Abstract
Background
Access to primary care was hindered by the coronavirus disease 2019 (COVID-19) pandemic.
Objective
Evaluate changes in health screening rates before and during the pandemic.
Design
Retrospective analysis of health maintenance and disease management screening rates among primary care patients before and during the pandemic.
Participants
Over 150,000 patients of a large, academic health system.
Main Measures
Six quality measures were analyzed: colon cancer, breast cancer, cervical cancer, diabetes Hgb A1C, diabetes eye, and diabetes nephropathy monitoring. Based on US Preventative Services Task Force screening guidelines, we determined which patients were due for at least one of the quality measures. We tracked completion rates during three time periods: pre-pandemic (January 1–March 3, 2020), stay-at-home (March 4–May 8, 2020), and phased reopening (May 9–July 8, 2020). Differences in quality measure completion rates were evaluated using mixed-effects logistic regression models.
Key Results
Compared to pre-pandemic rates, completion of all health screenings declined during the stay-at-home period: mammograms (OR: 0.34; 95% CI: 0.31–0.37), cervical cancer (OR: 0.83; 95% CI: 0.76–0.91), colorectal cancer (OR: 0.25; 95% CI: 0.23–0.28), diabetes eye (OR: 0.34; 95% CI: 0.29–0.41), diabetes Hgb A1c (OR: 0.41; 95% CI: 0.37–0.46), and diabetes nephropathy (OR: 0.46, 95% CI: 0.41–0.53). During phased reopening, completion of all quality measures increased compared to the stay-at-home period, except for cervical cancer screening (OR: 0.83; 95% CI: 0.76–0.92). There was a persistent reduction in completion of all quality measures, except for diabetic nephropathy monitoring (OR: 0.99; 95% CI: 0.89–1.09), during phased reopening compared to pre-pandemic.
Conclusions
Healthcare screening rates were reduced during the early part of the COVID-19 pandemic and did not fully recover to pre-pandemic rates by July 2020. Future research should aim to clarify the long-term impacts of delayed health screenings. New interventions should be considered for expanding remote preventative health services.
KEY WORDS: COVID-19, primary care, preventative screening, chronic disease management
INTRODUCTION
The novel coronavirus disease 2019 (COVID-19) is a highly infectious acute respiratory illness.1 On March 19, 2020, a California-wide shelter-in-place order mandated that all non-essential personnel stay at home.2 In accordance with this executive order and to contain the spread of COVID-19 within the healthcare system, many outpatient clinics stopped all in-person visits and quickly transitioned to telehealth encounters.3–5
Among clinics at the University of California Los Angeles (UCLA) Department of Medicine, telehealth visits encompassed fewer than 1% of all visits on March 9, 2020, and in just 50 days rose to 55% of all visits.5 While large healthcare systems like UCLA Health were well equipped with the technology and infrastructure to navigate the necessary transition to telehealth, these changes were much more difficult to adopt among rural communities.6 As many in-person visits were postponed, access to preventative health screenings including mammograms, pap smears, and colonoscopies was also suspended.
A potential consequence of postponing medical care is delayed diagnosis and treatment of critical illnesses.7,8 These effects have already been seen in the Netherlands, where cancer diagnoses have notably declined since the beginning of the COVID-19 pandemic likely due to undiagnosed disease.8 In the UK, the national cancer screening program, which accounts for 5% of all cancer diagnoses, was temporarily suspended during the pandemic.7 Though necessitated by reallocation of resources towards combating the current pandemic, these postponements in health screenings may similarly impede the progress made in recent decades towards more proactive preventative healthcare in the USA.
Understanding the impact of the COVID-19 pandemic on both preventative care and disease management screenings can aid the health system and policymaking in the event of a COVID-19 resurgence or other future epidemics. In this report, we analyze changes in the rates of six key health screenings among patients of the UCLA Health Department of Medicine before and during the COVID-19 pandemic.
METHODS
Study Design
UCLA Health is a large, nonprofit academic health system in Southern California. The UCLA Health Department of Medicine (DOM) encompasses 13 clinical divisions in over 180 outpatient practice settings. There are over 650 DOM physicians who provide nearly 1.5 million outpatient visits annually. Inclusion criteria for this analysis were patients over the age of 18 who were followed by a UCLA Health primary care provider in the Divisions of Internal Medicine, Family Medicine, and Internal Medicine and Pediatrics.
