Abstract
Background
We investigated the effect of platelet-rich plasma (PRP) on tendon–bone healing and intra-articular graft (IAG) maturation after anterior cruciate ligament (ACL) reconstruction.
Methods
In this prospective randomized controlled study, 60 patients with ruptured ACLs were divided one-to-one into two groups (study and control). Patients were treated using single-bundle autologous hamstring autografts. Only patients in the study group were administered PRP. Knee function (pre-operative and three-, six-, and 12-month post-operative Lysholm activity, Tegner and International Knee Documentation Committee scores, femoral tunnel (FT) and tibial tunnel (TT) diameters measured with computed tomography (post-operative follow-up at 4 days and at 12 months), and magnetic resonance imaging signal/noise quotients of the IAG and graft in the FT (at 12 months) were used to evaluate tendon–bone healing and graft maturation.
Results
Patients’ knee function scores improved after ACL reconstruction, but there were no significant differences between groups. At 12 months, FT (study, 8.88 ± 1.46 mm; control, 8.42 ± 2.75 mm) and TT (study, 9.50 ± 1.07 mm; control, 9.99 ± 1.91 mm) diameters were larger than FT (study, 6.91 ± 0.74 mm; control, 7.30 ± 1.17 mm) and TT (study, 9.31 ± 0.83 mm; control, 9.36 ± 0.88 mm) diameters at 4 days; however, differences between groups were not significant (FT, P = 0.67; TT, P = 0.52). There were no significant differences between groups for signal/noise quotients of the IAG (study, 1.38 ± 0.70; control, 2.01 ± 0.62; P = 0.06) and FT-portion of the graft (study, 2.39 ± 1.22; control, 2.46 ± 0.83; P = 0.89).
Conclusion
PRP had no significant effect on reducing bone tunnel widening, accelerating tendon–bone healing, or improving knee function; however, PRP may improve IAG maturation.
Trial registration
Our study was first registered at Clinicaltrials.gov with registration No. NCT04659447 on 12/09/2020.
Keywords: Platelet-rich plasma, Anterior cruciate ligament, Signal/noise quotient, Tendon–bone healing, Graft maturity
Background
Anterior cruciate ligament (ACL) rupture is a common sports injury [1] and ACL ruptures account for approximately 80% of knee injuries in sports [2]. An ACL rupture can affect the stability of the knee and is associated with injury to the meniscus and articular cartilage. Such injuries may lead to osteoarthritis; therefore, patients with ACL ruptures should undergo anatomical ACL reconstruction to restore the stability of the knee joint.
In anatomical ACL reconstruction, bone tunnels are drilled at the centres of the femoral and tibial ACL stumps, and the ends of a woven autologous or allogeneic tendon are implanted and fixed into the bone tunnels [3, 4]. Accelerating tendon–bone healing and graft maturation is important for early functional rehabilitation.
Platelet-rich plasma (PRP) is plasma in which the concentration of platelets is more than 1.0 × 106 platelets/μl [5]. Because PRP is rich in growth factors, with concentrations several times higher than those in normal plasma, PRP can promote tissue healing [6, 7]. This treatment adjunct is currently widely used in ACL reconstruction to promote graft maturation and allow early rehabilitation; however, to date, findings and conclusions from studies examining the effect of PRP have not been uniform [8, 9].
Thus, we designed a prospective randomized controlled study to evaluate the effect of PRP used during the anatomical ACL reconstruction with autologous hamstring on tendon–bone healing and intra-articular graft (IAG) maturation. In the study group, PRP was used in ACL reconstruction. Gelatin sponge filled with PRP was affixed in the graft and the remaining PRP was injected into the bone tunnels and graft. Nothing was used in the control group.
Materials and Methods
Study Design
This study was approved by the Ethics Committee of our hospital (No. 16152-0110) and conformed to the principles of the Declaration of Helsinki. Informed consent was obtained from each participant. Patients at out hospital being treated for ACL rupture were eligible to be included. Inclusion criteria were (1) patient age 15–50 years old and (2) diagnosis ACL injury confirmed using magnetic resonance imaging (MRI), physical examinations, and arthroscopy. Exclusion criteria were patients (1) with concomitant posterior cruciate ligament, lateral collateral ligament, or medial collateral ligament injury above grade III; (2) who had undergone previous knee operations, (3) with moderate to severe knee joint injury or articular cartilage injury, (4) with neurovascular injury, (5) with concomitant intra-articular and periarticular fractures of the knee, (6) with instability of the contralateral knee, (7) with rheumatoid arthritis or related diseases, or (8) with decreased physical activity levels due to other diseases.
