Visual Abstract
Keywords: dialysis, arteriovenous access, barriers, hemodialysis, mixed methods, quality improvement, vascular access
Key Points
• Targeting barriers to arteriovenous access through education, needs assessment, peer support, care navigation, and electronic supports was acceptable.
• The program yielded improvements in patient self-efficacy and knowledge, and trends toward improvements in patient and provider confidence.
Abstract
Background
Guidelines recommend pre-emptive creation of arteriovenous (AV) access. However, <20% of US patients initiate hemodialysis (HD) with a functional AV access. We implemented a quality improvement (QI) program to improve pre-HD vascular access care.
Methods
After conducting qualitative research with key informants, we implemented a 7-month vascular access support QI program at Geisinger Health. The program targeted patient and health system barriers to AV access through education, needs assessment, peer support, care navigation, and electronic supports. We performed pre-, intra-, and postprogram stakeholder interviews to identify program barriers and facilitators and to assess acceptability. In a research substudy, we compared pre- and postprogram self-efficacy, knowledge, and confidence navigating vascular access care.
Results
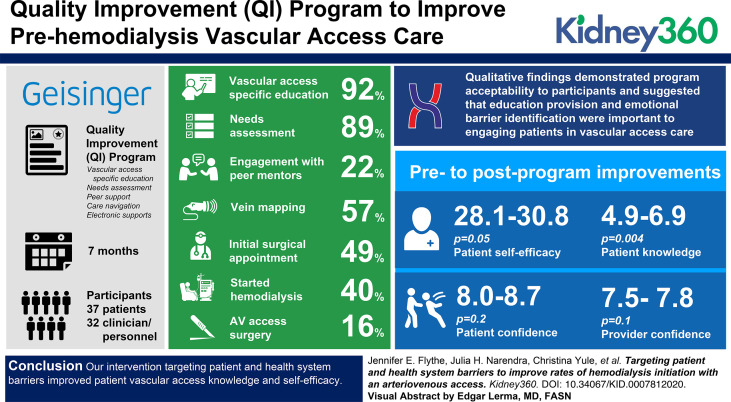
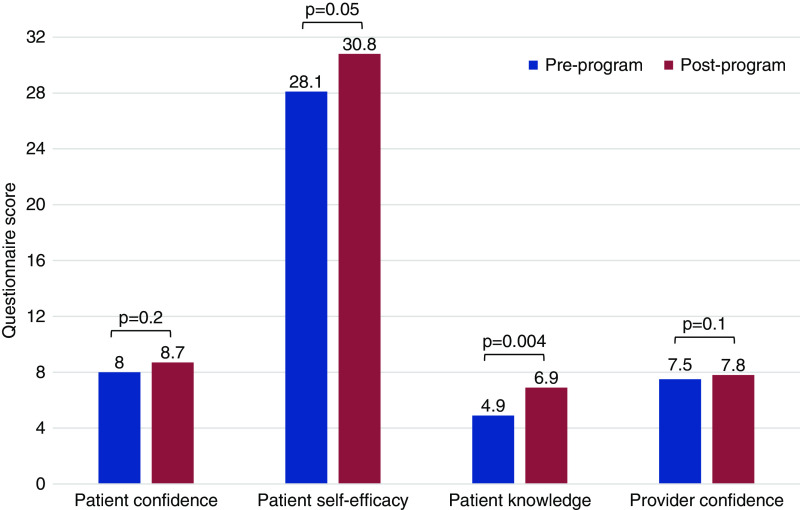
There were 37 patient and 32 clinician/personnel participants. Of the 37 patients, 34 (92%) completed vascular access–specific education, 33 (89%) underwent needs assessment, eight (22%) engaged with peer mentors, 21 (57%) had vein mapping, 18 (49%) had an initial surgical appointment, 15 (40%) underwent AV access surgery, and six (16%) started HD during the 7-month program. Qualitative findings demonstrated program acceptability to participants and suggested that education provision and emotional barrier identification were important to engaging patients in vascular access care. Research findings showed pre- to postprogram improvements in patient self-efficacy (28.1–30.8, P=0.05) and knowledge (4.9–6.9, P=0.004), and trends toward improvements in confidence among patients (8.0–8.7, P=0.2) and providers (7.5–7.8, P=0.1).
Conclusions
Our intervention targeting patient and health system barriers improved patient vascular access knowledge and self-efficacy.
Clinical Trial registry name and registration number:
Breaking Down Care Process and Patient-level Barriers to Arteriovenous Access Creation Prior to Hemodialysis Initiation, NCT04032613
Introduction
Vascular access is one of the most challenging and expensive aspects of hemodialysis (HD) care. Use of an arteriovenous (AV) access (fistula or graft) is associated with lower sepsis risk, lower hospitalization rates, and 50% lower mortality rates as compared with use of a catheter (1–6). Experts have estimated that a 50% increase in AV access–based HD initiation would lead to an annual savings of $1 billion (7). However, <20% of individuals in the United States start maintenance HD with a functional AV access (8).
Many prior efforts to increase AV access use focused on access creation after HD initiation. Encouragingly, the Fistula-First Initiative (9) led to a 30% increase in fistula use among prevalent patients on HD by targeting healthcare organizational deficiencies and inadequate patient education (10). However, there has been markedly less success in the predialysis period. Health system barriers (e.g., complex care processes, insufficient interprovider communication) (11–13) and patient barriers (e.g., fear, inadequate education) (14,15) likely contribute to low rates of AV access–based HD initiation. Prior research suggests that assistance with care navigation improves aspects of vascular access care, but care navigation alone has not been shown to improve AV access–based HD initiation in the United States (16,17). Such findings suggest barriers exist beyond those related to care processes. Prior interventions have not sufficiently addressed patient-level barriers, such as fear, dialysis reluctance, and worries about body disfigurement and needles, which also impede care (14–16,18–23).
We conducted a quality improvement (QI) project targeting both patient and health system barriers to AV access–based HD initiation, with the goal of improving pre-HD vascular access care outcomes and patient experiences. Simultaneously, we conducted a research substudy to assess the intervention's effect on patient- and care team–reported outcomes.
Materials and Methods
Overview
Figure 1 depicts project activities. To inform intervention development, we conducted qualitative research to elicit perceived barriers to pre-HD AV access creation from key stakeholders. We then developed our intervention and implemented it as a QI project at Geisinger Health. During implementation, we collected data on participant experiences and clinical processes, responsively updating the intervention to optimize its implementation. In addition, we offered QI participants the opportunity to enroll in a research substudy examining changes in patient self-efficacy, knowledge, and confidence and medical provider/personnel confidence.
Figure 1.
