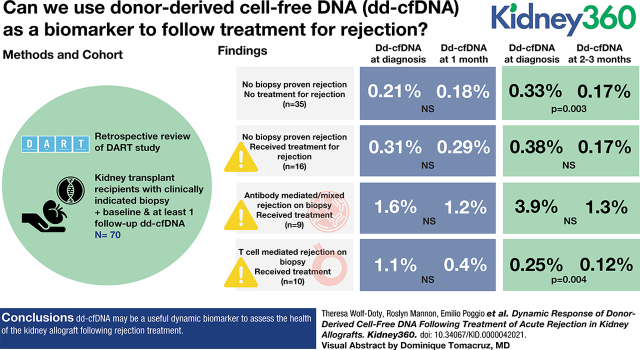
Visual Abstract
Keywords: transplantation, allografts, cell-free nucleic acids, kidney, tissue donors
Key Points
Donor-derived cell-free DNA (dd-cfDNA) changes dynamically after treatment for rejection.
dd-cfDNA allows for frequent assessments after episodes of rejection.
Persistently elevated dd-cfDNA levels could indicate incomplete recovery of rejection episodes.
Abstract
Background
The quantification of rejection treatment efficacy has been insufficient using traditional markers due, in part, to the lagging response of serum creatinine and histologic alterations on biopsy specimens. Donor-derived cell-free DNA (dd-cfDNA) is a molecular marker of injury that may assess allograft injury after rejection.
Methods
Retrospective review of the DART study identified 70 patients who had a clinically indicated biopsy, simultaneous dd-cfDNA measurement, and at least one follow-up dd-cfDNA within 3 months post-treatment. Thirty-five patients had no biopsy–proven rejection and no rejection treatment (NR), 16 patients had no biopsy–proven rejection but did receive rejection treatment (CR), 9 patients had diagnosis of ABMR/mixed rejection on biopsy and received rejection treatment (ABMR), and 10 patients had diagnosis of TCMR and received rejection treatment (TCMR). The CR, ABMR, and TCMR groups combined to form a rejection (R) group.
Results
In the R group, median dd-cfDNA values at baseline and 1 month were 0.62% and 0.35% (n=21 pairs, p=0.34), and at baseline and 2-3 months were 0.77% and 0.21% (n=23 pairs, p=0.002). In TCMR, median dd-cfDNA values at baseline and 1 month were 1.13% and 0.37% (n=5 pairs, p=0.63), and at baseline and 2-3 months were 0.25% and 0.12% (n=9 pairs, p=0.004). In ABMR, median dd-cfDNA values at baseline and 1 month were 1.61% and 1.2 % (n=6 pairs, p>0.99), and at baseline and 2-3 months were 3.85% and 1.32% (n=6 pairs, p=0.09). In CR, median dd-cfDNA values at baseline and 1 month were 0.31% and 0.29% (n=10 pairs, p=0.38), and at baseline and 2-3 months were 0.38% and 0.17% (n=8 pairs, p=0.31). Lastly, in NR, median dd-cfDNA values at baseline and 1 month were 0.23% and 0.18% (n=21 pairs, p=0.10), and at baseline and 2-3 months were 0.33% and 0.17% (n=26 pairs, p=0.003). Changes in serum creatinine across 1 month and 2-3 months following rejection were similar.
Conclusions
dd-cfDNA may be a useful dynamic biomarker to assess the health of the kidney allograft following rejection treatment.
Introduction
Early detection and management of allograft injury for kidney transplant recipients can improve long-term allograft survival. Despite improvements in 1-year survival, approximately one in five deceased-donor and one in ten living-donor kidney allografts fail within the first 5 years post-transplantation (1,2). Primary causes of allograft failure include T cell–mediated rejection (TCMR) and antibody-mediated rejection (ABMR). ABMR can be particularly deleterious due to potential diagnostic challenges and inconsistent treatment efficacy (3), resulting in four-fold increased allograft loss (1,4) compared with patients without ABMR. This may be further confounded in patients who progress to chronic rejection with persistent donor-specific antibodies (DSA) (1).
The current standard to assess for acute rejection is to perform a renal allograft biopsy for histologic analysis. Biopsies are often performed in response to clinical changes in allograft function, such as an increase in serum creatinine or proteinuria; however, these are nonspecific, late markers of injury that often prompt further investigation (5). Other tools used for detecting risk of injury and rejection include de novo DSA, non-HLA antibodies, serum BK virus (BKV) quantitative PCR, gene-expression profiling, and protocolized surveillance biopsies. Unfortunately, many of these tools are limited in their ability to detect injury early in the disease process, and this delay is hypothesized to result in higher grade or refractory rejection and allograft dysfunction.
Donor-derived cell-free DNA (dd-cfDNA) is a novel tool that aids in the detection of molecular allograft injury and has been analytically and clinically validated for assessment of kidney and heart allograft injury (6,7). dd-cfDNA discriminates and quantifies the level of circulating DNA that emanates from the allograft as a percentage of all circulating cell-free DNA levels in transplant recipients (5,6,8,9). In kidney transplantation, median values of dd-cfDNA in healthy, stable kidney recipients are 0.21% (10), and levels <1% have a high negative predictive value of 85%–95% for acute rejection (11,12).
The quantification of treatment efficacy and adequacy for biopsy–proven acute rejection (BPAR) is unclear. Repeat biopsies or monitoring changes in serum creatinine have limitations that implore for an alternate way to monitor response to treatment and highlight the potential for dd-cfDNA as a molecular marker of global allograft recovery. Initial data from a small, three-patient series of BPAR described a decrease in dd-cfDNA levels back to baseline as early as the final day of treatment (13). However, this finding has not been evaluated in a large cohort of patients from multiple centers. In our current analysis of patients enrolled in the Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Acute Rejection in Kidney Transplant Recipients (DART) study, we report on the performance of dd-cfDNA (AlloSure) in patients receiving treatment for rejection.
