Abstract
Despite continued advances in medical treatment, pediatric CKD remains an unremitting, burdensome condition characterized by decreased quality of life and earlier death. These burdens underscore the need for integration of pediatric palliative care (PPC) into nephrology practice. PPC is an evolving field that strives to (1) relieve physical, psychologic, social, practical, and existential suffering; (2) improve quality of life; (3) facilitate decision making; and (4) assist with care coordination in children with life-threatening or life-shortening conditions. Integration of palliative care into routine care has already begun for adults with kidney disease and children with other chronic diseases; however, similar integration has not occurred in pediatric nephrology. This review serves to provide a comprehensive definition of PPC, highlight the unmet need in pediatric nephrology and current integration efforts, discuss the state of palliative care in adult nephrology and analogous chronic pediatric disease states, and introduce future opportunities for study.
Keywords: geriatric and palliative nephrology, chronic kidney disease, dialysis, end stage kidney disease, kidney supportive care, pediatric nephrology, pediatric palliative care
Introduction
Despite advances in the understanding and treatment of pediatric kidney disease, children with CKD continue to face diminished life span, reduced quality of life, significant symptom burden, and generally withstand considerable suffering (1–7). These findings reinforce the need for continued research to improve treatment of kidney disease, but also highlight the need for integration of palliative care to improve the lives of children with CKD, and their families, now. Unfortunately, there has been limited utilization of palliative care principles and subspecialty palliative care consultation for this population (8,9). As emerging studies demonstrate the benefits of palliative care for children with other chronic diseases, and adults with kidney disease, it is imperative to critically examine the physical, emotional, social, and spiritual needs of children with CKD, and their families. In this article, we will define palliative care, highlight the unmet demand in pediatric nephrology and current state of integration efforts, examine the experience of routine integration of palliative care in adult nephrology and analogous chronic pediatric disease states, and, finally, offer suggestions to facilitate this process in pediatric nephrology.
Defining Palliative Care
The World Health Organization (WHO) defines palliative care as “an approach that improves the quality of life of patients and their families who are facing problems associated with life-threatening illness” attending to body, mind, and spirit by using meticulous symptom control, superior communication, and team-based support, without intending to speed or delay death (10). Palliative care provision, using a multidisciplinary approach, is endorsed early in the disease course, regardless of therapeutic goal (10). The current American Academy of Pediatrics (AAP) policy statement on pediatric palliative care (PPC) further describes PPC as an approach focused on relieving suffering, improving quality of life, enabling informed decision making, and coordinating care across realms (11). Similarly, the International Children’s Palliative Care Network defines PPC as an “active and total” care paradigm “embracing physical, emotional, social, and spiritual” components (12,13).
PPC grew from hospice and was initially synonymous with care provided at end of life (14,15,16). As the field has evolved, a broader scope of PPC has been embraced, which emphasizes comprehensive care through all disease stages (Figure 1) (14,16). Currently, the AAP Section on Palliative Care recommends PPC for all children with life-threatening illnesses, beginning at diagnosis and continuing irrespective of whether disease-directed treatment is ongoing (11). Conditions considered “appropriate” for PPC have also shifted (8). Whereas PPC was initially limited to patients with terminal illnesses, pediatric societies increasingly recommend PPC for all children with a “life-threatening” condition (11,13,16,17). This encompasses children with life-limiting conditions, where cure is not possible, and children with life-threatening conditions, where cure is possible and disease-directed treatments are ongoing (14,16,17). Importantly, many children with CKD fall into one of those two groups. Even in resource-limited health system settings, the Worldwide Palliative Care Alliance identifies early integration of palliative care as a human right for children (18). The AAP summarized this initiative as follows: “The goal is to add life to the child’s years, not simply years to the child’s life” (19).
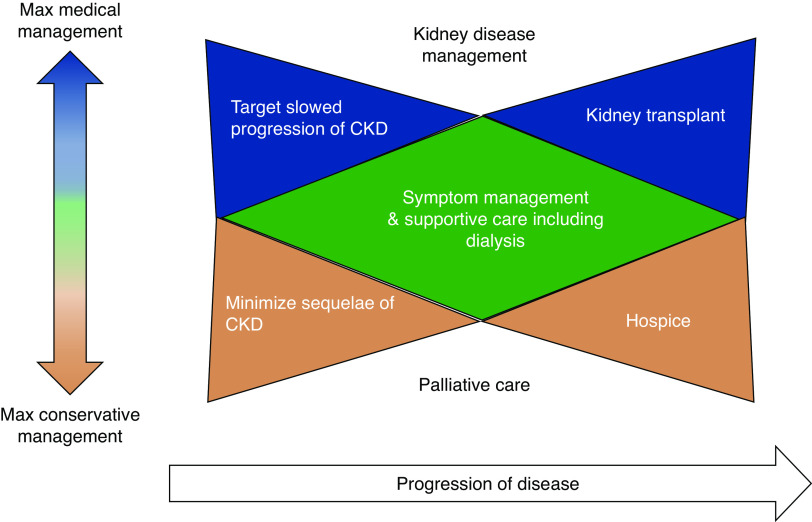
Figure 1.
Bow-tie model of palliative care integration in kidney disease. The bow-tie model highlights support of the patient and their family, concurrent with treatment of the disease, and the role of early integration of primary and/or subspecialty palliative care, while not diminishing the hope for survival. Dependent on patient and family goals, a range of complementary disease-targeted and palliative-focused care is available, with maximum (max) disease-targeted therapy represented by the top of the figure, and maximum conservative therapy represented by the bottom of the figure. Progression of the disease from diagnosis to realization of ultimate care goal is delineated from left to right. As the patient and their family move through their disease experience, their goals evolve, and kidney disease progresses, primary and subspecialty palliative care may take a larger role, ultimately with a transition toward kidney transplant and survivorship, or hospice and bereavement. Adapted from ref. (15), with permission.
In defining the scope and objectives of palliative care, it is vital to denote its varying degrees (Table 1). PPC is classified as primary or subspecialty PPC (20). Primary PPC is a responsibility of all clinicians within the multidisciplinary team, whereas subspecialty PPC requires advanced training in palliative care domains and concentrates on management of increasingly complicated issues (17,20). The domains of palliative care include (1) physical symptoms; (2) mental, emotional, social, and spiritual distress; and (3) patient- and family-centered communication (20,21). All clinicians should be equipped to manage physical symptoms, such as pain, sleep disturbance, painful edema, pruritis, and dyspnea. Beyond physical concerns, existential distress should be managed by diagnosing and treating depression and anxiety, exploring spiritual views, and addressing common psychosocial struggles. Patient- and family-centered communication requires exploration of patient and family goals, integration of multidisciplinary recommendations, and development of a care plan reflective of the goals of care. As these realms become increasingly complex, subspecialty PPC consultation is indicated (20). For example, subspecialty PPC consultation is indicated for refractory pain management, challenging existential suffering, or major communication conflicts among teams or families (17,20,21). Although the development of PPC offers the opportunity to improve the lives of children and their families, these benefits have largely been unrealized in pediatric nephrology.
