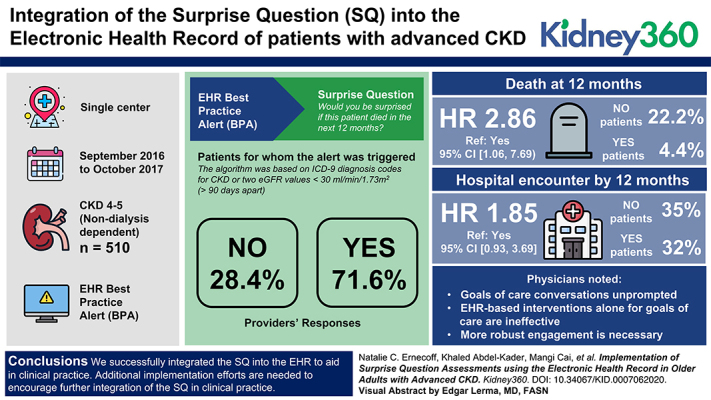
Visual Abstract
Keywords: geriatric and palliative nephrology, advance care planning, chronic kidney disease, electronic health records, health services research, palliative care
Key Points
The Surprise Question can be successfully integrated into the electronic health record for routine collection to aid in clinical practice.
A low response rate indicates additional implementation efforts are needed to encourage integration of the Surprise Question into clinical practice.
Assessment of reasons for nonuptake highlighted improving technical implementation and providing additional decision making support.
Abstract
Background
The Surprise Question (SQ; “Would you be surprised if this patient died in the next 12 months?”) is a validated prognostication tool for mortality and hospitalization among patients with advanced CKD. Barriers in clinical workflows have slowed SQ implementation in practice.
Objectives
The aims of this study were: (1) to evaluate implementation outcomes after the use of electronic health record (EHR) decision support to automate the collection of the SQ; and (2) to assess the prognostic utility of the SQ for mortality and hospitalization/emergency room (ER) visits.
Methods
We developed and implemented a best practice alert (BPA) in the EHR to identify nephrology outpatients ≥60 years of age with an eGFR <30 ml/min per 1.73 m2. At appointment, the BPA prompted the physician to answer the SQ. We assessed the rate and timeliness of provider responses. We conducted a post-hoc open-ended survey to assess physician perceptions of SQ implementation. We assessed the SQ’s prognostic utility in survival and time-to-hospital encounter (hospitalization/ER visit) analyses.
Results
Among 510 patients for whom the BPA triggered, 95 (19%) had the SQ completed by 16 physicians. Among those completed, nearly all (98%) were on appointment day, and 61 (64%) the first time the BPA fired. Providers answered “no” for 27 (28%) and “yes” for 68 (72%) patients. By 12 months, six (22%) “no” patients died; three (4%) “yes” patients died (hazard ratio [HR] 2.86, ref: yes, 95% CI, 1.06 to 7.69). About 35% of “no” patients and 32% of “yes” patients had a hospital encounter by 12 months (HR, 1.85, ref: yes, 95% CI, 0.93 to 3.69). Physicians noted (1) they had goals-of-care conversations unprompted; (2) EHR-based interventions alone for goals-of-care are ineffective; and (3) more robust engagement is necessary.
Conclusions
We successfully integrated the SQ into the EHR to aid in clinical practice. Additional implementation efforts are needed to encourage further integration of the SQ in clinical practice.
Introduction
Nearly half of older adults in the United States have CKD, and prevalence is rising because people are living longer with the disease (1,2). Further, people with CKD have high rates of comorbidity and frailty, which may predispose them to the burdens of treatments such as dialysis, including loss of function, high rates of hospitalization, and increased mortality (2,3). Recognizing patients who may experience these adverse outcomes can prompt earlier goals-of-care conversations and treatment decisions that align with patient goals, values, and priorities (4–6). Identifying such patients can be particularly difficult for clinicians, such as nephrologists, who deliver longitudinal care for patients with chronic disease and may not notice deterioration over time (7). Therefore, prognostic tools are necessary for delivering goal-concordant care among those with late-stage CKD (8,9).
Many patients feel they were not given a choice before initiating dialysis (10). Yet for many of these patients, their values and preferences may align with conservative management that focuses on quality of life and symptom management (11). Barriers remain to the discussion of conservative management, including reluctance to discuss the end of life, time constraints, and poor care coordination (12). Despite its limitations in prediction, the Surprise Question (SQ) is a simple way to prompt timelier goals of care discussions, including advance care planning (13,14, 15,16–18). Easy-to-use tools that prompt advance care planning have utility in nephrology settings in which physicians routinely cite a lack of time and competing priorities as reasons for not having goals-of-care discussions (19). Interventions that promote goals-of-care discussions may help patients match their values and preferences to treatment decisions, including whether to initiate dialysis or manage their kidney disease conservatively (20,21).
