ABSTRACT
The public health burden caused by influenza virus infections is not adequately addressed with existing vaccines and antivirals. Identifying approaches that interfere with human-to-human transmission of influenza viruses remains a pressing need. The importance of neuraminidase (NA) activity for the replication and spread of influenza viruses led us to investigate whether broadly reactive human anti-NA monoclonal antibodies (MAbs) could affect airborne transmission of the virus using the guinea pig model. In that model, infection with recent influenza virus clinical isolates resulted in 100% transmission from inoculated donors to recipients in an airborne transmission setting. Anti-NA MAbs were administered either to the inoculated animals on days 1, 2, and 4 after infection or to the naive contacts on days 2 and 4 after donor infection. Administration of NA-1G01, a broadly cross-reactive anti-NA MAb, to either the donor or recipient reduced transmission of the A/New York City/PV02669/2019 (H1N1) and A/New York City/PV01148/2018 (H3N2) viruses. Administration of 1000-3C05, an anti-N1 MAb, to either the donor or recipient reduced transmission of A/New York City/PV02669/2019 (H1N1) virus but did not reduce transmission of A/New York City/PV01148 (H3N2) virus. Conversely, 229-2C06, an anti-N2 MAb, reduced transmission of A/New York City/PV01148 (H3N2) but did not impact transmission of A/New York City/PV02669/2019 (H1N1) virus. Our work demonstrates that anti-NA MAbs could be further developed into prophylactic or therapeutic agents to prevent influenza virus transmission to control viral spread.
IMPORTANCE The burden of influenza remains substantial despite unremitting efforts to reduce the magnitude of seasonal influenza epidemics and prepare for pandemics. Although vaccination remains the mainstay of these efforts, current vaccines are designed to stimulate an immune response against the viral hemagglutinin. Interest in the role immunity against neuraminidase plays in influenza virus infection and transmission has recently surged. Human antibodies that bind broadly to neuraminidases of diverse influenza viruses and protect mice against lethal viral challenge have previously been characterized. Here, we show that three such antibodies inhibit the neuraminidase activity of recent isolates and reduce their airborne transmission in a guinea pig model. In addition to contributing to the accumulating support for incorporating neuraminidase as a vaccine antigen, these findings also demonstrate the potential of direct administration of anti-neuraminidase antibodies to individuals infected with influenza virus and to individuals for postexposure prophylaxis to prevent the spread of influenza virus.
KEYWORDS: influenza, transmission, guinea pig, NA antibodies
INTRODUCTION
Seasonal influenza virus infections cause significant global morbidity and mortality annually. In addition, pandemics occur at irregular and unpredictable intervals and can claim millions of lives. Current seasonal influenza virus vaccines induce narrow, strain-specific immune responses and have variable effectiveness, ranging from low to moderate (1), depending on how well they match circulating strains (2). Although, effectiveness against influenza B viruses is generally higher than effectiveness observed for influenza A virus strains (3). Antivirals can also be prescribed to limit influenza symptoms and reduce viral spread. However, the threat of drug resistance remains a constant concern. With the exception of baloxavir marboxil, all antiviral medications currently used for influenza prevention and treatment are small molecule neuraminidase (NA) inhibitors (NAIs). Although this near-monopoly validates the clinical prophylactic and therapeutic effectiveness of agents that have NA inhibition (NI) activity, it also leaves us extremely vulnerable if influenza viruses with drug resistance begin to spread extensively among humans. As demonstrated by the amantadines, a class of antivirals that specifically inhibits influenza A viruses by disrupting the function of the M2 ion channel, this phenomenon can occur rapidly and render an entire class of drugs obsolete from one influenza season to the next (4). Until the first global approval in 2018 of baloxavir marboxil, a first-in-class small molecule inhibitor of the influenza virus cap-dependent endonuclease, an enzyme function of the polymerase acidic (PA) subunit of the viral polymerase, NAIs were our sole option for influenza prophylaxis and treatment for years, and NAI-resistant H1N1 strains began to surge in prevalence shortly before they were completely replaced by 2009 pandemic H1N1 viruses (4, 5). The emergence of drug-resistant strains constitutes a looming threat that can be ameliorated by using combination therapy rather than monotherapy and by diversifying our antiviral arsenal.
Monoclonal antibodies (MAbs) as biopharmaceuticals are a rapidly emerging class of drugs and have a robust pipeline due to increasing research and development in innumerable areas of biomedical science. The ongoing coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in rapid development, testing, and emergency use authorization of antibody-based treatments, including convalescent plasma and MAbs (6). Advances in MAb engineering and production technologies have made this surge in antibody-based therapeutics possible. MAbs demonstrate significant promise in alleviating the burden of influenza. The increasing portfolio of broadly protective MAbs that target various conserved epitopes of influenza virus proteins combined with the substantial resources and knowledge developed in other fields represent novel opportunities to discover urgently needed breakthroughs. Using therapeutics that exert distinct pressures on the influenza virus will limit the available mechanisms of viral escape. Anti-NA MAbs have antiviral functions that correlate with their ability to protect against influenza viruses. They play a major role at the later stages of viral replication, specifically when the influenza virion buds off from an infected cell (7). During the final stages of viral replication, the NA enzymatically cleaves off sialic acid residues on the host cell surface, releasing virus progeny. It is at this point that most of the anti-NA MAbs inhibit viral egress (8, 9). Since NA MAbs are mostly effective during viral egress, virus titer is not generally affected in an in vitro plaque reduction assay (10–13). However, plaque diameter is significantly reduced in the presence of anti-NA MAbs (11–13). Therefore, most of the MAbs against the NA are considered non-neutralizing but prevent release and spread of virions from the host cell by inhibiting the enzymatic activity of NA (14). Furthermore, some NA-specific MAbs also activate antibody effector functions, such as antibody-dependent cellular cytotoxicity (ADCC), through the engagement of their fragment crystallizable (Fc) regions with the Fc receptors of immune cells (15–18). These attributes indicate the untapped promise of anti-NA MAbs in the control of influenza virus infection.
