Abstract
Background
With the rising incidence of hepatocellular carcinoma (HCC), ongoing efforts should be focused on providing equitable and state-of-the-art care to patients.
Purpose
The aim of this study was to determine the survival of patients with HCC seen at a high-proportion Safety Net Hospital (h-SNH), where loco-regional therapy and hepatology services are available and liver transplantation (LT) is referred to outside facilities.
Patients and Methods
A retrospective cohort study was conducted on all patients with HCC seen at Valley Wise Health Center (VWHC) over a ten-year period. Clinical variables, treatment modalities, survival duration, hospice, and LT referrals of 161 patients were collected from the medical records. Survival analysis was used to determine the relationship of clinically relevant variables and survival among patients with HCC. A Log rank test was used to compare univariate variables. A Cox regression analysis was used to compare and control for multiple variables.
Results
Of the 161 patients included in the study, 33% were uninsured. The median age was 59 (21 to >89) years with 47% Hispanic, 31% Caucasian, 15% African American and 7% other races included for the analysis. The median survival for the cohort was 20.1 months. In the multivariate model, insurance status, final MELD, tumor within the Milan criteria and having received treatment for HCC were associated with survival. Surveillance for HCC was associated with HCC in the univariate analysis, but not in the multivariable model. Thirty percent of patients were referred for LT and 1.25% of the entire cohort received it.
Conclusion
Despite the availability of treatment modalities available for HCC at VWHC and the option of liver transplantation for appropriate candidates at outside centers, OS was less than reported from programs with on-site liver transplant programs. Reasons for lower survival in centers without liver transplant programs should be further studied.
Keywords: liver cancer, cirrhosis, health disparities, health outcomes
Introduction
The survival of patients with hepatocellular carcinoma (HCC) is dependent upon establishing an early diagnosis when tumor burden is small, disease is localized to the liver, liver function is preserved and the provision of timely and curative treatment options including liver transplantation, liver resection and/or thermal ablation are possible.1 The cornerstone upon which lays the foundation of the best HCC outcomes is having access to affordable medical care, which allows for regular surveillance of the cirrhotic liver, thus making a timely referral to a team of specialists possible, who can coordinate the delivery of the best therapeutic modality to an individual patient diagnosed with HCC.2
Over the past few years, as incidence of HCC is rising,3 disparities in HCC outcomes due to insurance status, gender, race and treatments have also been highlighted.4,5 In the United States (US), a significant population remains uninsured with limited access to medical care.6,7 Safety Net Hospitals (SNH) have been created in the US to provide subsidized medical care to patients with limited resources.8 Low-proportion SNHs (l-SNHs) and high-proportion SNHS (h-SNHs) are classified based on the percentage of uninsured and Medicaid (MA) patients they serve.6
Results reported from Veteran Hospitals and Surveillance, Epidemiology and End results (SEER) databases have shown that HCC outcomes for commercially insured patients are better than those with government sponsored insurance, while patients without insurance do the worst.9,10 The reasons for relatively poor outcomes in those with government insurance compared to those with commercial insurance remain unclear.7
The primary aims of this study were to assess the survival duration and its associations in a cohort of HCC patients followed over a ten-year period in a single SNH where dedicated gastroenterology, hepatobiliary and interventional services are available, but liver transplant option is unavailable, relying on referring patients to liver transplant programs within Arizona.
Patients and Methods
The Valleywise Health Center (VWHC) billing database was searched from November 2010 to June 30, 2020 by the Department of Medical Informatics for patients 18 or over who had an encounter in surgical, GI, oncology, or radiology department with an ICD-9 or 10 coded diagnosis of 155.0 or C22.0 respectively over the study period, leading to the enrollment of 181 patients in the study. This list was further reviewed by the primary author to include only those patients who had biopsy proven diagnosis of HCC and/or typical characteristic of HCC demonstrated on the liver protocol CT scan as recommended by American Association of Liver Disease 2011 guidelines.11 20 patients were excluded after establishing their diagnoses to be benign liver lesions, cholangiocarcinoma, or metastatic liver disease. The primary and corresponding authors reviewed all pathology and radiologic studies to confirm the HCC diagnosis.