Investigators, including from our group, have shown the SQ (“Would you be surprised if this patient died in the next 12 months?”) is a validated prognostication tool for mortality and hospitalization among patients with advanced CKD (13,14,15,22,23,24). It can also be a useful screen for identifying frailty and disability in the older patients with CKD (14). Integration of SQ in the electronic health record (EHR) in the inpatient setting for other disease states has shown to be highly feasible, acceptable, and facilitate advance care planning discussions (16). This single question is likely to be more acceptable and less burdensome to clinicians than other real-time prognostic calculators that require manual tabulation or structured clinical data. The SQ is one step on a path to providing treatments to people living with CKD that are consistent with their goals.
To enhance clinical utilization of the SQ, the next logistic step is to integrate it into the outpatient clinical workflow of nephrologists, and determine its feasibility and acceptability. We used EHR informatics techniques to integrate the SQ into outpatient assessment during nephrology clinic visits with two objectives: (1) to evaluate implementation outcomes after use of EHR decision support to automate collection of the SQ, and (2) to assess the prognostic utility of the SQ for mortality and hospitalization/emergency room (ER) visits.
Materials and Methods
Study Setting and Participants
We conducted a prospective single-center study from September 2016 to October 2017 among adults ≥60 years of age with advanced, nondialysis-dependent CKD (stages 4–5), and a mean eGFR <30 ml/min per 1.73 m2 >90 days apart, before an outpatient follow-up nephrology clinic appointment.
Outpatient nephrology physicians (attendings and fellows) in one outpatient nephrology clinic affiliated with an academic medical center were educated about the EHR best-practice alert (BPA) and corresponding completion of the SQ via a link to a REDCap database. We used a REDCap database for data entry due to Institutional Review Board (IRB) concerns that the sensitive prognostic information for research purposes may otherwise be available to patients on heath record request. Physicians provided written informed consent; patients were exempt from consent. All study procedures were approved by either the University of Pittsburgh Medical Center Quality Review Committee (open-ended survey) or the University of Pittsburgh IRB (all other study procedures).
Automated Patient Identification
We built and integrated a structured algorithm into the EHR to identify eligible patients. The algorithm was on the basis of International Classification of Diseases, Ninth Revision diagnosis codes for CKD or two eGFR values <30 ml/min per 1.73 m2 more than 90 days apart. It excluded patients with AKI or kidney transplant diagnosis codes. During the office visit, an active or “pop-up” BPA was triggered once per patient appointment, per assigned physician (i.e., attending and fellow), with a message to the patient’s nephrologist, prompting the physician to click a link out to a deidentified REDCap form where they were asked, “Would you be surprised if this patient died in the next 12 months?” and yes/no response. This BPA was only triggered for patients with a follow-up visit, and not for new patients, to ensure physicians had an established relationship and understanding of the patient’s condition before answering the SQ. The BPA was discrete and could fire before or while the provider was with the patient. Physicians self-reported completion via a checkbox on the BPA in the EHR, upon which time prompts ceased; that is, physicians could check this box to cease prompts without actually completing the SQ in REDCap. To streamline responses and workflow, if the fellow completed the SQ, the attending did not get the trigger. If the physician did not indicate completing of the SQ via the BPA checkbox, the BPA would continue to fire at each subsequent appointment for that patient until the physician indicated otherwise.
Evaluation of Implementation
To evaluate implementation, we assessed the accuracy of the EHR algorithm to identify eligible patients, rate, and timeliness of BPA completion. We assessed provider characteristics among those who completed the BPA: age, sex, race, Hispanic/Latinx ethnicity, attending versus fellow, years since fellowship, US medical school location (binary), percent clinical effort, and number of weekly half-day clinics. Physicians provided demographic and clinical practice characteristics for themselves. We were unable to assess clinician characteristics of noncompleters. Patient demographic and clinical characteristics were obtained from EHR data.
To assess implementation process measures and outcomes, we present time from BPA trigger to completion (including percent complete on the same day as the trigger), number of patients for whom the BPA fired, fires per patient, and the same variables stratified for only those who had the SQ completed. We also report the number of REDCap forms completed among all triggers.
Variables for Assessment of Prognostic Utility
Physicians provided their own demographic and clinical practice characteristics. Patient data were obtained from the University of Pittsburgh clinical data warehouse. Patient sociodemographic and clinical characteristics were abstracted from structured EHR data. Laboratory values represent the value nearest the patient’s appointment from 12 months before the clinic appointment to 1 month after. We assessed the SQ’s predictive ability using survival and time-to-hospital encounter (hospitalization/ER visit) analyses for a follow-up period until January 31, 2020. Survival data were also crosschecked with obituaries until April 1, 2020.
Physician Perspective Survey
To provide additional post-hoc insight into acceptability, perceived usefulness, and reasons for nonuptake, we conducted a brief open-ended survey of physicians who either participated or did not participate in the initial implementation. Questions included feedback about patient communication (i.e., decision making, advance care planning, barriers to communication) and the implementation of the SQ in this project, specifically (Supplemental Appendix). An investigator (N.C.E.) with extensive experience conducting qualitative research identified patterns in the brief responses and reported areas participants highlighted for improvement of SQ uptake; responses and results were assessed and validated for consistency by two other investigators (M.J. and K.A.K.) (25,26).