Public Health Measures to Address COVID-19 in California and the Study Period
The pre-pandemic period was defined as 1/1/20 to 3/3/20 (weeks 1–9). On 3/4/20, both California and the Los Angeles County formally announced a state of emergency; therefore, 3/3/20 was set as the end of the pre-COVID period. The stay-at-home period was defined as 3/4/20 to 5/8/20 (weeks 10–18). Reopening of lower-risk workplaces occurred on 5/8/20, which marked the end of the strictest period of shelter-in-place orders and the start of stage 2 reopening in California. The phased reopening period was defined as 5/9/20 to 7/8/20 (weeks 19–27). This nine-week period following the 5/8/20 start of stage 2 reopening in California was set as a point of comparison for the pre-pandemic and stay-at-home periods.
Quality Metrics
The quality measures analyzed weekly include colon cancer, breast cancer, cervical cancer screening, diabetes Hgb A1C, diabetes eye, and diabetes nephropathy monitoring. Completion rates of each quality measure were defined as the percentage of patients up to date on the measure per all screening-eligible patients. Patient eligibility for screening exams was based upon recommendations from the US Preventative Services Task Force (USPSTF), which provides guidance for health screens by age group and individual risk factors. For diabetes management and screening, guidelines provided by the American Diabetes Association were used, since USPSTF does not provide specific recommendations. For breast cancer screening, USPSTF recommends biennial mammograms for women ages 50–74 years, but screening varies by institution. For this study, the UCLA Health recommendation of biennial screening for women ages 40–74 years was followed.
Data Extraction
The EPIC Electronic Health Record’s Health Maintenance Module (EPIC Systems, Verona, WI) was used to maintain and track quality measure completion rate at the patient level. The logic within UCLA Health’s Health Maintenance module is updated regularly based on USPSTF Guidelines and local expert opinion. For example, colorectal cancer screening is recommended by USPSTF for patients ages 50–75 years and can be addressed through any one of the following modalities: FIT (fecal immunohistochemical test), Cologuard, colonoscopy, flexible sigmoidoscopy, and others. Patients who are due-for-screening at the start of each period (pre-pandemic, stay-at-home, phased reopening) were monitored for completion of their open orders during that period.
In addition to general patient demographics, a COVID Risk Score was calculated for each patient. The COVID Risk Score was developed at UCLA Health to identify patients at risk for severe COVID-19 complications. A score was assigned to each patient based on CDC-reported risk factors for COVID-19: age > 65 years, chronic lung disease, serious heart conditions, immunocompromised status, BMI > 40, poorly controlled diabetes, liver disease, and end-stage kidney disease on dialysis. A COVID Risk Score of zero indicated the lowest risk and eight indicated the highest risk for developing COVID-19-related complications.
Statistical Analysis
Patient characteristics were summarized by study period using means and standard deviations for quantitative variables, and frequencies and percentages for categorical variables. Weekly provider-level completion rates for each quality measure were computed, and average completion rates across providers were plotted by week during the study period, as well as for the parallel period from the prior calendar year.
We used mixed effects logistic regression models to evaluate changes in completion rates between study periods. The outcome was a binary indicator of quality measure completion at the patient level. Patients were included in each study period if they were due for the measure in week 1 of the period and were classified as complete if they were up to date by the final week of that period. The analysis was limited to patients of a set of providers who saw at least some patients in all three periods. Models included fixed effects for the study period (pre-pandemic vs stay-at-home vs phased reopening), and random provider effects to account for clustering of patients by the provider. Models were adjusted for patient age, race/ethnicity, enrollment in the UCLA patient portal (MyChart), COVID risk score, and provider specialty (medicine-pediatrics/family medicine vs internal medicine). Models were also adjusted for patient sex, except in the cases of mammogram and cervical cancer screening, where analyses were restricted to female patients. Differences between periods were summarized using odds ratios (OR) and 95% confidence intervals (95% CI). P values less than 0.05 were considered statistically significant. Regression analyses were performed using SAS v. 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Demographic Characteristics
The study included 154 internal medicine–boarded physicians and 100 medicine-pediatrics and family medicine–boarded physicians. At the start of the pre-pandemic period, 146,281 patients were due for at least 1 screening per USPSTF guidelines. At the start of the stay-at-home period, 150,393 patients were due for at least one screening, and by the start of phased reopening, 154,961 patients were due for at least one screening.
Age, sex, race, and ethnicity did not vary significantly between study periods (Table 1). The average COVID Risk Score among these patients was 0.6 out of 8.
Table 1.