Person-time was calculated in days from the first encounter until time of last encounter, death, or end of the study period. The first day of inclusion in the study was based on the encounter of a patient at VWHC GI clinic, oncology, or radiology when the initial diagnosis of HCC was established, whether the patient was receiving a follow-up at VWHC for their chronic liver disease and undergoing surveillance for HCC or was seen spontaneously from the emergency department or through referral from an outside hospital with an already established diagnosis of HCC. Age, insurance status, ethnicity, and sex were self-reported. Insurance status of each patient was obtained from the registration sheet and ultimately patients were grouped into those with or without insurance. Those patients without insurance received assistance from VWHC based on their level of poverty. Initial Model for End-Stage Liver Disease (MELD) score and Milan criteria were calculated from the laboratory and imaging data which were available immediately after the first encounter of a patient with an established diagnosis of HCC. The final MELD score was recorded from the last available laboratory values. The care plan of HCC patients was discussed at multidisciplinary tumor board, following which treatment plan consisting of interventional radiology (IR) or laparoscopically administered radiofrequency ablation (RFA), IR guided Transarterial chemoembolization (TACE) Sorafenib therapy and/or surgical resection as well as referral for liver transplantation were agreed upon. Any patient who received one of these treatments was categorized as being treated for HCC. Uninsured patients received Sorafenib, from the drug company through its compassionate program as long as they were US citizens.
Patients who underwent ultrasound of the liver every six months from the diagnosis of cirrhosis until the HCC diagnosis was established were categorized as the surveillance group, while those patients who received a diagnosis of HCC without surveillance at VWHC were categorized as spontaneous. Any patient deemed to be a liver transplant candidate had a referral placed and was instructed to make an appointment at one of the three liver transplant programs available in Arizona, wherever their insurance was accepted.
Data were collected by the authors who are included in the study from September 2019 to June 2020 when the study period terminated. The first and corresponding authors implemented the plan for uniform data collection by each investigator. The first author reviewed all charts after the data were collected to ensure reliability and the corresponding authors also randomly reviewed charts to ensure that accurate data were collected.
Patient demographic and clinical characteristics were reported as medians and interquartile ranges for continuous variables, and frequencies, percentages for categorical variables. Categorical variables were compared using Chi-squared tests and continuous variables were compared using Wilcoxon rank-sum test and Student’s t-test. Log rank test estimated the risk of death during follow-up period relative to each covariate. Additionally, covariates with p < 0.20 in the univariate analysis were included in a second model where a multivariable Cox regression analysis ascertained which covariates best predict mortality risk. A Harrell’s C concordance statistic was completed to indicate a good model fit. Finally, The Kaplan-Meier Survival function and the Log Rank Test were used to estimate 1, 2, 3, 4, and 5-year survival followed by survival comparisons relative to patient characteristics. All two-sided p-values < 0.05 were considered statistically significant. All data analyses were conducted using STATA version 14 (STATA Corp; College Station, TX).
Results
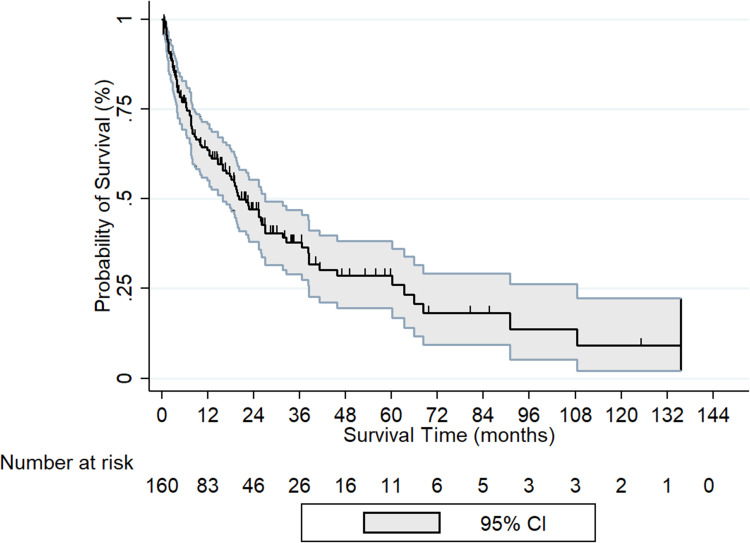
161 patients were enrolled during the study period. The median follow-up time was 19.82 (Range: 6.28–62.43) months. The baseline characteristics of the patients are summarized in Table 1. 53%, 13%, 2%, 33% had MA, Medicare, commercial insurance or had no insurance respectively. HBV, HCV and alcohol consumption were documented as 14%, 68%, and 62% respectively. 46% of patients were referred to hospice care. 60% of patients received at least 1 treatment for HCC. 24%, 31%, 14%, 3%, 6% of patients received Interventional Radiology (IR) directed radiofrequency ablation (RFA), IR- TACE, Sorafenib, surgeon guided laparoscopic RFA, or surgical resection of the liver tumor respectively. The median survival of the entire cohort was 20.1 months. One, two, three, four and five-year OS was 63%, 47%, 38%, 28% and 26% respectively (Figure 1).
