ABSTRACT
The low abundance of envelope spikes and the inability of IgG to aggregate virions render HIV-1 an inadequate target for antibody-mediated clearance by phagocytes. In an attempt to improve the ability of antibody to mediate the internalization of HIV-1 virions, we generated multimers of the broadly neutralizing HIV-1-specific monoclonal antibody (MAb) VRC01 using site-directed mutagenesis of the Fc segment. We then measured virion internalization using primary human monocytes and neutrophils. We found that, in the absence of complement, immune complexes consisting of HIV-1 virions and VRC01 multimers were slightly more efficiently internalized than were complexes formed with monomeric VRC01. The presence of complement, however, greatly augmented internalization of immune complexes formed with the multimeric MAb but had little impact on monomeric MAb-mediated internalization. Multimerization and the presence of complement overcome the limited ability of monomeric antibody to mediate internalization of HIV-1 virions and may thus provide a therapeutic approach to clearing virus.
IMPORTANCE Antibody-mediated internalization of HIV-1 by phagocytes, a potential mechanism for clearing virus, is very inefficient. In an effort to improve viral clearance, we produced a multimeric form of the broadly neutralizing monoclonal antibody VRC01. We found that VRC01 antibody multimers (primarily hexamers) were only slightly more efficient in mediating HIV-1 internalization than was monomeric VRC01. However, the addition of complement resulted in substantially greater internalization of multimer-opsonized virus. In contrast, complement had little if any impact on internalization of monomer-opsonized virus. Therefore, antibody multimerization in combination with complement may overcome the limited ability of monomeric antibody to mediate internalization of HIV-1 virions. Our findings may provide a therapeutic approach to clearing virus.
KEYWORDS: antibody, complement, Fc receptors, HIV-1, phagocytosis
INTRODUCTION
Antibodies play a pivotal role in preventing and controlling human immunodeficiency virus 1 (HIV-1) infection (1). Besides neutralization, antibodies mediate various effector functions through their Fc domain by interacting with Fcγ receptors (FcγRs) or with complement (2). Examples include antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis (2). Both effector functions have been studied in different experimental settings, including nonhuman primate models and a human HIV-1 efficacy trial (RV144) (3–6).
In the setting of viral infections, antibody-dependent internalization occurs when antibodies opsonizing an infected cell or a virion cross-link FcγRs or complement receptors on phagocytes such as monocytes, macrophages, and neutrophils. Such cross-linking results in the uptake and, generally, the inactivation of the virions or infected cells within the phagolysosome. For several viruses, antibody-dependent internalization is thought to play a major role in vivo in clearing virus (7). However, a beneficial in vivo role has not been confirmed with respect to preventing or controlling HIV-1 infection (8).
As reported earlier by our group, immune complexes made from HIV-1 virions and HIV-1-specific antibodies are inadequate targets for phagocytosis (9). Limiting factors such as particle size and the paucity of envelope spikes on the virion surface underlie the inefficient uptake of opsonized HIV-1 virions. We had also found that experimentally aggregating virus or increasing the abundancy of HIV-1 envelope spikes substantially enhanced the internalization of HIV-1 particles opsonized with antibody (9). However, these in vitro manipulations required to improve internalization cannot be accomplished in vivo.
In this study, we sought to improve the therapeutic potential of antibodies by producing multimers of the broadly neutralizing monoclonal antibody (MAb) VRC01 and testing the ability of the multimers to mediate internalization of HIV-1 virions.
RESULTS
Anti-HIV-1-specific antibodies capture virus but mediate limited internalization by monocytes or neutrophils.
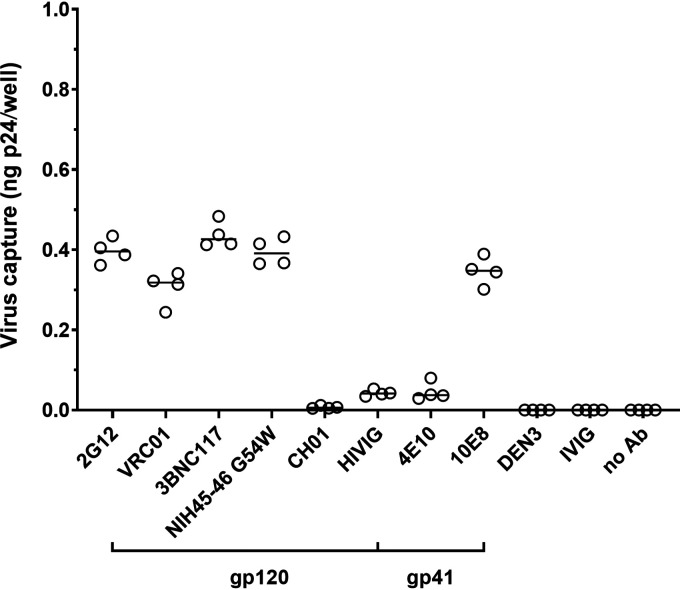
We first assessed the ability of a panel of HIV-1-specific antibodies to capture HIV-1iGFP/JRFL virions using a p24 readout as a measure of total virus captured. As reported previously (9), we found distinct capture profiles of the HIV-1 gp120- and gp41-specific antibodies (Fig. 1). As expected, the HIV-1-specific MAb CH01 was not able to capture HIV-1iGFP/JRFL virions, consistent with its previously reported inability to neutralize HIV-1JRFL (10). The negative-control antibodies DEN3 (anti-dengue NS1 human IgG1 MAb) and intravenous immunoglobulin (IVIG) did not capture HIV-1iGFP/JRFL virions.
FIG 1.
Envelope-specific antibodies differentially capture HIV-1iGFP/JRFL virions. Virus capture was measured by determining the total p24 content of captured HIV-1iGFP/JRFL virions by p24 ELISA. All envelope-specific antibodies (gp120-specific and gp41-specific) as well as controls were tested at 2 μg/mL. Experiments were performed in triplicate and repeated four times. Lines represent median p24 capture. HIVIG is IgG from pooled HIV-positive donors; IVIG (intravenous immunoglobulin) is IgG from pooled HIV-negative donors.