Pre-operative Procedures
After enrolment, each patient was randomly allocated to one of two groups—study or control—using a random number table. Each patient underwent single-bundle anatomical ACL reconstruction; PRP was administered during the operation to patients in the study group (30 patients) but not to those in the control group (30 patients).
After enrolment, we collected gender, age, injured side, body mass index (BMI) and injury time data, and Lysholm activity, Tegner and International Knee Documentation Committee (2000IKDC) scores were used to evaluate knee function.
Surgical Procedures
Doctors involved in ACL reconstruction operation did not participate in follow-up; follow-up examinations and imaging evaluation were performed by other professionals.
We used Platelet Rich Plasma Preparation Kits (WEGO; Beijing, China) and a centrifuge (WEGO; Beijing, China) to prepare PRP. Before each patient’s operation, we used a 50-ml syringe to draw 4 ml anticoagulant citrate dextrose solution A, and then drew 36 ml of peripheral blood from the patient’s median cubital vein. After fully mixing its contents, we placed the barrel of the syringe into a special centrifugal tube from the kit and performed the first centrifugation. Afterwards, we removed the lower layer, which comprised red blood cells, and performed centrifugation again on the upper layer, which comprised plasma. Finally, we extracted the platelet-poor top layer of plasma, and approximately 4 ml of PRP remained.
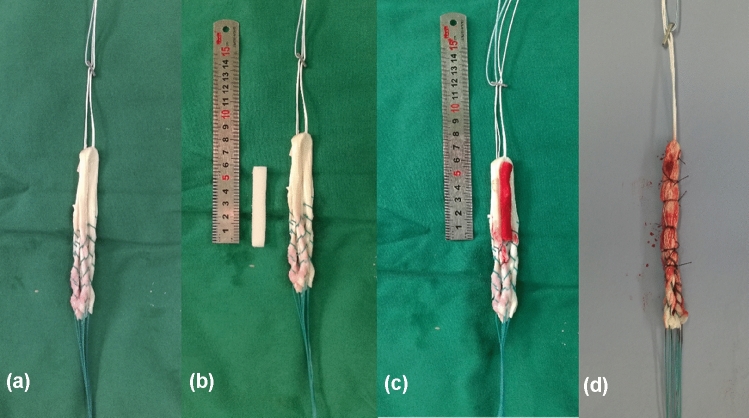
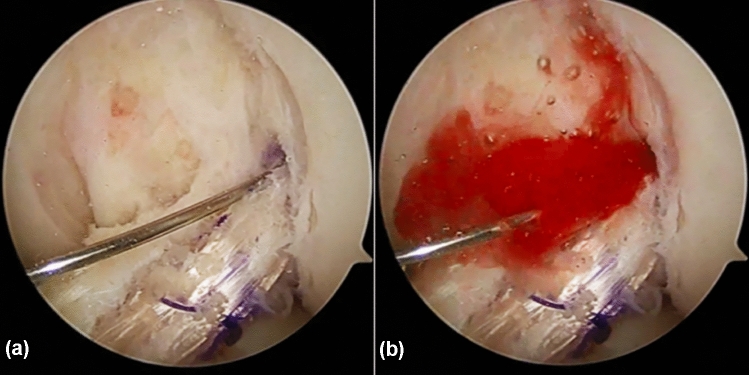
All ACL reconstruction operations were performed by the same surgeon, who had more than 20 years of experience in ACL reconstructions. Standard single-bundle anatomical ACL reconstruction was conducted for all patients [10]. The femoral tunnel (FT) was drilled into the centre of the ACL’s point of origin, with knee flexed 95°; the tibial tunnel (TT) was created at the centre of the ACL’s point of insertion, with the knee flexed 90°, using an ACL tibial tunnel director guide (DePuy Mitek; Raynham, MA, USA) set at 55°. Semitendinosus and gracilis tendons were harvested, cleaned of remaining soft tissue, and trimmed into four strands. The free ends of tendon that would be distally attached were woven with no. 2 braided polyester Syneture TI-Cron (Covidien, Mansfield, MA, USA) using the whip-stitch technique. The graft was attached to the Endobutton (Smith & Nephew; London, UK), and for patients in the study group, a 6-cm-long gelatin sponge filled with PRP was affixed in the centre of the four strands of tendon with 4–0 absorbable suture (Ethicon, Johnson & Johnson; New Brunswick, USA) (Fig. 1). The graft was pulled into the bone tunnels for fixation: the femoral end of the graft was fixed with the Endobutton, and the tibial end was fixed with an Intrafix Tibial Device (DePuy Mitek), with the knee flexed 20° by forced posterior drawer. For patients in the study group, the remaining PRP was then injected into the bone tunnels and graft (Fig. 2).
