Abstract
Sperm drastically change their flagellar movement in response to the surrounding physical and chemical environment. Testicular sperm are immotile; however, they gain the competence to initiate motility during passage through the male reproductive tract. Once ejaculated, the sperm are activated and promptly initiate motility. Unlike mammals, ejaculated sperm in birds are stored in specialized tubular invaginations referred to as sperm storage tubules (SSTs), located between the vagina and uterus, before fertilization. The resident sperm in the SSTs are in a quiescent state and then re-activated after release from the SSTs. It is thought that avian sperm can undergo motility change from quiescent to active state twice; however, the molecular mechanism underlying sperm motility regulation is poorly understood. In this short review, we summarize the current understanding of sperm motility regulation in male and female bird reproductive tracts. We also describe signal transduction, which regulates sperm motility, mainly derived from in vitro studies.
Keywords: avian sperm, sperm motility, sperm storage
Introduction
In vertebrates, sperm are produced as self-mobile tiny cells that carry male haploid DNA for successful fertilization. After production in testes, sperm initiate a journey to reach ovulated oocytes, which are localized deep inside the female reproductive tract. Before fertilization, sperm descend the male genital tract and sperm maturation proceeds. Then, they are ejaculated together with seminal plasma and transferred into the female genital tract by copulation. After copulation, sperm then ascend higher into the female genital tract and finally reach the oocyte fertilization site. Although as many as one hundred million sperm are ejaculated into the female reproductive tract during each copulation, only a small fraction that arrives at the site of fertilization is capable of fusing with ovulated ova (reviewed by Matsuzaki and Sasanami, 2017). From this perspective, the fertilization process can be considered highly competitive for sperm compared to oocytes. To compete with rival sperm, sperm motility is one of the most important traits because highly motile sperm can swim faster than less motile sperm (Rosengrave et al., 2008; Kim et al., 2017). It is known that sperm motility is generated by axonemal motor protein dynein, which hydrolyzes ATP to drive microtubule sliding (Gibbons, 1988; Inaba, 2003).
In internal fertilizers, successful fertilization also depends on the timely arrival of both gametes at the site of fertilization; however, de-coupling of ovulation timing and insemination is common in the majority of species. Females of such species can store sperm in reproductive tracts until eggs are ready to be fertilized. This phenomenon is common in many non-mammalian animals, including insects, fish, amphibians, reptiles, and birds (Birkhead and Møller, 1993; Holt, 2011). In birds, sperm storage tubules (SSTs), which are simple tubular invaginations located between the vagina and uterus, serve as sperm storage sites (Bakst et al., 1994; Sasanami et al., 2013; Matsuzaki and Sasanami, 2017). Once ejaculated, sperm migrate to and are subsequently stored in the lumen of SSTs, where they can remain without loss of fertilization capacity for long periods of time (up to 15 weeks) at normal body temperatures (41°C) (Birkhead and Møller, 1992; Bakst, 2011). Of particular interest in sperm motility regulation is that avian sperm can undergo motility alteration from quiescent to active state twice. First, sperm motility initiation occurs after ejaculation and is then inactivated while sperm reside in the SSTs. Motility is then thought to be re-activated after release from the SSTs. Therefore, a molecular switch, which controls motility activation and inactivation in response to extracellular stimuli, may operate in avian sperm; however, the underlying mechanism is yet to be elucidated.
In this review, we summarize the current understanding of sperm motility regulation in avian reproductive tracts (Fig. 1). We also describe signal transduction pathways that regulate sperm motility, mainly reported in vitro studies.
Fig. 1.
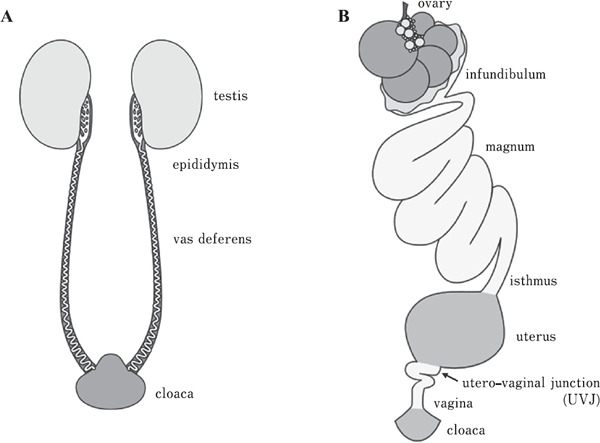
Schematic representation of the male and female reproductive tracts of the Japanese quail during the breeding season. (A) Male reproductive tract is composed of the testes, epididymis, vas deferens, and cloacal gland. Sperm are stored, and motility is arrested in the vas deferens until ejaculation. (B) Female reproductive tract is composed of the ovary and oviduct, and the ovulated oocyte passes through the oviduct. Several kinds of extracellular matrices, such as the outer layer of the vitelline membrane, albumen, shell membrane, and eggshell, are deposited on the surface of the oocyte. The ejaculated sperm are stored in sperm storage tubules located in the uterovaginal junction until the time of ovulation. Fertilization takes place in the infundibulum part of the oviduct.
