Key Points
Glucocorticoid Toxicity Index provides a global quantifiable assessment tool to assess glucocorticoid associated morbidity.
Cumulative doses of steroids in ANCA associated vasculitis leads to worse glucocorticoid related toxicity.
Whilst glucocorticoids remain the mainstay of AAV treatment, the narrow therapeutic window supports the need for GC-sparing treatments.
Keywords: clinical nephrology, ANCA, ANCA-associated vasculitis, glucocorticoid, glucocorticoid toxicity, Glucocorticoid Toxicity Index, health care outcome assessment, prednisolone, steroid, vasculitis
Abstract
Background
ANCA-associated vasculitis (AAV) is an autoimmune disease. Induction remission and maintenance treatment typically includes high-dose, tapering glucocorticoids (GC), in addition to other immunosuppressive medication. The use of theGlucocorticoid Toxicity Index (GTI) provides a global, quantifiable assessment tool in which clinicians can assess GC-associated morbidity. Recent trials in AAV have exposed the need for systemic assessment of GC burden. In this small cohort study, we look to address these issues and the justification of newer GC sparing agents, such as C5a inhibitors.
Methods
A retrospective cohort study of 43 patients with biopsy AAV was constructed from a single center between 2012–2016, and followed up for 48 months. The GTI table made up of adverse features was used to quantify patients’ GC toxicity. Electronic patient records were reviewed and scores calculated according to published methods. GTI scores were compared with cumulative steroid doses at separate intervals and incidences of adverse features in relation to the treatment timeline.
Results
The mean age was 65.9 (±11.06) years and treatment regimens consisted of glucocorticoids alongside cyclophosphamide or rituximab. Our results showed statistical significance in the association of cumulative GC doses and GTI scores (P=0.008; 95% CI, 1.31 to 8.05). Adverse features relating to mood disturbance and GC-induced psychosis occurred early, in contrast to adrenal insufficiency, which typically presented later in the follow-up. Infection-related adverse events were consistent throughout.
Conclusions
We demonstrated that higher cumulative doses of steroids in AAV lead to worse glucocorticoid-related toxicity. Using the GTI creates the potential to individualize and quantify the adverse effects patients experience as a result of GC treatment and permits more patient-centered management. Although glucocorticoids remain the main adjunctive immunosuppression of AAV treatment, the narrow therapeutic window supports the need for GC-sparing treatments.
Introduction
ANCA-associated vasculitis (AAV) is a complex autoimmune inflammatory disorder. It is characterized by inflammation and necrosis of small and medium-sized blood vessels, leading to tissue destruction and organ dysfunction. Immunosuppression is the mainstay of treatment.
The European Vasculitis Society has conducted several large, randomized control trials addressing various aspects of AAV treatment (1). The role of B cell depletion, cytotoxic and antimetabolite therapy in remission induction, and remission maintenance therapy is well supported (1,2). Induction and maintenance treatment typically involves adjuvant glucocorticoid (GC) treatment and, as a result, many patients with AAV receive long-term, high cumulative doses of GC as management of their AVV and any relapsed disease.
With the exceptionof the recent Plasma Exchange and Glucocorticoids for Treatment of ANCA Associated Vasculitis(3) and Avacopan for the Treatment of ANCA -Associated Vasculitis (ADVOCATE) (4) trials, there is a lack of evidence substantiating the role of GC dosing and duration, with significant evidence of treatment-related harm (5). The detrimental consequence of increasing GC doses has long been cited and the effects were recognized back in the 1950s, when steroids were given for many systemic diseases (6–8). Many of the side effects seen in autoimmune conditions such as AAV are often related to the duration of GC therapy (5,9). Adverse features can include steroid-induced diabetes, hypertension, osteoporosis, cataracts, and adrenal insufficiency (6,7,9).
In more recent years, there has been a gradual trend toward minimizing exposure to high-dose corticosteroids, including pulsed methylprednisolone, especially in patients who are high risk or frail (10,11). This is also seen in other areas of nephrology, for example, where GC treatment was once the mainstay in the prevention of rejection in solid organ transplant medicine. However, steroid-free transplantation is becoming increasingly common to reduce GC-related toxicity (12,13). These practice-based changes and recent studies have in part shaped the way we approach GC prescribing, but it remains a relatively evidence-free area, especially with regards to the use of methylprednisolone.
More recently, the term glucotoxicity has been coined, and this has brought about the introduction of the Glucocorticoid Toxicity Index (GTI) (6). The GTI is a global assessment tool that clinicians can use to quantitively assess the toxic effects of GC therapy and its associated morbidity at various time intervals (6). Although the adverse effects of GC are well known, there is a lack of substantial data quantifying adverse outcomes or incidence of steroid-related side effects (14). In 2014, Robson et al. (5) demonstrated significant adverse effects relating to the duration of GC use in AAV. GC treatment contributed to the vasculitis damage index, and this was of particular significance given those with a high index had an associated increased mortality (5,15). They demonstrated worsening outcomes over long-term follow-up and recommended clinicians to reduce GC use (5).
The measurement of GC-related toxicity varies significantly in published data (6,14), and is often confounded by the effect of other concurrently administered immunosuppression. This study aims to evaluate the GTI in AAV as a comprehensive and quantitative tool of assessing the steroid-associated treatment burden in current management strategies. Recent trials in AAV have exposed the need for systemic assessment of GC burden. In this small cohort study, we look to address these issues and the justification of newer GC sparing agents, such as C5a inhibitors.
