Abstract
Cytomegalovirus (CMV) and BK virus (BKV) are common viral infections after kidney transplant. Their negative effects on patient and graft outcomes have been well described. However, despite improvement in screening and prophylaxis strategies, CMV and BKV continue to negatively affect both short- and long-term graft survival. Adequate cell-mediated immunity is essential for the control and prevention of opportunistic viral infections, such as CMV and BKV. Therefore, immune reconstitution, in particular T cell recovery, is a key factor in antiviral control after kidney transplantation. Cell-based immunotherapy offers an attractive alternative approach to traditional interventions. Adoptive T cell transfer, via infusions of allogeneic virus-specific T lymphocytes is capable of restoring virus-specific T cell immunity, and are safe and effective in the treatment of viral infections after hematopoietic stem cell transplantation. In this article, we review the emerging role of virus-specific T cell therapy in the management of CMV and BKV after kidney transplantation. On the basis of the available data, virus-specific T cell therapy may be a promising addition to the antiviral treatment armamentarium after kidney transplantation. Future studies are needed to more clearly define the efficacy and risks of virus-specific T cell therapy in the kidney transplant population.
Keywords: transplantation, BK infection, cell- and tissue-based therapy, CMV, cytomegalovirus infections, kidney transplantation, viral-specific T cell therapy
Introduction
Cytomegalovirus (CMV) and BK virus (BKV) are common viral infections associated with significant morbidity and mortality after kidney transplantation (1–3). Despite an improvement in screening and prophylaxis strategies, CMV and BKV continue to negatively affect both short- and long-term graft survival (4,5). Adequate cell-mediated immunity is essential for the control and prevention of these opportunistic viral infections (6). Therefore, immune reconstitution after kidney transplantation, particularly T cell recovery, is paramount. However, the degree of cell-mediated immunity is inversely correlated to the intensity of the immunosuppressive regimen used to prevent allograft rejection, with lymphocyte depletion playing a key role in delayed reconstitution and viral risk (7). In this article, we discuss the potential role of allogeneic virus-specific T cell therapy (VST) for the management of CMV and BKV in kidney transplant recipients.
CMV after Kidney Transplantation
CMV is a ubiquitous herpes virus present in 40%–70% of the population (8). It is not associated with disease in the immunocompetent host; however, in the setting of immunosuppression, such as after kidney transplant, CMV can cause systemic illness. Risk of CMV infection is dependent on serostatus at transplant, and is highest in the first year after transplant. Although reactivation can lead to disease, patients without previous exposure (seronegative, R-) who receive allografts from exposed donors (seropositive, D+) are at the highest risk of severe CMV infectious sequelae due to primary infection. Primary infection has been associated with a myriad of negative infectious outcomes, including persistence, recurrence, and resistance, which in turn affect long-term graft survival (9). These negative outcomes are thought to be related to host inability to mount sufficient CMV-specific T cell immunity to adequately control viral replication (10). The drug of choice for CMV prevention and treatment is intravenous ganciclovir and its oral prodrug, valganciclovir (11). The market availability of valganciclovir improved the prophylaxis of CMV, given enhanced bioavailability over oral ganciclovir (12). Additionally, the extension of valganciclovir prophylaxis from 100 to 200 days after kidney transplant has been shown to reduce late-onset CMV disease in patients who are high risk. However, valganciclovir is associated with fairly significant myelosuppressive effects, which may be counterproductive to immune reconstitution, and cost can be a barrier to its use (13). Additionally, postprophylaxis late-onset CMV remains a common complication in the 3–6 months after antiviral drug withdrawal, particularly when potent induction and intense immunosuppression is used in a high-risk individual (14). Indeed, the literature suggests CMV serologic mismatch is still negatively affecting patient and graft survival after kidney transplant, even in the era of modern prophylaxis schemes (15). Of additional concern is the emergence of ganciclovir resistance in CMV, which is associated with significant morbidity and mortality. After ganciclovir-resistant CMV disease, the literature describes rates of virologic failure and recurrence range from 20% to 30%, along with 50% rejection incidence, 27% graft loss, and 20%–30% mortality (16,17). Although there are some antiviral agents in the drug pipeline, such as brincidofovir, a prodrug of cidofovir, and maribavir, a UL97 inhibitor, overall these have been plagued by efficacy concerns (17). Indeed, after phase 3 trials of brincidofovir failed to meet goal end points in hematopoietic stem cell transplantation (HSCT) recipients, concurrent phase 3 trials in renal transplant recipients were halted (18). Additionally, maribavir was shown to be inferior to ganciclovir for the prevention of CMV disease after liver transplantation, although this was thought to be due to suboptimal dosing (19). When maribavir was specifically studied as a treatment for refractory and resistant CMV infection in a phase 2 trial, including 120 solid organ transplant and HSCT recipients, results were favorable; however, the incidence of disease recurrence and adverse effects were high (20). These unapproved agents are also difficult to obtain for clinical use, with significant restrictions on compassionate use. The most recently available agent, letermovir, a 3,4-dihydro-quinazoline-4-yl-acetic acid derivative that inhibits viral terminase complex inhibitor encoded by UL56 is FDA approved for CMV prophylaxis in allogeneic HSCT recipients (21). However, its role in treatment and prophylaxis of CMV infection in solid organ transplant recipients has not been completely elucidated, and is still under active investigation (22).
BKV after Kidney Transplantation
BKV is a ubiquitous human polyomavirus. Although it does not cause disease in immunocompetent individuals, it is an important viral pathogen after a kidney transplant (23). Unlike CMV viremia, BK viremia is usually not associated with systemic symptoms. The concern with BK viremia is progression to BK nephropathy (BKN) (24,25). In a study by Drachenberg et al. (26), a BKV level of more than 10,000 copies/ml was significantly associated with BKN. Similarly, a study by Korth et al. (27) found that a high-level BKV of >10,000 copies/ml was associated with worse graft function, rejection, and higher rates of BKN. BKN is estimated to occur in 2% to 10% of all kidney transplant recipients, and is as nephrotoxic as acute rejection (28,29). Graft survival after diagnosis ranges from 10% to 60%, making the prevention of BKN as important as the prevention of acute rejection and calcineurin-inhibitor nephrotoxicity (30). There are multiple risk factors for the development of BKN; however, the intensity of the immunosuppressive regimen is the most widely accepted risk factor (31). Currently, there is no effective antiviral therapy for prophylaxis or treatment of BK viremia. Although there is rationale to suggest a possible role of cidofovir, its lipid conjugate brincidofovir, leflunomide, and intravenous Ig, well-designed studies demonstrating efficacy of these therapies are lacking (32). Pipeline antiviral agents with activity against BK are in development; however, these are mostly in preclinical phases. The most advanced candidate, currently in phase 2, is MAU868 (Amplyx Pharmaceuticals), a mAb directed against VP1, the major viral capsid protein of BKV (ClinicalTrials.gov identifier NCT04294472). Consensus guidelines recommend the prevention of BKN by protocolized screening for BK viremia. The consensus on the management of BKV detected with a positive viral-screen PCR is to decrease immunosuppression to facilitate the clearance and prevent deleterious outcomes, such as BKN (32,33). This presents a real challenge in post-transplant management, because immunosuppression is indicated for the prevention of graft rejection (28). In our institutional experience, stepwise immunosuppression reduction after diagnosis of BK viremia was effective in inducing resolution of viremia only in around half of patients; approximately one quarter of the remaining progressed to detrimental outcomes of either severe BK viremia or BKN, and the remaining one quarter developed de novo donor-specific antibody against HLA or experienced acute rejection (34).