Fig. 1.
a Tendon weaving, b gelatin sponge, c gelatin sponge with PRP affixed on the tendon, d getting the graft
Fig. 2.
The remaining PRP was administered into the tunnel and graft by a placing the tip of the needle in the tendon and b injecting the PRP
Post-operative Rehabilitation
The observation period was 12 months. All patients received the same post-operative treatment and rehabilitation protocol, which emphasized early restoration of motion and improvement of muscle strength. Pain was evaluated daily for 3 days after operation, using visual analogue scale scores. Each patient was given a functional knee brace immediately after their operation. Partial weight-bearing was permitted with the brace locked in full extension within 4 weeks after operation. Immediately after their operation, patients began isometric quadriceps exercises such as ankle pump and leg lifting to improve muscle strength. Patients were not discharged from the hospital until the knee could be flexed 90°. Full weight-bearing with the brace was permitted at the seventh week after operation; full weight-bearing without the brace was permitted when sufficient quadricep strength was regained, approximately at the third month after operation. Walking and swimming were permitted after 3 months, running was permitted after 6 months, and contact sports and heavy work were permitted after 9 months.
Post-operative Follow-Up and Assessments
On the fourth post-operative day, computed tomography (CT) scans and three-dimensional reconstruction without medial condyle were performed. Tunnel positions were evaluated, and FT and TT diameters were measured. Knee proprioception and function were evaluated at three-, six- and 12-month follow-ups. At the 12-month follow-up, CT was performed to re-evaluate tunnel diameters, MRI was performed to evaluate tendon–bone healing and IAG maturation, and anterior tibial translation (ATT) was evaluated with KT-2000.
Signal/noise quotients (SNQ) of regions of interest (ROIs) in the MRI images were used to evaluate tendon–bone healing and graft maturation. We searched T2-weighted coronal image layers to find one in which the IAG and FT were clearly visible, and 5.4-mm-diameter circular ROIs were selected to obtain signal values for the IAG and FT. The background signal value was obtained from approximately 3-cm regions of the medial knee (Fig. 3). Each ROI signal value was divided by the background signal value to yield SNQ for the IAG, and FT. Smaller SNQs represented higher tendon–bone healing or graft maturation. Each measurement was performed independently by three experienced radiologists, and mean values were used for analysis.
Fig. 3.
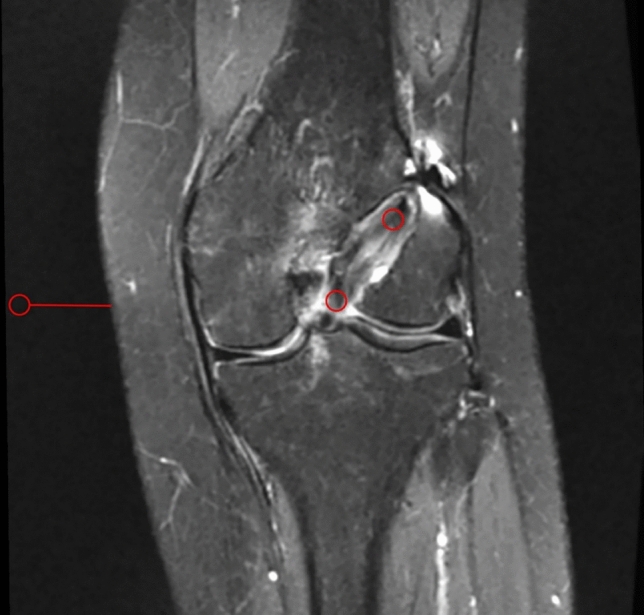
Signal values were obtained from regions of interest, indicated by 5.4-mm diameter circles on the MRI
Knee proprioception was measured by having patients attempt to stand for one minute on a single leg with both upper limbs abducted to 90° (one point), with both upper limbs abducted to 90° with one eye closed (two points), with both upper limbs abducted to 90° with two eyes closed (three points), and on a log (four points). The higher the score, the better the proprioception.