Materials and Methods
Data Collection
A cohort study of 43 patients who were ANCA positive with biopsy proven pauci-immune GN was constructed from a single center between 2012 and 2016. Each patient was followed up for a total of 48 months. Patients who did not have a biopsy, or where the biopsy was nondiagnostic or demonstrated significant dense deposit disease, were excluded. In addition, those with positive anti–glomerular basement membrane antibodies, dual pathology, and secondary vasculitis were also excluded. Data were collected retrospectively and GTI scores were collated from electronic patient records and telephone calls. Cumulative doses of GC were calculated alongside GTI scores at seven separate intervals: 1, 3, 6, 12, 24, 36, and 48 months. Pulsed methylprednisolone at induction and relapse were reviewed, and doses were converted to oral prednisolone dose equivalents, with all subsequent cumulative doses presented as prednisolone (mg).
The primary outcome measures include the GTI scores alongside the cumulative steroid doses. Secondary outcome measures include the incidence of each adverse event and the timeline in which each event occurred in relation to commencing GC therapy.
GTI Scoring System
The GTI is a tool that clinicians can use to validate and quantify the toxic effects of GC therapy at various time intervals. Patient information from our cohort was compared against the GTI in which there is a composite and specific list (Table 1). Each patient was given a score relating to features on the composite list and this exercise was repeated at seven separate intervals. This allowed for the measurement of change in the GTI over the 48-month follow-up period.
Table 1.
The Glucocorticoid Toxicity Index (6).
| Composite Glucocorticoid Toxicity Index | Item Weight | Specific List |
| BMI | ||
| Improvement in BMI | −8 | Major increase in BMI |
| No change in BMI | 0 | |
| Moderate increase in BMI | 21 | |
| Major increase in BMI | 36 | |
| Glucose tolerance | ||
| Improvement in glucose tolerance | −8 | Diabetic retinopathy |
| No change in glucose tolerance | 0 | Diabetic nephropathy |
| Worsening of glucose tolerance | 32 | Diabetic neuropathy |
| Worsening of glucose tolerance despite treatment | 44 | |
| Blood pressure | ||
| Improvement in blood pressure | −10 | Hypertensive emergency |
| No change in blood pressure | 0 | Posterior reversible encephalopathy syndrome |
| Worsening hypertension | 19 | |
| Worsening hypertension despite treatment | 44 | |
| Lipids | ||
| Improvement in lipids | −9 | |
| No change in lipids | 0 | |
| Worsening hyperlipidemia | 10 | |
| Worsening hyperlipidemia despite treatment | 30 | |
| Bone density | ||
| Improvement in bone density | −1 | Major decrease in bone density |
| No change in bone density | 0 | Insufficiency fracture |
| Decrease in bone density | 29 | |
| Steroid myopathy | ||
| No steroid myopathy | 0 | Severe steroid myopathy |
| Mild steroid myopathy | 9 | |
| Moderate steroid myopathy or greater | 63 | |
| Skin Toxicity | ||
| No skin toxicity | 0 | Severe skin toxicity |
| Mild skin toxicity | 8 | |
| Moderate skin toxicity or greater | 26 | |
| Neuropsychiatric toxicity | ||
| No neuropsychiatric symptoms | 0 | Psychosis |
| Mild neuropsychiatric symptoms | 11 | GC-induced violence |
| Moderate neuropsychiatric symptoms or greater | 74 | Other severe neuropsychiatric symptoms |
| Infection | ||
| No significant infection | 0 | Grade IV infection |
| Oral/vaginal candidiasis or uncomplicated zoster | 19 | Grade V infection |
| Grade 3 infection or greater | 93 | |
| Endocrine | Adrenal insufficiency | |
| Gastrointestinal | Perforation, peptic ulcer disease | |
| Musculoskeletal | Avascular necrosis, tendon rupture | |
| Ocular | Central serous retinopathy, intraocular pressure elevation, posterior subcapsular cataract. | |
The composite list includes features of glucocorticoid toxicity that are weighted and given numerical scores. The specific list includes adverse features of glucocorticoid treatment but considered significant but are not given a numerical score. Scores can range from −36 to 439, with increasing scores relating to an increase in glucocorticoid toxicity burden and negative scores reflecting an improvement in toxicity (16).
BMI, body mass index; GC, glucocorticoid.
The items listed relate to commonly recognized adverse events as a result of cumulative steroid exposure. Scores range from −36 to 439 with cumulative worsening score relating to an increase in GC toxicity burden. The Aggregate Improvement Score (AIS) is a negative score, reflecting an improvement in toxicity (16). The full details of the GTI scoring system, categories, and components are described in the paper by Miloslavsky et al. (6).
Statistical Analysis
Differences of quantitative parameters between groups were assessed using both t and rank-sum tests. The 95% confidence intervals are reported and the threshold for statistical significance throughout was P<0.05. Repeated measures of GTI and steroid doses were summarized by the area under the curve and modeled using linear regression with backward selection. All statistical analyses were conducted in STATA V.16. For continuous variables, means and standard deviations were calculated, and the median and interquartile range (IQR) for continuous variables with skewed distribution.
Ethical Approval
Approval for this study was obtained by The Centre for Health Research and Innovation on behalf of Lancashire Teaching Hospitals National Health Service Foundation Trust (Ref: SE-317). The study was approved as service evaluation, therefore formal ethics committee review was not required.