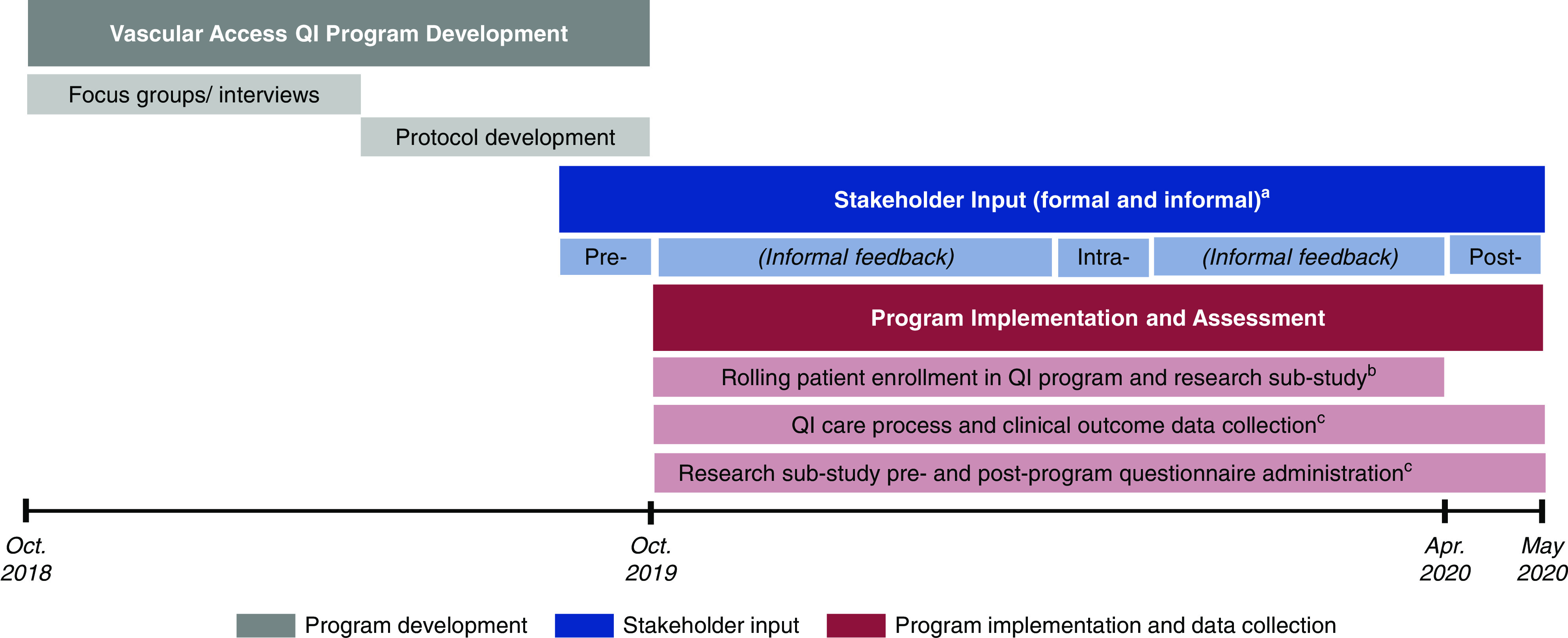
The figure displays project development, implementation, and assessment activities. aFormal stakeholder input was obtained pre-, intra-, and post-QI program implementation via semistructured interviews with key stakeholders. Informal feedback was collected throughout the program through in-person and email interactions with program participants. These interviews were conducted as part of QI program implementation and assessment, and were approved as QI by Geisinger Health. bEligible patients were enrolled in the QI program on a rolling basis for the first 6 months of the program (15 in October 2019, two in November 2019, one in December 2019, seven in January 2020, seven in February 2020, and five in March 2020). All QI participants were offered the opportunity to enroll in the research substudy at the time of QI program enrollment. cClinical outcome data (e.g., number of completed vascular access care steps, number and type of patient-level barriers to vascular access care, number and type of peer mentoring contacts, and vascular access type at HD initiation) were collected on all QI program patient participants. Research substudy participants completed pre- and postprogram questionnaires assessing patient self-efficacy, knowledge, and confidence, and medical provider/personnel confidence. EHR, electronic health record; QI, quality improvement.
This was an ancillary project to the PREPARE NOW study, a cluster-randomized controlled trial designed to quantify the effectiveness of integrated health system interventions to improve patients’ preparation for kidney failure treatments (24). The QI project was approved by Geisinger Health, and all protocols were approved by the Duke Health Institutional Review Board (IRB), the central IRB for PREPARE NOW (IRB Pro00075488). All participants provided consent to access their electronic health records (EHRs). We performed, analyzed, and reported the QI project in accordance with the Standards for Quality Improvement Reporting Excellence Guidelines (Supplemental Table 1) (25). We conducted our intervention as QI to support a systematic, clinic-level approach to implementation and evaluation. We assessed knowledge, self-efficacy, and confidence in a research subset so as not to burden all QI participants with additional questionnaires. Research substudy participants provided additional consent.
QI Intervention: Vascular Access Support Program
We designed the vascular access support program to support patients through pre-emptive AV access creation by targeting patient and health system barriers. The program consisted of (1) vascular access–specific patient education, (2) needs and barriers assessments, (3) peer mentoring, (4) care navigation, and (5) a vascular access–specific electronic dashboard for navigators (Table 1). Nurse navigators used a previously described CKD EHR registry (24) to identify patients potentially eligible for the program and, after obtaining agreement from the treating nephrologist, enrolled patients. Upon program enrollment, patients were scheduled for an education session and a needs and barriers assessment. During the education session, patients were offered the opportunity to connect with a peer mentor. The navigators then supported each patient through AV access processes by issuing appointment reminders, assisting with scheduling, and providing resources. Throughout, they used a vascular access–specific electronic dashboard to monitor progress.
Table 1.
Vascular access clinical support program components
| Component | Purpose | Description |
| Targeting patient barriers | ||
| Vascular access–specific education | Improve vascular access knowledge | Format: Individual or small group sessions dedicated to vascular access education (in person, telephone) |
| Content: Vascular access types and related terms, assocated care process steps, acknowledgement of common emotions and complications | ||
| Materials: investigator-developed and AAKP brochures | ||
| Needs and barriers assessment | Identify barriers to vascular access care and provide targeted resources | Assessment of financial, transportation, emotional, and other barriers to vascular access care; assessment of preferred communication mode; and provision of appropriate resources (referrals, support, etc.) |
| Opportunity to reinforce vascular access education content | ||
| Peer mentoring | Provide emotional support and practical care navigation tips | Peer-to-peer supportive interaction (in person, telephone, email) |
| Frequency and mode of interaction selected by mentees and mentors | ||
| Mentors underwent vascular access education and peer mentorship training before mentee connection | ||
| Targeting health system barriers | ||
| Vascular access care navigation | Provide care coordination support | Identify patients in need of vascular access planning and secure nephrologist agreement for patient enrollment |
| Facilitate care coordination, appointment scheduling and reminders, and peer mentor program enrollment | ||
| Prompt providers to communicate with each other | ||
| Address patient questions and concerns and provide education reinforcement | ||
| Vascular access–specific electronic dashboard | Enable efficient patient monitoring and care planning | Enable monitoring of patients through vascular access care steps |
| Organized by care step to facilitate identification of upcoming or missed appointments, unscheduled referrals, and study results | ||
AAKP, American Association of Kidney Patients.
Setting and Participants
Geisinger Health provides care for >4 million people in 45 Pennsylvania counties and seven southern New Jersey counties. The QI intervention was implemented at the Geisinger Danville Nephrology Clinic, a subspecialty clinic with ten nephrologists who care for approximately 1500 individuals with kidney disease. We selected this clinic because it was not randomized in the PREPARE NOW study.
Danville Nephrology Clinic patients who had an eGFR ≤25 ml/min per 1.73 m2 and a 2-year Kidney Failure Risk Equation (KFRE) (26,27) of >10% were eligible to participate in the QI program with agreement from their nephrologist. Patients were excluded if they were planning for peritoneal dialysis (PD) or conservative care, had already initiated the AV access creation process, or had cognitive impairment. Navigators identified potentially eligible patients from the CKD registry (24) and assessed eligibility via chart review. All patients participating in the QI program were eligible for the research substudy. Medical providers and personnel at the participating clinic and associated surgery and radiology clinics were also eligible for the research.