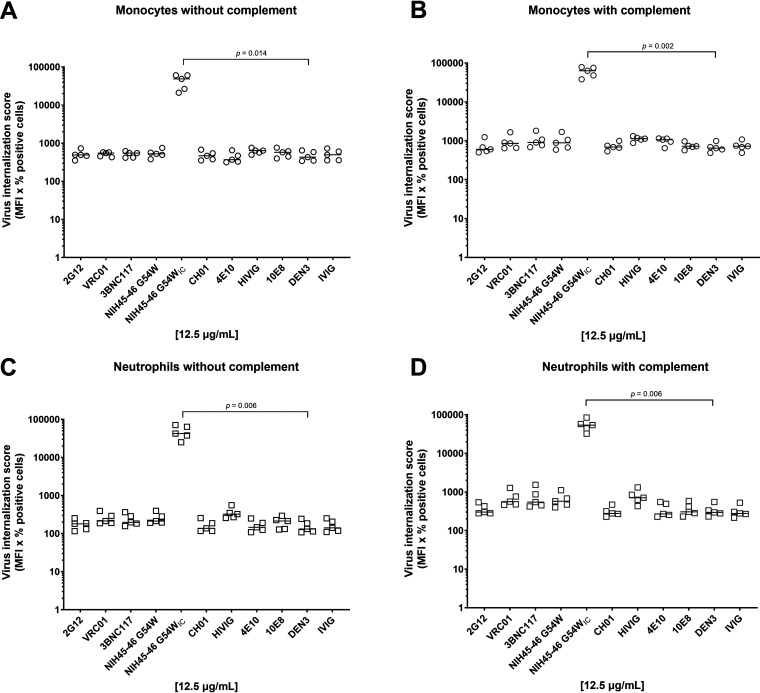
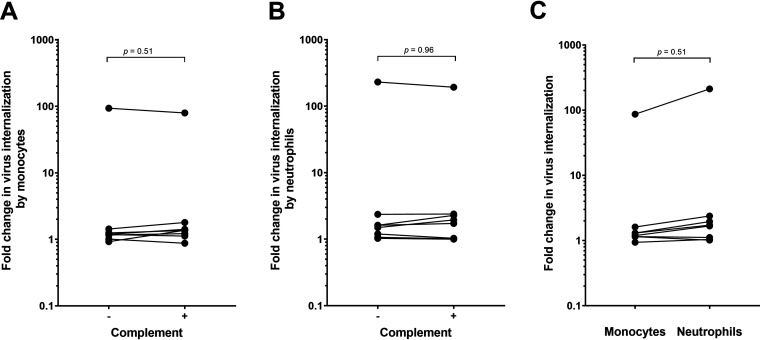
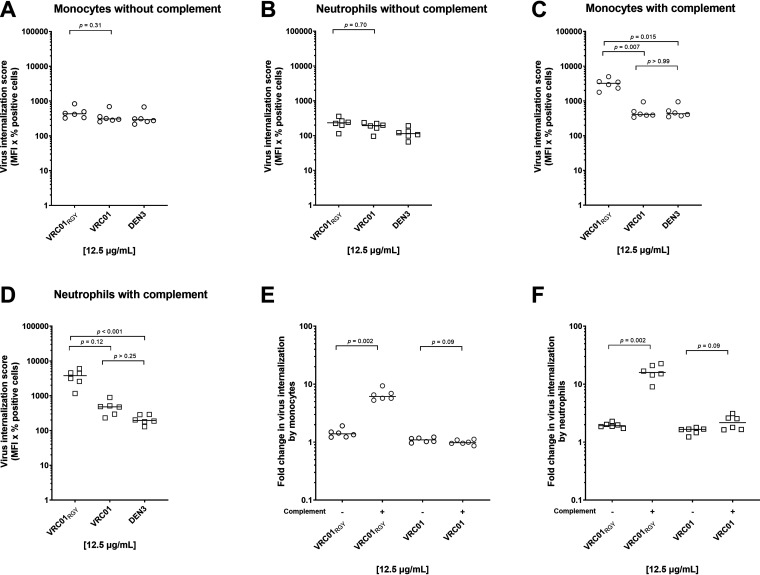
We next investigated whether virus captured by antibody was internalized by peripheral blood monocytes or neutrophils in either the presence or absence of complement. Neutrophils were incubated with diphenyleneiodonium chloride (DPI) prior to incubation with antibody-virus complexes to block neutrophil extracellular trap (NET) formation. The latter mechanism has been shown to actively capture and eliminate HIV-1 virions in vitro (11), thus potentially interfering with our internalization assay. As previously reported by our group, we observed no significant antibody-mediated internalization of virions (P > 0.05 for all HIV-specific MAbs compared with the DEN3 control or for HIVIG compared with IVIG; Kruskal-Wallis with Dunn’s correction) by monocytes (Fig. 2A) (9) or by neutrophils (Fig. 2C). Note, however, that immune complexes formed by cross-linking antibody-opsonized virions with a goat anti-human antibody (NIH45-46 G54WIC) resulted in >80-fold internalization compared with the DEN3 control (P < 0.05; Kruskal-Wallis with Dunn’s correction), thus confirming that cellular internalization function was intact (Fig. 2A to D). Addition of normal guinea pig serum as a source of complement did not improve monomer-mediated internalization of virions in monocytes (Fig. 2B and Fig. 3A) or neutrophils (Fig. 2D, Fig. 3B). In addition, overall antibody-mediated internalization by the monomeric MAbs, when expressed as a fold change compared to the negative-control MAb, did not differ between monocytes and neutrophils (Fig. 3C).
FIG 2.
Antibody opsonized HIV-1iGFP/JRFL virions are inefficient targets for primary phagocytes. (A to D) Uptake of HIV-1iGFP/JRFL virions by monocytes (A) or neutrophils (C) in the absence of complement or by monocytes (B) or neutrophils (D) in the presence of complement was measured using MAbs at a final concentration of 12.5 μg/mL and the polyclonal antibodies HIVIG and IVIG at a final concentration of 50 μg/mL. NIH45-46 G54WIC was included as a positive control. All experiments were performed in triplicate and were repeated five times with different healthy donors. Solid lines indicate median virus internalization.
FIG 3.
(A and B) Monomeric MAb-mediated internalization of virions is similar with and without complement on monocytes (A) and on neutrophils (B). Each data point represents the median fold change of the internalization score for the seven antibodies (Abs) that capture HIV-1iGFP/JRFL (2G12, VRC01, 3BNC117, NIH46 G54W, HIVIG, 10E8, and 4E10) as well as for the immune complexes formed by cross-linking NIH45-46 G54W with a goat anti-human antibody (data points with the highest values; Fig. 1). When expressed as the median fold change (combining median values with and without complement), antibody-mediated internalization by monocytes is similar to internalization by neutrophils for the seven monomeric Abs, though there may be slightly better internalization of the anti-human antibody-cross-linked immune complex on neutrophils (C). Mann-Whitney tests were used to calculate the P values.
Multimerization of VRC01.
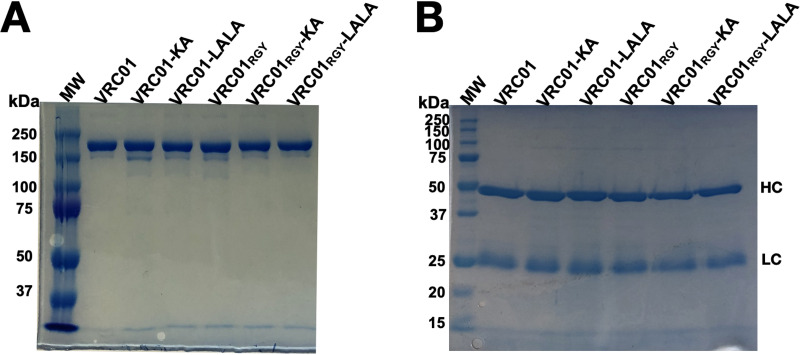
We had previously demonstrated that poor antibody-mediated internalization of HIV-1 was due to the scarcity of Env spikes on HIV-1 virions and the inability of IgG antibody to aggregate virions (9). In an attempt to overcome these factors, we generated multimers of VRC01 by mutating key amino acids in Fc. Using site-directed mutagenesis, we changed three amino acids (E345R, E430G, and S440Y) known to result in IgG multimerization (12). We also introduced mutations to alter FcγR and complement binding (LALA) or complement binding only (KA) (13). All VRC01 antibody variants were tested for integrity and purity under nonreducing and reducing conditions using SDS-PAGE. Under nonreducing conditions, we found that all antibody variants demonstrated a predominant band between 150 kDa and 250 kDa representing intact IgG (Fig. 4A). Addition of dithiothreitol as a reducing agent to the antibody variants resulted in bands representing the heavy-chain (50 kDa) and light-chain (25 kDa) fragments (Fig. 4B).
