Introduction
Acute interstitial nephritis (AIN) is a well-known cause of acute kidney disease (AKD) and CKD and is associated with progression to ESKD (1–6). Accordingly, it represents an important problem for clinicians caring for these patients. AIN is primarily an immune-mediated kidney injury triggered by use of certain medications, in particular antibiotics, PPIs, NSAIDs, and immune checkpoint inhibitors (ICPIs), or by autoimmune diseases, such as Sjogren syndrome, sarcoidosis, IgG4-related tubulointerstitial disease, and TINU. In developed countries, medications are the most common cause of AIN (>70%), whereas the number approximates 50% in developing countries. Infectious agents are a less common cause of AIN, except in developing countries.
The overall incidence of AIN in kidney biopsy registries is 2%–5%, whereas AIN is observed in approximately 15%–20% of patients with AKI or AKD who undergo kidney biopsy (1–3). The actual number is likely higher as many patients with AKI/AKD do not undergo biopsy and are presumed to have acute tubular injury (ATI). Importantly, diagnosing AIN clinically is often quite challenging, making kidney biopsy a frequent requirement to definitively confirm the diagnosis and guide therapy (Figure 1). Furthermore, delayed or missed AIN diagnosis leads to ongoing inflammation with resulting interstitial fibrosis, tubular atrophy, and permanent kidney damage, which may be the explanation for CKD occurring in 40%–60% of patients after an episode of AIN (4,5). Approximately 2% of CKD is considered to be due to AIN, which is equivalent to 10 million prevalent worldwide cases. Furthermore, AIN is the primary cause of ESKD in 3%–4% of incident patients (6). It is one of the few potentially treatable causes of AKI if identified and treated early. In view of these data, three key challenges that limit the diagnosis and management of patients suspected of having AIN are discussed.
Figure 1.
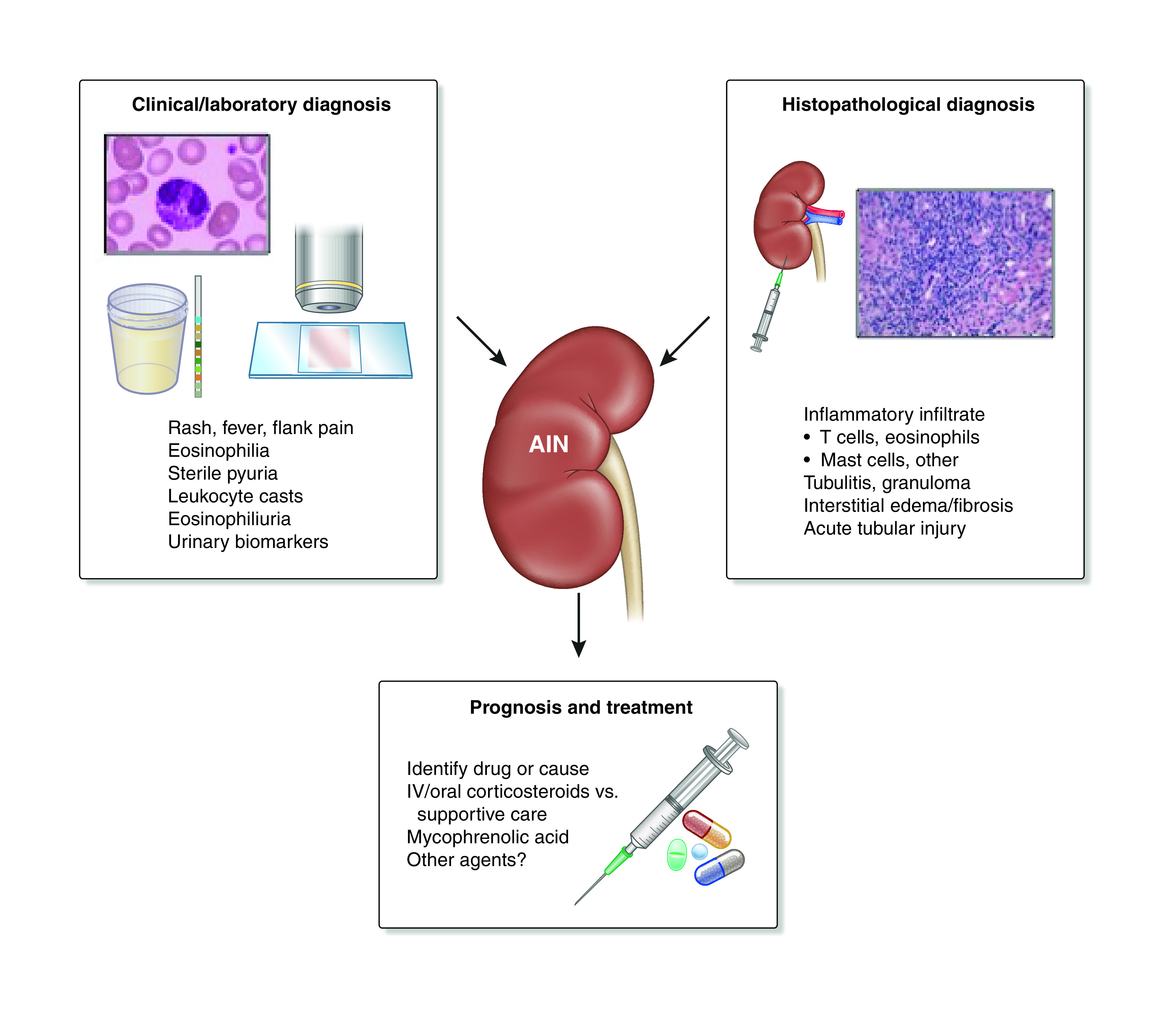
Clinical and histopathologic diagnosis and therapy of acute interstitial nephritis (AIN). Clinical symptoms and signs and laboratory and imaging tests are sometimes useful to make a diagnosis of AIN. However, histopathology obtained through kidney biopsy is frequently required to make a diagnosis of AIN and ultimately, guide management. IV, intravenous.
Clinical Diagnostic Challenges of AIN
Because the clinical diagnosis of AIN is difficult, delayed or missed diagnosis frequently occurs. Most patients with AIN do not have any characteristic systemic symptoms or signs, such as rash, fever, or flank pain (7–10). Most often, they manifest nonspecific constitutional symptoms, symptoms of kidney failure (when advanced), or no symptoms at all. Currently available diagnostic tests, including serum and urine eosinophils, and urine sediment examination for leukocytes and leukocyte casts have poor sensitivity and specificity