Statistical Analysis
SPSS 22.0 software (IBM, Armonk, NY, USA) was used to perform statistical analysis. The Kolmogorov–Smirnov test was used to test normality. Normally distributed continuous data were described with mean ± standard deviation (SD). If data were normally distributed, the independent-sample t-test or one-way analysis of variance was used; otherwise the Mann–Whitney test was used. The level of significance was set to P ≤ 0.05.
Results
There were 39 male patients (65%) and 21 female patients (35%) included in this study, and the average age was 34.53 ± 9.27 years. Demographic variables such as gender, BMI and age and pre-operative knee function scores were similar between groups (Table 1).
Table 1.
Demographic and clinical data of the patientsa
| Study group (n = 30) | Control group(n = 30) | P | |
|---|---|---|---|
| Gender, male/female | 18/12 | 21/9 | 0.42 |
| Side, left/right | 17/13 | 11/19 | 0.12 |
| Age (years old) | 33.5 ± 8.97 | 34.9 ± 9.68 | 0.58 |
| BMI (kg/m2) | 25.02 ± 3.43 | 25.50 ± 4.08 | 0.70 |
| Lysholm score | 58.91 ± 4.77 | 53.56 ± 4.86 | 0.44 |
| Tegner score | 4.13 ± 0.57 | 3.33 ± 0.60 | 0.34 |
| 2000IKDC score | 54.35 ± 2.83 | 54.44 ± 3.04 | 0.98 |
aBMI body mass index, 2000IKDC International Knee Documentation Committee
Mid-study, one patient in the study group was excluded because of post-operative tibial plateau fracture, and one patient in the control group was excluded because of post-operative joint infection. Furthermore, as a result of disruptions caused by the coronavirus disease 2019 (COVID-19) pandemic, we lost 5 patients (study 2; control 3). In total, 3 patients in the study group and 4 patients in the control group were not followed up at 12 months.
Pain scores decreased over the first three post-operative days, and there were no significant differences between the groups (P > 0.05) (Table 2). Over the course the 12-month follow-up period, knee function and proprioception scores of all patients improved. There were no significant differences between two groups at different time points (P > 0.05) (Table 2).
Table 2.
VAS score of patients 3 days after operation and the function of knee at clinical follow-upa
| Study group (n = 30) | Control group (n = 30) | P | |
|---|---|---|---|
| VAS | |||
| 1 day | 3.86 ± 1.41 (30) | 3.96 ± 1.77 (30) | 0.82 |
| 2 days | 2.72 ± 1.49 (30) | 3.00 ± 1.73 (30) | 0.53 |
| 3 days | 1.90 ± 1.47 (30) | 2.04 ± 1.46 (30) | 0.71 |
| Lysholm score | |||
| Pre-operation | 58.91 ± 22.87 (30) | 53.56 ± 20.60 (30) | 0.44 |
| 3 months | 76.12 ± 16.78 (30) | 77.58 ± 9.72 (29) | 0.75 |
| 6 months | 81.83 ± 14.83 (29) | 85.18 ± 12.89 (26) | 0.57 |
| 12 months | 83.10 ± 11.25 (27) | 85.62 ± 7.46 (26) | 0.53 |
| Tegner score | |||
| Pre-operation | 3.13 ± 2.72 (30) | 3.33 ± 2.54 (30) | 0.34 |
| 3 months | 3.40 ± 1.32 (30) | 4.00 ± 1.89 (29) | 0.08 |
| 6 months | 3.92 ± 1.68 (29) | 3.64 ± 1.29 (26) | 0.66 |
| 12 months | 4.10 ± 1.60 (27) | 4.19 ± 1.65 (26) | 0.08 |
| 2000IKDC | |||
| Pre-operation | 54.35 ± 13.59 (30) | 54.44 ± 12.89 (30) | 0.98 |
| 3 months | 56.53 ± 10.94 (30) | 60.84 ± 11.08 (29) | 0.25 |
| 6 months | 65.58 ± 10.74 (29) | 65.55 ± 10.28 (26) | 0.99 |
| 12 months | 68.90 ± 8.72 (27) | 67.85 ± 8.17 (26) | 0.77 |
| Proprioception | |||
| 3 months | 2.24 ± 0.83 (30) | 2.26 ± 0.81 (29) | 0.92 |
| 6 months | 2.67 ± 1.23 (29) | 2.45 ± 0.93 (26) | 0.65 |
