Introduction
Sodium-glucose cotransporter-2 inhibitors (SGLT2is) have emerged as an effective therapy for improving outcomes in diabetic and nondiabetic kidney disease (1,2). Clinical trials have demonstrated the benefits of SGLT2is for secondary prevention of adverse cardiovascular (CV) effects in patients with established atherosclerotic disease and/or heart failure with reduced ejection fraction (3–7). It is imperative for clinicians to assess the use of SGLT2is in medically eligible patients and prescribe these agents when appropriate.
Despite the overwhelming evidence of the benefits of SGLT2i therapy, the prescription rate remains dismally low, particularly among patients most likely to benefit from cardiorenal protective effects (8). Several potential factors contribute to low SGLT2i prescription rate, including prescriber hesitancy, treatment inertia, and high drug cost. In this article, we review clinical indications for SGLT2i use, therapeutic and adverse effects, and our approach to handling concomitant medications.
Clinical Indications for SGLT2i Use
The use of SGLT2is is clinically indicated in the following circumstances.
Type 2 diabetes mellitus (T2DM) and albuminuric kidney disease (albuminuria of ≥200 mg/g of creatinine plus eGFR of 25–90 ml/min per 1.73 m2). In the CREDENCE Trial, canagliflozin decreased the primary cardiorenal end point by 30%, compared with placebo, in patients with diabetic kidney disease (1). In the DAPA-CKD trial, dapagliflozin reduced the primary cardiorenal end point by 39%, compared with placebo, in patients with diabetic and nondiabetic kidney disease (2).
Nondiabetic albuminuric kidney disease (albuminuria ≥200 mg/d plus eGFR of 25–75 ml/min per 1.73 m2). In the DAPA-CKD trial, a third of the patients did not have T2DM, and the cardiorenal benefits of dapagliflozin were similar among patients with nondiabetic and diabetic kidney disease (2).
T2DM with CV disease. In the EMPA-REG and CANVAS trials, empagliflozin and canagliflozin demonstrated a 14% reduction in the primary end point of major adverse cardiac events in patients with a history of CV disease (3,4). In DECLARE-TIMI, dapagliflozin reduced the risk of CV death and hospitalization for heart failure by 17% in patients who had, or were at risk for, atherosclerotic heart disease (5).
Heart failure with reduced ejection fraction. In the DAPA-HF trial, dapagliflozin reduced worsening of heart failure and CV death by 26% in patients with an ejection fraction of ≤40% (6). In the EMPEROR-Reduced trial, empagliflozin reduced the primary outcome of CV death and hospitalization for heart failure by 25% in patients with an ejection fraction of ≤40% (7).
T2DM and hyperglycemia. Several professional society guidelines recommend using SGLT2is as either first-line therapy or as an add-on therapy to metformin for the management of hyperglycemia in patients with T2DM (9–11).
Once the appropriate clinical indications are identified, patients should be screened for conditions that increase risk for adverse effects of SGLT2is. Such conditions include cognitive impairment, advanced age, limited mobility, recurrent urinary tract infections (UTIs), indwelling Foley catheter, neurogenic bladder, poor genital hygiene, solitary kidney, bilateral renal-artery stenosis, immunosuppressive therapy, and history of diabetic ketoacidosis (DKA). Patients with these underlying conditions must be assessed on an individual basis, weighing the risks and benefits of SGLT2i therapy. The safety of SGLT2is have not been established in kidney transplant recipients, or in pregnant or lactating women.
Therapeutic and Adverse Effects of SGT2is
SGLT2is cause glucosuria and moderate natriuresis by blocking the sodium-glucose cotransporter-2 in the proximal tubule, causing reduction in BP, blood glucose, proximal tubule workload, and weight. SGLT2is confer CV, renal, and metabolic benefits, although the precise underlying mechanistic underpinnings remain to be fully elucidated.
Before initiation of SGLT2is, the patient's BP, volume status, and blood-glucose control must be assessed. SGLT2is must not be initiated in patients who are hypotensive/hypovolemic. In patients who are hypotensive, the antihypertensive medications, including diuretics, may need to be stopped or reduced to restore normotension. If the patient is hypervolemic/hypertensive, then SGLT2i therapy can be initiated without adjusting the dose of other antihypertensive medications. If the patient is euvolemic/normotensive then the antihypertensive agents, including diuretics, may need to be reduced or stopped if the BP decreases. The BP-lowering and natriuretic effect of SGLT2is is modest, resulting in a reduction of systolic BP of approximately 3–5 mm Hg (Figure 1). In the SGLT2i CV and kidney outcome trials, patients were required to be on the maximally tolerated dose of renin-angiotensin system (RAS) blockers before randomization to the SGLT2i or placebo groups. A similar strategy to continue RAS blockers must be adopted in clinical practice. Monotherapy with SGLT2i is reasonable in patients who are unable to tolerate RAS blockers.
