Abstract
Ophthalmoplegia following cosmetic facial filler injections is a rare but serious complication. The authors report two cases of ophthalmoplegia following filler injection. In the first case, a 54-year-old female presented with acute onset headache, vomiting, and diplopia during malar and temporal injection of hyaluronic acid. In the second case, a 37-year-old female presented with binocular diplopia that developed following injection of an unknown filler to the upper face. Neither of the two patients had skin necrosis or ocular abnormalities other than motility deficits. To our knowledge, there have been no other cases of isolated ophthalmoplegia without evidence of other ocular injuries following facial filler.
Précis
Presentation of two cases of isolated ophthalmoplegia following facial filler injection. To the authors’ knowledge, these are the first cases reported of ophthalmoplegia related to filler without evidence of other ocular injuries.
Facial filler injections have become increasingly more common over the last decade. They are now the second most commonly performed non-surgical aesthetic procedure following injection of botulinum toxin, with 2.6 million people undergoing soft tissue filler injection in 2018.1 As use has expanded, so has the number of patients impacted by adverse reactions. Although facial soft tissue fillers are generally considered low risk when performed properly, they can, in rare cases, cause serious complications, including ophthalmoplegia. Cases of ophthalmoplegia related to filler in the literature have been largely reported in conjunction with retinal artery occlusion and vision loss.2–7 Here, we present two cases of isolated acute ophthalmoplegia following filler injections. The collection and evaluation of protected patient health information were HIPAA compliant. This study adhered to the ethical tenets outlined in the Declaration of Helsinki as amended in 2013.
Case Presentation
Case 1
A 54-year-old female underwent cosmetic hyaluronic acid filler injections to the temples and periorbital region by an experienced nurse injector at an outside facility. During the malar injections, the patient began experiencing severe frontal headache and nausea. She was treated immediately with hyaluronidase around the left temple and periorbital region without improvement in symptoms. At no point was there blanching or bleeding at the injection site (Figure 1). The patient then developed dizziness, binocular diplopia, and vomiting and was subsequently taken to the emergency room. Ocular exam, including visual acuity, pupils, anterior segment and dilated fundus exam, was unremarkable, except for hypotropia and limited supraduction, -3, of the left eye (Figure 2). Magnetic resonance imaging (MRI) of the head and orbit and angiography (MRA) of the head were unremarkable. Specifically, there were no findings of an acute infarct, hemorrhage or mass effect to explain the patient’s ocular motility deficit. After discharge, the patient began to have pain over the left eye.
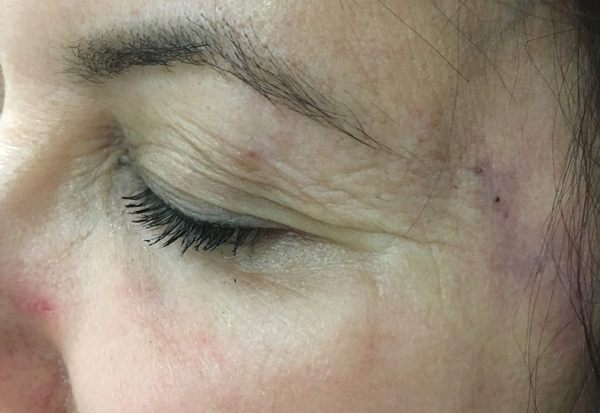
Figure 1.
Case 1 demonstrating a lack of local ischemia following injections. Mild ecchymosis is seen.
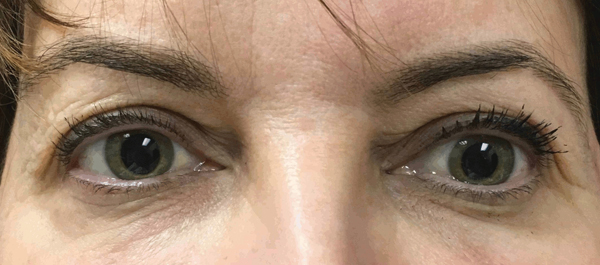
Figure 2.
Left hypotropia and mild esotropia in Case 1.
Seventy-two hours after facial filler injection the patient was started on a one-week methylprednisolone taper (Medrol Dosepak) and aspirin 81 mg to treat empirically for possible ischemic cranial nerve palsy. The patient reported improvement in her eye pain 6–7 hours after she started steroids. The ocular motility deficit and diplopia resolved over the next 8 weeks. Although ischemic third nerve palsy has been reportedly related to cosmetic hyaluronic acid filler injection, this patient’s findings were more consistent with an isolated superior rectus palsy given lack of ptosis.