Materials and Methods
The DART study (ClinicalTrials.gov identifier, NCT02424227) was an observational, prospective, 14-center trial that distinguished quiescence from active rejection in kidney transplant recipients (11). A total of 395 patients were enrolled into the DART study and 102 for-cause biopsy samples were obtained. Of these 102 clinically indicated biopsy samples, there was a cohort of 70 patients that had a matched dd-cfDNA level taken at the time of the biopsy (or up to 3 days prior) and at least one follow-up dd-cfDNA level taken between 1 and 3 months (16–105 days) after the biopsy.
The patients were divided into four groups (Figure 1). The no rejection (NR) group (n=35) had no BPAR and did not receive rejection treatment. Patients with suspicious changes on histology that did not meet the Banff criteria for TCMR grade IA or greater or ABMR were included in this group when no treatment was given. The clinical rejection (CR) group (n=16) had no BPAR but did receive rejection treatment. Patients with suspicious changes on histology that did not meet Banff criteria for TCMR grade IA or greater or ABMR were included in this group when rejection treatment was given. The ABMR group (n=9) had a diagnosis of ABMR or mixed rejection on biopsy and received rejection treatment. The TCMR group (n=10) had a diagnosis of TCMR grade IA or greater on biopsy and received rejection treatment. The CR, ABMR, and TCMR groups were also combined to comprise a rejection (R) group. A control cohort was created from DART (n=127) consisting of patients demonstrating stable function who had NR treatment, or clinically indicated biopsy, to delineate the natural course of dd-cfDNA in comparison with those with clinically indicated events. For each control group patient, a randomly chosen visit with a dd-cfDNA level was assigned as the initial visit, or equivalent to month 0. A total of 72 control group patients had at least one follow-up dd-cfDNA level obtained between 1 and 3 months after this index visit. Two patients in the control group had surveillance biopsies during this time and were found to be negative for rejection.
Figure 1.
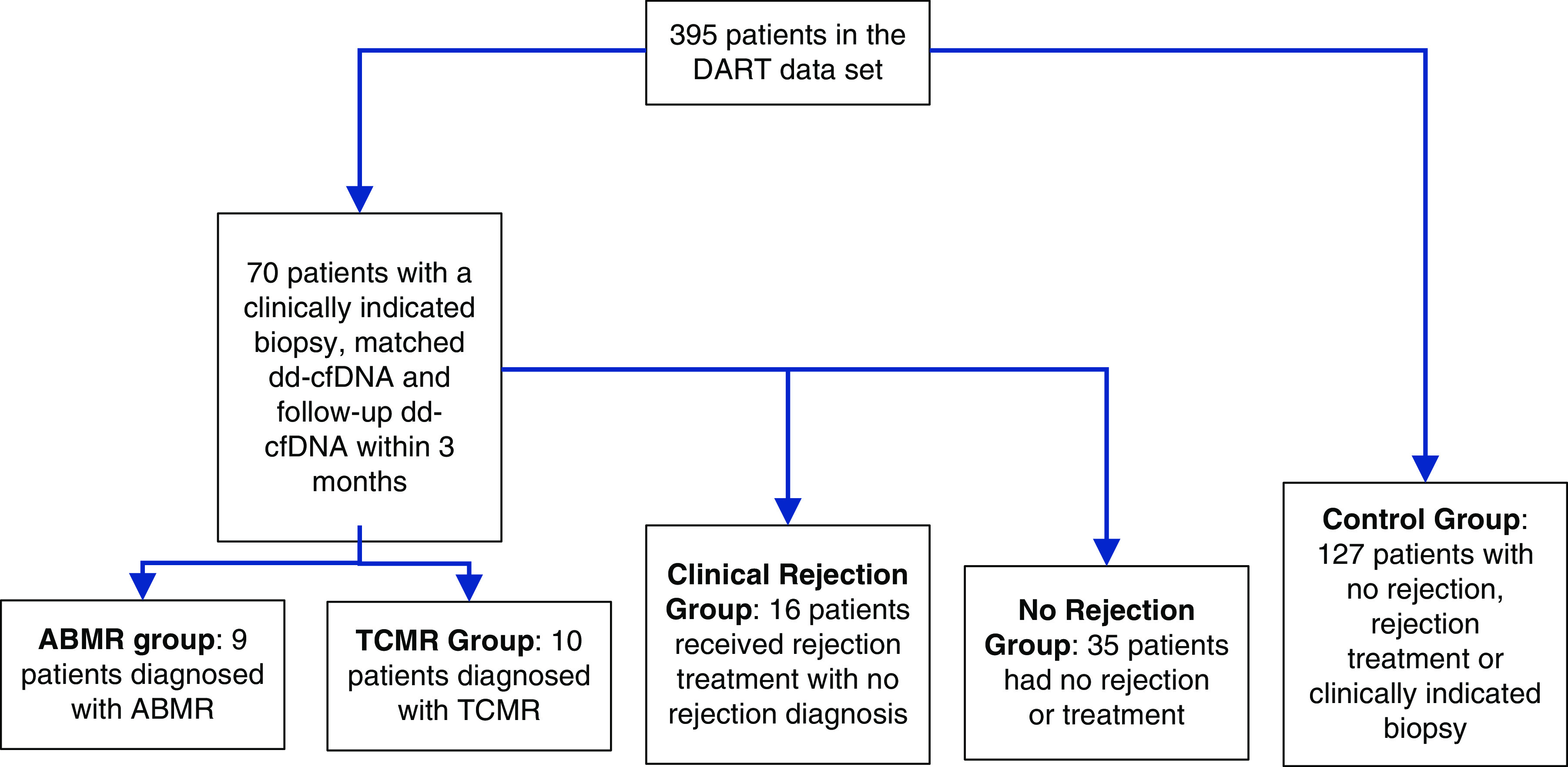
The groups used in this study were defined from the whole DART database and include ABMR (antibody-mediated rejection), TCMR (T cell-mediated rejection), clinical rejection, no rejection, and a control group. dd-cfDNA, donor-derived cell-free DNA.
