Abstract
Cancer-associated fibroblasts (CAFs) found in primary and metastatic tumours are highly versatile, plastic and resilient cells that are actively involved in cancer progression through complex interactions with other cell types in the tumour microenvironment. As well as generating extracellular matrix components that contribute to the structure and function of the tumour stroma, CAFs undergo epigenetic changes to produce secreted factors, exosomes and metabolites that influence tumour angiogenesis, immunology and metabolism. Because of their putative pro-oncogenic functions, CAFs have long been considered an attractive therapeutic target; however, clinical trials of treatment strategies targeting CAFs have mostly ended in failure and, in some cases, accelerated cancer progression and resulted in inferior survival outcomes. Importantly, CAFs are heterogeneous cells and their characteristics and interactions with other cell types might change dynamically as cancers evolve. Studies involving single-cell RNA sequencing and novel mouse models have increased our understanding of CAF diversity, although the context-dependent roles of different CAF populations and their interchangeable plasticity remain largely unknown. Comprehensive characterization of the tumour-promoting and tumour-restraining activities of CAF subtypes, including how these complex bimodal functions evolve and are subjugated by neoplastic cells during cancer progression, might facilitate the development of novel diagnostic and therapeutic approaches. In this Review, the clinical relevance of CAFs is summarized with an emphasis on their value as prognosis factors and therapeutic targets.
Cancer initiation, progression and metastasis elicit a wide spectrum of dynamic alterations in host tissues, leading to the formation of a complex tumour stroma, also known as the tumour microenvironment (TME)1–9. In certain cancer types, such as pancreatic and breast cancers, the tumour stroma develops with a profound desmoplastic reaction resulting in an abundance of fibrous and/or connective tissue. In general, the tumour stroma is composed of extracellular matrix (ECM) components and a variety of cell populations, including immune cells, fibroblasts and vascular endothelial cells7. Essential elements of the tumour stroma, such as immune responses, inflammation, angiogenesis, metabolism, hypoxia, ECM remodelling and fibroblast heterogeneity, have been widely investigated as potential therapeutic targets2,3,10.
Fibroblast populations found in primary and metastatic cancers, collectively referred to as cancer-associated fibroblasts (CAFs), have been widely studied and are implicated in tumour initiation, progression and metastasis11–13. CAFs are now known to be highly heterogeneous mesenchymal lineage cells with diverse putative functions as revealed in studies utilizing single-cell RNA sequencing (scRNA-seq) of different cancers14–28. Whether CAFs constitute a distinct class of mesenchymal cells with unique functions will remain an active area of research for years to come. In this Review, the proposed functional roles and clinical relevance of CAFs are discussed and summarized with particular emphasis on their heterogeneity, prognostic value and therapeutic potential. In addition, we provide insights and perspectives on future research and clinical studies involving CAFs.
The definition and origin of CAFs
Fibroblasts were first defined as cells that reside in connective tissues and synthesize collagens, especially type I collagen29. At present, fibroblasts are typically defined as interstitial cells of a mesenchymal lineage that are not epithelial, endothelial or immune cells30. However, the precise cellular origins and functions of fibroblasts remain ambiguous and challenging to determine owing to the substantial phenotypic and functional heterogeneity of these cells and, thus, a lack of definitive biomarkers11,13. Fibroblasts are arguably the most resilient and versatile cells contributing to the structural maintenance of tissues and participating in the wound healing processes of most organs. Quiescent fibroblasts become activated in response to tissue damage, during wound healing and as a consequence of neoplasia31–34. Such activation is usually defined based on ECM synthesis and remodelling abilities, secretory profiles, proliferative status, and/or the expression of certain markers such as α-smooth muscle actin (αSMA).
In the context of cancer, the definition of CAFs can be simply stated as fibroblasts (non-epithelial, non-cancerous, non-endothelial and non-immune cells) that are located within or adjacent to a tumour. The many putative origins of CAFs include quiescent tissue-resident fibroblasts and pancreatic or hepatic stellate cells35–37 (FIG. 1). Other major cellular origins of CAFs identified in different studies include bone marrow-derived mesenchymal stem cells23,38–41, endothelial cells42,43 and adipocytes44. Nevertheless, the precise origins of CAFs and CAF subpopulations remain elusive, partially owing to the phenotypic and functional plasticity of these cells and the lack of well-defined lineage biomarkers. The identification of new biomarkers for non-malignant-tissue fibroblasts and CAFs through the interrogation of scRNA-seq datasets might facilitate subsequent lineage-tracing studies using specific Cre-recombinase drivers to precisely determine the cellular origins of CAFs in the context of spontaneous cancers in relevant genetically engineered mouse models (GEMMs).
Fig. 1 |. Activation of CAFs.