We previously published the first characterization of broadly reactive MAbs that target the NA, describing murine MAbs that bound to influenza B virus NAs across more than 70 years of antigenic evolution and conferred potent protection from lethal influenza B virus challenge in mice (19). Subsequently, broadly protective anti-NA human Abs have also been identified (15, 17, 19). Given the attractive therapeutic potential of anti-NA antibodies in protecting against influenza virus infection and the previous association of anti-NA immunity with reduced shedding (20–22), we sought to understand the potential of therapeutic administration of anti-NA MAbs in preventing influenza virus transmission. In this study, we evaluated the potential of three human broadly reactive anti-NA MAbs in reducing airborne influenza virus transmission in the guinea pig model of influenza virus transmission. The NA-1G01 MAb displays universal reactivity against a diverse range of NAs, including NAs from influenza B viruses (17). The 229-2C06 and 1000-3C05 MAbs have more limited binding profiles but cover N2 and N1 NAs, respectively (15, 28). We tested the impact of intranasal administration of these MAbs in donors after infection with recent human isolates A/New York City/PV02669/2019 (H1N1) and A/New York City/PV01148/2018 (H3N2) and in recipients after exposure to donors infected with these viruses. We found that donor and recipient administration of a relevant anti-NA MAb reduces airborne transmission of these recent clinical isolates in the guinea pig model. Our work promotes the further investigation of anti-NA MAbs as potential therapeutics to control and limit influenza virus transmission.
RESULTS
Broad anti-NA MAbs inhibit NA activity of recent H1N1 and H3N2 isolates.
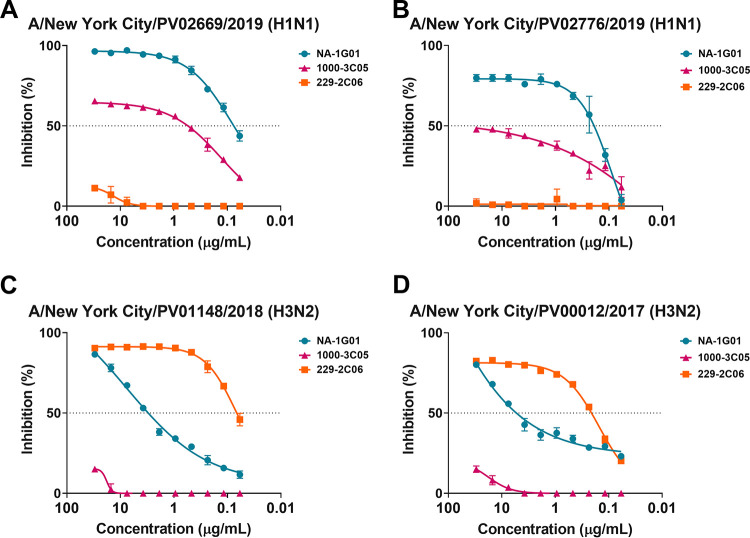
Human anti-NA MAbs that have broad binding and potent NI activity should be further investigated for their therapeutic potential against recently circulating influenza virus strains. The Personalized Virology Initiative, a component of the Pathogen Surveillance Program at the Icahn School of Medicine at Mount Sinai, provided four recent human influenza A virus isolates (A/New York City/PV02669/2019 (H1N1), A/New York City/PV02676/2019 (H1N1), A/New York City/PV01148/2018 (H3N2, H3 HA clade 3c.2a.2), and A/New York City/PV00012/2018 (H3N2, H3 HA clade 3c.2a.3)) for these studies. Of note, the H3N2 virus isolates had an N-linked glycosylation site at position 245 which has been shown to partially block antibody binding (22). To assess the potential applicability of the broad anti-NA MAbs NA-1G01 (pan-NA), 229-2C06 (pan-N2), and 1000-3C05 (pan-N1) in the context of viruses circulating recently in humans, we examined their ability to inhibit NA activity of these viruses. As previous studies suggest (15, 17), the NA-1G01 (pan-NA) and 1000-3C05 (anti-N1) MAbs retained the ability to inhibit the N1 of the recent A/New York City/PV02669/2019 (H1N1) (Fig. 1A) and A/New York City/PV02676/2019 (H1N1) (Fig. 1B) viruses. When testing NI of the N2-containing viruses A/New York City/PV01148/2018 (H3N2) and A/New York City/PV00012/17 (H3N2), we found that the NA-1G01 and 229-2C06 MAbs retained the ability to inhibit the activity of recent N2 NAs (Fig. 1C and D). The NI results for the N2-containing viruses complement previous work addressing the binding/inhibition of recent antigenically distinct H3N2 viruses by NA-1G01 (Stadlbauer et al., unpublished) (23).