Cell-based Immunotherapy
Improvement in the treatment of CMV and BKV after kidney transplant is needed. Cell-based immunotherapy offers an attractive alternative approach to traditional interventions. The use of adoptive allogeneic T cell transfer is a therapeutic option capable of restoring virus-specific T cell immunity with infusions of VST from donor-derived VSTs (35). VSTs are safe and effective in the treatment of viral infections post-HSCT over the last two decades (36–39).
The isolation of VST is time and labor intensive, and has been limited to a few specialized centers with a Good Manufacturing Practice–grade cell manufacturing facility. The CliniMACS cytokine capture system, which has been available for several years, allows for rapid isolation of IFN-γ–producing antigen-specific T cells, but remains labor intensive and presents significant operator-dependent variability.
Briefly, this method allows for the direct enrichment of virus-specific CD4+ and CD8+ T cells after incubation with the respective viral antigens (40). The method was first described in 1999 and exploits the natural mechanism that antigen-specific memory T cells produce IFN-γ on incubation with the specific antigen (41). In the first step of the selection process, unfractionated PBMCs from donors, typically collected by leukapheresis, are incubated with a peptide pool spanning defined viral protein antigens (MACS GMP PepTivator Peptide Pools). The peptides are presented by monocytes with APC competency part of PBMC mix, triggering the intracellular production of IFN-γ by memory lymphoid cells with peptide specificity. They are then labeled with two different IFN-γ–specific antibodies in a stepwise procedure. The first binding step uses the CliniMACS IFN-γ Catchmatrix reagent. The second binding step uses the CliniMACS IFN-γ enrichment reagent. The Catchmatrix reagent forms a cytokine affinity matrix on the cell plasma membrane, which then will “trap” all cytokines subsequently produced by the cells on specific stimulation (40). The enrichment reagent then binds to the trapped cytokines, thus enhancing the signal. The enrichment antibody is conjugated to superparamagnetic particles and the final selection of the antibody/cell complexes is performed using the long-established magnetic-activated cell sorting (MACS) technology. The final product captured is predominantly constituted by IFN-γ+ T cells (both CD4 and CD8), whereas IFN-γ–negative peptide unreactive T cells are discarded. The depletion of unreactive T cells is in itself of material importance because it allows removal of immunologically naïve T cells that may be theoretically causative of graft versus host disease (GVHD) when transfused in HLA-mismatched, immune-suppressed allogeneic recipients (42–45).
Clinical Use of Viral-Specific T Cell Therapy
The greatest degree of clinical experience with viral-specific T cell therapy has been with CMV among HSCT recipients and is summarized in Table 1 (46). However, VST has also been used in the HSCT population for several other opportunistic viral infections with limited alternative treatment options including adenovirus, Epstein-Barr virus (EBV), human herpesvirus 6, and BKV (47). On the basis of the relative safety and perceived efficacy of these therapies, expansion to the solid organ transplant population has been suggested as the next logical step. Although limited, accumulating evidence supports the potential use of virus-specific T cells after a solid organ transplant (Table 2) (48–54).
Table 1.
Clinical studies of donor-derived cytomegalovirus-specific T cells in patients after hematopoietic stem cell transplantation
| Cell Therapy | No. of Patients | Date of Study | Activation | Acute Graft Versus Host Disease | Dose | Cytomegalovirus-related Outcome | Refa |
| CD8 cell clones | 14 | 1995 | Autologous fibroblasts pulsed with CMV virion proteins | 3 grade 1–2 GVHD | Intrapatient dose escalation: range 33 × 106/m2 to 1 × 109/m2 | Reconstitution CMV immunity in 14/14 | (36) |
| Polyclonal T cell lines | 8 | 2002 | CMV lysate | None | 1 × 107 T cells/m2 | 5/8 cleared after dose 1; 1/8 cleared after dose 2; 1/8 nonevaluable | (68) |
| Polyclonal T cell lines | 16 | 2003 | CMV Ag-pulsed DCs | 3 Grade 1 skin GVHD | 1 × 105/kg | 8/16 patients did not require antiviral drugs; further reactivation in 2/16 only | (38) |
| CD8 cells (multimer selected) | 9 | 2005 | NLV-HLA-A02 pentamers | 2 Grade 1–2 GVHD | 1.23 × 103/kg to 3.3 × 104/kg | 8/9 cleared viremia | (69) |
| CD4 T cell clones | 25 | 2005 | CMV Ag | 1 Grade 2 GVHD | 1 × 105/kg to 1 × 106/kg | 7/25 reactivated CMV; 5/25 disease (2 died) | (70) |
| Polyclonal T cell lines | 9 | 2007 | NLVPMVATV (HLA-A2 restricted) pulsed DCs | 3 Grade 3 GVHD (fatal in 1) | Target dose 2 × 107/m2 | Transient detection CMV-specific T cells by NLV-tetramer staining; 2/9 reactivated CMV without requirement antiviral drugs | (71) |
| Polyclonal T cell lines | 12 | 2008 | DCs transduced with Ad5f35pp65 adenoviral vector encoding CMV-pp65 | 2 Grade 3; 2 Grade 2 GVHD | 2 × 107/m2 | Reconstitution CMV immunity in 12/12; no requirement for antiviral drugs | (72) |
| Polyclonal CD4 and CD8 T cells (gamma catch) | 18 | 2010 | CMV-pp65 protein | 1 possible GVHD | Mean dose 21 × 103/kg –65-specific T cells | Partial or complete viral clearance in 15/18 | (56) |
| Polyclonal CD4 and CD8 T cells (gamma catch) | 18 (11 preemptive, 7 prophylactic) | 2011 | Recombinant pp65 or overlapping pp65 peptide pool | 5 Grade 1; 2 Grade 2; 1 Grade 3 GVHD | Target dose 1 × 104/CD3 T cells/kg | CMV-reactive T cells in 11/11 preemptively treated patients; 7/7 patients treated prophylactically did not reactivate CMV | (73) |
| CD8 T cells (multimer selected) | 2 | 2011 | Peptides derived from pp65 | None | 0.37 × 105 to 2.2 × 105 CMV-pp65-CTL/kg | 2/2 complete responses | (74) |
| Polyclonal T cell lines | 7 | 2012 | Peptides derived from IE-1 and pp65 | None | 2.5 × 105 to 5 × 105 CD3+CMV CTL/kg | 5/7 developed CMV-specific CTL activity in the blood; 2/7 no response | (75) |
| Polyclonal CD4 and CD8 T cells (gamma catch) | 6 | 2012 | Two CMV-pp65 peptides | None | 0.6 × 106 to 17 × 106 T cells (comprising 54%–96% CMV-pp65-specific CD8 T cells | 6/6 cleared viremia | (76) |
| CD8 T cells (multimer selected) | 2 | 2012 | NLV-containing HLA-A02 pentamers | None | 0.8 × 104 to 10.8 × 104 cells/kg | 2/2 complete responses | (77) |
| Polyclonal T cell lines | 16 | 2015 | 15-mer peptides spanning pp65 | None | 5 × 105/kg (x 1 dose) to 1 × 106/kg (×3 weekly doses) | 14/16 cleared viremia (including 2 with CMV disease) | (78) |
CMV, cytomegalovirus; GVHD, graft versus host disease; Ag, antigen; DC, dendritic cell; NLV, NLVPMVATV; CTL, cytotoxic T lymphocytes; VST, virus-specific T lymphocyte.