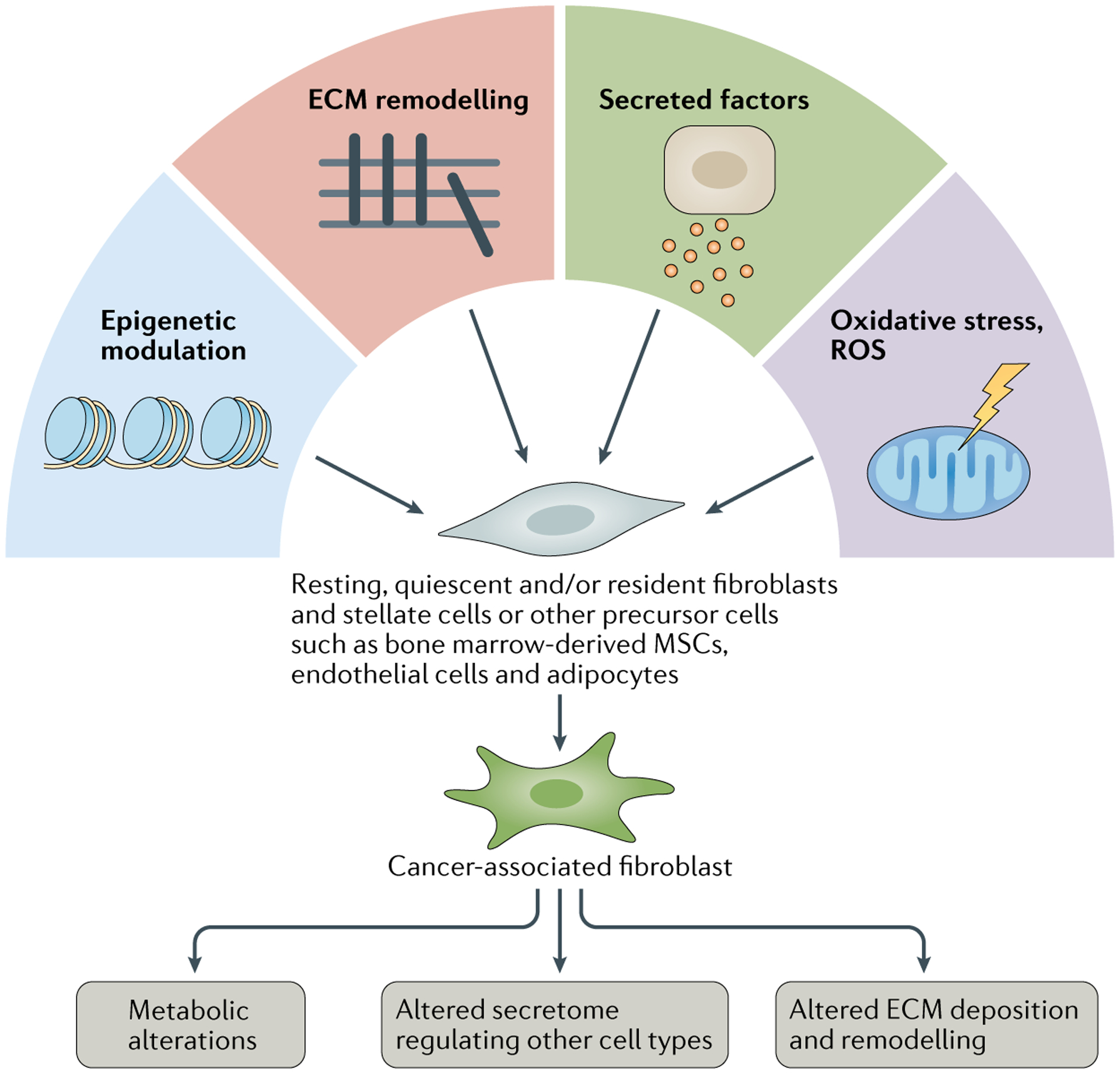
Schematic illustration of various mechanisms involved in cancer-associated fibroblast (CAF) activation. Potential cellular origins of CAFs include quiescent, resting or specific tissue-resident fibroblasts (stellate cells), bone marrow-derived mesenchymal stem cells (MSCs), endothelial cells and other cell types. ECM, extracellular matrix; ROS, reactive oxygen species.
CAFs share many basic characteristics, such as a secretory phenotype and capacity to synthesize ECM, with fibroblasts found in non-malignant tissues or in the setting of wound healing but can also have important alterations in epigenetic and transcriptional profiles11,13,45. The precise function of CAFs in cancer progression has remained elusive. Evidence from some preclinical studies suggests an initial role of fibroblast activation as part of the host response and defence mechanism against neoplasia46,47. Such defensive responses by CAFs can involve ECM-related physical barriers and encapsulation resulting from the desmoplasic reaction48. Findings from other studies suggest that, following tumour initiation, fibroblast activation and wound healing mechanisms are hijacked by cancer cells to support tumour growth45,49. Thus, fibroblasts probably have both tumour-supporting and tumour-suppressive functions that are context dependent and/or reflect the functional heterogeneity of CAFs.
CAF heterogeneity
A number of mesenchymal cell biomarkers have been used to identify CAFs, including but not limited to αSMA, fibroblast-specific protein 1 (FSP1, also known as S100A4), fibroblast activation protein (FAP), platelet-derived growth factor receptor-α (PDGFRα), PDGFRβ, desmin, discoidin domain-containing receptor 2 (DDR2) and vimentin. Heterogeneity in CAF biomarker expression was first demonstrated in co-staining studies50. More recently, the advent of scRNA-seq has enabled a deeper understanding of CAF heterogeneity across a wide range of tumour types, leading to the identification of a variety of biomarker genes defining different potential subpopulations of CAFs14–17,19–28,51–54 (TABLE 1). Such studies involve the initial selection of CAFs via flow cytometry sorting and/or analyses based on the absence of exclusion markers (epithelial, immune and endothelial markers)15,22,23,25 or the presence of putative cell-surface biomarkers, such as CD29, FAP, PDGFRα or CD90 (Thy1)25,55. Alternatively, CAFs have been identified based on their distinct transcriptional profile compared with epithelial and other stromal cells through scRNA-seq analysis14,56,57. Despite variability in CAF biomarkers used in different studies involving scRNA-seq, an αSMA-expressing myofibroblast subset has been consistently identified as one of the key subpopu lations of CAFs across multiple cancer types15,16,19,21,25,58. Other CAF subpopulations expressing biomarkers such as FAP (encoded by FAP), decorin (encoded by DCN) and/or podoplanin (encoded by PDPN) have also been identified (TABLE 1; Supplementary Table 1).
Table 1 |.