Results
Study Population
In total, 43 patients were identified, 23 female and 20 males, with a mean age of 65.9 (±11.06) years at the time of presentation. All patients were ANCA positive: 44% (n=19) were positive for proteinase 3 (PR3) ANCA antibodies, and 56% (n=24) were positive for myeloperoxidase (MPO) autoantibodies, and had a renal biopsy at diagnosis demonstrating evidence of pauci-immune GN. Baseline patient characteristics with subgrouping according to ANCA serology is outlined in Table 2, with no meaningful difference in age or sex across the two groups. One patient was lost to follow-up, five patients died, and one patient received a renal transplant during the follow-up period.
Table 2.
The differences between patients with PR3 and MPO positive vasculitis, showing demographics, induction treatment, relapse, and mortality results associated with each cohort.
| Demographics | Total | PR3 | MPO |
| (n=43) | (n=19) | (n=24) | |
| Age, years (SD) | 65.9 (±11.06) | 58.8 (±10.75) | 71.6 (±7.97) |
| Sex (M:F) | 20:23 | 9:10 | 11:13 |
| eGFR at diagnosis, median (IQR), ml/min | 18 (10.5–27.5) | 19 (9–28) | 18 (12.5–26.3) |
| Induction treatment | |||
| Plasma exchange | 12 | 8 | 4 |
| IV methylprednisolone | 16 | 7 | 9 |
| Cyclophosphamide | 39 | 17 | 22 |
| Rituximab | 4 | 2 | 2 |
| Cumulative steroid dose, mg, median (IQR) | |||
| 1 month | 1260 (1032.5–2380) | 1365 (1032.5–2487.5) | 1155 (1042–2115) |
| 6 months | 3870 (2826.3–4412.5) | 4060 (3432.5–4502.5) | 3360 (2795–4200) |
| 1 year | 4970 (4000–5555) | 5247 (4660–5587.5) | 4340 (3698.8–5357.5) |
| 2 years | 6795 (5092.5–7724) | 7072 (6485–7856.5) | 5291.3 (4678.5–7337.8) |
| 4 years | 9072 (5565–11126) | 9330.5 (8121.8–11,109.5) | 5665 (5223–11407.5) |
| Patients scoring on the composite GTI list, n (%) | |||
| 1 month | 3 (7.0) | 1 (5.3) | 2 (8.3) |
| 6 months | 14 (35) | 7 (36.8) | 7 (32) |
| 1 year | 20 (51.3) | 11 (57.9) | 9 (42.9) |
| 2 years | 26 (66.7) | 14 (73.7) | 13 (61.9) |
| 4 years | 27 (71.1) | 14 (73.7) | 13 (65) |
| Relapsed disease, n | 8 | 5 | 3 |
| ESRF, n | 2 | 2 | 0 |
| Mortality, n | 5 | 0 | 5 |
Cumulative steroid doses are demonstrated as median cumulative doses with interquartile range in milligrams. In addition, the number of patients suffering for glucocorticoid-related toxicity at separate time intervals are also shown. Percentages of patients scoring for features of glucocorticoid toxicity as per the composite list in the Glucocorticoid Toxicity Index are presented. All percentages are calculated on the basis of the number of patients alive at each time point. M, male; F, female; IV, intravenous; GTI, Glucocorticoid Toxicity Index; ESRF, end stage renal failure.
All patients had AAV with renal involvement and 70% had more than one system involvement, with three patients having more than five systems involved. The mean eGFR (ml/min per 1.73m2) at presentation was 23.3ml/min (±18.1ml/min), with eight patients requiring initial renal replacement therapy. Of these eight patients, six (75%) regained independent renal function later in the study. Eight patients experiencedrelapsing disease during the follow-up period and two patients reported worsening ear, nose, and throat disease on weaning GCs.
Treatment
Induction therapy consisted of either intravenous cyclophosphamide or rituximab alongside daily oral GC. Pulsed intravenous methylprednisolone and plasma exchange were administered according to physician discretion. The dosing regimen of cyclophosphamide was adjusted for age and renal function in line with recommendations made by the European Vasculitis Study Group (2). The median cumulative dose of cyclophosphamide given was 5.4 g (IQR 2.4–2.9 g). Rituximab was administered at a dose of 1 g every 2 weeks for two doses. The dose taper of oralGC followed the regimen outlined by the Cyclophosphamide Oral versus Pulsed trial with variation according to physician discretion. Within the overall patient cohort, two patients died during the induction period, one as result of vasculitis and the other from a cerebral vascular event.
Initial doses of oral prednisolone ranged between 20 mg and 60 mg daily. Intravenous pulsed methylprednisolone was given to 37% (n=16) of which most (n=12) received a total dose of 1.5 g over 3 days. Two thirds (66%) of patients had a 50% reduction in oral GC dose by week 4. Of those receiving intravenous pulsed methylprednisolone the mean eGFR was 21.6 ml/min per 1.73m2 (8–22.3 ml/min) at presentation and the majority (63%) had more than one system involvement.
In total, 12 patients received adjuvant plasma exchange with an average of 5.3 sessions. Within this subgroup, ten received concomitant pulsed intravenous methylprednisolone as part of remission induction therapy. The cumulative dose of prednisolone at 12 months was higher among patients receiving plasma exchange therapy, 5203.6 mg versus 4667.3 mg. Remission maintenance therapy in the surviving 41 patients consisted of rituximab (n=17), azathioprine (n=26), mycophenolic acid (n=3), methotrexate (n=3), and cyclophosphamide (n=1). In total, 95% of those on maintenance therapy received concurrent daily maintenance oral GC.