| 12 months | 2.80 ± 0.70 (27) | 2.69 ± 0.86 (26) | 0.39 |
aVAS Visual Analogue Scale, 2000IKDC International Knee Documentation Committee
Between CT scans performed 4 days and those taken 12 months after operation, FT and TT diameters increased significantly for all patients (P < 0.05). There were no significant differences between the study and control groups at the two time points (P > 0.05) (Table 3).
Table 3.
Comparison of the FT and TT among different groupsa
| Study group, n | Control group, n | P | |
|---|---|---|---|
| FT (mm) | |||
| 4 days | 6.91 ± 0.74 (n = 30) | 7.30 ± 1.17 (n = 30) | 0.20 |
| 12 months | 8.88 ± 1.46 (n = 27) | 8.42 ± 2.75 (n = 26) | 0.67 |
| P | 0.00b | 0.03b | |
| TT (mm) | |||
| 4 days | 9.31 ± 0.83 (n = 30) | 9.36 ± 0.88 (n = 30) | 0.84 |
| 12 months | 9.50 ± 1.07 (n = 27) | 9.99 ± 1.91 (n = 26) | 0.52 |
| P | 0.03b | 0.00b | |
aFT femoral tunnel, TT tibial tunnel
bP < 0.05
At the 12-month follow-up, SNQs for the FT (tendon–bone healing) and IAG (graft maturation) in the study group were lower than those of the control group, but the differences were not significant (FT, P = 0.89; IAG, P = 0.06). The P value for IAG was close to the critical value (Table 4). Less ATT was measured in the study group than that measured in the control group, but there was no significant difference between groups (P > 0.05) (Table 4).
Table 4.
Comparison of the SNQ and ATT among different groups at 12 monthsa
| Study group, 27 | Control group, 26 | P | |
|---|---|---|---|
| SNQ (LAG) | 1.38 ± 0.70 | 2.01 ± 0.62 | 0.06 |
| SNQ (FT) | 2.39 ± 1.22 | 2.46 ± 0.83 | 0.89 |
| ATT (mm) | 0.85 ± 0.99 | 1.39 ± 1.98 | 0.62 |
aSNQ Signal/noise quotient, LAG intra-articular graft, FTG femoral tunnel graft, ATT anterior tibial translation
Discussion
We found that, though knee joint function improved over time, there were no significant differences between two groups at time points; therefore, PRP had no apparent positive effects on knee function after ACL reconstruction. This finding is consistent with that of another study [11]. While some researchers [12] have concluded that PRP had no effect on either post-operative knee pain or functional improvement, others found that, while PRP did not appear to improve post-operative knee function, it could relieve post-operative pain [13, 14]. Our results did not indicate that pain relief was greater in the study group compared with that in the control group. This inconsistency in findings may be related to differences in PRP dose, PRP concentration, PRP control and operation quality, and sample size. PRP had no apparent effect on either proprioception or anterior stability of the knee.
This issue—promoting tendon–bone healing and graft maturity after ACL reconstruction—is not new. In 1998, Radice et al. [15] investigated healing after ACL reconstruction and found that, 6 months after ACL reconstruction, MRI showed that the IAG region had low signal values, bone plugs in the tunnel had high signal values, and diffuse oedema was evident around the tunnels—findings consistent with the integration of local tissue; at 9 months, the signal of the graft was non-homogeneous, which indicated few cells, irregular collagen fibres, and blood vessels in the tissue; and at 12 months, grafts had high signal values and appeared homogeneous, with no oedema around the tunnels, but graft tissues appeared similar to normal ACL tissue.