FIG 4.
Coomassie staining of purified VRC01 variants. (A and B) All VRC01 variants were examined with SDS-PAGE under nonreducing (A) and reducing (B) conditions. The percentage of acrylamide in the nonreducing gel (7.5%) differs from that in the reducing gel (10%). A total amount of 3 μg of each antibody was loaded per well. Lane MW contains the All Blue Prestained Protein Standard (Bio-Rad). Heavy-chain (HC) and light-chain (LC) bands are indicated.
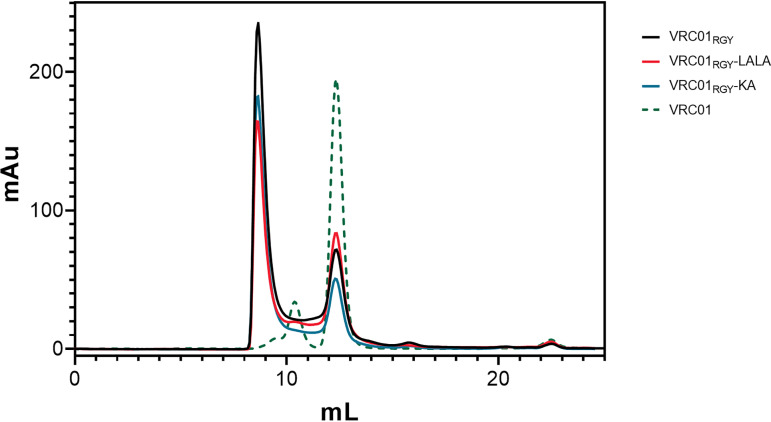
Size-exclusion chromatography (SEC) revealed that the multimeric antibodies VRC01RGY, VRC01RGY-LALA, and VRC01RGY-KA formed hexamers (1,030-kDa peaks) in solution (Fig. 5). Smaller peaks at 150 kDa indicated residual monomers. Both peaks were connected by a trace above the baseline level, suggesting a dynamic interconversion between the different oligomeric states (14). In contrast, the chromatogram of the parental monomeric VRC01 exhibited a predominant peak at 150 kDa (monomers) and a smaller peak around 412 kDa, indicating some degree of aggregation (Fig. 5).
FIG 5.
E345R, E430G, and S440Y triple mutation promotes VRC01 multimerization. Size exclusion chromatography of purified multimeric VRC01 variants (VRC01RGY, VRC01RGY-LALA, and VRC01RGY-KA) was performed on a Superdex 200 increase 10 300 GL column. Absorbance at 214 nm was plotted against elution volume for each antibody. A protein standard with known molecular mass was loaded as a control to determine the size distribution of the peaks. Monomeric VRC01, obtained through the NIH AIDS Reagent Program, was included as a reference.
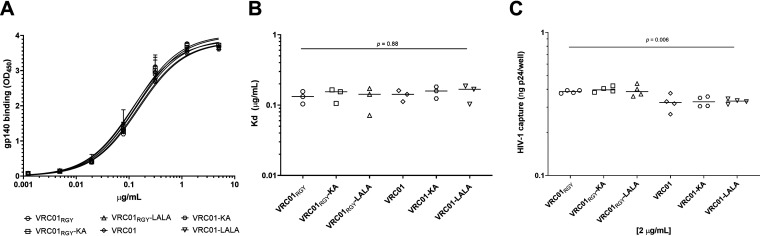
Next, we analyzed the ability of the antibodies to bind recombinant HIV-1 gp140 in an enzyme-linked immunosorbent assay (ELISA) and to capture HIV-1iGFP/JRFL virions. As expected, antibody binding to gp140 was not affected by the Fc mutations resulting in VRC01 multimers or resulting in multimers and monomers with altered FcγR and complement binding (Fig. 6A). We also found no significant difference between the median dissociation constant (Kd) values of any of the VRC01 variants (Fig. 6B). The multimeric VRC01RGY variants were slightly better able to capture HIV-1 virions than were the monomeric VRC01 variants (P = 0.006, Kruskal-Wallis; Fig. 6C), although differences were nonsignificant when applying Dunn’s multiple-comparison test.
FIG 6.
VRC01 Fc fragment mutations do not impact gp140 binding; however, VRCO1 multimers capture slightly more virus than monomers. VRC01 multimers and monomers were tested for HIV-1iGFP/JRFL gp140 binding in an ELISA (A). Curve fitting was used to calculate Kd values of each antibody variant at half-maximum binding. Kd values of VRC01 variants did not differ (P = 0.88; Kruskal-Wallis test) (B). All ELISA binding experiments were performed in triplicate and repeated three times. Lines represent median Kd values. All VRC01 variants were able to capture free HIV-1iGFP/JRFL virions (C). VRC01 multimers captured slightly more virus than monomers (P = 0.006; Kruskal-Wallis test). However, when analyzed for differences between the antibody groups (Dunn’s test), we found no significant difference between the antibodies. Virus capture was measured by p24 readout. All VRC01 variants were tested at 2 μg/mL. Capture assays were carried out in triplicate and repeated four times. Solid lines represent median p24 capture.
In total, these results indicate that multimerization of VRC01 did not affect HIV-1 gp140 binding or virus capture, nor did the introduction of Fc-binding-site mutations or complement-binding site mutations.
VRC01 multimerization enhances internalization of HIV-1 by primary monocytes and neutrophils.
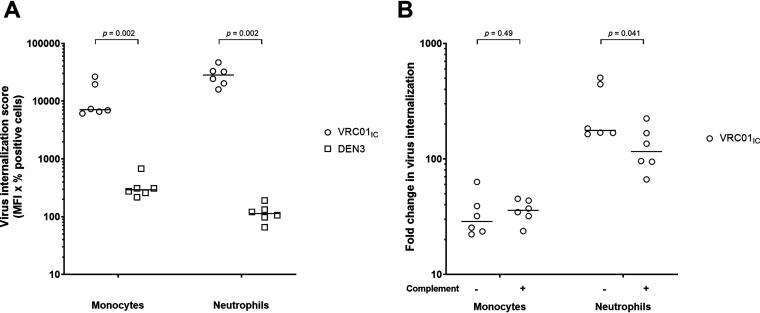
To evaluate the ability of the engineered antibody variant VRC01RGY to mediated internalization, we carried out additional assays with primary monocytes and neutrophils in the presence and absence of complement. We found that in the absence of complement, VRC01RGY and VRC01 mediated little internalization by monocytes compared to the negative-control antibody DEN3, although VRC01RGY was slightly, but not significantly, more effective than VRC01 (1.5-fold increase over DEN3 for VRC01RGY versus 1.1-fold increase over DEN3 for VRC01; P = 0.31; Fig. 7A). Similarly, with neutrophils, VRC01RGY resulted in a 2.1-fold increase and VRC01, a 1.7-fold increase in internalization compared with DEN3 (P = 0.70; Fig. 7B). The slightly increased internalization by the multimers may be due to their slightly increased ability to capture virions (Fig. 6C). In contrast, the immune complexes made by cross-linking monomeric VRC01-opsonized virions with a goat anti-human antibody (VRC01IC) induced a substantial uptake of virions in both cell types compared to the negative MAb (P = 0.002; Fig. 8A).