Patient Demographics in Pre-pandemic, Stay-at-Home, and Phased Reopening Periods
| Pre-pandemic (N = 146,281) | Stay at home (N = 150,393) | Phased reopening (N = 154,961) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 49.7 | 16.0 | 49.6 | 15.9 | 49.9 | 15.9 |
| BMI | 26.5 | 6.1 | 26.5 | 11.2 | 26.5 | 14.5 |
| COVID Risk Score (0–8) | 0.6 | 0.9 | 0.6 | 0.9 | 0.6 | 0.9 |
| Gender | ||||||
| Female | 113,125 (77.3%) | 116,540 (77.5%) | 119,324 (77.0%) | |||
| Male | 33,156 (22.7%) | 33,853 (22.5%) | 35,637 (23.0%) | |||
| Ethnicity | ||||||
| Not Hispanic or Latino | 109,086 (74.6%) | 111,185 (73.9%) | 114,258 (73.7%) | |||
| Hispanic or Latino | 15,053 (10.3%) | 15,551 (10.3%) | 16,115 (10.4%) | |||
| Unknown | 22,142 (15.1%) | 23,657 (15.7%) | 24,588 (15.9%) | |||
| Race | ||||||
| White | 82,833 (56.6%) | 84,690 (56.3%) | 86,979 (56.1%) | |||
| Black | 5,849 (4.0%) | 5,906 (3.9%) | 6,128 (4.0%) | |||
| Asian | 15,275 (10.4%) | 15,525 (10.3%) | 16,127 (10.4%) | |||
| Native American | 566 (0.4%) | 584 (0.4%) | 612 (0.4%) | |||
| Other/multiple/unknown | 41,758 (28.5%) | 43,688 (29.0%) | 45,115 (29.1%) | |||
| MyChart user | ||||||
| Yes | 124,180 (84.9%) | 128,182 (85.2%) | 132,520 (85.5%) | |||
| No | 22,101 (15.1%) | 22,211 (14.8%) | 22,441 (14.5%) | |||
Among patients due for mammogram screening at the start of the stay-at-home period, the odds of completion by the end of this period were reduced by 66% (OR: 0.34; 95%CI: 0.31–0.37) compared to the reference pre-pandemic period. Similarly, completion rates during the same period decreased for all other screenings (Tables 2 and 3).
Table 2.
Odds Ratios for Breast, Cervical, and Colorectal Cancer Screening
| Breast cancer screening | Cervical cancer screening | Colorectal cancer screening | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Period | ||||||
| Stay at home vs. pre-pandemic | 0.34 (0.31,0.37) | < 0.001 | 0.83 (0.76, 0.91) | < 0.001 | 0.25 (0.23, 0.28) | < 0.001 |
| Phased reopening vs. pre-pandemic | 0.49 (0.45, 0.53) | < 0.001 | 0.69 (0.63, 0.76) | < 0.001 | 0.49 (0.45, 0.53) | < 0.001 |
| Phased reopening vs stay-at-home | 1.44 (1.31, 1.58) | < 0.001 | 0.83 (0.76, 0.92) | < 0.001 | 1.93 (1.73, 2.15) | < 0.001 |
| Age (+ 5y) | ||||||
| 1.02 (1.00, 1.04) | 0.022 | 1.06 (1.04, 1.08) | < 0.001 | 0.94 (0.92, 0.97) | < 0.001 | |
| Female | ||||||
| N/A | N/A | N/A | N/A | 0.87 (0.81, 0.94) | < 0.001 | |
| Ethnicity (Ref = not Hispanic or Latino) | ||||||
| Hispanic or Latino | 1.28 (1.15, 1.43) | < 0.001 | 1.28 (1.13, 1.44) | < 0.001 | 1.24 (1.09, 1.40) | < 0.001 |
| Unknown | 0.87 (0.77, 0.98) | 0.024 | 0.83 (0.73, 0.94) | 0.004 | 0.77 (0.67, 0.88) | < 0.001 |
| Race (Ref = White) | ||||||
| Black | 1.15 (0.99, 1.34) | 0.076 | 1.14 (0.93, 1.39) | 0.202 | 1.06 (0.89, 1.26) | 0.513 |
| Asian | 1.27 (1.14, 1.40) | < 0.001 | 1.25 (1.11, 1.40) | < 0.001 | 1.23 (1.09, 1.39) | < 0.001 |
| Native American | 0.91 (0.55, 1.53) | 0.733 | 1.01 (0.53, 1.94) | 0.975 | 0.47 (0.22, 0.99) | 0.046 |
| Other/multiple/unknown | 0.95 (0.87, 1.05) | 0.304 | 1.05 (0.95, 1.16) | 0.364 | 0.93 (0.84, 1.02) | 0.137 |
| MyChart user | ||||||
| 2.54 (2.24, 2.89) | < 0.001 | 3.25 (2.68, 3.93) | < 0.001 | 2.20 (1.95, 2.47) | < 0.001 | |
| COVID Risk Score (+ 1) | ||||||
| 1.03 (0.99, 1.07) | 0.195 | 1.03 (0.97, 1.10) | 0.343 | 1.08 (1.03, 1.12) | < 0.001 | |
| Med-peds/family medicine (Ref = internal medicine) | ||||||
| 0.85 (0.75, 0.96) | 0.011 | 0.91 (0.76, 1.09) | 0.323 | 0.83 (0.73, 0.94) | 0.003 | |
Bolded values indicate statistically significant results with p-value < 0.05
Table 3.