Table 1.
Characteristics of HCC
| Insurance | No | 53 | 32.92% |
| Yes | 108 | 67.08% | |
| Age | Median (IQR) | 59 (9.25) | |
| Sex | Female | 24 | 14.91% |
| Male | 137 | 85.09% | |
| Ethnicity | African American | 24 | 15.38% |
| Asian | 5 | 3.21% | |
| Hispanic | 73 | 46.79% | |
| Native American | 6 | 3.85% | |
| White | 48 | 30.77% | |
| BMI | Median (IQR) | 27.27 (6.89) | |
| Surveillance | No | 86 | 53.42% |
| Yes | 75 | 46.58% | |
| Initial MELD | Median (IQR) | 11 (7.25) | |
| Final MELD | Median (IQR) | 16 (11) | |
| Liver Biopsy | No | 104 | 64.60% |
| Yes | 57 | 35.40% | |
| Milan criteria | No | 93 | 57.76% |
| Yes | 68 | 42.24% | |
| Transplant referral | No | 106 | 69.28% |
| Yes | 47 | 30.72% | |
| Listed for Transplant | No | 148 | 94.87% |
| Yes | 8 | 5.13% | |
| Treatment for HCC | No | 64 | 39.75% |
| Yes | 97 | 60.25% | |
| IR RFA | No | 122 | 75.78% |
| Yes | 39 | 24.22% | |
| IR TACE | No | 110 | 68.32% |
| Yes | 51 | 31.68% | |
| Sorafenib | No | 146 | 90.68% |
| Yes | 15 | 9.32% | |
| Surgical RFA | No | 156 | 96.89% |
| Yes | 5 | 3.11% | |
| Surgical Resection | No | 151 | 94.38% |
| Yes | 9 | 5.63% | |
| Survival | Dead | 92 | 57.50% |
| Alive | 68 | 42.50% | |
| Hospice care | No | 79 | 53.74% |
| Yes | 68 | 46.26% | |
Abbreviations: BMI, body mass index; HCC, hepatocellular carcinoma; IQR, interquartile range; MELD, Model for End Stage Liver Disease; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Figure 1.
Overall survival of HCC cohort; Kaplan Meier curve.
Table 2 summarizes the differences between insured and uninsured patients. There was no significant difference in surveillance for HCC or transplant listings between uninsured and insured patients. Insured patients had a lower final MELD, were within the Milan criteria, were more likely to receive treatment and/or referred for liver transplantation. Table 3 summarizes the survival duration of patients focusing on important variables recorded in the study. Insurance, surveillance, referral for liver transplantation, tumor within the Milan criteria, normal BMI, no hospice referral, lower Initial MELD, lower Final MELD, exposure to 1 or more treatments for HCC were found to be associated with longer survival duration.
Table 2.