for AIN diagnosis. Imaging tests, such as ultrasonography, CT scan, gallium scan, and PET/CT scan, are also suboptimal. In a retrospective analysis of 76 patients, of which 23 were considered to have AIN, renal 67Ga uptake showed an AUC of 0.75. However, only 20 of 76 patients were biopsied to confirm or exclude AIN, and those who determined diagnostic outcome were likely not blinded to 67Ga results (11). Thus, the diagnosis of AIN currently relies entirely on maintaining a high index of clinical suspicion for this disease and often requires confirmation by a kidney biopsy. Biopsy may not be feasible or delayed during optimization in some patients due to underlying bleeding risk (12,13). The lack of a diagnostic biomarker for AIN and the need for a kidney biopsy to establish AIN diagnosis often delay diagnosis, which is associated with permanent kidney damage. Unfortunately, delay in diagnosis and management of AIN is associated with lower recovery of kidney function (4,14–16).
A potential solution to this diagnostic challenge has recently been identified. On the basis of the fact that CD4+ T cells play an important role in the pathogenesis of AIN (17–19), 12 cytokines in the Th1 (IFN-γ, IL-2, and IL-12), Th2 (IL-4, IL-5, and IL-13), and Th9 (IL-9) pathways as well as other inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8, and IL-10) were measured in the urine and plasma in patients with biopsy-proven AIN and various other diagnoses. In this prospective study, urine TNF-α and IL-9 levels were consistently higher in participants with biopsy-proven, adjudicated AIN compared with other causes of AKD (20). These cytokine biomarkers were higher in AIN than ATI, glomerular diseases, and diabetic kidney disease, as well as in participants without kidney disease. Urine TNF-α and IL-9 improved discrimination for AIN diagnosis as compared with the clinical nephrologist’s prebiopsy AIN diagnosis and a model consisting of currently available blood and urine tests. Overall, these results suggest that concomitantly elevated levels of urine TNF-α and IL-9 are specific to AIN and may be a useful biomarker to distinguish AIN from other clinical causes of AKD. Furthermore, a higher ratio of urinary M1 (proinflammatory) to M2 (anti-inflammatory) macrophages was shown to differentiate between AIN and other kidney pathologies (21). Importantly, new insights into the pathogenesis of AIN as well its diagnosis and therapy may be garnered from this study. A recent study measured urinary retinol-binding protein/Cr in patients with ICPI-associated AKI (14 of 37 had biopsy-proven AIN) and 13 patients with non–ICPI-associated AKI (two of four with biopsy-proven ATI) (22). In a subgroup of patients, urinary retinol-binding protein/Cr was statistically increased in the ICPI AKI group versus the ICPI non-AIN group. All of these data offer hope for a noninvasive diagnostic test for AIN.