Blood Samples and dd-cfDNA Measurements
Whole venous blood was collected in Streck Cell-Free DNA BCT tubes and shipped to the laboratory at CareDx, Inc. (Brisbane, CA), which is certified by the Clinical Laboratories Improvements Act (CLIA). Prior publications have detailed the analytical specifications and blood-processing methods used to quantify dd-cfDNA (6). Because DART was a research study, physicians were blinded to dd-cfDNA results at time of testing; therefore, these results were not used for decisions regarding patient care.
Rejection Treatment
Measurements of dd-cfDNA were obtained at 1 month (3–35 days) and 2–3 months (36–100 days) post–rejection treatment. If more than one dd-cfDNA test occurred in the time interval, the earliest test result was chosen. The dd-cfDNA test must have been within 3 days before the time of biopsy, with month 0 as the time of first rejection treatment for the R group and time of biopsy for control group. Patients may have had additional biopsies or rejections after the initial biopsy. Due to different protocols at each center, some patients were followed up only at 1 month post–rejection treatment, other patients were followed up only at 2–3 months post–rejection treatment, and other patients were followed up at both time points. Therefore, patients with 1-month follow-up dd-cfDNA results were grouped together, and patients with 2- to 3-month follow-up dd-cfDNA results were grouped together for the analyses. Rejection treatment was defined as pulse glucocorticoids, plasmapheresis, intravenous Ig (IVIG), rabbit anti–thymocyte globulin (rATG), rituximab, or alemtuzumab, given in response to biopsy diagnosis of rejection or suspicion of rejection. Treatment had to be given within 15 days of a clinically indicated biopsy to be considered rejection treatment for the purpose of this analysis.
Diagnosis of Biopsy–Proven Rejection and Laboratory Tests
This study used the 2013 Banff Classification of Renal Allograft Pathology (14). In DART, all biopsy reports were independently reviewed to confirm correspondence with the Banff 2013 consensus criteria (14). Study results are based on center clinical management or treatment of rejection episode. All other laboratory test results, such as DSA and BKV PCR viral loads, were done locally at the center. DSA results were graded as present or absent depending on the individual center's mean fluorescence intensity (MFI) threshold.
Statistical Analyses
Descriptive statistics were used to describe patient baseline characteristics and demographics. Comparisons within rejection groups used ANOVA for continuous variables and the chi-squared test for discrete variables. We defined the 1-month visit to be within 16–45 days of the biopsy or index visit. The 2–3-month visit was defined as within 46–105 days. Wilcoxon tests were used for nonparametric analysis of dd-cfDNA and creatinine.
Within each group, the Wilcoxon signed rank test was performed to detect a change in dd-cfDNA level from the time of the biopsy to month 1, and separately from time of biopsy to the earliest dd-cfDNA measurement in months 2–3 after a rejection event.
As a control, parallel computations were used in the control group to assess changes in dd-cfDNA from the randomly selected index visit to visits at 1 month and 2–3 months after the index visit, using the Wilcoxon signed rank test. Wilcoxon rank sum tests were used to compare the distribution of dd-cfDNA at control index visits to the distribution at time of biopsy, 1 month later, and 2–3 months later, for each group.
Finally, to assess dd-cfDNA after a rejection event as compared with serum creatinine, Wilcoxon signed rank tests were used to compare serum creatinine levels at time of biopsy with levels obtained 1 month and 2–3 months later, for each group. We used the programming language R (R Core Team) for all analyses.
Results
Patient Demographics
Patient demographics stratified by rejection group are provided in Table 1. No significant differences were noted with respect to sex, race, primary renal disease, donor type, cytomegalovirus infection, age at time of enrollment, or HLA mismatches. There was an imbalance among the groups comparing DSA (P≤0.001), BKV infection (P=0.002), and days post-transplant from time to biopsy (P≤0.001). DSA was more prevalent among the ABMR group, BKV was more common among the CR and NR groups, and time post-transplant tended to be later among the R group.
Table 1.