CAF subpopulations identified in indicated cancer types
| Tumour type | Sample type | CAF subpopulation | Biomarkers or characteristics of CAF subpopulation |
|---|---|---|---|
| Pancreatic cancer14–18,56,59,75 | Patient samples; KPC, KIC and KPfloxC mouse tumours | Myofibroblast (myCAF) | αSMA, THY1, TAGLN, CTGF, IGFBP3, COL12A1, THBS2 and LRRC15 |
| Inflammatory CAF (iCAF) | CLEC3B, COL14A1 and LY6C | ||
| Antigen-presenting CAF | CD74, SLPI, SAA3, MHCII and FSP1 | ||
| Colorectal cancer19,20 | Patient samples | CAF-A (FAP-CAF) | FAP, MMP2 and DCN |
| CAF-B (αSMA-CAF) | αSMA, TAGLN and PDGFA | ||
| Head and neck cancer21 | Patient samples | Myofibroblast | αSMA, MYLK and MYL9 |
| CAF1 | FAP, PDPN, COL1A2, THY1, VIM, CAV1 and MMP11 | ||
| CAF2 | FAP, PDPN, FOS, JUN, FGF7 and TGFB2 | ||
| Lung cancer26 | Patient samples | Cluster-1 | Expression of ECM-related genes |
| Cluster-2 (myofibroblast) | αSMA | ||
| Cluster-4 | Enriched at the leading edge of tumours | ||
| Cluster-5 | High mTOR gene signature; enriched in the tumour core | ||
| Cluster-7 | High mTOR signature; enriched at the leading edge of tumours | ||
| Melanoma27 | B16-F10 mouse tumours | S1 | Immune CAFs; CD34 |
| S2 | Desmoplastic CAFs; TNC | ||
| S3 | Contractile CAFs; αSMA | ||
| Breast cancer and ovarian cancer24,25,65,66 | Patient samples | CAF-S1 | FAP-high,αSMA-high; contains 8 subclusters of CAFs; enriched in TNBC |
| CAF-S2 | Low expression of most detected markers; enriched in luminal A tumours | ||
| CAF-S3 | αSMA-low, FSP1+ and PDGFRβ+ | ||
| CAF-S4 | CD29-high,αSMA-high and FAP-low; enriched in TNBC | ||
| Breast cancer57 | Patient samples | iCAF | CXCL12 |
| myCAF | ACTA2, FAP, PDPN, COL1A1 and COL1A2 | ||
| Breast cancer22 | MMTV-PyMT mouse tumours | Vascular CAF | αSMA and PDGFRβ |
| Matrix CAF | Fibulin 1 and PDGFRα | ||
| Cycling CAF | PDGFRβ | ||
| Developmental CAF (dCAF) | SCRG1andSOX9 | ||
| Breast cancer23 | 4T1 mouse tumours | PDPN-CAF | Contains 6 subclusters: immune reg E (CXCL12), immune reg L (SAA3), ECM (fibrillin 1), wound healing (αSMA), inflammatory A (CXCL1) and inflammatory B (IL-6) |
| S100A4-CAF | Includes 2 subclusters: protein folding (HSPD1) and antigen presenting (SPP1) | ||
| Bladder cancer28 | Patient samples | myo-CAF | RGS5, MYL9 and MYH11 |
| iCAF | PDGFRα, CXCL12, IL-6, CXCL14, CXCL1 and CXCL2 | ||
| Prostate cancer53 | Patient samples | CAF-S1 | αSMA and PDGFRβ |
| CAF-S2 | PDGFRα, CREB3L1 and PLAGL1 | ||
| CAF-S3 | αSMA, HOXB2 and MAFB | ||
| Cholangiocarcinoma54 | Patient samples; KRAS/p19 and YaP/AKT mouse tumours | myCAF | COL1A1, αSMA, COL8A1, COL15A1 and SERPINF1 |
| iCAF | CXCL12, HGF and RGS5 | ||
| mesCAF | Mesothelin |
αSMA, α-smooth muscle actin; CAF, cancer-associated fibroblast; ECM, extracellular matrix; TNBC, triple-negative breast cancer.
In the context of pancreatic cancer, scRNA-seq analyses of mouse and human tumours have been performed to identify CAF subpopulations14–16. Three distinct subsets of fibroblasts have been identified in the non-malignant mouse pancreas, two of which were also observed in several GEMMs of advanced-stage pancreatic cancer16. These CAF subsets overlapped with two major CAF subpopulations previously identified in both KPC (LSL-KrasG12D/+;Trp53R172H/+;Pdx1-Cre) mice and patients with pancreatic cancer15,59: ECM-producing CAFs, termed myofibroblastic CAFs (myCAFs) and inflammatory CAFs (iCAFs)59 (TABLE 1). Immunostaining of tumour organoids revealed differences in the spatial distribution of these two CAF subpopulations. Specifically, myCAFs were located in proximity to cancer cells whereas iCAFs were more distant, potentially indicating that myCAFs and iCAFs interact in a juxtacrine and paracrine manner, respectively, with cancer cells59. A smaller subpopulation of antigen-presenting CAFs (apCAFs), characterized by MHC class II and CD74 expression but a lack of classical costimulatory molecules expressed by professional antigen-presenting cells, has also been identified in pancreatic tumours from GEMMs15 (TABLE 1). The antigen-presenting function of fibroblasts in various physiological and pathological conditions was discovered decades ago and has received renewed interest in the past few years60–64. Nevertheless, precise identification of apCAFs, especially in human cancers, requires further in-depth investigation and functional validation of this CAF subset. Heterogeneous CAF subpopulations have also been observed in scRNA-seq analyses of human pancreatic tumour specimens, although whether apCAFs are present as a distinct subpopulation in patients with this disease remains unclear14,15,17. LRRC15 has been described as a novel biomarker of myofibroblasts in human pancreatic tumours and high expression of a gene signature of LRRC15+ CAFs is correlated with a lack of response to immunotherapy18. LRRC15 is also expressed in a subset of CAFs identified in human breast tumours24.
Distinct sets of biomarkers and different classification strategies have been used to define various CAF subpopulations in other cancer types, including melanoma, cholangiocarcinoma, and colorectal, head and neck, breast and lung cancers19–26,53,54 (TABLE 1). Puram et al.21 reported a differential prevalence of CAF subsets in lymph node metastases compared with primary human head and neck tumours, which suggests that CAF heterogeneity might be tissue-specific and/or altered by the metastatic microenvironment. Notably, ex vivo culturing of the CAFs led to loss of expression of activation markers and ligands such as complement component 3 (C3), IL-6 and hepatocyte growth factor (HGF)21. In the context of breast cancer and ovarian cancer, four CAF subsets, CAF-S1 to CAF-S4, have been defined25,65,66. In a subsequent study by Kieffer et al.24, further analysis of the FAP+ CAF-S1 subset using scRNA-seq revealed eight subclusters, three of which expressed genes related to ECM production and transforming growth factor-β (TGFβ) signalling and were correlated with the presence of CD4+ T cells expressing PD-1 and/or CTLA4. Ex vivo coculturing of these subsets of CAF-S1, referred to as ECM-myCAFs and TGFβ-myCAFs, with T cells resulted in the upregulation of PD-1 and CTLA4 in the T cells and expansion of the TGFβ-myCAFs and ECM-myCAFs24. Similarly to Puram et al.21, Kieffer et al.24 also reported that CAF subtype identity ex vivo was dependent on the cell isolation and culture methodology used. Together, these studies highlight the need to study CAFs in physiologically relevant models.