Statistical Analyses
Categorical and continuous variables were presented using frequencies with percentages and means with SDs (or medians with interquartile range for skewed distributions), respectively. Patient characteristics between those whose providers answered “yes” and “no” were compared using a t test and chi-squared or Fisher’s exact test depending on the variable type.
Time to mortality was calculated from the first time the BPA fired. Observations were censored by March 31, 2020 if no death record was found. Kaplan-Meier curves for mortality were examined by SQ response groups. Unadjusted and adjusted Cox models were used to test whether SQ response was associated with mortality. Because of the limited size of the SQ “no” group (n=27), covariates were adjusted for one at a time. Covariate-adjusted models included age, sex, mean eGFR, mean albumin, Charlson Comorbidity Index, or number of hospitalizations in the prior year. These were selected on the basis of clinical rather than statistical reasons.
Similar to mortality, time to first hospitalization or ER utilization was calculated. To account for death as a competing event, cumulative incidence curves by SQ response groups were examined. Unadjusted and adjusted cause-specific Cox proportional hazards models were fitted with the SQ response as primary covariate, adjusting for one additional covariate as performed in the mortality analysis.
All statistical analyses were carried out in R (version 3.6.1) (27–30).
Results
Participants
Patients were 74.7 years old on average (SD 8.4), about half were female (n=50, 53%), and 31% were Black (n=29), consistent with the clinic’s patient population. Patients had a mean Charlson Comorbidity Index of 5.7 (SD 2.9), indicating moderate-high comorbidity burden (31) (Table 1).
Table 1.
Patient characteristics, stratified by clinician’s binary Surprise Question response
| Characteristic, n (%) Unless Otherwise Noted | Total Completed REDCap | SQ “Yes” | SQ “No” | P Value |
| (n=95) | (n=68) | (n=27) | ||
| Age, mean (SD) | 74.7 (8.4) | 72.1 (7.0) | 81.1 (8.3) | <0.001 |
| Female | 50.0 (53%) | 41.0 (60%) | 9.0 (33%) | 0.032 |
| Race | 0.329 | |||
| White | 64.0 (67) | 44.0 (65) | 20.0 (74) | |
| Black | 29.0 (31) | 23.0 (34) | 6.0 (22) | |
| Marital status | 0.556 | |||
| Married | 48.0 (51) | 31.0 (46) | 17.0 (63) | |
| Divorced/separated | 14.0 (15) | 12.0 (18) | 2.0 (7) | |
| Widow/widower | 14.0 (15) | 10.0 (15) | 4.0 (15) | |
| Single | 18.0 (19) | 14.0 (21) | 4.0 (15) | |
| Charlson comorbidity index, mean (SD) | 5.7 (2.9) | 5.6 (3.0) | 6.1 (2.7) | 0.449 |
| Hypertension | 86.0 (91) | 63.0 (93) | 23.0 (85) | 0.268 |
| Diabetes | 40.0 (42) | 32.0 (47) | 8.0 (30) | 0.186 |
| Dyslipidemia | 74.0 (78) | 54.0 (79) | 19.0 (70) | 0.501 |
| Coronary artery disease | 67.0 (71) | 47.0 (69) | 20.0 (74) | 0.819 |
| Congestive heart failure | 34.0 (36) | 22.0 (32) | 12.0 (44) | 0.383 |
| Laboratory tests, mean (SD) | ||||
| eGFR (ml/min per 1.73 m2) | 19.5 (7.4) | 20.7 (7.5) | 16.3 (6.2) | 0.004 |
| Serum creatinine (mg/dl) | 2.7 (0.8) | 2.5 (0.8) | 3.0 (1.0) | 0.032 |
| Serum albumin (g/dl) | 3.8 (0.5) | 3.8 (0.4) | 3.6 (0.5) | 0.023 |
| Hemoglobin (g/dl) | 11.1 (1.6) | 11.4 (1.7) | 10.4 (1.2) | 0.001 |
| Hospitalization prior 1 yr | 33.0 (35) | 18.0 (27) | 15.0 (56) | 0.013 |
| 0 visit | 62.0 (65) | 50.0 (74) | 12.0 (44) | |
| 1 visit | 21.0 (22) | 13.0 (19) | 8.0 (30) | |
| 2+ visits | 12.0 (13) | 5.0 (7) | 7.0 (26) |
Physicians were a mean 40 years of age (SD 10); 40% female; 27% Asian Indian and 23% Asian; 60% attending physicians, a mean 12 years out of fellowship (40% of physicians were fellows); and had a mean 70% (SD 30) clinical effo A () Q8rt (Table 2).
Table 2.