To inform QI program development, we performed focus groups and interviews with patients, caregivers, and providers/personnel. Focus groups and interviews were held at Geisinger Health (a semiclosed system integrated with a single insurer serving a rural population) and University of North Carolina Health System (an open, network-based system serving a socioeconomically and racially diverse, midsized, metropolitan population). Patients with nondialysis-dependent advanced CKD (eGFR ≤20 ml/min per 1.73 m2) with a preference for HD (and caregivers), and patients with dialysis-dependent kidney failure who have initiated HD within 1 year (and caregivers), were eligible to participate in focus groups. Interview participants included nephrologists, surgeons, and interventionalists, along with nephrology, surgery, and radiology clinic nurses, managers, and schedulers. We used purposive sampling to recruit individuals with differing vascular access and professional experiences.
Data Collection
Focus Groups and Interviews
Trained research assistants conducted the preintervention focus groups and semistructured interviews, using standardized moderator and interview guides (Supplemental Table 2). Focus groups were 60–90 minutes, and interviews were 30–45 minutes. Both were recorded and professionally transcribed. Participants provided informed consent.
After drafting the intervention protocol, we conducted preimplementation interviews with clinic stakeholders to assess resource availability, program perceptions, and intervention fit. We then conducted intraimplementation interviews with participating patients and care team members to assess program barriers, facilitators, and acceptability. We captured participants’ perceptions of program effect and potential for sustainability with postimplementation interviews. Trained interviewers used interview guides and structured note templates to conduct and document all interviews. Interviews were conducted under QI protocols.
EHR
During the QI program, we collected relevant EHR data, including demographics, comorbidities, laboratory results, and inpatient and outpatient encounters and procedures, and vascular access type at HD initiation, on all enrolled patients. The prespecified, exploratory clinical outcomes of the QI program included number of completed vascular access care steps, number and type of patient-level barriers to vascular access care, number and type of peer mentoring contacts, and vascular access type at HD initiation.
Patient- and Care Team–Reported Outcomes
Research substudy participants completed pre- and postprogram questionnaires (Supplemental Table 3). Patients completed the eight-item Perceived Kidney Disease Self-Management Scale (28), and investigator-developed questionnaires on vascular access knowledge (eight items) and confidence in navigating vascular access care (three items). Care team members completed an 11-item, investigator-developed questionnaire assessing confidence in helping patients navigate vascular access care.
Analysis
Qualitative Data
We analyzed preprogram qualitative data to identify barriers to AV access–based HD initiation. We imported transcriptions into ATLAS.ti software (version 7; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany) and analyzed the data using directed content analysis (29,30). Three team members (S. Manivannan, S. Murphy, J.H.N) used line-by-line coding to group text according to prespecified barrier types (patient, health system), adding new codes when text could not be categorized within the initial coding scheme. Through triangulation, researchers (S. Manivannan., S. Murphy, J.H.N, J.E.F.) resolved discrepancies and developed a final coding framework. Findings were used to inform QI program development.
We analyzed data from pre-, intra-, and postintervention interviews to characterize program perceptions and identify opportunities for refinement. Data were compiled into tables organized by interview timing, interviewee type, and program component. When considering refinements to the QI program on the basis of implementation challenges identified during stakeholder interviews, we weighted information provided by two or more interviewees.
Quantitative Data
We used descriptive statistics (e.g., count [%], median [interquartile range]) to report program participant characteristics and program component completion. We compared pre- and postprogram questionnaire scores using paired t tests. Analyses were performed in SAS version 9.4 (31) and R version 3.3.2 or later (32).
Results
Pre-QI Program Findings and Intervention Refinement
Barriers to AV Access–Based HD Initiation
We conducted four focus groups with 18 patients and six caregivers, and 16 individual interviews with providers/personnel (Supplemental Table 4). Table 2 displays the identified barriers to AV access–based HD initiation.
Table 2.
Illustrative quotations about barriers to predialysis vascular access care from focus group and interview participants
| Theme | Quotation |
| Patient barriers | |
| Experiencing negative emotions | |
| Fear | Patient with CKD: “I was worried and angry as all heck [when I found out I needed dialysis].” |
| Patient with CKD: “The thing is I don't really want to talk about it. No, I haven’t talked about it yet. I had an appointment at one time. I just didn't go because I was scared.” | |
| Patient on dialysis: “There's worry your life is changing. There's worry about the surgery itself.” | |
| Denial | Patient with CKD: “When I first heard about [needing AV access], it actually made me not want to come back. So, I just shut it off. I just didn’t want to face it, so I didn’t go.” |
| Patient with CKD: “I’m trying to avoid [follow-up for his AV access].” | |
| Surgeon: “[Patients] kind of want to ignore it until they’re admitted with a catheter. They might’ve even been someone we saw, who's like: ‘Yeah, I don’t know, I’ll call you back. I’m not sure I want surgery next week. I’ll get back to you about that.’ They typically don’t get back to us till they’re on dialysis.” | |
| Uncertainty | Patient with CKD: “Anything unfamiliar to you, you tend to worry and dread it coming. You know it's got to happen, but it doesn’t make it any better.” |
| Patient with CKD: “I constantly worry that I’m going to, like, in life, you know, you just bump my arm, or fall. I don't know how fragile that [fistula] is, like how much I should be worried.” | |
| Patient on dialysis: “Oh man, just the stress, like to kill me. Because, you just don’t know really what's going to happen.” | |
| Nephrology clinic nurse: “It's very difficult for people to make those decisions, and there's always the hope that their kidneys are going to recover.” | |
| Having inadequate vascular access and dialysis knowledge | Patient with CKD: “If I could have gotten more information on what to expect after the fistula was done. Maybe that would have helped me feel a little more reassured.” |
| Patient on dialysis: “I took the Kidney Smart Class, and they did touch on [AV access], but not enough to really let you know what you were getting into.” | |
| Caregiver: “See his [fistula] was in, but his kidneys went that fast. There wasn’t time for it to mature, and he had to get the catheter. There was no head's up that something like that might happen… Things happen. There's going to be hiccups along the way—having the conversation about things like that is good. But you don’t hear that.” | |
| Surgery nurse: “A large majority of the time, [patients] have no idea why they’re here. They have no idea what a fistula is. They have no idea what a graft is.” | |
| Access center nurse: “When they get to me [vessel mapping], they don’t even know the difference between a fistula and a graft, and some people think they will have stuff hanging out of their arm. There's very little education.” | |
| Health system barriers | |
| Poor interprovider communication | Surgery nurse: “There needs to be a single person who can help facilitate all these patients that is in the know versus us doing our thing, and nephrology doing their thing. I think it would help if there was a single person monitoring all these patients and has a means of getting in touch with them.” |
| Access center nurse: “You’ve got the nephrologists in this medical record, the access center in that record, the surgeon in another. You’ve got a lot of turning wheels in one patient, and the doctors are not on the same page.” | |
| Interventionalist: “I think multiple specialties working collaboratively to deliver a service is important.” | |
| Lack of a centralized approach to care navigation and patient monitoring | |
| Patient with CKD: “I’ll tell you though, it's hard getting through to anybody in the hospital anymore. You call, and you get switched all around.” | |
| Care partner: “His vascular surgeon is at so many different sites, he never has time to keep track of you.” | |
| Nephrology clinic nurse: “I think the biggest barrier is that there's not someone guiding them through the process… there's a lot of follow-up that has to happen, and there's not one person guiding that sort of journey.” | |
| Surgeon: “They have a hard time navigating the healthcare system. It's often a multistep process. You see your nephrologist, you get your mapping, you see your surgeon, you get a surgery date, you might have something else in between. So, all those little things, you know, if there's someone who is identifying all these little bumps in the road and getting the patients through them, that would be helpful.” |
Focus group participants included patients and care partners. Interview participants included nephrologists; surgeons; interventionalists; and nephrology, surgery, and radiology clinic nurses and schedulers. AV, arteriovenous.
