Introduction
Worldwide, ESKD prevalence per million population (PMP) has steadily increased from 2003 to 2016 (1), with the greatest proportional increases occurring in lower- and middle-income countries (2). Although dialysis is a lifesaving therapy, it is also extraordinarily expensive, so its use is limited in lower-income countries with less resources available for healthcare. Specifically, the prevalence of dialysis in 2010 was 1176 PMP in higher-income countries, 688 PMP in upper-middle-income countries, 170 PMP in lower-income countries, and 16 PMP in lower-income countries (2). The most common modality of kidney replacement therapy globally is dialysis (78%) and, among patients receiving dialysis, only 11% receive peritoneal dialysis (3).
The Kidney360 Global Dialysis Perspective series launched in 2020 and showcases how dialysis is practiced, delivered, and financed in different countries across the world. To date, we have featured perspectives from 17 countries in six continents: Africa (Senegal, South Africa), Asia (India, Israel, Japan, Korea, Singapore, Thailand, Vietnam), Australia, Europe (Spain), North America (Canada, Mexico, United States), and South America (Argentina, Brazil, Guatemala) (4–20). Authors of each global perspective were asked to report standard information about their dialysis populations, including general characteristics of the dialysis system and its treatments, such as percentage of patients by dialysis modality, dialysis-unit financing (for profit versus nonprofit), reimbursement (public or private insurance, or self-pay), unit location (hospital versus freestanding), staffing (proportion of nurses versus patient-care technicians and nurse/patient ratios), hemodialysis frequency and session length, and frequency of nephrologist visits. Authors also discussed key challenges and needs unique to their countries, with many discussing potential strategies to improve care moving forward. These perspectives provide fascinating insights about dialysis care in individual countries.
Although the availability of dialysis correlates roughly with a country's wealth, there are substantial variations in specific attributes of dialysis delivery and financing that cannot be explained only by wealth differences (Figure 1). These discrepancies suggest the existence of additional factors, such as government policy and local practice patterns, that may be as important as overall healthcare expenditures. Many of these country-specific factors are highlighted in individual global perspectives. This review focuses on some of the most salient observations raised by these global perspectives.
Figure 1.
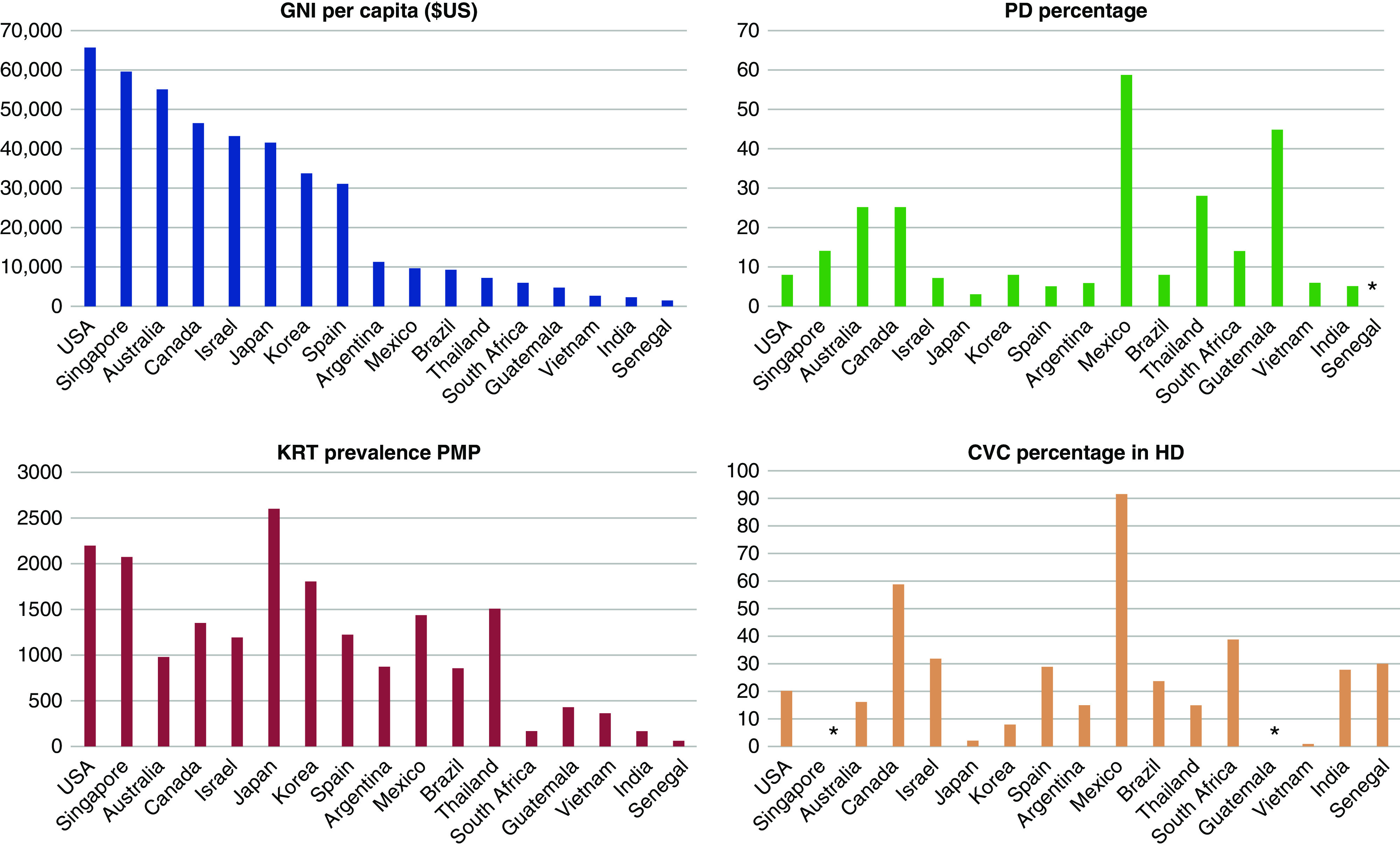