Some cited studies incorporate results of other published studies.
Table 2.
Clinical studies of virus-specific T cells in solid organ transplantation
| Cell Therapy | Indication | N | Date Study | Solid organ transplant | Virus-Specific T Cell Donor | Activation | Dose | Graft Versus Host Disease | Acute Rejection | Adverse Events | Disease-related Outcome |
| Autologous polyclonal EBV-specific Cytotoxic T cells | EBV+ PTLD | 1 | 1999 | Lung | Autologous | EBV infected lymphoblastoid cell lines | 35 × 106 T cells×2 + 60×106 T cells×2 | 0 | 0 | Death (pulmonary vein invasion with necrosis and hemorrhage) | PTLD near resolution |
| Allogeneic polyclonal EBV- specific cytotoxic T cells | EBV+ PTLD | 5 | 2002 | Liver (4), kidney (1) small bowel (3) | Frozen bank of CTLs derived from healthy blood donors | EBV infected lymphoblastoid cell lines | 106/kg x one to six times | 0 | 0 | 0 | 3/5 complete remission 2/5 no response |
| Autologous CMV-specific CD4+ and CD8+ cells | CMV | 1 | 2009 | Lung | Autologous | Overlapping IE‐1/pp65 peptide pools | 107 T cells/m2 x 2 | 0 | 1 | Death (rejection) | CMV resolution |
| Autologous CMV-specific CD4+ and CD8+ cells | CMV | 1 | 2015 | Lung | Autologous | Autologous PBMC coated with HLA class I restricted CMV peptide epitopes | Four infusions (total of 12 × 107 cells) | 0 | 0 | 0 | Persistent negative CMV PCR |
| Allogeneic Polyclonal CMV- specific CD4+ and CD8+ cells | CMV | 1 | 2015 | Kidney | 3/6 HLA matched third-party donor | Overlapping peptides covering pp65 | 1.6 × 107 T cells/m2 | 0 | 0 | Mild fever post VST infusion | At 1 yr CMV viral load declined from 5.5 M to 73 copies/ml |
| Allogenic BK- specific VST | BKV | 3 | 2017 | Kidney (1) kidney + heart (1) heart (1) | Adult volunteers | IFN-γ production in response to repeat stimulation with BKV pepmixes | 5 × 107T cells/m2 | 0 | 0 | 0 | BKV cleared (1) BK partial response or reduced (2) |
EBV, Epstein-Barr virus; PTLD, post-transplant lymphoproliferative disorder; CTL, cytotoxic T lymphocytes; CMV, cytomegalovirus; BKV, BK virus.
In kidney transplantation, there are few reported patients of adoptive allogeneic T cell immunotherapy for the treatment of EBV+ post-transplant lymphoproliferative disorder (49) and CMV (48) infection. In the earlier report (49), a 60-year-old kidney transplant recipient with widespread drug-resistant non-Hodgkin’s lymphoma 15 years after transplant failed allogeneic cytotoxic T lymphocytes therapy from a pool of healthy blood donors after six infusions. The second report described ganciclovir-resistant CMV disease in a renal transplant recipient, manifested by thrombotic microangiopathy and glomerulopathy, and adoptive T cell immunotherapy using CMV-specific T cells from a donor bank was safely used as salvage therapy (48). Finally, in a recent report by Nelson et al. describing the use of VST for BKV an “off-the-shelf” third-party VST was successful in clearing BK viremia <10,000 copies in two kidney transplant recipients who had failed cidofovir, leflunomide, and IV Ig therapy. One patient had a complete response after ten infusions, the second had a partial response after a single infusion (54). In aggregate, there have been no reported patients with acute rejection, GVHD, or death associated with VST in kidney transplantation. Together, these data present a proof-of-concept of the clinical and operational feasibility of this therapy in solid organ transplant recipients. There are few clinical trials registered on ClinicalTrials.gov that propose to study the role of VST against CMV in solid organ transplantation (Table 3). All these trials utilize VST from allogeneic, partially matched donors.
Table 3.
Registered clinical trials of virus-specific T cells in solid organ transplantation
| ID | Cell Therapy | Targets | Indication | N | Solid organ transplant | Virus-specific T Lymphocyte Donor | Primary Endpoint | Site |
| NCT02779439 | CTL | CMV, EBV, ADV | Resistance to Rx >2 wk | 25 | Any | Allogeneic | Infusion related safety at 1 wk | Sydney |
| NCT02532452 | CTL | EBV, CMV, ADV, or BKV | Any Infection | 100 | Any | Allogeneic | Infusion related toxicity | Cincinnati |
| NCT03010332 | CTL | CMV | Intolerant to or failed Rx | N/A | Any | Allogeneic (ATA230) | N/A | Atara Biotherapeutics |
| NCT03950414 | CTL | CMV | Intolerant to or failed Rx | 20 | Kidney | Allogenic | Safety and tolerability | Madison, Wisconsin |
CTL, cytotoxic T lymphocytes; CMV, cytomegalovirus; EBV, Epstein-Barr virus; ADV, adenovirus.
Potential Obstacles and Risks of VST in Kidney Transplant
GVHD
Although the T cells infused are selected to be antigen specific, a small amount of nonspecific alloreactive T cells may be infused, carrying a risk for increased GVHD. Also, cross-reactivity with other antigens, although unlikely, cannot be predicted. The resultant effect would be the development or worsening of GVHD. To date, multiple groups have reported the safety of infusion of virus-specific T cells in HSCT. There have been no infusion-related side effects, and GVHD reactivation has been reported in very few instances (55,56). However, this potential risk is met with significant trepidation in solid organ transplant, because unlike in HSCT, GVHD is almost universally fatal in this population (57).
T Cell Transfer Toxicity
There are toxicity concerns associated with the use of T cells to treat active infection, including the overstimulation of antigen-specific T cells that could potentially result in cytokine release syndrome (CRS) and tissue damage. CRS is a potential risk of the infusion of virus-specific, antigen-selected T cells. Most patients with CRS will see it occur within 48–72 hours of infusion, but it can occur later (58).
Lack of Efficacy Related to Ongoing Immunosuppression
Although HSCT and kidney transplant recipients are at risk for many of the same opportunistic viral infections, the etiology of their immunosuppressed state is inherently different. In the setting of HSCT, the duration of immunocompromise is not anticipated to be lifelong. Additionally, beyond the conditioning regimen, immunosuppressive therapies are reserved for complications such as GVHD. Conversely, patients who have had a kidney transplant are maintained lifelong on immunosuppressive drugs. These regimens typically include potent T cell inhibitors, such as tacrolimus and mycophenolate. The effect of iatrogenic immunosuppression on the efficacy of VST has not been completely elucidated (59). There are limited studies in HSCT that report a lack of impairment of VST efficacy in the setting of glucocorticoid doses of 1 mg/kg, used to treat GVHD in HSCT (39,60). However, the negative effect of T cell–depleting antibodies on VST expansion would be anticipated to be similar to its profound effects on host cell–mediated immunity to viral infection (59,61). Furthermore, the role of steroids in the absence of T cell–specific immunosuppression in impairment of T cell–mediated immunity to viral infections is thought to be fairly minimal (61,62). With standard transplant immunosuppressive potency falling somewhere between T cell depletion and glucocorticoid monotherapy, the anticipated effect on VST efficacy is unknown.