Table 1.
Primary and subspecialty palliative care skills
| Primary Palliative Care | Subspecialty Palliative Care |
| Management of basic symptoms, such as pain, edema, pruritis, sleep disturbance | Management of refractory symptoms |
| Diagnosis and treatment of depression and anxiety, attendance to psychosocial challenges | Management of complex depression, anxiety, and existential suffering |
| Delivery of patient- and family-centered communication addressing topics such as goals of care, prognostic discussion, advanced care planning | Delivery of complex, serious illness communication, such as addressing disagreements in goals of care between patient and family, family and clinicians, or care teams |
The Unmet Need for PPC in Pediatric Nephrology
Although the burden intensity of CKD varies throughout the disease course for each patient and family, many children with CKD have life-limiting or life-threatening conditions. Consequently, routine primary and, in select cases, subspecialty PPC intervention, beginning at diagnosis and continuing regardless of therapeutic goal, is recommended for this population by the WHO, AAP, and others (Figure 2) (10,11). Additionally, the Renal Physicians Association (RPA) Clinical Practice Guideline on Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis endorses development of a palliative care plan for all children with ESKD, from the time of diagnosis, and children with AKI who forgo dialysis (22). Despite the positions of these professional organizations, there has been limited integration of PPC into nephrology.
Figure 2.
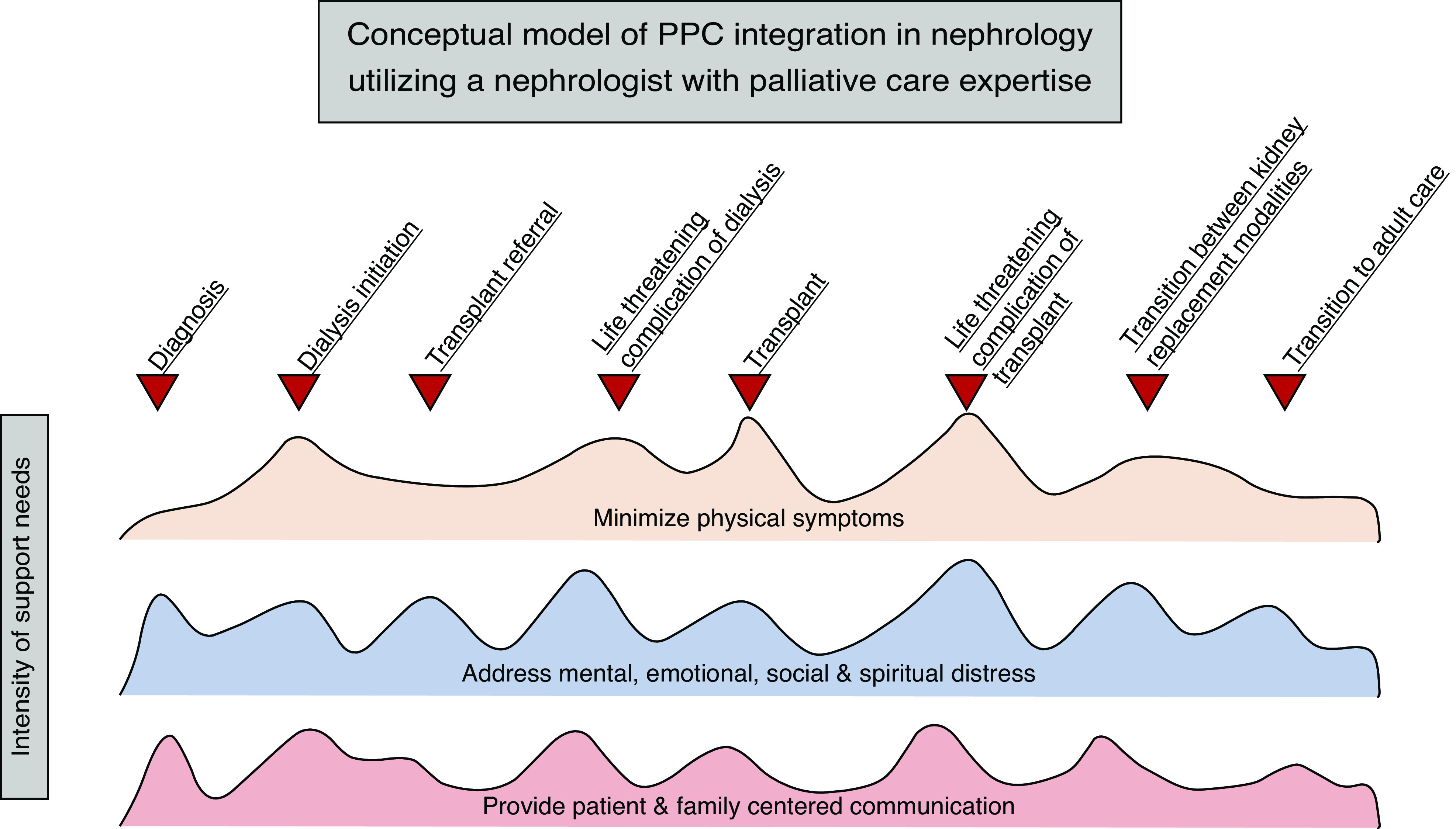
Conceptual model of pediatric palliative care (PPC) integration in nephrology utilizing a nephrologist with palliative care expertise. The domains of PPC should be addressed by the primary nephrologist and multidisciplinary team throughout the disease course, with the intensity of support individualized to the patient and family’s needs at that time in their care. A nephrologist with palliative care expertise is utilized at inflection points in care, when the patient and their family may experience significant changes in symptoms, experiences, burdens, and—ultimately—prognosis. With significant changes, goals of care may need to be revisited or revised, further reinforcing the benefit of additional PPC expertise. Although not every patient and family will experience each of these events, individualized inflection points of care may be identified that require additional burden alleviation. One example of an inflection point, where needs and burdens of patient and family can be more effectively addressed through integration of a nephrologist with palliative care expertise, includes diagnosis of CKD. The patient may already be experiencing resulting sleep disturbance. The patient and family may find it difficult to accept the diagnosis and feel grief that their hopes, goals, and dreams may be altered in the setting of CKD. Extensive questions about prognosis and care planning are likely. Another example of an inflection point that would benefit from integration of a nephrologist with palliative care expertise is at the time of transplant referral. A patient receiving a living, related kidney transplant may endorse emotional distress related to donation from a parent. The family may be anticipating significant financial burden associated with a parent’s donation, resulting in negative effects to the patient, parent, and siblings’ mental health and overall quality of life. Additionally, the patient and family may be overwhelmed with considering the potential complications of transplant, necessitating extra opportunities for patient- and family-centered communication. In both examples, the needs and burdens of the patient, and their family, can be more effectively met through enhanced palliative care provision by a nephrologist with PPC expertise.
