Abstract
Benzo[a]pyrene (BaP) is formed by incomplete combustion of organic materials (petroleum, coal, tobacco, etc.). BaP is designated by the International Agency for Research on Cancer as a group 1 known human carcinogen; a classification supported by numerous studies in preclinical models and epidemiology studies of exposed populations. Risk assessment relies on toxicokinetic and cancer studies in rodents at doses 5–6 orders of magnitude greater than average human uptake. Using a dose-response design at environmentally relevant concentrations, this study follows uptake, metabolism, and elimination of [14C]-BaP in human plasma by employing UPLC - accelerator mass spectrometry (UPLC-AMS). Volunteers were administered 25, 50, 100, and 250 ng (2.7–27 nCi) of [14C]-BaP (with interceding minimum 3-week washout periods) with quantification of parent [14C]-BaP and metabolites in plasma measured over 48 hours. [14C]-BaP median Tmax was 30 minutes with Cmax and area under the curve (AUC) approximating dose-dependency. Marked inter-individual variability in plasma pharmacokinetics following a 250 ng dose was seen with 7 volunteers as measured by the Cmax (8.99 ± 7.08 ng × mL−1) and AUC0–48hr (68.6 ± 64.0 fg × hr−1 × mL−1). Approximately 3–6% of the [14C] recovered (AUC0–48 hr) was parent compound, demonstrating extensive metabolism following oral dosing. Metabolite profiles showed that, even at the earliest time-point (30 min), a substantial percentage of [14C] in plasma was polar BaP metabolites. The best fit modeling approach identified non-compartmental apparent volume of distribution of BaP as significantly increasing as a function of dose (p=0.004). Bay region tetrols and dihydrodiols predominated, suggesting not only was there extensive first pass metabolism but also potentially bioactivation. AMS enables the study of environmental carcinogens in humans with de minimus risk, allowing for important testing and validation of physiologically based pharmacokinetic models derived from animal data, risk assessment, and the interpretation of data from high-risk occupationally exposed populations.
Keywords: Benzo[a]pyrene, Polycyclic Aromatic Hydrocarbons, Human Carcinogen Dosing, Accelerator Mass Spectrometry, Toxicokinetics in Humans
Graphical Abstract
1. Introduction
1.1. BaP formation and carcinogenicity
Incomplete combustion of carbon based materials such as petroleum products (Sadiktsis et al., 2012; Titaley et al., 2016), coal (Simoneit et al., 2007), wood (Li et al., 2011), and tobacco (Hecht, 1999; Staretz et al., 1997; Stepanov et al., 2010) produces hundreds of polycyclic aromatic hydrocarbons (PAHs) including benzo[a]pyrene (BaP). The International Agency for Research on Cancer classifies BaP as a Group 1 known carcinogen for humans (IARC, 2021). Found at 524 hazardous waste sites on the National Priorities List (NPL), BaP is ranked number 8 out of 275 chemicals on the Priority List of Hazardous Substances (ATSDR, 2020). While frequently occurring at NPL sites, BaP is ubiquitous in the environment and often used as an indicator compound to measure PAH exposure in humans and other animals (ATSDR, 2020; Bostrom, et al., 2002; Usenko et al., 2010, 2007). BaP is also included in U.S. EPA’s Persistent Bioaccumulative and Toxic Chemical Program where it is used as an index chemical for deriving Relative Potency Factors (BaP = 1) to estimate carcinogenicity of other individual PAHs or PAH mixtures (U.S. EPA, 2017, 2010, 2013). Many types of cancers are linked to BaP exposure along with developmental, immunological, cardiovascular, reproductive, and neurological toxicities (Miller and Ramos, 2001; Nebert, 1989; U.S. EPA, 2017).
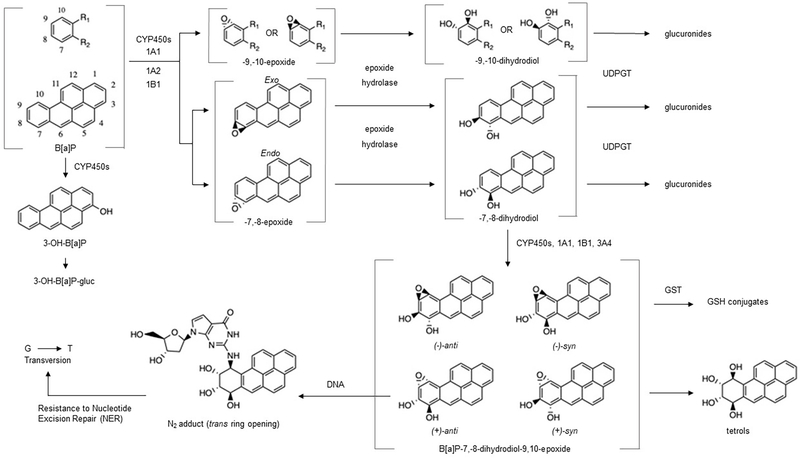
1.2. BaP metabolism and DNA adduction
Risk following exposure is largely determined by metabolism as BaP is bioactivated or detoxified by a number of phase 1 and phase 2 enzymes in various tissues (Bauer et al., 1995; Cavalieri and Rogan, 1995; Conney et al., 1994; Kim et al., 1998; Penning et al., 1996; Shimada et al., 1999; Shimada and Fujii-Kuriyama, 2004). Cytochromes P-450 in the 1 family (CYP1A1, 1A2, and 1B1) are the most efficient of the CYPs in activation of BaP by oxygenation at the bay region to form BaP-7,8-epoxide (endo or exo) (Bauer et al., 1995; Hecht et al., 2010; Kim et al., 1998; Shimada et al., 1999; Shimada and Fujii-Kuriyama, 2004). After action by epoxide hydrolase to form two trans-BaP-7,8-dihydrodiols ((±)-trans-BaP-DHD), a second epoxygenation by CYP1 produces (±)-trans/cis-BaP-7,8-dihydrodiol-9,10-epoxides (BaP-DHDE, 4 enantiomers with the (+)-anti-BaP-DHDE being the most carcinogenic (Figure 1)). The electrophilic BaP-DHDE covalently binds to deoxyguanine (dG) or deoxyadenine with a preference for the N2 position of dG resulting in G to T transversion (Bauer et al., 1995; Cavalieri and Rogan, 1995; Conney et al., 1994; Hecht et al., 2010; Kim et al., 1998; Meinl et al., 2008; Melendez-Colon et al., 1999; Penning et al., 1996; Shimada et al., 1999, 1997; Shimada and Fujii-Kuriyama, 2004). BaP is also an aryl hydrocarbon receptor (AHR) agonist and up-regulates CYP1, increasing the likelihood of CYP1-dependent bioactivation. Further, BaP-mediated AHR activation also stimulates the mitogen activated protein kinase (MAPK) signaling cascade, altering cellular processes including those fundamental to mitigating effects like BaP-induced DNA damage (Vázquez-Gómez et al., 2018).
Figure 1.
Metabolism of benzo[a]pyrene and adduction to DNA (formation of diones not depicted).
1.3. BaP levels in humans
Concentrations of BaP in blood, urine, and tissue, as well as covalent adducts, are used as biomarkers of BaP exposure and risk (Lodovici et al., 1998; Obana et al., 1981; Ross et al., 1995). Human exposure can occur as a result of environmental contamination, like an oil spill or wildfire, through ingestion, smoking, or occupational exposures such as asphalt paving or iron work (Brandt and Watson, 2003; Pastorelli et al., 2000; Tuominen et al., 2002). Roofers are exposed to coal tar dust as well as hot roofing tar. A study by Herbert et al. (1990) reported that BaP exposure (as per personal air monitors and skin wipes) in roofers averaged 0.8 μg/m3 resulting in a 10 fold increase in DNA adducts when compared to controls (1.3/108 nucleotides compared to 0.13/108 nucleotides, respectively). BaP has been identified in drinking water (4–24 ng/L; IPCS, 1998; Mumtaz, 1995), prepared meals (mean of 0.14 and a maximum of 1.15 μg/day; Butler et al., 1993)) and human milk (mean of 6.5 ng/kg, n = 51 in Japan; IPCS, 1998). Dietary intake from packaging, processing/cooking, and deposition from the atmosphere to food and water are the greatest contributors of BaP intake in the general human population (Bansal and Kim, 2015; Domingo and Nadal, 2015; Kazerouni et al., 2001). Human liver and fat samples from autopsied individuals have shown average concentrations of BaP to be 0.020 μg/kg (or 200 ng/kg, wet weight), with no significant differences noted between tissue, age, or sex (n=6; Obana et al., 1981). Rodent PBPK models have previously been published by our group (Crowell et al., 2011) and AMS studies suggest these can be applied for human low dose exposures (Madeen et al., 2015, 2016, 2019).
1.4. Accelerator Mass Spectrometry and dosing humans with carcinogens
Graphite AMS has been employed to determine DNA adduction in target tissues of numerous [14C]-labeled carcinogens, including aflatoxin B1 (AFB1), BaP, dibenzo[def,p]chrysene (DBC), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazole[4,5-b]pyridine (PhIP) (Table S1). Preliminary studies employing AMS with [14C]-BaP in human subjects suggested considerable metabolism as determined by plasma levels of the parent PAH following oral micro-dosing (Madeen et al., 2019). These studies measured only total [14C], however the coupling of UPLC and AMS by development of a “moving wire” interface now allows for the identification of metabolites (Thomas et al., 2011) and with UPLC–AMS the metabolic profile of [14C]-BaP can be determined. Further, the sensitivity of this method of detection allows for human studies with minimal risk, providing data on a known carcinogen at environmental levels.