Pathologic Diagnostic Challenges of AIN
Kidney biopsy with histology is considered the “gold standard” for diagnosis of AIN. However, in the absence of consensus guidelines regarding histologic diagnosis of AIN, there is significant heterogeneity in reporting by pathologists. Currently, histologic diagnosis of AIN is on the basis of two major components. These include (1) an interstitial infiltrate consisting of lymphocytes, monocytes, macrophages, plasma cells, and sometimes, eosinophils and (2) the presence of tubulitis, which represents the extension/invasion of the inflammatory cells into tubules. ATI, interstitial edema, and interstitial fibrosis often may accompany AIN. Unfortunately, the presence and severity of these findings are often interpreted subjectively without a standard approach.
It is increasingly recognized that the reliability of kidney biopsy reports by a single pathologist has limitations. In a prospective observational study, we noted that a majority of adjudicating pathologists reclassified clinically reported AIN cases into non-AIN controls in a third of cases. This reclassification was lower when AIN was listed as the first diagnosis (18%) than when it was listed as second or later diagnosis (41%) (20). In addition, there was low inter-rater agreement among pathologists. We noted a low κ for agreement for AIN diagnosis (0.35), as well as features of interstitial infiltrate (0.22), tubulitis (0.20), and eosinophils (0.39). Furthermore, an acute interstitial infiltrate is commonly associated with other diagnoses on the biopsy, including ATI, diabetic kidney disease, lupus nephritis, and ANCA-associated vasculitis. It is unclear when AIN is thought to be secondary to these associated diagnoses, which would not warrant management changes directed at AIN or a separate diagnosis that would require therapeutic intervention. This poses a significant challenge for treating clinicians in making management decisions, particularly if a kidney pathologist is not available on site for discussion, which is increasingly common at many centers. Clinicians in our study seemed to understand the uncertainty in histologic diagnosis. Nineteen percent of AIN diagnoses were subsequently reclassified as not AIN; this reclassification was lower when AIN was listed as first diagnosis (8%) than when it was listed as second or later diagnosis (29%).
Pathologists are often asked to determine if AIN is due to a drug or other etiology, such as a systemic disease (Sjogren syndrome, sarcoidosis, IgG4 disease, TINU, etc.), infection, or idiopathic. An eosinophil-predominant infiltrate often raises the possibility of drug-induced AIN; however, several drugs do not have an eosinophilic infiltrate, and nondrug causes may have eosinophils. In some cases, the histology can identify nondrug causes, such as IgG4 disease (IgG4 staining plasma cells and storiform fibrosis) and sarcoidosis with lymphocyte-dominant infiltrate and noncaseating granuloma. Most important to determining etiology is an ongoing discussion of the patient’s clinical data and histologic data by the nephrologist and pathologist. Unfortunately, this approach may not be common practice for many clinicians and pathologists.
There are a few solutions to the challenges with histopathologic diagnosis of AIN, which at this time appears to be a suboptimal “gold standard.” First, pathologists in the NEPTUNE study improved concordance on glomerular diagnoses through an iterative adjudication process using a description-based scoring system (23). Thus, it might be possible to improve the agreement among pathologists by establishing consensus criteria for AIN. Certainly, such criteria exist for the histopathology in many other kidney diseases. Second, reporting of interstitial features should be undertaken in a standardized manner (e.g., percentages or percentage ranges). Terms, such as mild, moderate, minimal, and severe, should be avoided, as they are subjective and not helpful to the clinician. This approach would improve patient care and research by allowing comparison across centers, studies, and pathologists; help develop models to diagnose AIN using interstitial features; and allow application of machine learning techniques to biopsy slides. Finally, identification of etiology-specific subsets of immune cells involved in AIN may lead to improved histologic diagnosis as well as guide treatment. For example, recent studies have shown involvement of mast cells and Th17 cells in AIN, which are not routinely tested in clinical histopathology (18,24).