Characteristics of HCC by Insurance Status
| Uninsured | Insured | P value | ||||
|---|---|---|---|---|---|---|
| Insurance status | 53 | 32.92% | 108 | 67.08% | ||
| Age | Median (IQR) | 58 (8.25) | 59 (10) | 0.0717 | ||
| Sex | Female | 10 | 18.87% | 14 | 12.96% | 0.3229 |
| Male | 43 | 81.13% | 94 | 87.04% | ||
| Ethnicity | African American | 8 | 15.69% | 16 | 15.24% | 0.4981 |
| Asian | 2 | 3.92% | 3 | 2.86% | ||
| Hispanic | 26 | 50.98% | 47 | 44.76% | ||
| Native American | 0 | 0.00% | 6 | 5.71% | ||
| White | 15 | 29.41% | 33 | 31.43% | ||
| BMI | Median (IQR) | 27 (5.49) | 27.45 (7.68) | 0.2829 | ||
| Surveillance | Surveillance | 19 | 35.85% | 56 | 51.85% | 0.0558 |
| Spontaneous | 34 | 64.15% | 52 | 48.15% | ||
| Initial MELD | Median (IQR) | 12 (6.5) | 10 (6) | 0.1386 | ||
| Final MELD | Median (IQR) | 20 (11.75) | 15 (10.25) | 0.0053 | ||
| Liver Biopsy | No | 33 | 62.26% | 71 | 65.74% | 0.6647 |
| Yes | 20 | 37.74% | 37 | 34.26% | ||
| Milan criteria | No | 41 | 77.36% | 52 | 48.15% | 0.0004 |
| Yes | 12 | 22.64% | 56 | 51.85% | ||
| Transplant referral | No | 43 | 84.31% | 63 | 61.76% | 0.0023 |
| Yes | 8 | 15.69% | 39 | 38.24% | ||
| Listed for Transplant | No | 51 | 98.08% | 97 | 93.27% | 0.2071 |
| Yes | 1 | 1.92% | 7 | 6.73% | ||
| Treatment for HCC | No | 28 | 52.83% | 36 | 33.33% | 0.0175 |
| Yes | 25 | 47.17% | 72 | 66.67% | ||
| IR RFA | No | 48 | 90.57% | 74 | 68.52% | 0.0022 |
| Yes | 5 | 9.43% | 34 | 31.48% | ||
| IR TACE | No | 42 | 79.25% | 68 | 62.96% | 0.0369 |
| Yes | 11 | 20.75% | 40 | 37.04% | ||
| Sorafenib | No | 44 | 98.11% | 94 | 87.04% | 0.4935 |
| Yes | 9 | 1.89% | 14 | 12.96% | ||
| Surgical RFA | No | 52 | 92.45% | 104 | 96.30% | 0.5323 |
| Yes | 1 | 7.55% | 4 | 3.70% | ||
| Surgical Resection | No | 49 | 92.45% | 102 | 95.33% | 0.5924 |
| Yes | 4 | 7.55% | 5 | 4.67% | ||
| Hospice care | No | 18 | 38.30% | 61 | 61.00% | 0.01 |
| Yes | 29 | 61.70% | 39 | 39.00% | ||
Abbreviations: BMI, body mass index; d, days; HCC, hepatocellular carcinoma; IQR, interquartile range; MELD, Model for End Stage Liver Disease; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Table 3.
Survival Duration Based on Variables Associated with HCC
| Variable | Survival Duration | ||
|---|---|---|---|
| Median (Months) | Range (Months) | ||
| Insurance | Uninsured | 8.35 | 3.28–18.61 |
| Insured | 32.02 | 8.71–89.62 | |
| Initial MELD | ≤10 | 26.63 | 12.10–106.91 |
| >10 | 14.3 | 3.75–59.28 | |
| Final MELD | ≤13 | 62.43 | 19.36–106.91 |
| >13 | 12.1 | 3.85–31.04 | |
| Milan criteria | No | 12.23 | 3.75–37.87 |
| Yes | 36 | 15.65–89.62 | |
| Treatment for HCC | No | 9.8 | 3.48–37.87 |
| Yes | 24.95 | 9.53–64.87 | |
| Transplant Referral | No | 12.66 | 3.75–62.43 |
| Yes | 26.63 | 17.46–64.87 | |
| Hospice care | No | 45.04 | 3.85–45.04 |
| Yes | 9.8 | 12.23–62.43 | |
| Surveillance | No | 14.3 | 3.85–45.04 |
| Yes | 26.63 | 12.23–62.43 | |
Abbreviations: HCC, hepatocellular carcinoma; MELD, Model for End Stage Liver Disease.
Table 4 further describes the differences between the surveillance and spontaneous groups. It was shown that the Milan criteria, referral for transplant, initial and final MELD were significant. However, patients received treatment regardless of whether they were in the spontaneous or surveillance group.
Table 4.