Cumulative steroid doses were calculated at seven separate time intervals. At the end of the 48-month follow-up period, over half (54%) of patients were steroid free. Of those who completed steroid therapy, the median GC treatment period was 34.5 months (IQR, 24.2–41.8 months). Among the 17 patients still receiving GC treatment at the end of the study period, one was receiving 10 mg/day prednisolone as a part of a reducing course after recent relapse and the remaining nine patients were on ≤5 mg/day of prednisolone daily.
GTI Scores
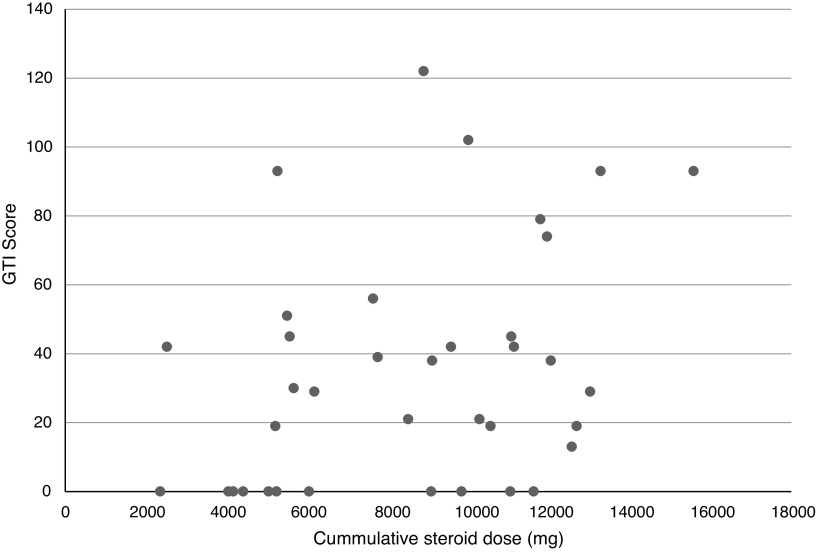
GTI scores and cumulative steroid doses were calculated at fixed intervals. Scores ranged from 0 to 123 over the 4-year follow-up period. Of the 37 patients included at the end of the study, only 12 patients (28%) had a GTI score of 0. Figure 1 shows the cumulative GC dose and GTI scores of individual patients at the end of the study period. With the exception of one patient, this demonstrates that at 48 months, patients with a GTI score >60 had received >9000 mg of GCs. Using linear regression with backward selection, there was a statistically significant association demonstrated between the cumulative GC dose and GTI score with a P value of 0.008 and 95% confidence interval for the regression coefficient of 1.31 to 8.05. There was no association between the use of pulsed intravenous methylprednisolone and GTI score at the end of the study period (P=0.8). Similarly, there was no difference in cumulative GC dose and GTI scores in those that were treated with cyclophosphamide (P=0.72) or rituximab (P=0.36).
Figure 1.
The cumulative glucocorticoid (GC) dose and Glucocorticoid Toxicity Index (GTI) scores of individual patients at the end of the study period. The graph shows that at the 4-year follow-up, for those still included in the study, a patient with a GTI score >60 had received >9000 mg of GCs (with the exception of one patient).
Overall, patients who were PR3 positive received higher cumulative doses of GCs than patients who were MPO positive at the end of the study period, 9330.5 mg versus 5665 mg. In line with this, a larger proportion of patients in the PR3 group had evidence of GC toxicity at follow-up compared with those with MPO positivity, 74% versus 65%, respectively. There was no significant difference in relapse rates, but higher mortality was associated with MPO-positive disease (Table 2).
Incidence of Toxicity
The incidence of adverse features related to steroid therapy both from the composite and specific list increased over the follow-up period. Three patients (7%) demonstrated GC toxicity as early as 4 weeks. At the end of the follow-up period, 27 patients (72%) demonstrated GC toxic effects (Table 2).
Infections, reduced bone density, and increasing body mass index were the most common adverse effects of GC therapy. Nearly a quarter (23%) had an improvement in their GTI score (AIS) during the follow-up period, with the majority seeing an improvement in weight, glucose tolerance, and blood pressure. The AIS occurred after the first year of treatment and after a reduction in the daily dose of GC.
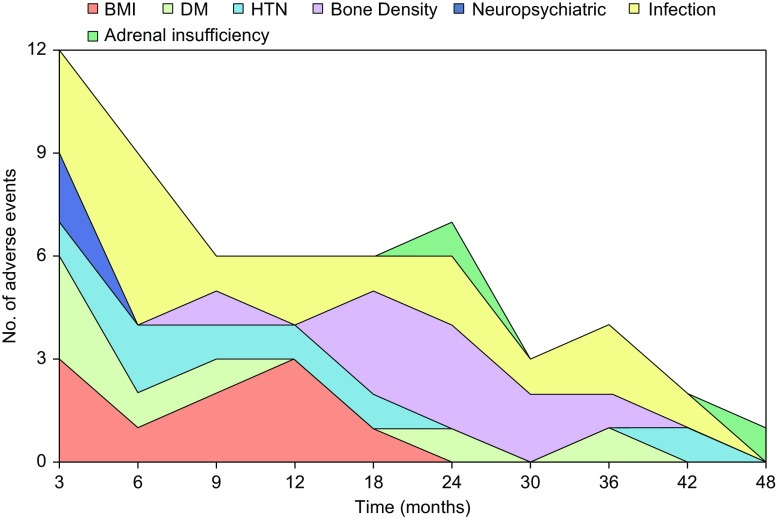
Figure 2 outlines the distribution of adverse events related to GC toxicity along the follow-up period. As expected, certain dose-dependent adverse features such as mood disturbance and steroid-induced psychosis occurred early in the treatment course. This is in contrast to adrenal insufficiency that occurred later in the follow-up period, with a median time of 21 days (IQR, 18–24) and 1059 days (IQR, 895–1224), respectively.