Participants identified the patient barriers of negative emotions (fear, uncertainty, denial) and inadequate education, and the health system barriers of poor interprovider communication and lack of a centralized approach to care navigation as important obstacles to pre-HD vascular access care. Specifically, patients reported that feelings of fear and uncertainty about starting dialysis often led to denial and, subsequently, inertia and poor engagement in their care. Low engagement was compounded by inadequate education about vascular access, leaving many to arrive at appointments ill-equipped to engage in shared decision making and unprepared for potential complications and/or extra procedures that often arose. Both providers and patients noted that these patient barriers were exacerbated by health system challenges, such as poor interprovider communication and lack of coordinated systems. As a result, many patients started the AV access creation process, but never finished before HD initiation. Participants of all types suggested that strong patient-provider relationships, dedicated vascular access education, peer support, care partner involvement, and individualized care navigation may help overcome these barriers.
Responsive Updates to the QI Intervention
We refined our intervention in response to these findings. To address the concern that existing vascular access educational materials were overwhelming and provided too late, we developed an appropriate health literacy–level educational brochure and scheduled educational sessions early in AV access evaluation (Supplemental Table 5). To better incorporate emotional and educational support, we bolstered our peer mentoring program, encouraged care navigators to involve care partners, and updated our needs and barriers assessment. Finally, we updated the electronic dashboard to be sortable by care step to support providers who lacked systematic approaches to monitoring patients.
QI Program and Research Participant Characteristics
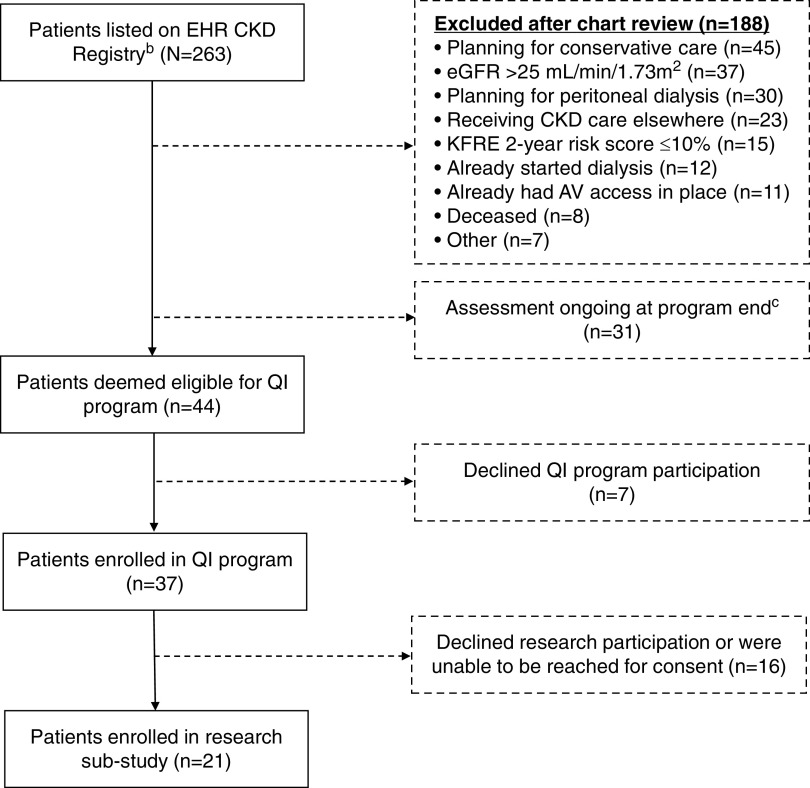
Figure 2 and Table 3 display QI program and research substudy participants. Of the 263 patients on the CKD registry during the project period, 44 patients met program eligibility criteria. Planning for conservative care (n=45), eGFR >25 ml/min per 1.73 m2 (n=37), and planning for peritoneal dialysis (n=30) were the most common exclusion reasons, and 31 (12%) remained under review at program end (i.e., awaiting nephrologist approval of program enrollment). Of the 44 eligible patients, 37 (84%) and 21 (48%) patients were enrolled in the QI program and research substudy, respectively. The mean±SD age of QI patient participants 64±14 years, 12 (32%) participants were female, and 36 (97%) were White. At program entry, the mean±SD eGFR was 18±5 ml/min per 1.73 m2, and the mean±SD 2-year KFRE score was 43%±29%. Patient research participants had similar characteristics. Overall, 32 providers/personnel participated in the QI program (eight nephrologists, 14 surgeons, six interventionalists, two navigators, and two clinic personnel), of whom 25 participated in the research substudy.
Figure 2.
The figure displays a flowchart of QI program and research substudy patient participants. aIn addition to the displayed patient QI and research substudy participants, there were 32 medical provider/personnel QI participants and 25 medical provider/personnel research substudy participants. bThe EHR population-based kidney disease registry (i.e., continually updated electronic list, called the “Kidney Transitions Registry”) incorporates an automated risk prediction tool (KFRE) alongside the Geisinger EHR platform. Outpatient data from the EHR are processed nightly to identify qualifying patients. The KFRE is a well-validated algorithm designed to help providers identify individuals with a high predicted risk of developing kidney failure within 2 years on the basis of their age, sex, eGFR, urine albumin-creatinine ratio, calcium, phosphate, albumin, and bicarbonate) (26,27). cAssessment ongoing at the end of the program indicates the navigator was awaiting nephrologist approval for program enrollment. In many cases, nephrologists were waiting for an upcoming appointment to discuss the program with the patient before agreeing to program enrollment. AV, arteriovenous; KFRE, Kidney Failure Risk Equation.
Table 3.
Characteristics of the vascular access quality improvement program participants
| Characteristic | QI Program | Research Substudy |
| Patients, N | 37 | 21 |
| Age (yr) | 63 (58–74) | 67 (55–74) |
| Female | 12 (32) | 5 (24) |
| Race | ||
| Black | 1 (3) | 0 |
| White | 36 (97) | 21 (100) |
| Highest level of education completed | — | |
| Less than high school | 1 (5) | |
| High school graduate or GED | 10 (47) | |
| Some college | 5 (23) | |
| 4-Year college degree or more | 4 (20) | |
| Missing | 1 (5) | |
| Insurance type | ||
| Geisinger Health Plan | 18 (49) | 8 (38) |
| Other private or commercial plan | 6 (16) | 3 (14) |
| Medicare | 11 (30) | 8 (38) |
| Self-pay | 1 (3) | 1 (5) |
| Other | 1 (3) | 1 (5) |
| Charlson comorbidity index(37) | 7 (5,8) | 7 (5, 8) |
| Diabetes | 24 (65) | 15 (71) |
| KFRE score (%) | 40 (10–60) (n=34) | 0.4 (0.1–0.6) (n=20) |
| eGFR (ml/min per 1.73 m2) | 20 (17–22) | 19 (17–23) |
| Distance from home to nephrology clinic (miles) | 18 (13–23) | 18 (13–23) |
| Duration of nephrology care (mo) | 37 (14–93) | 66 (25–147) |
| Hospitalized in 90 d prior to program enrollment | 5 (14) | 4 (19) |
| Medical providers and clinic personnel, N | 32 | 25 |
| Age (yr) | — | 38 (35–42) (n=24) |
| Female | 14 (44) | 12 (48) |
| Race | ||
| Black | 0 | 0 |
| White | 26 (81) | 22 (88) |
| Other | 6 (19) | 3 (12) |
| Professional role | ||
| Nephrologist | 8 (25) | 8 (32) |
| Surgeon | 14 (44) | 10 (40) |
| Interventional radiologist | 6 (19) | 3 (12) |
| Nephrology clinic nurse | 2 (6) | 2 (8) |
| Case manager | 2 (6) | 2 (8) |
| Time in current role | — | |
| ≤1 yr | 7 (28) | |
| 2–4 yr | 7 (28) | |
| ≥5 yr | 11 (44) | |
| Time working with vascular access | — | |
| ≤1 yr | 2 (8) | |
| 2–4 yr | 6 (24) | |
| ≥5 yr | 17 (68) |
Participant characteristics at time of QI program enrollment. Values are presented as n (%) or median (interquartile range). QI, quality improvement; GED, general educational development; KFRE, Kidney Failure Risk Equation.