FIG 7.
VRC01 multimerization augments HIV-1 internalization in the presence of complement. (A to D) VRC01RGY- and VRC01-mediated uptake was measured in the absence of complement with monocytes (A) or neutrophils (B) and in the presence of complement with monocytes (C) or neutrophils (D). (E and F) VRC01RGY-mediated significantly greater HIV-1iGFP-JRFL internalization in the presence of complement compared to VRC01 in both monocytes (E) and neutrophils (F). Results are reported as virus internalization score (percent positive cells × MFI) or fold change compared to the DEN3 control. Experiments were performed in triplicate and were repeated with cells from the blood of six healthy donors. Solid lines indicate median internalization score or fold change in viral uptake. P values were calculated using Kruskal-Wallis with Dunn’s multiple-comparisons tests (panels A to D) or using Mann-Whitney tests (panels E and F).
FIG 8.
Enhanced internalization of VRC01 opsonized virions cross-linked with anti-human antibody. Immune complexes made by cross-linking VRC01-opsonized virions with a goat anti-human antibody (VRC01IC) induced a substantial uptake of virions in both monocytes and neutrophils compared to DEN3 (A). Addition of complement slightly decreased (P = 0.041) the uptake of HIV-1iGFP-JRFL virions by neutrophils but not by monocytes (B). P values were calculated using Mann-Whitney tests. Results are reported as virus internalization score (percent positive cells × MFI) or fold change compared to the DEN3 control. Experiments were performed in triplicate and were repeated using cells from six healthy donors. Solid lines indicate medians.
Unlike the minimally increased virion uptake observed in the absence of complement, its addition substantially augmented VRC01RGY-mediated internalization by monocytes (8.0-fold increase compared to VRC01; P = 0.007 and a 7.5-fold increase compared to DEN3 Fig. 7C) and by neutrophils (7.8-fold increase compared to VRC01; P = 0.12 and a 19.4-fold increase compared to DEN3; Fig. 7D). Moreover, VRC01RGY mediated significantly greater internalization with complement than without on both monocytes (P = 0.002; Fig. 7E) and neutrophils (P = 0.002; Fig. 7F). For monomeric VRC01, the addition of complement resulted in no increased internalization compared to DEN3 by monocytes (Fig. 7C) and a 2.2-fold, nonsignificant increase by neutrophils (Fig. 7D). There was no statistically significant increase in internalization by VRC01 with complement compared to no complement on monocytes (P = 0.09; Fig. 7E) or neutrophils (P = 0.09; Fig. 7F). Internalization of virions by the VRC01IC control was little changed by the addition of complement with monocytes, whereas with neutrophils, we found a slight decrease in the presence of complement (P = 0.041; Fig. 8B).
These results indicate that multimeric antibody can augment phagocyte-cell internalization of opsonized virions, but substantial increases in antibody-mediated internalization by multimeric antibody depend on the presence of complement.
Fc engagement with complement is critical for augmented antibody-mediated internalization by multimeric VRC01.
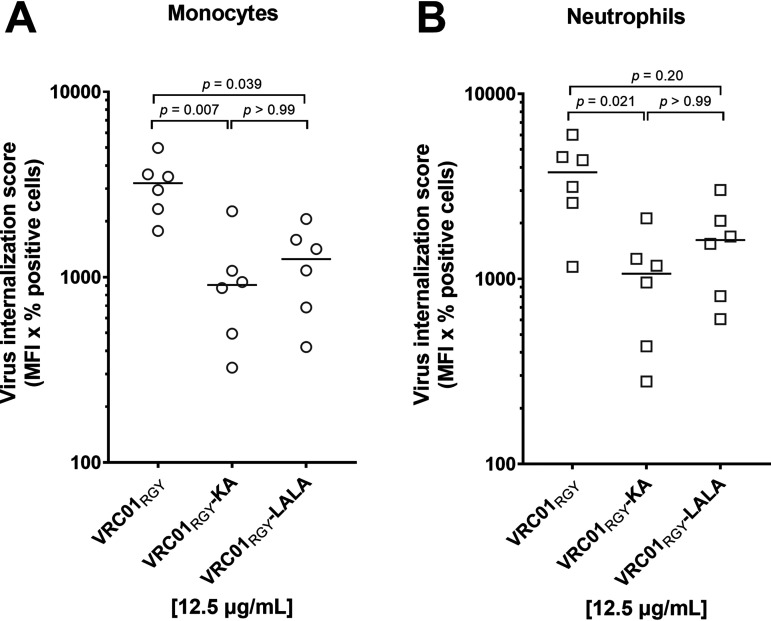
To decipher whether FcγR- or complement-binding site mutations impact VRC01RGY-mediated internalization, we compared VRC01RGY with VRC01RGY-LALA and with VRC01RGY-KA. We found that both mutations resulted in lower uptake by monocytes and by neutrophils (Fig. 9A and B). Compared to VRC01RGY, VRC01RGY-KA reduced uptake 3.5-fold on monocytes (P = 0.007) and 3.5-fold on neutrophils (P = 0.021). VRC01RGY-LALA also resulted in less uptake than VRC01RGY on monocytes and neutrophils, but the reductions were smaller (2.6-fold and 2.3-fold on monocytes and neutrophils, respectively), and the difference was only significant on monocytes (P = 0.039). There was no significant difference between VRC01RGY-KA and VRC01RGY-LALA on either cell type (Fig. 9A and B). Since both the KA and LALA mutations affect complement binding, these findings suggest an important role of complement in the improved VRC01RGY-mediated internalization of HIV-1iGFP/JRFL but do not rule out a role of Fc-FcγR engagement as well.
FIG 9.
Reduced complement binding results in a decrease in VRC01RGY-mediated virus internalization. (A and B) The VRC01 multimer variants VRC01RGY, VRC01RGY-KA, and VRC01RGY-LALA were tested for their ability to mediate HIV-1iGFP-JRFL internalization in monocytes (A) and neutrophils (B). Results are reported as virion internalization score (percent positive cells × MFI). Experiments (performed in triplicate) were repeated six times with cells from different healthy donors. Median internalization scores are indicated by a solid line. P values were calculated using Kruskal-Wallis with Dunn’s multiple-comparisons tests.
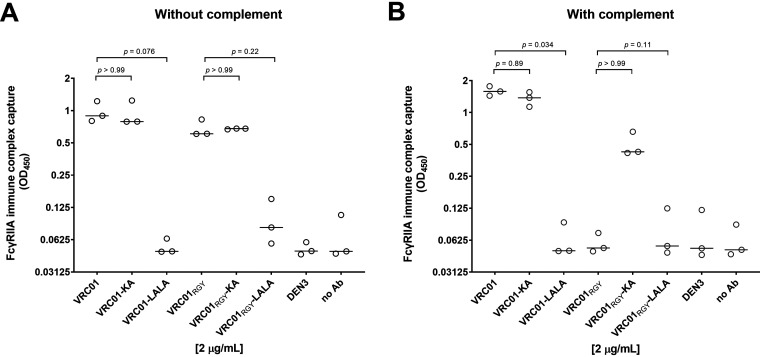
To investigate the impact of multimerization on binding of immune complexes to FcγRIIA (a key activating FcγR on both monocytes and neutrophils [15]), we developed an in-solution capture assay to measure the amount of HIV-1iGFP/JRFL bound by VRC01 variants in complex with soluble FcγRIIA in the presence or absence of complement. Virus bound to VRC01 and VRC01-KA was similarly captured by FcγRIIA, with or without complement, consistent with the fact that the KA mutation does not affect FcγRIIA binding (13) (Fig. 10A and B). Also consistent with the known ability of the LALA mutation to greatly reduce FcγRIIA binding, little if any virus bound to VRC01-LALA was captured by FcγRIIA with or without complement (Fig. 10A and B).