Only 69% of children’s hospitals in the United States feature a subspecialty PPC team (23). Yet even in those centers benefiting from such a subspecialty PPC provision, there may be under-recognition of the burdens experienced by children with CKD, and their families, by PPC specialists, or underutilization of PPC by pediatric nephrologists to address these burdens. PPC specialists serve a diverse population of children, but those with kidney disease compose a fraction of consultations (9). In an influential review by palliative care experts, only kidney failure, in which dialysis and transplantation were unavailable or not indicated, was listed as an “appropriate” condition for PPC specialist consultation among children with kidney disease (8). Transplant surgeons may view PPC consultation as contraindicated to transplant, further complicating the fraught equation of subspecialty PPC involvement (24). In a review of PPC integration in pediatric nephrology, Thumfart et al. (25) recommended that, at a minimum, a specialized PPC team should provide care for pediatric nephrology patients with multiple comorbidities, in conservative treatment settings, and in patients suffering acute, life-threatening complications. Although adoption of this recommendation would increase specialist PPC consultation for some children with CKD, this would still potentially exclude a larger group of children with CKD facing life-limiting or life-threatening conditions that stands to benefit from primary PPC, at a minimum. These stances are outdated and overlook the burdens experienced by a range of children with CKD and their families.
CKD is an incurable, life-long, and life-shortening condition, which imposes significant burden on the child and family. Globally, survival of children with ESKD remains about 30 times worse than that of children without kidney disease (1). Additionally, after kidney transplant, the relative risk of death remains 12.7 times higher than that of peers (4). With a median age at transplant of 13 years, half of all pediatric kidney transplant recipients will need a second graft before the age of 25 years, reinforcing that there is no cure for CKD (4).
Beyond shortened life span, the burdens associated with CKD manifest in a variety of detriments. Children with CKD currently on dialysis, or who previously received a kidney transplant, experience poorer health-related quality of life (HRQoL) compared with healthy peers (3,5–7,26–30). Additionally, children with ESKD report significantly worse HRQoL compared with children with other chronic illnesses (31). CKD negatively affects mental health and well-being (32–35). For example, the prevalence of psychiatric disorders in prior studies of children with CKD ranged from 40% to 50% (36,37). Even among transplant recipients, negative effects on well-being may persist. Endén et al. (38) found higher depression inventory scores, worse general health, and more pain among young adult men who received kidney transplants during childhood compared with healthy controls and survivors of childhood leukemia. Further, the pain needs of children with CKD may go under-recognized. Research using the Patient-Reported Outcomes Measurement Information System demonstrated that about half of children with CKD, regardless of stage or transplant history, had experienced pain within the last week which interfered with daily activities (39). Children in this study who were hospitalized within the past 6 months also reported poorer scores for depression, anxiety, fatigue, and mobility (39). Children with CKD also experience impaired sleep, with studies showing high rates of sleep disturbances in children with nondialysis-dependent (37%) and dialysis-dependent CKD (86%), with resultingly impaired HRQoL (40,41). The importance of recognizing and developing strategies to address these burdens was highlighted by a recent qualitative study in which adolescents with CKD shared their desire that outcomes such as fatigue, lifestyle restrictions, and physical activity be prioritized (42).
The burdens associated with pediatric CKD reach beyond the patient and affect others, including siblings and parents. Siblings of kidney transplant recipients demonstrate worse physical well-being and lower scores on financial-resource dimensions of quality-of-life measures than healthy controls (43). Siblings of children with CKD report anxiety around their siblings’ medical needs, and frustration with limitations in their own lives (44). Parents of children with CKD have higher rates of depression and anxiety, poorer HRQoL, and increased financial strain; and although these burdens evolve, they do not resolve with transplantation (45–51).
The challenges experienced by children with CKD and their families require intentional, thoughtful, shared decision making incorporating the patient, parent, and clinician. Patients and parents value information sharing with nephrologists and desire to serve as experts in their lived experience, but both parties report feelings of censorship in interactions at times (52). This is compounded by the constantly evolving communication needs of the patient depending on their developmental stage (52). In a study of audio-recorded visits involving adolescents, young adults, and nephrologists, Coburn et al. demonstrated that nephrologists often struggle to engage in shared decision making and in incorporating the patient’s perspective (53).
The hardships of CKD are extensive, and patients and families express a clear desire to have their needs better met (42,52,54). PPC integration in pediatric nephrology affords an opportunity to realize this desire and need.
Current State of PPC in Pediatric Nephrology
To date, there has been limited utilization or study of PPC in nephrology. In a single-center analysis, Keefer et al. found that fewer than half of specialist PPC consults for children with ESKD were requested by the nephrology service (55). In a survey of multidisciplinary nephrology care team members, Thumfart et al. (56) found respondents identified a need for increased PPC resources, but disagreed about which patients should routinely receive subspecialty PPC. Both studies reinforce the need for integration of primary and subspecialty PPC in pediatric nephrology, but highlight that nephrologists, as stakeholders, may be unsure how best to meet this need. Given the limited integration of PPC into pediatric nephrology, it is important to consider examples of successful integration, including “kidney supportive care” and other pediatric chronic diseases.
Integration of Palliative Care within Adult Nephrology
There has been increasing recognition in adult nephrology of the multidimensional morbidity and mortality associated with CKD (57). Consequently, integration of palliative care within adult nephrology, termed “kidney supportive care,” has become a rising field (58,59). The previously discussed RPA Guideline recommends providing effective palliative care to all patients with AKI, CKD, and ESKD who “suffer from burdens of their disease.” (22) Similarly, authors of the report of a 2013 Kidney Disease Improving Global Outcomes Controversies Conference called for delivery of kidney supportive care to all patients with advanced CKD, recognizing this area as a core competency of nephrology in need of focused research efforts (58). Subsequently, developments in kidney supportive care have progressed in education, clinical care, and health systems. New educational programs, such as NephroTalk, equip nephrology providers with primary palliative care communication skills, including delivering bad news, establishing goals of care, and managing conflict (21,60,61). Many centers have adopted novel models to integrate kidney supportive care within the nephrology practice. In some dialysis units, this is achieved by employing nursing staff or social workers with additional palliative training focused on advanced care planning (21). Other clinics feature a specialist, dually trained in palliative care and nephrology, to lead the team (62,63). Mobile and home-based interventions have also been developed to increase patient access (21). In Canada and Australia, kidney supportive care has been integrated into governmental health systems, enabling provision as a routine component of delivered healthcare (21). Finally, there has been a general shift in approach, recognizing that adhering to a strict choice of transplant, dialysis, or conservative care may not fit the goals of all patients with advanced CKD, and rather that a continuum of care should be considered and individualized (64). Whereas kidney supportive care is an emerging field, integration of palliative care within clinical practice has already become standard in certain pediatric chronic disease states.