Seven human volunteers were given escalating doses of [14C]-BaP (25 to 250 ng) to further investigate potential bioactivation and metabolism of the compound. Plasma extracts (0–48 hours) analyzed by UPLC-AMS revealed the extent of [14C]-BaP metabolism and inter-individual variability, plus identification and quantitation of ten metabolites. With a dose range of 25–250 ng we observed considerable first pass metabolism and metabolic profiles suggested bioactivation over detoxification. These observations offer insights into in situ human metabolism of a key PAH at actual environmental exposure concentrations, providing important information for future risk assessment and subsequent models.
2. Materials and methods
2.1. Human subjects
2.1.1. Recruitment, enrollment, and pre-dosing protocol
Seven subjects (5 males and 2 females, Table S2) ranging from 26 to 65 years old with BMIs of 19.0–33.3 were recruited and enrolled under the guidelines of an FDA-IND (#117175) as well as IRBs at both Oregon State University (OSU; #8233) and Lawrence Livermore National Laboratory (LLNL; Protocol #2017–008). The study details can be found at ClinicalTrials.gov (NCT03318978). Inclusion and exclusion criteria have been previously described in detail (Madeen et al., 2019). This study protocol required abstaining from consumption of charcoal-broiled meats, smoked meats and cheeses, as well as cruciferous vegetables and condiments for two weeks prior to administration of [14C]-BaP and throughout the 48 hour study duration. Dietary questionnaires (designed to assess total BaP consumption) were collected for the 3 days immediately preceding dosing through the final blood draw. Just prior to ingestion of [14C]-BaP, blood was drawn for determination of background levels of 62 PAHs by GC-MS/MS (Anderson et al., 2015). Doses were separated with a washout period of three weeks minimum with all four doses administered in less than one year.
2.1.2. Micro-dosing with [14C]-BaP, timed blood collection, and preparation of plasma
Participants fasted overnight prior to ingesting the morning capsule containing 25, 50, 100, or 250 ng of [14C]-BaP (2.7, 5.4, 10.8, or 27 nCi, respectively) with 200–250 ml water. Preparation of the food-grade [14C]-BaP capsule, as well as quality control of the amount and purity of [14C]-BaP, has been described previously (Hummel et al., 2018). Fasting continued for 2 hours following dosing. Blood (8–10 mL) was drawn into heparinized tubes prior to dosing (time 0) and at 0.25, 0.5, 1, 1.5, 2, 3, and 4 hours post-dose using an in-dwelling catheter placed in a vein in the antecubital area. Later blood collections at 8, 24, and 48 hours post-dose were done by needle stick. Blood was centrifuged (rcf`~1000 for 10 min) immediately following the draw and plasma extracted within 2 hours of collection.
2.2. Extraction of plasma and UPLC-AMS at LLNL
Following centrifugation, samples were held at 4°C until extraction with established methods (Crowell et al., 2011; Hummel et al., 2018). Briefly, plasma aliquots (1.5 mL) were acidified and vortexed with ethyl acetate (3x at 1.5 mL) in the presence of K2SO4. The ethyl acetate layers of each sample were pooled and, following evaporation to dryness under nitrogen, reconstituted with 100 μL ethyl acetate and stored at −80°C until shipped on dry ice to the BioAMS Center at LLNL or GC-MS/MS analysis by the Analytical Chemistry Core of the Oregon State University Superfund Research Program.
Just prior to UPLC-AMS analysis, plasma samples stored at −80°C were evaporated to dryness under vacuum and reconstituted in 50 or 100 μL acetonitrile depending on initial dose of [14C]-BaP (25 and 50 ng into 50 μL with 100 and 250 ng in 100 μL, respectively). Samples were maintained in the autosampler of a Waters Acquity UPLC H-Class System at 20°C, the column at 28°C, and injection volume set at 10 μL. A Waters Acquity UPLC BEH C18 1.7 μm 2.1 × 50 mm column with a Vanguard HSS PFP 1.8 μm 2.1 × 5 mm guard column was used for separation with 0.3% formic acid in water (A) and PEG-free acetonitrile (B). Elution of [14C]-BaP and metabolites was achieved over 20 min at 0.25 mL/minute utilizing a gradient (0.0 min 70:30 A:B; 0.1 60:40; 10.0 54:46; 10.1 43:57; 14.3 to 16.3 0:100; 16.4 to 20 min 70:30). [14C]-BaP metabolites were identified by retention time using analytical standards obtained from Oregon State University Superfund Program PAH repository (http://limsweb.science.oregonstate.edu/).
Elution times of metabolites and parent compound included in the analytical standard are in Table 1.
Table 1.
Elution time of BaP and BaP metabolite standards.
| Compounds | Elution Time (min) | Compounds | Elution Time (min) |
|---|---|---|---|
|
| |||
| BaP tetrols (mix) | 2.16 | 6,12 - dione BaP | 9.50 |
| 9,10 - dihydrodiol BaP | 2.68 | 7,8 - dione BaP | 10.23 |
| 4,5 - dihydrodiol BaP | 4.20 | 9 - OH BaP | 12.59 |
| 7,8 - dihydrodiol BaP | 4.56 | 3 - OH BaP | 12.95 |
| 1,6 - dione BaP | 7.40 | [14C] - BaP / BaP | 15.01 |
| 3,6 - dione BaP | 8.16 | ||
After passing through a PDA detector, the UPLC eluate was split with 0.12 mL/min (48%) deposited onto the moving wire interface for AMS analysis with the rest sent to waste. The eluate was carried by periodically indented nickel wire through a drying oven then a combustion oven, evaporating solvent and combusting analytes to [14C]-CO2, allowing transfer to the CO2-gas-accepting ion source of the 250 kV AMS (Ognibene et al., 2019; Thomas et al., 2011). Measurements of 13C and 14C with known isotope ratios allow for calculations of [14C]-BaP and [14C]-metabolite concentrations. Prior to injection of each sample the precision and accuracy of the moving wire interface and AMS were confirmed using an aqueous sucrose reference solution (Keck et al., 2010; Ognibene et al., 2015). Established validation parameters include accuracy (1–3%), precision (expressed as coefficient of variation, 1–6%), and sensitivity expressed as limit of detection (LOD) of 1 attomole of [14C] (0.58 fg for [14C]-BaP) (Keck et al., 2010). The lower limit of quantitation (LOQ, 0.60 fg/mL) was established using extracted plasma blanks from each individual and measurements at the known elution times for each compound.
2.2.1. UPLC-AMS separation, detection, and identification
Biological samples naturally contain carbon isotopes that can change background levels of AMS measurements of 12C, 13C, and 14C and these vary by source article, individual, and sample type (Keck et al., 2010; Salehpour et al., 2008). AMS counts individual 14C atoms in relation to 12C and/or 13C ion currents with extremely high sensitivity and any variation in the background signal of isotopes can change the degree of confidence of LOQ. To increase confidence, a matrix-matched LOQ was determined using the standard deviation of the signal in the blank samples (t = 0 h) from each participant. These extracted blanks have the same matrix effects as study samples and by time-matching the signal to noise ratio from blanks and study samples the LOQ takes into account whether the peaks seen are the compounds of interest or are part of the background noise.
Subsequently, the calculated LOQ for each compound showed little variation and values were rounded up to a single method LOQ of 0.60 fg/mL plasma (calculated range 0.55–0.59 fg/mL for all compounds) that was consistent and uniform across all participants. With AMS analysis the 14C naturally present in the plasma blanks is measured with the same precision as a low-signal sample, supporting this method of determining the LOQ. (FDA, 1995; Keck et al., 2010)
The radiolabeled carbon detected is either part of the naturally occurring [14C] in humans or from the dosed [14C]-BaP. Due to the unavailability of radiolabeled BaP metabolite standards, chromatography and metabolite identification cannot be confirmed with AMS directly. In order to use BaP metabolite standards with known retention times to identify metabolites, the UPLC eluate went to a PDA detector and a moving wire interface for AMS analysis, however not all peaks seen using UPLC-AMS could be identified using the analytical standard mix.
Unknown A eluted at the beginning of the chromatography run (0.48 min) and is likely composed of conjugated metabolites. BaP phenols, dihydrodiols, dihydrodiol-epoxides and diones are all subject to conjugation (glucuronides, sulfates, and glutathione) and would be the most polar BaP metabolites in plasma. Further research is in progress to separate and identify conjugates in both the urine and plasma of participants. Based on retention time Unknowns B (3.16 min) and C (4.85 min) are possibly additional dihydrodiols. Unknown D (5.39 min) is either a late eluting dihydrodiol or a dione. The peak identified as the 3-phenol may also contain small amounts of the minor metabolites 7- and 1-BaP phenols as these hydroxylated compounds co-elute if present.
2.3. Pharmacokinetic analysis
Pharmacokinetics of [14C]-BaP, metabolites, and unknowns were evaluated using non-compartmental analyses. The area under the curve (AUC) of [14C]-BaP and metabolite concentrations in plasma from time zero to the last measured time point or extrapolated to infinity ((AUC0−∞) using the last 3 time points) were calculated using the trapezoidal rule (Gibaldi and Perrier, 1982). Mean residence times were calculated as a ratio of AUC under the first moment curve extrapolated to infinity to AUC extrapolated to infinity. Non-compartmental half-lives were calculated as the product of mean residence times with the natural log of two.