Demographics
| Variable | All | ABMR | TCMR | Clinical | None | Control | P |
| No. of patients | 197 | 9 | 10 | 16 | 35 | 127 | 0.59 |
| Male sex, n (%) | 116 (59) | 5 (56) | 6 (60) | 8 (50) | 24 (69) | 73 (57) | |
| Race, n (%) | |||||||
| American Indian or Alaskan native | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0.09 |
| Asian | 5 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (4) | |
| Black | 63 (32) | 6 (67) | 1 (10) | 6 (38) | 12 (34) | 38 (30) | |
| Hispanic/Latino | 22 (11) | 0 (0) | 0 (0) | 2 (12) | 3 (9) | 17 (13) | |
| Native Hawaiian or other Pacific Islander | 2 (1) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 1 (1) | |
| Other | 12 (6) | 0 (0) | 0 (0) | 0 (0) | 5 (14) | 7 (6) | |
| White | 90 (46) | 3 (33) | 7 (70) | 8 (50) | 13 (37) | 59 (46) | |
| Donor type, n (%) | 0.27 | ||||||
| Deceased donor | 127 (64) | 6 (67) | 7 (70) | 10 (62) | 19 (54) | 85 (67) | |
| Living related | 27 (14) | 3 (33) | 1 (10) | 2 (12) | 3 (9) | 18 (14) | |
| Living unrelated | 41 (21) | 0 (0) | 1 (10) | 4 (25) | 12 (34) | 24 (19) | |
| CMV infection, n (%) | 3 (2) | 1 (11) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 0.17 |
| BKV infection, n (%) | 13 (7) | 0 (0) | 1 (10) | 4 (25) | 5 (14) | 3 (2) | 0.002 |
| DSA, n (%) | 18 (9) | 9 (100) | 2 (20) | 2 (12) | 5 (14) | 0 (0) | <0.001 |
| Age at enrollment, mean±SD | 50±14 | 52±13 | 38±17 | 52±15 | 51±12 | 50±13 | 0.11 |
| Days post-transplant at time of biopsy, mean±SD | 151±153.2 | 1024±880.5 | 207±281 | 726.5±446.5 | 108.5±132.4 | 135±119 | <0.001 |
| HLA class 1 mismatches, mean±SD | 2.9±1.1 | 2.7±1.5 | 2.9±1.5 | 2.4±1.1 | 3.1±1 | 2.9±1 | 0.28 |
| HLA class 2 mismatches, mean±SD | 1.3±0.7 | 1.1±0.8 | 1.2±0.7 | 1.1±0.9 | 1.4±0.7 | 1.2±0.7 | 0.48 |
Demographic and clinical information for each of the four biopsy groups plus the control group is provided. ABMR, antibody-mediated rejection; TCMR, T cell–mediated rejection; CMV, cytomegalovirus; BKV, BK virus; DSA, donor-specific antibodies.
Rejection and Rejection Treatment
In the NR group, 23 of 35 patients had biopsies for elevated serum creatinine, five for proteinuria, and seven for other reasons. Three biopsies (9%) were suspicious for TCMR (one of these was downgraded from a local biopsy grade of IA due to not meeting the Banff 2013 criteria for IA), but no treatment was given.
In the CR group 12 of 16 patients received their biopsy for elevated creatinine, one for proteinuria, and three for other reasons. Ten (63%) of these biopsies were suspicious for TCMR, and three had a diagnosis of BKV. A total of 15 of 16 patients had pulse methylprednisolone and one patient receiving plasmapheresis as the primary rejection therapy. Eight of the 16 patients received a single treatment, and eight patients required additional therapy: three had IVIG, three had pulse prednisone, two had rATG, and two received rituximab.
Of the ten patients in the TCMR group, eight had their biopsy because of elevated creatinine and two were for other reasons. Four patients had a TCMR grade IA rejection, five had TCMR grade IB rejection, and one had TCMR IIA rejection. All ten patients received rejection treatment: nine had pulse glucocorticoids as the primary treatment. Seven patients received a single primary rejection treatment, and three required additional therapy: one underwent plasmapheresis, one had IVIG, two had pulse prednisone, three had rATG, one received rituximab, and five patients had a follow-up biopsy.
Of the nine patients in the ABMR group, six received a biopsy because of elevated creatinine, two because of proteinuria, and one for other reasons. Two had mixed rejection (the TCMR diagnoses were IB and IIA), and two were suspicious for TCMR. Five of the patients had treatment within 15 days. One had pulse prednisone only, but the other four all had multiple treatments (pulse methylprednisolone, plasmapheresis, IVIG, rituximab, rATG). Four patients had no documented rejection treatment within 15 days. However, two patients started rejection treatment after 15 days: one with IVIG and pulse methylprednisolone, and one with IVIG only. One of these two patients started treatment late because of insurance; no reason was stated for the other patient. Of the remaining two patients who never received rejection treatment, one was switched from cyclosporine to tacrolimus because of the ABMR diagnosis. The other was not treated as a rejection episode by the transplant center despite satisfying the requirements for ABMR per the 2013 Banff criteria. This patient was switched from everolimus to mycophenolate and prednisone because of proteinuria.
Longitudinal Changes in dd-cfDNA and Serum Creatinine
Figure 2, A–D, and Tables 2 and 3 present the distribution of dd-cfDNA and serum creatinine values over 1 month and 2–3 months post-biopsy. For the combined R group, dd-cfDNA trended down in patients with 1 month follow-up (0.62% at month 0 to 0.35%; P=0.34) in addition to patients with 2–3 months follow-up (0.77% at month 0 to 0.21%; P=0.002). In the TCMR group, patients with a 1-month follow-up had elevations in dd-cfDNA at the time of diagnosis (1.13%) that improved after treatment ( 0.37%; P=0.63), and the 2–3-month follow-up group reported values of 0.25% at the time of diagnosis and 0.12% after treatment (P=0.004). In the ABMR group, dd-cfDNA trended down after 1 month follow-up (1.61% at month 0 to 1.20%; P>0.99) and in the 2–3-month follow-up cohort (3.85% at month 0 to 1.32%; P=0.09). For the CR group, dd-cfDNA levels were low at the time of biopsy and the intrapatient improvement was not significant for both the 1-month follow-up cohort (0.31% at month 0 to 0.29%; P=0.375) and the 2–3-month follow-up cohort (0.38% at month 0 to 0.17%; P=0.31). In addition, dd-cfDNA values in the NR group were 0.21% at the index visit and 0.18% after 1-month follow-up (P=0.10), and 0.33% at the index visit and 0.17% after 2–3-month follow-up (P=0.003). Lastly, in the control group, dd-cfDNA levels were low during and subsequent to the index visit in the 1-month follow-up cohort (0.37% at month 0 to 0.25%; P=0.27) and in the 2–3-month follow-up cohort (0.24% at month 0 to 0.15%; P=0.004).
Figure 2.