Other studies [16] found that the signal of the graft tissue first increased then decreased. Therefore, the signal on MRI is significantly correlated with the tissue healing on histology. However, the process of tendon–bone healing has three main stages—necrosis, proliferation and ligamentization [17]—which occur slowly. This may be related to the formation of Sharpey fibre at the tendon–bone interface; however, Silva et al. [18] found that PRP cannot promote Sharpey fibre integration and tendon–bone healing.
We found no significant differences in SNQ of the IAG and FT (P > 0.05). However, for IAG, P = 0.06; therefore, with an increased sample size or prolonged follow-up time, the difference may be significant. Therefore, it is possible that PRP promotes graft maturation, which has been supported by the findings of some studies [19].
Additionally, in a study [20] that divided the signal value in ROIs into different levels, transforming the signal values into categorical data, results showed improved healing in the PRP group (healing grades) compared with those in the control group. However, in another study [21] using a similar method, PRP did not appear to promote tendon–bone healing. Our SNQ (FT) data agree with the findings of the latter.
Existing evaluation method for graft maturation, including MRI scoring, grading, computer simulation and computerized three-dimensional reconstruction, are based on signal value of graft, shape or continuity [20, 22–25]. SNQ has been used to evaluate tendon–bone healing in ACL reconstruction studies because this method is simple and can be adjusted for different picture archiving and communication systems by using ROIs outlined by a specific shape instead of using circles [16]. This method has some problems: SNQ relies on researchers’ subjective selection of ROIs, and inhomogeneous signals are distributed on many layers from which ROIs must be selected. Therefore, measurement errors may be large, and conclusions may be inaccurate. Histology and arthroscopy are more accurate methods, but for ethical reasons, must be mostly used in animal experiments. A satisfactory method of assessing tendon–bone healing and graft maturation does not yet exist. Further interdisciplinary research is needed to find a reliable standard for verifying the effect of PRP.
Almost all patients exhibited bone tunnel enlargement after operation. The earlier necrosis, proliferation and ligamentization are completed, the better the clinical effect achieved [26]. Ran et al. [27] concluded that the enlargement of bone tunnels affects tendon–bone healing; if tunnels are enlarged by 4 mm, the effect on the stability of graft would require reoperation. Starantzis et al. [28] believed that PRP was a safe and effective choice in ACL reconstruction and could offset biological factors influencing tunnel enlargement but also that the effect of PRP may be masked by mechanical factors and cause inevitable enlargement of bone tunnels. However, other researchers [29, 30] thought PRP did little for that. We also found no significant differences between the group administered PRP and the control group: both groups exhibited bone tunnel enlargement, but PRP had no significant effect.
Limitations
There are some limitations to our research that may affect the results, such as a small sample size, short follow-up period and missing follow-up data because of the COVID-19 pandemic. In particular, because of individual differences with respect to concentration and platelet activity in autogenous PRP applications, we could not precisely control PRP quality. We made conclusions just based on CT, MRI and knee function scores, but we did not explore the effects of PRP at the cellular and molecular level.
Conclusion
PRP had no significant effect in reducing bone tunnel enlargement, accelerating tendon–bone healing or improving knee function but may have the ability to accelerate IAG maturation after ACL reconstruction.
Acknowledgements
Thank my friend Xinrong Qiao and my girlfriend Danyu Chen for their advice and help to this study.
Author Contributions
HG contributed to the article writing and statistics. BH contributed to the article writing and data collection. ZZ contributed to read images. LF contributed to follow up. LC performed surgeries, designed the study and was responsible for the paper.
Funding
This study was funded by Capital Characteristic Clinic Project, China (NO. 12017B3003).
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
This study was approved by the Ethical Committee of Beijing Tsinghua Changgung Hospital (No. 16152-0110).