Sperm Motility Regulation in the Male Reproductive Tract
In natural mating or artificial insemination, sperm motility is integral for the success of fertilization. Howarth (1983) reported that testicular sperm showed limited motility, and no fertilized egg was obtained when they were inseminated into vaginas (Howarth, 1983). On the other hand, epididymal sperm showed medium motility (49% of sperm were motile) and those obtained from the vas deferens exhibited equivalent motility (88%) to ejaculated sperm when suspended in a sperm extender. Considering this, sperm descending the male reproductive tract gradually acquire motility competence in the epididymis and fully develop this trait after reaching the vas deferens. However, the factors/mechanisms that lead to sperm motility competence in the male reproductive tract are not understood. Few studies are available, and our current understanding is extremely limited. Ashizawa and his colleague tested the effects of seminal plasma on the motility of washed sperm in chickens. They demonstrated that the addition of different doses of seminal plasma enhanced sperm motility in vitro (Ashizawa and Wishart, 1987). Sperm motility reduction in the absence of seminal plasma is not thought to be due to a reduction in energy metabolism because ATP contents in the cells showed similar levels irrespective of the presence or absence of seminal plasma (Ashizawa and Wishart, 1987). These results indicate the presence of factors in seminal plasma that regulate sperm motility. Although it is not known whether these substances alter sperm motility competence or simply enhance sperm motility itself, fractionation studies on chicken seminal plasma indicated that the factor affecting sperm motility was a low molecular weight substance (Ashizawa and Okauchi, 1984; Ashizawa and Wishart, 1987). Unfortunately, the nature of this substance is un- known.
As mentioned above, sperm stored in vas deferens have motility competence; however, the motility initiation is arrested. We also confirmed that vas deferens sperm in quails are immotile inside; however, they quickly gain motility when suspended in sperm extender (Matsuzaki et al., 2017). Currently, it is unclear how sperm motility is arrested in the vas deferens. In external fertilizers such as marine invertebrates and fish, sperm are immotile before spawning, but they are immediately activated by specific chemicals or environmental cues, such as osmolality changes (Inaba, 2003; Yoshida et al., 2008). In ascidians, a sulfated steroid, referred to as sperm activating and attracting factor (SAAF), is released from the eggs and the sperm sense the SAAF via plasma membrane Ca2+-ATPase to initiate motility (Yoshida et al., 2002; Nomura et al., 2004). In freshwater teleosts, sperm motility is initiated when sperm are diluted in a hypotonic solution, whereas a hypertonic solution effectively activates sperm motility in marine teleosts (Morisawa and Suzuki, 1980). In the newt Cynops pyrrhogaster, a protein localized in the outer layer of the egg jelly, referred to as a sperm motility-initiating substance, was reported to initiate sperm motility (Watanabe et al., 2011). Our preliminary experiments in Japanese quail showed that the ATP contents in vas deferens sperm were very low, but immediately (within 30 seconds) increased as sperm motility was initiated (data not shown). These results indicate the possibility that ATP production in vas deferens sperm is inhibited by an unknown mechanism and that such inhibitory effects are not observed when the sperm are released outside the body. However, further studies are required to elucidate the initiation system in avian sperm motility.