Generation of calcineurin inhibitor–resistant VST by gene-transfer technology has been explored (63,64). This would allow coadministration with these drugs and increase potential utility of VST in the solid organ transplant population if clinical studies demonstrate impaired in vivo VST expansion in the setting of immunosuppressive therapy. Further clinical studies are needed to better explore the effect of iatrogenic immunosuppression on the in vivo efficacy of VSTs.
Methods and Challenges in The Preparation of VST
FDA-Compliant VST Manufacturing within Academic Health Centers
VSTs are regulated as more-than-minimally manipulated products, as defined by Public Health Service 351, and require an investigational new drug license issued by the FDA Center for Biologics Evaluation and Research for use as investigational cell pharmaceuticals in human clinical trials before marketing approval. Both CMV and BKV VST manufactured by the CliniMACS Prodigy present similar challenges for the accurate determination of the viability of the final cell collection. VST cells are purified by the cytokine capture system and eluted from the MACS column by an automated process. The intrinsic nature of the automated processes favors the coelution of VST cells and nonviable cells, rendering the determination of the final product by the hemocytometer–trypan blue enumeration method inadequate. In addition, the concentration of cells in the final cell elution is low and often does not meet standard system suitability of ≥10 cells/hemocytometer quadrant, resulting in an inaccurate determination of both the live and nonviable cell count. In addition, the presence of visual cellular debris results in method interference, confounding the specificity of cell product identity. As a result, cell count and percent viability are determined by flow cytometry using CD45 as a marker.
As per the FDA guidance for investigational new drug chemistry, manufacturing, and control for human somatic cell therapy, ≥70% is the generally accepted viability criteria (65). A lower viability limit must accompany data demonstrating nonviable cells do not influence the safe administration of the product and/or the therapeutic effect. The CliniMACS Prodigy procedure is an automated process. However, once the instrument has eluted cells from the MACS column, the procedure for quality control (QC) testing and preparation of the final product is a continuous process without interruption to deliver the product to the clinic within a 6-hour time limit from collection to infusion. As a result, a high level of risk management, readiness, and coordinated, sequential events must be precisely executed. Time management is critical for the resolution of QC test system suitability issues, final product preparation, and quality assurance, and medical director authorization for infusion. Quality assurance must be present throughout the entire QC testing and final product preparation process for real-time review of in-process, check-point procedures, and accompanying documentation, and approval/rejection of product specification results.
Cell collection from the CliniMACS Prodigy gives an approximate final volume of 7 ml, and from that volume, 1 ml is removed for QC testing purposes, leaving approximately 6 ml available for the clinic. From the 1 ml QC sample, only minimum volumes are available to complete endotoxin, gram stain, sterility, and flow cytometry test methods. There is little or no extra final product for repeat testing, if necessary and justified. The 1 ml volume is removed from the bag and tested and approved as a drug substance. The final product is prepared from the drug substance by drawing the remaining drug substance into a syringe and sealing it with a sterile cap.
Donor Selection
The Miltenyi Biotec Blood Donor Eligibility Test is a flow cytometry–based method designed, manufactured, and marketed specifically for the purpose of VST cell donor eligibility (66). PBMCs are incubated with either CMV or BKV Miltenyi Biotec virus-specific PepTivators. Negative controls are incubated in the absence of PepTivators, and positive controls are prepared and analyzed for system suitability purposes. For our CMV clinical trials, leukapheresis donor selection for CMV VST cell manufacturing is on the basis of a percent of CD3+IFNγ+, CD4+INFγ+, and CD8+IFNγ+ positive response. Table 4 summarizes the results of 16 CMV seropositive donors and 14 BKV seropositive donors that were screened for process development and donor selection at our institution. These data support the published findings that BKV VST cells are a rare cell type (67). For this reason, BKV VST leukapheresis donors are selected On the basis of BKV seropositivity only. QC testing flow cytometry results from CliniMACS Prodigy manufactured VST cells support the donor pretest data collected from blood donor eligibility tests. Figures 1 and 2 show example results comparing CMV and BKV VST blood donor eligibility test and manufactured VSTs.
Table 4.
Blood Donor Eligibility Test data summary of 16 cytomegalovirus seropositive donors and 14 BK seropositive donors
| Marker % | Cytomegalovirus Donor Pre-test Summary (Mean of n=16 donors) | BK Donor Pre-test Summary (Mean of n=14 donors) | ||
| Negative Control (n=1/analysis) | CMV PepTivator-stimulated Mean (n=3/analysis) | Negative Control (n=1/analysis) | BK PepTivator-stimulated Mean (n=3/analysis) | |
| %CD3+IFNγ+ | 0.08 | 0.42 | 0.08 | 0.07 |
| %CD4+IFNγ+ | 0.07 | 0.22 | 0.09 | 0.08 |
| %CD8+IFNγ+ | 0.13 | 0.69 | 0.08 | 0.07 |
Figure 1.
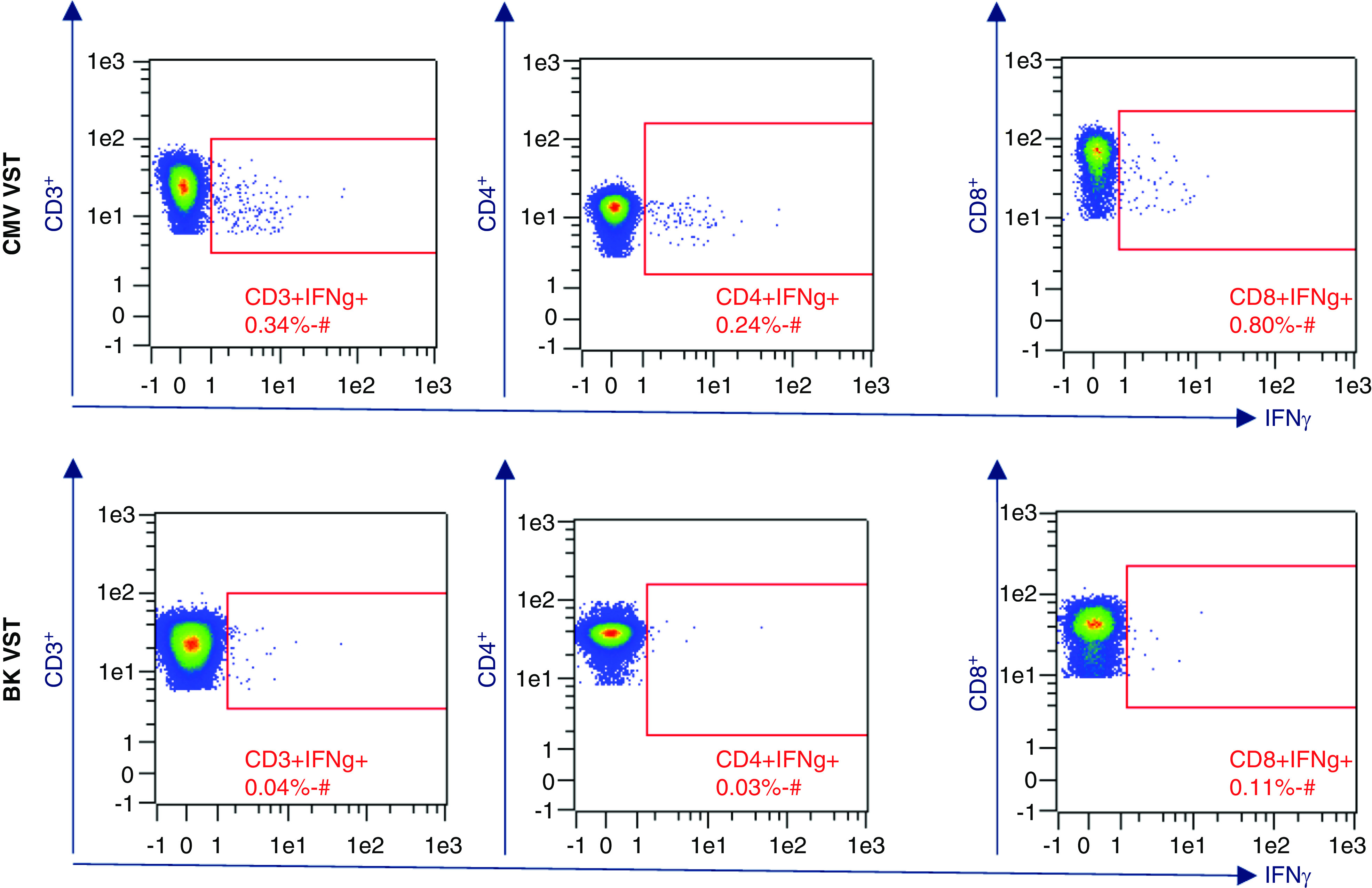
Example Blood Donor Eligibility Test flow cytometry data analysis of Ficoll-isolated PBMCs after 4-hour stimulation with virus-specific peptides. Frequency comparison of CMV versus BK virus–specific INFγ+ T cells. CMV, cytomegalovirus; BKV, BK virus; VST, virus-specific T lymphocytes.