PPC in Pediatric Chronic Disease States
Subspecialty PPC consultation has been demonstrated to have positive effects in a variety of pediatric disease states (16,65–67). Subsequently, the value of early primary and subspecialty PPC has been demonstrated in some specific pediatric chronic disease populations, including oncology, pulmonology, and cardiology.
It is well established that children with cancer experience substantial disease burdens, although most will ultimately survive their illness (68–70). In a study of children with cancer and their parents, few expressed negative attitudes toward early PPC consultation, and the majority reported desirability (71). A randomized controlled trial evaluating the effect of a novel, PPC-based intervention targeting resilience in adolescents and young adults with cancer demonstrated improved psychosocial outcomes compared with participants receiving usual care (72). It is now standard of care at many pediatric oncology programs to consult specialty palliative care at time of diagnosis of malignancy, and for pediatric oncologists to routinely engage in primary PPC (68).
Similarly, although the development of new therapies has dramatically altered the disease course of cystic fibrosis (CF), there is increasing recognition of the long-term burdens of the disease on patients and families. Emerging research supports integration of primary and subspecialty PPC involvement to alleviate these burdens (73,74). Parents of adolescents with CF have expressed a desire for integration of PPC principles (75). Promisingly, a pilot study of a primary PPC intervention for patients with CF demonstrated improvement in CF-associated symptoms, decreased depressive symptoms, and diminished psychologic suffering (76). On the basis of these findings, there has been a call for routine integration of PPC as standard of care for children with CF (77).
Finally, a similar shift to integrating PPC has taken place in the care of children with hypoplastic left heart syndrome. These children experience high burdens of care throughout their lives, particularly concentrated in the first year of life (78). Early integration of PPC in this population has been demonstrated to decrease maternal anxiety and improve communication in a randomized clinical trial (79).
In each of these disease states, PPC has been shown to be desired, beneficial for patients and families, and increasingly integrated as standard of care. Children with advanced CKD and their families suffer comparable burdens. It is essential to consider steps for similar evidence-based integration of PPC to improve the experience of children with CKD and their families.
Directions for Future Opportunities To Integrate PPC into Pediatric Nephrology
Successful integration of PPC into pediatric nephrology will require a continued paradigm shift from the association of PPC with end-of-life care to holistic care intended to mitigate suffering and promote flourishing, an increased awareness of the needs of patients and their families, fostering of primary PPC knowledge and skills, integration of PPC within clinical care models, and dedicated research.
There is an outdated perception equating palliative care with hospice or only end-of-life care that overlooks the myriad benefits that PPC provision provides to patients and families across a variety of points in life-threatening or life-limiting diseases (Figure 2) (8,24,56). Rebranding, as demonstrated in adult nephrology, may be worthwhile to support this shift in stakeholder perceptions (58). Continued transition from this antiquated perception is critical to ensure the benefits of PPC are not denied to children with CKD and their families.
It is also vital to increase clinician awareness of the effect of their patients’ burdens, and those of their families. Efforts must focus on nephrologists, and palliative care specialists, transplant surgeons, and other multidisciplinary team members (8,9,24,55,56). In addition to continued study of burden, pain, HRQoL, and other outcomes, allowing patients and families to share experiences “in their own words,” through qualitative research, may provide the most persuasive means of achieving clinician recognition (80).
There is a need to further develop PPC capacity. Only a portion of children’s hospitals in the United States feature a subspecialty PPC team, reinforcing the need to increase the workforce of PPC specialists, but also highlighting the critical need to increase capabilities for primary PPC provision through nephrology care (23). Pediatric nephrologists serve a privileged, intimate role in the care of children with CKD and their families, often spanning decades, uniquely positioning them to deliver primary PPC. Clinician inexperience was found to be a barrier to integration of primary palliative care in adult nephrology (60). Training within residency and fellowship programs has been proposed to equip physicians with primary PPC skills, but, until this is achieved, clinicians will need to fill this gap in knowledge using alternative means (11,60). Available resources to assist in PPC proficiency range from formal courses to just-in-time smartphone applications (21,81–83).
As nephrologists attain competency, a structured approach for routine integration and evaluation of PPC in the care of children with CKD is imperative. The skill sets of multidisciplinary team members—including social workers, child life specialists, and psychologists—should be leveraged, but, ultimately, routine primary PPC provision is best applied under the direction of the pediatric nephrologist. Ideally, an embedded care model mirrored on kidney supportive care, using a physician with PPC expertise in the nephrology clinic, is warranted (Figure 2) (21,84). These tailored supports should remain as children move through care phases from advanced CKD, to dialysis, to transplant, and back, and through transition from pediatric to adult care.
Ongoing research must seek to better understand the experience and priorities of patients with CKD and their families, effectively intervene on their burdens, and provide a means for routine integration of PPC within nephrology clinical care. Adjusting clinical research to include patient-focused outcomes is foundational to understanding the needs and hopes of patients and families across disease, cultural, and developmental states (80,85–87). Additionally, there is a need to develop and study targeted interventions focused on patient-reported and -prioritized outcomes, including symptoms present in CKD, such as pain, pruritis, edema, and sleep disturbances. Finally, effective models for routine integration of PPC into clinical care for children with CKD must be developed, applied, and investigated.
Conclusion
Children with CKD face tremendous suffering that affects their entire family. PPC strives to relieve the suffering in physical, emotional, and existential realms experienced by children with life-threatening conditions, beginning at diagnosis and continuing throughout the child’s life. There is growing evidence demonstrating successful integration in adult nephrology, and areas of pediatric disease comparable with CKD. To add life to our patients’ years, we must hasten to understand and implement PPC within pediatric nephrology.
Disclosures
Dr. House is supported by a National Institutes of Health training grant (5T32DK007662-30, P I Hingorani). The remaining author has nothing to disclose.
Funding
None.
Author Contributions
T.R. House wrote the original draft and was responsible for project administration; T.R. House and A. Wightman conceptualized the study and reviewed and edited the manuscript; and A. Wightman provided supervision.