Figure 1.
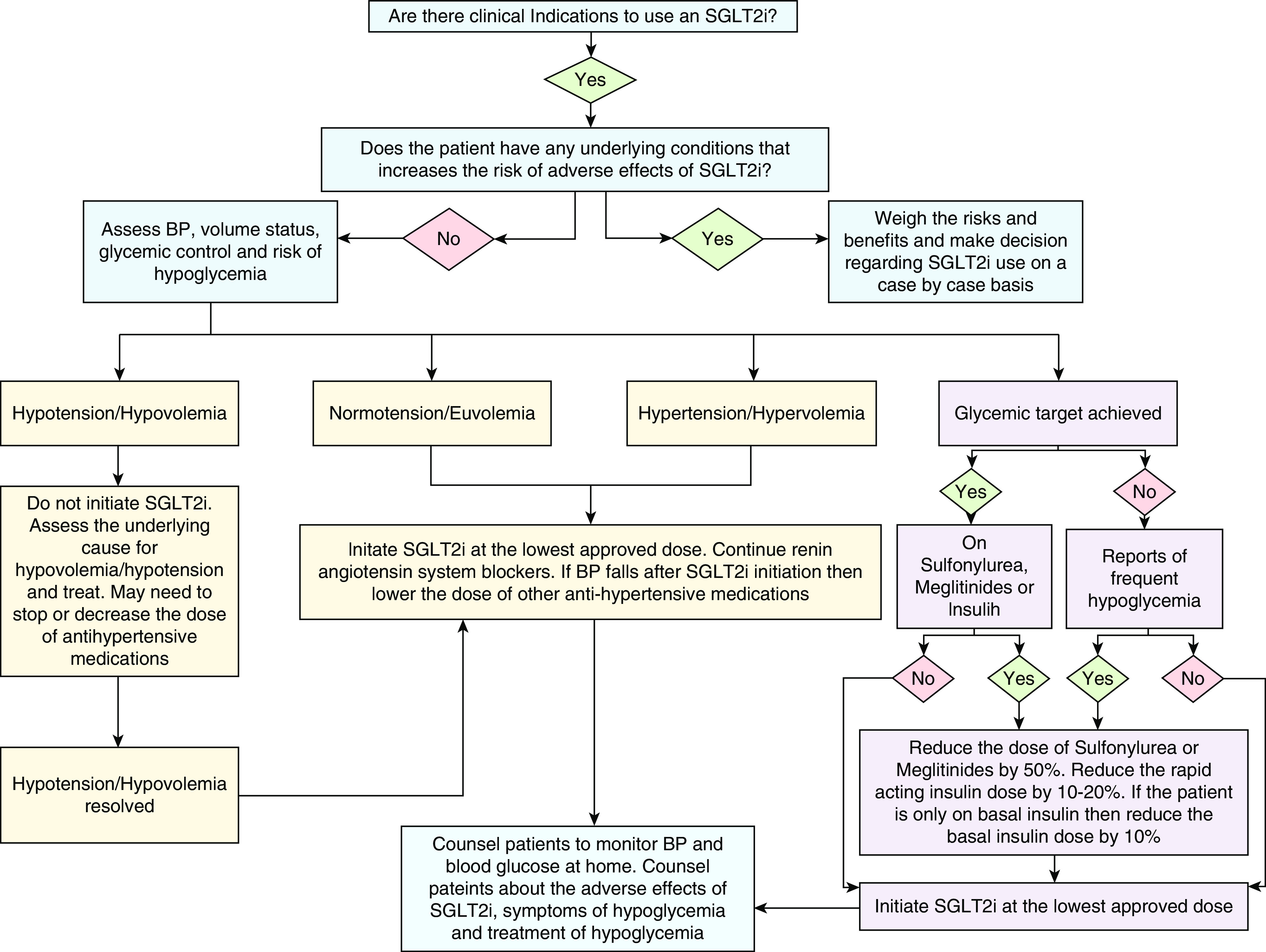
Algorithm to assess BP, volume status and glycemic control at the time of sodium-glucose cotransporter-2 inhibitor (SGLT2i) initiation.
SGLT2i therapy can cause an acute drop in eGFR; however, the decline in eGFR is subsequently attenuated with continuation of SGLT2i therapy. In the absence of hemodynamic instability or an alternate cause of AKI, the initial decline in eGFR of up to 30% after SGLT2i initiation is likely due to reduction in intraglomerular pressure. It may be difficult to distinguish whether the decline in eGFR is due to the hemodynamic effect of SGLT2i or due to AKI. Periodic monitoring of the kidney function over 2–4 weeks can help in distinguishing these two entities because the decline in eGFR due to the hemodynamic effects of SGLT2is is typically not progressive. In the absence of hemodynamic instability, SGTL2is do not increase the risk of AKI. In fact, an overall reduction in AKI has been observed with SGLT2i use (12).