Clinical and Patient- and Care Team–Reported Outcomes
Patients were enrolled in the QI program on a rolling basis between October 1, 2019 and March 31, 2020, with 15, two, one, seven, seven, and five patients enrolled in October, November, December, January, February, and March, respectively. The mean±SD time in the program was 4.9±2.1 months. Overall, of the 37 enrolled patients, 34 (92%) completed vascular access–specific education, 33 (89%) underwent needs and barriers assessment, eight (22%) engaged peer mentors, 21 (57%) completed vein mapping, 18 (49%) had an initial surgical appointment, 15 (40%) underwent AV access surgery, and six (16%) started HD during the 7-month program period. Of the 18 patients who were enrolled in the first 3 months of the program, 17 (94%) completed vascular access–specific education, 17 (94%) underwent needs and barriers assessment, ten (56%) completed vein mapping, ten (56%) had an initial surgical appointment, and nine (50%) underwent AV access surgery.
The median (interquartile range) time between vein mapping and first surgical appointment, first surgical appointment and AV access surgery, and AV access surgery and HD initiation was 0 (0–0), 26 (10–40), and 52 (20–56) days, respectively. Of the six patients who started HD, two had a functioning AV access, two had a nonmature AV access, one had AV access surgery within 5 days of HD initiation, and one had a catheter without known plans for AV access creation.
Of the 37 program participants, ten (27%) participants expressed interest in peer mentorship, and eight (22%) participants connected with a mentor during the program. There were a total of 30 mentor interactions (two in person and 28 by telephone) with a mean±SD of 4±2 encounters per dyad and a mean±SD relationship duration of 68±56 days. The most common reasons for disinterest in peer mentorship were having a friend or family member on dialysis from whom they preferred to seek information, and not wanting to talk about HD until closer to initiation. Among the 33 patients who underwent needs and barriers assessment, 20 (61%), 12 (36%), 16 (48%), and seven (21%) patients met the criteria for low education, low health literacy, financial difficulty, and limited transportation, respectively.
Research findings showed significant pre- to postprogram improvements in patient self-efficacy (P=0.05) and knowledge (P=0.004), and trends toward improvements in patient (P=0.2) and provider (P=0.1) confidence (Figure 3).
Figure 3.
Patient- and care team–reported outcomes before and after program implementation. aScores from the 16 patients and 23 providers/clinic personnel who completed both pre- and postprogram surveys are reported as means. Differences in pre- and postprogram scores were assessed with paired t tests.
QI Program Implementation Findings and Responsive Changes
We conducted 14 preprogram interviews (eight patients and six providers/personnel), 34 intraprogram interviews (29 patients and six providers/personnel), and 28 postprogram interviews (11 patients, four peer mentors, and 13 providers/personnel). During the interviews, we identified a number of potential barriers to effective implementation, made responsive changes to the program, and developed recommendations for future implementations (Table 4).
Table 4.
Barriers to QI program implementation, responsive changes, and future recommendations
| Identified Barriers (pre- and intraprogram) | Responsive Changes | Remaining Barriers (postprogram) | Future Recommendations | |
| Program eligibility criteria and enrollment | ||||
| [Pre-] Eligibility threshold of eGFR <20 ml/min per 1.73 m2 felt to be too restrictive and could lead to missed opportunities for early education | Changed threshold to eGFR <25 ml/min per 1.73m2 | [Post-] Eligibility threshold of eGFR <25 ml/min per 1.73 m2 identified some patients for whom nephrologists thought vascular access planning premature, and some patients were reluctant | Continue to alert nephrologists to patients with eGFR <25 ml/min per 1.73 m2 to prompt consideration | |
| Use referral to modality-education class as trigger to enroll patients in the vascular access program | ||||
| [Intra-] Nephrologists wanted to speak to patients about vascular access before navigator contacting patients | Ensured that navigators contacted nephrologists before approaching patients about program enrollment | |||
| [Intra-] Difficult for navigators to meet in person with nephrologists to discuss program-eligible patients | ↑ EHR message use and ↓ in-person meetings | [Post-] Paper notices improved communication/provider awareness, but more communication needed | Obtain stronger nephrologist buy-in preprogram | |
| Placed paper notices on exam room doors of eligible patients to remind nephrologists to discuss | Give medical providers access to electronic dashboard | |||
| Vascular access–specific patient education | ||||
| [Pre-] Need for standardized education | Developed education session facilitator guide | [Post-] Patients and care partners desired supplemental education video and more patient testimonials | Develop video to complement written materials | |
| Encourage use of peer mentoring program | ||||
| [Intra-] Need for supplemental resources | Developed handout with resource weblinks | |||
| [Intra-] COVID-19 pandemic interrupted in-person education sessions | Shifted to telephone-based education sessions | |||
| Peer mentoring | ||||
| [Pre-] Concern that a national peer mentoring program (with mentor telephone access) would not be used | Developed local peer mentor program | [Post-] Mentor training did not have enough time for mock mentee interactions | Increase time for peer mentor training | |
| Tailored program to vascular access by equipping mentors with lists of common barriers to vascular access care and frequently asked questions | ||||
| [Intra-] Need for vascular access education for mentors | Developed vascular access education “refresher” that was provided to mentors before mentee matching | [Post-] One mentee did not “match” with a mentor | Add option to participate in a national peer program | |
| Needs and barriers assessment | ||||
| [Pre-] Potential overlap with existing needs assessment | Adapted existing assessment to incorporate barriers relevant to vascular access and established thresholds of responses for resource provision | |||
| Vascular access care navigation | ||||
| [Pre-] Concern about a heavy workload for navigators | Trained three clinic personnel (case managers, nurses) so duties could be shared, and back-up provided | [Post-] Duties can be time consuming if added to additional non-navigator job responsibilities | Incorporate vascular access navigator responsibilities with those of a CKD navigator (1 FTE) | |
| Vascular access–specific electronic dashboard | ||||
| [Intra-] Difficult and time consuming to prioritize potentially eligible patients | Created filters by which list could be sorted (eligible, ineligible, need further review, etc.) | [Post-] Only the navigators used the dashboard | Give medical providers access to dashboard | |
Barriers were ascertained from pre-, intra-, and post-QI program implementation interviews with patients, care partners, medical providers, and clinic personnel participating in the program. [Pre-], [Intra-], and [Post-] denote timing of barrier identification. QI, quality improvement; EHR, electronic health record; COVID-19, coronavirus disease 2019; FTE, full-time equivalent.