Vascular CAFs, matrix CAFs, cycling (proliferative) CAFs and developmental CAFs have been described as being present in breast tumours22 (TABLE 1). These CAF subsets are proposed to have disparate origins within the tumour, putatively including the perivascular niche, resident fibroblasts and transformed epithelium22, potentially informing on their putative functions. Other studies have demonstrated that myCAFs (characterized by the expression of PDPN, FAP, ACTA2, COL1A1 and COL1A2) are located at the invasive front of human breast tumours, whereas iCAFs (marked by the expression of CXCL12) are localized in distal areas of the tumour stroma and are associated with a higher number of lymphocytes57. Friedman et al.23 profiled the dynamics of CAF subpopulations throughout breast tumour progression in mice and found that the transcriptional profiles of CAF subsets shift from a presumed immunoregulatory programme to wound healing and antigen-presentation programmes, suggesting that CAF functions change during tumour progression. Data from a study using mouse models of melanoma similarly showed that the abundance of three CAF subclusters, denoted as Stromal 1 (S1; expressing high levels of Pdpn, Pdgfra and Cd34), S2 (expressing high levels of Pdpn and Pdgfra and low levels of Cd34 and Acta2) and S3 (expressing high levels of Acta2), changed during tumour progression, with the S3 subpopulation dominating at later stages of tumour growth27. Together, these findings suggest that CAF subsets have distinct origins and functions, with the caveat that they are derived from correlative analyses of scRNA-seq and immunostaining data with extrapolation of CAF functions from transcriptional data and in vitro studies. Advances in imaging enabling highly multiplexed spatial analysis coupled with functional studies54 might provide better insight into the precise origins and functional roles of CAF subsets.
The observation that the CAF subsets and their gene signatures can be very different across various cancer types is unsurprising considering their distinct cellular origins and microenvironments. One can therefore expect that the identification of a universal CAF biomarker system that is applicable to multiple cancer types will be difficult (BOX 1). Such differences will probably also necessitate rational biomarker-guided strategies for future CAF-targeted therapeutics, which will be discussed in later sections of this Review.
Box 1 |. Does a pan-CAF biomarker exist?
The question of whether a single biomarker can be used to identify all cancer-associated fibroblasts (CAFs) really starts with the question of whether a specific biomarker can exclusively define fibroblasts. The simple answer is no and this also extends to CAFs. For a long time, α-smooth muscle actin (αSMA) was considered an ideal biomarker to identify ‘activated’ fibroblasts32,163. This paradigm has now been demonstrated to be oversimplified14–16,18,19,21–27,53,54 and many other biomarkers have been proposed (TABLE 1; Supplementary Table 1), clearly illustrating the heterogeneity of CAFs. Despite numerous different unbiased analyses of CAF populations, including proteomics, single-cell RNA sequencing, flow cytometry and multiplex staining techniques, a single biomarker that can identify all CAFs in a given tumour has not been identified. What we are left with are some biomarkers that might identify a percentage of CAFs and combinations of two or more biomarkers that could potentially identify all CAFs. Therefore, the search for an absolute biomarker of CAFs continues.
The observed plasticity and heterogeneity of CAFs could have several possible explanations: (1) dynamic and interchangeable shifts of individual classes of CAFs between either tumour-promoting or tumour-restraining phenotypes (as discussed below) (FIG. 2), depending on the complex context of the surrounding TME; (2) the existence of a wide spectrum of CAF subpopulations with distinct functions (FIG. 3); and (3) a diversity of CAF subpopulations that is likely to exceed the 3–4 major subpopulations defined in previous studies (TABLE 1; FIG. 3). Pseudotime mapping of scRNA-seq data suggests that CAFs from breast tumours are phenotypically plastic and can transition across different transcriptional states57. Similarly, Ohlund et al.59 showed that pancreatic CAFs can transition between the myCAF and iCAF states in vitro. Notwithstanding, comprehensive in vivo lineage-tracing studies are needed to definitively prove the existence of transitions between CAF subsets during tumour progression. In addition, the transcriptome signatures and classifications of various CAF subpopulations based on scRNA-seq data require functional validation (BOX 2).
Fig. 2 |. The heterogeneity and plasticity of CAFs with both tumour-restraining and tumour-promoting functions.
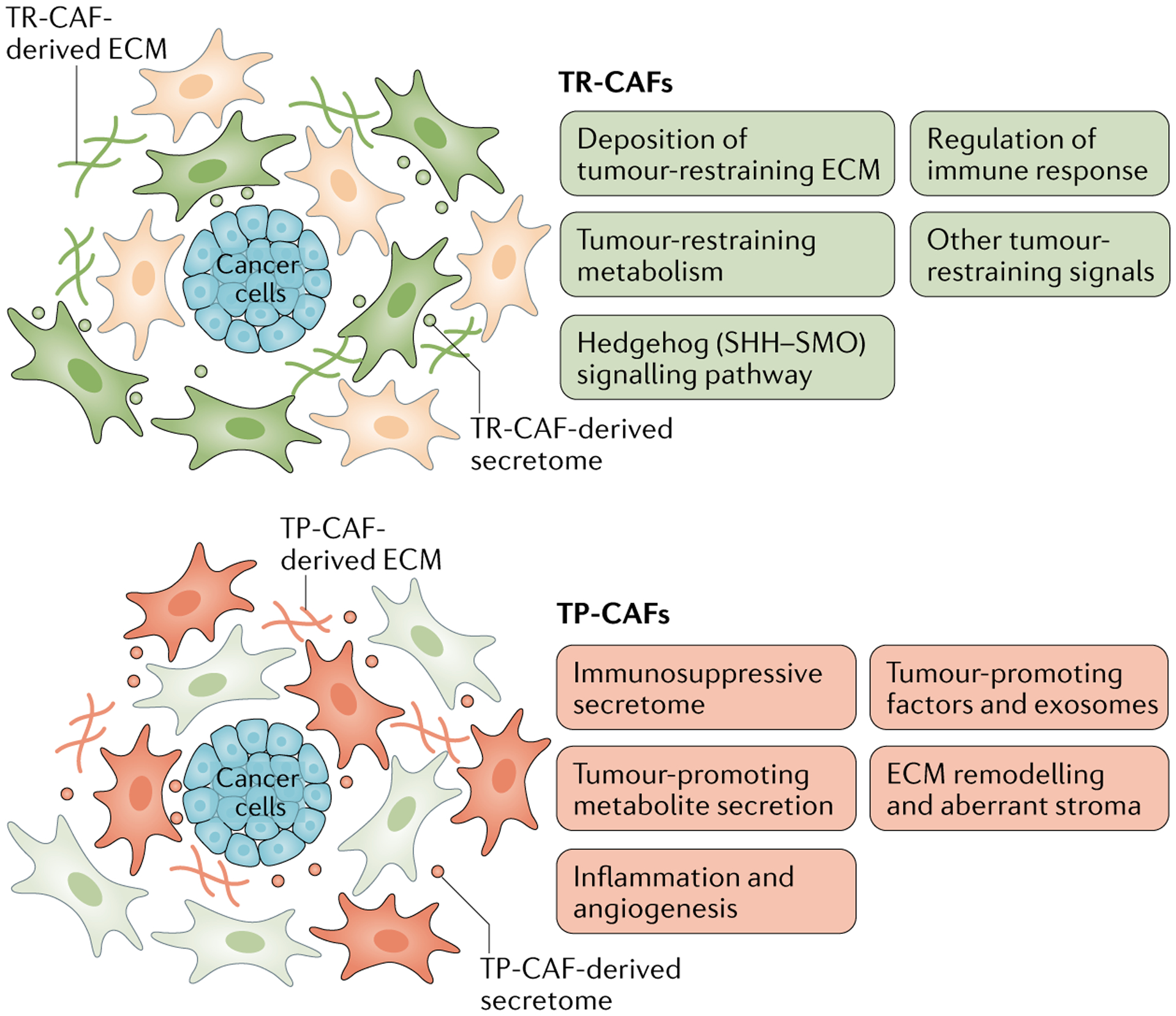
Schematic illustration of heterogeneous cancer-associated fibroblast (CAF) subpopulations with potential tumour-restraining (TR) functions and tumour-promoting (TP) functions through various indicated mechanisms. However, the spectrum of CAF heterogeneity is likely to be non-binary, without distinct polarization, and therefore should not be oversimplified as either tumour restraining or tumour promoting, and might exhibit context-dependent plasticity that needs to be further elucidated. ECM, extracellular matrix; SHH–SMO, sonic hedgehog–smoothened.