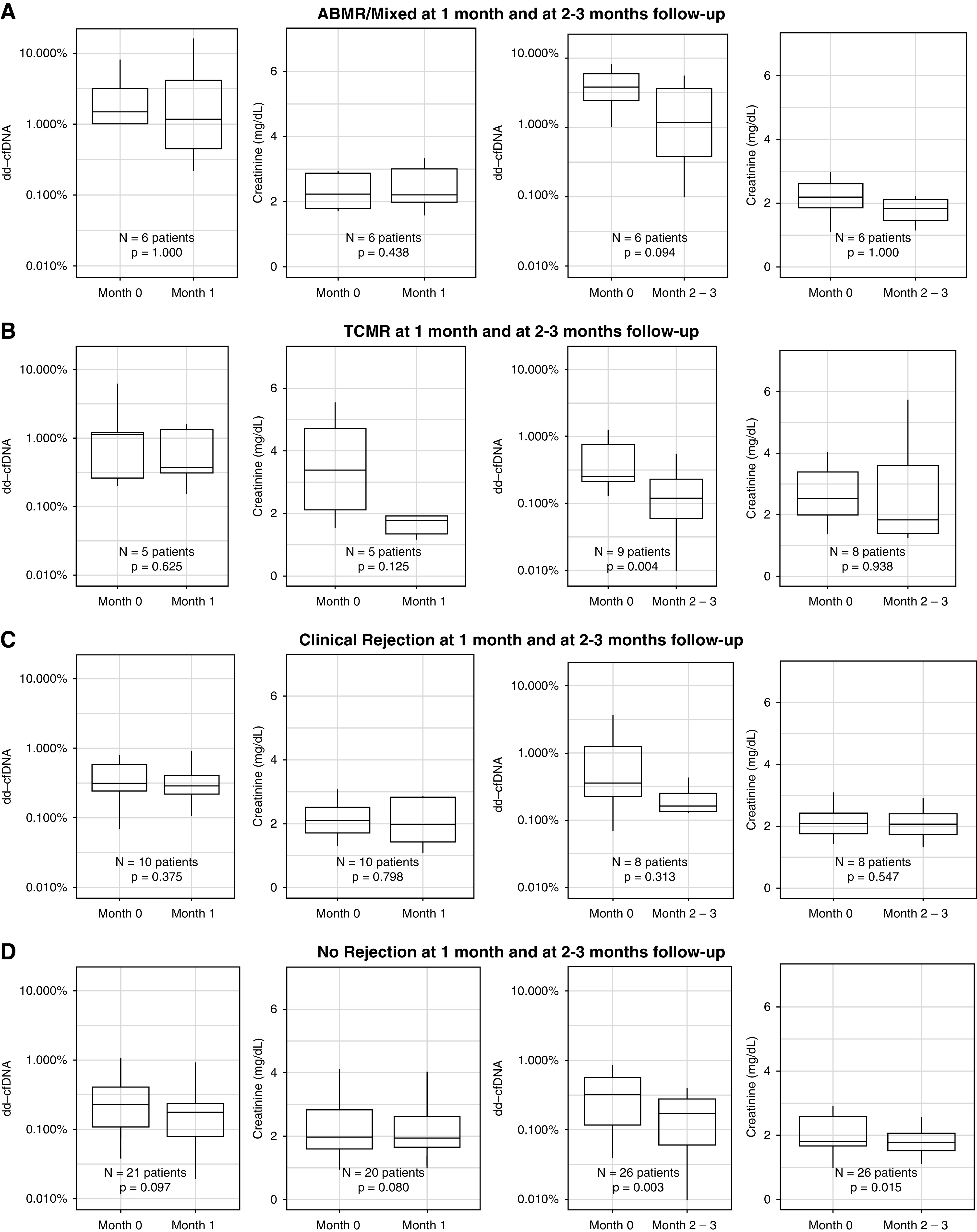
Distribution and change of dd-cfDNA and serum creatinine by group post-transplantation. For each time interval, and for each of the four groups, the within-patient change in dd-cfDNA and serum creatinine after 1 and 2–3 months of follow-up are shown. (A) ABMR/mixed at 1-month and at 2–3-month follow-up. (B) TCMR at 1-month and at 2–3-month follow-up. (C) Clinical Rejection at 1-month and at 2–3-month follow-up. (D) No Rejection at 1-month and at 2–3-month follow-up.
Table 2.
Changes in dd-cfDNA post-biopsy by type of rejection
| Group | 1-Month Follow-up | 2–3-Month Follow-up | ||||||
| N | Month 0, Median (25th, 75th), % | Month 1, Median (25th, 75th), % | P | N | Month 0, Median (25th, 75th), % | Month 2–3, Median (25th, 75th), % | P | |
| BPAR or treated rejection | 21 | 0.62 (0.26, 1.23) | 0.35 (0.23, 0.96) | 0.34 | 23 | 0.77 (0.23, 2.91) | 0.21 (0.11, 0.63) | 0.002 |
| Clinical (no BPAR) | 10 | 0.31 (0.25, 0.60) | 0.29 (0.22, 0.41) | 0.38 | 8 | 0.38 (0.23, 1.26) | 0.17 (0.14, 0.26) | 0.31 |
| ABMR | 6 | 1.61 (1.02, 3.28) | 1.20 (0.50, 4.94) | >0.99 | 6 | 3.85 (2.54, 6.10) | 1.32 (0.42, 3.87) | 0.09 |
| TCMR | 5 | 1.13 (0.26, 1.23) | 0.37 (0.31, 1.35) | 0.63 | 9 | 0.25 (0.21, 0.77) | 0.12 (0.06, 0.23) | 0.004 |
| No BPAR or treated rejection | 21 | 0.23 (0.11, 0.41) | 0.18 (0.08, 0.24) | 0.10 | 26 | 0.33 (0.12, 0.57) | 0.17 (0.06, 0.28) | 0.003 |
| Control | 36 | 0.37 (0.21, 0.51) | 0.25 (0.17, 0.52) | 0.27 | 62 | 0.24 (0.12, 0.42) | 0.15 (0.08, 0.23) | 0.004 |
The study groups are listed in the first column. For each group, distribution of dd-cfDNA at time of biopsy, at first test at 1 mo, and after 2–3 mo is given, along with the number of patients in each group with a dd-cfDNA measurement at that time point. Finally, a P value is given, showing whether dd-cfDNA exhibits a statistically significant change from time of biopsy to follow-up time for two different follow-up times: at 1 mo and at 2–3 mo. dd-cfDNA, donor-derived cell-free DNA; BPAR, biopsy–proven acute rejection; ABMR, antibody-mediated rejection; TCMR, T cell–mediated rejection.