Prescriber hesitancy due to concern for hypoglycemia can be decreased by following a simple SGLT2i initiation strategy (Figure 1). Patients already on glucose-lowering agents require assessment of current glycemic control, along with the underlying risk factors for hypoglycemia. Glycemic control is typically determined by hemoglobin A1C (HbA1C) levels, but patients with CKD, liver disease, erythrocyte disorders, and those with frequent hypoglycemic episodes can have falsely low HbA1C values, rendering the test unreliable. In such cases, fructosamine levels, self-monitored blood-glucose readings, or continuous glucose-monitor data must be used. In most nonpregnant adult patients, an HbA1C target of <7% is reasonable, but for those at high risk of hypoglycemia, a less-stringent HbA1C target of <8% can be used (13,14).
The risk of hypoglycemia is not increased in patients only taking metformin, dipeptidyl peptidase 4 inhibitors, glucagon-like peptide-1 receptor agonists, and thiazolidinediones; hence, these agents do not require dose adjustment at the time of SGLT2i initiation. On the other hand, insulin, sulfonylurea, and meglitinides increase the risk of hypoglycemia and require dose adjustment at the time of SGLT2i initiation in patients with optimal glycemic control or those already experiencing hypoglycemia. In such patients, sulfonylurea/meglitinides must either be stopped or the dose must be decreased by 50%, and insulin doses—particularly the rapid-acting (bolus) insulin for patients on a traditional basal-bolus insulin regimen—must be reduced by 10%–20%. Patients only using basal insulin can have their doses reduced by 10%. A stepwise dose-reduction strategy of insulin doses is preferred, rather than complete discontinuation, to mitigate the risk of DKA. Patients who are above their glycemic target without frequent hypoglycemia, should not require dose adjustment of other antiglycemic agents at the time of SGLT2i initiation. All patients should be counseled on the risk, identification, and treatment of hypoglycemia. It is worth noting that SGLT2is have not been shown to cause hypoglycemia in patients without diabetes (2,6).
In the SGLT2i trials, the absolute risk of DKA in T2DM is low, but the relative risk of DKA associated with SGLT2is has been two to 11 times higher compared with placebo (1,3–5). The DKA associated with SGLT2is can be euglycemic (blood glucose of <250 mg/dl) and its diagnosis is established by the presence of a high anion gap metabolic acidosis and ketonemia. The absence of hyperglycemia can cause a diagnostic delay; any patient on SGLT2is with symptoms of DKA, such as nausea, vomiting, and abdominal pain, should be evaluated for euglycemic DKA. The pathogenesis of SGLT2i-induced euglycemic DKA is thought to be due to SGLT2i-induced suppression of insulin, an increase in glucagon, and masking of hyperglycemia due to enhanced glucosuria (15). It is important to note that DKA has not been observed in patients without diabetes in the large SGLT2i trials.
Patients must be counseled to monitor their BP, weight, and blood glucose at home when initiating SGLT2is (Table 1). SGLT2is must not be initiated or continued in patients who are hypovolemic. Patients must be advised to hold SGLT2is when their oral intake of food and water is restricted due to a planned surgery or due to an underlying illness to prevent DKA, hypovolemia, and hypotension—this is also referred to as the “sick-day rule.”
Table 1.