Fig. 3 |. Proposed models explaining the diverse functions and phenotypes of CAFs in cancer.

Activation of cancer-associated fibroblasts (CAFs) might lead to heterogeneous CAF compositions according to various possible scenarios (A–D). These distinct models of CAF subset differentiation can be interchangeable and dynamically regulated by the tumour microenvironment. ECM, extracellular matrix.
Box 2 |. A cautious approach to phenotypic classification of CAFs.
The possibility that cancer-associated fibroblasts (CAFs) constitute a heterogeneous population of cells was first described in 2006 (REF.50). More recently, single-cell RNA sequencing has been used to identify multiple distinct subsets of CAFs in different tumour types. Using basic bioinformatic tools, such CAF clusters were classified using a presumptive functional nomenclature, for example, as myofibroblastic, inflammatory, matrix, cycling or developmental CAFs. The concern with this approach is that transcriptional patterns might not actually define cell phenotypes and their purported functions. For example, naming a CAF cluster ‘inflammatory’ implies that all CAFs in this subset have inflammatory properties. Moreover, thorough analyses suggest that CAFs in other clusters also have gene-expression profiles reflecting a putative inflammatory phenotype. Certain cytokines that have been used to define inflammatory CAFs (iCAFs), such as IL-6, can be expressed not only by iCAFs but also by myofibroblastic CAFs. In addition, extracellular matrix components can be expressed robustly not only by matrix-producing myofibroblastic CAFs but also by iCAFs. Thus, the nomenclatures of CAF subpopulations based on their transcriptomic profiles without functional validation might lead to invalid presumptions in this field of research. Such over-interpretation is analogous to endowing a functional nomenclature to an immune cell cluster without confirming their definitive functional biomarkers or precise functions. Moreover, this type of analysis assumes that function is solely dictated by transcript abundance and does not account for post-transcriptional regulation or spatial proximity of cell types for signalling. Unless careful functional analyses are performed, ascribing phenotypic names to CAF subsets can be misleading.
Despite the advances made with single-cell analysis techniques, measures should be taken in future studies to ensure correct interpretation of the functional roles of CAFs based on scRNA-seq datasets. Moreover, gene signatures of a given CAF subtype can vary across different cancer types, different stages of a given cancer type or between patients, thus underscoring the need to identify both universal and specific biomarkers for CAFs (or CAF subtypes) using samples from large cohorts of patients and multiple model systems with a unified and/or standardized classification system.
CAFs with tumour-promoting functions
Mouse model systems commonly utilized to investigate CAF functions include (1) transgenic models using thymidine kinase or diphtheria toxin receptor systems that ablate certain CAF lineages; (2) Cre-loxP-based transgenic models that enable the genetic deletion of target genes in particular CAF lineages; and (3) conventional xenograft models using co-injection of cancer cells with CAFs to investigate the contributions of CAFs to tumour formation and progression33,67–70. The majority of studies demonstrating tumour-promoting roles of CAFs utilized co-implantation models that require ex vivo culturing of fibroblasts, which can induce transcriptional alterations and changes in CAF biomarker expression and, thus, loss of their in vivo identity21,24. Therefore, whether many of the proposed mechanisms by which CAFs promote tumour progression are actually conserved in autochthonous tumours and pathological contexts remains to be determined.
CAFs are the major producers of ECM components and various other secreted factors15,59,71–75. As CAFs dynamically evolve along with cancers, their secretome can modulate cancer progression and tumour immunity, both directly and indirectly, in a context-dependent manner (Fig. 2). Such regulatory functions can be exerted via growth factors, cytokines and chemokines, including CC-chemokine 2 (CCL2), CCL5, colony-stimulating factor 1 (CSF1), CXC-chemokine 5 (CXCL5), CXCL9, CXCL10, CXCL12 (also known as stromal cell-derived factor 1 (SDF1)), HGF, insulin-like growth factor 1 (IGF1), connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), IL-1, IL-4, IL-6, IL-6, IL-8, IL-10, leukaemia inhibitory factor (LIF), prostaglandin E2 (PGE2) and TGFβ11,13,33,68,76–79
Preclinical studies have revealed an immunosuppressive role of CAF-derived TGFβ, which can strongly influence the functions of various immune cell types, including T cells, macrophages and neutrophils80–83. In addition, CAFs have been reported to promote tumour progression via the secretion of CXCL12 (REF.33) and VEGFA84, which promote angiogenesis. Other factors secreted by CAFs, such as LIF, IGF1, HGF, IL-6, WNT5α and bone morphogenic protein 4 (BMP4), have been shown to induce reciprocal signalling interactions from CAFs to cancer cells that promote tumour growth and progression40,76,85,86.