References
- 1.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ: Epidemiology of chronic kidney disease in children [published correction appears in Pediatr Nephrol 27: 507, 2012]. Pediatr Nephrol 27: 363–373, 2012. 10.1007/s00467-011-1939-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsnefes MM: Cardiovascular complications of pediatric chronic kidney disease. Pediatr Nephrol 23: 27–39, 2008. 10.1007/s00467-006-0359-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein SL, Gerson AC, Goldman CW, Furth S: Quality of life for children with chronic kidney disease. Semin Nephrol 26: 114–117, 2006. 10.1016/j.semnephrol.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 4.Rees L: Long-term outcome after renal transplantation in childhood. Pediatr Nephrol 24: 475–484, 2009. 10.1007/s00467-007-0559-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buyan N, Türkmen MA, Bilge I, Baskin E, Haberal M, Bilginer Y, Mir S, Emre S, Akman S, Ozkaya O, Fidan K, Alpay H, Kavukcu S, Sever L, Ozçakar ZB, Dogrucan N: Quality of life in children with chronic kidney disease (with child and parent assessments). Pediatr Nephrol 25: 1487–1496, 2010. 10.1007/s00467-010-1486-1 [DOI] [PubMed] [Google Scholar]
- 6.Tjaden LA, Grootenhuis MA, Noordzij M, Groothoff JW: Health-related quality of life in patients with pediatric onset of end-stage renal disease: State of the art and recommendations for clinical practice. Pediatr Nephrol 31: 1579–1591, 2016. 10.1007/s00467-015-3186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Splinter A, Tjaden LA, Haverman L, Adams B, Collard L, Cransberg K, van Dyck M, Van Hoeck KJ, Hoppe B, Koster-Kamphuis L, Lilien MR, Raes A, Taylan C, Grootenhuis MA, Groothoff JW: Children on dialysis as well as renal transplanted children report severely impaired health-related quality of life. Qual Life Res 27: 1445–1454, 2018. 10.1007/s11136-018-1789-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Himelstein BP, Hilden JM, Boldt AM, Weissman D: Pediatric palliative care. N Engl J Med 350: 1752–1762, 2004. 10.1056/NEJMra030334 [DOI] [PubMed] [Google Scholar]
- 9.Feudtner C, Kang TI, Hexem KR, Friedrichsdorf SJ, Osenga K, Siden H, Friebert SE, Hays RM, Dussel V, Wolfe J: Pediatric palliative care patients: a prospective multicenter cohort study. Pediatrics 127: 1094–1101, 2011. 10.1542/peds.2010-3225 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO): WHO definition of palliative care. Available at: https://www.who.int/news-room/fact-sheets/detail/palliative-care. Accessed September 24, 2020
- 11.Section on Hospice and Palliative Medicine and Committee on Hospital Care: Pediatric palliative care and hospice care commitments, guidelines, and recommendations. Pediatrics 132: 966–972, 2013. 10.1542/peds.2013-2731 [DOI] [PubMed] [Google Scholar]
- 12.Marston J, Boucher S, Downing J: International Children’s Palliative Care Network: A global action network for children with life-limiting conditions. J Pain Symptom Manage 55: S104–S111, 2018. 10.1016/j.jpainsymman.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 13.International Children’s Palliative Care Network: What is children’s palliative care? Available at: http://www.icpcn.org/about-icpcn/what-is-childrens-palliative-care/. Accessed October 30, 2020
- 14.Hawley P: Barriers to access to palliative care. Palliat Care 10: 1178224216688887, 2017. 10.1177/1178224216688887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley PH: The bow tie model of 21st century palliative care. J Pain Symptom Manage 47: e2–5, 2014. 24321509 [DOI] [PubMed] [Google Scholar]
- 16.Kang TI, Munson D, Hwang J, Feudtner C: Integration of palliative care into the care of children with serious illness. Pediatr Rev 35: 318–325, quiz 326, 2014. 10.1542/pir.35-8-318 [DOI] [PubMed] [Google Scholar]
- 17.Liben S, Papadatou D, Wolfe J: Paediatric palliative care: Challenges and emerging ideas. Lancet 371: 852–864, 2008. 10.1016/S0140-6736(07)61203-3 [DOI] [PubMed] [Google Scholar]
- 18.Ezer T, Lohman D, de Luca GB: Palliative care and human rights: A decade of evolution in standards. J Pain Symptom Manage 55: S163–S169, 2018. 10.1016/j.jpainsymman.2017.03.027 [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics Committee on Bioethics and Committee on Hospital Care: Palliative care for children. Pediatrics 106: 351–357, 2000 [PubMed] [Google Scholar]
- 20.Quill TE, Abernethy AP: Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med 368: 1173–1175, 2013. 10.1056/NEJMp1215620 [DOI] [PubMed] [Google Scholar]
- 21.Lam DY, Scherer JS, Brown M, Grubbs V, Schell JO: A conceptual framework of palliative care across the continuum of advanced kidney disease. Clin J Am Soc Nephrol 14: 635–641, 2019. 10.2215/CJN.09330818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renal Physicians Association: Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis: Clinical Practice Guideline Recommendation Summary, 2nd Ed., Rockville, MD, Renal Physicians Association, 2010 [Google Scholar]
- 23.Feudtner C, Womer J, Augustin R, Remke S, Wolfe J, Friebert S, Weissman D: Pediatric palliative care programs in children’s hospitals: A cross-sectional national survey. Pediatrics 132: 1063–1070, 2013. 10.1542/peds.2013-1286 [DOI] [PubMed] [Google Scholar]
- 24.Fowler A, Freiberger D, Moonan M: Palliative and end-of-life care in pediatric solid organ transplantation. Pediatr Transplant 19: 11–17, 2015. 10.1111/petr.12387 [DOI] [PubMed] [Google Scholar]
- 25.Thumfart J, Reindl T, Rheinlaender C, Müller D: Supportive palliative care should be integrated into routine care for paediatric patients with life-limiting kidney disease. Acta Paediatr 107: 403–407, 2018. 10.1111/apa.14182 [DOI] [PubMed] [Google Scholar]