Characteristics of HCC by Surveillance
| Spontaneous | Surveillance | P value | ||||
|---|---|---|---|---|---|---|
| Surveillance | 86 | 53.42% | 75 | 46.58% | ||
| Milan criteria | No | 59 | 68.60% | 33 | 44.59% | 0.002 |
| Yes | 27 | 31.40% | 41 | 55.41% | ||
| Referred for Transplant | No | 67 | 79.76% | 39 | 54.93% | <0.001 |
| Yes | 17 | 20.24% | 32 | 45.07% | ||
| Treatment for HCC | No | 36 | 41.86% | 27 | 36.49% | 0.488 |
| Yes | 50 | 58.14% | 47 | 63.51% | ||
| Initial MELD | Median (IQR) | 12 (6) | 10 (5) | <0.001 | ||
| Final MELD | Median (IQR) | 17 (9) | 15 (12) | <0.001 | ||
Abbreviations: HCC, hepatocellular carcinoma; IQR, interquartile range; MELD, Model for End Stage Liver Disease.
Univariate Analysis
BMI, insurance status, initial and final MELD, surveillance, Milan criteria, HCC treatment, transplant referral as well as listed for liver transplantation (LT) were associated with HCC survival (Table 5).
Table 5.
Univariable Analysis of Variables Associated with HCC
| Variable | Hazard Ratio | 95% CI | P value | |
|---|---|---|---|---|
| Insurance Status | Uninsured | Reference | - | - |
| Insured | 0.49 | 0.31-0.78 | 0.003 | |
| Age (Years) | <60 | Reference | - | - |
| >60 | 1.07 | 0.71-1.63 | 0.731 | |
| Sex | Female | Reference | - | - |
| Male | 1.07 | 0.61-1.9 | 0.804 | |
| Ethnicity | White | Reference | - | - |
| African American | 0.78 | 0.41-1.55 | 0.865 | |
| Hispanic | 0.84 | 0.53-1.33 | 0.865 | |
| Other Ethnicity | 0.84 | 0.29-2.41 | 0.865 | |
| BMI | <25 | Reference | - | - |
| 25.0-<30 | 0.53 | 0.33-0.87 | 0.02 | |
| >30 | 0.57 | 0.34-0.97 | 0.02 | |
| Surveillance | Spontaneous | Reference | - | - |
| Surveillance | 0.66 | 0.43–0.99 | 0.047 | |
| Initial MELD | <10 | Reference | - | - |
| >10 | 1.83 | 1.2–2.79 | 0.005 | |
| Final MELD | <13 | Reference | - | - |
| >13 | 3.04 | 1.87–4.93 | <0.001 | |
| Hepatitis B | No | Reference | - | - |
| Yes | 1.00 | 0.57–1.75 | 1.00 | |
| Hepatitis C | No | Reference | - | - |
| Yes | 1.00 | 0.64–1.55 | 1.00 | |
| Milan criteria | No | Reference | - | - |
| Yes | 0.44 | 0.28–0.68 | 0.002 | |
| Transplant Referral | No | Reference | - | - |
| Yes | 0.59 | 0.38–0.93 | 0.02 | |
| Listed for Transplant | No | Reference | - | - |
| Yes | 0.09 | 0.01–0.67 | 0.003 | |
| Treatment for HCC | No | Reference | - | - |
| Yes | 0.56 | 0.37–0.85 | 0.006 | |
| Hospice Care | No | Reference | - | - |
| Yes | 2.85 | 1.85–4.38 | <0.001 |
Abbreviations: BMI, body mass index; HCC, hepatocellular carcinoma; MELD, Model for End Stage Liver Disease.
Multivariate Analysis
Table 6 summarizes the multivariable Cox regression analysis. HCC surveillance, transplant referral, initial MELD, sex and ethnicity, BMI, and listed for LT became insignificant. Uninsured patients, those out of Milan Criteria, those without exposure to HCC treatment, patients referred to hospice, and those with a higher final MELD were associated with lower survival in the model.
Table 6.