Clinician characteristics among those who completed the Surprise Question
| Provider Characteristics, n (%) Unless Otherwise Noted | Total |
| (N=30) | |
| Age, mean (SD) | 40 (10) |
| Female | 12 (40) |
| Race | |
| American Indian/Alaska Native | 1 (3) |
| Asian Indian: Bangladesh, India, Pakistan | 8 (27) |
| Asian: Chinese, Cambodian, Filipino, Japanese, Korean, Laotian, Thai, Vietnamese | 7 (23) |
| White | 10 (33) |
| Other | 4 (13) |
| Hispanic/Latinx ethnicity | 3 (10) |
| Attending (versus fellow) | 18 (60) |
| Yr since fellowship (among attendings), mean (SD) | 12 (10) |
| US medical school location | 15 (50) |
| Percent clinical effort, mean (SD) | 70 (30) |
| Weekly half-d clinics | |
| ≤1 | 12 (40) |
| ≥1.5 | 18 (60) |
Implementation
Among 510 unique patients for whom the BPA triggered, 95 had the SQ completed (19%) by 16 unique providers; the physician identification field was missing for six observations. Among those patients with completed SQ, nearly all providers (98%) completed the SQ on the clinic appointment day, and 61 (64%) the first time the BPA fired (Table 3). On reviewing the patients manually, 69 patients (14%) had an eGFR >30 ml/min per 1.73 m2 proximal to their clinic visit. Physicians completed the SQ for more patients for whom their answer was “yes” than those for whom the answer was “no.”
Table 3.
Implementation outcomes
| Implementation Outcome Mean (SD) | Total |
| Time from trigger to completion (d) | 0.8 (5.3) |
| Completed on same d, n (%) | 93 (98) |
| Patients for whom the BPA fired, n | 510 |
| Fires per patient | 1.8 (1.2) |
| Patients for whom BPA fired >1×, n (%) | 212 (42) |
| Among those who completed the Surprise Question | 95 |
| Fires per patient | 1.7 (1.2) |
| Patients for whom BPA fired >1×, n (%) | 34 (36) |
| REDCap form completion, n (%) | 95 (19) |
BPA, best-practice alert.
Physician Perceptions
In total, 14 physicians completed the post-hoc open-ended survey, highlighting the perceived effectiveness and reasons for nonuptake; half (n=7) recalled completing the SQ. Of those who completed the SQ, most generally found it low burden and effective in improving communication.
Qualitatively, physicians cited (1) barriers to goals-of-care and advance care planning discussions, (2) facilitators thereof, (3) benefits of the SQ, and (4) critiques and recommendations for improving the SQ, each of which is detailed below (Supplemental Appendix, Supplemental Table 1).
Barriers to Goals-of-Care and Advance Care Planning Discussions
The most recurring barrier cited by physicians was time and time tradeoffs in short clinic visits: “Time consuming and not feasible within the time allotted to patients in clinic and during busy hospital service.” Physicians also cited that “all [goals-of-care] discussions are hard,” and that it is a “challenging topic to talk about considering gravity of outcomes.” Patient and family factors were added barriers according to physicians, including readiness (“Sometimes the patients are just not ready, so you have to slowly approach the topic over several visits”) and understanding (“Ensuring that the patient has an adequate understanding about conservative kidney care versus dialysis—this requires multiple visits and conversations”). Clinicians also noted the importance of systems-level factors, including difficulties communicating within and between EHRs, and timely access to specialty palliative care services.
Facilitators of Goals-of-Care and Advance Care Planning Discussions
Physicians emphasized that a longitudinal relationship with a patient is a facilitator of discussions: “Those with the best relationship, especially a long-term trusting relationship are best to do this.” Physicians felt that multidisciplinary teams, generally led by nephrologists, facilitated comprehensive goals-of-care discussions: “There should be an integrated approach to modality, transplant, and/or conservative therapy involving the nephrologist, an educator, and, when appropriate, a palliative physician. [It] ideally starts with the nephrologist and then gets directed appropriately.” Patient education was also identified by physicians, including attention to the delivery mechanism, such as “renal care educators and social workers,” “nurse education that’s integrated in our care flow,” and “printed materials easily accessible by a link.” From a system-level perspective, clinicians noted potential facilitation in telemedicine and designating specific clinic time to goals-of-care discussion.
Benefits of the SQ
Physicians stated the SQ prompted them to have discussions, provided systematic identification of patients who were high risk, and reminded them of the big picture (providing a “reality check”), all while it “took minimal time as [it was] built into the system.”
Critiques and Recommendations for Improving the SQ
Those physicians who did not find it effective cited (1) they already had such conversations unprompted; (2) EHR-based interventions do not affect care and take additional time; and (3) that even more robust engagement is often necessary, including referral to palliative care. One respondent noted the role of their own judgement and competing limitations, including time, “Seeing this question doesn’t change the fact that I may not feel it’s the appropriate time to discuss end-of-life care wishes with the patient. Also, it does not change the fact that there may not be time to discuss it with the patient at that visit.”
Participants provided suggestions for improving implementation of the SQ, including having the pop-up when the note is started (before the visit starts), incorporating a next step or suggestion when the SQ is answered “no,” not making the SQ mandatory, pairing it with printed material or education to support decision making, and utilizing advanced practice providers.
Prognostic Utility
Providers answered “no” for 27 (28%) and “yes” for 68 (72%) patients. Compared with “yes” responses, patients with “no” responses were older (81.1 versus 72.1 years, P<0.001) on average, and less likely to be female (33% versus 60%). There were no statistically significant differences by race. With respect to laboratory values, compared with patients with “yes” responses, patients with “no” responses had lower eGFR (16.3 versus 20.7 ml/min per 1.73 m2; P=0.004), higher serum creatinine (3.0 versus 2.5 mg/dl; P=0.03), lower serum albumin (3.6 versus 3.8 g/dl; P=0.02), and lower hemoglobin (10.4 versus 11.4 g/dl; P=0.001). Patients who received a “no” response had more hospitalizations in the prior year (≥1 hospitalization: 56% versus 27% among those with “yes” responses; P=0.01) (Table 1 and Table 4).