FIG 1.
Anti-NA MAbs inhibit NA activity of recent clinical isolates. NI of A/New York/City/PV02669/2019 (H1N1) (A), A/New York/City/PV02676/2019 (H1N1) (B), A/New York City/PV01148/2018 (H3N2) (C), and A/New York City/PV00012/17 (H3N2) (D) by NA-1G01 (pan-NA), 1000-3C05 (anti-N1), and 229-2C06 (anti-N2) as measured in NI assays.
Airborne transmission of recent H1N1 and H3N2 isolates occurs in the guinea pig model.
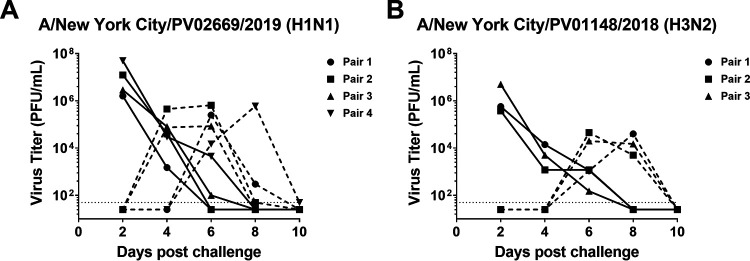
Our ultimate aim in these studies was to further define the therapeutic potential of anti-NA MAbs by assessing their ability to inhibit transmission of more recent influenza virus strains. After determining that the selected anti-NA MAbs retained inhibition of the recent clinical isolates (Fig. 1), we next wanted to assess the ability of these viruses to transmit in the guinea model of influenza virus transmission, as transmission of more recent influenza virus strains in this model has not yet been described. We elected to assess the transmission of the A/New York City/PV02669/2019 (H1N1) and A/New York City/PV01148/2018 (H3N2) viruses in the first instance as these viruses grew to higher titers in Madin-Darby canine kidney (MDCK) cells, allowing the generation of viral stocks with higher quantities of infectious material for animal infection studies. These viruses were tested without prior adaptation in a guinea pig model of airborne influenza virus transmission where the donor and recipients are housed in separate cages that do not allow physical contact but provide lateral airflow between cages (24–26). Productive infection was observed in all donor guinea pigs directly inoculated with 104 PFU of A/New York City/PV02669/2019 (H1N1) virus (n = 4) (Fig. 2A) or 105 PFU of A/New York City/PV01148/2018 (H3N2) (Fig. 2B) virus (n = 3) and resulted in subsequent transmission to all exposed recipients.
FIG 2.
Recent H1N1 and H3N2 clinical isolates exhibit airborne transmission in the guinea pig model of influenza virus transmission. Guinea pigs were intranasally infected with A/New York City/PV02669/2019 (H1N1) (n = 4 transmission pairs) (A) or A/New York City/PV01148/2018 (H3N2) (n = 3 transmission pairs) (B) and transmission from donor guinea pigs to recipient guinea pigs was determined by assessing virus titers in nasal wash samples collected at days 2, 4, 6, 8, and 10 postinfection. Complete lines represent donors. Dashed lines represent recipients.
Administration of human anti-NA MAbs to infected donor guinea pigs reduces airborne transmission to exposed recipients.
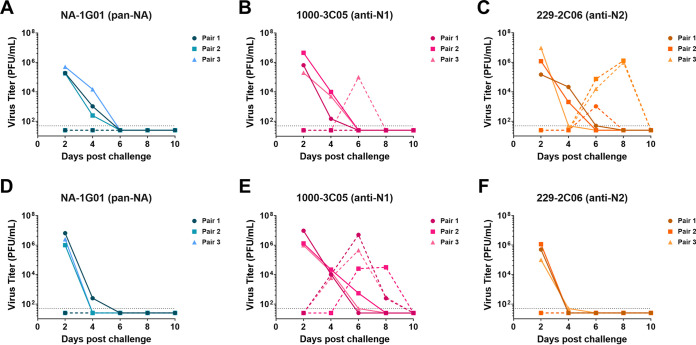
After confirming the ability of human anti-NA MAbs NA-1G01 (pan-NA), 229-2C06 (pan-N2), and 1000-3C05 (pan-N1) to inhibit the NA activity of the recent clinical isolates (Fig. 1), and determining that recent clinical isolates A/New York City/PV02669/2019 (H1N1) and A/New York City/PV01148/2018 (H3N2) transmit in the guinea pig model of airborne influenza virus transmission (Fig. 2), we next wanted to evaluate the therapeutic potential of these MAbs in preventing influenza virus transmission. Here, donor guinea pigs were intranasally inoculated with 104 PFU of A/New York City/PV02669/2019 (H1N1) virus or 105 PFU of A/New York City/PV01148/2018 (H3N2) virus. Donor guinea pigs were then intranasally administered 10 mg/kg of MAb NA-1G01 (pan-NA), 1000-3C05 (anti-N1), or 229-2C06 (anti-N2) on days 1, 2, and 4 postinfection. Transmission pairs were set up 1 day postdonor infection with recipient guinea pigs housed in cages that precluded physical contact between donor and recipient animals while allowing lateral airflow from the donor cages to the recipient cages.
