The cardiorenal benefits of sodium-glucose cotransporter 2 (SGLT2) inhibitors (SGLT2is) are transforming the care of CKD. An inherent challenge of practice-changing advances on this scale is how to best incorporate them into daily practice. In this vein, although there are four SGLT2is commercially available in the United States, the question remains whether these SGLT2is may be viewed as interchangeable or if specific agents offer unique benefits or harms in specific clinical settings. The more similarities between the agents within a pharmacologic class (e.g., chemical structure, pharmacodynamics, and pharmacokinetics), the greater the likelihood of shared class effects. Preclinical data, starting with largely comparable chemical structures, provide a foundation where class effects might be anticipated. Nevertheless, on- and off-target effects may be expected to modify the response to any drug—an area of particular interest when considering the potential for drug, rather than class-specific, effects (1). Although translational, toxicologic, and pharmacologic investigations carried out in model systems may shed light into class- versus drug-specific effects, the ultimate adjudicator of this question is data from sufficiently powered, rigorously controlled, human, randomized clinical trials (RCTs). Before delving into the trial data, it may be helpful to briefly review the preclinical data regarding SGLT2is to explore the a priori bio-plausibility of class- versus drug-specific effects.
The origins of SGLT2is can be linked to two earlier tracks of research: familial renal glucosuria (FRG) and phlorizin rodent studies (2). The presence of patients with a familiar tendency to produce elevated levels of glucose in the urine, despite normal blood glucose, had been identified for some time and, as early as the 1920s, work had begun to identify the pattern of inheritance (2,3). The precise molecular mechanism for this clinical finding would take considerably longer to come to light (4). One observation from these patients that is directly applicable to the SGLT2i era is that individuals with FRG tend to have little in the way of adverse effects attributable to this defect (2), except the tendency of these patients to develop ketosis when stressed perioperatively. Phlorizin, a compound extracted from unripe apple, had been shown to induce glucosuria as early as the 19th century. Starting in the late 1980s, a series of studies involving rat models of diabetes used phlorizin to gain additional insights into the antiglycemic effects of SGLT2 blockade (5). Further studies found that, in blocking glucose absorption in the proximal tubule, this agent was able to mitigate hyperfiltration and tubuloglomerular feedback was felt to be the likely mechanism of this action (6). Investigations marrying the evidence of FRG and the phlorizin rodent studies demonstrated that, in SGLT2-knockout mice given diabetes, hyperfiltration was attenuated (7), providing the rationale for testing these agents in (diabetic) CKD.
Notwithstanding these encouraging observations, phlorizin (which is a dual inhibitor of SGLT1 and SGLT2) was never developed for human use because of two unappealing properties. First, it has a limited oral bioavailability, necessitating the use of rather high doses to achieve a systemic exposure, and a significant potential for gastrointestinal side effects as a result of the inhibition of the glucose absorption in the small bowel (8). To overcome the limited bioavailability, drug development shifted away from O-glycoside structures to C-glycoside ones because of the latter’s resistance to hydrolysis by intestinal glucosidases. All SGLT2is interact with the SGLT2 at the luminal side after filtration in the glomerulus. All SGLT2is are highly selective inhibitors of the cotransporter, with selectivity for SGLT2 over SGLT1 ranging from 1:414 for canagliflozin to 1:2500 for empagliflozin. This variable selectivity is unlikely to be of physiologic or clinical importance because the SGLT2is are concentrated in the luminal side due to proximal filtrate fluid reabsorption, thus saturating their target. Furthermore, they exhibit broad pharmacokinetic similarities: very fast absorption with time to peak within 2 hours, extremely high bioavailability (ranging from 65% in the case of canagliflozin to nearly 100% for ertugliflozin), high protein binding (>85%), large volume of distributions (e.g., 74 L for empagliflozin to 119 L for canagliflozin), and t1/2s between 12 and 17 hours. Elimination of metabolites is roughly equal between renal and gastrointestinal routes (except for dapagliflozin, in which urinary excretion predominates); of the four SGLT2is currently approved in the United States, only empagliflozin is recovered intact in the urine in a substantial amount (20% of administered dose versus <2% for all others). All SGLT2is variably activate the AMP-activated protein kinase, although the precise role this shared off-target effect plays on the cardiorenal benefit of these drugs is still not clear (9,10). Although these differences in pharmacokinetic, selectivity, and off-target effects are of scientific interest, they are unlikely to explain the variation in the results of the RCTs of these agents, as we detail below.