Program Eligibility
Medical providers acknowledged the need to balance the potential challenges of overly liberal selection criteria (i.e., enrollment of patients who never start HD), and the potential harms of overly restrictive criteria (i.e., missed opportunities for education, inadequate time to create functional AV access). Nephrologists found the initially proposed eGFR threshold of <20 ml/min per 1.73 m2 too low, reporting that many patients begin vascular access planning at higher eGFRs. As such, we implemented the program using an eGFR threshold <25 ml/min per 1.73 m2 and a 2-year KFRE >10%. However, some nephrologists were reluctant to discuss vascular access with patients with eGFRs >20 ml/min per 1.73 m2, regardless of their KFRE scores, citing uncertainty about disease progression. A navigator noted, “The nephrologists aren’t always ready to hand off their patients.” Other nephrologists were comfortable initiating dialysis planning at eGFRs >25 ml/min per 1.73 m2 and had already done so. In fact, some patients with eGFRs of 20–30 ml/min per 1.73 m2 had already been referred to surgery and were thus ineligible for the program. This resulted in enrolling many program-eligible patients who were reluctant to start dialysis planning or were generally harder to engage in care. This is evidenced by the low mean±SD eGFR (18±5 ml/min per 1.73 m2) and high mean±SD KFRE (43%±29%) of program participants. In postprogram interviews, navigators and nephrologists suggested the dialysis-modality education class would be an optimal time to enroll patients in the vascular access program, because class referral signified nephrologist agreement that patients were ready for kidney failure planning. Regardless, providers considered it important to use EHR tools to identify patients at risk of kidney disease progression and prompt nephrologist planning.
Program Components
Vascular Access-Specific Education
Overall, participants found the education effective in preparing patients for clinical encounters, resulting in more efficient and meaningful appointments. Specifically, surgeons and interventionalists reported that patients arrived at appointments more prepared to ask questions and were more likely to express their concerns than they had been before program implementation. To ensure delivery of consistent education, we developed guides to facilitate education sessions. Several patients suggested a complementary educational video with patient testimonials would strengthen future program implementations.
Peer Mentoring
In preprogram interviews, patients emphasized the importance of learning from other patients’ experiences. In response, we bolstered the peer mentoring program by recruiting local mentors and providing them with training and vascular access education. Mentees valued the peer-to-peer interactions, describing support that could only be provided by those with lived experiences. Participants suggested adding an option for connection to a national peer program to support patients who did not find a good match in the local program.
Needs and Barriers Assessment
In response to preprogram concerns that the needs and barriers assessment overlapped with an existing Geisinger assessment, we modified the existing assessment to incorporate evaluation of vascular access–specific barriers (e.g., understanding of the vascular access–specific education). The assessments offered opportunities to not only identify patients who needed resources, but also reinforce education and answer lingering questions. Navigators viewed the educational reinforcement aspect of the encounter as particularly important, with one noting, “This topic is emotional and there is a lot of information to digest. Patients have questions about how it will affect their life—it's hard for them to digest in one sitting.”
Care Navigation and Electronic Supports
Providers found the health system–focused program components integral to supporting patients. To enhance efficiency, we made the dashboard sortable by care step and added fields for text updates. To better engage nephrologists and surgeons in future program implementations, participants suggested giving medical providers access to the dashboard. Overall, the program was acceptable to medical providers/personnel; however, the navigator tasks were sometimes time consuming.
Discussion
Our findings demonstrate that targeting both patient and health system barriers may improve predialysis vascular access care. Specifically, we showed that a multifaceted intervention designed to improve patient knowledge, address resource gaps, support emotional needs, and assist with care navigation and monitoring improves patient vascular access–related self-efficacy and knowledge and has the potential to improve clinical outcomes.
Pre-emptive vascular access creation requires teamwork among nephrologists, surgeons, and interventionalists. It is thus reasonable to posit that interventions facilitating care navigation and collaboration may improve AV access–based initiation rates. Yet, prior studies of such interventions have yielded mixed results. Two Australian trials showed increased rates of AV access–based HD initiation with an access coordinator (20) and, separately, a multidisciplinary team intervention (19). However, US studies of similar interventions resulted in higher rates of AV access creation predialysis, but no significant change in functional AV access at HD initiation (16,21,22). These data suggest that health system interventions, on their own, may be insufficient to increase AV access–based HD initiation.
Qualitative studies involving >1000 patients found that patients often experience emotional vulnerability, unpreparedness, and loss of control during vascular access planning (14). A study of 190 patients with CKD found that >50% of participants intended to delay access creation despite early referral (33). Patient perspectives from our work support these findings and underscore the emotional weight that accompanies vascular access planning. In our intervention, we addressed patient-level barriers from several angles. For example, we incorporated vascular access–specific questions about emotional and educational needs into an existing resource assessment. In addition, we provided focused vascular access education, directly acknowledging common emotions, and explaining the care steps and potential complications (including high primary failure rates). Finally, interested patients received peer support. Postprogram improvements in patient self-efficacy and vascular access knowledge suggest these strategies were helpful in preparing patients to engage in vascular access care and may have addressed patients’ psychologic and planning needs. Our qualitative findings also supported the importance of these patient-focused program components. Providers found patients to be more prepared, ultimately making encounters more meaningful and efficient. Patients also expressed appreciation for the transparent and timely communication about potential vascular access–related complications.
Our findings also highlight the importance of health system barriers, including those at the nephrologist level. Overall, the implemented eGFR and KFRE thresholds were acceptable to nephrologists, but they did identify some patients who had not yet discussed dialysis planning with their nephrologists. In some cases, navigator prompts to nephrologists led to earlier dialysis discussions, but, in others, nephrologists felt planning was premature and cited concern that patients might interpret these discussions to mean that dialysis was inevitable. These practice differences are consistent with those reported elsewhere (34) and reflect the tension nephrologists may face when balancing the benefits of pre-emptive access creation with the potential harm of creating unused accesses and associated complications. Expert society guidelines reveal community uncertainty about this issue (3,6,35). In addition, most nephrologists preferred to discuss dialysis with their patients before involvement of the navigator. However, this often led to delays in care. Better supporting nephrologists in these conversations, potentially by earlier involvement of the broader care team (navigator, nurses, case managers), may improve predialysis vascular access care.
We used the health system supports of a CKD registry to identify patients potentially ready for vascular access planning, thresholds of eGFR, and the 2-year KFRE to establish program eligibility, and a complementary electronic dashboard to monitor patients. Although experts have suggested the KFRE may be valuable in vascular access planning (36), the KFRE does not account for competing risks, such as death and transplant. However, the KFRE does incorporate markers of disease progression (e.g., albuminuria) that offer information beyond eGFR. Use of objective laboratory thresholds to identify eligible patients was key for program implementation because it identified some individuals earlier than if nephrologists were left to act on their own. In addition, the electronic dashboard helped navigators follow up on missed appointments and new results, facilitating proactive rescheduling and care advancement (37).
Whereas our study was not designed to assess the relative importance of the intervention components, nor powered to detect their effect (individual or collective) on clinical outcomes, our qualitative data suggest the systematic approach to patient identification and individualized patient education may have been the most influential program elements. The education component not only addressed patient health information needs, but its related encounters (i.e., educational session, needs and barriers assessment, and navigator follow-ups) offered opportunities to address questions and concerns, often unearthing emotional barriers. Despite these program successes, less than half of participants underwent AV access creation during the project. Although reasons for this are likely multifactorial, we suspect the main drivers were (1) rolling program enrollment and consequent short program exposure for some patients, (2) short project duration (7 months), and (3) scheduling challenges associated with holidays (November/December 2019) and the coronavirus disease 2019 pandemic (March 2020). Longer study of this intervention or select components is needed.