- 26.Anthony SJ, Hebert D, Todd L, Korus M, Langlois V, Pool R, Robinson LA, Williams A, Pollock-BarZiv SM: Child and parental perspectives of multidimensional quality of life outcomes after kidney transplantation. Pediatr Transplant 14: 249–256, 2010. 10.1111/j.1399-3046.2009.01214.x [DOI] [PubMed] [Google Scholar]
- 27.Kiliś-Pstrusińska K, Medyńska A, Chmielewska IB, Grenda R, Kluska-Jóźwiak A, Leszczyńska B, Niedomagała J, Olszak-Szot I, Miklaszewska M, Szczepańska M, Tkaczyk M, Urzykowska A, Wasilewska A, Zachwieja K, Zajączkowska M, Ziółkowska H, Zagożdżon I, Zwolińska D: Perception of health-related quality of life in children with chronic kidney disease by the patients and their caregivers: Multicentre national study results. Qual Life Res 22: 2889–2897, 2013. 10.1007/s11136-013-0416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopes M, Ferraro A, Koch VH: Health-related quality of life of children and adolescents with CKD stages 4-5 and their caregivers. Pediatr Nephrol 29: 1239–1247, 2014. 10.1007/s00467-014-2769-8 [DOI] [PubMed] [Google Scholar]
- 29.Kilicoglu AG, Bahali K, Canpolat N, Bilgic A, Mutlu C, Yalçın Ö, Pehlivan G, Sever L: Impact of end-stage renal disease on psychological status and quality of life. Pediatr Int (Roma) 58: 1316–1321, 2016. 10.1111/ped.13026 [DOI] [PubMed] [Google Scholar]
- 30.Clavé S, Tsimaratos M, Boucekine M, Ranchin B, Salomon R, Dunand O, Garnier A, Lahoche A, Fila M, Roussey G, Broux F, Harambat J, Cloarec S, Menouer S, Deschenes G, Vrillon I, Auquier P, Berbis J: Quality of life in adolescents with chronic kidney disease who initiate haemodialysis treatment. BMC Nephrol 20: 163–173, 2019. 10.1186/s12882-019-1365-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varni JW, Limbers CA, Burwinkle TM: Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 5: 43–58, 2007. 10.1186/1477-7525-5-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira JM, Bouissou Morais Soares CM, Teixeira AL, Simões E Silva AC, Kummer AM: Anxiety, depression, resilience and quality of life in children and adolescents with pre-dialysis chronic kidney disease. Pediatr Nephrol 30: 2153–2162, 2015. 10.1007/s00467-015-3159-6 [DOI] [PubMed] [Google Scholar]
- 33.Bailey PK, Hamilton AJ, Clissold RL, Inward CD, Caskey FJ, Ben-Shlomo Y, Owen-Smith A: Young adults’ perspectives on living with kidney failure: A systematic review and thematic synthesis of qualitative studies. BMJ Open 8: e019926, 2018. 10.1136/bmjopen-2017-019926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kogon AJ, Vander Stoep A, Weiss NS, Smith J, Flynn JT, McCauley E: Depression and its associated factors in pediatric chronic kidney disease. Pediatr Nephrol 28: 1855–1861, 2013. 10.1007/s00467-013-2497-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kogon AJ, Kim JY, Laney N, Radcliffe J, Hooper SR, Furth SL, Hartung EA: Depression and neurocognitive dysfunction in pediatric and young adult chronic kidney disease. Pediatr Nephrol 34: 1575–1582, 2019. 10.1007/s00467-019-04265-z [DOI] [PubMed] [Google Scholar]
- 36.Bakr A, Amr M, Sarhan A, Hammad A, Ragab M, El-Refaey A, El-Mougy A: Psychiatric disorders in children with chronic renal failure. Pediatr Nephrol 22: 128–131, 2007. 10.1007/s00467-006-0298-9 [DOI] [PubMed] [Google Scholar]
- 37.Senses Dinc G, Cak T, Cengel Kultur E, Bilginer Y, Kul M, Topaloglu R: Psychiatric morbidity and different treatment modalities in children with chronic kidney disease. Arch Pediatr 26: 263–267, 2019. 10.1016/j.arcped.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 38.Endén K, Tainio J, Jalanko H, Jahnukainen K, Jahnukainen T: Lower quality of life in young men after pediatric kidney transplantation when compared to healthy controls and survivors of childhood leukemia-a cross-sectional study. Transpl Int 31: 157–164, 2018. 10.1111/tri.13040 [DOI] [PubMed] [Google Scholar]
- 39.Selewski DT, Massengill SF, Troost JP, Wickman L, Messer KL, Herreshoff E, Bowers C, Ferris ME, Mahan JD, Greenbaum LA, MacHardy J, Kapur G, Chand DH, Goebel J, Barletta GM, Geary D, Kershaw DB, Pan CG, Gbadegesin R, Hidalgo G, Lane JC, Leiser JD, Song PX, Thissen D, Liu Y, Gross HE, DeWalt DA, Gipson DS: Gaining the Patient Reported Outcomes Measurement Information System (PROMIS) perspective in chronic kidney disease: A Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 29: 2347–2356, 2014. 10.1007/s00467-014-2858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha R, Davis ID, Matsuda-Abedini M: Sleep disturbances in children and adolescents with non-dialysis-dependent chronic kidney disease. Arch Pediatr Adolesc Med 163: 850–855, 2009. 10.1001/archpediatrics.2009.149 [DOI] [PubMed] [Google Scholar]
- 41.Davis ID, Baron J, O’riordan MA, Rosen CL: Sleep disturbances in pediatric dialysis patients. Pediatr Nephrol 20: 69–75, 2005. 10.1007/s00467-004-1700-0 [DOI] [PubMed] [Google Scholar]
- 42.Hanson CS, Gutman T, Craig JC, Bernays S, Raman G, Zhang Y, James LJ, Ralph AF, Ju A, Manera KE, Teixeira-Pinto A, Viecelli AK, Alexander SI, Blydt-Hansen TD, Dionne J, McTaggart S, Michael M, Walker A, Carter S, Wenderfer SE, Winkelmayer WC, Bockenhauer D, Dart A, Eddy AA, Furth SL, Gipson DS, Goldstein SL, Groothoff J, Samuel S, Sinha A, Webb NJA, Yap HK, Zappitelli M, Currier H, Tong A: Identifying important outcomes for young people with CKD and their caregivers: A nominal group technique study. Am J Kidney Dis 74: 82–94, 2019. 10.1053/j.ajkd.2018.12.040 [DOI] [PubMed] [Google Scholar]
- 43.Velasco J, Ferraris V, Eymann A, Coccia PA, Ghezzi LR, Sánchez MC, De Cunto CL, D’Agostino D, Ferraris JR: Quality of life among siblings of patients with chronic conditions. Arch Argent Pediatr 118: 252–257, 2020 [DOI] [PubMed] [Google Scholar]
- 44.Agerskov H, Thiesson HC, Pedersen BD: Everyday life experiences in families with a child with kidney disease. J Ren Care 45: 205–211, 2019. 10.1111/jorc.12297 [DOI] [PubMed] [Google Scholar]