Handout for patients when initiating sodium-glucose cotransporter-2 inhibitor therapy
| It is recommended that the patients follow the recommendations stated below and must contact their provider if they have any questions or concerns |
| Increase in urine output |
| You may notice an increase in your urine output after starting this medication |
| Monitor your weight at home |
| BP |
| Monitor your BP at home because this medicine may lower BP |
| Inform your doctor if your BP is too low, or if you experience light headedness or dizziness |
| Blood glucose |
| Monitor your blood glucose level at home because this medicine may lower blood glucose |
| Inform your doctor if your blood glucose is low |
| Follow the “sick-day rule” |
| Do not take this medicine on days that you are unable to eat because you are feeling sick due to fever, infection, poor appetite, nausea, vomiting, or diarrhea |
| You can resume the medicine once you are able to eat and drink |
| If you continue to feel sick, then call your doctor because you may need to have blood tests to rule out diabetic ketoacidosis |
| Stop the medication 3–4 d before a scheduled surgery that requires you to be “nothing by mouth” (meaning you are instructed to not eat or drink anything for several h before your surgery) |
| Avoid very low carbohydrate and keto diets because they may increase the risk of diabetic ketoacidosis |
| Wound on your feet or legs |
| If you notice a wound, ulcer, or skin breakdown on your feet or legs, then hold this medicine and inform your doctor |
| Burning or pain during urination |
| If you experience pain or burning on urination, then inform your doctor because you may need further evaluation |
| Redness or itching in the genital area, or foul-smelling vaginal or penile discharge |
| Keep the genital area clean |
| If you notice redness or itching in the genital area, or foul-smelling vaginal or penile discharge, then inform your doctor; you may need a cream or oral medication to treat an underlying infection |
SGLT2i therapy must be initiated at the lowest recommended daily dose (10 mg empagliflozin, 100 mg canagliflozin, 10 mg dapagliflozin, or 5 mg ertugliflozin). SGLT2i titration to a higher dose is not necessary for maximal cardiorenal benefits (3). Although a higher dose of SGLT2i can be used to improve glycemic control, it is important to recognize that the glucose-lowering effect of SGLT2i declines at lower eGFR. On the basis of the evidence from CREDENCE and DAPA-CKD trials, once the SGLT2i therapy is initiated at the recommended level of eGFR, it can be continued until the patient initiates dialysis therapy.
Due to its glucosuric effect, SGLT2is increase the risk of genital mycotic infections by three- to four-fold in patients with diabetes. The vast majority of the SGLT2i-related genital mycotic infections are treatable with topical antifungal agents or a single oral dose of fluconazole, and do not necessitate discontinuation of SGLT2i therapy. Clinicians must counsel the patients regarding maintenance of genital hygiene (Table 1). It is not known if SGLT2is increase the risk of genital mycotic infections among patients who are nondiabetic.
Fournier gangrene is a serious medical condition, and it remains unclear whether SGLT2is increase the risk of Fournier gangrene or not; however, it is worth noting that such an association has not been observed in the any of the large SGLT2i clinical trials.
SGLT2is do not increase the risk of UTIs; however, their use in patients at high risk for UTIs, such as those with an indwelling Foley catheter, recurrent UTIs, or neurogenic bladder, has not been studied.
SGLT2is and their association with bone fractures and lower-extremity amputation remains weak because this association was only been observed with the use of canagliflozin in the CANVAS trial, and not in the other large SGLT2i trials.
Conclusions
SGLT2is offer cardiorenal protection for patients with and without T2DM. Using a simple strategy for assessing the risks and modifying antihypertensive, diuretic, and antiglycemic agents can mitigate the potential adverse effects of SGLT2is. With the growing evidence for safe use and renal-protective effects of SGLT2is, nephrologists now have a therapeutic agent to combat the pandemic of diabetic kidney disease.
Disclosures
D. Lam reports receiving research funding from Insulet Corporation, outside the submitted work. A. Shaikh reports being a member of the educational and communication committee of American Society of Diagnostic and Interventional Nephrology. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
D. Lam and A. Shaikh wrote the original draft and reviewed and edited the manuscript.
References
- 1.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 2.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde A-M, Wheeler DC; DAPA-CKD Trial Committees and Investigators: Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 3.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group: Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators: Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators: Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995–2008, 2019. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 7.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators: Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383: 1413–1424, 2020. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 8.McCoy RG, Dykhoff HJ, Sangaralingham L, Ross JS, Karaca-Mandic P, Montori VM, Shah ND: Adoption of new glucose-lowering medications in the U.S.-The case of SGLT2 inhibitors: Nationwide cohort study. Diabetes Technol Ther 21: 702–712, 2019. 10.1089/dia.2019.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE: Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm – 2018 executive summary. Endocr Pract 24: 91–120, 2018. 10.4158/CS-2017-0153 [DOI] [PubMed] [Google Scholar]
- 10.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group: 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 41: 255–323, 2020. 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes Diabetes Work Group, KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease 2020. Available at: 10.1016/j.kint.2020.06.019. Accessed February 11, 2021 [DOI]
- 12.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ: SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 7: 845–854, 2019. 10.1016/S2213-8587(19)30256-6 [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association: 6. Glycemic targets: Standards of medical care in diabetes-2021. Diabetes Care 44[Suppl 1]: S73–S84, 2021. 10.2337/dc21-S006 [DOI] [PubMed] [Google Scholar]
- 14.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41: 2669–2701, 2018. 10.2337/dci18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barski L, Eshkoli T, Brandstaetter E, Jotkowitz A: Euglycemic diabetic ketoacidosis. Eur J Intern Med 63: 9–14, 2019. 10.1016/j.ejim.2019.03.014 [DOI] [PubMed] [Google Scholar]