Figure 2.
The number of adverse events relating to each category of the GTI over the 48-month follow-up period. Each adverse event is scored once and the graph shows the distribution of onset for each adverse feature. The graph shows the number of infections was highest in the first few months and continued throughout the follow-up period. Neuropsychiatric presentations occurred early in GC treatment in contrast to adrenal insufficiency, which appeared to present later, typically after 18 months of GC treatment. BMI, body mass index; DM, diabetes mellitus; HTN, hypertension.
Figure 3 shows the percentage distribution of specific GC-related adverse effects. Weight gain, increased body mass index, steroid-induced diabetes, and gastrointestinal issues such as peptic ulcer disease occurred mostly within the first year of treatment, whereas reduced bone density and osteoporotic fractures occurred on average between 2 and 3 years into GC therapy.
Figure 3.
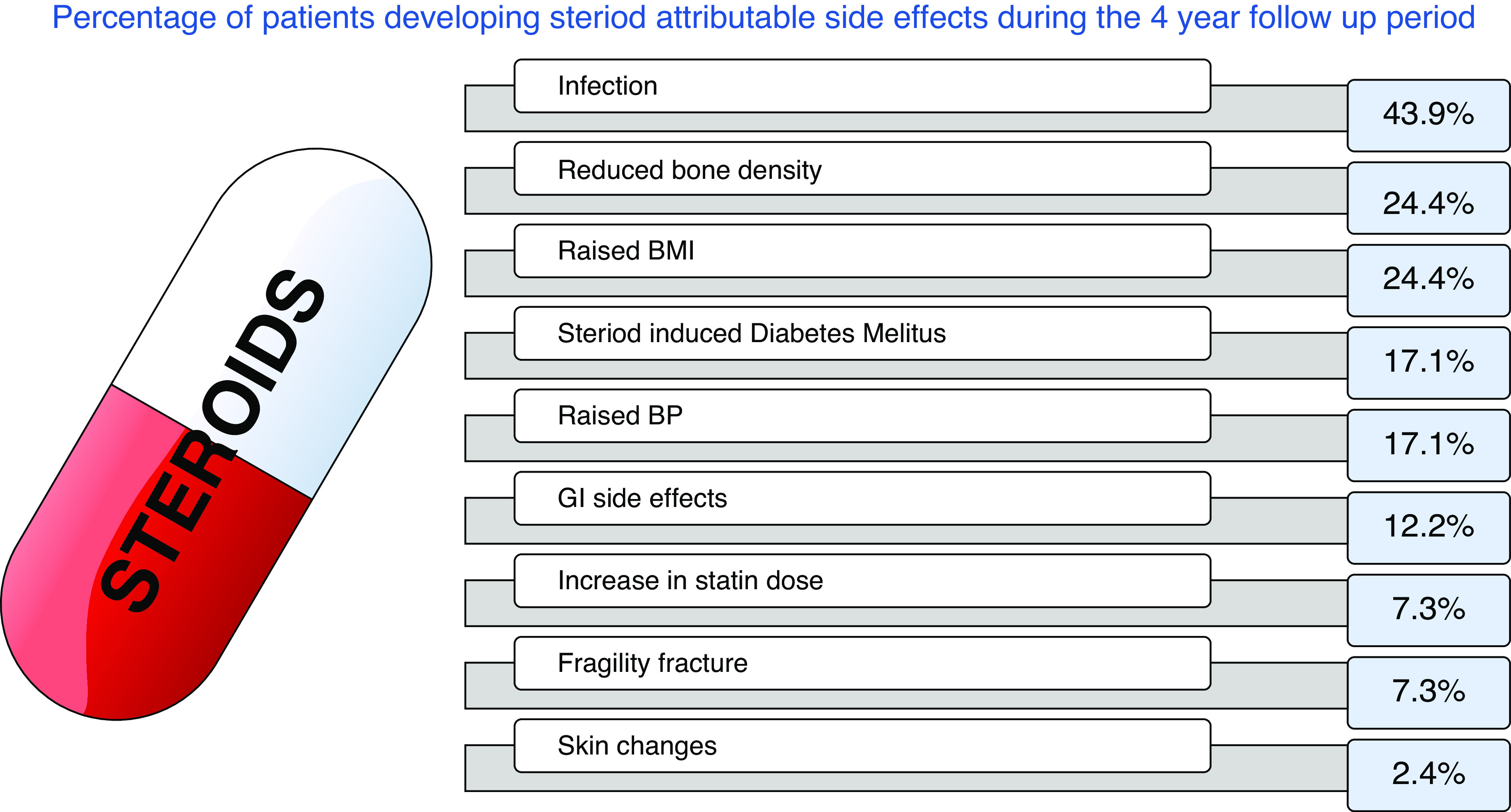
The percentage of adverse effects related to GC treatment within our cohort of patients over the 4-year follow-up period. Changes in BMI were determined by increases in weight >5 kg, raised blood pressure was assessed on the basis of a rise >20 mmHg from baseline, or needing up titration of antihypertensive medications to keep BP in range. Infections included anything from oral thrush to sepsis as explained in the composite GTI list. Gastrointestinal (GI) side effects include gastritis and peptic ulcer disease.