- 45.Tong A, Lowe A, Sainsbury P, Craig JC: Parental perspectives on caring for a child with chronic kidney disease: An in-depth interview study. Child Care Health Dev 36: 549–557, 2010. 10.1111/j.1365-2214.2010.01067.x [DOI] [PubMed] [Google Scholar]
- 46.Medway M, Tong A, Craig JC, Kim S, Mackie F, McTaggart S, Walker A, Wong G: Parental perspectives on the financial impact of caring for a child with CKD. Am J Kidney Dis 65: 384–393, 2015. 10.1053/j.ajkd.2014.07.019 [DOI] [PubMed] [Google Scholar]
- 47.Geense WW, van Gaal BGI, Knoll JL, Cornelissen EAM, van Achterberg T: The support needs of parents having a child with a chronic kidney disease: A focus group study. Child Care Health Dev 43: 831–838, 2017. 10.1111/cch.12476 [DOI] [PubMed] [Google Scholar]
- 48.Wightman A, Zimmerman CT, Neul S, Lepere K, Cedars K, Opel D: Caregiver experience in pediatric dialysis. Pediatrics 143: e20182102, 2019. 10.1542/peds.2018-2102 [DOI] [PubMed] [Google Scholar]
- 49.Wightman A: Caregiver burden in pediatric dialysis. Pediatr Nephrol 35: 1575–1583, 2020. 10.1007/s00467-019-04332-5 [DOI] [PubMed] [Google Scholar]
- 50.Darwish MM, Hassan SH, Taha SF, Abd El-Megeed HS, Ismail TAM: Family impact and economic burden among caregivers of children with chronic kidney disease in Assiut, Egypt. J Egypt Public Health Assoc 95: 27–34, 2020. 10.1186/s42506-020-00058-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ong ZH, Ng CH, Tok PL, Kiew MJX, Huso Y, Shorey S, Ng YPM: Sources of distress experienced by parents of children with chronic kidney disease on dialysis: A qualitative systematic review. J Pediatr Nurs 57: 11–17, 2021. 10.1016/j.pedn.2020.10.018 [DOI] [PubMed] [Google Scholar]
- 52.Gutman T, Hanson CS, Bernays S, Craig JC, Sinha A, Dart A, Eddy AA, Gipson DS, Bockenhauer D, Yap HK, Groothoff J, Zappitelli M, Webb NJA, Alexander SI, Goldstein SL, Furth S, Samuel S, Blydt-Hansen T, Dionne J, Michael M, Wenderfer SE, Winkelmayer WC, Currier H, McTaggart S, Walker A, Ralph AF, Ju A, James LJ, Carter S, Tong A: Child and parental perspectives on communication and decision making in pediatric CKD: A focus group study. Am J Kidney Dis 72: 547–559, 2018. 10.1053/j.ajkd.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 53.Coburn SS, Callon WA, Eakin MN, Pruette CS, Brady TM, Mendley SR, Tuchman S, Fivush BA, Riekert KA: Evaluating provider communication in pediatric chronic kidney disease care using a global coding system. Patient Educ Couns 103: 1358–1365, 2020. 10.1016/j.pec.2020.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clementi MA, Zimmerman CT: Psychosocial considerations and recommendations for care of pediatric patients on dialysis. Pediatr Nephrol 35: 767–775, 2020. 10.1007/s00467-019-04227-5 [DOI] [PubMed] [Google Scholar]
- 55.Keefer P, Lehmann K, Shanley M, Woloszyk T, Khang E, Luckritz K, Saul D: Single-center experience providing palliative care to pediatric patients with end-stage renal disease. J Palliat Med 20: 845–849, 2017. 10.1089/jpm.2016.0353 [DOI] [PubMed] [Google Scholar]
- 56.Thumfart J, Bethe D, Wagner S, Pommer W, Rheinländer C, Müller D: A survey demonstrates limited palliative care structures in paediatric nephrology from the perspective of a multidisciplinary healthcare team. Acta Paediatr 108: 1350–1356, 2019. 10.1111/apa.14688 [DOI] [PubMed] [Google Scholar]
- 57.Abdel-Kader K, Unruh ML, Weisbord SD: Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 4: 1057–1064, 2009. 10.2215/CJN.00430109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kidney Disease Improving Global Outcomes: Supportive/Palliative Care in CKD, 2013. Available at: https://kdigo.org/home/conferences/supportivecare. Accessed May 14, 2021 [Google Scholar]
- 59.Moss AH, Lupu DR, Armistead NC, Diamond LH, editors: Palliative Care in Nephrology, New York, Oxford University Press, 2020. 10.1093/med/9780190945527.001.0001 [DOI] [Google Scholar]
- 60.Schell JO, Green JA, Tulsky JA, Arnold RM: Communication skills training for dialysis decision-making and end-of-life care in nephrology. Clin J Am Soc Nephrol 8: 675–680, 2013. 10.2215/CJN.05220512 [DOI] [PubMed] [Google Scholar]
- 61.Schell JO, Cohen RA, Green JA, Rubio D, Childers JW, Claxton R, Jeong K, Arnold RM: NephroTalk: Evaluation of a palliative care communication curriculum for nephrology fellows. J Pain Symptom Manage 56: 767–773.e2, 2018. 10.1016/j.jpainsymman.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 62.Brown MA, Collett GK, Josland EA, Foote C, Li Q, Brennan FP: CKD in elderly patients managed without dialysis: Survival, symptoms, and quality of life. Clin J Am Soc Nephrol 10: 260–268, 2015. 10.2215/CJN.03330414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scherer JS, Wright R, Blaum CS, Wall SP: Building an outpatient kidney palliative care clinical program [published correction appears in J Pain Symptom Manage 55: e12, 2018]. J Pain Symptom Manage 55: 108–116.e2, 2018. 10.1016/j.jpainsymman.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 64.Kalantar-Zadeh K, Wightman A, Liao S: Ensuring choice for people with kidney failure - dialysis, supportive care, and hope. N Engl J Med 383: 99–101, 2020. 10.1056/NEJMp2001794 [DOI] [PubMed] [Google Scholar]
- 65.Mack JW, Wolfe J: Early integration of pediatric palliative care: For some children, palliative care starts at diagnosis. Curr Opin Pediatr 18: 10–14, 2006. 10.1097/01.mop.0000193266.86129.47 [DOI] [PubMed] [Google Scholar]
- 66.Vollenbroich R, Duroux A, Grasser M, Brandstätter M, Borasio GD, Führer M: Effectiveness of a pediatric palliative home care team as experienced by parents and health care professionals. J Palliat Med 15: 294–300, 2012. 10.1089/jpm.2011.0196 [DOI] [PubMed] [Google Scholar]