The first SGLT2i was approved by the Food and Drug Administration (FDA) for the treatment of diabetes in 2013, and three others soon followed. The overall effectiveness of SGLT2is as antiglycemics are broadly similar. The average benefit in treatment-naive patients with diabetes was approximately a 1% (0.81%–1.02%) reduction in hemoglobin A1C and, when SGLT2is are added to metformin, the A1C only improves by an additional 0.6% (range of point estimates for individual SGLT2i 0.57%–0.63%) (11). The approval of SGLT2is as antiglycemics was followed by a series of trials to document the cardiovascular safety of these agents. Such studies have been mandated by the FDA since 2008 because of the increased rate of cardiovascular adverse events seen in the phase 2/3 studies of the antiglycemic, proliferator-activated receptor agonist rosiglitazone, and in the aftermath of the ACCORD trial, in which intense glycemic control was associated with a 22% higher risk of death. The first cardiovascular outcomes trials of empagliflozin (empagliflozin, EMPA-REG OUTCOME; canagliflozin, CANVAS/CANVAS-R) established the superiority of SGLT2is over the standard of care against the composite outcome of cardiovascular death, nonfatal myocardial infarction, or stroke. Although dapagliflozin and ertugliflozin only achieved noninferiority in their cardiovascular outcomes trials (DECLARE-TIMI-58 and VERTIS-CV), studies in heart failure with reduced ejection fraction (dapagliflozin’s DAPA-HF and empagliflozin’s EMPEROR-Reduced) and kidney disease (canagliflozin’s CREDENCE and dapagliflozin’s CKD) documented the broad efficacy of SGLT2is as cardiorenal protective agents.
So, is the cardiorenal benefit of SGLT2is a class or a drug effect? This is a reasonable question to ask, considering the numerically variable outcomes with SGLT2is noted in the large RCTs. A recent, systematic meta-analysis (12) of the eight randomized controlled trials of the available SGLT2is was undertaken to explore this question. The open data and software code of this meta-analysis are reused here to illustrate there is little heterogeneity in the cardiorenal benefits of SGLT2is.
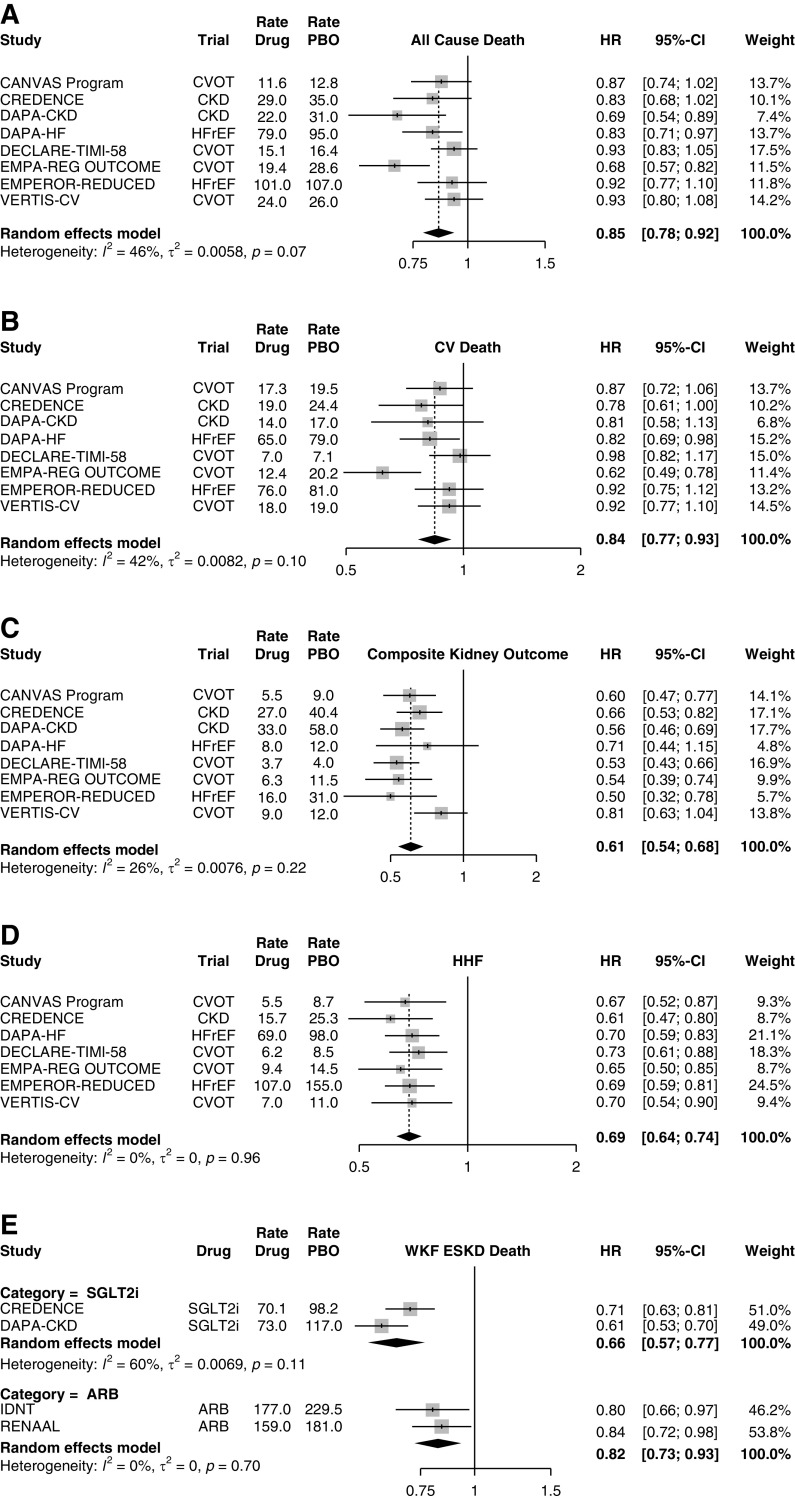
SGLT2is reduce all-cause mortality (Figure 1A) by 15% (P<0.001) and cardiovascular mortality (Figure 1B) by 16% (P=0.0006), with little evidence for heterogeneity (for the Q test, P=0.07 and 0.10, respectively). When analyzing the composite kidney outcome (Figure 1C) of worsening kidney function (defined variably as doubling of serum creatinine or >40% drop in the eGFR), ESKD, or need for RRT, SGLT2is reduced the outcomes by 39% (P<0.001), with no evidence of heterogeneity by study drug. All trials reported consistent decreases in the number of hospitalizations for heart failure (Figure 1D), with hazard ratios in