Sperm Motility Regulation in Female Reproductive Tract
During copulation, males release fully mature sperm into the female vagina. In birds, spermatozoa initially reside in oviductal sperm storage sites, SSTs, before encountering an ovulated ovum. Sperm are stored in the SSTs located in the uterovaginal junction (UVJ) and released according to the timing of ovulation. Before entering SSTs, ejaculated sperm are selected in the vagina, most likely via sperm ejection through female defecation. In turkeys, more than 80% of the sperm are ejected from the vagina soon after mating (Howarth, 1983). In addition, less than 1% of sperm inseminated into the vagina enter the SST (Bakst et al., 1994). Sperm artificially introduced into the vagina of a chicken reached the SST within an hour (Das et al., 2009), and the intrinsic motility of sperm may be an important factor in the uptake into the SST (Froman, 2003). In fact, it was reported that sperm with poor motility showed less ability to fill the SSTs, and the paternity of embryos was biased towards males with high sperm motility when a sperm mix with similar sperm numbers from two males was artificially inseminated into females (unpublished data). Although there is no doubt that sperm motility is essential for better fertilization success, we have no information as to whether sperm motility is regulated by factors derived from the female reproductive tract.
We, and others, observed that sperm residing in SSTs are quiescent in motility (Bakst, 1987; Matsuzaki et al., 2015), and this phenomenon is a long-standing enigma in the field of avian reproduction. We recently demonstrated that the luminal environment of SSTs is acidic due to the presence of large amounts of lactic acid (approximately 13 mM) released from the SSTs under hypoxic conditions (Matsuzaki et al., 2015). This acidic condition inhibits the ATPase activity of dynein, the motor protein of sperm flagellum (Matsuzaki et al., 2015). In this condition, the pHof sperm cytosol also declines to around 5.4, coinciding with the extracellular pH. The hypoxic condition created by the SSTs also decreases sperm mitochondrial activity (Matsuzaki et al., 2015). Thus, sperm motility in the SSTs is highly arrested due to less energy production and consumption. This system appears reasonable regarding sperm storage for longer periods of time because reduced sperm respiration in the SSTs leads to less production of reactive oxygen species and thus enables minimal sperm damage. In contrast to our experimental evidence regarding arrested sperm motility in the SSTs, Froman (2003) suggested another model in which sperm maintained their motility inside of SSTs in chickens (Froman, 2003). He suggested that sperm sustain their location against the reverse flow (from the base of the SSTs to orifice) generated by SSTs, and are egressed from the SSTs when the sperm swimming velocity falls below the flow. Furthermore, Ahammad et al. (2011) reported that chicken sperm gain the ability to bind with SST epithelial cells during the passage though the male reproductive tract, and this binding plays a role in sperm storage (Ahammad et al., 2011). Although the speculation of such a ligand-receptor interaction model is interesting to investigate, we, and others, observed that sperm residing in SSTs form a bundle (Bakst, 1983; Sasanami et al., 2013), and the cells located in the center of the bundle are unable to contact the SST epithelium. We have no explanation for this inconsistency between chicken and quail sperm.
The sperm stored in the SSTs are released according to the time of ovulation and reach the fertilization site. Females have been shown to actively control the timing of sperm release. When females with sperm-filled SSTs were intravenously injected with progesterone, most of the sperm were released within 1 h post-injection (Ito et al., 2011). The circulating progesterone in poultry, including Japanese quail, reaches a peak around 4 to 6 h before the next ovulation (Mashaly et al., 1976; Doi et al., 1980; Etches and Cheng, 1981). Therefore, it is reasonable to suppose that the two events, sperm release from the SST and ovulation, are stimulated simultaneously by progesterone to achieve efficient fertilization. Furthermore, we found that the surface epithelial cells of UVJ express heat shock protein 70 (HSP70), and we hypothesize that this protein stimulates sperm motility to facilitate sperm migration in the oviduct after its release from SSTs (Hiyama et al., 2014). Additionally, we found that bacterially expressed HSP70 activates flagellar movement in sperm and that recombinant HSP70 binds to the surface of sperm by interacting with voltage-dependent anion channel protein 2 (VDAC2) (Hiyama et al., 2014). Furthermore, injection of anti-HSP70 antibody into the vagina significantly inhibited fertilization in vivo (Hiyama et al., 2014). Thus, these results suggest that HSP70 binds to the sperm surface by binding with VDAC2, and this binding appears to play an important role in sperm migration within the oviduct. Because direct observation of sperm migration inside the oviduct is technically difficult due to the opaque and thick oviductal wall, we are unable to conclude that sperm motility is the sole important factor in the migration of cells to the fertilization site. Currently, we are producing infrared fluorescent sperm using a CRISPR-Cas-based gene knock-in system that facilitates live imaging of sperm in vivo/exvivo. Such experiments will help to advance our understanding of how sperm reach the fertilization site in birds.