Odds Ratios for Diabetes Health Maintenance Screenings
| Diabetes: eye exam | Diabetes: Hgb A1C screening | Diabetes: nephropathy monitoring | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Period | ||||||
| Stay at home vs. pre-pandemic | 0.34 (0.29, 0.41) | < 0.001 | 0.41 (0.37, 0.46) | < 0.001 | 0.46 (0.41, 0.53) | < 0.001 |
| Phased reopening vs. pre-pandemic | 0.64 (0.56, 0.74) | < 0.001 | 0.91 (0.83, 0.98) | 0.019 | 0.99 (0.89, 1.09) | 0.834 |
| Phased reopening vs stay-at-home | 1.87 (1.54, 2.27) | < 0.001 | 2.20 (1.98, 2.45) | < 0.001 | 2.13 (1.88, 2.42) | < 0.001 |
| Age (+ 5y) | ||||||
| 1.04 (1.02, 1.07) | 0.002 | 1.02 (1.01, 1.04) | 0.004 | 0.99 (0.97, 1.01) | 0.272 | |
| Female | ||||||
| 0.97 (0.85, 1.11) | 0.649 | 0.95 (0.87, 1.02) | 0.156 | 0.87 (0.79, 0.96) | 0.004 | |
| Ethnicity (Ref = not Hispanic or Latino) | ||||||
| Hispanic or Latino | 1.29 (1.07, 1.55) | 0.007 | 1.06 (0.94, 1.18) | 0.352 | 1.17 (1.02, 1.35) | 0.022 |
| Unknown | 0.64 (0.47, 0.87) | 0.004 | 0.72 (0.61, 0.85) | < 0.001 | 0.86 (0.71, 1.05) | 0.144 |
| Race (Ref = White) | ||||||
| Black | 1.36 (1.07, 1.72) | 0.013 | 1.10 (0.96, 1.27) | 0.179 | 0.98 (0.81, 1.17) | 0.796 |
| Asian | 1.31 (1.08, 1.58) | 0.005 | 1.10 (0.98, 1.23) | 0.107 | 1.19 (1.04, 1.37) | 0.013 |
| Native American | 1.24 (0.55, 2.81) | 0.600 | 0.87 (0.53, 1.44) | 0.593 | 1.20 (0.67, 2.16) | 0.542 |
| Other/multiple/unknown | 1.19 (1.00, 1.43) | 0.055 | 0.99 (0.89, 1.10) | 0.838 | 0.95 (0.83, 1.08) | 0.454 |
| MyChart user | ||||||
| 1.69 (1.40, 2.04) | < 0.001 | 2.09 (1.88, 2.31) | < 0.001 | 1.83 (1.62, 2.08) | < 0.001 | |
| COVID Risk Score (+ 1) | ||||||
| 1.11 (1.05, 1.18) | < 0.001 | 1.16 (1.12, 1.21) | < 0.001 | 1.17 (1.12, 1.22) | < 0.001 | |
| Med-peds/family medicine (ref = internal medicine) | ||||||
| 0.91 (0.73, 1.13) | 0.398 | 0.86 (0.78, 0.94) | 0.001 | 0.99 (0.86, 1.14) | 0.910 | |
Bolded values indicate statistically significant results with p-value < 0.05
In the subsequent phased reopening period, odds of mammogram completion increased by 44% compared to the stay-at-home period (OR: 1.44; 95% CI: 1.31–1.58). Similarly, most other screenings saw a rise in completion during the phased reopening period (Tables 2 and 3). Only cervical cancer screening continued to decline during phased reopening compared to stay-at-home (OR: 0.83; 95%CI: 0.76–0.92). Screening rate trends comparing 2019 to 2020 data are seen in Figures 1 and 2.
Fig. 1.