FIG 10.
Interactions between FcγRIIA and monomeric and multimeric VRC01 are differentially affected by complement. (A and B) Interactions of monomeric and multimeric VRC01/HIV-1iGFP/JRFL immune complexes with FcγRIIA were measured in the absence (A) and presence (B) of guinea pig complement. Virus capture was evaluated by subsequent p24 ELISA. All VRC01 variants and nonspecific controls were tested at 2 μg/mL. Experiments were performed in triplicate and repeated three times. Lines represent median OD450.values. P values were calculated using Kruskal-Wallis with Dunn’s multiple-comparisons tests.
In the absence of complement, multimerized VRC01 variants revealed slightly less virus capture than the monomers. Most strikingly, VRC01RGY did not interact with FcγRIIA in the presence of complement (Fig. 10B). However, the KA mutation on VRC01RGY multimers restored much of the binding to FcγRIIA, suggesting that complement shields the multimer’s Fc regions from interaction with FcγRIIA. This shielding effect was not observed with VRC01 monomers, presumably because monomeric IgG1 is not efficient at binding complement. As expected, both the monomeric and multimeric LALA mutants exhibited similarly poor interactions with FcγRIIA.
DISCUSSION
We previously reported that antibody-mediated internalization of HIV-1 by monocytes is ineffective in vitro (9). By aggregating virus or by increasing Env density on the surface of virions, more efficient internalization resulted (9). However, such manipulations of virions in vitro would have little relevance in clinic settings. In the current investigation, we first determined if neutrophils might be better able to internalize opsonized virus. In addition, we evaluated the ability of a multimeric IgG1 to mediate internalization on monocytes and neutrophils in the presence or absence of complement, reasoning that multimerization might form bigger complexes, increase complement activation, or improve FcγR cross-linking. We found that in the absence of complement, neutrophils were slightly better, albeit not significantly, at internalizing HIV-1 opsonized by either monomeric or multimeric VRC01 than were monocytes. Moreover, on both monocytes and neutrophils, multimeric VRC01 was slightly more effective in mediating internalization than was monomeric MAb. In all cases absent complement, internalization remained at low levels, with a maximum 2-fold increase in internalization over a non-HIV-1-specific negative-control MAb observed with multimeric VRC01 added to neutrophils. The addition of complement had little effect on uptake of monomeric VRC01-opsonized HIV-1. However, complement markedly enhanced the ability of multimeric VRC01 to mediate uptake of virions on both monocytes and neutrophils.
In accordance with our previously published observations using monomeric MAbs in the absence of complement (9), uptake of opsonized virions by monocytes was low even though antibodies were fully capable of capturing HIV-1iGFP/JRFL virions. We now observe slightly increased internalization with neutrophils compared to monocytes. Nonetheless, the level of antibody-mediated internalization by neutrophils remains quite low. This inefficient uptake is perhaps best appreciated by comparing uptake of monomeric or multimeric VRC01-opsonized virions with the uptake of immune complexes formed by adding a goat anti-human antibody to virions opsonized with either NIH45-45 G54W (Fig. 2 and Fig. 3) or with monomeric VRC01 (Fig. 8). The large aggregates formed in this manner (9) result in an over 200-fold increase in uptake on neutrophils compared to the negative antibody control. In any case, it is possible that the increased uptake of NIH45-45 G54WIC- and VRC01IC-opsonized virions by neutrophils compared with monocytes is a result of the unique repertoire and higher expression levels of Fc gamma receptors, including FcγRIIA, on the cell surface of neutrophils (16, 17). Furthermore, monocytes are composed of a variety of lineages and subsets whose activities may be unique at each stage of maturation (15).
We observed only minimally improved internalization mediated by monomeric VRC01 after addition of complement. The limited effect of complement may be due to the fact that monomeric IgG1 only weakly interacts with a single globular head of the complement factor C1q hexamer (18).
It has been reported that antibody multimerization enhances effector functions, including antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity (12, 19). It is unknown whether such multimerization impacts antibody-mediated internalization of virus particles. In an attempt to develop a reagent capable of mediating more efficient phagocytic clearance of virus, we implemented an innovative Fc domain-multimerization approach using the CD4 binding site-specific MAb VRC01. In our hands VRC01RGY readily formed a high proportion of hexamers in solution as measured by SEC. This is in accordance with previous studies where introduction of the RGY triple mutation resulted in antibody hexamerization of other MAbs (14, 20). Although VRC01 hexamerization had no effect on gp140 binding in an ELISA, there was a slight increase in the ability of the multimers to capture HIV-1iGFP-JRFL virions compared with the monomers. Note that in all assays, monomeric and multimeric VRC01 were added in equal weight/volume concentrations so that the number of antigen-binding sites remained equivalent. The greater virion capture but equivalent gp140 binding by multimeric compared with monomeric VRC01 is likely the result of multivalency in the capture assay, where a hexamer can interact with one or more virus particles. The presence of detergent (i.e., Tween 20) in the gp140 ELISA, however, most likely prevents multimerization of VRC01 (as seen with our SDS-PAGE analysis).
Compared to monomeric VRC01, multimeric VRC01 in the absence of complement resulted in slight improvements in virus uptake by both monocytes and neutrophils. However, the addition of complement to multimeric VRC01 resulted in a substantial increase in virion uptake by both cell types. Confirming a role for complement in the enhanced virus uptake, we found that both the KA mutation (which only reduces complement binding [13]) and the LALA mutation (which reduces both complement and FcγR binding [13, 21]) greatly lowered antibody-mediated internalization by the multimeric VRC01RGY. In addition, the KA mutation had a slightly greater impact on internalization than did the LALA mutation, consistent with the fact that the KA mutation’s effect on complement binding is greater than that of the LALA mutation (13).
The increased uptake of HIV-1iGFP-JRFL virions by multimeric VRC01RGY in the presence of complement is likely C1q dependent, since C1q is the component of complement that binds to IgG and since the KA mutant decreases internalization (Fig. 9) (22). This is in accordance with the notion that VRC01RGY multimers represent clusters of IgG that are vital to induce the conformational change required to expose the C1q-binding site within the antibody Fc region (20, 23). Once bound, the C1q-antibody-virus complex can be readily internalized by phagocytes in a C3b-independent manner (24).
Based on our finding that binding of VRC01 multimers to FcγRIIA was nearly completely abrogated in the presence of complement and that the abrogated binding was mostly reversed by the KA mutation, it is likely that complement sterically hinders Fc-FcγRIIA interactions. Notably, the binding of monomeric VRC01 to FcγRIIA was not negatively impacted by complement or by the KA mutation, consistent with the limited ability of monomeric IgG to engage complement as discussed above. Thus, although multimerization improves virus internalization by phagocytes in the presence of complement, there appears to be a trade-off resulting in less FcγRIIA binding (Fig. 10). How this might translate in vivo remains to be seen.