the narrow range of 0.61–0.73. We also contrasted the composite outcomes of worsening kidney function, ESKD, and mortality (Figure 1E) between the two CKD- SGLT2i trials and the two pivotal angiotensin receptor blocker (ARB) trials in diabetic kidney disease: IDNT (irbesartan) and RENAAL (losartan). The comparison depicted a meta-regression with SGLT2is showing an effect that is 15% larger than that of the angiotensin receptor blocker (95% CI, 1% to 27%; P=0.04). Again, the beneficial effects of the SGLT2is were observed irrespective of the study drug. The reported common risks associated with SGLT2is—such as diabetic ketoacidosis, volume depletion, genital mycotic infections, and urinary tract infections—also appear to be similar between the individual drugs (12).
Figure 1.
SGLT2i reduce total and cardiovascular mortality, kidney outcomes, and heart failure. Effects of SGLT2is on (A) all-cause death and (B) cardiovascular (CV) death, (C) the composite kidney outcome (defined variably as doubling of serum creatinine or >40% drop in the eGFR, ESKD, or need for RRT), (D) hospitalizations for heart failure (HHF), and (E) comparison between SGLT2is and angiotensin receptor blockers (ARB). Random-effects model synthesizes the effect across all studies. Hazard ratio (HR) event rates (per 1000 patient years) are shown for both the SGLT2i and the placebo arms. CVOT, cardiovascular outcome trial; HFrEF, heart failure with reduced ejection fraction; PBO, placebo; SGLT2is, sodium-glucose cotransporter 2 inhibitors; WKF, worsening kidney function.
On the basis of the available clinical trial data thus far, and basic considerations from pharmacology and physiology, it can be inferred that both the benefit and the side effects of SGLT2is are part of their class features and not specific to individual drug members of the class. Although one may be tempted to attribute the variable outcomes noted in some of the trials (e.g., VERTIS-CV) to drug effects, highly variable outcomes were observed for all SGLT2is that completed more than one trial. RCTs, as with any other experiment, are subject to noise, and one should simply resist the tendency to follow the noise in the data. In summary, as a class, SGLT2is demonstrate cardiovascular and renal benefits in patients with diabetes mellitus type 2 at risk for cardiovascular disease, cardiovascular benefit in patients with diabetic or nondiabetic kidney disease, and heart failure benefit in patients with known heart failure with reduced ejection fraction or CKD with or without diabetes. The physician prescription practice at this time should be guided by the approved FDA indications which do differ among drugs (Table 1), insurance coverage, and patient affordability of the copay, because at the end of the day any SGLT2i is better than no SGLT2i.