In donors infected with A/New York City/PV0266/2019 (H1N1), we found that MAb administration of NA-1G01 (pan-NA) resulted in reduced virus titers in treated donors when compared with administration of 1000-3C05 (anti-N1) or 229-2C06 (anti-N2) (Fig. 3A to C). When assessing transmission of A/New York City/PV02669/2019 (H1N1) from infected donors to uninfected recipients, we found that donors administered N1-inhibiting MAbs transmitted virus at reduced rates to untreated recipients (Fig. 3A to C). In donors infected with A/New York City/PV01148/2018 (H3N2), we found that MAb administration of NA-1G01 (pan-NA) or 229-2C06 (anti-N2) resulted in reduced virus titers in treated donors at day 4 or at days 2 and 4 postinfection, respectively, compared with administration of 1000-3C05 (anti-N1) (Fig. 3D to F). When assessing transmission of A/New York City/PV01148/2018 (H3N2) from infected donors to uninfected recipients, we found that administration of N2-inhibiting MAbs to infected donors was able to prevent transmission to untreated recipients (Fig. 3D to F). Our results demonstrate that intranasal MAb administration to influenza virus-infected donor guinea pigs prevented airborne transmission to untreated recipient guinea pigs.
FIG 3.
MAb administration to influenza virus-infected donors reduces airborne influenza virus transmission to recipients. Donor guinea pigs were infected with either 104 PFU of A/New York City/PV02669/2019 (H1N1) (n = 3 transmission pairs) (A–C) or 105 PFU of A/New York City/PV01148/2018 (H3N2) (n = 3 transmission pairs) (D-F). Transmission pairs were set up on day 1 postinfection such that donors and recipients could not make physical contact while air flowed laterally from donor cages to recipient cages. On days 1, 2, and 4 postinfection, donor guinea pigs were intranasally administered NA-1G01 (pan-NA) (A, D), 1000-3C05 (anti-N1) (B, E), or 229-2C06 (anti-N2) (C, F) MAb. Transmission from donor guinea pigs to recipient guinea pigs was determined by assessing virus titers in nasal wash samples collected at days 2, 4, 6, 8, and 10 postinfection. Complete lines represent donors. Dashed lines represent recipients.
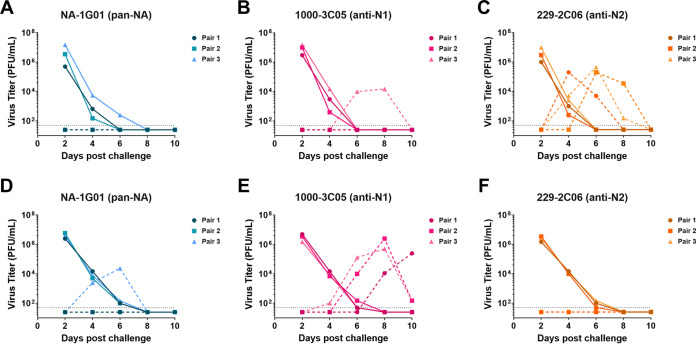
Administration of human anti-NA MAbs to exposed recipient guinea pigs reduces airborne transmission from infected donors.
After assessing the impact of anti-NA MAbs on influenza virus transmission from MAb-treated donors to untreated recipients (Fig. 3), we next wanted to determine if administration of MAbs to recipient guinea pigs would prevent these animals from becoming infected with influenza virus in an airborne transmission setting. Here we found that virus transmitted from A/New York City/PV02669/2019 (H1N1)-infected donors to recipient guinea pigs that intranasally received NA-1G01 (pan-NA), 1000-3C05 (anti-N1) or 229-2C06 (anti-N2) MAbs in 0/3, 1/3, and 3/3 transmission pairs, respectively (Fig. 4A to C). In addition, we found that virus transmitted from A/New York City/PV01148/2018 (H3N2)-infected donors to recipient guinea pigs that intranasally received NA-1G01 (pan-NA), 1000-3C05 (anti-N1), or 229-2C06 (anti-N2) MAbs in 1/3, 3/3, and 0/3 transmission pairs, respectively (Fig. 4D-F). Our results demonstrate that intranasal MAb administration to recipient guinea pigs housed adjacent to influenza virus-infected donors limits airborne transmission.
FIG 4.
Intranasal anti-NA MAb administration to recipient guinea pigs reduces airborne influenza virus transmission from infected donors. Donor guinea pigs were infected with either 104 PFU of A/New York City/PV02669/2019 (H1N1) (n = 3 transmission pairs) (A–C) or 105 PFU of A/New York City/PV01148/2018 (H3N2) (n = 3 transmission pairs) (D–F). Transmission pairs were set up on day 1 postinfection such that donors and recipients could not make physical contact while air flowed laterally from donor cages to recipient cages. On days 2 and 4 after donor infection, recipient guinea pigs were intranasally administered NA-1G01 (pan-NA) (A, D), 1000-3C05 (anti-N1) (B,E), or 229-2C06 (anti-N2) (C, F) MAb. Transmission from donor guinea pigs to recipient guinea pigs was determined by assessing virus titers in nasal wash samples collected at days 2, 4, 6, 8, and 10 postinfection. Complete lines represent donors. Dashed lines represent recipients.