Table 3.
Changes in creatinine post-biopsy by type of rejection
| Group | 1-Month Follow-up | 2–3-Month Follow-up | ||||||
| N | Month 0, Median (25th, 75th), mg/dl | Month 1, Median (25th, 75th), mg/dl | P | N | Month 0, Median (25th, 75th), mg/dl | Month 2–3, Median (25th, 75th), mg/dl | P | |
| BPAR or treated rejection | 21 | 2.3 (1.8, 2.8) | 1.9 (1.5, 2.8) | 0.75 | 23 | 2.2 (1.8, 2.9) | 1.9 (1.5, 2.7) | 0.92 |
| Clinical (no BPAR) | 10 | 2.1 (1.7, 2.5) | 2.0 (1.4, 2.8) | 0.80 | 8 | 2.1 (1.8, 2.4) | 2.1 (1.7, 2.4) | 0.55 |
| ABMR | 6 | 2.2 (1.8, 2.9) | 2.2 (2.0, 3.0) | 0.44 | 6 | 2.2 (1.9, 2.6) | 1.8 (1.5, 2.1) | >0.99 |
| TCMR | 5 | 3.4 (2.1, 4.7) | 1.8 (1.3, 1.9) | 0.13 | 8 | 2.5 (2.0, 34) | 1.8 (1.4, 3.6) | 0.94 |
| No BPAR or treated rejection | 20 | 2.0 (1.6, 2.8) | 1.9 (1.7, 2.6) | 0.08 | 26 | 1.8 (1.7, 2.6) | 1.8 (1.5, 2.1) | 0.02 |
The study groups are listed in the first column. For each group, distribution of creatinine at time of biopsy, at first test at 1 mo, and after 2–3 mo is given, along with number of patients in each group with a creatinine measurement at that time point. Finally, a P value is given, showing whether creatinine exhibits a statistically significant change from time of biopsy to follow-up time for two different follow-up times: at 1 mo and at 2–3 mo. BPAR, biopsy–proven acute rejection; ABMR, antibody-mediated rejection; TCMR, T cell–mediated rejection.
For serum creatinine, all values at the time of the clinically indicated biopsy were elevated (Table 3). However, longitudinal changes after the biopsy and rejection treatment did not significantly decline post-treatment except in the NR group. Serum creatinine in the TCMR group numerically declined to 1.8 mg/dl by 2–3-month follow-up but was not statistically significant (P=0.94).
Discussion
The value of dd-cfDNA as a noninvasive biomarker to discriminate active rejection from no active rejection has been analytically (6) and clinically validated (10), as demonstrated in the DART study (11). The efficacy and trajectory of dd-cfDNA as a monitoring tool after rejection treatment has not been previously shown across multiple centers. This report demonstrates the kinetics of dd-cfDNA as a tool for assessing allograft injury after treatment for rejection.
The shortcomings of serum creatinine, as a lagging indicator, were demonstrated in this study, with dd-cfDNA showing an expeditious decline when compared with conventional monitoring. Serum creatinine generally declines slowly, if at all, and is a crude, noninformative marker of rejection resolution. This is shown by elevations in dd-cfDNA and serum creatinine at the time of rejection, with dd-cfDNA differing after treatment, whereas serum creatinine remained relatively unchanged. Although the TCMR group showed some improvement in serum creatinine in line with a decrease in dd-cfDNA, there were no statistically significant changes in creatinine values among any of the other rejection groups.
The variation in dd-cfDNA values associated with biopsy–proven cellular rejection highlights the heterogeneity of TCMR. A statistically significant change in dd-cfDNA was found in the TCMR group that had a 2–3-month follow-up (0.25% at month 0 to 0.12%), but 0.25% is well below the published cutoff of 1% and not considered clinically significant. However, dd-cfDNA values were elevated (1.13%) at the time of diagnosis in the group with 2–3-month follow-up, which was clinically significant, then declined to a level of 0.37%. This may demonstrate that varying levels of allograft injury are associated with a comparable diagnosis of TCMR. Stites et al. (15) reported that Banff borderline and TCMR grade IA rejection episodes with dd-cfDNA >0.5% were associated with significant eGFR decline, recurrent rejections, and development of DSA when compared with those with the same histologic pattern where dd-cfDNA was ≤0.5%. Therefore, the value of dd-cfDNA as a continuous, rather than an absolute, variable may be important.