- 67.Voyles E: The development and outcomes of a pediatric palliative care program: A quality improvement process. J Pediatr Nurs 28: 196–199, 2013. 10.1016/j.pedn.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 68.Weaver MS, Heinze KE, Kelly KP, Wiener L, Casey RL, Bell CJ, Wolfe J, Garee AM, Watson A, Hinds PS: Palliative care as a standard of care in pediatric oncology. Pediatr Blood Cancer 62[Suppl 5]: S829–S833, 2015. 10.1002/pbc.25695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiener L, Kazak AE, Noll RB, Patenaude AF, Kupst MJ: Standards for the psychosocial care of children with cancer and their families: An introduction to the special issue. Pediatr Blood Cancer 62[Suppl 5]: S419–S424, 2015. 10.1002/pbc.25675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenberg AR, Weaver MS, Wiener L: Who is responsible for delivering palliative care to children with cancer? Pediatr Blood Cancer 65: 10.1002/pbc.26889, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levine DR, Mandrell BN, Sykes A, Pritchard M, Gibson D, Symons HJ, Wendler D, Baker JN: Patients’ and parents’ needs, attitudes, and perceptions about early palliative care integration in pediatric oncology. JAMA Oncol 3: 1214–1220, 2017. 10.1001/jamaoncol.2017.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenberg AR, Bradford MC, McCauley E, Curtis JR, Wolfe J, Baker KS, Yi-Frazier JP: Promoting resilience in adolescents and young adults with cancer: Results from the PRISM randomized controlled trial. Cancer 124: 3909–3917, 2018. 10.1002/cncr.31666 [DOI] [PubMed] [Google Scholar]
- 73.Waldman E, Quinn M: Palliative care and cystic fibrosis: Opportunities for growth. Pediatr Pulmonol 55: 2179–2180, 2020. 10.1002/ppul.24953 [DOI] [PubMed] [Google Scholar]
- 74.Robinson WM: Palliative and end-of-life care in cystic fibrosis: What we know and what we need to know. Curr Opin Pulm Med 15: 621–625, 2009. 10.1097/MCP.0b013e3283304c29 [DOI] [PubMed] [Google Scholar]
- 75.Dellon EP, Helms SW, Hailey CE, Shay R, Carney SD, Schmidt HJ, Brown DE, Prieur MG: Exploring knowledge and perceptions of palliative care to inform integration of palliative care education into cystic fibrosis care. Pediatr Pulmonol 53: 1218–1224, 2018. 10.1002/ppul.24073 [DOI] [PubMed] [Google Scholar]
- 76.Friedman D, Linnemann RW, Altstein LL, Georgiopoulos AM, Islam S, Bach KT, St John A, Fracchia MS, Neuringer I, Lapey A, Sicilian L, Moskowitz SM, Yonker LM: Effects of a primary palliative care intervention on quality of life and mental health in cystic fibrosis. Pediatr Pulmonol 54: 984–992, 2019 [DOI] [PubMed] [Google Scholar]
- 77.Dellon EP, Goggin J, Chen E, Sabadosa K, Hempstead SE, Faro A, Homa K: Defining palliative care in cystic fibrosis: A Delphi study. J Cyst Fibros 17: 416–421, 2018. 10.1016/j.jcf.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 78.Bertaud S, Lloyd DF, Laddie J, Razavi R: The importance of early involvement of paediatric palliative care for patients with severe congenital heart disease. Arch Dis Child 101: 984–987, 2016. 10.1136/archdischild-2015-309789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hancock HS, Pituch K, Uzark K, Bhat P, Fifer C, Silveira M, Yu S, Welch S, Donohue J, Lowery R, Aiyagari R: A randomised trial of early palliative care for maternal stress in infants prenatally diagnosed with single-ventricle heart disease. Cardiol Young 28: 561–570, 2018. 10.1017/S1047951117002761 [DOI] [PubMed] [Google Scholar]
- 80.Hanson CS, Craig JC, Tong A: In their own words: The value of qualitative research to improve the care of children with chronic kidney disease. Pediatr Nephrol 32: 1501–1507, 2017. 10.1007/s00467-016-3526-y [DOI] [PubMed] [Google Scholar]
- 81.Center to Advance Palliative Care (CAPC): CAPC Curriculum Continuing Education Courses, 2018. Available at: https://www.capc.org/providers/courses/. Accessed September 24, 2020
- 82.Cambia Palliative Care Center of Excellence: Graduate Certificate in Palliative Care, 2018. Available at: http://uwpctc.org/. Accessed September 24, 2020
- 83.Harvard Medical School Center for Palliative Care: Palliative Care Education and Practice, 2018. Available at: https://pallcare.hms.harvard.edu/courses/pcep. Accessed September 24, 2020
- 84.Filler G, Lipshultz SE: Why multidisciplinary clinics should be the standard for treating chronic kidney disease. Pediatr Nephrol 27: 1831–1834, 2012. 10.1007/s00467-012-2236-3 [DOI] [PubMed] [Google Scholar]
- 85.Chong LSH, Sautenet B, Tong A, Hanson CS, Samuel S, Zappitelli M, Dart A, Furth S, Eddy AA, Groothoff J, Webb NJA, Yap HK, Bockenhauer D, Sinha A, Alexander SI, Goldstein SL, Gipson DS, Raman G, Craig JC: Range and heterogeneity of outcomes in randomized trials of pediatric chronic kidney disease. J Pediatr 186: 110–117.e11, 2017. 10.1016/j.jpeds.2017.03.034 [DOI] [PubMed] [Google Scholar]
- 86.Kalantar-Zadeh K, Li PK, Tantisattamo E, Kumaraswami L, Liakopoulos V, Lui SF, Ulasi I, Andreoli S, Balducci A, Dupuis S, Harris T, Hradsky A, Knight R, Kumar S, Ng M, Poidevin A, Saadi G, Tong A; World Kidney Day Steering Committee: Living well with kidney disease by patient and care-partner empowerment: Kidney health for everyone everywhere. Clin Nephrol 95: 115–122, 2021 [DOI] [PubMed] [Google Scholar]
- 87.Logeman C, Guha C, Howell M, Hanson CS, Craig JC, Samuel S, Zappitelli M, Matsuda-Abedini M, Dart A, Furth S, Eddy A, Groothoff J, Yap HK, Bockenhauer D, Sinha A, Alexander SI, Goldstein SL, Gipson DS, Michael M, Walker A, Kausman J, Gaillard S, Bacchetta J, Rheault MN, Warady BA, Neu A, Christian M, McTaggart S, Liu I, Teo S, Sautenet B, Gutman T, Carter S, Teixeira-Pinto A, Tong A: Developing consensus-based outcome domains for trials in children and adolescents with CKD: An international Delphi survey. Am J Kidney Dis 76: 533–545, 2020. 10.1053/j.ajkd.2020.03.014 [DOI] [PubMed] [Google Scholar]