Pharmacokinetics of parent [14C]-BaP was also evaluated using compartmental analysis. A two-compartment pharmacokinetic model was used to evaluate the time course of [14C]-BaP (Equations 1–3), where “A0”, “A1”, and “A2” represent the amount (fg) of [14C]-BaP in the absorption, central, and peripheral compartments, respectively; “ka”, “ke”, “k12”, and “k21” are first-order rate constants. The concentration of the central compartment (C1) was calculated by normalizing the amount with the apparent volume of distribution (V1, L, Equation 4). Optimizations of model parameters were obtained using a maximum log likelihood objective, and all data points were weighted equally when fitting. Initial values were set by adjusting parameters visually.
| (1) |
| (2) |
| (3) |
| (4) |
Pharmacokinetic parameters were evaluated for linearity using a best fit modeling approach. First, pharmacokinetic parameters were evaluated for significant change as a function of dose using a standard linear regression model including a fit y-intercept. Slopes were compared using a t-test and an alpha value of 0.05. If the parameter changed as a function of dose, we further evaluated the parameter with a linear regression model (Equation 5), where k is the slope through the origin, and a Michaelis-Menten model (Equation 6), which assumes saturation at Vmax and an affinity constant K, to individual parameters (P) as a function of external [14C]-BaP dose. The Bayesian information criterion (BIC) was used to judge the best-fit model and provide evidence of linear or saturable pharmacokinetics (Schwarz, 1978).
| (5) |
| (6) |
Software used to analyze data was “R: A language and environment for statistical computing” Version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results and discussion
3.1. Background levels of PAHs in plasma
Blood samples were drawn prior to each [14C]-BaP dose to assess background levels of 62 PAHs and alkylated PAHs commonly found in environmental mixtures including food (Anderson et al., 2015). Table 2 shows plasma levels of the 17 PAHs detected above the LOD in all seven participants. Variation was high, with no statistically significant differentiation of overall PAH concentration between participants. No PAHs with assigned RPFs for cancer were detected. Naphthalene and retene were the only PAHs present in all samples (7 subjects at 4 doses, n = 28) and below LOD in all quality control samples.
Table 2.
Plasma concentrations of background PAHs from all participants at the beginning of each cycle (n = 28). Plasma samples were collected between 9:00–10:00 AM following an overnight fast. The GC-MS/MS method employed quantifies 62 parent and alkylated PAHs; 49/62 PAHs were below the LOD (each similar to the ranges shown for detected PAHs). Non-detect compounds and those below the LOD were omitted. Full list of compounds, CAS numbers, and LODs provided in Table S3.
| Compound | Range (ng/mL) | Mean (ng/mL) | Number of samples above LOD (n=28) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|
| 1-methylnaphthalene | 0.07 – 0.29 | 0.13 ± 0.07 | 8 (28.57%) | 0.06 | 0.3 |
| 1-methylpyrene | 0.32 – 4.73 | 0.79 ± 0.97 | 19 (67.86%) | 0.08 | 0.4 |
| 2,6-dimethylnaphthalene | 1.00 – 4.01 | 2.17 ± 1.01 | 11 (37.29%) | 0.2 | 0.9 |
| 2-methylanthracene | 1.58 | / | 1 (3.57%) | 0.09 | 0.5 |
| 2-methylnaphthalene | 0.16 – 0.99 | 0.41 ± 0.32 | 10 (35.71%) | 0.1 | 0.7 |
| 2-methylphenanthrene | 4.95 | / | 1 (3.57%) | 0.08 | 0.4 |
| 6-methylchrysene | 1.26 | / | 1 (3.57%) | 0.2 | 0.9 |
| 7,12-dimethylbenz[a]anthracene | 4.00 | / | 1 (3.57%) | 0.2 | 0.9 |
| 9-methylanthracene | 0.64 | / | 1 (3.57%) | 0.2 | 0.9 |
| benzo[c]fluorene | 0.53 | / | 1 (3.57%) | 0.06 | 0.3 |
| benzo[k]fluoranthene | 1.54 | / | 1 (3.57%) | 0.1 | 0.5 |
| fluoranthene | 0.9 | / | 1 (3.57%) | 0.1 | 0.5 |
| fluorene | 0.13 – 1.36 | 0.48 ± 0.32 | 12 (42.86%) | 0.2 | 0.8 |
| naphthalene | 0.16 – 2.29 | 0.84 ± 0.55 | 28 (100%) | 0.2 | 1.0 |
| phenanthrene | 0.64 | 0.47 ± 0.16 | 5 (17.86%) | 0.09 | 0.5 |
| pyrene | 0.13 – 6.8 | 1.94 ± 2.35 | 7 (25%) | 0.08 | 0.4 |
| perylene | 23.9 – 52.2 | 35.43 ± 12.23 | 4 (14.29%) | 1.0 | 5.0 |
| retene | 1.01 – 57.8 | 8.81 ± 10.66 | 28 (100%) | 0.2 | 0.8 |
PAHs in multiple plasma samples exhibited wide concentration ranges. For example, 1-methylpyrene ranged from 0.32 – 4.73 ng/mL (n = 19; 0.79 ± 0.97 ng/mL). Fluorene, perylene, alkyl-naphthalenes, phenanthrenes, and pyrenes were also present in a number of samples. In vitro studies and animal models have shown that previous exposure and/or co-exposure to other PAHs can alter the metabolism and excretion of BaP (Courter et al., 2007; Jarvis et al., 2014). Assessment of how participants were potentially impacted by previous or co-exposure of PAHs is out of the scope of this study and limited by size of the study pool. No BaP was detected in any background plasma samples (LOD 0.24 ng/mL; n = 28). For comparison, in study subjects dosed with an environmentally relevant dose of 250 ng [14C]-BaP, the Cmax averaged 8.99 ± 7.08 fg/mL (range, 2.16–23.29 fg/mL), or 36 ± 28 fM, 0.0009–0.01% of the LOD for background BaP as assessed by GC-MS/MS.
None of the known or probable carcinogenic PAHs, including BaP were detected perhaps due, in part, to our exclusion criteria and the dietary PAH restrictions required. For comparison, a study by Pleil et al. (2010) measured levels of 22 PAHs in two separate healthy populations. These study groups included non-smoking university students and non-smoking adults from the general population with no recent occupational or other known PAH exposures. The median (and range) of BaP was 0.004 (≤LOD-0.017) and 0.019 (≤LOD-0.195) ng/mL in plasma from the students and adult general population, respectively (Pleil et al., 2010).
3.2. [14C]-BaP pharmacokinetics
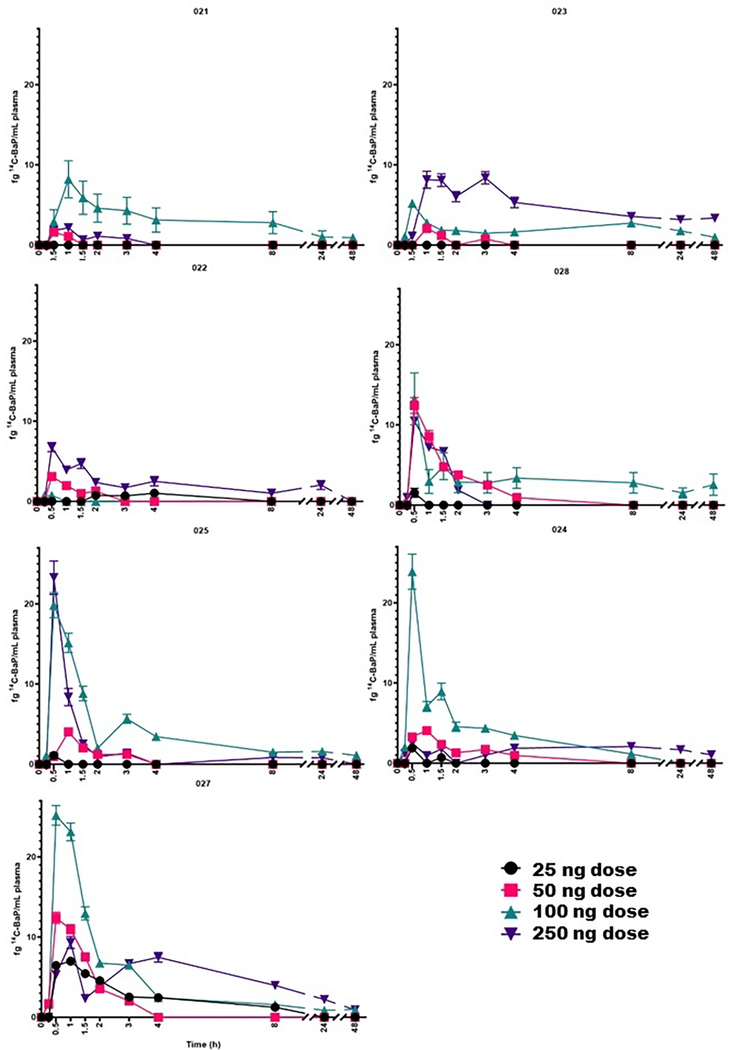
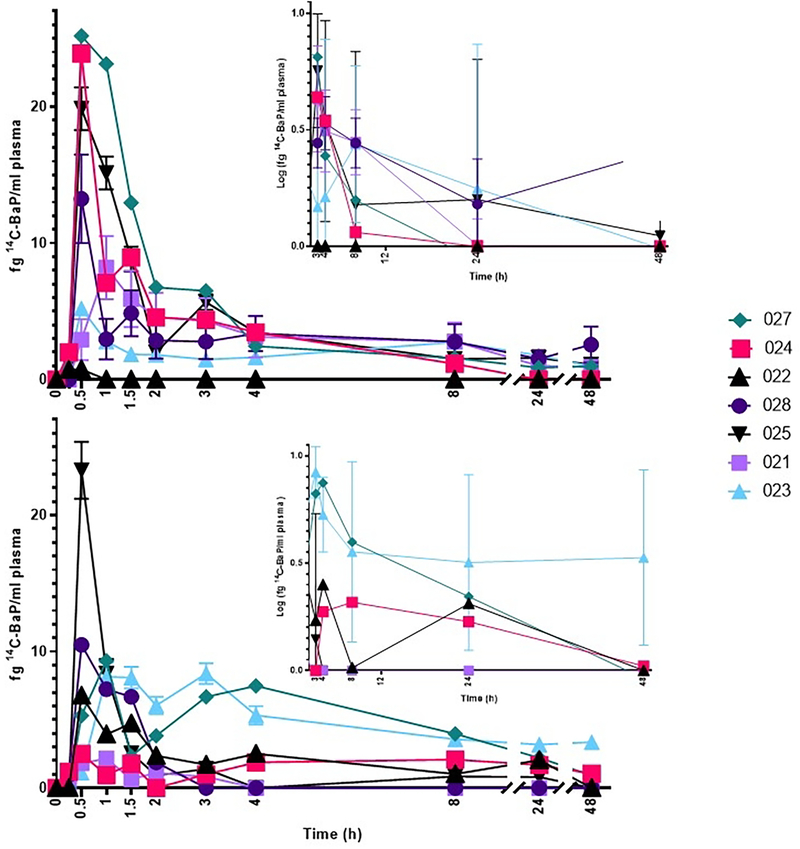
Absorption of BaP was rapid (Figure 2), with a median Tmax of 0.5 hours following each dose (Table 3). The BaP Cmax (Table 3) ranged from 2.51 ± 2.53 to 13.8 ± 9.52 fg BaP/mL) over the dose range of 25–250 ng. Interestingly, the largest Cmax and AUC0–48h (with AUC0–48h ranging from 12.2 ± 14.5 to 88.5 ± 14.1 fg BaP/mL x h, respectively in the non-compartmental model) is observed at the 100 ng dose and not the largest dose of 250 ng [14C]-BaP. In a preliminary study (2 subjects) employing UPLC-AMS, following a 46 ng dose, the Tmax and Cmax for [14C]-BaP was 0.75–1.0 h and 2.2–4.1 fg/mL, respectively. The closest comparable dose in the present study was 50 ng which gave a Tmax (0.5–1.0 h) and Cmax of (5.7 ± 4.7 fg/mL) which is in good agreement. A notable finding from the Madeen et al. (2019) study was, although as in this study a wide inter-individual variability between subjects was apparent, the intra-individual variability was low (even with over a year between doses). The large inter-individual variability is readily apparent at the two highest doses of 100 and 250 ng [14C]-BaP (Figure 3) where an order of magnitude difference between the highest and lowest Cmax and AUC0–48hr is apparent. As in previous AMS studies with both [14C]-BaP and [14C]-DBC, there was no obvious correlation to sex, age, or BMI, plus the sample size is too small for robust analysis to make strong conclusions (Hummel et al., 2018; Madeen et al., 2015, 2016, 2019). Comparison of the AUC (either AUC0–48 hr or AUC0−∞) values for [14C]-BaP (Table 3) to previous results for [14C]-BaPeq is more tenuous given that the latter encapsulates all metabolites and potential unknown metabolites in addition to parent [14C]-BaP.