Table 4.
Patient outcomes
| Outcome n (%) Unless Otherwise Noted | Total Completed REDCap | SQ “Yes” | SQ “No” | P Value |
| (n=95) | (n=68) | (n=27) | ||
| Death | 24.0 (25) | 9.0 (13) | 15.0 (56) | <0.001 |
| At 1 yr | 9.0 (10) | 3.0 (4) | 6.0 (22) | 0.014 |
| At 2 yr | 15.0 (16) | 5.0 (7) | 10.0 (37) | 0.001 |
| Utilization, time to first event, mean (SD) | ||||
| ED visits | 44.0 (46) | 29.0 (43) | 15.0 (56) | 0.363 |
| At 1 yra | 27.0 (28) | 18.0 (27) | 9.0 (33) | 0.677 |
| At 2 yra | 44.0 (46) | 29.0 (43) | 15.0 (56) | 0.363 |
| Time to first, mean (SD) | 306.5 (221.2) | 322.0 (227.0) | 276.0 (213.0) | 0.506 |
| Hospitalizations | 45.0 (47) | 31.0 (46) | 14.0 (52) | 0.746 |
| At 1 yra | 28.0 (30) | 17.0 (25) | 11.0 (41) | 0.205 |
| At 2 yra | 45.0 (47) | 31.0 (46) | 14.0 (52) | 0.746 |
| Time to first, mean (SD) | 280.7 (220.8) | 315.0 (222.0) | 206.0 (206.0) | 0.121 |
| Total hospital d, mean (SD) at 2 yr |
8.6 (16.7) | 6.6 (11.8) | 13.7 (24.8) | 0.163 |
| Total encounter d, mean (SD) | 9.1 (16.8) | 7.0 (11.9) | 14.3 (24.8) | 0.157 |
Raw percentages did not account for death as competing event nor censoring. P values were derived from the chi-squared test.
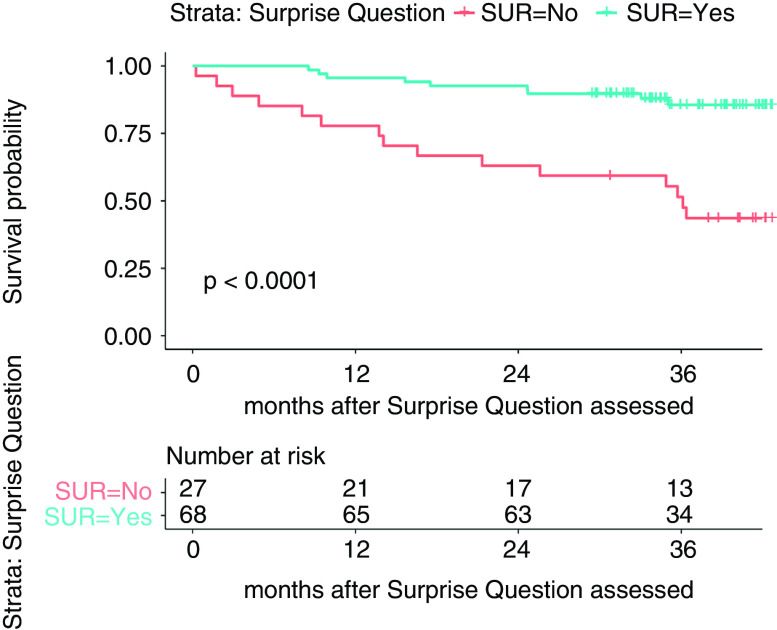
By 12 months, six (22%) patients with “no” responses died; three (4%) patients with “yes” responses died (age-adjusted hazard ratio, 0.35, 95% confidence interval, 0.13 to 0.94) (Figure 1, Table 3).
Figure 1.
Kaplan-Meier survival curve estimates by Surprise Question response. SUR, survive.