Infection-related adverse events were largely consistent throughout the duration of follow-up as demonstrated in Figure 2. This can be attributed to baseline immunosuppression rather than GC alone. Five patients scored 19 on the composite list (Table 1) for having oral candidiasis and/or uncomplicated varicella zoster infections. Oral fluconazole treatment was prescribed to 90% (n=37) of patients, and all patients that developed oral candidiasis received appropriate fluconazole prophylaxis. Six patients scored 93 points due to grade three infection, which was defined as needing hospitalization and or intravenous antibiotics. These infections occurred within the first year of commencing treatment. Cotrimoxazole prophylaxis was prescribed in 98% (n=40) of patients and there were no incidences of Pneumocystis jirovecii infection.
Reduced bone density and fragility fractures were recognized in 24% and 7% of patients, respectively. All three patients that suffered fragility fractures had evidence of reduced bone mineral density on Dual Energy X-ray Absorptiometry scan. Bone protection was prescribed in the form of bisphosphates, vitamin D replacement, and calcium supplements. In total, 81% (n=33) received calcium supplements and 37% (n=15) were treated with bisphosphonates. Of those that did not receive bisphosphonate treatment, the median eGFR was 26 ml/min per 1.73m2 (IQR, 13.5–46ml/min), which may have been a contributory factor.
Discussion
This retrospective study looks at the effect of prolonged exposure to GCs using a novel scoring system. Although the effects of GC toxicity are well recognized, there has not previously been a tool that enables clinicians to quantify the toxic effects of GC treatment at an individual level. There have been several studies demonstrating the correlation between steroid exposure and GC toxicity; however, with the exception of the recent ADVOCATE (4) trial, there have been no other studies that have evaluated GTI scores in the context of AAV. Our data demonstrated that higher cumulative doses of GCs led to more adverse effects of therapy and a quantitative increase in GC toxicity using this tool.
The concept of systematically measuring GC toxicity in the form of GTI is new and warrants further validation. A study in 2020 by McDowell et al. (17) looked at a variation of the GTI (GTI 2.0) in severe asthma. It demonstrated that GTI scores were not only associated with GC doses but also correlated with patient reported outcome measures. Using tools such as the Mini Asthma Quality of Life Questionnaire, they demonstrated strong correlation with GTI scores and patient-reported quality of life (17).
At present, steroids remain a cornerstone of AAV treatment, but more recent studies have looked at ways to reduce steroid exposure. McGovern et al. (10) looked at lower-dose GC in the management of AAV in elderly and frail groups. The median cumulative dose of prednisolone at 3 months in this study was slightly lower than the cumulative doses seen in our cohort; 2030 mg (IQR, 1785–2167) versus 2520 mg (IQR, 1995–3495), respectively. The outcomes reported by McGovern et al. (10) supported a low-dose GC regime in favor of higher daily doses or pulsed methylprednisolone at induction. This was further supported by retrospective, multi-center study of 114 patients in which pulsed intravenous methylprednisolone was associated with increased risk of infection and steroid-induced diabetes, and offered no benefit in the treatment of AAV compared with high-dose oral corticosteroids (18).
In this study, infection was the most common toxicity among our cohort with the incidence of infection occurring throughout the follow-up period. Many patients experienced oral candidiasis and varicella zoster infections, and some suffered more serious effects such as sepsis, requiring hospitalization. It is important to note that all patients in our cohort received steroids concurrent to other immunosuppressive therapies, and therefore the specific role of GC in infection-related toxicity is not in isolation. Furthermore, other vasculitic-contributing factors such as damaged sinorespiratory mucosa may have also had a role in the development of infections.
Steroid-induced mineral bone density disease was also noted in our cohort. Its incidence trended toward a delayed onset, correlating with a higher cumulative GC dose. One potential confounder is the demographic risk factors of our cohort (age >60 years and female sex). Similar features have been recognized in other publications (1) and the role of prophylactic bone protection is vital. Although over half of patients in our cohort were steroid free at 48 months, many continued on low-dose, maintenance steroids, further exposing them to collective side effects.
With larger trialssuch as Plasma Exchange and Glucocorticoids for Treatment of ANCA Associated Vasculitis identifying noninferiority of lower-dose GC treatment and potential reductions in the incidence of severe infection (3,11), it has made way for other trials to look at GC-sparing treatments. Avacopan (CCX168) is a selective C5a receptor inhibitor that has been used as an adjuvant to reduce GC exposure significantly in the management of AAV. The recent landmark trial, ADVOCATE (4), published earlier this year, has demonstrated that not only is avacopan effective in the management of AAV, but reports significantly fewer adverse effects related to GC treatment compared with standard steroid treatment. It suggests that avacopan precludes the need for high-dose GC treatment in these patients (4,19,20). Furthermore, the trial used GTI as a secondary endpoint, and demonstrated worsening GTI cumulative scores that were higher in prednisolone group compared with the avacopan group (4). Not only does this promote the use of GC-sparing agents and highlight the adverse effects of GC treatment supported by the findings in our cohort, but it also highlights the use of GTI in clinic trials and future research.