Figure 2.
Plasma levels of [14C]-BaP (fg/mL) as a function of dose in each participant as determined by UPLC-AMS over 0–48 hours. [14C]-BaP (fg/mL plasma) as a function of dose (25, 50, 100, and 250 ng) in each participant (023, 021, 024, 028, 026, 022, and 027; left to right, top to bottom) at each time point (0.25, 0.5, 1, 1.5, 2, 3, 4, 8, 24, and 48h).
Table 3.
Summary of [14C]-BaP pharmacokinetics as a function of dose, estimated using a non-compartmental model. The results are given as mean ± standard deviation except Tmax, which is the median Tmax for the dose and includes all seven participants and all quantifiable [14C]-BaP data points (n=170). The number listed under each parameter is the number of [14C]-BaP data curves used to estimate the parameter across all participants (potential n=7, one data curve for each participant). Curves were excluded due to an inadequate number of data points (less than 3) for a dose/individual.
| Dose (ng) | ||||
|---|---|---|---|---|
| Parameter | 25 | 50 | 100 | 250 |
|
| ||||
| Cmax (fg BaP/mL) | 2.51 ± 2.53 n = 5 |
5.68 ± 4.70 n = 7 |
13.8 ± 9.52 n = 7 |
8.99 ± 7.08 n = 7 |
|
| ||||
| Tmax (h) (Median) (Range) | 0.5 0.5 – 4.0 |
0.5 0.5 – 1.0 |
0.5 0.5 – 1.0 |
0.5 0.5 – 3.0 |
|
| ||||
| AUC (fg BaP/mL×h) | 12.2 ± 14.5 n= 2 |
19.6 ± 15.7 n = 5 |
88.5 ± 14.1 n = 6 |
68.6 ± 64.0 n = 7 |
|
| ||||
| AUC0-∞ (fg BaP/mL×h) | 30.8 ± NA n = 1 |
29.1 ± 22.5 n = 5 |
151 ± 54.0 n = 5 |
6,600 ± 1,260 n = 5 |
|
| ||||
| Half-life (h) | 4.31 ± NA n = 1 |
9.33 ± 12.7 n = 5 |
42.4 ± 21.0 n = 5 |
130 ± 243 n = 5 |
|
| ||||
| Clearance (L/h) | 811 ± NA n = 1 |
2,970 ± 2,270 n = 5 |
725 ± 230 n = 5 |
4,810 ± 6,950 n = 5 |
|
| ||||
| Vol. of Distribution (L) | 5,040 ± NA n = 1 |
20,000 ± 14,800 n = 5 |
39,200 ± 6,810 n = 5 | 77,000 ± 51,000 n = 5 |
Figure 3.
Individual variability in plasma levels of [14C]-BaP from 0.25–48 hours at doses of 100 (top) and 250 ng (bottom) as determined by UPLC-AMS. Inset figures at each dose illustrate the variability of the date with log concentration of 14C-BaP over time (3–48 hours). Time point 0 h had no [14C]-BaP in any participant at any dose. Legends are consistent, with each participant represented by the same color and shape in both figures.
The best fit modeling approach identified non-compartmental apparent volume of distribution of BaP as significantly increasing as a function of dose (p=0.004, Table S5). Mean non-compartmental apparent volume of distributions increased 15× from in 25 to 250 ng BaP dose groups. Although not statistically significant (p=0.051), compartmental model apparent volume of distributions also demonstrated an increasing trend with dose (Table S4), where apparent volume of distributions increased 4.6× in 25 to 250 ng BaP dose groups. Since volunteers were the same in each dose group, and volunteer body weights were not significantly altered over the duration of the experiment (6.7 kg maximum weight change, 2.31 ± 2.28 kg), this observation suggests the fraction of BaP absorbed decreased as a function of dose. BaP likely existed as a solid in the administered capsule also containing lactose. As such, the lipophilic BaP would need to be solubilized to absorb, and larger masses of BaP may have required more time for complete dissolution and absorption if the particle surface area did not increase in the same proportion as the BaP mass. Large apparent volumes of distribution (thousands of liters, Tables 3 and 4) calculated in both approaches indicated that BaP was not well absorbed. Another possible explanation for dose-dependent increases in apparent volume of distribution is saturating a plasma protein binding site for BaP, allowing for greater BaP distribution into tissues at higher doses and higher apparent volume of distribution. Due to the low measured concentrations of BaP in blood (fg/mL), this biding site would require high specificity for BaP (Kd <100 fM) and have relatively low abundance in plasma (~250 fmol total binding sites). Due to these unlikely theoretical constraints, we do not think saturation of plasma protein binding sites is plausible. The absorption rate (ka) of [14C]-BaP was slightly greater than in two different individuals examined in a previous study (Madeen et al., 2019), which may have limited precision due to the few number of data points defining the constant in this study. Future analyses of urinary BaP metabolites will provide additional evidence on extent of BaP oral absorption and implications of dose on fraction absorbed.
Table 4.
Summary of [14C]-BaP pharmacokinetics as a function of dose, estimated using a two-compartmental model. Includes all seven participants and all quantifiable [14C]-BaP data points (n=170). The number listed under each parameter is the number of [14C]-BaP data curves used to estimate the parameter across all participants (n=5, 6, or 7); curves were excluded due to an inadequate number of data points for a dose/individual. The results are given as the mean ± standard deviation except 25 ng dose (n = 1).
| Dose (ng) | ||||
|---|---|---|---|---|
| Parameter | 25 | 50 | 100 | 250 |
|
| ||||
| ka (1/h) | 592 ± NA | 1.30 × 107 ± 2.90 × 107 n = 5 |
3.52 ± 4.75 n = 6 |
43.2 ± 111 n = 7 |
|
| ||||
| k12 (1/h) | 0.331 ± NA | 0.83 ± 1.11 n = 5 |
2.60 ± 3.45 n = 6 |
4.27 ± 6.92 n = 7 |
|
| ||||
| k21 (1/h) | 0.028 ± NA | 0.270 ± 0.253 n = 5 |
0.190 ± 0.180 n = 6 |
0.290 ± 0.564 n = 7 |
|
| ||||
| k1e (1/h) | 0.0068 ± NA | 0.694 ± 0.412 n = 5 |
0.558 ± 0.897 n = 6 |
1.25 ± 1.71 n = 7 |
|
| ||||
| Vol. of Distribution (L) | 2944 ± NA | 4423 ± 2699 n = 4 |
2419 ± 2498 n = 5 |
10732 ± 7844 n = 6 |
|
| ||||
| α (1/h) | 0.366 ± NA | 1.69 ± 0.89 n = 5 |
3.33 ± 4.28 n = 6 |
5.80 ± 6.64 n = 7 |
|
| ||||
| α half-life (h) | 1.892 ± NA | 0.484 ± 0.187 n = 5 |
0.400 ± 0.212 n = 6 |
0.361 ± 0.407 n = 7 |
|
| ||||
| β (1/h) | 5.03 × 10−4 ± NA | 0.105 ± 0.164 n = 5 |
0.020 ± 0.021 n = 6 |
0.014 ± 0.019 n = 7 |
|
| ||||
| β half-life (h) | 1307 ± NA | 37.86 ± 43.24 n = 5 |
84.1 ± 88.0 n = 6 |
12070 ± 24100 n = 7 |
All other non-compartmental and compartmental pharmacokinetic parameters of BaP concentrations in plasma did not change significantly as a function of administered BaP dose (p≥0.10 Tables S4 and S5). This observation is counter to an expected increasing AUC and Cmax with dose. In contrast, for most of the individual BaP metabolites (Table S6) and a sum of all BaP metabolites (Table S7, a significant linear increase as a function of dose was observed for AUC and Cmax. This suggests that increasing BaP metabolism as a function of dose may play a role in the observed trends. Non-significant changes in BaP AUC and Cmax may be obscured by high variability of BaP concentration in blood data (especially at the high BaP dose (250 ng)), due to potentially prolonged and highly variable fraction of BaP absorbed (perhaps exacerbated at higher doses), and hypothesized dose-dependent decrease in fraction of BaP absorbed.