Multivariable Analysis of Associated Variables in HCC
| Effect | Hazard Ratio | 95% CI | P value | |
|---|---|---|---|---|
| BMI Category | 0.86 | 0.65 | 1.13 | 0.295 |
| Insurance N vs Y | 0.474 | 0.292 | 0.768 | 0.002 |
| Milan criteria N vs Y | 0.52 | 0.32 | 0.84 | 0.008 |
| Hospice N vs Y | 2.04 | 1.3 | 3.21 | 0.002 |
| Treatment for HCC N vs Y | 0.57 | 0.37 | 0.88 | 0.012 |
| Surveillance vs Spontaneous | 0.99 | 0.62 | 1.57 | 0.98 |
| Final MELD | 1.76 | 1.03 | 3.02 | 0.038 |
| Initial MELD cat | 1.22 | 0.79 | 1.9 | 0.35 |
| Referred | 1.11 | 0.67 | 1.83 | 0.67 |
| Listed | 0.18 | 0.023 | 1.4 | 0.103 |
| Sex | 0.98 | 0.51 | 1.88 | 0.97 |
| Ethnicity | 0.86 | 0.69 | 1.08 | 0.22 |
Abbreviations: BMI, body mass index; HCC, hepatocellular carcinoma; MELD, Model for End Stage Liver Disease.
Discussion
We have reported a 26% 5-year and 20-month median survival for predominantly Hispanic HCC patients, treated at a h-SNH without an on-site liver transplant program with large majority of cohort having state-sponsored insurance. A third of the patients who were uninsured were still offered subsidized services available at VWHC enabling 60% patients in the cohort to receive at least one treatment option possible for HCC.
It is not surprising that we found biological factors of HCC including extent of the HCC, severity of liver disease and whether treatment was given or not as significantly correlated with survival. These variables have been correlated with HCC survival from other studies conducted at a liver transplant program,12 veterans hospital system,10 SEER database9 and studies that have drawn on the data from multiple hospitals.13 Nonetheless, median survival of HCC patients reported from these studies has varied based on where the care was rendered, whether academic or non-academic setting,10 facilities available in the individual hospital12 and the collaboration that may or may not have occurred among different specialists.13 Ultimately, liver transplantation for HCC patients, which simultaneously cures the HCC in carefully selected patients and replaces the diseased liver with a healthy graft, remains the best management option. However, even at Stanford University Hospital with an onsite liver transplant option available, only 13% of those who were referred for HCC treatment consideration ultimately received a liver transplantation despite 40% of their patients within the Milan criteria and 81% with insurance.12,14
In this context, it is intriguing to highlight that despite having a team of dedicated physicians participating in caring for HCC patients at VWHC; and referral of a third of the patients for liver transplantation, only 5% were listed for it; and 1.25% received a liver transplant. Nonetheless, despite negligible liver transplant rates, the median OS for HCC patients in our database was 8.5 and 32 months among uninsured and insured patients respectively. Those patients with last MELD recorded in the database of ≤13 and who were within Milan criteria had median OS of 63 and 36 months respectively. 47% of patients were referred to hospice, more often those who were uninsured and who died, in line with previously published data showing higher hospice referral for those HCC patients who did not receive treatment.15
A wide range of survival durations has been reported for HCC patients from liver transplant programs,12 and from a mixture of hospitals with or without liver transplant programs on site.6,13,16 Data on HCC outcomes ascertained from SEER database have limitations of under reporting treatment modalities like TACE or non-reporting of key indicators like HCC surveillance that are associated with early diagnosis and prognosis of HCC.7 Nonetheless, compared to Adler et al’s report and the 20-month survival of our cohort, a lower median survival rate of 18 vs 11 vs 8 months was reported for commercial, Medicaid and uninsured patients respectively from the SEER database. Moreover, Adler et al documented that 17% and 39% more Medicaid and uninsured patients with localized HCC died compared to those with commercial insurance.9 Similarly, Wang et al reported, based on SEER database analysis, a five-year survival of 21% for Medicare/Commercial insurance; 17.6% for MA patients and 8.3% for uninsured patients. Liver resection or liver transplant was also received more often by commercially insured compared to Medicaid patients at 16% and 9% respectively.7 In yet another report, median survival for hospitals designated h-SNH was 3 months with 7% and 1% of them receiving liver resection and liver transplantation respectively.6 A median survival of 30 months has been reported when three, 6-year long, cohorts of HCC patients were evaluated and treated at Stanford University Hospital program with or without undergoing liver transplantation.12