Consistent with prior publications, there was a significant difference in the behavior of TCMR and ABMR with respect to treatment (16,17). This was reflected in the pattern demonstrated by dd-cfDNA, with a decline after TCMR, whereas median values of dd-cfDNA in ABMR remained elevated >1%, or continued to increase, despite treatment. This highlights potential inadequate treatment for ABMR and ongoing injury to the graft. Further characterization of ABMR and DSA (MFI) to assess the response of dd-cfDNA and serum creatinine across various MFI thresholds would be important in future studies. This may elucidate the association between antibody strength and level of injury, or resolving injury, to the allograft. Thus, serial monitoring of dd-cfDNA during and after rejection treatment could allow expedited adjustments in therapy for persistent evidence of graft injury and potentially inadequate treatment, highlighting additional research opportunities.
Several strengths of this study demonstrate the performance of dd-cfDNA in response to rejection treatment. First, dd-cfDNA has been shown to distinguish graft injury from quiescence in a large, multi-center, prospective study (6,10,11). This CLIA approved laboratory test, with a turnaround time of approximately 48–72 hours, permits expedited analysis of graft injury (6). Next, this study used a large, independent reference group as a control (10). This confirms the dd-cfDNA levels associated with stable, healthy kidney transplant patients and is consistent with the prior analysis of DART (10,11). The dd-cfDNA results were also blinded to clinicians and did not bias management decisions. In addition, the t1/2 of dd-cfDNA is approximately 30 minutes, which provides a real-time assessment of ongoing damage or recovery from allograft injury, because the donor material is continuously released and cleared (12). Finally, dd-cfDNA has a 95% negative predictive value for rejection when ≤0.21% (10). All rejection groups in this study, with exception of the ABMR group, saw a change in dd-cfDNA with follow-up values <0.21% at 2–3 months post-treatment. Therefore, a clinician can be confident that they are not missing an important ongoing episode of rejection when dd-cfDNA results are <0.21%.
We acknowledge there are certain limitations in this study. First, the sample size in this study was small, affecting statistical significance; however, our protocol required that patients had assessment with a dd-cfDNA measurement concurrent with the clinically indicated biopsy, and a follow-up level obtained within 3 months. Although the number of patients who received rejection treatment is modest, the necessary subsets of rejection were required due to inherent differences in the natural history of TCMR and ABMR with response to treatment. Sample-size limitations were also due to lack of available dd-cfDNA levels for individual patients for both the 1 month and 2–3-month post-treatment analyses. Nevertheless, improvement in dd-cfDNA was evident for specific categories of rejection. In addition, most patients were enrolled at the time of a clinically indicated biopsy, without preceding baseline levels for comparison. Therefore, baseline levels were established from a separate control group. There was also a lack of histologic data, or uniformity in the use of biopsies, after rejection treatment was administered. Some centers perform biopsy and others relying on laboratory values (i.e., serum creatinine) to follow patients post–rejection treatment. Although repeat biopsies allow for comparison of histologic architecture to assess whether there is true resolution of pathology, they are invasive with increased hemorrhagic risk and findings can be patchy in distribution. In this study, only six of the 70 patients in this study had a follow-up biopsy at 1 month, and six patients had a follow-up biopsy in the 2–3-month range, making the analysis limited for histologic response to treatment. However, only seven of 35 patients received further rejection treatment at 1 month and four patients at 2–3 months; therefore, we suspect most patients had clinically recovered from the antecedent rejection events. Lastly, dd-cfDNA is a molecular marker of injury and there are other causes of allograft injury aside from rejection. Other non-rejection sources of injury, such as BK virus-associated nephropathy or de novo DSA, were not assessed in this study and may have contributed to the elevations in dd-cfDNA.
Inter-observer variability among histopathologic assessment of rejection has also been shown and tissue molecular markers align closely with dd-cfDNA compared with traditional histology, lending to increased precision as an adjunct to traditional histopathology (18). From these data, decreased dd-cfDNA could confirm recovery from a rejection event, whereas persistently elevated dd-cfDNA could signal incomplete recovery and the need for closer monitoring and/or additional treatment. However, future studies should be performed to validate these findings in a larger, prospective cohort that includes consensus on markers of rejection resolution, standardized dd-cfDNA and serum creatinine assessments, and longer follow-up to determine the effect on long-term kidney allograft health.
In summary, longitudinal monitoring of dd-cfDNA may be useful as a dynamic biomarker to assess the health of the kidney allograft after rejection treatment. Future studies may allow dd-cfDNA to differentiate adequate versus inadequate response to current treatment regimens and suggest the need to provide alternative therapeutic options or augment treatment practices. We speculate that serial surveillance of dd-cfDNA after rejection events may support strategies for precision medicine and immunosuppression, thereby improving post-transplant health.
Disclosures
D. Brennan reports being a consultant for Amplyx, CareDx, Medeor, Natera, and Sanofi; receiving grants/research support from CareDx; and receiving honoraria for participating in a speakers bureau for CareDx and Veloxis. J. Bromberg reports receiving research grant support from CareDx. D. Hiller reports being an employee of CareDx. R. Hinojosa reports receiving research grant support from CareDx, and receiving honoraria for participating in a speakers bureau for CareDx. R. Mannon reports receiving grant support from CareDx and Transplant Genomics. E. Poggio reports receiving honoraria for participating in a speakers bureau for CareDx. T. Wolf-Doty reports being an employee of CareDx.
Funding
The original DART study was funded by CareDx; no additional funding provided for this study.
Author Contributions
D.C. Brennan, J.S. Bromberg, R.B. Mannon, and E.D. Poggio were responsible for methodology; D. Hiller was responsible for formal analysis; T.K. Wolf-Doty conceptualized the study and wrote the original draft; and all authors reviewed and edited the manuscript.