3.3. [14C]-BaP metabolite profiles
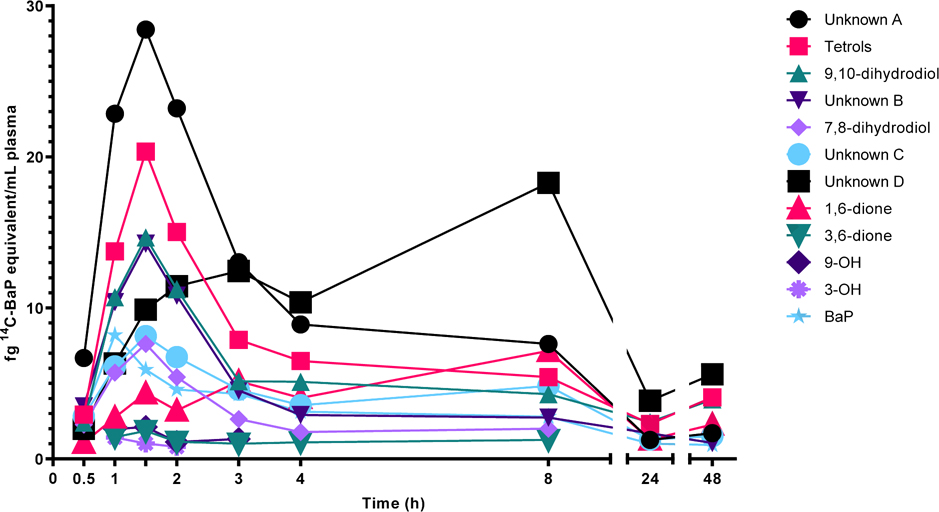
Plasma [14C]-BaP metabolites were measured even at the earliest time point assessed (0.25 hours). A representative plasma metabolite profile from a single individual over 0–48 hours following administration of the 100 ng dose is shown in Figure 4. Plasma [14C]-BaP metabolites were first detected at 0.5 hours. The greatest yield of metabolites were tetrols (specific stereoisomers unknown) and dihydrodiols (7,8- and 9,10- with perhaps some 4,5-dihydrodiol). The small amount of 3-hydroxy-[14C]-BaP (in addition to the 4,5-dihydrodiol which is possibly unknown peak C) suggest that metabolism at the bay region predominated. Formation of the mutagenic and carcinogenic bay region 7,8-dihydrodiol-9,10-epoxide and subsequent covalent binding to DNA is a biomarker of risk. In our previous study (46 ng [14C]-BaP dose) we found 2/4 subjects had detectable (6–7 adducts per 1011 bp, LOD of 0.5 per 1011 bp) DNA adduction in PBMCs at 2–3 hours but little or no adduction at 48–72 hours (Madeen et al., 2019). For comparison, BaP-DNA adducts in occupationally exposed populations are typically 1–10 per 108 bp (Pavanello et al., 2005). Tetrols, the result of hydrolysis of BaP-7,8-DHD-9,10-E have been used as a urinary biomarker of BaP cancer risk (Zhong et al., 2011). The 1,6-dione was present along with 2 unknown peaks that eluted in the region of the chromatogram corresponding to the 3,6-, 6,12- and possibly the 7,8-dione. [14C]-BaP and metabolites were markedly reduced in plasma 24–48 hours after dosing (Figures 3 and 4). Inter-individual variation in metabolism is shown in Figure S1. If glucuronide and sulfate conjugation were a significant fraction of metabolites, one could expect enterohepatic recirculation following bacterial β-glucuronidase and/or sulfatase cleavage and reuptake of the phenol, dihydrodiol, or tetrol into plasma. The only metabolite exhibiting what could be interpreted as reuptake is unknown D. At these very low concentrations there may not be active deconjugation in intestine. Individual pharmacokinetic constants for [14C]-BaP metabolites are given in Table 5.
Figure 4.
Plasma levels of [14C]-BaP and [14C]-BaP metabolites from a single representative participant at 0.5–48 hours following a dose of 100 ng [14C]-BaP. No parent compounds were quantified prior to 0.5 h in plasma extractions from this participant.
Table 5.
Summary of pharmacokinetic parameter estimates of [14C]-BaP metabolites in volunteers, estimated using a non-compartmental model. Results are listed as mean ± standard deviation except Tmax, which is the median Tmax for the dose/compound and includes all seven participants and all quantifiable data points.
| Compound | Dose (ng) | Cmax (fg BaP/mL) | Tmax (h) | AUC (fg BaP/mL×h) | AUC0-∞ (fg BaP/mL×h) | Half-life (h) |
|---|---|---|---|---|---|---|
|
| ||||||
| Unknown A | 25 | 5.06 ± 3.24 | 1.5 | 29.8 ± 20.8 | 45.9 ± 30.8 | 9.79 ± 5.23 |
| 50 | 11.3 ± 9.47 | 1.5 | 73.1 ± 63.9 | 187 ± 267 | 69.3 ± 114 | |
| 100 | 38.7 ± 16.2 | 1.0 | 352 ± 201 | 514 ± 296 | 28.1 ± 23.0 | |
| 250 | 54.9 ± 36.1 | 1.5 | 522 ± 477 | 653 ± 614 | 16.7 ± 7.71 | |
|
| ||||||
| Tetrols | 25 | 3.14 ± 0.84 | 2.0 | 43.1 ± 39.2 | 85.0 ± 53.8 | 31.4 ± 26.4 |
| 50 | 7.93 ± 5.23 | 1.5 | 64.5 ± 54.6 | 110.0 ± 83.2 | 25.7 ± 22.4 | |
| 100 | 32.2 ± 20.6 | 1.5 | 416 ± 316 | 676 ± 429 | 45.3 ± 54.2 | |
| 250 | 33.9 ± 19.4 | 1.5 | 475 ± 432 | 672 ± 598 | 24.4 ± 22.0 | |
|
| ||||||
| 9,10-dihydrodiol | 25 | 2.33 ± 0.90 | 2.0 | 18.6 ± 14.6 | 25.9 ± 25.3 | 19.1 ± 14.5 |
| 50 | 6.88 ± 4.01 | 1.5 | 47.7 ± 33.9 | 105 ± 58.2 | 43.2 ± 38.3 | |
| 100 | 21.7 ± 14.5 | 1.0 | 249 ± 175 | 2040 ± 4130 | 336 ± 724 | |
| 250 | 24.6 ± 11.9 | 1.0 | 298 ± 173 | 505 ± 474 | 33.3 ± 40.2 | |
|
| ||||||
| Unknown B | 25 | 2.12 ± 0.42 | 1.5 | 5.93 ± 2.60 | 12.9 ± 7.86 | 5.22 ± 2.79 |
| 50 | 7.39 ± 7.02 | 1.5 | 26.7 ± 29.1 | 107 ± 221 | 27.9 ± 57.7 | |
| 100 | 21.4 ± 16.1 | 1.0 | 156 ± 124 | 185 ± 129 | 19.4 ± 9.58 | |
| 250 | 24.8 ± 14.7 | 1.0 | 176 ± 141 | 215 ± 169 | 14.2 ± 8.23 | |
|
| ||||||
| 7,8-dihydrodiol | 25 | 1.24 ± 1.24 | 1.5 | 3.90 ± 2.11 | 13.8 ± 11.0 | 8.13 ± 5.96 |
| 50 | 3.25 ± 3.25 | 1.5 | 24.7 ± 23.2 | 24.1 ± 18.8 | 9.08 ± 8.41 | |
| 100 | 13.9 ± 13.9 | 1.0 | 127 ± 125 | 184 ± 142 | 25.1 ± 17.9 | |
| 250 | 17.2 ± 17.2 | 1.5 | 155 ± 155 | 186 ± 176 | 13.3 ± 6.93 | |
|
| ||||||
| Unknown C | 25 | 1.40 ± 0.34 | 3.0 | 17.6 ± 19.8 | 31.9 ± 30.7 | 14.3 ± 11.1 |
| 50 | 3.29 ± 2.21 | 2.0 | 44.4 ± 36.4 | 80.8 ± 77.3 | 27.5 ± 23.3 | |
| 100 | 12.8 ± 10.5 | 1.5 | 250.0 ± 241 | 401 ± 326 | 35.7 ± 27.4 | |
| 250 | 16.3 ± 10.7 | 1.5 | 264 ± 261 | 500.4 ± 639 | 27.0 ± 33.1 | |
|
| ||||||
| Unknown D | 25 | 2.63 ± 1.15 | 6.0 | 56.5 ± 41.0 | 97.1 ± 50.9 | 22.8 ± 9.01 |
| 50 | 5.75 ± 3.33 | 3.0 | 138 ± 88.8 | 209 ± 107 | 34.7 ± 26.5 | |
| 100 | 18.08 ± 10.0 | 4.0 | 451 ± 269 | 136 ± 148 | 60.4 ± 63.8 | |
| 250 | 16.6 ± 10.6 | 2.0 | 389 ± 337 | 426 ± 390 | 25.4 ± 11.6 | |
|
| ||||||
| 1,6-dione | 25 | 1.17 ± 0.24 | 4.0 | 14.3 ± 16.0 | 21.4 ± 13.4 | 14.4 ± 6.69 |
| 50 | 2.63 ± 1.96 | 3.0 | 44.7 ± 38.5 | 124 ± 45.7 | 69.2 ± 57.1 | |
| 100 | 6.56 ± 3.06 | 1.5 | 90.6 ± 46.8 | 208 ± 172 | 57.4 ± 61.0 | |
| 250 | 7.88 ± 4.63 | 1.0 | 107 ± 27.0 | 363 ± 459 | 108 ± 188 | |
|
| ||||||
| 3,6-dione | 25 | NA ± NA | NA | NA ± NA | NA ± NA | NA ± NA |
| 50 | 0.85 ± 0.24 | 1.5 | 10.3 ± 12.7 | 350 ± 485 | 315 ± 439 | |
| 100 | 1.62 ± 0.694 | 1.5 | 17.09 ± 23.8 | 12.0 ± 7.65 | 4.79 ± 1.74 | |
| 250 | 2.22 ± 1.09 | 1.0 | 9.66 ± 6.54 | 30.6 ± 44.6 | 12.6 ± 20.4 | |
|
| ||||||
| 9-OH | 25 | 0.66 ± 0.06 | 0.5 | NA ± NA | NA ± NA | NA ± NA |
| 50 | 1.90 ± 2.28 | 1.0 | 9.53 ± NA | 10.79 ± NA | 1.19 ± NA | |
| 100 | 8.13 ± 6.96 | 1.0 | 21.9 ± 17.9 | 104 ± 213 | 67.6 ± 167 | |
| 250 | 6.26 ± 3.64 | 0.75 | 32.3 ± 18.9 | 65.05 ± 64.9 | 30.3 ± 49.2 | |
|
| ||||||
| 3-OH | 25 | 1.47 ± 0.74 | 1.0 | 1.88 ± 0.47 | 21.04 ± 27.9 | 14.02 ± 20.5 |
| 50 | 3.12 ± 2.83 | 1.25 | 8.50 ± 6.07 | 11.0 ± 6.38 | 2.28 ± 0.51 | |
| 100 | 9.48 ± 6.63 | 1.0 | 20.3 ± 18.4 | 30.7 ± 19.6 | 8.030 ± 11.2 | |
| 250 | 9.34 ± 6.91 | 1.5 | 35.7 ± 28.9 | 63.09 ± 62.0 | 17.02 ± 25.8 | |
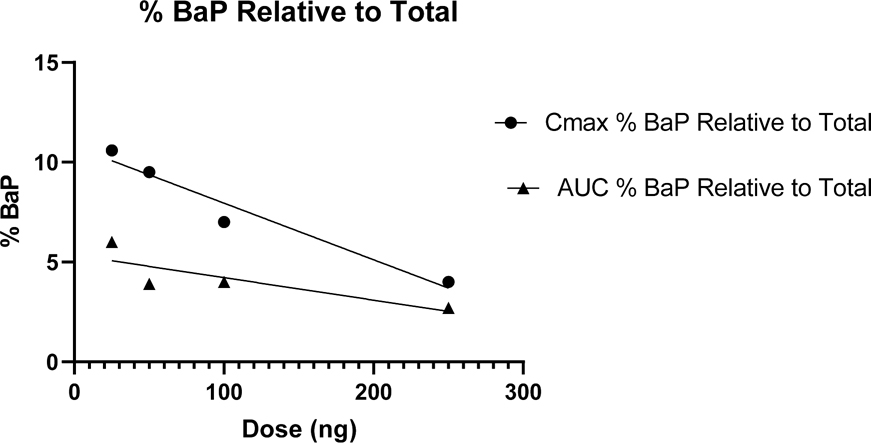