Within the world of nephrology, the use of steroids is not limited to AAV. They are used as standard of care in the management of other conditions, such as minimal change disease, and membranous and IgA nephropathy. Although GC remain imperative to treatment in many of these conditions, the narrow therapeutic index means close observation and monitoring is essential.
The findings of our study should be considered within the context of its limitations. Firstly, we recognize the observational nature of the study and other confounds, such as immunosuppressive medication, plasma exchange, and active vasculitis, are potentially contributory to the development of adverse effects. It is not possible to fully attribute all of the adverse effects discussed to GC treatment alone. However, a panel of scientific experts who developed the GTI defined the features to be included in the composite GTI list on the basis of different domains (6). These included the likelihood of adverse features occurring in over 5% in patients exposed to GCs, with toxicity being more likely a result of the GC therapy than the disease itself. Additionally, any measurements and scores were not dependent on or requiring invasive procedures or imaging (6). Other adverse features that were considered significant but had the potential be confounded by other features such as underlying disease or other treatments were included in the specific list. Secondly, the retrospective nature of our study may also lend itself to inaccuracies in the reporting and documentation of some mild side effects, such as mood disturbance or skin changes. This is likely to have led to an under representation of the adverse features. Thirdly, our relatively small sample size may restrict the accuracy of our results.
Progressing the roleof GTI in the assessment and management of patients with kidney disease treated with GC will be of significant value. Calculating a baseline GTI score at induction with prospective monitoring, will help identify those patients at increased risk of developing toxic effects from prolonged steroid exposure. It is well known that there is a degree of genetic predisposition to GC toxicity (21). The role of pharmacogenetics alongside tools such as the GTI will allow for more patient-centered management and tailored treatment plans (22).
In spite of a relatively small cohort, this study has demonstrated the use of measuring GTI in patients with AAV. We have shown that cumulative doses of steroids in AAV leads to worsening GC-related toxicity. Using the GTI tool allows us to individualize and quantify the adverse effects patients experience, which in turn will improve patient-centered management. We have also shown the incidence of adverse features relating to GC exposure occur at different stages, with a persistent risk of infection. These data support the need for further work evaluating GC-sparing treatments, the assessment of steroid toxicity and its relation to clinical outcomes and patient-reported outcome measures. This pertinent need is not only restricted to AAV; although GC remains imperative in the treatment of a range of nephrological conditions, the narrow therapeutic index means that close observation and monitoring is essential.
Disclosures
All authors have nothing to disclose.
Funding
None.
Acknowledgments
The authors would like to acknowledge the support of the Renal Department at Royal Preston Hospital, Lancashire National Health Service Foundation Trust and the National Institute for Health Research Lancashire Clinical Research Facility.
Author Contributions
L. Floyd was responsible for data curation; M. Joshi and L. Floyd were responsible for statistical analysis; A. Dhaygude provided supervision; L. Floyd and A. Morris wrote the original draft; and A. Dhaygude, L. Floyd, M. Joshi, and A. Morris reviewed and edited the paper.
References
- 1.Jayne D: Evidence-based treatment of systemic vasculitis. Rheumatology (Oxford) 39: 585–595, 2000. 10.1093/rheumatology/39.6.585 [DOI] [PubMed] [Google Scholar]
- 2.Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, Hauser T, Hellmich B, Jayne D, Kallenberg CG, Merkel PA, Raspe H, Salvarani C, Scott DG, Stegeman C, Watts R, Westman K, Witter J, Yazici H, Luqmani R; European Vasculitis Study Group: EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 68: 310–317, 2009. 10.1136/ard.2008.088096 [DOI] [PubMed] [Google Scholar]
- 3.Walsh M, Merkel PA, Peh C-A, Szpirt WM, Puéchal X, Fujimoto S, Hawley CM, Khalidi N, Floßmann O, Wald R, Girard LP, Levin A, Gregorini G, Harper L, Clark WF, Pagnoux C, Specks U, Smyth L, Tesar V, Ito-Ihara T, de Zoysa JR, Szczeklik W, Flores-Suárez LF, Carette S, Guillevin L, Pusey CD, Casian AL, Brezina B, Mazzetti A, McAlear CA, Broadhurst E, Reidlinger D, Mehta S, Ives N, Jayne DRW; PEXIVAS Investigators: Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis. N Engl J Med 382: 622–631, 2020. 10.1056/NEJMoa1803537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group: Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med 384: 599–609, 2021. 10.1056/NEJMoa2023386 [DOI] [PubMed] [Google Scholar]