References
- 1.Davis S, Cooper JE: Acute antibody-mediated rejection in kidney transplant recipients. Transplant Rev (Orlando) 31: 47–54, 2017. 10.1016/j.trre.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 2.Eikmans M, Gielis EM, Ledeganck KJ, Yang J, Abramowicz D, Claas FFJ: Non-invasive biomarkers of acute rejection in kidney transplantation: Novel targets and strategies. Front Med (Lausanne) 5: 358, 2019. 10.3389/fmed.2018.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong AS, Rothstein DM, Safa K, Riella LV: Outstanding questions in transplantation: B cells, alloantibodies, and humoral rejection. Am J Transplant 19: 2155–2163, 2019. 10.1111/ajt.15323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan SC, Bunnapradist S, Bromberg JS, Langone AJ, Hiller D, Yee JP, Sninsky JJ, Woodward RN, Matas AJ: Donor-derived cell-free DNA identifies antibody-mediated rejection in donor specific antibody positive kidney transplant recipients. Transplant Direct 4: e379, 2018. 10.1097/TXD.0000000000000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knight SR, Thorne A, Lo Faro ML: Donor-specific cell-free DNA as a biomarker in solid organ transplantation. A systematic review. Transplantation 103: 273–283, 2019. 10.1097/TP.0000000000002482 [DOI] [PubMed] [Google Scholar]
- 6.Grskovic M, Hiller DJ, Eubank LA, Sninsky JJ, Christopherson C, Collins JP, Thompson K, Song M, Wang YS, Ross D, Nelles MJ, Yee JP, Wilber JC, Crespo-Leiro MG, Scott SL, Woodward RN: Validation of a clinical-grade assay to measure donor-derived cell-free DNA in solid organ transplant recipients. J Mol Diagn 18: 890–902, 2016. 10.1016/j.jmoldx.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 7.De Vlaminck I, Valantine HA, Snyder TM, Strehl C, Cohen G, Luikart H, Neff NF, Okamoto J, Bernstein D, Weisshaar D, Quake SR, Khush KK: Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med 6: 241ra77, 2014. 10.1126/scitranslmed.3007803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck J, Oellerich M, Schulz U, Schauerte V, Reinhard L, Fuchs U, Knabbe C, Zittermann A, Olbricht C, Gummert JF, Shipkova M, Birschmann I, Wieland E, Schütz E: Donor-derived cell-free DNA is a novel universal biomarker for allograft rejection in solid organ transplantation. Transplant Proc 47: 2400–2403, 2015. 10.1016/j.transproceed.2015.08.035 [DOI] [PubMed] [Google Scholar]
- 9.Crespo-Leiro M, Hiller D, Woodward R, Grskovic M, Marchis C, Song M, Collins J, Zuckermann A: Analysis of donor-derived cell-free DNA with 3-year outcomes in heart transplant recipients. J Heart Lung Transplant 36: S69–S70, 2017. 10.1016/j.healun.2017.01.172 [DOI] [Google Scholar]
- 10.Bromberg JS, Poggio E, Bunnapradist S, Langone A, Sood P, Matas AJ, Mannon RB, Mehta S, Sharfuddin A, Fischbach B, Narayanan M, Jordan SC, Cohen DJ, Zaky ZS, Hiller D, Woodward RN, Grskovic M, Sninsky JJ, Yee JP, Bloom RD: Biological variation of donor-derived cell-free DNA in renal transplant recipients: Clinical implications. JALM 2: 309–321, 2017. 10.1373/jalm.2016.022731 [DOI] [PubMed] [Google Scholar]
- 11.Bloom RD, Bromberg JS, Poggio ED, Bunnapradist S, Langone AJ, Sood P, Matas AJ, Mehta S, Mannon RB, Sharfuddin A, Fischbach B, Narayanan M, Jordan SC, Cohen D, Weir MR, Hiller D, Prasad P, Woodward RN, Grskovic M, Sninsky JJ, Yee JP, Brennan DC; Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients (DART) Study Investigators: Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol 28: 2221–2232, 2017. 10.1681/ASN.2016091034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thongprayoon C, Vaitla P, Craici IM, Leeaphorn N, Hansrivijit P, Salim SA, Bathini T, Rivera FHC, Cheungpasitporn W: The use of donor-derived cell-free DNA for assessment of allograft rejection and injury status. J Clin Med 9: 1480, 2020. 10.3390/jcm9051480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinojosa RJ, Chaffin K, Gillespie M, Villarreal VH Jr: Donor-derived cell-free DNA may confirm real-time response to treatment of acute rejection in renal transplant recipients. Transplantation 103: e61, 2019. 10.1097/TP.0000000000002579 [DOI] [PubMed] [Google Scholar]
- 14.Haas M: The revised (2013) Banff classification for antibody-mediated rejection of renal allografts: Update, difficulties, and future considerations. Am J Transplant 16: 1352–1357, 2016. 10.1111/ajt.13661 [DOI] [PubMed] [Google Scholar]
- 15.Stites E, Kumar D, Olaitan O, John Swanson S, Leca N, Weir M, Bromberg J, Melancon J, Agha I, Fattah H, Alhamad T, Qazi Y, Wiseman A, Gupta G: High levels of dd-cfDNA identify patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am J Transplant 20: 2491–2498, 2020. 10.1111/ajt.15822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts DM, Jiang SH, Chadban SJ: The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation 94: 775–783, 2012. 10.1097/TP.0b013e31825d1587 [DOI] [PubMed] [Google Scholar]
- 17.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012. 10.1111/j.1600-6143.2011.03840.x [DOI] [PubMed] [Google Scholar]
- 18.Moinuddin I, Kumar D, Kamal L, King A, Kang L, Levy M, Bobba S, Massey D, Halloran P, Gupta G: Calibration of Donor-Derived Cell-Free DNA Criteria for Rejection with Molecular Diagnoses of Kidney Transplant Biopsies. Am J Transplant 20[suppl 3]: 2020 [Google Scholar]