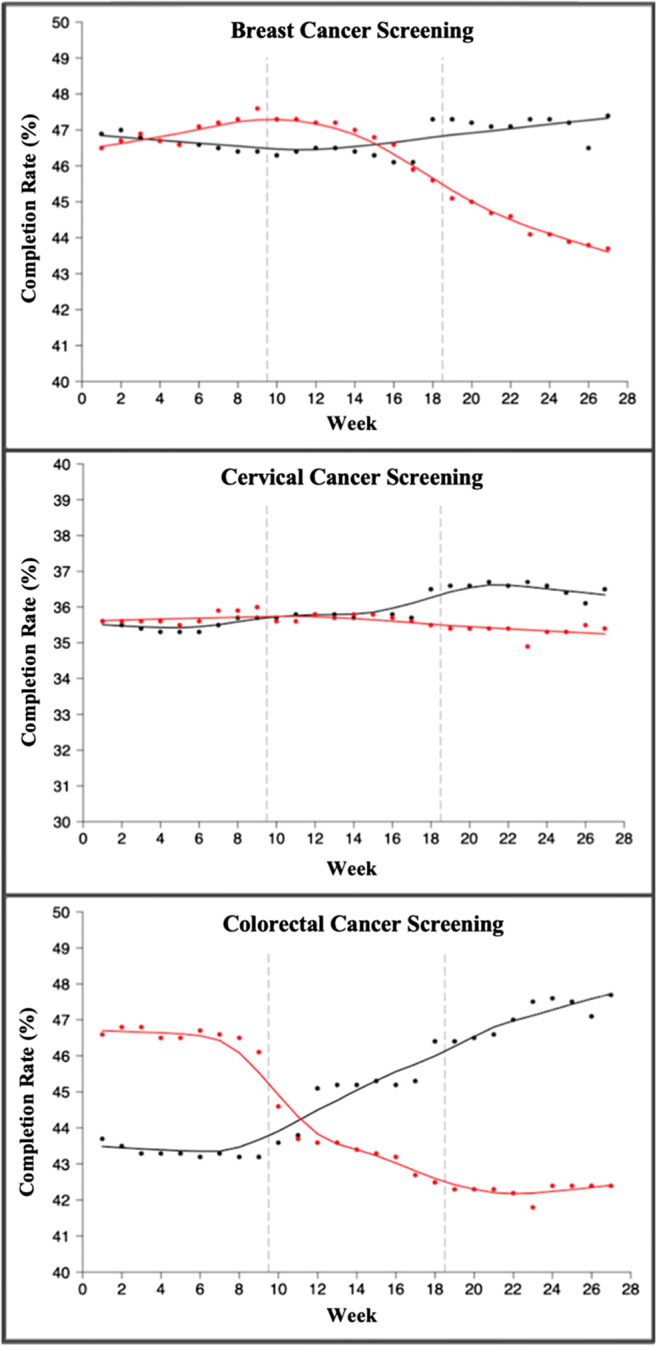
Trends in breast, cervical, and colorectal cancer screenings by week (weeks 1–27 in 2019 vs 2020). Black line: average weekly completion rate (%) for each screening in 2019. Red line: average weekly completion rate (%) for each screening in 2020. For each graph, dashed line at 9 weeks indicates start of stay-at-home order, and dashed line at 19 weeks indicates start of phased reopening during the COVID-19 pandemic.
Fig. 2.
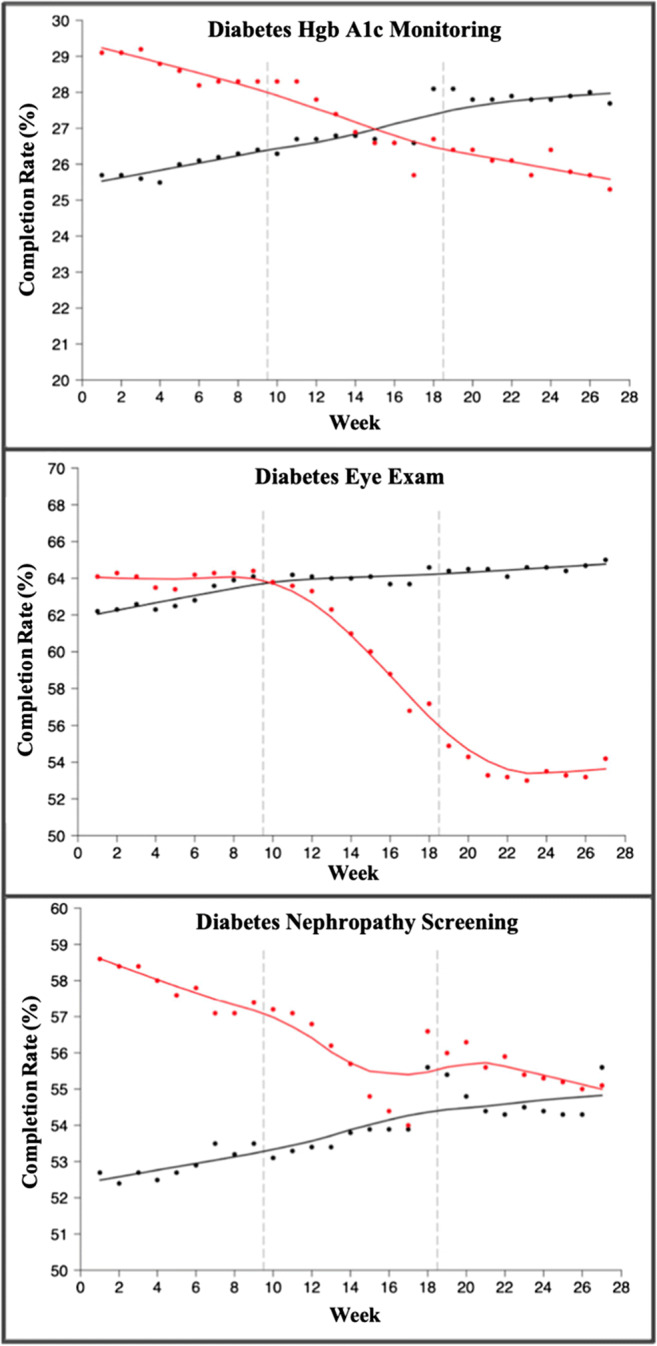
Trends in diabetes Hgb A1c, diabetes eye, and diabetes nephropathy monitoring by week (weeks 1–27 in 2019 vs 2020). Black line: average weekly completion rate (%) for each screening in 2019. Red line: average weekly completion rate (%) for each screening in 2020. For each graph, dashed line at 9 weeks indicates start of stay-at-home order, and dashed line at 19 weeks indicates start of phased reopening during the COVID-19 pandemic.
Demographic Predictors of Screening Completion
Female patients were less likely to complete colorectal cancer screening (OR: 0.87; 95%CI: 0.81–0.94) and diabetic nephropathy monitoring (OR: 0.87; 95%CI: 0.79–0.96). Older age increased the likelihood of completing screenings for most quality measures (Tables 2 and 3). However, colorectal cancer screening completion decreased with age (OR: 0.94; 95%CI: 0.92–0.97). Hispanic or Latino patients were more likely to be up to date on screenings compared to non-Hispanic/Latino patients (Tables 2 and 3).
DISCUSSION
We found that completion of all six primary care quality measures fell during the stay-at-home order compared to pre-pandemic rates. While the screening rates did increase for most quality measures upon lifting of the stay-at-home orders, the rates did not reach pre-pandemic levels by the end of phased reopening.
In the past several decades, a rise in preventative health measures has greatly reduced morbidity and mortality, improving patient outcomes.9 In our analysis of preventative health screening completion rates during the COVID-19 pandemic, significantly fewer patients successfully completed overdue health screenings during the stay-at-home period compared to the pre-pandemic period. Most notable was the change was for colorectal cancer (CRC) screening. In the nine-week stay-at-home period, the likelihood of completing CRC screening was 25% compared to pre-pandemic. While CRC screening during phased reopening did increase compared to stay-at-home, there remained a significant gap in completion rates compared to pre-pandemic–—patients were only 49% as likely to complete their due CRC screening (Table 2). At UCLA Health during the pandemic, colonoscopy suites were closed from 3/18/2020 to 5/8/2020.9 Upon reopening, COVID testing was mandatory prior to any procedure which may have posed additional barriers to preventative screening.