Prognosis and Management Challenges of AIN
There are no evidence-based guidelines available to aid clinicians in the management of patients with AIN. This results in a substantial variation in practice. For example, although it is accepted that withdrawal of the offending drug is the best first step after diagnosis of drug-induced AIN, prescription of corticosteroid therapy is more controversial. Observational studies of corticosteroid use in AIN show conflicting results in terms of benefit for kidney function recovery, potentially indicating heterogenous treatment effects (16). It is possible that there are certain subgroups of patients with AIN who derive the most benefit from corticosteroids (e.g., those with highly active immune responses), whereas others gain little benefit and only experience treatment side effects. However, there are currently no guidelines around which patients are best suited to this therapy.
Recent data suggest that urine biomarkers may help select appropriate patients for therapy (25). In a prospective cohort of participants with biopsy-proven, adjudicated AIN, higher urine IL-9 levels were associated with lower kidney function only in patients who did not receive corticosteroid therapy (25). Corticosteroid therapy was noted to be most beneficial in the patient subgroup with higher urine IL-9 levels and higher baseline eGFR before the onset of AIN. These findings provide a potential framework for IL-9–guided clinical trials to test the efficacy of immunosuppressive therapy in patients with AIN. In addition, higher interstitial fibrosis is associated with lower kidney function recovery, whereas higher interstitial inflammation is associated with greater kidney function recovery (25). These findings could assist clinicians in providing a more accurate estimate of prognosis to patients with AIN. However, it remains unclear what the appropriate dose, route of delivery (oral versus intravenous), and duration of therapy should be for patients. Thus, clinicians rely on expert opinion, and local practices vary by center. In addition to corticosteroids, other agents with potential utility for AIN, such as mycophenolic acid, infliximab, and other agents, should undergo study in biopsy-proven AIN.
Approach to Challenges Associated with AIN
We propose that the key diagnostic and management issues relevant to patients with AIN be the focus of basic research and clinical investigation. This area of acute tubulointerstitial disease needs evidence-based guidelines and/or expert consensus opinion to assist clinicians in their care of these patients. Focus on key aspects of clinical and histologic diagnosis and management of patients suspected of having AIN is an important first step. The field sorely needs useful clinical and laboratory criteria to confirm a clinical diagnosis of AIN. In the same vein, consensus histologic criteria are needed to determine a pathologic diagnosis of AIN. Additionally, a consensus approach for prognosis and treatment of AIN that addresses issues of patient selection for immunosuppressive therapy, dose, and duration of therapy as well as predictors of prognosis is required. Finally, knowledge gaps and areas of needed research in each of the three AIN domains must be identified. Table 1 lists areas and challenges that must be addressed.
Table 1.
Specific challenges to be addressed in acute interstitial nephritis
| Clinical diagnostic challenges of AIN |
| Utility of clinical features of immune and drug-induced causes of AIN |
| Accuracy of currently available clinical features for AIN |
| Accuracy of currently available blood tests for AIN |
| Accuracy of currently available urinary tests for AIN |
| The role of imaging tests |
| The role of novel biomarkers |
| Features that establish the diagnosis of AIN |
| Pathologic diagnostic challenges of AIN |
| Relevance of histologic features, such as inflammatory interstitial infiltrate (lymphocytes, eosinophils, plasma cells, etc.), tubulitis, and granuloma |
| Relevance of associated diagnoses, including acute tubular injury, glomerular diseases, and other pathology |
| Utility of novel studies of the kidney tissue, including T cell subsets, mast cells, and macrophages |
| Prognosis and management challenges of AIN |
| Accuracy of data in determining the prognosis of AIN |
| Approach to identifying and discontinuing the offending agent in drug-induced AIN |
| Appropriate use of corticosteroids in the treatment of AIN |
| Intravenous versus oral corticosteroids to initially treat AIN |
| Oral steroids alone to treat AIN |
| Combination of intravenous and oral corticosteroids to treat AIN |
| Appropriate duration of corticosteroid therapy for AIN |
| Appropriate discontinuation and tapering of corticosteroids prior to reaching the intended duration |
| Utility of other drugs appropriate for the treatment of AIN (e.g., mycohenolic acid, azathioprine, etc.) |
| Utility of potential novel, targeted therapies to treat AIN (anti–TNF-α drugs, antihistamines, etc.) |
AIN, acute interstitial nephritis.