As mentioned above, antibody multimerization enhances effector functions, including antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity (12, 19). It will be of interest to ascertain the overall effect of antibody hexamerization on the clearance of infected cells in vitro and on the clearance of infected cells and virions in vivo in animal models of HIV-1 infection. In addition, to further study the role of complement in antibody-mediated virus internalization, HIV-1 envelope-specific IgM multimers could prove useful. IgM can recruit complement more efficiently than IgG (23), and IgM does not engage FcγRs. It is also possible that lower-valency IgG multimers, including dimers, could be sufficient to augment internalization of virions in the presence of complement. Finally, by affecting antigen uptake and presentation, it is possible that hexameric antibodies could enhance anti-lentiviral immune responses in infected animals and humans.
We note that although the multimeric VRC01 consisted primarily of hexamers, the monomers present may have had an adverse effect on virion internalization. A more uniform reagent consisting solely of hexamers might further enhance uptake.
In conclusion, we confirmed our previous finding that antibody-mediated internalization of free HIV-1 virions by monocytes is very limited. In addition, we found that uptake of opsonized virions by neutrophils is also limited, though somewhat better than that by monocytes. By multimerizing the neutralizing MAb VRC01, further augmentation in virion uptake was noted, but substantial improvements only occurred when complement was added to multimeric VRC01. In vivo studies will be needed to determine if multimeric neutralizing antibodies will be therapeutically useful.
MATERIALS AND METHODS
Ethical statement.
Peripheral blood from anonymous healthy donors was obtained expressly for this research (through the University of California, Irvine, Normal Blood Donors Program) with informed, written consent in accordance with the institutional review board at the University of California, Irvine.
Reagents.
The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 JRFL gp140 recombinant protein (B.JRFL gp140CF) from Barton F. Haynes and Hua-Xin Liao, VRC01 MAb heavy-chain and VRC01 MAb light-chain expression vectors from John Mascola, HIV Gag-iGFP-JRFL expression vector from Benjamin Chen, and the HIV-1-specific antibodies, VRC01, 10E8, 4E10, 3BNC117, NIH45-46 G54W, CH01, and HIVIG. The control human IgG1 monoclonal antibody (MAb), DEN3 (anti-dengue NS1), was provided by Dennis Burton (13, 25).
Antibody mutagenesis.
The VRC01 heavy-chain expression vector was used as a DNA template for generating all VRC01 heavy-chain variants described in this study, including VRC01RGY, VRC01RGY-KA, VRC01RGY-LALA, VRC01-KA, and VRC01-LALA. Mutagenesis primers are listed in Table 1. Numbering of the amino acid positions in the antibody Fc region is according to the European Union numbering system (26). All amino acid substitutions, K322A, L234A, L235A, E345R, E430G, and S440Y, were introduced by site-directed mutagenesis using the QuikChange XL mutagenesis kit according to the manufacturer’s instructions (Agilent). Amino acid substitutions of each VRC01 heavy-chain expression vector variant were confirmed by Sanger sequencing (Genewiz) prior to antibody expression and purification.
TABLE 1.
Primers used for VRC01 heavy-chain mutagenesis
| Mutation | Primer name | Primer sequence (5′ to 3′) |
|---|---|---|
| KA | K322A sense | 5′-gcaaggagtacaagtgcgcggtctccaacaaagccc-3′ |
| K322A antisense | 5′-gggctttgttggagaccgcgcacttgtactccttgc-3′ | |
| LALA | L234A L235A_sense | 5′-accgtgcccagcacctgaagccgcggggggaccg-3′ |
| L234A L235A _antisense | 5′-cggtccccccgcggcttcaggtgctgggcacggt-3′ | |
| RGY | E345R sense | 5′-tgtacacctgtggtcttcggggctgccctttgg-3′ |
| E345R antisense | 5′-ccaaagggcagccccgaagaccacaggtgtaca-3′ | |
| E430G sense | 5′-ggttgtgcagagccccatgcatcacggag-3′ | |
| E430G antisense | 5′-ctccgtgatgcatggggctctgcacaacc-3′ | |
| S440Y sense | 5′-cccggagacagggagagatacttctgcgtgtagtggt-3′ | |
| S440Y antisense | 5′-accactacacgcagaagtatctctccctgtctccggg-3′ |
Antibody expression and purification.
Adherent human embryonic kidney (HEK) 293T cells were cultured in serum-free medium (BalanCD HEK293, 1× Pen/Strep, 20 mM glutamine, and 1× insulin-transferrin-selenium) prior to transfection. Cells were cotransfected with VRC01 heavy- and light-chain-plasmid/polyethylenimine (PEI) complexes at a DNA/PEI ratio of 1:3. Four days posttransfection, antibody-containing supernatants were collected, cleared (2,500 rpm for 5 min), filtered (0.45 μm), and concentrated with a 50-kDa Amicon Ultra-15 centrifugal filter device. VRC01 variants were then purified over a protein A Sepharose spin column (Thermo Scientific) as previously reported (27). Eluted fractions containing the purified VRC01 antibody variants were concentrated, and buffer was exchanged using Amicon Ultra 0.5 mL centrifugal filter (50 kDa) units (Millipore). Antibody concentrations were measured at an optical density of 280 nm (OD280) with a NanoDrop spectrophotometer (Thermo Scientific).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
VRC01 antibody variants were separated by SDS-PAGE as previously described (28). Antibodies were examined under nonreducing (7.5% [wt/vol]) and reducing conditions (10% [wt/vol]) followed by a SimplyBlue SafeStain (Thermo Fisher) Coomassie G-250 staining procedure according to the manufacturer’s instructions.
Size exclusion chromatography (SEC).
For SEC analysis, a Superdex 200 increase 10/300 GL column (GE Healthcare) was washed at room temperature with a down-flow of 2.0 column volumes (CV) of 0.22-μm-filtered, sterile water at 0.500 mL/min. Next, the column was equilibrated with 2.0 CV of 0.22-μm-filtered, sterile phospate-buffered saline (PBS), pH 7, at 0.750 mL/min. All VRC01 samples were filtered by centrifugation through a 0.22-μm cellulose acetate filter (Costar) at 10,000 × g, for 1 min at room temperature prior to loading onto a 0.1-mL sample loop (GE Healthcare). After a brief column wash with 0.05 CV of PBS at 0.750 mL/min, the sample loop was emptied with 2.00 mL of PBS at 0.750 mL/min onto the column. The column was eluted with 1.0 CV of PBS at 0.750 mL/min, and any eluted proteins were detected at UV 214 nm. The first 0.1 CV of eluate was wasted, and the remaining 0.9 CV of eluate was collected into 200-μL fractions in 96-deep-well plates. Between each sample application, the column was rewashed and equilibrated. For protein size estimation, the column was calibrated with a protein standard (Bio-Rad) containing thyroglobulin (669 kDa), gamma globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa), and vitamin B12 (1.35 kDa).
Antibody enzyme-linked immunoassay (ELISA).