Mechanism Regulating Sperm Motility
It is known that the flagellar movement of sperm is driven by the sliding of microtubules with motor protein dynein ATPase (Gibbons, 1988, 1996; Inaba, 2007). However, much is unresolved regarding the regulatory mechanism of sliding events in the axoneme. Ashizawa and colleagues observed a reversible immobilization of chicken sperm motility in which sperm suspended in a simple salt solution without Ca2+ were immotile at body temperature (40°C), but motility was instantly restored when the incubation temperature decreased to 30°C (Munro, 1938; Ashizawa and Nishiyama, 1978). Another group reported that this reversible activation/inactivation of motility is also observed in drake sperm and partially in turkey sperm, but not in quail sperm (Wishart and Wilson, 1999). In chicken sperm, motility inhibition at 40°C was quickly recovered when Ca2+ was included in the incubation mixture (Ashizawa et al., 1989a). The depletion of Ca2+ from the medium by adding Ca2+ chelating reagents disturbed sperm motility even at 30°C. Therefore, it is considered that Ca2+ is essential for the maintenance of chicken sperm motility, as also reported in the sperm of many animals (Ashizawa et al., 1994b). Later, it was found that motility inactivation at body temperature was also canceled by an increase in intracellular pH(pH i) of the sperm (Ashizawa et al., 1994c). At 30°C, sperm motility was activated in a medium with various extracellular pH (pHe) ranging from 7.3–10.1, whereas sperm were in a quiescent state at 40°C in a medium with pHe below 8.1 (Ashizawa et al., 1994c). Because pHi at 40°C is approximately 0.3 units lower than pHi at 30°C, an acidic pHi at 40°C may play a role in motility immobilization at body temperature. Alternatively, the alkalization of sperm pHi is important for sperm motility activation in chickens. To further understand this unique phenomenon in chicken sperm, the authors investigated flagellar motility in demembraned sperm in which sperm were treated with the non-ionic detergent, TritonX-100. They found that the de-membranated sperm expressed similar motility patterns to intact sperm regarding reversible immobilization of motility (Ashizawa et al., 1989b), indicating that de-membraned spermatozoa are a suitable model for investigating the direct effects of various inhibitors/activators that are not able to pass through the plasma membrane by sperm flagellar movements in vitro. Using this system, it was found that complex signal transduction machinery exists in sperm motility regulation in chickens. For instance, the addition of recombinant protein phosphatase type 1 (PP-1), the specific activator of protein kinase C (PKC), or the substrate peptides for mitogen-activated protein kinase (MAPK) or p34cdc2 kinase markedly decreased the motility of de-membraned spermatozoa at 30°C, indicating that the protein phosphorylation/de-phosphorylation may be involved in sperm motility regulation (Ashizawa et al., 1994d, 1997a, 2006). A marked difference in the phosphorylation status of 116-, 86-, 79-, 50-, and 29-kDa proteins was observed after the addition of these inhibitors (Ashizawa et al., 1995, 1997a, b). Recently, we also studied the effects of various inhibitors of protein kinases in quail spermatozoa (Matsuzaki et al., 2017). We employed four protein kinase inhibitors: bisindolylmaleimide II (BisII), a potent competitive protein kinase C (PKC) inhibitor (Mahata et al., 2002); bisindolylmaleimide V (BisV), a weak inhibitor of PKC (Mahata et al., 2002); H-89, a potent cell-permeable inhibitor of protein kinase A (PKA) (Chijiwa et al., 1990); and LY294002, a selective inhibitor of phosphatidylinositol 3-kinase (PI3-kinase) (Vlahos et al., 1994). When we incubated the intact spermatozoa with these inhibitors, only BisII inhibited the sperm motility. In addition, when the phosphorylated substrate proteins by PKC were detected by Western blot analysis, the intensity of the band in sperm incubated in the presence of BisII decreased. Moreover, immunoreactive PKCι and µ isoforms in the sperm lysates were detected. Therefore, these results indicated that the PKC signaling pathway may be involved in sperm motility regulation, and protein phosphorylation by PKC may be required to maintain flagellar movement in the Japanese quail. Thus, the signal transduction system regulating sperm motility appears to differ between chickens and quails, though the details of this discrepancy are not known. For instance, evidence has shown that PKC activation may contribute to a decrease in the flagellar movement of fowl spermatozoa (Ashizawa et al., 1994a), which is contrary to our observations in the Japanese quail. Because the structure of quail sperm in terms of midpiece size is different from that of chickens (Fig. 2), we suspect there are many differences, including the signal transduction system, regulating flagellar movement (Table 1). Further studies are required to advance our understanding of motility regulation machinery in avian spermatozoa.