Global comparisons of gross national income (GNI) per capita, prevalence of kidney replacement therapy (KRT) per million population (PMP), relative use of peritoneal dialysis (PD), and frequency of central vein catheter (CVC) use among prevalent patients receiving hemodialysis. Whereas higher KRT correlates roughly with GNI per capita, PD and CVC use have poor correlations with GNI per capita. *not reported.
Dialysis Modalities
Although hemodialysis is the predominant form of kidney replacement therapy across the world, there are notable outliers. One might also expect richer countries to have a greater utilization of peritoneal dialysis. However, there is poor correlation between a country's wealth and the extent of its use of peritoneal dialysis (Figure 1). Among countries with a gross national income (GNI) per capita of >$40,000, the proportion of patients receiving peritoneal dialysis ranges from a high of 25% in Canada (5) and Australia (6), to a low of 3% in Japan (9). Similarly, whereas most low-income countries (GNI per capita of <$10,000) have <10% utilization of peritoneal dialysis, three countries in this income category (Mexico, Guatemala, and Thailand) use peritoneal dialysis in 28%–59% of their patients receiving dialysis (Figure 1). One possible reason that there is less peritoneal-dialysis usage in some of these countries is because of the continued lack of training in peritoneal dialysis for nephrology trainees.
Mexico has mandated a “peritoneal dialysis–first” policy, under which all patients with ESKD (with rare exceptions) initiate peritoneal dialysis first, and are only permitted to switch to hemodialysis if peritoneal dialysis fails (19). This policy has been extremely successful, with 59% of patients on dialysis using this modality, resulting in substantial healthcare savings (19). A similar policy in Guatemala has resulted in 45% of patients receiving dialysis being treated with peritoneal dialysis (7). Most recently, Thailand introduced a peritoneal dialysis–first policy in 2007 (12). In the first decade, the number of Thai patients on peritoneal dialysis grew exponentially, from 1198 to 26,450 patients (12).
Elsewhere, peritoneal-dialysis utilization has declined precipitously, in parallel with the proliferation of hemodialysis units in close proximity to patients’ homes. For example, in Israel, peritoneal dialysis use decreased from 34% in 1990 to 7% in 2015 (10). The authors attribute this decline to a growing elderly population that enjoys the social aspects of in-center hemodialysis, family preference for elderly relatives to dialyze while being monitored in a healthcare setting, high rates of multidrug-resistant peritonitis, and proliferation of outpatient units that offer accessibility and convenience (10). Similarly, in Korea, the proportion of patients receiving peritoneal dialysis decreased from 22% to 7% between 2006 and 2018, whereas the number of hemodialysis units doubled from 487 to 983 during the same time period (13).