Surveillance of a cirrhotic liver is the best strategy for early HCC detection17 and 46% of our patients underwent surveillance, 55% of those surveyed were within the Milan criteria compared to 31% who presented spontaneously and 45% of the surveyed patients were referred for liver transplantation compared to 21% of those who presented spontaneously. However, surveillance was not significant in determining survival in the multivariable analysis. We believe that HCC surveillance would have been significant in the multivariable analysis had there been a higher sample size as in the univariate analysis the 95% confidence interval for surveillance or spontaneous group was narrow at 0.43 to 0.99. Inconsistent surveillance of HCC with only 13% receiving it annually has been reported in literature with African Americans receiving even less HCC surveillance. Moreover, those who are well off have been reported to undergo surveillance for HCC more frequently, although contribution of income or education to regular surveillance was not discerned.17 Scaglione et al reported important data from 2 tertiary and 2 SNHs with a 42% surveillance of HCC in their cohort and an overall median survival of 25.7 months. Early tumor detection was predicted by HCC surveillance and seeing a GI specialist, but not by insurance status. Curative treatment was associated with HCC stage within Milan criteria and having commercial insurance.13 We did not find race to be associated with either insurance or living/dead status. Reports in literature have18,19 or have not found13 significant association of HCC survival with race; although race has been shown to predict the type of insurance acquired.7
Overall, we report 60% locoregional therapy and 1.2% liver transplantation for the entire cohort consisting of 42% patients reported within the Milan criteria. In a large database of 3988 veterans, those who were treated at an academically affiliated hospital or received a multi-specialist evaluation were more likely to receive both curative and non-curative options for HCC including liver transplantation, liver resection and loco-regional therapies.10 Treatment at a comprehensive cancer center and/or a solid organ transplant program has also been reported to have better overall survival.20 Similarly, it has been reported that type of insurance and sub-specialty consultation from gastroenterology are predictive of a higher survival for HCC patients.13 In yet another report, consultation from hepatology was predictive of receipt of therapy for HCC but not when gastroenterology consultation alone was obtained.10
Being a retrospective study where data gathering of ten-year clinical care has been conducted, our report has weaknesses. Many variables were included in the analysis, patients entered or exited the study at different time points and received a variety of treatments at the discretion of the health care providers and availability of such treatments at VWHC. Surveillance for HCC was more common for insured patients; however, we cannot comment on the reasons for spontaneous presentation of HCC in our patients and the type of follow up they received outside VWHC. All these factors could have affected OS. Moreover, we cannot offer further input as to what happened to patients after they were referred for liver transplant, whether they showed up for their appointments, or their SES despite having MA, and/or lack of family support or ongoing drug abuse might have contributed to not making it to the list for liver transplantation. System-level differences in care delivery based on SES and race have been attributed to poor prognosis of HCC at h-SNHs in Texas6 and our patients who were treated at h-SNH had a higher median survival compared to those reported from other SNHs,6,7 but lower than reported from hospitals with indigenous liver transplant programs.12
Conclusion
Despite having a team of specialists at VWHC, HCC outcomes were not on par with the outcomes reported from established liver transplant programs like Stanford University where all patients whether transplanted or not were included in analysis. The inferior survival duration at VWHC could be related to the fact that only 2/161 patients received a liver transplant, which is the definitive treatment for HCC. Medical literature has documented that multidisciplinary input of gastroenterologists, hepatologists and oncologists to the tertiary centers where they do not work but are referring their patients to, could potentially increase the listing for liver transplantation and/or availability of other curative options like liver resection, even for those patients who may be socially disadvantaged. Our report emphasizes the need for efforts to improve the coordination of care in various hospital systems to overcome the disparity of care rendered to the increasing number of HCC patients in the US.
Acknowledgments
We would like to acknowledge Dr. Robert Richards for his thoughtful comments throughout the preparation of this manuscript as a senior researcher.