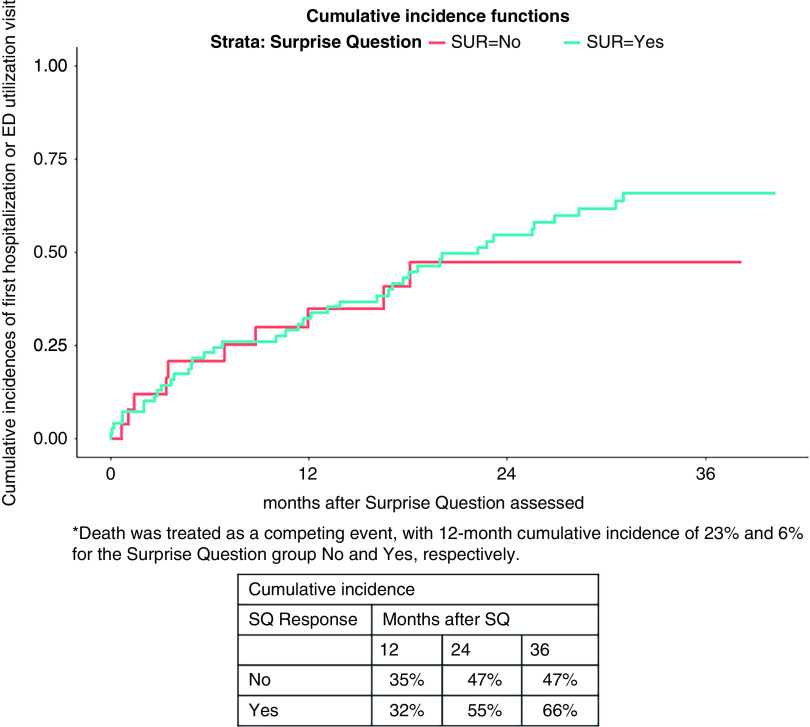
About 40% of “no” patients and 25% of patients with “yes” responses had a hospital encounter by 12 months (adjusted hazard ratio, 1.85; 95% confidence interval, 0.93 to 3.69) (Figure 2).
Figure 2.
Cumulative incidence of hospitalization or emergency room utilization estimates by Surprise Question response. Death was included in the analysis as a competing event. SQ, Surprise Question.
Discussion
We successfully integrated the SQ into the EHR for routine collection to aid in clinical practice. Our low (<20%) response rate indicates additional implementation efforts are needed to encourage further integration of the SQ in clinical practice patterns. Post-hoc assessment of reasons for nonuptake provided insights for next steps, including additional barriers and suggestions for improving technical implementation in the EHR, and facilitating additional decision making support.
Although the SQ has been shown to be effective in identifying patients appropriate for advance care planning conversations in CKD, automated implementation for research and clinical practice have proved difficult (13,14). The technical mechanism for this project was motivated by one specific IRB concern for sharing information with patients: including the SQ directly in the patient’s health record—an explicit question about prognosis—raised concerns that patients could have adverse reactions if they requested their medical records and that information was included. Therefore, the EHR BPA linked to a separate REDCap database where the question could be answered honestly, assuaging regulatory concern. Separately, knowing BPA fatigue is well documented, we intentionally built the BPA as a pop-up, although users reported this feature was inhibitory on elicitation of barriers to physicians (32). Differential physician completion of the SQ may imply physicians were less likely to complete the question for patients for whom they felt the SQ was less applicable; reading the question may prompt some physicians to quickly assess prognosis, and triage completion of the REDCap only for those patients for whom they find the question applicable, arguably appropriately saving time by not documenting for patients they perceive to have a longer prognosis.
In addition to technical suggestions, physicians recommended incorporating a next step and pairing it with printed material and education to support actionable decision making on a “no” answer to the SQ. Desire for such facilitation materials is well documented in advance care planning literature, and this feedback indicates many providers desired more engagement, rather than less (33). Even with general clinician acceptability, barriers in clinical workflow have slowed basic implementation of the SQ into practice; making the corresponding resources more robust may improve uptake with improved perceived utility (34). One study of emergency medicine and inpatient clinicians cited little difficulty using the SQ, although they expressed concern their responses were not accurate (16).
Limitations
This single-center study produced a sample size too small to conduct a comprehensive adjustment for confounding, and limits generalizability. However, from an implementation perspective, this study provides one method for effectively implementing the SQ in the EHR as a template starting point for other institutions. Although our center had little clinical turnover during the study period, physicians received only one brief orientation session to the project and had voluntary participation. More robust implementation efforts will be required to improve uptake by physicians. Low completion rates may also have resulted from the multistep process of leaving the EHR to enter REDCap to answer the question; integration of the SQ into the EHR itself may reduce clinician burden. To streamline responses to the SQ and minimize duplication, if the fellow responded to SQ, the attending did not get the trigger; therefore, we were not able to compare responses among fellows and attendings. We did not assess the false-negative rate due to limitations in our ability to screen all clinic patients to determine those who met inclusion criteria but were neglected by the algorithm.
Future Directions
This study provides insight about implementation considerations for incorporating the SQ into clinical decision making, including a desire for more robust information and decision support. From a technical perspective, the SQ can effectively be incorporated into the EHR, although special attention must be paid to workflow and perceived utility. Enhancing support surrounding SQ use and implementation may increase attention to the intersection of prognosis and decision making for clinicians caring for people living with CKD.
Disclosures
J. Schell reports receiving honoraria from Uptodate; and reports other interests/relationships with Dialysis Clinic, Inc., Palliative Care Advisor, and Salary support. K. Abdel-Kader reports being a scientific advisor or member of BioMed Central Nephrology and CJASN; and reports other interests/relationships with the National Kidney Foundation Education committee. M. Jhamb reports receiving research funding from Arbor Research Collaborative for Health, Dialysis Clinic, Inc., and the National Institutes of Health; and reports other interests/relationships as a member of ASN and National Kidney Foundation. All remaining authors have nothing to disclose.