Within-Country Variations in Dialysis Use
Some perspectives’ authors also describe substantial in-country variations in dialysis services and use. For example, in Brazil, the prevalence of dialysis is lower in the northern region as compared with the southern region (17). Despite universal insurance coverage in Brazil, access to healthcare is more limited in the northern region due to reduced health services in more rural areas. Another example is in Guatemala, where the majority of patients with ESKD are located near Guatemala City (7). The prevalence is markedly lower in rural highland areas with predominantly indigenous populations (7). Similarly, Australia has experienced challenges in staffing remote dialysis units for indigenous patients (6). Access to dialysis is very limited in rural India, where 60% of patients have to travel >50 km and 25% >100 km to the closest unit (4).
Hemodialysis Treatment Duration and Frequency
Treatment duration and frequency of hemodialysis also varies substantially across the world. The most commonly prescribed hemodialysis treatment time is 3–4 hours, and the most common frequency is two to three treatments per week. However, there are several notable exceptions. In Mexico, the average number of hemodialysis treatments per week is 1.2, with only 2% of patients undergoing hemodialysis treatments three times a week (19). In Guatemala, many patients receive hemodialysis just weekly, with the frequency varying by type of healthcare funding (7). Specifically, patients with one type of health-insurance coverage receive hemodialysis thrice weekly, whereas those with a different coverage are dialyzed only once weekly (7). Locations of dialysis units also vary, with the majority of countries in this series having both hospital-based and freestanding units. Guatemala is an exception, with 100% freestanding units (7).
Dialysis Staffing
Dialysis-unit staffing differs substantially by country. Many countries use a combination of dialysis nurses and technicians. However, countries such as Canada, Korea, Australia, Thailand, Israel, Japan, and Spain use dialysis nurses exclusively (6,9,12,13,16). In contrast, Guatemala uses patient-care technicians exclusively (7). Furthermore, the nursing staff/patient ratio varies markedly from 1:3 in Mexico (19) and Australia (6), to 1:35 in Brazil (17). The frequency of patient visits by a nephrologist is once monthly in most countries, but is every dialysis session in a few countries (e.g., Korea, Japan, Brazil, and Spain).
Vascular Access
Given that central vein catheters (CVCs) are considered the least desirable type of vascular access, one might expect the richest countries to have the lowest rates of CVC use. In fact, there is a disconnect between national income and CVC use among patients receiving hemodialysis across the globe (Figure 1). Among those countries with a GNI per capita of >$40,000, CVC use varies from a high of 59% in Canada (5), to a low of 2% in Japan (9). Similarly, among countries with a GNP per capita of <$10,000, CVC use varies from a high of 92% in Mexico (19), to a low of 15% in Thailand (12) (Figure 1). Interestingly, the frequency of catheter-related bloodstream infections is exceptionally low in Canada (5), despite the very high rate of CVC use.
Financing of Dialysis
The majority of countries featured in the Kidney360 Global Dialysis Series have a combination of for-profit and nonprofit dialysis units. At the two extremes, Guatemala and Korea have only for-profit dialysis units (7,13), whereas Canada and Japan have only not-for-profit dialysis units (5,9). Financing for dialysis also differs by country. The majority of countries have both public and private health-insurance coverage for dialysis, or use a combination of insurance coverage and individual out-of-pocket payments. However, several countries, such as Australia, Israel, and Korea, have only public insurance (government insurance) (6,10,13). Private insurance is forbidden in Korea (13). These insurance differences often correlate with observed practice-pattern differences, with those countries without insurance-covered dialysis care having shorter hemodialysis treatment times and lower frequencies of treatments.
Access to healthcare is a significant to barrier to receiving dialysis in developing countries. For example, in Mexico (19), only 49% of the population has health insurance, and uninsured patients only receive dialysis if they can pay for treatment out of pocket. As a consequence, many Mexican patients with kidney failure die without receiving dialysis (19). Similarly, in India, a quarter of patients receive dialysis once a week or “as needed” due to financial constraints (4).
Summary
Kidney failure requiring dialysis continues to increase worldwide. In many cases, the growth is outpacing the capacity for kidney replacement therapy, particularly in developing countries. Hemodialysis remains the most common form of kidney replacement therapy. However, several countries use a peritoneal dialysis–first policy to conserve resources and mitigate costs. The global perspectives featured in Kidney360 highlight the wide range of health-system characteristics, dialysis practice patterns, and outcomes across the globe. Further study regarding whether and how the reported differences affect morbidity and mortality is warranted. Establishment of more robust and uniform registries to collect these data will help address these questions and guide resource allocation and policy development for the dialysis population globally.