Table 1.
FDA-approved SGLT2is commercially available in the United States as of January 2021
| Indication | Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin |
| Antiglycemic | As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus | |||
| Cardiovascular disease | Reduce the risk of major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease | Reduce the risk of hospitalization for heart failure in adults with type 2 diabetes mellitus and established cardiovascular disease or multiple risk factors | Reduce the risk of cardiovascular death in adult patients with type 2 diabetes mellitus and established cardiovascular disease | |
| Heart failure | Partial (see below) | Reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure with reduced ejection fraction (NYHA classification II–IV) | ||
| Renal disease | Reduce the risk of ESKD, doubling of serum creatinine, cardiovascular death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria ˃300 mg/d | Breakthrough therapy designation in the United States for patients with CKD with and without type 2 diabetes (indication pending) | ||
FDA, Food and Drug Administration; SGLT2is, sodium-glucose cotransporter 2 inhibitors; NYHA, New York Heart Association.
Disclosures
C. Argyropoulos reports receiving consulting fees from Bayer, Baxter Healthcare, and Health Services Advisory Group, and research support from Dialysis Clinic, Inc. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
Dr. Christos Argyropoulos would like to thank multiple social media users who reached out to him and convinced him to convert his “tweetorials” about SGLT2i (https://twitter.com/ChristosArgyrop/status/1301706984379482113?s=20 and https://twitter.com/ChristosArgyrop/status/1301736105688014849?s=20) to publication formats, and Dr. Steven Coca for inviting our group to contribute to Kidney360.
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
C. Argyropoulos was responsible for visualization; and all authors conceptualized the study, wrote the original draft, and reviewed and edited the manuscript.
References
- 1.Papoian T, Chiu H-J, Elayan I, Jagadeesh G, Khan I, Laniyonu AA, Li CX, Saulnier M, Simpson N, Yang B: Secondary pharmacology data to assess potential off-target activity of new drugs: A regulatory perspective. Nat Rev Drug Discov 14: 294, 2015. 10.1038/nrd3845-c1 [DOI] [PubMed] [Google Scholar]
- 2.Santer R, Calado J: Familial renal glucosuria and SGLT2: From a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 5: 133–141, 2010. 10.2215/CJN.04010609 [DOI] [PubMed] [Google Scholar]
- 3.Hjarne U: A study of orthoglycemic glycosuria with particular reference to its inheritability. Acta Med Scand 67: 495–571, 1927. 10.1111/j.0954-6820.1927.tb18537.x [DOI] [Google Scholar]
- 4.Kanai Y, Lee WS, You G, Brown D, Hediger MA: The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93: 397–404, 1994. 10.1172/JCI116972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA: Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79: 1510–1515, 1987. 10.1172/JCI112981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H: Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T: Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304: F156–F167, 2013. 10.1152/ajprenal.00409.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faillie J-L: Pharmacological aspects of the safety of gliflozins. Pharmacol Res 118: 71–81, 2017. 10.1016/j.phrs.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 9.Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG: The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 65: 2784–2794, 2016. 10.2337/db16-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyani CN, Plastira I, Sourij H, Hallström S, Schmidt A, Rainer PP, Bugger H, Frank S, Malle E, von Lewinski D: Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol Res 158: 104870, 2020. 10.1016/j.phrs.2020.104870 [DOI] [PubMed] [Google Scholar]
- 11.Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, Liakos A, Matthews DR, Bekiari E: Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: A systematic review and network meta-analysis. Ann Intern Med 173: 278–286, 2020. 10.7326/M20-0864 [DOI] [PubMed] [Google Scholar]
- 12.Johansen ME, Argyropoulos C: The cardiovascular outcomes, heart failure and kidney disease trials tell that the time to use sodium glucose cotransporter 2 inhibitors is now. Clin Cardiol 43: 1376–1387, 2020. 10.1002/clc.23508 [DOI] [PMC free article] [PubMed] [Google Scholar]