Informed Consent
All patients signed informed consent regarding publishing their data and photographs.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lai CCH, Ardern CL, Feller JA, Webster KE. Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: a systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. British Journal of Sports Medicine. 2018;2018(52):128–138. doi: 10.1136/bjsports-2016-096836. [DOI] [PubMed] [Google Scholar]
- 2.Gianotti S, Marshall S, Hume P, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. Journal of Science and Medicine in Sport. 2009;2009(12):622–627. doi: 10.1016/j.jsams.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. American Journal of Sports Medicine. 2010;2010(38):189–199. doi: 10.1177/0363546509356530. [DOI] [PubMed] [Google Scholar]
- 4.Mohtadi N, Chan D, Barber R, Oddone PE. A randomized clinical trial comparing patellar tendon, hamstring tendon, and double-bundle acl reconstructions: patient-reported and clinical outcomes at a minimal 2-year follow-up. Clinical Journal of Sport Medicine. 2015;2015(25):321–331. doi: 10.1097/JSM.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 5.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 1998;1998(85):638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 6.Wu W, Zhang J, Dong Q, Liu Y, Mao T, Chen F. Platelet-rich plasma—a promising cell carrier for micro-invasive articular cartilage repair. Medical Hypotheses. 2009;2009(72):455–457. doi: 10.1016/j.mehy.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi A, Doumas C, O'Connor JP, Parsons JR, Lin SS. The effects of local platelet rich plasma delivery on diabetic fracture healing. Bone. 2006;2006(38):540–546. doi: 10.1016/j.bone.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Król W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. Journal of Bone and Joint Surgery. British Volume. 2007;2007(89):417–420. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 9.Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;2008(24):560–568. doi: 10.1016/j.arthro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Fu FH, van Eck CF, Tashman S, Irrgang JJ, Moreland MS. Anatomic anterior cruciate ligament reconstruction: a changing paradigm. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;2015(23):640–648. doi: 10.1007/s00167-014-3209-9. [DOI] [PubMed] [Google Scholar]
- 11.Davey MS, Hurley ET, Withers D, Moran R, Moran CJ. Anterior cruciate ligament reconstruction with platelet-rich plasma: a systematic review of randomized control trials. Arthroscopy. 2020;2020(36):1204–1210. doi: 10.1016/j.arthro.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Walters BL, Porter DA, Hobart SJ, Bedford BB, Hogan DE, McHugh MM, et al. Effect of intraoperative platelet-rich plasma treatment on postoperative donor site knee pain in patellar tendon autograft anterior cruciate ligament reconstruction: a double-blind randomized controlled trial. American Journal of Sports Medicine. 2018;2018(46):1827–1835. doi: 10.1177/0363546518769295. [DOI] [PubMed] [Google Scholar]
- 13.de Almeida AM, Demange MK, Sobrado MF, Rodrigues MB, Pedrinelli A, Hernandez AJ. patellar tendon healing with platelet-rich plasma. American Journal of Sports Medicine. 2012;2012(40):1282–1288. doi: 10.1177/0363546512441344. [DOI] [PubMed] [Google Scholar]
- 14.de Andrade ALL, Sardeli AV, Garcia TA, Livani B, Belangero WD. Time-dependent effect of platelet-rich plasma in reducing donor-site pain after anterior cruciate ligament reconstruction. American Journal of Sports Medicine. 2020;2020:363546520968289. doi: 10.1177/0363546520968289. [DOI] [PubMed] [Google Scholar]
- 15.Radice F, Gutiérrez V, Ibarra A. Arthroscopic, histologic and MRI correlation in the maturation process of the graft in ACL reconstruction in humans. Arthroscopy. 1998;14:S201998. [Google Scholar]
- 16.Zhang Y, Liu S, Chen Q, Hu Y, Sun Y, Chen J. Maturity progression of the entire anterior cruciate ligament graft of insertion-preserved hamstring tendons by 5 years: a prospective randomized controlled study based on magnetic resonance imaging evaluation. American Journal of Sports Medicine. 2020;2020(48):2970–2977. doi: 10.1177/0363546520951507. [DOI] [PubMed] [Google Scholar]