DISCUSSION
In this study, we evaluated the impact of three broadly reactive anti-NA MAbs on the airborne transmission of two recent influenza A virus isolates in the guinea pig model of influenza virus transmission (27). We demonstrate for the first time that administration of a relevant anti-NA MAb can reduce airborne transmission of influenza viruses. In our guinea pig model of airborne transmission, we found that NI activity predicted the ability of an anti-NA MAb to prevent transmission between infected donors and exposed recipients. Treatment of either donor or recipient with NA-1G01 reduced transmission of both viruses tested, treatment with 229-2C06 reduced transmission of the A/New York City/PV01148/2018 (H3N2) virus, and treatment with 1000-3C05 reduced transmission of the A/New York City/PV02669/2019 (H1N1) virus. Treatment with an irrelevant MAb, 229-2C06 in the context of the A/New York City/PV02669/2019 (H1N1) virus and 1000-3C05 in the context of the A/New York City/PV01148/2018 (H3N2) virus, allowed transmission between 100% of the pairs (3/3 for each virus), while treatment with a relevant MAb reduced transmission events to 0/3 or 1/3.
This effect occurred with postinfection administration of anti-NA MAbs in donors, even though treated animals continued to demonstrate comparable viral titers as those seen in donors receiving an irrelevant MAb. Additional work needs to be conducted to examine the mechanisms by which delayed anti-NA MAb delivery to infected donors reduces airborne transmission even after the establishment of robust infection as was observed in this study. Perhaps, intranasal MAb administration decreases or otherwise impacts the egress of influenza virus particles and subsequent airborne release by inhibiting NA activity (27). Many studies describe the effect of anti-NA MAbs on plaque size (i.e., reducing the amount of virus that is able to infect adjacent cells) (18), however the translation of these observations into, e.g., reduction of infectious virus per respiratory droplet have not been described. The similar decreases observed in airborne transmission when treating recipient animals with relevant anti-NA MAbs 24 h after exposure also warrant further investigation. According to our data, at the time when recipients received the first MAb treatment, the infected donors had already reached peak levels of viral shedding. As anti-NA antibodies are active in some neutralization, NI assays and ADCC reporter assays, how the anti-NA MAbs provide protection from transmission in this context may provide useful insights that have implications on the use of current prophylactic agents, many of which function by inhibiting NA activity, or reveal a unique and useful feature of anti-NA MAbs.
Influenza virus transmission in animals is commonly assessed in airborne or contact settings. In airborne transmission studies, such as the ones described in this study, the donor and recipients are housed in cages that do not allow physical contact but provide lateral airflow between paired animals. In contact transmission studies, the donor and recipient animals are co-caged and allowed to directly interact with each other. Unsurprisingly, enhanced influenza virus transmission is more frequently observed in transmission studies conducted in contact settings (24). Whether postinfection anti-NA MAb treatment of donors or postexposure anti-MAb treatment of recipients is sufficient in reducing influenza virus transmission between co-caged animals requires further investigation.
Our study assessed the administration of anti-NA MAbs in a therapeutic setting when MAbs were given at multiple time points postinfection to donor or recipient guinea pigs. The administration of MAbs over multiple time points may be too costly for appreciating their full potential as therapeutic agents. As such, the therapeutic development of anti-NA MAbs in the prevention of influenza virus transmission experiments could be strengthened by assessing different dosing regimens. In our current study, we administered MAb at 10 mg/kg at days 1, 2, 4, and 6 postchallenge in the donors (and days 2, 4, and 6 in the recipients). Using this concentration and number of time points could prove a costly affair for the further development of anti-NA MAbs as therapeutics. Different dosing regimens could examine the minimal dose required to prevent transmission. A single administration of MAb at a certain time point (e.g., day 2) may also sufficient to prevent transmission from a MAb administered donor to a recipient guinea pig, or to a MAb administered recipient guinea pig. In addition, prophylactic administration of MAbs where MAbs are given prior to infection of donor guinea pigs or prior to recipient guinea pig exposure to infected donors could be studied. Evaluating this would allow us to determine if a single dose could prevent recipient guinea pigs from becoming infected from donor guinea pigs. Although we did not address these points in our current study, assessing these could help further promote the development of therapeutic MAbs that prevent influenza virus transmission.
Anti-NA MAbs that can inhibit the NA activity of circulating viruses might be leveraged in reducing airborne transmission of influenza viruses both by treating infected individuals even at the peak of viral shedding and by treating individuals as a pre- or postexposure prophylaxis. For example, the use of nasal sprays to administer MAbs at the beginning of and during the influenza season could protect individuals from influenza virus infection, particularly if they are unable to be vaccinated or in the case of vaccine-mismatch. Our results also strengthen the case for using NA as a vaccine target that may induce broad protection and reduce viral transmission. With the constant threat that drug-resistant strains may begin to circulate widely, diversifying the repertoire of agents that can be used for the prevention of influenza has obvious benefits.
MATERIALS AND METHODS
Cells.
MDCK cells were grown and maintained at 37°C in 5% CO2 in complete Dulbecco’s modified Eagle’s medium, comprising Dulbecco’s modified Eagle’s medium (Gibco, Cat #11965) supplemented with penicillin-streptomycin (100 U/ml penicillin, 100 μg/ml streptomycin) (Gibco, Cat #15140), 10% fetal bovine serum (FBS) (Gibco, Cat #10082147), and 0.01M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (Gibco, Cat #15630). Expi293F cells (Gibco, Cat #A14527) were grown and maintained in Expi293 Expression Medium (Gibco Cat #A1435101) in a shaking incubator (37°C, 8% CO2, 125 RPM).
Viruses.