Figure 2.
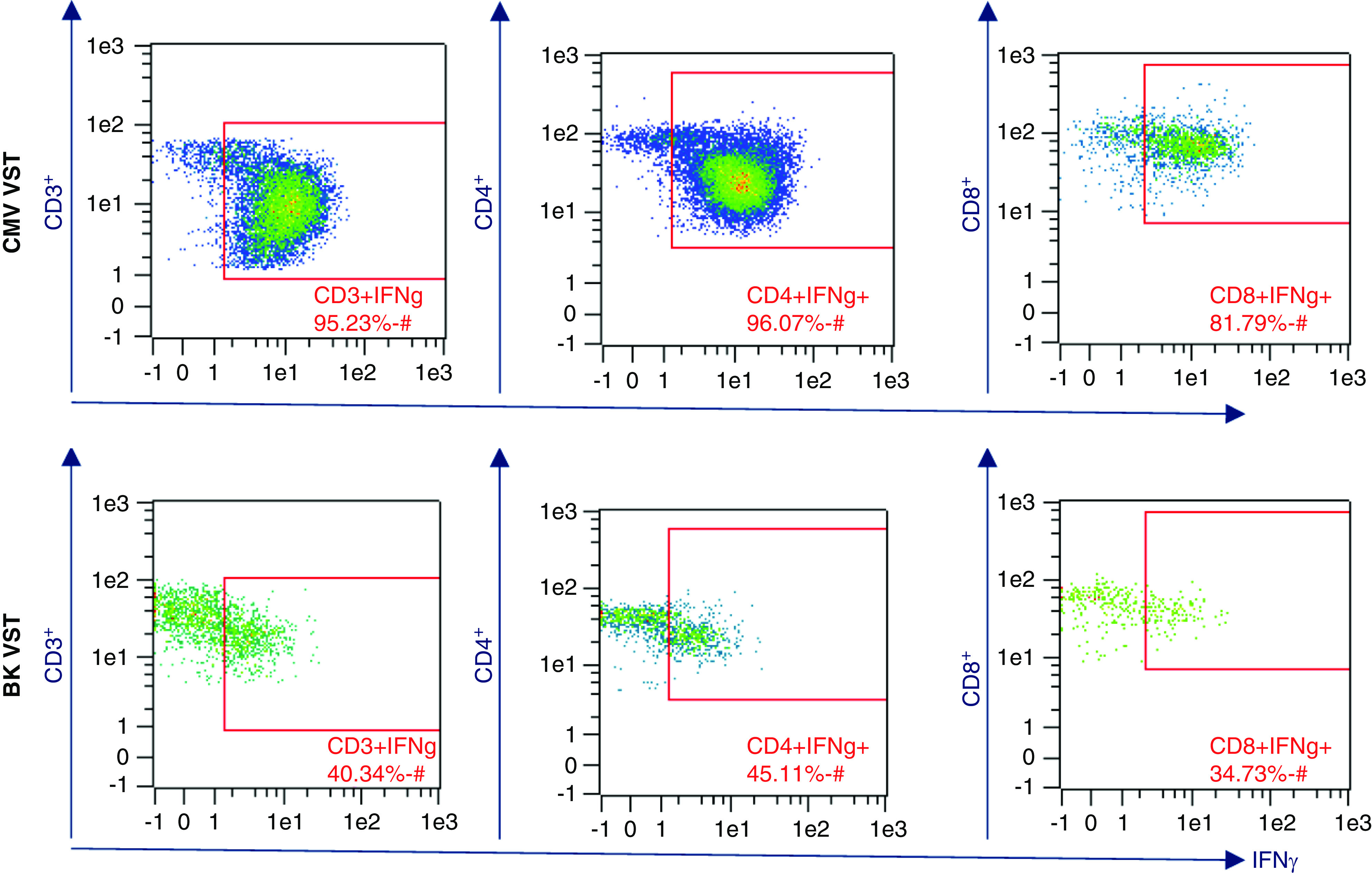
Cell therapy product release data example. Flow cytometry analysis of enriched virus-specific T cells post automated processing by CliniMACS Prodigy. Comparison of frequency of CMV versus BKV-specific INFγ+ T cells.
Summary
In summary, CMV and BK are common viral infections in kidney transplant recipients with imperfect treatment strategies, and are associated with negative patient and graft outcomes. VST may be a promising novel therapeutic approach for these viral infections in kidney transplant recipients. With emerging data mainly from HSCT and very limited studies in the solid organ transplant population, more evidence is needed exploring the therapeutic efficacy and potential risks of VST in kidney transplant recipients, taking into account the unique aspects of this population over HSCT recipients and bespoke FDA-compliant cell manufacturing outcomes.
Disclosures
A. Djamali reports having consultancy agreements with CSL and CareDx; reports receiving research funding from CareDx and Takeda; reports receiving honoraria from CSL and CareDx; and reports being a scientific advisor or member of CSL and CareDx. S. Parajuli reports receiving research funding from Veloxis. All remaining authors have nothing to disclose.
Funding
The University of Wisconsin Program for Advanced Cell Therapy is supported by the UW School of Medicine and Public Health, UW Health, and UW Carbone Cancer Center with a partnered unrestricted grant.
Acknowledgments
The authors are thankful to Ms. Olga Ganz for her technical contributions.
Author Contributions
R.O. Meyers was responsible for the validation; S. Parajuli wrote the original draft; and all authors reviewed and edited the manuscript.