Funding
This work was supported by Dr. Abdel-Kader’s Satellite Healthcare Norman S Koplon Grant.
Author Contributions
N.C. Ernecoff and M. Jhamb conceptualized the study; M. Cai was responsible for the data curation; K. Abdel-Kader, M. Cai, N.C. Ernecoff, and J. Yabes were responsible for the formal analysis; M. Jhamb and J. Yabes were responsible for the methodology; K. Abdel-Kader, M. Jhamb, and N. Shah were responsible for the investigation; M. Jhamb provided supervision; M. Cai and N.C. Ernecoff wrote the original draft; and K. Abdel-Kader, M. Jhamb, J.O. Schell and N. Shah reviewed and edited the manuscript.
Footnotes
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/K360/2021_06_24_KID0007062020.mp3
Supplemental Material
This article contains the following supplemental material online at https://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0007062020/-/DCSupplemental.
Open-ended survey to assess physicians’ acceptability, perceived usefulness, and reasons for nonuptake of the SQ. Download Supplemental Appendix, PDF file, 155 KB (154KB, pdf)
Themes and representative quotes from physician feedback on the SQ implementation. Download Supplemental Table 1, PDF file, 155 KB (154KB, pdf)
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007. 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System: USRDS 2018 annual data report: End-Stage Renal Disease (ESRD) in the United States, 2018. Available at: https://www.usrds.org/2018/view/Default.aspx. Accessed September 20, 2019
- 3.Brown EA, Johansson L: Epidemiology and management of end-stage renal disease in the elderly. Nat Rev Nephrol 7: 591–598, 2011. 10.1038/nrneph.2011.113 [DOI] [PubMed] [Google Scholar]
- 4.O’hare AM, Song M-K, Tamura MK, Moss AH: Research priorities for palliative care for older adults with advanced chronic kidney disease. J Palliat Med 20: 453–460, 2017. 10.1089/jpm.2016.0571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hare AM, Armistead N, Schrag WLF, Diamond L, Moss AH: Patient-centered care: An opportunity to accomplish the “three aims” of the national quality strategy in the medicare ESRD program. Clin J Am Soc Nephrol 9: 2189–2194, 2014. 10.2215/CJN.01930214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson DS, Kapoian T, Taylor R, Meyer KB: Going upstream: Coordination to improve CKD care. Semin Dial 29: 125–134, 2016. 10.1111/sdi.12461 [DOI] [PubMed] [Google Scholar]
- 7.Butler CR, Taylor JS, Reese PP, O’Hare AM: Thematic analysis of the medical records of patients evaluated for kidney transplant who did not receive a kidney. BMC Nephrol 21: 300, 2020. 10.1186/s12882-020-01951-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galla JH: Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The renal physicians association and the American society of nephrology [published correction appears in J Am Soc Nephrol 11: 2 p following 1788, 2000]. J Am Soc Nephrol 11: 1340–1342 [DOI] [PubMed] [Google Scholar]
- 9.Moss AH: Revised dialysis clinical practice guideline promotes more informed decision-making. Clin J Am Soc Nephrol 5: 2380–2383, 2010. 10.2215/CJN.07170810 [DOI] [PubMed] [Google Scholar]
- 10.Ladin K, Lin N, Hahn E, Zhang G, Koch-Weser S, Weiner DE: Engagement in decision-making and patient satisfaction: A qualitative study of older patients’ perceptions of dialysis initiation and modality decisions. Nephrol Dial Transplant 32: 1394–1401, 2017. 10.1093/ndt/gfw307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison SN: End-of-life care preferences and needs: Perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 195–204, 2010. 10.2215/CJN.05960809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladin K, Pandya R, Kannam A, Loke R, Oskoui T, Perrone RD, Meyer KB, Weiner DE, Wong JB: Discussing conservative management with older patients with CKD: An interview study of nephrologists. Am J Kidney Dis 71: 627–635, 2018. 10.1053/j.ajkd.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javier AD, Figueroa R, Siew ED, Salat H, Morse J, Stewart TG, Malhotra R, Jhamb M, Schell JO, Cardona CY, Maxwell CA, Ikizler TA, Abdel-Kader K: Reliability and utility of the surprise question in CKD stages 4 to 5. Am J Kidney Dis 70: 93–101, 2017. 10.1053/j.ajkd.2016.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baddour NA, Robinson-Cohen C, Lipworth L, Bian A, Stewart TG, Jhamb M, Siew ED, Abdel-Kader K: The surprise question and self-rated health are useful screens for frailty and disability in older adults with chronic kidney disease. J Palliat Med 22: 1522–1529, 2019. 10.1089/jpm.2019.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramer SJ, Baddour NA, Siew ED, Salat H, Bian A, Stewart TG, Wong SPY, Jhamb M, Abdel-Kader K: Nephrology provider surprise question response and hospitalizations in older adults with advanced CKD. Am J Nephrol 51: 641–649, 2020. 10.1159/000509046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haydar SA, Almeder L, Michalakes L, Han PKJ, Strout TD: Using the surprise question to identify those with unmet palliative care needs in emergency and inpatient settings: What do clinicians think? J Palliat Med 20: 729–735, 2017. 10.1089/jpm.2016.0403 [DOI] [PubMed] [Google Scholar]