Nasopharyngeal swabs from patients with influenza virus infections seeking care at the Mount Sinai Health System were collected by the Mount Sinai Pathogen Surveillance Program (IRB HS#13-00981). Whole viral genome sequencing was performed as part of the Centers of Excellence for Influenza Virus Research and Surveillance.
Selected clinical isolates A/New York City/PV02669/2019 (H1N1pdm09), A/New York City/PV02676/2019 (H1N1pdm09), A/New York City/PV01148/2018 (H3N2) (H3 HA clade 3c.2a.2), and A/New York City/PV00012/2017 (H3N2) (H3 HA clade 3c.2a.3) were grown in MDCK cells. The H3N2 virus isolates had an N-linked glycosylation at position 245 which has been shown to partially block antibody binding (22). Briefly, MDCK cells were infected with virus at a multiplicity of infection (MOI) of 0.01 in 1× minimum essential medium (MEM) comprising 10% 10× MEM (Gibco, Cat #11430030), 2 mM l-glutamine (Gibco, Cat #25030), 0.1% wt/vol sodium bicarbonate (Corning, Cat #25-035-Cl), 0.01 M HEPES buffer, penicillin-streptomycin (100 U/ml penicillin, 100 μg/ml streptomycin), and 0.2% bovine serum albumin (BSA) (MP Biomedical, Cat #ICN810063); 1 μg/ml tolylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Sigma-Aldrich, Cat #T6763); and 0.1% (wt/vol) diethylaminoethanol (DEAE)-dextran (MP Biomedicals, Cat #195133). Infected cells were incubated at 33°C with 5% CO2 for 72 h. After 72 h, the supernatant was collected, clarified by centrifugation, aliquoted, and stored at −80°C prior to determining stock titers via plaque assay as described below.
MAb production.
MAbs were produced by co-expressing heavy and light chain plasmids in Expi293F cells using the ExpiFectamineTM 293 Transfection Kit (Gibco, Cat #A14525) as per the manufacturer’s instructions. Supernatant was harvested 7 days after transfection, centrifuged at 4,000 × g at 4°C for 30 min, and filtered. Supernatant was then applied to a HiTrap Protein G HP column (Cytiva, Cat #17-0405-03) using an ÄKTA purification system for antibody purification.
Airborne transmission experiments.
Five- to 6-week-old female guinea pigs were purchased from Charles River Laboratory. Three transmission pairs (one donor and one recipient) were used for each MAb administration and virus challenge. Donor guinea pigs were randomly selected and anesthetized with ketamine (30 mg/kg) (KetaVed) and xylazine (5 mg/kg) (AnaSed Injection, Cat #NDC 59399-110-20) before being infected with 104 PFU of A/New York City/PV02669/2019 (H1N1) or 105 PFU of A/New York City/PV01148/2018 (H3N2) delivered intranasally in 300 μl sterile phosphate-buffered saline (PBS) (Gibco, Cat #10010) before being transferred into a climate-controlled transmission chamber (20°C, 20% relative humidity). Transmission pairs were set up the following day with recipient guinea pigs housed in cages that precluded physical contact between donor and recipient animals while allowing lateral airflow from the donor cages to the recipient cages to occur. On days 2, 4, 6, 8, and 10 postdonor infection, all guinea pigs were anesthetized with ketamine (30 mg/kg) and xylazine (5 mg/kg) and nasal washed with 1 ml sterile PBS. All guinea pigs were anesthetized with ketamine (44 mg/kg) and xylazine (5 mg/kg) and terminally bled 14–18 days postdonor infection.
The antibodies NA-1G01 (pan-NA) (16), 229-2C06 (anti-N2) (14), or 1000-3C05 (anti-N1) (14) were delivered intranasally to anesthetized animals at 10 mg/kg in 350 μl sterile PBS. To test antibody administration in donor guinea pigs, the infected donor guinea pigs received antibody on days 1, 2, and 4 after infection and were paired with untreated recipients. To test antibody administration in recipient guinea pigs, the recipient guinea pigs were given antibody on days 2 and 4 after the donor animals were infected (days 1 and 3 after exposure to the donor animal); in these experiments, donor guinea pigs did not receive MAbs.
All animal procedures were performed in accordance with the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee.
Plaque assays.
Virus titers were measured by performing plaque assays on MDCK cells seeded at 4 × 105 cells/ml in 12-well plates (Corning, Cat #3513). The plates were incubated overnight at 37°C with 5% CO2. The following day, nasal wash samples diluted serially by a factor of 10 were added to MDCK monolayers and incubated at 33°C for 1 h with shaking every 15 min before the inoculum in each well was replaced with an overlay containing 0.64% agar (Oxoid, Cat # LP0028); 1xMEM; 1 μg/ml TPCK-treated trypsin; and 0.1% (wt/vol) DEAE-dextran. The cells were then incubated for 72 h at 33°C with 5% CO2. Plaques were visualized by immunostaining with guinea pig polyclonal sera raised against either A/New York City/PV02669/2019 (H1N1) or A/New York City/PV01148/2018 (H3N2). The limit of detection for these plaque assays was 50 PFU/ml.
NA and NI assay.