Disclosures
M. Allon reports having consultancy agreements with CorMedix. J. E. Flythe reports serving on the editorial board for American Journal of Kidney Diseases (2017–), on the editorial board for CJASN (2017–), as associate editor for Kidney360 (2019–), on the Kidney Disease Improving Global Outcomes Executive Committee (2020–), on the Kidney Health Initiative (KHI) Board of Directors (2019–), as the KHI Patient Preferences Project Chairperson (2019–), on the editorial board for Kidney Medicine (2019–), and on the editorial board for Nephrology Dialysis and Transplantation as the hemodialysis theme editor (2018–); receiving honoraria from the American Renal Associates, American Society of Nephrology, Baxter, Dialysis Clinic, Inc., Fresenius Medical Care North America, National Kidney Foundation, Renal Ventures, and numerous universities; having consultancy agreements with AstraZeneca and NxStage Medical Advisory Board; and receiving research funding from National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH/National Heart, Lung, and Blood Institute (NHLBI), Patient-Centered Outcomes Research Institute, Robert Wood Johnson Foundation, and the Renal Research Institute (subsidiary of Fresenius Medical Care North America). T. Lee reports serving as the chair of the research committee for the American Society of Diagnostic and Interventional Nephrology; serving on the editorial board for CJASN and as associate editor for Kidney360; and having consultancy agreements with, and being a coscientific consultant for, Merck.
Funding
T. Lee is supported by NIDDK grant R44DK109789, NHLBI grant R01HL139692, and U.S. Department of Veterans Affairs Merit Award I01BX003387. J.E. Flythe is supported by NHLBI grant R01 HL152034 and NIDDK grant K23 DK109401. M. Allon is supported by National Institute on Minority Health and Health Disparities grant R01 MD013818.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
M. Allon and J.E. Flythe reviewed and edited the manuscript; M. Allon, J.E. Flythe, and T. Lee conceptualized the study and were responsible for visualization; J.E. Flythe and T. Lee wrote the original draft; and T. Lee was responsible for methodology.
References
- 1.United States Renal Data System . Available at: https://www.usrds.org/. Accessed January 15, 2021
- 2.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015. 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 3.Pecoits-Filho R, Okpechi IG, Donner JA, Harris DCH, Aljubori HM, Bello AK, Bellorin-Font E, Caskey FJ, Collins A, Cueto-Manzano AM, Feehally J, Goh BL, Jager KJ, Nangaku M, Rahman M, Sahay M, Saleh A, Sola L, Turan Kazancioglu R, Walker RC, Walker R, Yao Q, Yu X, Zhao MH, Johnson DW: Capturing and monitoring global differences in untreated and treated end-stage kidney disease, kidney replacement therapy modality, and outcomes. Kidney Int Suppl (2011) 10: e3–e9, 2020. 10.1016/j.kisu.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharati J, Jha V: Global dialysis perspective: India. Kidney360 1: 1143–1147, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake P: Global dialysis perspective: Canada. Kidney360 1: 115–118, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damasiewicz M, Polkinghorne K: Global dialysis perspective: Australia. Kidney360 1: 48–51, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia P, Sánchez-Polo V: Global dialysis perspective: Guatemala. Kidney360 1: 1300–1305, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y, Saran R: Global dialysis perspective: United States. Kidney360 1: 1137–1142, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanafusa N, Fukagawa M: Global dialysis perspective: Japan. Kidney360 1: 416–419, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haviv Y, Glolan E: Global dialysis perspective: Israel. Kidney360 1: 119–122, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jardine T, Davids M: Global dialysis perspective: South Africa. Kidney360 1: 1432–1436, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanjanbuch T, Takkavatakarn K: Global dialysis perspective: Thailand. Kidney360 1: 671–675, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim Y, Jin D: Global dialysis perspective: Korea. Kidney360 1: 52–57, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niang A, Lemrabott A: Global dialysis perspective: Senegal. Kidney360 1: 538–540, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orias M, Diez G: Global dialysis perspective: Argentina. Kidney360 1: 676–679, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roca-Tey R, Ibeas J, Sànchez Alvarez JE: Global dialysis perspective: Spain. Kidney360 2: 344–349, 2020. 10.34067/KID.0005722020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sesso R, Lugon J: Global dialysis perspective: Brazil. Kidney360 1: 216–219, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van B, Duc C: Global dialysis perspective: Vietnam. Kidney360 1: 974–976, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vazquez-Jimenez E, Madero M: Global dialysis perspective: Mexico. Kidney360 1: 534–537, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leo CCH, Chan GC: Global dialysis perspective: Singapore. Kidney360 1: 1306–1309, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