Case 2
A 37-year-old healthy female frequently attended “filler and Botox parties.” After one event, the patient developed binocular diplopia almost immediately after filler injections to the nasal bridge and periorbital region. There was no written documentation of the event, but the patient thought she had received hyaluronic acid and possibly polymethylmethacrylate (Artefill) injections. She denied any vision loss or skin changes at the time the diplopia began. Due to personal tragedies near the time of the event, she did not seek medical attention until 3–4 months later, when she was noted to have severe limitation of adduction and moderate limitation in supraduction in the left eye, with a large angle left exotropia and left hypotropia, consistent with a partial third nerve palsy (Figure 3). Pupils and the rest of the ocular exam were unremarkable. Specifically, there was no evidence of central or branch retinal artery occlusion, and she had no history of vascular or cardiac disease. There was no improvement of the third nerve palsy at 10 months. She underwent two strabismus surgeries with partial correction of the motility deficit.
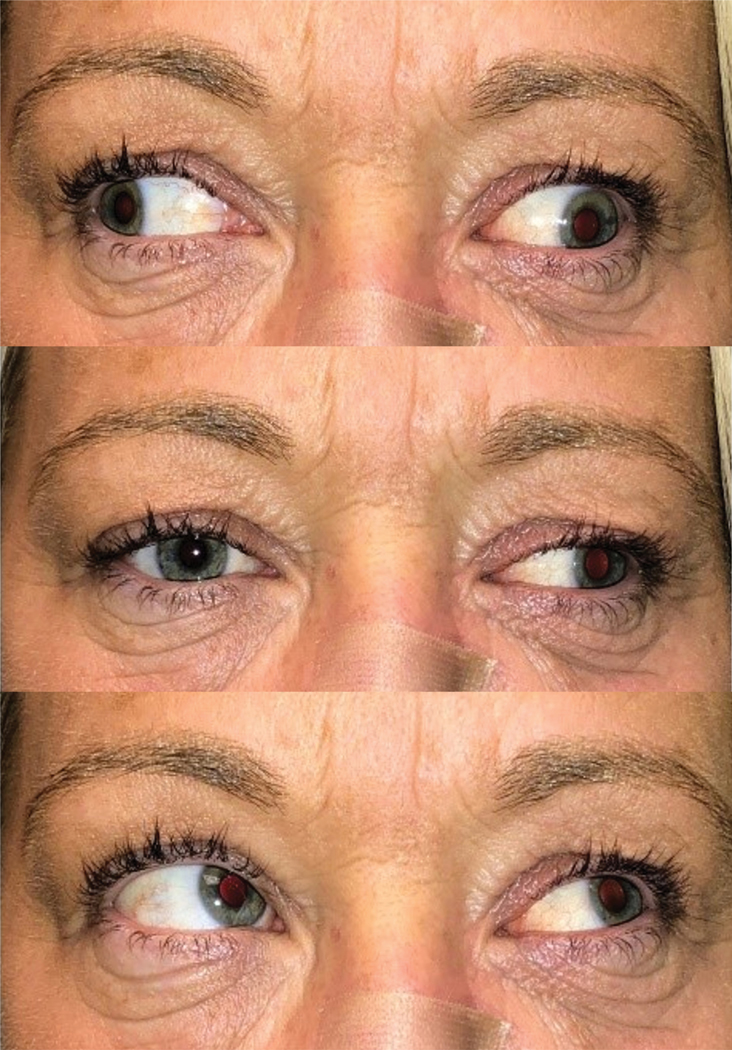
Figure 3.
Left eye motility deficits in right gaze, primary gaze and left gaze in Case 2.
Discussion
Complications following periorbital facial filler injections are rare, but when they do occur they can result in serious or even vision threatening complications. The central retina artery or ophthalmic artery may be occluded by retrograde migration of filler particles, more commonly following injections in the periocular area, nasal dorsum and nasolabial fold.3,8–10 In addition to vision loss, facial filler injection has also been associated with ophthalmoplegia.6 In a survey by the Korean Retina Society, it was found that ophthalmoplegia was present in 50 percent of patients who presented with occlusion of the ophthalmic artery or its branches following facial filler injection.7 In another study by Yang et al6, cases of 21 patients with central retinal artery occlusion (CRAO) and ophthalmic artery occlusion after filler injection were reviewed. They found that ophthalmoplegia occurred in 71 percent of patients with iatrogenic artery occlusion, with the average number of involved rectus muscles being 2.8. Possible mechanisms for ophthalmoplegia were presumed to be ischemia to either the cranial nerves or the extraocular muscles.2,6
To our knowledge, there have been no cases of ophthalmoplegia reported that were not associated with vision loss, evidence of anterior segment ischemia, or both. In Case 1, the patient presented to our institution immediately following the injection of hyaluronidase and did not have any evidence of ocular abnormality other than a limitation in elevation of the left eye. In Case 2, the patient had a delayed presentation, so it is unknown whether she had any findings of ocular ischemia at the time she developed diplopia, although she did not report any vision loss.