CAFs are also important producers of ECM-degrading proteases, such as matrix metalloproteinases (MMPs), in addition to ECM-crosslinking enzymes and ECM components, which indicate their crucial roles in ECM remodelling in the TME87,88. The cytokines and chemokines, along with the remodelled ECM, produced by CAFs actively regulate immune responses and angiogenesis in tumours, thereby contributing to an immunosuppressive TME that is permissive for tumour progression20,25,65,89,90 and therapy resistance mediated by various mechanisms, including via the regulation of interstitial fluid pressure, cell adhesion and cancer cell survival67,91–95. Genetic deletion of tissue inhibitor of metalloproteinase (TIMP) expression in fibroblasts is associated with increased ECM deposition and tumour growth as well as secretion of promigratory exosomes containing disintegrin and metalloproteinase domain-containing protein 10 (ADAM10)96. In mouse models of cholangiocarcinoma, depletion of αSMA+ or Lrat lineage-traced CAFs is associated with a decreased tumour burden54. Genetic deletion of hyaluronan synthase 2 (Has2) or HGF (Hgf) in the putative majority of cholangiocarcinoma CAFs also reduced tumour growth54; however, whether survival is also affected remains to be determined. Expression of the deleted genes is largely restricted to specific CAF subpopulations, with myCAFs and iCAFs predominantly expressing Has2 and Hgf, respectively. Nevertheless, other CAF subtypes also have some expression of these genes and the functional roles of the various CAF subsets remain to be further elucidated.
Data from preclinical models and patient samples also suggest connections between certain CAF subsets, tumour immune responses and immunotherapeutic efficacy18,20,24,97. Specifically, FAP-expressing CAFs can have an immunosuppressive function, which might present an opportunity for improving immunotherapies via depletion of these CAFs or targeting CAF-derived factors such as CXCL12 or CCL2 (REFS98–100). In addition, CAFs can actively influence other cell types, including cancer cells and immune cells, through the production of various metabolites, including alanine, aspartate, lactate, deoxycytidine and lipids (such as lysophosphatidic acid)101–107. In particular, CAFs can provide various types of metabolic support to cancer cells through the release of alanine101,102, deoxycytidine103, proline108 and lipid species106, especially in the context of a nutrient-deprived TME. Exosomes derived from CAFs have also been shown to contain various surface proteins (such as WNT) and metabolites (such as TCA cycle intermediates) that can influence other cell populations in the TME, thus affecting cancer progression, stromal remodelling and drug resistance109–113.
CAFs with tumour-restraining functions
Evidence from numerous studies underscores the fact that CAFs (or certain subtypes of CAFs) have context-dependent functions, imparting both tumour-promoting and tumour-restraining influences11,12,47,50,58,59,75,114–120. The mechanisms underlying the tumour-restraining functions of CAFs are probably dependent on promotion of anticancer immunity, a pro-inflammatory secretome, tumour-inhibitory signalling and the production of certain ECM components as barriers to tumour cell invasion and dissemination (FIG. 2).
In mouse models of pancreatic cancer, cell lineage-based depletion of αSMA+ CAFs accelerates tumour progression, reduces fibrotic reactions and decreases survival58. In line with these findings, higher tumoural αSMA levels and stromal densities are associated with favourable overall survival outcomes in patients with pancreatic cancer58,121. Other studies have revealed that targeting the Sonic hedgehog (SHH)-Smoothened (SMO) signalling axis increases cancer cell proliferation and tumour formation owing to inhibition of SHH–SMO-mediated activation of the tumour-restraining phenotypes of myofibroblasts117,120. Tumour-restraining functions of SHH signalling and the fibrotic tumour stroma have also been identified in the context of bladder cancer122. Together, these findings indicate that αSMA+ CAFs, namely myofibroblasts, possess tumour-restraining functions that are partially mediated through the SHH–SMO signalling pathway. However, definitive connections between transcriptional signatures of specific CAF subpopulations and these tumour-restraining functions remain to be demonstrated.
In preclinical models of pancreatic cancer, JAK inhibitors suppress IL-1-induced LIF signalling in inflammatory iCAFs, thereby skewing these cells towards an ECM-producing myofibroblast (myCAF) phenotype, increasing the myCAF to iCAF ratio and facilitating ECM deposition, which in turn results in a reduction in cancer cell proliferation and tumour growth75. We have shown that αSMA+ myofibroblasts can exert tumour-restraining functions through the deposition of type I collagen, the most abundant protein in the TME of pancreatic cancers. Specifically, mouse models with genetic deletion of collagen I (Col1a1) in αSMA+ myofibroblasts using a system have markedly decreased collagen deposition, leading to accelerated pancreatic tumour development and progression56. This effect is associated with a more immunosuppressive stroma characterized by upregulation of CXCL5 in cancer cells, which results in increased recruitment of myeloid-derived suppressor cells and suppression of CD8+ T cells56. Other studies have shown that deletion of Col1a1 in CAFs promotes liver colonization of pancreatic and colorectal cancers123 but does not affect cholangiocarcinoma growth54. These results, in combination with those of previous studies56,58,75,117,120,122–124, underscore the specific tumour-restraining functions of myofibroblasts and the associated tumour stroma. By contrast, tumour-promoting functions of collagen in regulating cancer cell survival, proliferation, invasion and metabolism have also been reported125–128. Future studies are therefore required to further dissect the mechanisms underlying the context-dependent effects of stromal components such as collagen I.
The discovery of the tumour-restraining functions of CAFs provides a potential explanation for the unsuccessful clinical trials of therapeutic agents targeting CAFs or stromal components129–134. These observations suggest that future therapeutic strategies should avoid generic targeting of tumour-restraining CAF subpopulations in favour of precise reprogramming and normalization of tumour-promoting CAF subsets (preferably to non-malignant-tissue fibroblasts or tumour-restraining CAF subpopulations).