ELISA plates were coated overnight at 4°C with 250 ng/well of a goat anti-human Fc fragment. Plates were then washed three times with wash buffer (Dulbecco’s phosphate-buffered saline [DPBS]-containing 0.05% Tween 20) and incubated for 1 h at 37°C with 5% blocking buffer (wash buffer containing 5% nonfat dry milk). After washing the plates three times, serial dilutions (1:5 in 1% blocking buffer) of VRC01 antibody variants starting at 1 μg/mL were added. Plates were further incubated for 1 h at 37°C. Unbound antibodies were removed by three washes, and bound antibodies were detected with a horseradish peroxidase conjugated goat anti-human F(ab′)2 antibody. After 1 h of incubation at 37°C, plates were washed four times, developed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate, and stopped with H2SO4. Optical densities (at 450 nm) were measured with a Synergy 2 plate reader (BioTek). The IgG concentrations of the VRC01 variants were calculated based on a standard curve generated with a parental VRC01 obtained from the NIH AIDS Reagent Program.
HIV-1JRFL gp140-binding ELISA.
HIV-1JRFL gp140 ELISA was carried out as previously reported (29) with some modifications. In brief, ELISA plates (Costar) were coated with 100 ng/well of recombinant JRFL gp140. Plates were washed, blocked, and incubated with serial dilutions (1:4) of MAbs starting at a concentration of 5 μg/mL. After 1 h at 37°C, unbound antibodies were removed, and bound antibodies were detected by a horseradish peroxidase (HRP)-labeled goat anti-human Fc gamma-specific conjugate (Sigma-Aldrich). Plates were washed, developed, stopped, and read as described above. All antibodies were tested in duplicate, and binding assays were performed three times. Equilibrium dissociation constants (Kd) at half-maximum binding were calculated using GraphPad Prism 9.2 software.
Virus production and infectivity.
Full-length molecular clones of HIV-1iGFP/JRFL were generated and tested for infectivity as previously reported (9). In brief, HEK 293T cells were transfected with the HIV-1 plasmid HIV Gag-iGFP-JRFL using PEI as a transfection reagent at a DNA/PEI ratio of 1:3. After 3 days, virus-containing cell culture-supernatant fluid was harvested, concentrated, and stored at −80°C. Infectious units per mL of HIV-1iGFP/JRFL virions were determined by virus titration on TZM-bl luciferase reporter cells. Luminescence in relative light units (RLU) was measured using a Synergy 2 microplate luminometer (BioTek).
Virion capture assay.
HIV-1 capture assays were carried out as previously described (9). Briefly, ELISA plates were coated with 250 ng/well of a goat anti-human IgG gamma chain-specific antibody (Jackson ImmunoResearch). Plates were then washed, blocked, and further incubated with 100 ng/well of HIV-1-specific antibodies. After washing, HIV-1iGFP/JRFL (10 ng of p24/well) was added for 4 h, and the p24 content captured on the plate was directly measured with a p24 ELISA. Samples were analyzed in triplicate, and assays were repeated three times. Monomeric and multimeric VRC01 variants were compared using equal weight/volume concentrations to keep the number of available binding sites proportional.
Fc gamma receptor IIA (FcγRIIA) virion capture assay.
ELISA plates were coated with a mouse anti 6×-His tag-specific antibody (250 ng per well). VRC01 variants (2 μg/mL) were mixed with HIV-1 virions (10 ng of p24/well) diluted in medium with guinea pig serum (10%) or heat-inactivated guinea pig serum and incubated at 37°C for 30 min to form MAb-HIV-1 immune complexes. The immune complexes were further incubated for 90 min at 37°C with soluble His-tagged human FcγRIIA (4 μg/mL). Next, the immune complex/FcγRIIA mixture was transferred into blocked (5% blocking buffer) and washed anti-His tag antibody-coated plates and incubated for 90 min at 37°C. Plates were then washed five times with DPBS. Bound HIV-1 virions were lysed with viral RNA lysis buffer and quantified by HIV-1 p24 ELISA. All antibodies were tested in triplicate. The assay was repeated three times.
HIV-1 p24 ELISA.
The HIV-1 p24 content of all viral preparations was determined using the RETRO-TEK HIV-1 p24 antigen ELISA kit (ZeptoMetrix) following the manufacturer’s instructions. Levels of p24 were calculated by a point-to-point algorithm.
Human polymorphonuclear neutrophil isolation.
Human peripheral neutrophils were isolated as previously described (30). In brief, blood from healthy HIV-1-negative donors was layered onto neutrophil isolation medium (Lympholyte-Poly; Cedarlane). After centrifugation, neutrophil-containing layers were isolated, washed, and resuspended in red cell lysis buffer (Santa Cruz). Neutrophils were washed once more and resuspended in the respective assay medium (RPMI, Pen/Strep, 20 mM glutamine) with active or heat-inactivated (1 h at 56°C) 10% guinea pig serum for subsequent experiments. To inhibit neutrophil extracellular trap (NET) release, cells were pretreated with 50 μM diphenyleneiodonium chloride (DPI) 30 min prior to phagocytosis assays. Neutrophils were used in the antibody-dependent internalization assays the same day as isolation.
Human monocyte isolation.
Human peripheral blood mononuclear cells were isolated from healthy, HIV-1-negative donors with a density gradient medium (Lymphoprep) according to the manufacturer’s instructions (StemCell). Monocytes were separated using human CD14 MicroBeads according to the manufacturer’s instructions (Miltenyi). Positively selected monocytes were subsequently resuspended in assay medium (RPMI, Pen/Strep, 20 mM glutamine) supplemented with active or inactive guinea pig serum (10%) and used the same day in antibody-dependent internalization assays.
Antibody-dependent internalization assay.
Note that we use the term “internalization,” rather than “endocytosis” or “phagocytosis” to refer to the uptake of opsonized virus particles, since we do not know the size of the particles. Phagocytosis usually refers to the internalization of particles >0.5 μm, whereas endocytosis refers to the internalization of particles <0.5 μm (31). Internalization assays were performed as previously described with some minor modifications (9). Assay medium (RPMI, Pen/Strep, 20 mM glutamine) containing active or inactive guinea pig serum (10%) was used for all internalization assays. HIV-1iGFP/JRFL virions (20 ng p24/reaction) were opsonized for 120 min at 37°C with monoclonal (50 μg/mL) or polyclonal antibody preparations (200 μg/mL) in a 1:1 (vol/vol) ratio. For immune complex formation with MAbs NIH45-46 G54W (NIH45-46 G54WIC) and VRC01 (VRC01IC), 5 μg of a goat anti-human Fc gamma fragment was added after 30 min to the respective wells. Subsequently, the antibody-opsonized HIV-1 virions were incubated with DPI-treated neutrophils or monocytes (150,000 cells per well) for 75 min at 37°C. After internalization, cells were washed three times with ice-cold DPBS and fixed in 4% paraformaldehyde (PFA). Neutrophils and monocytes were analyzed by flow cytometry on a NovoCyte flow cytometer (ACEA) for internalized HIV-1iGFP/JRFL virions. A virion internalization score was determined by gating the samples on events representing live cells and calculating as follows: percent green fluorescent protein (GFP) positive × median fluorescence intensity (MFI) GFP positive. Virus internalization experiments were performed at least five times with antibody samples tested in triplicate.
Statistical analysis.
All statistics were performed using Graph Pad Prism 9.2. For paired-group analyses, we used Wilcoxon matched-pair signed rank tests. For unpaired group analyses, we used Mann-Whitney rank tests. For multiple-group comparisons, Kruskal-Wallis tests followed by Dunn’s correction were used.