- 17.Pauzenberger L, Syré S, Schurz M. “Ligamentization” in hamstring tendon grafts after anterior cruciate ligament reconstruction: a systematic review of the literature and a glimpse into the future. Arthroscopy. 2013;2013(29):1712–1721. doi: 10.1016/j.arthro.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Silva A, Sampaio R. Anatomic ACL reconstruction: Does the platelet-rich plasma accelerate tendon healing? Knee Surgery, Sports Traumatology, Arthroscopy. 2009;2009(17):676–682. doi: 10.1007/s00167-009-0762-8. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Fu SC, Cheuk YC, Ong TY, Feng H, Yung SH. The effect of thermosensitive hydrogel platelet-rich-plasma complex in the treatment of partial tear of anterior cruciate ligament in rat model. J Orthop Translat. 2020;2020(24):183–189. doi: 10.1016/j.jot.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seijas R, Ares O, Catala J, Alvarez-Diaz P, Cusco X, Cugat R. Magnetic resonance imaging evaluation of patellar tendon graft remodelling after anterior cruciate ligament reconstruction with or without platelet-rich plasma. Journal of Orthopaedic Surgery (Hong Kong) 2013;2013(21):10–14. doi: 10.1177/230949901302100105. [DOI] [PubMed] [Google Scholar]
- 21.Orrego M, Larrain C, Rosales J, Valenzuela L, Matas J, Durruty J, et al. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy. 2008;2008(24):1373–1380. doi: 10.1016/j.arthro.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Nin JR, Gasque GM, Azcárate AV, Beola JD, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;2009(25):1206–1213. doi: 10.1016/j.arthro.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Ahn JH, Lee SH, Choi SH, Lim TK. Magnetic resonance imaging evaluation of anterior cruciate ligament reconstruction using quadrupled hamstring tendon autografts: comparison of remnant bundle preservation and standard technique. American Journal of Sports Medicine. 2010;2010(38):1768–1777. doi: 10.1177/0363546510368132. [DOI] [PubMed] [Google Scholar]
- 24.Ge Y, Li H, Tao H, Hua Y, Chen J, Chen S. Comparison of tendon-bone healing between autografts and allografts after anterior cruciate ligament reconstruction using magnetic resonance imaging. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;2015(23):954–960. doi: 10.1007/s00167-013-2755-x. [DOI] [PubMed] [Google Scholar]
- 25.Lee A, Chung W, Kim D, Lee K, Chung D, Do S, et al. Anterior cruciate ligament reconstruction in a rabbit model using canine small intestinal submucosa and autologous platelet-rich plasma. Journal of Surgical Research. 2012;2012(178):206–215. doi: 10.1016/j.jss.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 26.Claes S, Verdonk P, Forsyth R, Bellemans J. The, “ligamentization” process in anterior cruciate ligament reconstruction: What happens to the human graft? A systematic review of the literature. American Journal of Sports Medicine. 2011;2011(39):2476–2483. doi: 10.1177/0363546511402662. [DOI] [PubMed] [Google Scholar]
- 27.Ran S. The Relevant Study of Bone Tunnel Enlargement after Anterior Cruciate Ligament Reconstruction. Hebei Medical University; 2007. [Google Scholar]
- 28.Starantzis KA, Mastrokalos D, Koulalis D, Papakonstantinou O, Soucacos PN, Papagelopoulos PJ. The potentially positive role of PRPs in preventing femoral tunnel widening in ACL reconstruction surgery using hamstrings: a clinical study in 51 patients. Journal of Sports Medicine (Hindawi Publ Corp). 2014;2014(789317):2014. doi: 10.1155/2014/789317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirzatolooei F, Alamdari MT. Khalkhali HR (2013) The impact of platelet-rich plasma on the prevention of tunnel widening in anterior cruciate ligament reconstruction using quadrupled autologous hamstring tendon: a randomised clinical trial. The Bone and Joint Journal. 2013;95:65–69. doi: 10.1302/0301-620X.95B1.30487. [DOI] [PubMed] [Google Scholar]
- 30.Vadalà A, Iorio R, De Carli A, Ferretti M, Paravani D, Caperna L, et al. Platelet-rich plasma: does it help reduce tunnel widening after ACL reconstruction? Knee Surgery, Sports Traumatology, Arthroscopy. 2013;2013(21):824–829. doi: 10.1007/s00167-012-1980-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.