The presumed mechanism of ocular complications due to filler injection is inadvertent intra-arteriolar injection. Given that ophthalmoplegia cases in the literature have been associated with other ocular complications, and our patients had no evidence of other ocular manifestations related to their filler, it is possible that their exam findings were not due to vascular occlusion. Hyaluronic acid has been associated with a potential inflammatory response, including intermittent swelling and severe granulomatous allergic reactions11,12. Although speculative, it is possible that the dysmotility in Case 1 may, in part, be related to filler toxicity to the extraocular muscles, causing a myositis, which could explain the patient’s rapid improvement in eye pain after starting steroids. However, given her near immediate onset of diplopia, an inflammatory mechanism cannot be the sole cause of her ophthalmoplegia. The mechanism underlying the partial third nerve palsy in Case 2 is unclear given a lack of clear history and examination at the onset of the double vision, due to poor follow-up and patient cooperation. Possible mechanisms could include either ischemia or direct filler infiltration to the intraorbital divisions of the third nerve.
Conclusions
Isolated ophthalmoplegia without evidence of retinal artery occlusion or skin necrosis appears to be extremely rare. Here we present two cases of ophthalmoplegia occurring after filler injection to the upper face. One manifested as an isolated superior rectus palsy and the other as a persistent partial third nerve palsy. Individuals who develop ophthalmoplegia related to facial filler may recover function over time, as seen in the majority of cases presented in the literature.6 However, in some cases, the injury is permanent. Patients need to be aware of this risk prior to injection.
Acknowledgments
Financial support: National Institute of Health Core grant P30EY016665, and unrestricted funds from Research to Prevent Blindness, Inc., NY, NY to the University of Wisconsin Department of Ophthalmology and Visual Sciences.
Footnotes
The authors have no financial interests related to this manuscript to declare.
References
- 1.2018 Plastic Surgery Statistics Report. Plast Surg. 2018:25. [Google Scholar]
- 2.Kim A, Kim S-H, Kim H-J, et al. Ophthalmoplegia as a complication of cosmetic facial filler injection. Acta Ophthalmol. 2016;94(5):e377–e379. [DOI] [PubMed] [Google Scholar]
- 3.Kwon SG, Hong JW, Roh TS, et al. Ischemic Oculomotor Nerve Palsy and Skin Necrosis Caused by Vascular Embolization After Hyaluronic Acid Filler Injection: A Case Report. Ann Plast Surg. 2013;71(4):333–334. [DOI] [PubMed] [Google Scholar]
- 4.Bae IH, Kim MS, Choi H, et al. Ischemic oculomotor nerve palsy due to hyaluronic acid filler injection. J Cosmet Dermatol. 2018;17(6):1016–1018. [DOI] [PubMed] [Google Scholar]
- 5.Sung MS, Kim HG, Woo KI, Kim Y-D. Ocular ischemia and ischemic oculomotor nerve palsy after vascular embolization of injectable calcium hydroxylapatite filler. Ophthal Plast Reconstr Surg. 2010;26(4):289–291. [DOI] [PubMed] [Google Scholar]
- 6.Yang HK, Lee Y, Woo SJ, et al. Natural Course of Ophthalmoplegia after Iatrogenic Ophthalmic Artery Occlusion Caused by Cosmetic Filler Injections. Plast Reconstr Surg. 2019;144(1). [DOI] [PubMed] [Google Scholar]
- 7.Park KH, Kim Y-K, Woo SJ, et al. Iatrogenic occlusion of the ophthalmic artery after cosmetic facial filler injections: a national survey by the Korean Retina Society. JAMA Ophthalmol. 2014;132(6):714–723. [DOI] [PubMed] [Google Scholar]
- 8.Hong J-H, Ahn SJ, Woo SJ, et al. Central retinal artery occlusion with concomitant ipsilateral cerebral infarction after cosmetic facial injections. J Neurol Sci. 2014;346(1–2):310–314. [DOI] [PubMed] [Google Scholar]
- 9.Roberts SAI, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthal Plast Reconstr Surg. 2012;28(3):e68–70. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh YH, Lin CW, Huang JS, Yeh PT. Severe ocular complications following facial calcium hydroxylapatite injections: Two case reports. Taiwan J Ophthalmol. 2015;5(1):36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olenius M. The first clinical study using a new biodegradable implant for the treatment of lips, wrinkles, and folds. Aesthetic Plast Surg. 1998;22(2):97–101. [DOI] [PubMed] [Google Scholar]
- 12.Hönig JF, Brink U, Korabiowska M. Severe granulomatous allergic tissue reaction after hyaluronic acid injection in the treatment of facial lines and its surgical correction. J Craniofac Surg. 2003;14(2):197–200. [DOI] [PubMed] [Google Scholar]