Our results were in line with similar studies that showed a decline in colorectal cancer screening during the late-winter and early-spring 2020 period of the COVID-19 pandemic.10,11 Based on a multitude of factors (patient fear of contracting SARS-CoV-2, reallocation of resources, and decreased access to primary care, imaging, and laboratory services), there have been delays in preventative colorectal cancer screening and thus delayed cancer detection. One potential solution being implemented by Kaiser Permanente of Southern California is mailing self-sampling fecal immunohistochemical tests (FIT) to patients.12 The USPSTF recommends colorectal cancer screening for adults aged 50 to 75 years through stool-based tests like FIT or direct visualization tests.13 In a randomized, controlled trial of 26,703 subjects, those assigned to a FIT were more likely to complete screening than were those assigned to a colonoscopy, though colonoscopies were able to identify more adenomas.14 As a temporary solution to postponing direct visualization tests during the pandemic, primary care providers may consider recommending FIT as an alternative for their patients, with colonoscopies prioritized for those who screen positive with FIT.15,16.
As hypothesized, the likelihood of patients completing most screenings increased from stay-at-home to phased reopening. Among the six quality measures, only cervical cancer screening rates continued to decline during phased reopening compared to stay-at-home. Per USPSTF guidelines, cervical cancer screening includes a combination of cervical cytology alone or cytology with high-risk human papillomavirus testing, depending on patient age. This recommended screening has significantly reduced mortality from cervical cancer in the USA.17 The rates of cervical cancer screening at our institution did not decrease as dramatically with the start of the stay-at-home period (OR 0.83 stay-at-home vs pre-pandemic) compared to the drop in completion seen with the other health screenings. A possible explanation for this trend is that compared to more frequent screenings like biennial mammograms, cervical cancer screening is every 3–5 years, which is a larger interval and may be less impacted by the pandemic.