We acknowledge our project has limitations. The QI program was implemented in a semiclosed health system; open systems may face different challenges. However, care and communication are even more cumbersome in open systems, emphasizing the need for structured support programs. Second, enrollment during our 7-month program was rolling, resulting in a relatively short program experience for some participants, limiting our conclusions about outcomes. Third, selection bias may have led to inclusion of patients with weaker care engagement. Specifically, the program included many patients who were eligible for vascular access referral who had either not followed through or declined such a referral. Patients willing to proceed with planning had already had their surgical evaluations and were thus excluded. However, inclusion of patients with lower engagement renders our findings of improved patient education and self-efficacy even more encouraging. Fourth, we assessed patient knowledge and confidence, and care team confidence, with investigator-developed questionnaires. Results should be interpreted with caution until confirmed in larger studies using validated instruments. Fifth, there may be additional barriers to AV access creation that were not identified in this study. Finally, this was a pilot study without a control group and, therefore, was not designed nor powered to evaluate clinical outcomes. Larger randomized controlled studies investigating the program's effect on clinical outcomes are needed.
In conclusion, we demonstrated that implementing a program targeting both patient and health system barriers to AV access–based HD initiation was acceptable to participants and may have the potential to improve outcomes. Longer implementations in more diverse health systems are needed.
Disclosures
L.E. Boulware reports serving as a scientific advisor for or member of the Association for Clinical and Translational Science, on the editorial board for JAMA and JAMA Network Online, and on the Robert Wood Johnson Clinical Scholars National Advisory Committee; and receiving honoraria from the Robert Wood Johnson Clinical Scholars Program and from various universities for visiting professorships. In the last 2 years, J.E. Flythe reports receiving speaking honoraria from the American Society of Nephrology and multiple universities; receiving consulting fees from AstraZeneca and Fresenius Kidney Care North America; serving on the medical advisory board for NxStage Medical, now owned by Fresenius Kidney Care North America; and receiving investigator-initiated research funding from the Renal Research Institute, a subsidiary of Fresenius Kidney Care North America. J. F. Pendergast reports receiving honoraria from the National Institutes of Health National Institute on Aging/National Institute of Mental Health Advanced Research Institute for training junior scholars to get their first R01 ($1000 for 3 days of mentoring). S. Peskoe reports serving as a statistical reviewer for JAMA Network Open. All remaining authors have nothing to disclose.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R21 DK116115 (to J.E. Flythe, L.E. Boulware, J.A. Green, J.F. Pendergast, and S. Peskoe). In addition, J.E. Flythe is supported by NIDDK grant K23 DK109401.
Acknowledgments
The results presented in this article have not been published previously in whole or part, except in abstract form.
Author Contributions
L.E. Boulware, J.E. Flythe, J.A. Green, S.-Y.D. Lee, J.F. Pendergast, and S. Peskoe were responsible for methodology; L.E. Boulware, J.E. Flythe, J.A. Green, and J.F. Pendergast provided supervision; L.E. Boulware, J.E. Flythe, J.A. Green, J.F. Pendergast, and T.S. Strigo were responsible for funding acquisition; J.E. Flythe wrote the original draft; J.E. Flythe and J.A. Green conceptualized the study; J.E. Flythe, J.A. Green, J.H. Narendra, and C. Yule were responsible for data curation; J.E. Flythe, J.H. Narendra, T.S. Strigo, and C. Yule were responsible for project administration; J.E. Flythe, J.A. Green, and C. Yule were responsible for investigation; J.A. Green was responsible for validation; S. Manivannan, S. Murphy, J.H. Narentra, and S. Peskoe were responsible for formal analysis; and all authors reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0007812020/-/DCSupplemental.
Standards for Quality Improvement Reporting Excellent Guidelines and manuscript section with relevant content. Download Supplemental Table 1, PDF file, 285 KB (284.6KB, pdf)
Focus group and interview guides. Download Supplemental Table 2, PDF file, 285 KB (284.6KB, pdf)
Research sub-study questionnaires. Download Supplemental Table 3, PDF file, 285 KB (284.6KB, pdf)
Characteristics of the pre-intervention focus group and interview participants. Download Supplemental Table 4, PDF file, 285 KB (284.6KB, pdf)
Overview of vascular access educational brochure and education session content. Download Supplemental Table 5, PDF file, 285 KB (284.6KB, pdf)
References
- 1.Vascular Access 2006 Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S247, 2006. 10.1053/j.ajkd.2006.04.029 [DOI] [PubMed] [Google Scholar]
- 2.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF; Canadian Society of Nephrology Committee for Clinical Practice Guidelines: Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol 17[Suppl 1]: S1–S27, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on vascular access. Nephrol Dial Transplant 22[Suppl 2]: ii88–ii117, 2007. 10.1093/ndt/gfm021 [DOI] [PubMed] [Google Scholar]
- 4.Ravani P, Gillespie BW, Quinn RR, MacRae J, Manns B, Mendelssohn D, Tonelli M, Hemmelgarn B, James M, Pannu N, Robinson BM, Zhang X, Pisoni R: Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J Am Soc Nephrol 24: 1668–1677, 2013. 10.1681/ASN.2012121234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, Pannu NI, Thomas C, Hemmelgarn BR, Craig JC, Manns B, Tonelli M, Strippoli GF, James MT: Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol 24: 465–473, 2013. 10.1681/ASN.2012070643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, Graham J, Moist LM, Rajan DK, Roberts C, Vachharajani TJ, Valentini RP; National Kidney Foundation: KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 75[Suppl 2]: S1–S164, 2020. 10.1053/j.ajkd.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Allon M, Dinwiddie L, Lacson E Jr, Latos DL, Lok CE, Steinman T, Weiner DE: Medicare reimbursement policies and hemodialysis vascular access outcomes: A need for change. J Am Soc Nephrol 22: 426–430, 2011. 10.1681/ASN.2010121219 [DOI] [PubMed] [Google Scholar]
- 8.United States Renal Data System Annual Data Report, 2018. Available at: https://www.usrds.org/annual-data-report/previous-adrs/. Accessed December 30, 2020
- 9.End Stage Renal Disease National Coordinating Center: Fistula first catheter last. Available at: https://esrdncc.org/en/fistula-first-catheter-last. Accessed December 15, 2020
- 10.Lok CE: Fistula first initiative: Advantages and pitfalls. Clin J Am Soc Nephrol 2: 1043–1053, 2007. 10.2215/CJN.01080307 [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Vargas PA, Craig JC, Gallagher MP, Walker RG, Snelling PL, Pedagogos E, Gray NA, Divi MD, Gillies AH, Suranyi MG, Thein H, McDonald SP, Russell C, Polkinghorne KR: Barriers to timely arteriovenous fistula creation: A study of providers and patients. Am J Kidney Dis 57: 873–882, 2011. 10.1053/j.ajkd.2010.12.020 [DOI] [PubMed] [Google Scholar]