A comparison of Cmax and AUC0–48 hr for the sum of all plasma metabolites relative to parent BaP plus metabolites as a function of dose is shown in Figure 5. At the highest dose of 250 ng parent BaP makes up 2.7% of the total [14C] (AUC0–48 hr) in plasma. The significant linear inverse relationship of Cmax with dose raises the possibility of predicting plasma levels across a wide environmental exposure range which includes daily estimates of exposure for non-smokers in the U.S. This would certainly be of value in BaP risk assessment. From the Cmax data, the x-intercept (100% metabolites) for dose of BaP administered would be 380 ng with a 95% confidence level of 258–1019 ng which is in the range of average daily dietary BaP exposure in the U.S. (U.S. EPA, 2017). There is, of course, the caveat that our results are from a single bolus dose in fasted individuals which does not represent daily dietary exposure. Our previous study with co-administration of food (smoked salmon) found marked differences in pharmacokinetics between fasted and fed individuals (Hummel et al., 2018). In addition, we speculate that the majority of metabolism is occurring at the bay region of BaP (tetrols from hydrolysis of BaP-7,8-DHD-9,10-E, 7,8- and 9,10-DHDs and the 9-phenol). However, until the identity of the conjugates (unknown A) and unknowns B, C, and D is established the ratio of bioactivation to detoxication is uncertain. We are currently attempting identification of these unknown metabolites in plasma and in urine. This extensive metabolism of BaP at ng levels, seen in this study following oral ingestion, may not apply for exposures through inhalation or dermal contact characteristic of smokers or individuals with high occupational exposures.
Figure 5.
Pharmacokinetic constants Cmax and AUC0–48hr for [14C]-BaP in plasma as a percentage of Cmax and AUC0–48hr for total [14C] (BaP plus metabolites) as a function of dose. The percent of [14C]-BaP in plasma decreased with increasing dose for both Cmax (r2=0.950, p=0.025) and AUC0–48hr (r2=0.691, p=0.169) although the latter was not significant.
3.4. Limitations
There are limitations associated with this study, including the unknown metabolites that cannot be further identified due to a lack of standards. The small sample size limited assessment of potential confounders and sources of the large inter-individual variability. Further, the inclusion and exclusion criteria for recruitment and enrollment of so few participants creates a group that may not be representative of the wider population. Dietary questionnaires collected for the three days immediately preceding dosing through the final blood draw may be less useful than quantitative data for exposure and consumption assessments; however, given the limited sample pool any potential variations introduced by diet would not be statistically significant. Third, variability in background levels of PAHs, including BaP, at levels lower than the GC-MS/MS LOD, could impact pharmacokinetics. Again, potential effects are beyond the scope of this study and limited by sample size. Fourth, participants received a low oral bolus dose of a single PAH, which is not how humans are typically exposed. People are continuously exposed to PAH mixtures at various concentrations primarily (≥ 95%) through diet.
3.5. Conclusions
The IARC class 1 known human environmental carcinogen, BaP, when given as a microdose to humans, exhibited rapid uptake (Tmax, 0.5–1 hours), extensive metabolism, and rapid elimination (average half-life across doses, 46.5 ± 58.2 hours). Parent [14C]-BaP was a minor component (based on Cmax and AUC) in plasma relative to total metabolites and declined with dose. The pharmacokinetic parameters for most of the metabolites exhibited a linear dose-response relationship. As these doses (25–250 ng) cover the range of likely human dietary exposure, our results should be of use in risk assessment not just for BaP but for carcinogenic PAH mixtures as levels of BaP in environmental samples are predictive of concentrations of other carcinogenic PAHs (Schneider et al., 2002). Our finding that most metabolism is likely occurring at the bay region also has implications for risk assessment. As in previous studies employing AMS and [14C]-PAHs, there is a large inter-individual variability in [14C]-BaP pharmacokinetics. The interface of AMS to UPLC via the moving wire (Thomas et al., 2011) allows for tentative identification of [14C]-BaP metabolites based on coelution with known standards. This is a unique dataset on the uptake, metabolism and disappearance from plasma of an important environmental carcinogen. The extensive metabolism of [14C]-BaP, as reflected in the time course of plasma levels, is presumably a consequence of robust “first-pass” metabolism by the liver (with perhaps a significant contribution from intestine) and should be incorporated into revisions of current PBPK models. The high inter-individual variability in pharmacokinetics may be due in part to a wide range in expression of CYPs, GSTs, UGTs, SULTs, and transporters (and allelic variants) important in the ADME of BaP and similar environmental carcinogens.
Supplementary Material
Highlights.
Human dose-response study of carcinogen BaP at environmental levels with UPLC-AMS.
UPLC-AMS of [14C]-BaP and metabolites over 4 doses in human plasma was determined.
Inter-individual differences in pharmacokinetics with 25–250 ng doses were observed.
UPLC-AMS analysis showed extensive metabolism predominantly at the BaP bay region.
This metabolic profile at relevant exposures should impact cancer risk assessment.
Acknowledgements
The authors would like to thank the participants who volunteered to be part of this study. Special thanks are also given to Danny Chen and Peter Scruggs for their contributions with sample extractions and preparation.
Funding sources
This study was funded by Public Health Service NIH grants P42ES016465, R01ES028600, T32ES07060, and P30ES030287. Work performed in part at the National User Resource for Biological Accelerator Mass Spectrometry, which is operated at LLNL under the auspices of the U.S. Department of Energy under contract DE-AC52-07NA27344. The User Resource is supported by the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS) under grant R24GM137748.