References
- 1.Moss P, Rickinson A: Cellular immunotherapy for viral infection after HSC transplantation. Nat Rev Immunol 5: 9–20, 2005. 10.1038/nri1526 [DOI] [PubMed] [Google Scholar]
- 2.Fishman JA, Rubin RH: Infection in organ-transplant recipients. N Engl J Med 338: 1741–1751, 1998. 10.1056/NEJM199806113382407 [DOI] [PubMed] [Google Scholar]
- 3.Weikert BC, Blumberg EA: Viral infection after renal transplantation: Surveillance and management. Clin J Am Soc Nephrol 3[Suppl 2]: S76–S86, 2008. 10.2215/CJN.02900707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garces JC: BK virus-associated nephropathy in kidney transplant recipients. Ochsner J 10: 245–249, 2010 [PMC free article] [PubMed] [Google Scholar]
- 5.Ho M: The history of cytomegalovirus and its diseases. Med Microbiol Immunol (Berl) 197: 65–73, 2008. 10.1007/s00430-007-0066-x [DOI] [PubMed] [Google Scholar]
- 6.Kotton CN, Fishman JA: Viral infection in the renal transplant recipient. J Am Soc Nephrol 16: 1758–1774, 2005. 10.1681/ASN.2004121113 [DOI] [PubMed] [Google Scholar]
- 7.Duncan MD, Wilkes DS: Transplant-related immunosuppression: A review of immunosuppression and pulmonary infections. Proc Am Thorac Soc 2: 449–455, 2005. 10.1513/pats.200507-073JS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishman JA: Infection in solid-organ transplant recipients. N Engl J Med 357: 2601–2614, 2007. 10.1056/NEJMra064928 [DOI] [PubMed] [Google Scholar]
- 9.Gardiner BJ, Chow JK, Brilleman SL, Peleg AY, Snydman DR: The impact of recurrent cytomegalovirus infection on long-term survival in solid organ transplant recipients. Transpl Infect Dis 21: e13189, 2019. 10.1111/tid.13189 [DOI] [PubMed] [Google Scholar]
- 10.Asberg A, Jardine AG, Bignamini AA, Rollag H, Pescovitz MD, Gahlemann CC, Humar A, Hartmann A; VICTOR Study Group: Effects of the intensity of immunosuppressive therapy on outcome of treatment for CMV disease in organ transplant recipients. Am J Transplant 10: 1881–1888, 2010. 10.1111/j.1600-6143.2010.03114.x [DOI] [PubMed] [Google Scholar]
- 11.Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group: The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 102: 900–931, 2018. 10.1097/TP.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 12.Asberg A, Rollag H, Hartmann A: Valganciclovir for the prevention and treatment of CMV in solid organ transplant recipients. Expert Opin Pharmacother 11: 1159–1166, 2010. 10.1517/14656561003742954 [DOI] [PubMed] [Google Scholar]
- 13.Kalil AC, Freifeld AG, Lyden ER, Stoner JA: Valganciclovir for cytomegalovirus prevention in solid organ transplant patients: An evidence-based reassessment of safety and efficacy. PLoS One 4: e5512, 2009. 10.1371/journal.pone.0005512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD: Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 355: 2032–2036, 2000. 10.1016/S0140-6736(00)02350-3 [DOI] [PubMed] [Google Scholar]
- 15.Leeaphorn N, Garg N, Thamcharoen N, Khankin EV, Cardarelli F, Pavlakis M: Cytomegalovirus mismatch still negatively affects patient and graft survival in the era of routine prophylactic and preemptive therapy: A paired kidney analysis. Am J Transplant 19: 573–584, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Young PG, Rubin J, Angarone M, Flaherty J, Penugonda S, Stosor V, Ison MG: Ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients: A single-center retrospective cohort study. Transpl Infect Dis 18: 390–395, 2016. 10.1111/tid.12537 [DOI] [PubMed] [Google Scholar]
- 17.Rolling KE, Jorgenson MR, Descourouez JL, Mandelbrot DA, Redfield RR, Smith JA: Ganciclovir-resistant cytomegalovirus infection in abdominal solid organ transplant recipients: Case series and review of the literature. Pharmacotherapy 37: 1258–1271, 2017. 10.1002/phar.1987 [DOI] [PubMed] [Google Scholar]
- 18.Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, Brundage TM, Robertson AT, Godkin S, Momméja-Marin H, Boeckh M; CMX001-201 Clinical Study Group: CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med 369: 1227–1236, 2013. 10.1056/NEJMoa1303688 [DOI] [PubMed] [Google Scholar]
- 19.Winston DJ, Saliba F, Blumberg E, Abouljoud M, Garcia-Diaz JB, Goss JA, Clough L, Avery R, Limaye AP, Ericzon BG, Navasa M, Troisi RI, Chen H, Villano SA, Uknis ME; 1263-301 Clinical Study Group: Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: A randomized, double-blind, multicenter controlled trial [published correction appears in Am J Transplant 13: 529, 2013]. Am J Transplant 12: 3021–3030, 2012. 10.1111/j.1600-6143.2012.04231.x [DOI] [PubMed] [Google Scholar]
- 20.Papanicolaou GA, Silveira FP, Langston AA, Pereira MR, Avery RK, Uknis M, Wijatyk A, Wu J, Boeckh M, Marty FM, Villano S: Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic-cell or solid-organ transplant recipients: A randomized, dose-ranging, double-blind, phase 2 study. Clin Infect Dis 68: 1255–1264, 2019. 10.1093/cid/ciy706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lischka P, Hewlett G, Wunberg T, Baumeister J, Paulsen D, Goldner T, Ruebsamen-Schaeff H, Zimmermann H: In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother 54: 1290–1297, 2010. 10.1128/AAC.01596-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Helou G, Razonable RR: Letermovir for the prevention of cytomegalovirus infection and disease in transplant recipients: An evidence-based review. Infect Drug Resist 12: 1481–1491, 2019. 10.2147/IDR.S180908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice: BK polyomavirus in solid organ transplantation. Am J Transplant 13[Suppl 4]: 179–188, 2013. 10.1111/ajt.12110 [DOI] [PubMed] [Google Scholar]
- 24.Vasudev B, Hariharan S, Hussain SA, Zhu YR, Bresnahan BA, Cohen EP: BK virus nephritis: Risk factors, timing, and outcome in renal transplant recipients. Kidney Int 68: 1834–1839, 2005. 10.1111/j.1523-1755.2005.00602.x [DOI] [PubMed] [Google Scholar]
- 25.Ahuja M, Cohen EP, Dayer AM, Kampalath B, Chang CC, Bresnahan BA, Hariharan S: Polyoma virus infection after renal transplantation. Use of immunostaining as a guide to diagnosis. Transplantation 71: 896–899, 2001. 10.1097/00007890-200104150-00013 [DOI] [PubMed] [Google Scholar]
- 26.Drachenberg CB, Hirsch HH, Papadimitriou JC, Gosert R, Wali RK, Munivenkatappa R, Nogueira J, Cangro CB, Haririan A, Mendley S, Ramos E: Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: A prospective evaluation. Transplantation 84: 323–330, 2007. 10.1097/01.tp.0000269706.59977.a5 [DOI] [PubMed] [Google Scholar]
- 27.Korth J, Widera M, Dolff S, Guberina H, Bienholz A, Brinkhoff A, Anastasiou OE, Kribben A, Dittmer U, Verheyen J, Wilde B, Witzke O: Impact of low-level BK polyomavirus viremia on intermediate-term renal allograft function. Transpl Infect Dis 20: 2018. 10.1111/tid.12817 [DOI] [PubMed] [Google Scholar]
- 28.Parajuli S, Astor BC, Kaufman D, Muth B, Mohamed M, Garg N, Djamali A, Mandelbrot DA: Which is more nephrotoxic for kidney transplants: BK nephropathy or rejection? Clin Transplant 32: e13216, 2018. 10.1111/ctr.13216 [DOI] [PubMed] [Google Scholar]