Fig. 2.
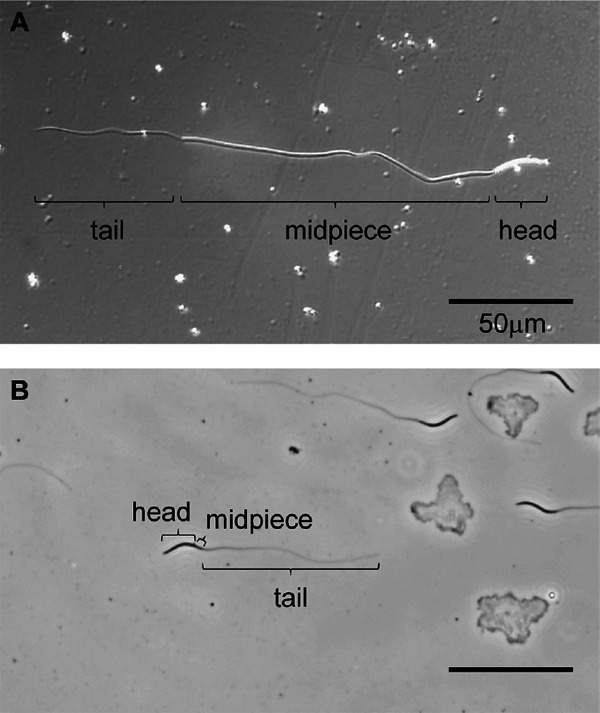
Light micrograph of the avian sperm head, midpiece, and tail. (A) Japanese quail sperm. The junction of the head and midpiece or midpiece and tail are clearly differentiated. (B) Chicken sperm. The junction of the head and midpiece is unclear. Bar=50 µm.
Table 1. Effects of various chemicals on chicken and quail sperm motility in the presence or absence of Ca2+ in vitro at 40°C.
| Chemicals | Effects | Chicken | Japanese quail | References | ||
|---|---|---|---|---|---|---|
| −Ca2+ | +Ca2+ | −Ca2+ | +Ca2+ | |||
| EGTA | Ca2++ chelator | − | + | +* | +* | Ashizawa et al., 1994b |
| BAPTA-AM | Intracellular Ca2++ chelator | − | − | +* | +* | Ashizawa et al., 1994b |
| PD 150606 | Ca2++-dependent calpain inhibitor | − | − | N.D. | N.D. | Ashizawa et al., 2006 |
| Y-27632 | Ca2++-dependent Rho-kinase inhibitor | − | + | N.D. | N.D. | Ashizawa et al., 2006 |
| W-7 | Calmodulin antagonist | − | − | N.D. | N.D. | Ashizawa et al., 1994b |
| W-5 | Calmodulin antagonist | − | − | N.D. | N.D. | Ashizawa et al., 1994b |
| Trifluoperazine | Calmodulin antagonist | − | − | N.D. | N.D. | Ashizawa et al., 1994b |
| Calyculin A | PP1 and PP2A inhibitor | + | + | N.D. | N.D. | Ashizawa et al., 1994d |
| Okadaic acid | PP1 and PP2A inhibitor | + | + | N.D. | N.D. | Ashizawa et al., 1994d |
| LY294002 | PI3-kinase inhibitor | − | − | N.D. | + | Ashizawa et al., 2009 |
| Matsuzaki et al., 2017 | ||||||
| 1L-6-Hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate) | Akt inhibitor | − | − | N.D. | N.D. | Ashizawa et al., 2009 |
| H89 | PKA inhibitor | N.D. | N.D. | N.D. | + | Matsuzaki et al., 2017 |
| SC-9 | PKC activator | − | − | N.D. | N.D. | Ashizawa et al., 1994a |
| OAG | PKC activator | − | − | N.D. | N.D. | Ashizawa et al., 1994a |
| H-7 | PKC inhibitor | + | + | N.D. | N.D. | Ashizawa et al., 1994a |
| Bis II | PKC inhibitor | N.D. | N.D. | − | − | Matsuzaki et al., 2017 |
−: immotile, +: motile, N.D.: not determined, *: author's unpublished data
Conclusion
In this short review, we summarized our current understanding regarding sperm motility regulation in birds. Avian fertilization systems are quite different from those of mammalian species because unique systems, such as polyspermic fertilization and oviductal sperm storage, are employed for successful fertilization in avian species. Several important phenomena and molecules that regulate avian fertilization have been discovered, but our understanding of the precise mechanism has not advanced significantly because there are no efficient methods to produce genemanipulated birds. With the use of modern technology, such as the CRISPR/Cas9 system, we expect the production of transgenic and gene-knockout birds in the near future. It is possible that reverse genetics will advance our understanding of the mechanisms of avian fertilization, including sperm motility regulation.