- 17.Aaronson EL, George N, Ouchi K, Zheng H, Bowman J, Monette D, Jacobsen J, Jackson V: The surprise question can Be used to identify heart failure patients in the emergency department who would benefit from palliative care. J Pain Symptom Manage 57: 944–951, 2019. 10.1016/j.jpainsymman.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White N, Kupeli N, Vickerstaff V, Stone P: How accurate is the ‘surprise question’ at identifying patients at the end of life? A systematic review and meta-analysis. BMC Med 15: 139, 2017. 10.1186/s12916-017-0907-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubbs V, Tuot DS, Powe NR, O’Donoghue D, Chesla CA: System-level barriers and facilitators for foregoing or withdrawing dialysis: A qualitative study of nephrologists in the United States and England. Am J Kidney Dis 70: 602–610, 2017. 10.1053/j.ajkd.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosansky SJ, Schell J, Shega J, Scherer J, Jacobs L, Couchoud C, Crews D, McNabney M: Treatment decisions for older adults with advanced chronic kidney disease. BMC Nephrol 18: 200, 2017. 10.1186/s12882-017-0617-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Wightman A, Liao S: Ensuring choice for people with kidney Failure - dialysis, supportive care, and hope. N Engl J Med 383: 99–101, 2020. 10.1056/NEJMp2001794 [DOI] [PubMed] [Google Scholar]
- 22.Moss AH, Ganjoo J, Sharma S, Gansor J, Senft S, Weaner B, Dalton C, MacKay K, Pellegrino B, Anantharaman P, Schmidt R: Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol 3: 1379–1384, 2008. 10.2215/CJN.00940208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salat H, Javier A, Siew ED, Figueroa R, Lipworth L, Kabagambe E, Bian A, Stewart TG, El-Sourady MH, Karlekar M, Cardona CY, Ikizler TA, Abdel-Kader K: Nephrology provider prognostic perceptions and care delivered to older adults with advanced kidney disease. Clin J Am Soc Nephrol 12: 1762–1770, 2017. 10.2215/CJN.03830417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang WF, Kwan BCH, Chow KM, Leung CB, Li PKT, Szeto CC: Predicting 12-month mortality for peritoneal dialysis patients using the “surprise” question. Perit Dial Int 33: 60–66, 2013. 10.3747/pdi.2011.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale RC, Wu J, Erhardt T, Bounthavong M, Reardon CM, Damschroder LJ, Midboe AM: Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the Veterans Health Administration. Implement Sci 14: 11, 2019. 10.1186/s13012-019-0853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beebe J: Rapid Qualitative Inquiry: A Field Guide to Team-Based Assessment, 2nd Ed., Lanham, MD, Rowman & Littlefield, 2014 [Google Scholar]
- 27.Wickham H, Francois R, Henry L, Muller K: dplyr: A grammar of data manipulation. R package version 0.7.6, 2018. Available at: https://dplyr.tidyverse.org/. Accessed June 29, 2020
- 28.Subirana I, Sanz H, Vila J: Building Bivariate tables: The compareGroups package for R. J Stat Softw 57: 1–16, 2014. 10.18637/jss.v057.i1225400517 [DOI] [Google Scholar]
- 29.Therneau TM: Survival Analysis [R package survival version 3.2-3]. Available at: https://cran.r-project.org/web/packages/survival/index.html. Accessed September 20, 2019
- 30.Wickham H: Ggplot2, New York, NY, Springer, 2009, 10.1007/978-0-387-98141-3 [DOI] [Google Scholar]
- 31.Jhamb M, Cavanaugh KL, Bian A, Chen G, Ikizler TA, Unruh ML, Abdel-Kader K: Disparities in electronic health record patient portal use in nephrology clinics. Clin J Am Soc Nephrol 10: 2013–2022, 2015. 10.2215/CJN.01640215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lofthus DM, Gadgil JY, De Lemos JA: Overriding concerns: The role of electronic medical record-based best practice alerts in reducing unnecessary laboratory testing. Clin Chem 61: 456–458, 2015. doi: 10.1373/clinchem.2014.236406 [DOI] [PubMed] [Google Scholar]
- 33.O’Halloran P, Noble H, Norwood K, Maxwell P, Shields J, Fogarty D, Murtagh F, Morton R, Brazil K: Advance care planning with patients who have end-stage kidney disease: A systematic realist review. J Pain Symptom Manage 56: 795–807.e18, 2018. 10.1016/j.jpainsymman.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DesRoches CM, Audet AM, Painter M, Donelan K: Meeting meaningful use criteria and managing patient populations: A national survey of practicing physicians. Ann Intern Med 158: 791–799, 2013. 10.7326/0003-4819-158-11-201306040-00003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Open-ended survey to assess physicians’ acceptability, perceived usefulness, and reasons for nonuptake of the SQ. Download Supplemental Appendix, PDF file, 155 KB (154KB, pdf)
Themes and representative quotes from physician feedback on the SQ implementation. Download Supplemental Table 1, PDF file, 155 KB (154KB, pdf)