Abbreviations
- AFB1
aflatoxin B1
- AMS
accelerator mass spectrometry
- AHR
aryl hydrocarbon receptor
- AUC
area under the curve
- ATSDR
Agency for Toxic Substances and Disease Registry
- BaP
benzo[a]pyrene
- BaP-DHD
(± trans-benzo[a]pyrene-7,8-dihydrodiol
- BaP-DHDE
BaP-DHD-9,10-epoxide
- BMI
body mass index
- Cmax
maximum concentration in plasma
- CYP
cytochrome P-450
- DBC
dibenzo[def,p]chrysene
- FDA-IND
U.S. Food and Drug Administration Investigative New Drug
- IARC
International Agency for Research on Cancer
- IPCS
International Programme on Chemical Safety
- IQ
2-amino-3-methylimidazo[4,5-f]quinolone
- IRB
Institutional Review Board
- LLNL
Lawrence Livermore National Laboratory
- LOD
limit of detection
- LOQ
limit of quantitation
- MeIQx
2-amino-3,8-dimethylimidazo[4,5-b]quinoxaline
- NPL
National Priorities List
- N2-dG
nitrogen at the 2 position of deoxyguanosine
- OSU
Oregon State University
- PAH
polycyclic aromatic hydrocarbon
- PBMCs
peripheral blood mononucleated cell
- PBPK
physiologically based pharmacokinetics
- PhIP
2-amino-3-methyl-6-phenylimidazo[4,5-b]pyridine
- RPF
Relative Potency Factor
- Tmax
time at Cmax
- UPLC
ultra-pressure liquid chromatography (Waters)
- U.S. EPA
United States Environmental Protection Agency
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KA, Szelewski MJ, Wilson G, Quimby BD, Hoffman PD, 2015. Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses. J. Chromatogr. A 1419, 89–98. 10.1016/j.chroma.2015.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR, 2020. ATSDR substance priority list [WWW Document]. Agency Toxic Subst. Dis. Regist. URL https://www.atsdr.cdc.gov/spl/index.html (accessed 3.23.21).
- Bansal V, Kim K-H, 2015. Review of PAH contamination in food products and their health hazards. Environ. Int. 84, 26–38. 10.1016/j.envint.2015.06.016 [DOI] [PubMed] [Google Scholar]
- Bauer E, Guo Z, Ueng YF, Bell LC, Zeldin D, Guengerich FP, 1995. Oxidation of benzo[a]pyrene by recombinant human cytochrome P450 enzymes. Chem. Res. Toxicol. 8, 136–142. 10.1021/tx00043a018 [DOI] [PubMed] [Google Scholar]
- Bostrom C, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Törnqvist M, Victorin K, Westerholm R, 2002. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 110, 451–488. 10.1289/ehp.110-1241197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt HCA, Watson WP, 2003. Monitoring human occupational and environmental exposures to polycyclic aromatic compounds. Ann. Occup. Hyg. 47, 349–378. 10.1093/annhyg/meg052 [DOI] [PubMed] [Google Scholar]
- Butler JP, Post GB, Lioy PJ, Waldman JM, Greenberg A, 1993. Assessment of carcinogenic risk from personal exposure to benzo(a)pyrene in the total human environmental exposure study (THEES). Air Waste 43, 970–977. 10.1080/1073161X.1993.10467179 [DOI] [PubMed] [Google Scholar]
- Cavalieri EL, Rogan EG, 1995. Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica 25, 677–688. 10.3109/00498259509061885 [DOI] [PubMed] [Google Scholar]
- Conney AH, Chang RL, Jerina DM, Wei S-JC, 1994. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab. Rev. 26, 125–163. 10.3109/03602539409029788 [DOI] [PubMed] [Google Scholar]
- Courter LA, Musafia-Jeknic T, Fischer K, Bildfell R, Giovanini J, Pereira C, Baird WM, 2007. Urban dust particulate matter alters PAH-induced carcinogenesis by Inhibition of CYP1A1 and CYP1B1. Toxicol. Sci. 95, 63–73. 10.1093/toxsci/kfl137 [DOI] [PubMed] [Google Scholar]
- Crowell SR, Amin SG, Anderson KA, Krishnegowda G, Sharma AK, Soelberg JJ, Williams DE, Corley RA, 2011. Preliminary physiologically based pharmacokinetic models for benzo[a]pyrene and dibenzo[def,p]chrysene in rodents. Toxicol. Appl. Pharmacol. 257, 365–376. 10.1016/j.taap.2011.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL, Nadal M, 2015. Human dietary exposure to polycyclic aromatic hydrocarbons: a review of the scientific literature. Food Chem. Toxicol. 86, 144–153. 10.1016/j.fct.2015.10.002 [DOI] [PubMed] [Google Scholar]
- FDA, 1995. International conference on harmonisation; guideline on validation of analytical procedures: definitions and terminology; availability [WWW Document]. Fed. Regist. URL https://www.federalregister.gov/documents/1995/03/01/95-4956/international-conference-on-harmonisation-guideline-on-validation-of-analytical-procedures (accessed 5.11.21).
- Gibaldi Perrier, 1982. Pharmacokinetics, 2nd ed. Marcel Dekker, New York, NY. [Google Scholar]
- Hecht SS, 1999. Tobacco smoke carcinogens and lung cancer. JNCI J. Natl. Cancer Inst. 91, 1194–1210. 10.1093/jnci/91.14.1194 [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Villalta PW, Hochalter JB, 2010. Analysis of phenanthrene and benzo[a]pyrene tetraol enantiomers in human urine: relevance to the bay region diol epoxide hypothesis of benzo[a]pyrene carcinogenesis and to biomarker studies. Chem. Res. Toxicol. 23, 900–908. 10.1021/tx9004538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert R, Marcus M, Wolff MS, Perera FP, Andrews L, Godbold JH, Rivera M, Stefanidis M, Lu XQ, Landrigan PJ, 1990. Detection of adducts of deoxyribonucleic acid in white blood cells of roofers by 32P-postlabeling. Relationship of adduct levels to measures of exposure to polycyclic aromatic hydrocarbons. Scand. J. Work. Environ. Health 16, 135–143. 10.5271/sjweh.1806 [DOI] [PubMed] [Google Scholar]
- Hummel JM, Madeen EP, Siddens LK, Uesugi SL, McQuistan T, Anderson KA, Turteltaub KW, Ognibene TJ, Bench G, Krueger SK, Harris S, Smith J, Tilton SC, Baird WM, Williams DE, 2018. Pharmacokinetics of [14C]-benzo[a]pyrene (BaP) in humans: impact of co-administration of smoked salmon and BaP dietary restriction. Food Chem. Toxicol. 115, 136–147. 10.1016/j.fct.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC, 2021. IARC monographs on the identification of carcinogenic hazards to humans [WWW Document]. Int. Agency Res. Cancer URL https://monographs.iarc.who.int/list-of-classifications (accessed 3.23.21). [Google Scholar]
- IPCS, 1998. Selected non-heterocyclic polycyclic aromatic hydrocarbons (EHC 202) [Monograph]. WHO Library Cataloguing in Publication Data. [Google Scholar]
- Jarvis IWH, Dreij K, Mattsson Å, Jernström B, Stenius U, 2014. Interactions between polycyclic aromatic hydrocarbons in complex mixtures and implications for cancer risk assessment. Toxicology 321, 27–39. 10.1016/j.tox.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N, 2001. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 39, 423–436. 10.1016/s0278-6915(00)00158-7 [DOI] [PubMed] [Google Scholar]
- Keck BD, Ognibene T, Vogel JS, 2010. Analytical validation of accelerator mass spectrometry for pharmaceutical development. Bioanalysis 2, 469–485. 10.4155/bio.10.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Stansbury KH, Walker NJ, Trush MA, Strickland PT, Sutter TR, 1998. Metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis 19, 1847–1853. 10.1093/carcin/19.10.1847 [DOI] [PubMed] [Google Scholar]
- Li Z, Sjödin A, Romanoff LC, Horton K, Fitzgerald CL, Eppler A, Aguilar-Villalobos M, Naeher LP, 2011. Evaluation of exposure reduction to indoor air pollution in stove intervention projects in Peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environ. Int. 37, 1157–1163. 10.1016/j.envint.2011.03.024 [DOI] [PubMed] [Google Scholar]
- Lodovici M, Akpan V, Giovannini L, Migliani F, Dolara P, 1998. Benzo[a]pyrene diol-epoxide DNA adducts and levels of polycyclic aromatic hydrocarbons in autoptic samples from human lungs. Chem. Biol. Interact. 116, 199–212. 10.1016/S0009-2797(98)00091-X [DOI] [PubMed] [Google Scholar]
- Madeen E, Corley RA, Crowell S, Turteltaub K, Ognibene T, Malfatti M, McQuistan TJ, Garrard M, Sudakin D, Williams DE, 2015. Human in vivo pharmacokinetics of [(14)C]dibenzo[def,p]chrysene by accelerator mass spectrometry following oral microdosing. Chem. Res. Toxicol. 28, 126–134. 10.1021/tx5003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeen E, Siddens LK, Uesugi S, McQuistan T, Corley RA, Smith J, Waters KM, Tilton SC, Anderson KA, Ognibene T, Turteltaub K, Williams DE, 2019. Toxicokinetics of benzo[a]pyrene in humans: extensive metabolism as determined by UPLC-accelerator mass spectrometry following oral micro-dosing. Toxicol. Appl. Pharmacol. 364, 97–105. 10.1016/j.taap.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeen EP, Ognibene TJ, Corley RA, McQuistan TJ, Henderson MC, Baird WM, Bench G, Turteltaub KW, Williams DE, 2016. Human microdosing with carcinogenic polycyclic aromatic hydrocarbons: in vivo pharmacokinetics of dibenzo[def,p]chrysene and metabolites by UPLC Accelerator Mass Spectrometry. Chem. Res. Toxicol. 29, 1641–1650. 10.1021/acs.chemrestox.6b00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinl W, Ebert B, Glatt H, Lampen A, 2008. Sulfotransferase forms expressed in human intestinal Caco-2 and TC7 cells at varying stages of differentiation and role in benzo[a]pyrene metabolism. Drug Metab. Dispos. 36, 276–283. 10.1124/dmd.107.018036 [DOI] [PubMed] [Google Scholar]