Acknowledgments
The corresponding author, Matsuzaki, M. was awarded the 2019 Young Scientist Prize by the Japan Poultry Science Association for “Studies on the mechanism of sperm storage and motility regulation”. The corresponding author would like to express her sincere gratitude to all her collaborators and students, as well as her family. This work was supported, in part, by a Grant-in-Aid for Young Scientist (20K15648 to MM), a Grant-in-Aid for Scientific Research (B) (General) (17H03902 to TS), and a Grant-in-Aid for Challenging Exploratory Research (20K21368 to TS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahammad MU, Nishino C, Tatemoto H, Okura N, Kawamoto Y, Okamoto S and Nakada T. Maturational changes in the survivability and fertility of fowl sperm during their passage through the male reproductive tract. Animal Reproduction Science, 128: 129-136. 2011. [DOI] [PubMed] [Google Scholar]
- Ashizawa K and Nishiyama H. Effects of temperature on the vigour of motility, oxygen consumption and duration of motility of fowl spermatozoa under aerobic conditions. Japanese Poultry Science, 15: 264-266. 1978. [Google Scholar]
- Ashizawa K and Okauchi K. Stimulation of sperm motility and oxygen consumption of fowl spermatozoa by a low molecular weight fraction of seminal plasma. Journal of Reproduction and Fertility, 71: 593-598. 1984. [DOI] [PubMed] [Google Scholar]
- Ashizawa K and Wishart GJ. Resolution of the sperm motility-stimulating principle of fowl seminal plasma into Ca2+ and an unidentified low molecular weight factor. Journal of Reproduction and Fertility, 81: 495-9. 1987. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Maeda S and Okauchi K. The mechanisms of reversible immobilization of fowl spermatozoa at body temperature. Journal of Reproduction and Fertility, 86: 271-276. 1989. a. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Suzuki Y and Okauchi K. Flagellar movement in demembranated preparations of ejaculated fowl spermatozoa. Journal of Reproduction and Fertility, 86: 263-270. 1989. b. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Katayama S, Kobayashi T and Tsuzuki Y. Possible role of protein kinase C in regulation of flagellar motility and intracellular free Ca2+ concentration of fowl spermatozoa. Journal of Reproduction and Fertility, 101: 511-517. 1994. a. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Tomonaga H and Tsuzuki Y. Regulation of flagellar motility of fowl spermatozoa: evidence for the involvement of intracellular free Ca2+ and calmodulin. Journal of Reproduction and Fertility, 101: 265-272. 1994. b. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Wishart GJ, Nakao H, Okino Y and Tsuzuki Y. Inhibition of temperature-dependent immobilization of fowl spermatozoa at body temperature by an increased intracellular pH. Journal of Reproduction and Fertility, 101: 593-598. 1994. c. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Wishart GJ, Tomonaga H, Nishinakama K and Tsuzuki Y. Presence of protein phosphatase type 1 and its involvement in temperature-dependent flagellar movement of fowl spermatozoa. FEBS Letters, 350: 130-134. 1994. d. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Wishart GJ, Hashimoto K and Tsuzuki Y. Dephosphorylation of a 30-kDa protein of fowl spermatozoa by the addition of myosin light chain kinase substrate peptide inhibits the flagellar motility. Biochemical and Biophysical Research Communications, 215: 706-712. 1995. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Hashimoto K, Higashio M and Tsuzuki Y. The addition of mitogen-activated protein kinase and p34cdc2 kinase substrate peptides inhibits the flagellar motility of demembranated fowl spermatozoa. Biochemical and Biophysical Research Communications, 240: 116-121. 1997. a. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Hashimoto K and Tsuzuki Y. Regulation of fowl sperm flagellar motility by protein phosphatase type 1 and its relationship with dephosphorylation of axonemal and/or accessory cytoskelet al. proteins. Biochemical and Biophysical Research Communications, 235: 108-112. 1997. b. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Wishart GJ, Katayama S, Takano D, Ranasinghe ARAH, Narumi K and Tsuzuki Y. Regulation of acrosome reaction of fowl spermatozoa: Evidence for the involvement of protein kinase C and protein phosphatase-type 1 and/or -type 2A. Reproduction, 131: 1017-1024. 2006. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Omura Y, Katayama S, Tatemoto H, Narumi K and Tsuzuki Y. Intracellular signal transduction pathways in the regulation of fowl sperm motility: evidence for the involvement of phosphatidylinositol 3-kinase (PI3-K) cascade. Molecular Reproduction and Development, 76: 603-610. 2009. [DOI] [PubMed] [Google Scholar]
- Bakst MR. Fate of turkey spermatozoa after intrainfundibular and intramagnal inseminations. Journal of Reproduction and Fertility, 67: 315-317. 1983. [DOI] [PubMed] [Google Scholar]
- Bakst MR. Anatomical basis of sperm-storage in the avian oviduct. Scanning Microscopy, 1: 1257. 1987. [PubMed] [Google Scholar]
- Bakst MR, Wishart G and Brillard J-P. Oviducal sperm selection, transport, and storage in poultry. Poultry Science Reviews, 5: 117-143. 1994. [Google Scholar]
- Bakst MR. Role of the oviduct in maintaining sustained fertility in hens. Journal of Animal Science, 89: 1323-1329. 2011. [DOI] [PubMed] [Google Scholar]
- Birkhead TR and Møller AP. Numbers and size of sperm storage tubules and the duration of sperm storage in birds: a comparative study. Biological Journal of the Linnean Society, 45: 363-372. 1992. [Google Scholar]
- Birkhead TR and Møller AP. Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biological Journal of the Linnean Society, 50: 295-311. 1993. [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T and Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma. Journal of Biological Chemistry, 265: 5267-5272. 1990. [PubMed] [Google Scholar]
- Das SC, Isobe N and Yoshimura Y. Changes in the expression of interleukin-1β and lipopolysaccharide-induced TNF factor in the oviduct of laying hens in response to artificial insemination. Reproduction, 137: 527-536. 2009. [DOI] [PubMed] [Google Scholar]
- Doi O, Takai T, Nakamura T and Tanabe Y. Changes in the pituitary and plasma LH, plasma and follicular progesterone and estradiol, and plasma testosterone and estrone concentrations during the ovulatory cycle of the quail (Coturnixcoturnix japonica). General and Comparative Endocrinology, 41: 156-163. 1980. [DOI] [PubMed] [Google Scholar]
- Etches R and Cheng K. Changes in the plasma concentrations of luteinizing hormone, progesterone, oestradiol and testosterone and in the binding of follicle-stimulating hormone to the theca of follicles during the ovulation cycle of the hen (Gallus domesticus). Journal of Endocrinology, 91: 11-22. 1981. [DOI] [PubMed] [Google Scholar]
- Froman D. Deduction of a model for sperm storage in the oviduct of the domestic fowl (Gallus domesticus). Biology of Reproduction, 69: 248-253. 2003. [DOI] [PubMed] [Google Scholar]
- Gibbons IR. Dynein ATPases as microtubule motors. Journal of Biological Chemistry, 263: 15837-15840. 1988. [PubMed] [Google Scholar]
- Gibbons IR. The role of dynein in microtubule-based motility. Cell Sructure and Function, 21: 331-342. 1996. [DOI] [PubMed] [Google Scholar]
- Hiyama G, Matsuzaki M, Mizushima S, Dohra H, Ikegami K, Yoshimura T, Shiba K, Inaba K and Sasanami T. Sperm activation by heat shock protein 70 supports the migration of sperm released from sperm storage tubules in Japanese quail (Coturnix japonica ). Reproduction, 147: 167-178. 2014. [DOI] [PubMed] [Google Scholar]
- Holt WV. Mechanisms of sperm storage in the female reproductive tract: An interspecies comparison. Reproduction in Domestic Animals, 46: 68-74. 2011. [DOI] [PubMed] [Google Scholar]
- Howarth B. Fertilizing ability of cock spermatozoa from the testis epididymis and vas deferens following intramagnal insemination. Biology of Reproduction, 28: 586-590. 1983. [DOI] [PubMed] [Google Scholar]
- Inaba K. Molecular architecture of the sperm flagella: Molecules for motility and signaling. Zoological Science, 20: 1043-1056. 2003. [DOI] [PubMed] [Google Scholar]