- 12.Woo K, Lok CE: New insights into dialysis vascular access: What is the optimal vascular access type and timing of access creation in CKD and dialysis patients? Clin J Am Soc Nephrol 11: 1487–1494, 2016. 10.2215/CJN.02190216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiaii M, MacRae JM: A dedicated vascular access program can improve arteriovenous fistula rates without increasing catheters. J Vasc Access 9: 254–259, 2008. 10.1177/112972980800900406 [DOI] [PubMed] [Google Scholar]
- 14.Casey JR, Hanson CS, Winkelmayer WC, Craig JC, Palmer S, Strippoli GF, Tong A: Patients’ perspectives on hemodialysis vascular access: A systematic review of qualitative studies. Am J Kidney Dis 64: 937–953, 2014. 10.1053/j.ajkd.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 15.Taylor MJ, Hanson CS, Casey JR, Craig JC, Harris D, Tong A: “You know your own fistula, it becomes a part of you”--Patient perspectives on vascular access: A semistructured interview study. Hemodial Int 20: 5–14, 2016. 10.1111/hdi.12340 [DOI] [PubMed] [Google Scholar]
- 16.Gale RC, Kehoe D, Lit YZ, Asch SM, Kurella Tamura M: Effect of a dialysis access coordinator on preemptive access placement among veterans: A quality improvement initiative. Am J Nephrol 45: 14–21, 2017. 10.1159/000452346 [DOI] [PubMed] [Google Scholar]
- 17.Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Nakhoul G, Konig V, Hyland J, Burrucker YK, Dann PD, Tucky BH, Sharp J, Nally JV: Pragmatic randomized, controlled trial of patient navigators and enhanced personal health records in CKD. Clin J Am Soc Nephrol 12: 1418–1427, 2017. 10.2215/CJN.02100217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosa SD, Bhola C, Lok CE: Hemodialysis patients’ satisfaction and perspectives on complications associated with vascular access related interventions: Are we listening? J Vasc Access 17: 313–319, 2016. 10.5301/jva.5000560 [DOI] [PubMed] [Google Scholar]
- 19.Owen JE, Walker RJ, Edgell L, Collie J, Douglas L, Hewitson TD, Becker GJ: Implementation of a pre-dialysis clinical pathway for patients with chronic kidney disease. Int J Qual Health Care 18: 145–151, 2006. 10.1093/intqhc/mzi094 [DOI] [PubMed] [Google Scholar]
- 20.Polkinghorne KR, Seneviratne M, Kerr PG: Effect of a vascular access nurse coordinator to reduce central venous catheter use in incident hemodialysis patients: A quality improvement report. Am J Kidney Dis 53: 99–106, 2009. 10.1053/j.ajkd.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 21.Glazer S, Diesto J, Crooks P, Yeoh H, Pascual N, Selevan D, Derose S, Farooq M: Going beyond the kidney disease outcomes quality initiative: Hemodialysis access experience at Kaiser Permanente Southern California. Ann Vasc Surg 20: 75–82, 2006. 10.1007/s10016-005-9110-8 [DOI] [PubMed] [Google Scholar]
- 22.Ackad A, Simonian GT, Steel K, Parisi C, Mancini S, Douglas C, Buckner D: A journey in reversing practice patterns: A multidisciplinary experience in implementing DOQI guidelines for vascular access. Nephrol Dial Transplant 20: 1450–1455, 2005. 10.1093/ndt/gfh818 [DOI] [PubMed] [Google Scholar]
- 23.Fishbane S, Agoritsas S, Bellucci A, Halinski C, Shah HH, Sakhiya V, Balsam L: Augmented nurse care management in CKD stages 4 to 5: A randomized trial. Am J Kidney Dis 70: 498–505, 2017. 10.1053/j.ajkd.2017.02.366 [DOI] [PubMed] [Google Scholar]
- 24.Green JA, Ephraim PL, Hill-Briggs FF, Browne T, Strigo TS, Hauer CL, Stametz RA, Darer JD, Patel UD, Lang-Lindsey K, Bankes BL, Bolden SA, Danielson P, Ruff S, Schmidt L, Swoboda A, Woods P, Vinson B, Littlewood D, Jackson G, Pendergast JF, St Clair Russell J, Collins K, Norfolk E, Bucaloiu ID, Kethireddy S, Collins C, Davis D, dePrisco J, Malloy D, Diamantidis CJ, Fulmer S, Martin J, Schatell D, Tangri N, Sees A, Siegrist C, Breed J Jr, Medley A, Graboski E, Billet J, Hackenberg M, Singer D, Stewart S, Alkon A, Bhavsar NA, Lewis-Boyer L, Martz C, Yule C, Greer RC, Saunders M, Cameron B, Boulware LE: Putting patients at the center of kidney care transitions: PREPARE NOW, a cluster randomized controlled trial. Contemp Clin Trials 73: 98–110, 2018. 10.1016/j.cct.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D: SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process. BMJ Qual Saf 25: 986–992, 2016. 10.1136/bmjqs-2015-004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011. 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 27.Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC, Chodick G, Collins AJ, Djurdjev O, Elley CR, Evans M, Garg AX, Hallan SI, Inker LA, Ito S, Jee SH, Kovesdy CP, Kronenberg F, Heerspink HJ, Marks A, Nadkarni GN, Navaneethan SD, Nelson RG, Titze S, Sarnak MJ, Stengel B, Woodward M, Iseki K; CKD Prognosis Consortium: Multinational assessment of accuracy of equations for predicting risk of kidney failure: A meta-analysis [published correction appears in JAMA 315: 822, 2016 10.1001/jama.2016.0342]. JAMA 315: 164–174, 2016. 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wild MG, Wallston KA, Green JA, Beach LB, Umeukeje E, Wright Nunes JA, Ikizler TA, Steed J, Cavanaugh KL: The Perceived Medical Condition Self-Management Scale can be applied to patients with chronic kidney disease. Kidney Int 92: 972–978, 2017. 10.1016/j.kint.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh HF, Shannon SE: Three approaches to qualitative content analysis. Qual Health Res 15: 1277–1288, 2005. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 30.Moser A, Korstjens I: Series: Practical guidance to qualitative research. Part 3: Sampling, data collection and analysis. Eur J Gen Pract 24: 9–18, 2018. 10.1080/13814788.2017.1375091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SAS Institute Inc.: SAS/STAT(c) 15.1 user's guide. Cary, NC, SAS Institute Inc., 2018 [Google Scholar]
- 32.R Code Team: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2020 [Google Scholar]
- 33.Chia JMX, Goh ZS, Seow PS, Seow TY, Choo JCJ, Foo MW, Newman S, Griva K: Psychosocial factors, intentions to pursue arteriovenous dialysis access, and access outcomes: A cohort study [published online ahead of print December 3, 2020]. Am J Kidney Dis 10.1053/j.ajkd.2020.09.019 [DOI] [PubMed] [Google Scholar]
- 34.Dumaine C, Kiaii M, Miller L, Moist L, Oliver MJ, Lok CE, Hiremath S, MacRae JM: Vascular access practice patterns in Canada: A national survey. Can J Kidney Health Dis 5: 2054358118759675, 2018. 10.1177/2054358118759675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ethier JH, Lindsay RM, Barre PE, Kappel JE, Carlisle EJ, Common A: Clinical practice guidelines for vascular access. Canadian Society of Nephrology. J Am Soc Nephrol 10[Suppl 13]: S297–S305, 1999 [PubMed] [Google Scholar]
- 36.Inston N, Lok CE: Improving precision in prediction: Using kidney failure risk equations as a potential adjunct to vascular access planning. J Vasc Access 20: 95–97, 2019. 10.1177/1129729818786630 [DOI] [PubMed] [Google Scholar]
- 37.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003. 10.1016/S0272-6386(03)00415-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standards for Quality Improvement Reporting Excellent Guidelines and manuscript section with relevant content. Download Supplemental Table 1, PDF file, 285 KB (284.6KB, pdf)
Focus group and interview guides. Download Supplemental Table 2, PDF file, 285 KB (284.6KB, pdf)
Research sub-study questionnaires. Download Supplemental Table 3, PDF file, 285 KB (284.6KB, pdf)
Characteristics of the pre-intervention focus group and interview participants. Download Supplemental Table 4, PDF file, 285 KB (284.6KB, pdf)
Overview of vascular access educational brochure and education session content. Download Supplemental Table 5, PDF file, 285 KB (284.6KB, pdf)