et al. The Differential Effect of Excess Aldosterone on Skeletal Muscle Mass by Sex. Front Endocrinol (Lausanne) (2019) 10:195. doi: 10.3389/fendo.2019.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee JY, Kim DA, Choi E, Lee YS, Park SJ, Kim BJ. Aldosterone Inhibits In Vitro Myogenesis by Increasing Intracellular Oxidative Stress via Mineralocorticoid Receptor. Endocrinol Metab (Seoul) (2021) 36(4):865–74. doi: 10.3803/EnM.2021.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Burniston JG, Saini A, Tan LB, Goldspink DF. Aldosterone Induces Myocyte Apoptosis in the Heart and Skeletal Muscles of Rats In Vivo . J Mol Cell Cardiol (2005) 39(2):395–9. doi: 10.1016/j.yjmcc.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 58. Chadwick JA, Hauck JS, Lowe J, Shaw JJ, Guttridge DC, Gomez-Sanchez CE, et al. Mineralocorticoid Receptors Are Present in Skeletal Muscle and Represent a Potential Therapeutic Target. FASEB J (2015) 29(11):4544–54. doi: 10.1096/fj.15-276782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lowe J, Kolkhof P, Haupt MJ, Peczkowski KK, Rastogi N, Hauck JS, et al. Mineralocorticoid Receptor Antagonism by Finerenone Is Sufficient to Improve Function in Preclinical Muscular Dystrophy. ESC Heart Fail (2020) 7(6):3983–95. doi: 10.1002/ehf2.12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feraco A, Marzolla V, Scuteri A, Armani A, Caprio M. Mineralocorticoid Receptors in Metabolic Syndrome: From Physiology to Disease. Trends Endocrinol Metab (2020) 31(3):205–17. doi: 10.1016/j.tem.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 61. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2016) 101(5):1889–916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 62. Jaffe G, Gray Z, Krishnan G, Stedman M, Zheng Y, Han J, et al. Screening Rates for Primary Aldosteronism in Resistant Hypertension: A Cohort Study. Hypertension (2020) 75(3):650–9. doi: 10.1161/hypertensionaha.119.14359 [DOI] [PubMed] [Google Scholar]
- 63. Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping Primary Aldosteronism in Mind: Deficiencies in Screening at-Risk Hypertensives. Surgery (2019) 165(1):221–7. doi: 10.1016/j.surg.2018.05.085 [DOI] [PubMed] [Google Scholar]
- 64. Chomsky-Higgins Menut K, Pearlstein SS, Conroy PC, Roman SA, Shen WT, Gosnell J, et al. Screening for Primary Aldosteronism in the Hypertensive Obstructive Sleep Apnea Population Is Cost-Saving. Surgery (2021) 171(1):96–103. doi: 10.1016/j.surg.2021.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rossi GP, Bisogni V, Bacca AV, Belfiore A, Cesari M, Concistrè A, et al. The 2020 Italian Society of Arterial Hypertension (SIIA) Practical Guidelines for the Management of Primary Aldosteronism. Int J Cardiol Hypertens (2020) 5:100029. doi: 10.1016/j.ijchy.2020.100029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pengo MF, Soranna D, Giontella A, Perger E, Mattaliano P, Schwarz EI, et al. Obstructive Sleep Apnoea Treatment and Blood Pressure: Which Phenotypes Predict a Response? A Systematic Review and Meta-Analysis. Eur Respir J (2020) 55(5):1901945. doi: 10.1183/13993003.01945-2019 [DOI] [PubMed] [Google Scholar]
- 67. Labarca G, Schmidt A, Dreyse J, Jorquera J, Enos D, Torres G, et al. Efficacy of Continuous Positive Airway Pressure (CPAP) in Patients With Obstructive Sleep Apnea (OSA) and Resistant Hypertension (RH): Systematic Review and Meta-Analysis. Sleep Med Rev (2021) 58:101446. doi: 10.1016/j.smrv.2021.101446 [DOI] [PubMed] [Google Scholar]
- 68. Rotenberg BW, Murariu D, Pang KP. Trends in CPAP Adherence Over Twenty Years of Data Collection: A Flattened Curve. J Otolaryngol Head Neck Surg (2016) 45(1):43. doi: 10.1186/s40463-016-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Posadas T, Campos-Rodriguez F, Sapiña-Beltrán E, Oscullo G, Torres G, Martinez-Garcia MA. Obstructive Sleep Apnea and Arterial Hypertension: Implications of Treatment Adherence. Curr Hypertens Rep (2020) 22(2):12. doi: 10.1007/s11906-020-1015-y [DOI] [PubMed] [Google Scholar]