- Inaba K. Molecular basis of sperm flagellar axonemes: Structural and evolutionary aspects. Annals of the New York Academy of Sciences, 1101: 506-526. 2007. [DOI] [PubMed] [Google Scholar]
- Ito T, Yoshizaki N, Tokumoto T, Ono H, Yoshimura T, Tsukada A, Kansaku N and Sasanami T. Progesterone is a sperm-releasing factor from the sperm-storage tubules in birds. Endocrinology, 152: 3952-3962. 2011. [DOI] [PubMed] [Google Scholar]
- Kim KW, Bennison C, Hemmings N, Brookes L, Hurley LL, Griffith SC, Burke T, Birkhead TR and Slate J. A sex-linked supergene controls sperm morphology and swimming speed in a songbird. Nature Ecology & Evolution, 1: 1168-1176. 2017. [DOI] [PubMed] [Google Scholar]
- Mahata M, Mahapatra NR, O'connor DT and Mahata SK. Chromaffin cell catecholamine secretion: Bisindolylmaleimide compounds exhibit novel and potent antagonist effects at the nicotinic cholinergic receptor in pheochromocytoma cells. Molecular Pharmacology, 61: 1340-1347. 2002. [DOI] [PubMed] [Google Scholar]
- Mashaly MM, Birrenkott GP, EL-Begearmi M and Wentworth BC. Plasma LHand progesterone concentrations in the turkey hen during the ovulatory cycle. Poultry Science, 55: 1226-1234. 1976. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Mizushima S, Hiyama G, Hirohashi N, Shiba K, Inaba K, Suzuki T, Dohra H, Ohnishi T, Sato Y, Kohsaka T, Ichikawa Y, Atsumi Y, Yoshimura T and Sasanami T. Lactic acid is a sperm motility inactivation factor in the sperm storage tubules. Scientific Reports, 5: 17643. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Mizushima S, Ichikawa Y, Shiba K, Inaba K and Sasanami T. Effects of a protein kinase inhibitor on sperm motility in the Japanese quail. Journal of Poultry Science, 54: 73-79. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M and Sasanami T. Sperm storage in the female reproductive tract: A conserved reproductive strategy for better fertilization success. In: Avian Reproduction (Sasanami, T., ed.). pp. 173-186. Springer Singapore, Singapore. 2017. [DOI] [PubMed] [Google Scholar]
- Morisawa M and Suzuki K. Osmolality and potassium ion: their roles in initiation of sperm motility in teleosts. Science, 210: 1145-1147. 1980. [DOI] [PubMed] [Google Scholar]
- Munro SS. Fowl sperm immobilization by a temperature-media interaction and its biological significance. Quarterly Journal of Experimental Physiology, 27: 281-291. 1938. [Google Scholar]
- Nomura M, Yoshida M and Morisawa M. Calmodulin/calmodul-independent protein kinase II mediates SAAF-induced motility activation of ascidian sperm. Cell Motility and the Cytoskeleton, 59: 28-37. 2004. [DOI] [PubMed] [Google Scholar]
- Rosengrave P, Gemmell NJ, Metcalf V, McBride K and Montgomerie R. A mechanism for cryptic female choice in chinook salmon. Behavioral Ecology, 19: 1179-1185. 2008. [Google Scholar]
- Sasanami T, Matsuzaki M, Mizushima S and Hiyama G. Sperm storage in the female reproductive tract in birds. Journal of Reproduction and Development, 59: 334-338. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY and Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). Journal of Biological Chemistry, 269: 5241-5248. 1994. [PubMed] [Google Scholar]
- Watanabe A, Takayama-Watanabe E, Vines CA and Cherr GN. . Sperm motility-initiating substance in newt egg-jelly induces differential initiation of sperm motility based on sperm intracellular calcium levels. Development Growth and Differentiation, 53: 9-17. 2011. [DOI] [PubMed] [Google Scholar]
- Wishart GJ and Wilson YI. Temperature-dependent inhibition of motility in spermatozoa from different avian species. Animal Reproduction Science, 57: 229-235. 1999. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Murata M, Inaba K and Morisawa M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proceedings of the National Academy of Sciences of the United States of America, 99: 14831-14836. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Kawano N and Yoshida K. Control of sperm motility and fertility: Diverse factors and common mechanisms. Cellular and Molecular Life Sciences, 65: 3446-3457. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