Ethics Statement
The Institutional Review Board (IRB) of Valley Wise Health Center waived the requirement for obtaining informed consent for this retrospective chart review (IRB; 2019-062) which involves no more than minimal risk to the subjects. Data were kept confidential, and no patient identifiable data were collected. The study was conducted in compliance with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Varela M, Bruix J. Hepatocellular carcinoma in the United States. Lessons from a population-based study in Medicare recipients. J Hepatol. 2006;44:8–10. doi: 10.1016/j.jhep.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 2.Mellinger JL, Moser S, Welsh DE, et al. Access to subspecialty care and survival among patients with liver disease. Am J Gastroenterol. 2016;111:838–844. doi: 10.1038/ajg.2016.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology. 2017;152:812–20 e5. doi: 10.1053/j.gastro.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greten TF. Gender disparity in HCC: is it the fat and not the sex? J Exp Med. 2019;216:1014–1015. doi: 10.1084/jem.20190441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng B, Zhu YJ, Wang HY, Chen L. Gender disparity in hepatocellular carcinoma (HCC): multiple underlying mechanisms. Sci China Life Sci. 2017;60:575–584. doi: 10.1007/s11427-016-9043-9 [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AA, Murphy CC, Pruitt SL, et al. Effect of hospital safety net designation on treatment use and survival in hepatocellular carcinoma. Cancer. 2018;124:743–751. doi: 10.1002/cncr.31066 [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Ha J, Lopez A, Bhuket T, Liu B, Wong RJ. Medicaid and uninsured hepatocellular carcinoma patients have more advanced tumor stage and are less likely to receive treatment. J Clin Gastroenterol. 2018;52:437–443. doi: 10.1097/MCG.0000000000000859 [DOI] [PubMed] [Google Scholar]
- 8.Sommers BD, Stone J, Kane N. Predictors of payer mix and financial performance among safety net hospitals prior to the affordable care act. Int J Health Serv. 2016;46:166–184. doi: 10.1177/0020731415586408 [DOI] [PubMed] [Google Scholar]
- 9.Adler Jaffe S, Myers O, Meisner ALW, Wiggins CL, Hill DA, McDougall JA. Relationship between insurance type at diagnosis and hepatocellular carcinoma survival. Cancer Epidemiol Biomarkers Prev. 2020;29:300–307. doi: 10.1158/1055-9965.EPI-19-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serper M, Taddei TH, Mehta R, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology. 2017;152:1954–1964. doi: 10.1053/j.gastro.2017.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. American Association for the study of liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim NG, Nguyen PP, Dang H, et al. Temporal trends in disease presentation and survival of patients with hepatocellular carcinoma: a real-world experience from 1998 to 2015. Cancer. 2018;124:2588–2598. doi: 10.1002/cncr.31373 [DOI] [PubMed] [Google Scholar]
- 13.Scaglione S, Adams W, Caines A, et al. Association between race/ethnicity and insurance status with outcomes in patients with hepatocellular carcinoma. Dig Dis Sci. 2020;65:1669–1678. doi: 10.1007/s10620-019-05890-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kardashian A, Florman SS, Haydel B, et al. Liver transplantation outcomes in a U.S. Multicenter Cohort of 789 patients with hepatocellular carcinoma presenting beyond Milan criteria. Hepatology. 2020;72:2014–2028. doi: 10.1002/hep.31210 [DOI] [PubMed] [Google Scholar]
- 15.Sanoff HK, Chang Y, Reimers M, Lund JL. Hospice utilization and its effect on acute care needs at the end of life in medicare beneficiaries with hepatocellular carcinoma. J Oncol Pract. 2017;13:e197–e206. doi: 10.1200/JOP.2016.017814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sellers CM, Uhlig J, Ludwig JM, et al. The impact of socioeconomic status on outcomes in hepatocellular carcinoma: inferences from primary insurance. Cancer Med. 2019;8:5948–5958. doi: 10.1002/cam4.2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C, Nguyen MH. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J Clin Gastroenterol. 2016;50:120–133. doi: 10.1097/MCG.0000000000000446 [DOI] [PubMed] [Google Scholar]
- 18.Singal AG, Li X, Tiro J, et al. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med. 2015;128:90 e1–7. doi: 10.1016/j.amjmed.2014.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanoff HK, Chang Y, Stavas JM, Sturmer T, Lund J. Effectiveness of initial transarterial chemoembolization for hepatocellular carcinoma among medicare beneficiaries. J Natl Compr Canc Netw. 2015;13:1102–1110. doi: 10.6004/jnccn.2015.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Hansen BE, Peppelenbosch MP, De Man RA, Pan Q, Sprengers D. Factors associated with ethnical disparity in overall survival for patients with hepatocellular carcinoma. Oncotarget. 2017;8:15193–15204. doi: 10.18632/oncotarget.14771 [DOI] [PMC free article] [PubMed] [Google Scholar]