To determine NA activity, samples were tested on flat-bottom Immunolon 4BX 96-well plates (ThermoScientific, Cat #3855) coated overnight at 4°C with 100 μl of fetuin (EMD Millipore, Cat #341506) at 25 μg/ml in PBS. Fetuin-coated plates were then washed 3x with PBS containing 0.1% Tween 20 (PBS-T) (Fisher, Cat #BP337). On a separate plate, viruses were serially diluted 2-fold in sample diluent (PBS supplemented with 0.9 mM CaCl2 (Sigma-Aldrich, Cat #C8106) and 0.5 mM MgCl2 (Sigma-Aldrich, Cat #M8266) supplemented with 1% BSA and 0.5% Tween 20 (Fisher Scientific)) and 100 μl of diluted virus samples were added to the washed fetuin-coated plates. The fetuin-coated plates were then incubated for 16–18 h at 37°C. Plates were washed 3× with PBS-T and 100 μl/well of horseradish peroxidase (HRP)-conjugated peanut agglutinin (PNA) (Sigma-Aldrich, Cat #L7759) in PBS were added to the plates. The plates were incubated for 2 h at room temperature before being washed 4× with PBS-T with shaking. To develop the plates, 100 μl of O-phenylenediamine dihydrochloride (OPD) substrate (Sigma-Aldrich, Cat #P9187) was added to each well. After a 10-minute incubation, the reaction was stopped by adding 50 μl of 3 M hydrochloric acid (Fisher Scientific, Cat #S25856) to each well. The optical density at 490 nm (OD490) was measured on a Synergy 4 plate reader (BioTek). The half maximal effective concentration (EC50) was determined using GraphPad Prism 8.
To measure NI, antibodies were serially diluted in sample diluent with a starting concentration of 30 μg/ml and incubated for 18 h at 37°C with an equal volume (50 μl) of the respective virus dilution in the fetuin-coated plates. The remainder of the assay was performed as described above. One column on the plate contained sample diluent without antibody and served as a positive (virus-only) control. Another column contained sample diluent only (no virus) and served as a negative (background) control. Data were analyzed in GraphPad Prism 8.
ACKNOWLEDGMENTS
This study was partially funded by the NIH/NIAID Centers of Excellence for Influenza Virus Research and Surveillance (CEIRS) under contract HHSN272201400008C.
The Icahn School of Medicine at Mount Sinai has filed patent applications regarding an influenza virus vaccine. F.K. is named as coinventor on these applications. Washington University has filed patent applications on the use of MAb 1G01, which name F.K. and A.E. as inventor.
M.M. and F.K. conceived the experimental questions; J.T., G.O., and M.M. conducted all animal experiments and assays; A.E., P.C.W., and V.S. provided antibodies or virus isolates; M.M. and J.T. analyzed data; J.T., F.K., and M.M. wrote the manuscript; all authors edited and reviewed the manuscript prior to submission.
Contributor Information
Florian Krammer, Email: florian.krammer@mssm.edu.
Meagan McMahon, Email: meagan.mcmahon@mssm.edu.
Stacey Schultz-Cherry, St. Jude Children's Research Hospital.
REFERENCES
- 1.Centers for Disease Control. 2020. CDC Seasonal Flu Vaccine Effectiveness Studies. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm?web=1&wdLOR=c05150D7B-8483-4D38-A562-E59CCC551B64.
- 2.Krammer F, Palese P. 2015. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov 14:167–182. 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 3.Flannery B, Chung JR, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Rolfes MA, Spencer S, Fry AM, US Flu VE Investigators. 2019. Influenza vaccine effectiveness in the United States during the 2016–2017 season. Clin Infect Dis 68:1798–1806. 10.1093/cid/ciy775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duwe S. 2017. Influenza viruses: Antiviral therapy and resistance. GMS Infect Dis 5:Doc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heo YA. 2018. Baloxavir: First global approval. Drugs 78:693–697. 10.1007/s40265-018-0899-1. [DOI] [PubMed] [Google Scholar]
- 6.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD. 2021. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 384:238–251. 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAuley JL, Gilbertson BP, Trifkovic S, Brown LE, McKimm-Breschkin JL. 2019. Influenza virus neuraminidase structure and functions. Front Microbiol 10:39. 10.3389/fmicb.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohlbold TJ, Krammer F. 2014. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 6:2465–2494. 10.3390/v6062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilchuk IM, Bangaru S, Gilchuk P, Irving RP, Kose N, Bombardi RG, Thornburg NJ, Creech CB, Edwards KM, Li S, Turner HL, Yu W, Zhu X, Wilson IA, Ward AB, Crowe JE. 2019. Influenza H7N9 virus neuraminidase-specific human monoclonal antibodies inhibit viral egress and protect from lethal influenza infection in mice. Cell Host Microbe 26:715–728. 10.1016/j.chom.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang L, Fantoni G, Couzens L, Gao J, Plant E, Ye Z, Eichelberger MC, Wan H. 2016. Comparative efficacy of monoclonal antibodies that bind to different epitopes of the 2009 pandemic H1N1 influenza virus neuraminidase. J Virol 90:117–128. 10.1128/JVI.01756-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan H, Gao J, Xu K, Chen H, Couzens LK, Rivers KH, Easterbrook JD, Yang K, Zhong L, Rajabi M, Ye J, Sultana I, Wan X-F, Liu X, Perez DR, Taubenberger JK, Eichelberger MC. 2013. Molecular basis for broad neuraminidase immunity: conserved epitopes in seasonal and pandemic H1N1 as well as H5N1 influenza viruses. J Virol 87:9290–9300. 