Disclosures
D.G. Moledina reports receiving research funding from National Institute of Diabetes and Digestive and Kidney Diseases grants K23DK117065, R01DK12681, UH3DK114866, P30DK079310 outside the submitted work; received honoraria from the British Medical Journal, the National Kidney Foundation, and Remedy Health Media; and scientific advisor or membership with Kidney360 as an editorial board member. D.G. Moledina is a coinventor of the pending patent application “Methods and Systems for Diagnosis of Acute Interstitial Nephritis” that is subject to an option for a license agreement with Renalytix AI Inc. M.A. Perazella reports royalties as an author for UpToDate and participation on the data safety monitoring board for the Yale study on Hospital Electronic Alerts for AKI (no compensation).
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
D.G. Moledina and M.A. Perazella conceptualized the manuscript; D.G. Moledina and M.A. Perazella wrote the original draft; and D.G. Moledina and M.A. Perazella reviewed and edited the manuscript.
References
- 1.Haas M, Spargo BH, Wit EJ, Meehan SM: Etiologies and outcome of acute renal insufficiency in older adults: A renal biopsy study of 259 cases. Am J Kidney Dis 35: 433–447, 2000. 10.1016/S0272-6386(00)70196-X [DOI] [PubMed] [Google Scholar]
- 2.Moledina DG, Perazella MA: PPIs and kidney disease: From AIN to CKD. J Nephrol 29: 611–616, 2016. 10.1007/s40620-016-0309-2 [DOI] [PubMed] [Google Scholar]
- 3.Nochaiwong S, Ruengorn C, Awiphan R, Koyratkoson K, Chaisai C, Noppakun K, Chongruksut W, Thavorn K: The association between proton pump inhibitor use and the risk of adverse kidney outcomes: A systematic review and meta-analysis. Nephrol Dial Transplant 33: 331–342, 2018. 10.1093/ndt/gfw470 [DOI] [PubMed] [Google Scholar]
- 4.Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, Nasr SH: Biopsy-proven acute interstitial nephritis, 1993-2011: A case series. Am J Kidney Dis 64: 558–566, 2014. 10.1053/j.ajkd.2014.04.027 [DOI] [PubMed] [Google Scholar]
- 5.Simpson IJ, Marshall MR, Pilmore H, Manley P, Williams L, Thein H, Voss D: Proton pump inhibitors and acute interstitial nephritis: Report and analysis of 15 cases. Nephrology (Carlton) 11: 381–385, 2006. 10.1111/j.1440-1797.2006.00651.x [DOI] [PubMed] [Google Scholar]
- 6.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, Kapke A, Kurtz V, Li Y, McCullough K, Modi Z, Morgenstern H, Mukhopadhyay P, Pearson J, Pisoni R, Repeck K, Schaubel DE, Shamraj R, Steffick D, Turf M, Woodside KJ, Xiang J, Yin M, Zhang X, Shahinian V: US Renal Data System 2019 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 75[Suppl 1]: A6–A7, 2020. 10.1053/j.ajkd.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 7.Perazella MA: Diagnosing drug-induced AIN in the hospitalized patient: A challenge for the clinician. Clin Nephrol 81: 381–388, 2014. 10.5414/CN108301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogazzi GB, Ferrari B, Garigali G, Simonini P, Consonni D: Urinary sediment findings in acute interstitial nephritis. Am J Kidney Dis 60: 330–332, 2012. 10.1053/j.ajkd.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 9.Muriithi AK, Nasr SH, Leung N: Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol 8: 1857–1862, 2013. 10.2215/CJN.01330213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perazella MA, Bomback AS: Urinary eosinophils in AIN: Farewell to an old biomarker? Clin J Am Soc Nephrol 8: 1841–1843, 2013. 10.2215/CJN.08620813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham F, Lord M, Froment D, Cardinal H, Bollée G: The use of gallium-67 scintigraphy in the diagnosis of acute interstitial nephritis. Clin Kidney J 9: 76–81, 2016. 10.1093/ckj/sfv129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moledina DG, Luciano RL, Kukova L, Chan L, Saha A, Nadkarni G, Alfano S, Wilson FP, Perazella MA, Parikh CR: Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol 13: 1633–1640, 2018. 