- Melendez-Colon VJ, Luch A, Seidel A, Baird WM, 1999. Comparison of cytochrome P450- and peroxidase-dependent metabolic activation of the potent carcinogen dibenzo[a,l]pyrene in human cell lines: formation of stable DNA adducts and absence of a detectable increase in apurinic sites. Cancer Res. 59, 1412–1416. [PubMed] [Google Scholar]
- Miller KP, Ramos KS, 2001. Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metab. Rev. 33, 1–35. 10.1081/DMR-100000138 [DOI] [PubMed] [Google Scholar]
- Mumtaz George, 1995. Toxicological profile for polycyclic aromatic hydrocarbons (PAHs). [PubMed] [Google Scholar]
- Nebert DW, 1989. The Ah Locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit. Rev. Toxicol. 20, 153–174. 10.3109/10408448909017908 [DOI] [PubMed] [Google Scholar]
- Obana H, Hori S, Kashimoto T, Kunita N, 1981. Polycyclic aromatic hydrocarbons in human fat and liver. Bull. Environ. Contam. Toxicol. 27, 23–27. 10.1007/BF01610981 [DOI] [PubMed] [Google Scholar]
- Ognibene TJ, Haack KW, Bench G, Turteltaub KW, 2019. Trials and tribulations in the first three years in operation of the SSAMS for biomedical 14C-AMS at LLNL. Nucl Instrum Methods Phys Res B 438, 166–171. 10.1016/j.nimb.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognibene TJ, Thomas AT, Daley PF, Bench G, Turteltaub KW, 2015. An Interface for the Direct Coupling of Small Liquid Samples to AMS. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At 361, 173–177. 10.1016/j.nimb.2015.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli R, Guanci M, Cerri A, Minoia C, Carrer P, Negri E, Fanelli R, Airoldi L, 2000. Benzo(a)pyrene diolepoxide-haemoglobin and albumin adducts at low levels of benzo(a)pyrene exposure. Biomarkers 5, 245–251. 10.1080/135475000413791 [DOI] [PubMed] [Google Scholar]
- Pavanello S, Pulliero A, Siwinska E, Mielzynska D, Clonfero E, 2005. Reduced nucleotide excision repair and GSTM1 -null genotypes influence anti -B[ a ]PDE–DNA adduct levels in mononuclear white blood cells of highly PAH-exposed coke oven workers. Carcinogenesis 26, 169–175. 10.1093/carcin/bgh303 [DOI] [PubMed] [Google Scholar]
- Penning TM, Ohnishi ST, Ohnishi T, Harvey RG, 1996. Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase. Chem. Res. Toxicol. 9, 84–92. 10.1021/tx950055s [DOI] [PubMed] [Google Scholar]
- Pleil JD, Stiegel MA, Sobus JR, Tabucchi S, Ghio AJ, Madden MC, 2010. Cumulative exposure assessment for trace-level polycyclic aromatic hydrocarbons (PAHs) using human blood and plasma analysis. J. Chromatogr. B 878, 1753–1760. 10.1016/j.jchromb.2010.04.035 [DOI] [PubMed] [Google Scholar]
- Ross JA, Nelson GB, Wilson KH, Rabinowitz JR, Galati A, Stoner GD, Nesnow S, Mass MJ, 1995. Adenomas induced by polycyclic aromatic hydrocarbons in strain A/J mouse lung correlate with time-integrated DNA adduct levels. Cancer Res. 55, 1039–1044. [PubMed] [Google Scholar]
- Sadiktsis I, Bergvall C, Johansson C, Westerholm R, 2012. Automobile Tires—A potential source of highly carcinogenic dibenzopyrenes to the environment. Environ. Sci. Technol. 46, 3326–3334. 10.1021/es204257d [DOI] [PubMed] [Google Scholar]
- Salehpour M, Possnert G, Bryhni H, 2008. Subattomole sensitivity in biological accelerator mass spectrometry. Anal. Chem. 80, 3515–3521. 10.1021/ac800174j [DOI] [PubMed] [Google Scholar]
- Schneider K, Roller M, Kalberlah F, Schuhmacher-Wolz U, 2002. Cancer risk assessment for oral exposure to PAH mixtures. J. Appl. Toxicol. 22, 73–83. 10.1002/jat.828 [DOI] [PubMed] [Google Scholar]
- Schwarz G, 1978. Estimating the dimension of a model. Ann. Stat. 6, 461–464. 10.1214/aos/1176344136 [DOI] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y, 2004. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 95, 1–6. 10.1111/j.1349-7006.2004.tb03162.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Gillam EM, Oda Y, Tsumura F, Sutter TR, Guengerich FP, Inoue K, 1999. Metabolism of benzo[a]pyrene to trans-7,8-dihydroxy-7, 8-dihydrobenzo[a]pyrene by recombinant human cytochrome P450 1B1 and purified liver epoxide hydrolase. Chem. Res. Toxicol. 12, 623–629. 10.1021/tx990028s [DOI] [PubMed] [Google Scholar]
- Shimada T, Gillam EMJ, Sutter TR, Strickland PT, Guengerich FP, Yamazaki H, 1997. Oxidation of xenobiotics by recombinant human cytochrome P450 1B1. Drug Metab. Dispos. 25, 617–622. [PubMed] [Google Scholar]
- Simoneit BRT, Bi X, Oros DR, Medeiros PM, Sheng G, Fu J, 2007. Phenols and hydroxy-PAHs (arylphenols) as tracers for coal smoke particulate matter: source tests and ambient aerosol assessments. Environ. Sci. Technol. 41, 7294–7302. 10.1021/es071072u [DOI] [PubMed] [Google Scholar]
- Staretz ME, Murphy SE, Patten CJ, Nunes MG, Koehl W, Amin S, Koenig LA, Guengerich FP, Hecht SS, 1997. Comparative metabolism of the tobacco-related carcinogens benzo[a]pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and N’- nitrosonornicotine in human hepatic microsomes. Drug Metab. Dispos. 25, 154–162. [PubMed] [Google Scholar]
- Stepanov I, Villalta PW, Knezevich A, Jensen J, Hatsukami D, Hecht SS, 2010. Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography-mass spectrometry. Chem. Res. Toxicol. 23, 66–73. 10.1021/tx900281u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AT, Ognibene T, Daley P, Turteltaub K, Radousky H, Bench G, 2011. Ultrahigh efficiency moving wire combustion interface for online coupling of high-performance liquid chromatography (HPLC). Anal. Chem. 83, 9413–9417. 10.1021/ac202013s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titaley IA, Chlebowski A, Truong L, Tanguay RL, Massey Simonich SL, 2016. Identification and toxicological evaluation of unsubstituted PAHs and novel PAH derivatives in pavement sealcoat products. Environ. Sci. Technol. Lett. 3, 234–242. 10.1021/acs.estlett.6b00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen R, Baranczewski P, Warholm M, Hagmar L, Möller L, Rannug A, 2002. Susceptibility factors and DNA adducts in peripheral blood mononuclear cells of aluminum smelter workers exposed to polycyclic aromatic hydrocarbons. Arch. Toxicol. 76, 178–186. 10.1007/s00204-002-0331-0 [DOI] [PubMed] [Google Scholar]
- U.S. EPA, 2017. Toxicological Review of Benzo[a]pyrene [CASRN 50–32-8].
- U.S. EPA, 2010. Development of a Relative Potency Factor (Rpf) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures (External Review Draft, Suspended) [WWW Document]. U. S. Environ. Prot. Agency Sci. Inventory. URL https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NCEA&dirEntryId=194584 (accessed 3.23.21). [Google Scholar]
- U.S. EPA, O., 2013. Persistent Bioaccumulative Toxic (PBT) Chemicals Covered by the TRI Program [WWW Document]. U. S. Environ. Prot. Agency Toxic Release Inventory. URL https://www.epa.gov/toxics-release-inventory-tri-program/persistent-bioaccumulative-toxicpbt-chemicals-covered-tri (accessed 3.23.21). [Google Scholar]
- Usenko S, Landers DH, Appleby PG, Simonich SL, 2007. Current and historical deposition of PBDEs, pesticides, PCBs, and PAHs to Rocky Mountain National Park. Environ. Sci. Technol. 41, 7235–7241. 10.1021/es0710003 [DOI] [PubMed] [Google Scholar]
- Usenko S, Simonich SLM, Hageman KJ, Schrlau JE, Geiser L, Campbell DH, Appleby PG, Landers DH, 2010. Sources and deposition of polycyclic aromatic hydrocarbons to Western U.S. National Parks. Environ. Sci. Technol. 44, 4512–4518. 10.1021/es903844n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Gómez G, Rocha-Zavaleta L, Rodríguez-Sosa M, Petrosyan P, Rubio-Lightbourn J, 2018. Benzo[a]pyrene activates an AhR/Src/ERK axis that contributes to CYP1A1 induction and stable DNA adducts formation in lung cells. Toxicol. Lett. 289, 54–62. 10.1016/j.toxlet.2018.03.012 [DOI] [PubMed] [Google Scholar]
- Zhong Y, Carmella SG, Hochalter JB, Balbo S, Hecht SS, 2011. Analysis of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in human urine: a biomarker for directly assessing carcinogenic polycyclic aromatic hydrocarbon exposure plus metabolic activation. Chem. Res. Toxicol. 24, 73–80. 10.1021/tx100287n [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.