10.1128/JVI.01203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohlbold TJ, Chromikova V, Tan GS, Meade P, Amanat F, Comella P, Hirsh A, Krammer F. 2016. Hemagglutinin stalk- and neuraminidase-specific monoclonal antibodies protect against lethal H10N8 influenza virus infection in mice. J Virol 90:851–861. 10.1128/JVI.02275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shcherbik S, Carney P, Pearce N, Stevens J, Dugan VG, Wentworth DE, Bousse T. 2018. Monoclonal antibody against N2 neuraminidase of cold adapted A/Leningrad/134/17/57 (H2N2) enables efficient generation of live attenuated influenza vaccines. Virology 522:65–72. 10.1016/j.virol.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couzens L, Gao J, Westgeest K, Sandbulte M, Lugovtsev V, Fouchier R, Eichelberger M. 2014. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J Virol Methods 210:7–14. 10.1016/j.jviromet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y-Q, Wohlbold TJ, Zheng N-Y, Huang M, Huang Y, Neu KE, Lee J, Wan H, Rojas KT, Kirkpatrick E, Henry C, Palm A-KE, Stamper CT, Lan LY-L, Topham DJ, Treanor J, Wrammert J, Ahmed R, Eichelberger MC, Georgiou G, Krammer F, Wilson PC. 2018. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 173:417–429. 10.1016/j.cell.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiLillo DJ, Palese P, Wilson PC, Ravetch JV. 2016. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest 126:605–610. 10.1172/JCI84428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadlbauer D, Zhu X, McMahon M, Turner JS, Wohlbold TJ, Schmitz AJ, Strohmeier S, Yu W, Nachbagauer R, Mudd PA, Wilson IA, Ellebedy AH, Krammer F. 2019. Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science 366:499–504. 10.1126/science.aay0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wohlbold TJ, Podolsky KA, Chromikova V, Kirkpatrick E, Falconieri V, Meade P, Amanat F, Tan J, tenOever BR, Tan GS, Subramaniam S, Palese P, Krammer F. 2017. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat Microbiol 2:1415–1424. 10.1038/s41564-017-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen A, Dai Y-N, McMahon M, Schmitz AJ, Turner JS, Tan J, Lei T, Alsoussi WB, Strohmeier S, Amor M, Mohammed BM, Mudd PA, Simon V, Cox RJ, Fremont DH, Krammer F, Ellebedy AH. 2020. Human antibodies targeting influenza B virus neuraminidase active site are broadly protective. Immunity 53:852–863.e7. 10.1016/j.immuni.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, Levine M, Katz JM, Ohmit SE. 2015. Antibody to influenza virus neuraminidase: An independent correlate of protection. J Infect Dis 212:1191–1199. 10.1093/infdis/jiv195. [DOI] [PubMed] [Google Scholar]
- 21.Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, Davey RT, Taubenberger JK. 2016. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 7:e00417-16. 10.1128/mBio.00417-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier HE, Nachbagauer R, Kuan G, Ng S, Lopez R, Sanchez N, Stadlbauer D, Gresh L, Schiller A, Rajabhathor A, Ojeda S, Guglia AF, Amanat F, Balmaseda A, Krammer F, Gordon A. 2020. Pre-existing antineuraminidase antibodies are associated with shortened duration of influenza A(H1N1)pdm virus shedding and illness in naturally infected adults. Clin Infect Dis 70:2290–2297. 10.1093/cid/ciz639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan H, Gao J, Yang H, Yang S, Harvey R, Chen Y-Q, Zheng N-Y, Chang J, Carney PJ, Li X, Plant E, Jiang L, Couzens L, Wang C, Strohmeier S, Wu WW, Shen R-F, Krammer F, Cipollo JF, Wilson PC, Stevens J, Wan X-F, Eichelberger MC, Ye Z. 2019. The neuraminidase of A(H3N2) influenza viruses circulating since 2016 is antigenically distinct from the A/Hong Kong/4801/2014 vaccine strain. Nat Microbiol 4:2216–2225. 10.1038/s41564-019-0522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon M, Kirkpatrick E, Stadlbauer D, Strohmeier S, Bouvier NM, Krammer F. 2019. Mucosal immunity against neuraminidase prevents influenza B virus transmission in guinea pigs. mBio 10:e00560-19. 10.1128/mBio.00560-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowen AC, Bouvier NM, Steel J. 2014. Transmission in the guinea pig model. Curr Top Microbiol Immunol 385:157–183. 10.1007/82_2014_390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seibert CW, Rahmat S, Krause JC, Eggink D, Albrecht RA, Goff PH, Krammer F, Duty JA, Bouvier NM, García-Sastre A, Palese P. 2013. Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J Virol 87:7793–7804. 10.1128/JVI.00979-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krammer F, Fouchier RAM, Eichelberger MC, Webby RJ, Shaw-Saliba K, Wan H, Wilson PC, Compans RW, Skountzou I, Monto AS. 2018. NAction! How can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio 9. 10.1128/mBio.02332-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkpatrick Roubidoux E, McMahon M, Carreño JM, Capuano C, Jiang K, Simon V, van Bakel H, Wilson P, Krammer F. 2021. Identification and characterization of novel antibody epitopes on the N2 neuraminidase. mSphere 6:e00958–20. 10.1128/mSphere.00958-20. [DOI] [PMC free article] [PubMed] [Google Scholar]