These findings differ from another recent study of a large integrated healthcare system in Southern California, which found that cervical cancer cytology rates similarly declined with the stay-at-home order but then rose to near-baseline levels once the order was lifted.12 In this study at Kaiser Permanente, the authors speculated that the health system’s organized screening program (with reminder systems and tracking patients lost-to-follow-up) may have contributed to their quick recovery of screening rates once stay-at-home orders were lifted. As the peak of this pandemic regresses, timely outreach by clinic staff to patients due for follow-up will be vital to increase screening levels. Another potential option, if approved by the US Food and Drug Administration, is the implementation of human papillomavirus self-testing kits which have been found to be nearly as effective as in-office testing.18 These kits and other at-home modalities for cancer screening may be cost-efficient and effective solutions in the future of preventative health.
Our findings differ from prior studies that found that racial/ethnic minorities in the USA had lower rates of cancer screening.19 In our analysis, we found that Hispanic/Latino patients (compared to non-Hispanic) and Asian patients (compared to white) were more likely to complete all cancer screens and several diabetes exams. We designed our study to assess whether there were differences between our study groups; however, we unfortunately are not able to assess why these differences are present. A possible explanation for this difference is that everyone in our cohort had baseline access to healthcare and established care with primary care physicians. This may negate some of the health disparities observed in other studies.20
We hypothesized that an increase in patient age would reduce the likelihood of maintaining health screenings during the COVID-19 pandemic, since increased age is associated with increased risk for COVID-related complications. However, this trend was observed only for colorectal cancer screening, where increased age was associated with a reduction in colorectal cancer screening completion. Conversely, each increase in patient age by 5 years was associated with an increased likelihood to complete all other analyzed health screens. The increase in health screening maintenance with each 5-year increase in age may be explained by greater intervention at both the patient and provider level for older patients with greater risk for comorbidities during the pandemic.
We also anticipated that higher COVID-19 Risk Scores would be associated with lower health screening completion rates, since these patients would be at greater risk of developing severe complications from COVID. However, we found that as COVID Risk Scores increased, the likelihood of completing health care screenings increased. This suggests that patients with a higher risk for COVID-related complications were more likely to complete the screenings they were due for, perhaps because they were more accustomed to engaging with the health care system. We speculate that the benefit of managing comorbidities and avoiding subsequent adverse outcomes outweighed the risk of COVID-19 exposure for many of our patients.
A potential limitation of our study is that we cannot directly evaluate how patient-specific visit behaviors may have impacted the performance of each primary care quality measure. Additionally, the number of primary care visits per patient throughout our study was not readily available; therefore, we were unable to assess whether patients with higher primary care exposure overall completed more screenings during the study period. We suspect that access to care, especially in-person care, during our study period is a primary driver of the identified changes in quality performance.
The COVID-19 pandemic and associated stay-at-home orders abruptly interrupted healthcare delivery to patients across the USA. The CDC’s framework for the provision of non-COVID-19 healthcare during the pandemic recommended deferring routine primary care visits and screenings for asymptomatic conditions if care could not be delivered remotely, at least until community transmission risk decreased.21 These policies may have been appropriate for the containment of COVID-19, but it is evident from our results that the pandemic and associated stay-at-home policies did indeed significantly lower health maintenance and disease prevention screening and quality performance. Even after the removal of these policies, many patients remain overdue.
As this pandemic progresses, primary care physicians and their patients will need to adopt different strategies to ensure that patients continue to receive the best possible care. Increasing utilization of at-home FIT colorectal cancer screening and incorporating effective HPV screening kits may increase healthcare accessibility during the pandemic and provide less invasive screening options. Further work is needed to develop, evaluate, and scale effective home self-screening technologies that would reduce the need for frequent in-person office visits. For screenings and care that still require in-person visits, active patient outreach at the clinic level would aid in the recovery of preventative health efforts. Health systems should also continue to advance the use of online patient portals to increase patient engagement through regular messaging on important health topics and patient-specific gaps in care. Research expanding upon our study period to include subsequent waves of the COVID-19 pandemic would provide additional insight into the long-term effects of the pandemic and its associated policies (i.e., stay-at-home order). In the event of COVID-19 resurgences or other similar global crises, we hope that our findings will guide policymakers in weighing the benefits of health resource reallocation with the cost of delaying important preventative health and disease management efforts.