Value of CAFs as prognostic biomarkers
Many different biomarkers have been used to identify CAFs and their subtypes; however, none of these biomarkers are specific for CAFs (Supplementary Table 1). Owing to the expression of CAF biomarkers in other cell types (such as immune cells, endothelial cells, pericytes, smooth muscle cells and/or cancer cells), confusing conclusions might be drawn regarding the prognostic value of a given CAF biomarker in various cancer types. Indeed, many of the CAF biomarkers do not have any clear prognostic value when examined as single biomarkers in most cancer types included in analyses of the Human Protein Atlas based on The Cancer Genome Atlas datasets135 (Supplementary Table 1). Nevertheless, numerous studies have investigated the prognostic value of commonly used CAF biomarkers in various cancer types (Supplementary Table 1). In addition, given the existence of distinct CAF subpopulations with both pro-tumour and antitumour functions, it is not surprising that contradictory phenotypes have been observed in different model systems using therapeutic or genetic strategies targeting a certain subset of CAFs harbouring a given biomarker set. Neither is it surprising to obtain contradictory results when attempting to generalize the prognostic value of the overall CAF population without differentiating the effects of heterogeneous CAF subpopulations.
Novel techniques, such as scRNA-seq and mass spectrometry-based cytometry by time of flight (CyTOF) as well as multiplex flow cytometry and multiplex immunostaining methods, have greatly facilitated the identification and validation of a variety of biomarkers for distinct CAF subpopulations at the transcriptional or protein level. Future studies elucidating the functional roles of distinct biomarker-defined CAF subpopulations using clinical samples and clinically relevant model systems are necessary to further evaluate the prognostic values of CAFs and various CAF biomarkers. Current data indicate that certain subpopulations of CAFs have similar features and gene-expression signatures across different cancer types, while many other subpopulations are likely to vary substantially owing to the heterogeneous cellular origins of CAFs between cancer types (TABLE 1). Thus, additional investigations are needed to identify whether the prognostic value of specific biomarker-defined CAF subpopulations is either universal or cancer-type specific (BOX 1). Multiple biomarkers will probably need to be evaluated simultaneously in order to better inform on CAF function and, therefore, their prognostic effect. In addition, a better understanding of biomarkers for non-cancer-associated or quiescent fibroblasts will aid in identifying cancer-specific biomarkers.
Another intriguing issue relates to potential genetic alterations present within CAFs. Studies performed over the past two decades have revealed that CAFs can harbour genetic mutations136–138, in contrast with the previous notion that CAFs are genetically stable. In 2020, a single-cell multiomics sequencing study demonstrated frequent somatic copy number alterations in CAFs from patients with colorectal cancer55,139. By contrast, data reported in 2021 demonstrate that CAFs from patients with breast cancer are copy number stable140, highlighting potential cancer-specific dependencies. Other recent studies using single-cell whole-genome sequencing or scRNA-seq integrated with somatic mutation detection indicate the potential for detection of somatic mutations in CAFs (and other cell types of the TME) at the single-cell level141–144. Further advances in multiomics sequencing technology will provide additional insights into the genetic alterations of CAFs and their implications.
CAFs as potential therapeutic targets
Studies of the tumour-promoting functions of CAFs have identified various mechanisms, thus presenting multiple potential therapeutic targets (FIG. 4). These findings have led to numerous clinical trials testing strategies targeting CAFs and/or related signalling pathways (Supplementary Table 2). However, several therapeutic strategies, such as approaches targeting SHH–SMO signalling or hyaluronic acid, did not have sufficient therapeutic efficacy or, in some contexts, even shortened patient survival in clinical trials129,130,133,134. These unexpected results underscore the importance of considering both the tumour-promoting and tumour-restraining subtypes of CAFs.
Fig. 4 |. Interactions between CAFs and other cell types in the tumour microenvironment.
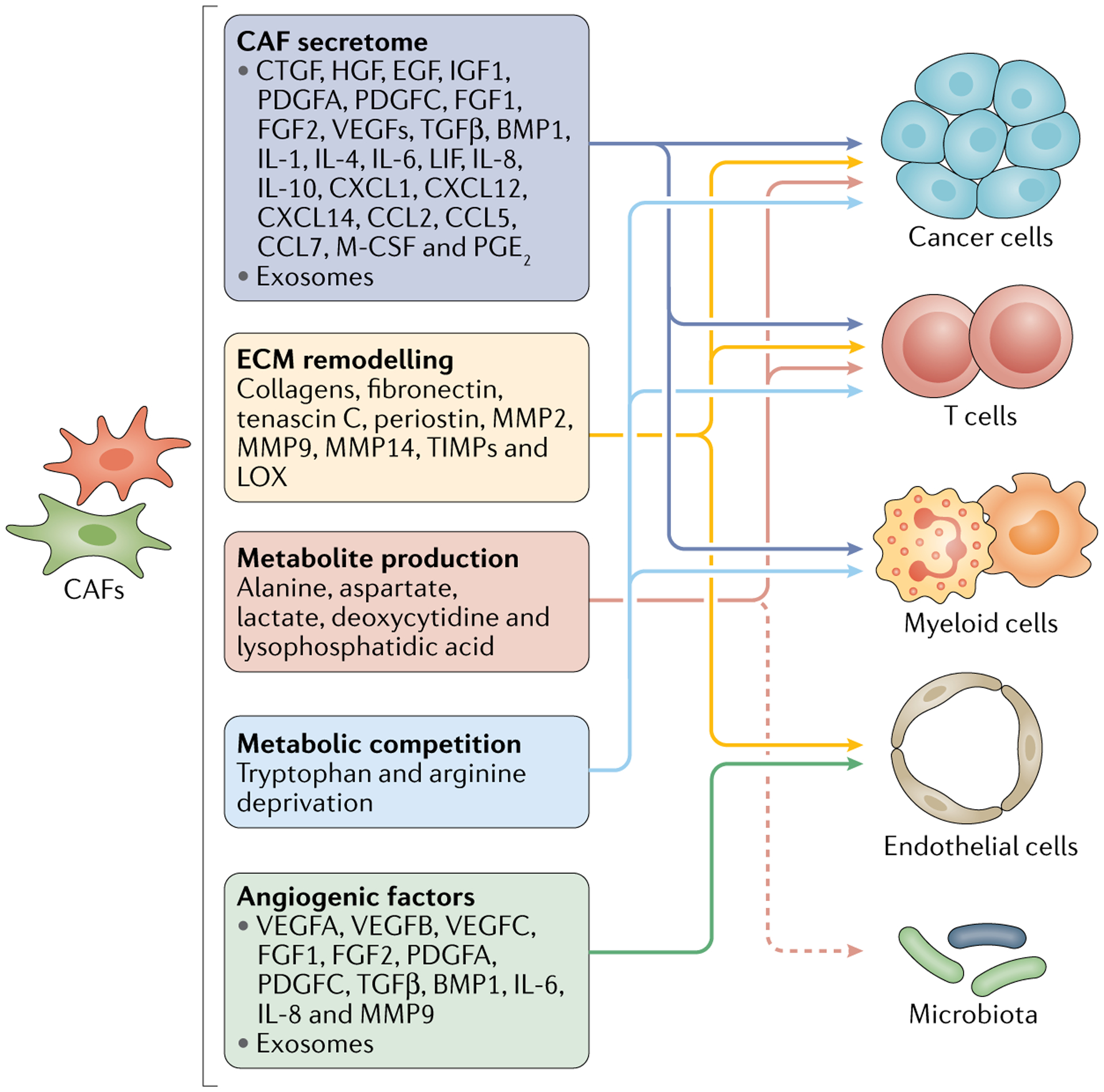
Schematic illustration of the potential regulatory effects of cancer-associated fibroblasts (CAFs) on other cell populations (including cancer cells, lymphocytes, myeloid cells and endothelial cells) and the microbiota within the tumour microenvironment. BMP, bone morphogenetic protein; CCL, CC motif chemokine; CTGF, connective tissue growth factor; CXCL, CXC motif chemokine; ECM, extracellular matrix; EGF, epidermal growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IGF, insulin-like growth factor; LOX, lysyl oxidase; M-CSF, macrophage colony-stimulating factor; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; PGE2, prostaglandin E2; TGFβ, transforming growth factor-β ; TIMPs, tissue inhibitors of metalloproteinases; VEGF, vascular endothelial growth factor.