10.2215/CJN.04910418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korbet SM, Gashti CN, Evans JK, Whittier WL: Risk of percutaneous renal biopsy of native kidneys in the evaluation of acute kidney injury. Clin Kidney J 11: 610–615, 2018. 10.1093/ckj/sfy048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moledina DG, Perazella MA: Drug-induced acute interstitial nephritis. Clin J Am Soc Nephrol 12: 2046–2049, 2017. 10.2215/CJN.07630717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moledina DG, Perazella MA: Treatment of drug-induced acute tubulointerstitial nephritis: The search for better evidence. Clin J Am Soc Nephrol 13: 1785–1787, 2018. 10.2215/CJN.12001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Juarez G, Perez JV, Caravaca-Fontán F, Quintana L, Shabaka A, Rodriguez E, Gadola L, de Lorenzo A, Cobo MA, Oliet A, Sierra M, Cobelo C, Iglesias E, Blasco M, Galeano C, Cordon A, Oliva J, Praga M; Spanish Group for the Study of Glomerular Diseases (GLOSEN): Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol 13: 1851–1858, 2018. 10.2215/CJN.01390118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spanou Z, Keller M, Britschgi M, Yawalkar N, Fehr T, Neuweiler J, Gugger M, Mohaupt M, Pichler WJ: Involvement of drug-specific T cells in acute drug-induced interstitial nephritis. J Am Soc Nephrol 17: 2919–2927, 2006. 10.1681/ASN.2006050418 [DOI] [PubMed] [Google Scholar]
- 18.Zand L, Monaghan M, Griffin BR, Wagner SJ, Criaci IM, Kamal A, Raissian Y, Grande JP, Lim KG, Garovic VD: The role of type I hypersensitivity reaction and IgE-mediated mast cell activation in acute interstitial nephritis. Clin Nephrol 84: 138–144, 2015. 10.5414/CN108254 [DOI] [PubMed] [Google Scholar]
- 19.D’Agati VD, Theise ND, Pirani CL, Knowles DM, Appel GB: Interstitial nephritis related to nonsteroidal anti-inflammatory agents and beta-lactam antibiotics: A comparative study of the interstitial infiltrates using monoclonal antibodies. Mod Pathol 2: 390–396, 1989 [PubMed] [Google Scholar]
- 20.Moledina DG, Wilson FP, Pober JS, Perazella MA, Singh N, Luciano RL, Obeid W, Lin H, Kuperman M, Moeckel GW, Kashgarian M, Cantley LG, Parikh CR: Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight 4: e127456, 2019. 10.1172/jci.insight.127456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun PP, Zhou XJ, Su JQ, Wang C, Yu XJ, Su T, Liu G, Wang SX, Nie J, Yang L: Urine macrophages reflect kidney macrophage content during acute tubular interstitial and glomerular injury. Clin Immunol 205: 65–74, 2019. 10.1016/j.clim.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 22.Isik B, Alexander MP, Manohar S, Vaughan L, Kottschade L, Markovic S, Lieske J, Kukla A, Leung N, Herrmann SM: Biomarkers, clinical features and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int Rep 6: P1022–P1031, 2021. 10.1016/j.ekir.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barisoni L, Troost JP, Nast C, Bagnasco S, Avila-Casado C, Hodgin J, Palmer M, Rosenberg A, Gasim A, Liensziewski C, Merlino L, Chien HP, Chang A, Meehan SM, Gaut J, Song P, Holzman L, Gibson D, Kretzler M, Gillespie BW, Hewitt SM: Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol 29: 671–684, 2016. 10.1038/modpathol.2016.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berney-Meyer L, Hung N, Slatter T, Schollum JB, Kitching AR, Walker RJ: Omeprazole-induced acute interstitial nephritis: A possible Th1-Th17-mediated injury? Nephrology (Carlton) 19: 359–365, 2014. 10.1111/nep.12226 [DOI] [PubMed] [Google Scholar]
- 25.Moledina DG, Wilson FP, Kukova L, Obeid W, Luciano R, Kuperman M, Moeckel GW, Kashgarian M, Perazella MA, Cantley LG, Parikh CR: Urine interleukin-9 and tumor necrosis factor-α for prognosis of human acute interstitial nephritis [published online ahead of print October 28, 2020]. Nephrol Dial Transplant 10.1093/ndt/gfaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]