- 5.Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Höglund P, Jayne D, Mahr A, Westman K, Luqmani R: Glucocorticoid treatment and damage in the anti-neutrophil cytoplasm antibody-associated vasculitides: Long-term data from the European Vasculitis Study Group trials. Rheumatology (Oxford) 54: 471–481, 2015. 10.1093/rheumatology/keu366 [DOI] [PubMed] [Google Scholar]
- 6.Miloslavsky EM, Naden RP, Bijlsma JWJ, Brogan PA, Brown ES, Brunetta P, Buttgereit F, Choi HK, DiCaire JF, Gelfand JM, Heaney LG, Lightstone L, Lu N, Murrell DF, Petri M, Rosenbaum JT, Saag KS, Urowitz MB, Winthrop KL, Stone JH: Development of a Glucocorticoid Toxicity Index (GTI) using multicriteria decision analysis. Ann Rheum Dis 76: 543–546, 2017. 10.1136/annrheumdis-2016-210002 [DOI] [PubMed] [Google Scholar]
- 7.Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D: Incidence and US costs of corticosteroid-associated adverse events: A systematic literature review. Clin Ther 33: 1413–1432, 2011. 10.1016/j.clinthera.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 8.Bollet AJ, Black R, Bunim JJ: Major undesirable side-effects resulting from prednisolone and prednisone. J Am Med Assoc 158: 459–463, 1955. 10.1001/jama.1955.02960060017005 [DOI] [PubMed] [Google Scholar]
- 9.Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Höglund P, Jayne D, Mahr A, Westman K, Luqmani R: Damage in the ANCA-associated vasculitides: Long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis 74: 177–184, 2015. 10.1136/annrheumdis-2013-203927 [DOI] [PubMed] [Google Scholar]
- 10.McGovern D, Williams SP, Parsons K, Farrah TE, Gallacher PJ, Miller-Hodges E, Kluth DC, Hunter RW, Dhaun N: Long-term outcomes in elderly patients with ANCA-associated vasculitis. Rheumatology (Oxford) 59: 1076–1083, 2020. 10.1093/rheumatology/kez388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris A, Geetha D: PEXIVAS challenges current ANCA-associated vasculitis therapy. Nat Rev Nephrol 16: 373–374, 2020. 10.1038/s41581-020-0269-6 [DOI] [PubMed] [Google Scholar]
- 12.El-Faramawi M, Rohr N, Jespersen B: Steroid-free immunosuppression after renal transplantation: Long-term experience from a single centre. Nephrol Dial Transplant 21: 1966–1973, 2006. 10.1093/ndt/gfl131 [DOI] [PubMed] [Google Scholar]
- 13.Kasiske BL, Chakkera HA, Louis TA, Ma JZ: A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol 11: 1910–1917, 2000 [DOI] [PubMed] [Google Scholar]
- 14.van der Goes MC, Jacobs JWG, Boers M, Andrews T, Blom-Bakkers MAM, Buttgereit F, Caeyers N, Cutolo M, Da Silva JA, Guillevin L, Kirwan JR, Rovensky J, Severijns G, Webber S, Westhovens R, Bijlsma JW: Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann Rheum Dis 69: 1913–1919, 2010. 10.1136/ard.2009.124958 [DOI] [PubMed] [Google Scholar]
- 15.Exley AR, Carruthers DM, Luqmani RA, Kitas GD, Gordon C, Janssen BA, Savage CO, Bacon PA: Damage occurs early in systemic vasculitis and is an index of outcome. QJM 90: 391–399, 1997. 10.1093/qjmed/90.6.391 [DOI] [PubMed] [Google Scholar]
- 16.McDowell PJ, Stone J, Honeyford K, Dunn L, Logan RJ, Butler CA, Heaney L: Evaluation of the steroid sparing effects of Mepolizumab using the Glucocorticoid Toxicity Index. Eur Respir J 54[suppl 63]: PA2513, 2019. 10.1183/13993003.congress-2019.PA2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDowell PJ, Stone JH, Zhang Y, Honeyford K, Dunn L, Logan RJ, Butler CA, McGarvey LPA, Heaney LG: Quantification of glucocorticoid-associated morbidity in severe asthma using the Glucocorticoid Toxicity Index. J Allergy Clin Immunol Pract 9: 365–372.e5, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Chanouzas D, McGregor JAG, Nightingale P, Salama AD, Szpirt WM, Basu N, Morgan MD, Poulton CJ, Draibe JB, Krarup E, Dospinescu P, Dale JA, Pendergraft WF, Lee K, Egfjord M, Hogan SL, Harper L: Intravenous pulse methylprednisolone for induction of remission in severe ANCA-associated vasculitis: A multi-center retrospective cohort study. BMC Nephrol 20: 58, 2019. 10.1186/s12882-019-1226-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayne DRW, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, Burst V, Grundmann F, Jadoul M, Szombati I, Tesař V, Segelmark M, Potarca A, Schall TJ, Bekker P: Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 28: 2756–2767. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merkel PA, Jayne DR, Wang C, Hillson J, Bekker P: Evaluation of the safety and efficacy of avacopan, a C5a receptor inhibitor, in patients with antineutrophil cytoplasmic antibody-associated vasculitis treated concomitantly with rituximab or cyclophosphamide/azathioprine: Protocol for a randomized, double-blind, active-controlled, phase 3 trial. JMIR Res Protoc 9: e16664, 2020. 10.2196/16664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens A, Ray DW, Zeggini E, John S, Richards HL, Griffiths CEM, Donn R: Glucocorticoid sensitivity is determined by a specific glucocorticoid receptor haplotype. J Clin Endocrinol Metab 89: 892–897, 2004. 10.1210/jc.2003-031235 [DOI] [PubMed] [Google Scholar]
- 22.Schijvens AM, Ter Heine R, de Wildt SN, Schreuder MF: Pharmacology and pharmacogenetics of prednisone and prednisolone in patients with nephrotic syndrome. Pediatr Nephrol 34: 389–403, 2019. 10.1007/s00467-018-3929-z [DOI] [PMC free article] [PubMed] [Google Scholar]