ACKNOWLEDGMENT
The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI118581.
Contributor Information
Johannes S. Gach, Email: jgach@uci.edu.
Donald N. Forthal, Email: dnfortha@uci.edu.
Guido Silvestri, Emory University.
REFERENCES
- 1.French MA, Tjiam MC, Abudulai LN, Fernandez S. 2017. Antiviral functions of human immunodeficiency virus type 1 (HIV-1)-specific IgG antibodies: effects of antiretroviral therapy and implications for therapeutic HIV-1 vaccine design. Front Immunol 8:780. 10.3389/fimmu.2017.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forthal DN. 2014. Functions of antibodies. Microbiol Spectr 2:AID-0019-2014. 10.1128/microbiolspec.AID-0019-2014. [DOI] [PubMed] [Google Scholar]
- 3.Bradley T, Pollara J, Santra S, Vandergrift N, Pittala S, Bailey-Kellogg C, Shen X, Parks R, Goodman D, Eaton A, Balachandran H, Mach LV, Saunders KO, Weiner JA, Scearce R, Sutherland LL, Phogat S, Tartaglia J, Reed SG, Hu SL, Theis JF, Pinter A, Montefiori DC, Kepler TB, Peachman KK, Rao M, Michael NL, Suscovich TJ, Alter G, Ackerman ME, Moody MA, Liao HX, Tomaras G, Ferrari G, Korber BT, Haynes BF. 2017. Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge. Nat Commun 8:15711. 10.1038/ncomms15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng'ang'a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. 2015. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349:320–324. 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corey L, Gilbert PB, Tomaras GD, Haynes BF, Pantaleo G, Fauci AS. 2015. Immune correlates of vaccine protection against HIV-1 acquisition. Sci Transl Med 7:310rv7. 10.1126/scitranslmed.aac7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tay MZ, Wiehe K, Pollara J. 2019. Antibody-dependent cellular phagocytosis in antiviral immune responses. Front Immunol 10:332. 10.3389/fimmu.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorny MK. 2020. Search for antiviral functions of potentially protective antibodies against V2 region of HIV-1. Hum Vaccin Immunother 16:2033–2041. 10.1080/21645515.2020.1787070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gach JS, Bouzin M, Wong MP, Chromikova V, Gorlani A, Yu KT, Sharma B, Gratton E, Forthal DN. 2017. Human immunodeficiency virus type-1 (HIV-1) evades antibody-dependent phagocytosis. PLoS Pathog 13:e1006793. 10.1371/journal.ppat.1006793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O'Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. 2011. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol 85:9998–10009. 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, Yamamoto N, Akira S. 2012. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 12:109–116. 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 12.de Jong RN, Beurskens FJ, Verploegen S, Strumane K, van Kampen MD, Voorhorst M, Horstman W, Engelberts PJ, Oostindie SC, Wang G, Heck AJ, Schuurman J, Parren PW. 2016. A novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLoS Biol 14:e1002344. 10.1371/journal.pbio.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, de Jong RN, van den Bremer ET, Beurskens FJ, Labrijn AF, Ugurlar D, Gros P, Schuurman J, Parren PW, Heck AJ. 2016. Molecular basis of assembly and activation of complement component C1 in complex with immunoglobulin G1 and antigen. Mol Cell 63:135–145. 10.1016/j.molcel.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Powell RLR, Fox A, Itri V, Zolla-Pazner S. 2019. Primary human neutrophils exhibit a unique HIV-directed antibody-dependent phagocytosis profile. J Innate Immun 11:181–190. 10.1159/000494371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivas-Fuentes S, García-García E, Nieto-Castañeda G, Rosales C. 2010. Fcgamma receptors exhibit different phagocytosis potential in human neutrophils. Cell Immunol 263:114–121. 10.1016/j.cellimm.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Kerntke C, Nimmerjahn F, Biburger M. 2020. There is (scientific) strength in numbers: a comprehensive quantitation of Fc gamma receptor numbers on human and murine peripheral blood leukocytes. Front Immunol 11:118. 10.3389/fimmu.2020.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg BS, Ackerman ME. 2020. Antibody-mediated complement activation in pathology and protection. Immunol Cell Biol 98:305–317. 10.1111/imcb.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook EM, Lindorfer MA, van der Horst H, Oostindie S, Beurskens FJ, Schuurman J, Zent CS, Burack R, Parren PW, Taylor RP. 2016. Antibodies that efficiently form hexamers upon antigen binding can induce complement-dependent cytotoxicity under complement-limiting conditions. J Immunol 197:1762–1775. 10.4049/jimmunol.1600648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, van de Winkel JG, Wilson IA, Koster AJ, Taylor RP, Saphire EO, Burton DR, Schuurman J, Gros P, Parren PW. 2014. Complement is activated by IgG hexamers assembled at the cell surface. Science 343:1260–1263. 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlothauer T, Herter S, Koller CF, Grau-Richards S, Steinhart V, Spick C, Kubbies M, Klein C, Umaña P, Mössner E. 2016. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng Des Sel 29:457–466. 10.1093/protein/gzw040. [DOI] [PubMed] [Google Scholar]
- 22.Hezareh M, Hessell AJ, Jensen RC, van de Winkel JG, Parren PW. 2001. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J Virol 75:12161–12168. 10.1128/JVI.75.24.12161-12168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. 2015. Complement system part I: molecular mechanisms of activation and regulation. Front Immunol 6:262. 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster SD, Galvan MD, Ferran E, Garzon-Rodriguez W, Glabe CG, Tenner AJ. 2001. Antibody-mediated phagocytosis of the amyloid beta-peptide in microglia is differentially modulated by C1q. J Immunol 166:7496–7503. 10.4049/jimmunol.166.12.7496. [DOI] [PubMed] [Google Scholar]
- 25.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5:e1000433. 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelman GM, Cunningham BA, Gall WE, Gottlieb PD, Rutishauser U, Waxdal MJ. 1969. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci USA 63:78–85. 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gach JS, Gorlani A, Dotsey EY, Becerra JC, Anderson CT, Berzins B, Felgner PL, Forthal DN, Deeks SG, Wilkin TJ, Casazza JP, Koup RA, Katlama C, Autran B, Murphy RL, Achenbach CJ. 2016. HIV-1-specific antibody response and function after DNA prime and recombinant adenovirus 5 boost HIV vaccine in HIV-infected subjects. PLoS One 11:e0160341. 10.1371/journal.pone.0160341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gach JS, Furtmüller PG, Quendler H, Messner P, Wagner R, Katinger H, Kunert R. 2010. Proline is not uniquely capable of providing the pivot point for domain swapping in 2G12, a broadly neutralizing antibody against HIV-1. J Biol Chem 285:1122–1127. 10.1074/jbc.M109.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dotsey EY, Gorlani A, Ingale S, Achenbach CJ, Forthal DN, Felgner PL, Gach JS. 2015. A high throughput protein microarray approach to classify HIV monoclonal antibodies and variant antigens. PLoS One 10:e0125581. 10.1371/journal.pone.0125581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh H, Siano B, Diamond S. 2008. Neutrophil isolation protocol. J Vis Exp 2008:745. 10.3791/745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rennick JJ, Johnston APR, Parton RG. 2021. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat Nanotechnol 16:266–276. 10.1038/s41565-021-00858-8. [DOI] [PubMed] [Google Scholar]