CONCLUSION
We observed a decline in health screening completion rates during the COVID-19 pandemic and associated stay-at-home orders at a large academic center in Southern California. Further research is needed to understand the long-term impacts of delayed health screenings and to develop new methods for implementing these screenings both in the clinic and the home. With continued surges of COVID-19, it is important for health care providers to develop strategies to continue providing the best care to their patients, even if they are unable to come to the clinic. Shifting care to telehealth when appropriate, utilizing at-home self-screenings, and expanding patient outreach and population health efforts are all critical to bridging care gaps during the pandemic and beyond.
Acknowledgements
The authors would like to acknowledge the UCLA Health Department of Medicine leadership and all the clinic teams for their dedication to this work.
Author Contribution
Conceptualization: D.M.C., E.K.; Data curation: D.M.C., S.V.; Formal analysis: S.V.; Investigation: E.K., D.M.C.; Methodology: E.K., N.K., D.M.C.; Project Administration: E.K., D.M.C.; Supervision: D.M.C.; Visualization: E.K., S.V., D.M.C.; Writing – Original Draft: E.K.; Writing – Reviewing & Editing: D.M.C., N.K., S.V., A.D., S.L., M.G., M.H.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Prior presentations: none
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. Published online January 29, 2020. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed]
- 2.Governor Gavin Newsom Issues Stay at Home Order. California Governor. Published March 20, 2020. Accessed February 14, 2021. https://www.gov.ca.gov/2020/03/19/governor-gavin-newsom-issues-stay-at-home-order/
- 3.Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation Guide for Rapid Integration of an Outpatient Telemedicine Program During the COVID-19 Pandemic. J Am Coll Surg. 2020;231(2):216–222.e2. doi: 10.1016/j.jamcollsurg.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler DM, Nikpay S, Huckman RS. The Business of Medicine in the Era of COVID-19. JAMA. 2020;323(20):2003. doi: 10.1001/jama.2020.7242. [DOI] [PubMed] [Google Scholar]
- 5.Croymans Daniel, Hurst Ian, Han Maria. Telehealth: The Right Care, at the Right Time, via the Right Medium. NEJM Catal Innov Care Deliv. Published online December 30, 2020. Accessed March 12, 2021. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0564
- 6.Hirko KA, Kerver JM, Ford S, et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020;27(11):1816–1818. doi: 10.1093/jamia/ocaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones D, Neal RD, Duffy SRG, Scott SE, Whitaker KL, Brain K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21(6):748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myint A, Roh L, Yang L, Connolly L, Esrailian E, May FP. Noninvasive Colorectal Cancer Screening Tests Help Close Screening Gaps During Coronavirus Disease 2019 Pandemic. Gastroenterology. 2021;161(2):712–714.e1. doi: 10.1053/j.gastro.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clin Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorin SNS, Jimbo M, Heizelman R, Harmes KM, Harper DM. The future of cancer screening after COVID-19 may be at home. Cancer. 2021;127(4):498–503. doi: 10.1002/cncr.33274. [DOI] [PubMed] [Google Scholar]
- 12.Miller MJ, Xu L, Qin J, et al. Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21–65 Years in a Large Integrated Health Care System — Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. Morb Mortal Wkly Rep. 2021;70(4):109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315(23):2576. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 14.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus Fecal Immunochemical Testing in Colorectal-Cancer Screening. N Engl J Med. 2012;366(8):697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 15.Corley DA, Jensen CD, Quinn VP, et al. Association Between Time to Colonoscopy After a Positive Fecal Test and Risk of Colorectal Cancer Stage at Diagnosis. JAMA. 2017;317(16):1631–1641. doi: 10.1001/jama.2017.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y-C, Fann JC-Y, Chiang T-H, et al. Time to Colonoscopy and Risk of Colorectal Cancer in Patients With Positive Results From Fecal Immunochemical Tests. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2019;17(7):1332–1340.e3. doi: 10.1016/j.cgh.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 17.US Preventive Services Task Force. Curry SJ, Krist AH, et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(7):674. doi: 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- 18.Arbyn M, Smith SB, Temin S, Sultana F, Castle P, Collaboration on Self-Sampling and HPV Testing Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823. doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124(2):315–332. doi: 10.1038/s41416-020-01038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benavidez GA, Zgodic A, Zahnd WE, Eberth JM. Disparities in Meeting USPSTF Breast, Cervical, and Colorectal Cancer Screening Guidelines Among Women in the United States. Prev Chronic Dis. 2021;18:E37. doi: 10.5888/pcd18.200315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. Healthcare Workers. Centers for Disease Control and Prevention. Published February 11, 2020. Accessed February 14, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/framework-non-COVID-care.html