- 29.Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, Mihatsch MJ, Nickeleit V, Ramos E, Randhawa P, Shapiro R, Steiger J, Suthanthiran M, Trofe J: Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation 79: 1277–1286, 2005. 10.1097/01.TP.0000156165.83160.09 [DOI] [PubMed] [Google Scholar]
- 30.Trofe J, Hirsch HH, Ramos E: Polyomavirus-associated nephropathy: Update of clinical management in kidney transplant patients [published correction appears in Transpl Infect Dis 10: 75, 2008]. Transpl Infect Dis 8: 76–85, 2006. 10.1111/j.1399-3062.2006.00166.x [DOI] [PubMed] [Google Scholar]
- 31.Borni-Duval C, Caillard S, Olagne J, Perrin P, Braun-Parvez L, Heibel F, Moulin B: Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation 95: 1498–1505, 2013. 10.1097/TP.0b013e3182921995 [DOI] [PubMed] [Google Scholar]
- 32.Hirsch HH, Randhawa PS; AST Infectious Diseases Community of Practice: BK polyomavirus in solid organ transplantation-guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant 33: e13528, 2019. 10.1111/ctr.13528 [DOI] [PubMed] [Google Scholar]
- 33.Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice: BK virus in solid organ transplant recipients. Am J Transplant 9[Suppl 4]: S136–S146, 2009. 10.1111/j.1600-6143.2009.02904.x [DOI] [PubMed] [Google Scholar]
- 34.Kharel A, Djamali A, Jorgenson MR, Alzoubi B, Swanson KJ, Garg N, Aziz F, Mohamed MA, Mandelbrot DA, Parajuli S: Risk factors for progression from low level BK dnaemia to unfavorable outcomes after BK management via immunosuppressive reduction [published online ahead of print January 5, 2021]. Transpl Infect Dis: e13561, 2021 [DOI] [PubMed] [Google Scholar]
- 35.Lamarche C, Orio J, Georges-Tobar V, Pincez T, Goupil M, Dahmani A, Carli C, Brasey A, Busque L, Delisle JS: Clinical-scale rapid autologous BK virus-specific T cell line generation from kidney transplant recipients with active viremia for adoptive immunotherapy. Transplantation 101: 2713–2721, 2017. 10.1097/TP.0000000000001698 [DOI] [PubMed] [Google Scholar]
- 36.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR: Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 333: 1038–1044, 1995. 10.1056/NEJM199510193331603 [DOI] [PubMed] [Google Scholar]
- 37.Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, Brenner MK, Heslop HE: Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 345: 9–13, 1995. 10.1016/S0140-6736(95)91150-2 [DOI] [PubMed] [Google Scholar]
- 38.Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, Mackinnon S: Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 362: 1375–1377, 2003. 10.1016/S0140-6736(03)14634-X [DOI] [PubMed] [Google Scholar]
- 39.Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, Hedvat C, Chou JF, Heller G, Barker JN, Boulad F, Castro-Malaspina H, George D, Jakubowski A, Koehne G, Papadopoulos EB, Scaradavou A, Small TN, Khalaf R, Young JW, O’Reilly RJ: Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 119: 2644–2656, 2012. 10.1182/blood-2011-08-371971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell JD: Detection and enrichment of antigen-specific CD4+ and CD8+ T cells based on cytokine secretion. Methods 31: 150–159, 2003. 10.1016/S1046-2023(03)00125-7 [DOI] [PubMed] [Google Scholar]
- 41.Brosterhus H, Brings S, Leyendeckers H, Manz RA, Miltenyi S, Radbruch A, Assenmacher M, Schmitz J: Enrichment and detection of live antigen-specific CD4(+) and CD8(+) T cells based on cytokine secretion. Eur J Immunol 29: 4053–4059, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Tischer S, Priesner C, Heuft HG, Goudeva L, Mende W, Barthold M, Kloeß S, Arseniev L, Aleksandrova K, Maecker-Kolhoff B, Blasczyk R, Koehl U, Eiz-Vesper B: Rapid generation of clinical-grade antiviral T cells: Selection of suitable T-cell donors and GMP-compliant manufacturing of antiviral T cells. J Transl Med 12: 336, 2014. 10.1186/s12967-014-0336-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunos M, Hümmer C, Wingenfeld E, Sorg N, Pfirrmann V, Bader P, Seifried E, Bönig H: Automated isolation of primary antigen-specific T cells from donor lymphocyte concentrates: Results of a feasibility exercise. Vox Sang 109: 387–393, 2015. 10.1111/vox.12291 [DOI] [PubMed] [Google Scholar]
- 44.Priesner C, Esser R, Tischer S, Marburger M, Aleksandrova K, Maecker-Kolhoff B, Heuft HG, Goudeva L, Blasczyk R, Arseniev L, Köhl U, Eiz-Vesper B, Klöß S: Comparative analysis of clinical-scale IFN-γ-Positive T-cell enrichment using partially and fully integrated platforms. Front Immunol 7: 393, 2016. 10.3389/fimmu.2016.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumaresan P, Figliola M, Moyes JS, Huls MH, Tewari P, Shpall EJ, Champlin R, Cooper LJ: Automated cell enrichment of cytomegalovirus-specific T cells for clinical applications using the cytokine-capture system. J Vis Exp 104: 52808, 2015. 10.3791/52808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roddie C, Peggs KS: Immunotherapy for transplantation-associated viral infections. J Clin Invest 127: 2513–2522, 2017. 10.1172/JCI90599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arasaratnam RJ, Leen AM: Adoptive T cell therapy for the treatment of viral infections. Ann Transl Med 3: 278, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macesic N, Langsford D, Nicholls K, Hughes P, Gottlieb DJ, Clancy L, Blyth E, Micklethwaite K, Withers B, Majumdar S, Fleming S, Sasadeusz J: Adoptive T cell immunotherapy for treatment of ganciclovir-resistant cytomegalovirus disease in a renal transplant recipient. Am J Transplant 15: 827–832, 2015. 10.1111/ajt.13023 [DOI] [PubMed] [Google Scholar]
- 49.Haque T, Wilkie GM, Taylor C, Amlot PL, Murad P, Iley A, Dombagoda D, Britton KM, Swerdlow AJ, Crawford DH: Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet 360: 436–442, 2002. 10.1016/S0140-6736(02)09672-1 [DOI] [PubMed] [Google Scholar]
- 50.Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJ: Epstein-barr virus-positive posttransplant lymphoproliferative disease after solid organ transplantation: Pathogenesis, clinical manifestations, diagnosis, and management. Transplant Direct 2: e48, 2015. 10.1097/TXD.0000000000000557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmes-Liew CL, Holmes M, Beagley L, Hopkins P, Chambers D, Smith C, Khanna R: Adoptive T-cell immunotherapy for ganciclovir-resistant CMV disease after lung transplantation. Clin Transl Immunology 4: e35, 2015. 10.1038/cti.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melendez D, Razonable RR: Immune-based monitoring for cytomegalovirus infection in solid organ transplantation: Is it ready for clinical primetime? Expert Rev Clin Immunol 10: 1213–1227, 2014. 10.1586/1744666X.2014.943190 [DOI] [PubMed] [Google Scholar]
- 53.Khanna R, Bell S, Sherritt M, Galbraith A, Burrows SR, Rafter L, Clarke B, Slaughter R, Falk MC, Douglass J, Williams T, Elliott SL, Moss DJ: Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci U S A 96: 10391–10396, 1999. 10.1073/pnas.96.18.10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson AS, Heyenbruch D, Rubinstein JD, Sabulski A, Jodele S, Thomas S, Lutzko C, Zhu X, Leemhuis T, Cancelas JA, Keller M, Bollard CM, Hanley PJ, Davies SM, Grimley MS: Virus-specific T-cell therapy to treat BK polyomavirus infection in bone marrow and solid organ transplant recipients. Blood Adv 4: 5745–5754, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feuchtinger T, Matthes-Martin S, Richard C, Lion T, Fuhrer M, Hamprecht K, Handgretinger R, Peters C, Schuster FR, Beck R, Schumm M, Lotfi R, Jahn G, Lang P: Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol 134: 64–76, 2006. 10.1111/j.1365-2141.2006.06108.x [DOI] [PubMed] [Google Scholar]
- 56.Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, Mohty M, Or R, Maschan M, Schumm M, Hamprecht K, Handgretinger R, Lang P, Einsele H: Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 116: 4360–4367, 2010. 10.1182/blood-2010-01-262089 [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Ruiz P: Solid organ transplant-associated acute graft-versus-host disease. Arch Pathol Lab Med 134: 1220–1224, 2010. 10.5858/2008-0679-RS.1 [DOI] [PubMed] [Google Scholar]
- 58.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL: Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood 126: 1048, 2015]. Blood 124: 188–195, 2014. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian C, Wang Y, Reppel L, D’aveni M, Campidelli A, Decot V, Bensoussan D: Viral-specific T-cell transfer from HSCT donor for the treatment of viral infections or diseases after HSCT. Bone Marrow Transplant 53: 114–122, 2018. 10.1038/bmt.2017.232 [DOI] [PubMed] [Google Scholar]
- 60.Feucht J, Opherk K, Lang P, Kayser S, Hartl L, Bethge W, Matthes-Martin S, Bader P, Albert MH, Maecker-Kolhoff B, Greil J, Einsele H, Schlegel PG, Schuster FR, Kremens B, Rossig C, Gruhn B, Handgretinger R, Feuchtinger T: Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood 125: 1986–1994, 2015. 10.1182/blood-2014-06-573725 [DOI] [PubMed] [Google Scholar]
- 61.Sia IG, Patel R: New strategies for prevention and therapy of cytomegalovirus infection and disease in solid-organ transplant recipients. Clin Microbiol Rev 13: 83–121, 2000. 10.1128/CMR.13.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis RM, Johnson PC, Golden D, Van Buren CT, Kerman RH, Kahan BD: The adverse impact of cytomegalovirus infection on clinical outcome in cyclosporine-prednisone treated renal allograft recipients. Transplantation 45: 353–359, 1988. 10.1097/00007890-198802000-00022 [DOI] [PubMed] [Google Scholar]
- 63.De Angelis B, Dotti G, Quintarelli C, Huye LE, Zhang L, Zhang M, Pane F, Heslop HE, Brenner MK, Rooney CM, Savoldo B: Generation of Epstein-Barr virus-specific cytotoxic T lymphocytes resistant to the immunosuppressive drug tacrolimus (FK506). Blood 114: 4784–4791, 2009. 10.1182/blood-2009-07-230482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brewin J, Mancao C, Straathof K, Karlsson H, Samarasinghe S, Amrolia PJ, Pule M: Generation of EBV-specific cytotoxic T cells that are resistant to calcineurin inhibitors for the treatment of posttransplantation lymphoproliferative disease. Blood 114: 4792–4803, 2009. 10.1182/blood-2009-07-228387 [DOI] [PubMed] [Google Scholar]
- 65.Food and Drug Administration: Guidance for FDA Reviewers and Sponsors Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs), 2008. Available at: https://www.fda.gov/media/73624/download. Accessed February 14, 2021
- 66.Kable K, Davies CD, O’connell PJ, Chapman JR, Nankivell BJ: Clearance of BK virus nephropathy by combination antiviral therapy with intravenous immunoglobulin. Transplant Direct 3: e142, 2017. 10.1097/TXD.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pello OM, Innes AJ, Bradshaw A, Finn SA, Uddin S, Bray E, Olavarria E, Apperley JF, Pavlů J: BKV-specific T cells in the treatment of severe refractory haemorrhagic cystitis after HLA-haploidentical haematopoietic cell transplantation. Eur J Haematol 98: 632–634, 2017. 10.1111/ejh.12848 [DOI] [PubMed] [Google Scholar]
- 68.Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Löffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H: Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99: 3916–3922, 2002. 10.1182/blood.V99.11.3916 [DOI] [PubMed] [Google Scholar]
- 69.Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, Assenmacher M, Billingham L, Steward C, Crawley C, Olavarria E, Goldman J, Chakraverty R, Mahendra P, Craddock C, Moss PA: Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med 202: 379–386, 2005. 10.1084/jem.20040613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, Capanni M, Urbani E, Mancusi A, Aversa F, Martelli MF, Romani L, Velardi A: Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood 106: 4397–4406, 2005. 10.1182/blood-2005-05-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Micklethwaite K, Hansen A, Foster A, Snape E, Antonenas V, Sartor M, Shaw P, Bradstock K, Gottlieb D: Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 13: 707–714, 2007. 10.1016/j.bbmt.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 72.Micklethwaite KP, Clancy L, Sandher U, Hansen AM, Blyth E, Antonenas V, Sartor MM, Bradstock KF, Gottlieb DJ: Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood 112: 3974–3981, 2008. 10.1182/blood-2008-06-161695 [DOI] [PubMed] [Google Scholar]
- 73.Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, Pang K, Mackinnon S, Lowdell MW: Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis 52: 49–57, 2011. 10.1093/cid/ciq042 [DOI] [PubMed] [Google Scholar]
- 74.Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M, Germeroth L, Ringhoffer M, Ringhoffer S, Wiesneth M, Greiner J, Michel D, Mertens T, Rojewski M, Marx M, von Harsdorf S, Döhner H, Seifried E, Bunjes D, Schmitt M: Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion 51: 591–599, 2011. 10.1111/j.1537-2995.2010.02940.x [DOI] [PubMed] [Google Scholar]
- 75.Bao L, Cowan MJ, Dunham K, Horn B, McGuirk J, Gilman A, Lucas KG: Adoptive immunotherapy with CMV-specific cytotoxic T lymphocytes for stem cell transplant patients with refractory CMV infections. J Immunother 35: 293–298, 2012. 10.1097/CJI.0b013e31824300a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meij P, Jedema I, Zandvliet ML, van der Heiden PL, van de Meent M, van Egmond HM, van Liempt E, Hoogstraten C, Kruithof S, Veld S, Marijt EW, von dem Borne PA, Lankester AC, Halkes CJ, Falkenburg JH: Effective treatment of refractory CMV reactivation after allogeneic stem cell transplantation with in vitro-generated CMV pp65-specific CD8+ T-cell lines. J Immunother 35: 621–628, 2012. 10.1097/CJI.0b013e31826e35f6 [DOI] [PubMed] [Google Scholar]
- 77.Uhlin M, Gertow J, Uzunel M, Okas M, Berglund S, Watz E, Brune M, Ljungman P, Maeurer M, Mattsson J: Rapid salvage treatment with virus-specific T cells for therapy-resistant disease. Clin Infect Dis 55: 1064–1073, 2012. 10.1093/cid/cis625 [DOI] [PubMed] [Google Scholar]
- 78.Koehne G, Hasan A, Doubrovina E, Prockop S, Tyler E, Wasilewski G, O’Reilly RJ: Immunotherapy with donor T cells sensitized with overlapping pentadecapeptides for treatment of persistent cytomegalovirus infection or viremia. Biol Blood Marrow Transplant 21: 1663–1678, 2015. 10.1016/j.bbmt.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]