Indeed, future therapeutic approaches targeting CAFs should differentiate the distinct subtypes and functions of CAFs, presumably based on a better-defined set of biomarkers (box 3). However, designing a therapeutic approach to specifically target tumour-promoting subtypes of CAFs is difficult owing to (1) the lack of definitive biomarkers and signalling pathways; (2) the dynamic interchangeability between CAF subtypes; and (3) the possibility that CAFs might have both tumour-promoting and tumour-restraining functions (FIG. 2). In other words, the spectrum of CAFs is typically nonbinary, with a lack of distinct cell polarization, and is dynamically regulated by a complex set of microenvironmental cues (FIG. 3).
Box 3 |. Is targeting of CAFs a feasible approach to cancer therapy?
Many different strategies to target cancer-associated fibroblasts (CAFs) or associated factors that mediate cancer progression have failed clinically. This lack of success primarily reflects a lack of stringent preclinical studies and an inadequate understanding of the functions of CAFs in cancer. Clear evidence indicates that CAFs can possess both tumour-restraining and tumour-promoting functions. Therefore, it is important to determine if targeting a putative CAF functional mediator affects different pathways and results in cancer acceleration in an unexpected manner owing to inadequate preclinical assessment of the drug target. Additionally, standard approaches for cancer drug screening are unlikely to be applicable to CAFs given that CAFs demonstrate a greater propensity to alter their phenotypes in cell culture as compared with cancer cells. Nevertheless, with careful analysis of the putative CAF-related drug target in appropriate and relevant in vivo models of cancer (preferably autochthonous genetic models of cancer with the natural evolution of CAFs) and detailed validation of the target using genetic gain-of-function and loss-of-function tools, the development of a CAF-targeted therapy with potential benefits in controlling cancer is possible. The following mechanistic and strategic guidelines could be considered before targeting CAFs in the clinic:
Identify the relevant CAFs with a specific biomarker.
Establish their precise function in cancer progression and metastasis using genetic mouse models, and establish whether a given CAF subset is in totality tumour restraining or tumour promoting.
Perform in-depth analyses of the transcriptome and proteome of the relevant CAF subset to identify the potential mediators that positively influence cancer progression and metastasis.
Perform genetic experiments to determine the precise function of the identified CAF mediators in cancer progression and metastasis.
Conduct preclinical efficacy studies in models relevant to the human disease in order to establish the CAF mediator as a specific experimental drug candidate.
Perform drug validation experiments using genetic mouse models to confirm target engagement in CAFs.
Establish a clinical development programme in conjunction with a CAF biomarker assessment.
Fine-tuning of specific CAF subpopulations might be important for therapeutic interventions targeting CAFs. For example, normalization and reprogramming of CAFs using vitamin D, calcipotriol or all-trans retinoic acid (ATRA) has been shown to restrain the tumour-promoting functions of CAFs and enhance the efficacy of pancreatic cancer therapy in preclinical models145,146. Combination therapy with paricalcitol, nivolumab and/or chemotherapy is currently being evaluated in a phase II clinical trial involving patients with pancreatic cancer (NCT02754726). As described previously, CAF reprogramming with JAK inhibitors can skew the iCAF subtype towards a myCAF subtype, which increases ECM deposition and restrains tumour growth75. A more comprehensive understanding of the tissue-specific gene-expression profiles and functions of physiological fibroblast populations as well as the precise mechanisms by which these characteristics are altered in cancer is urgently needed in order to design novel strategies for effective reprogramming of CAFs into a ‘normal’ or quiescent state.
The regulatory effects of CAFs and particular CAF subpopulations on tumour immunology and immunotherapy response have also been intensively investigated over the past few years. Several studies indicate connections between certain CAF subpopulations and immunosuppression, suggesting the potential for therapeutic interventions targeting these immunosuppressive CAF subpopulations in combination with immunotherapies18,20,24,60,99. For example, the CXCR4 antagonist motixafortide (BL-8040), which can inhibit the immunosuppressive CXCL12–CXCR4 axis driven by FAP-expressing CAFs, is currently being investigated in combination with pembrolizumab and/or chemotherapy in a phase II clinical trial involving patients with pancreatic cancer (NCT02826486). CAFs can directly or indirectly regulate, through complex interactions, the functions of other cell types within the TME and potentially even the microbiota, although more experimental evidence is needed regarding the latter effect (FIG. 4). Some of these interactions could present potential therapeutic targets following additional adequate and appropriate preclinical assessments (BOX 3).
Perspectives and future considerations
As we expand our understanding of CAF heterogeneity using the latest techniques and models, the clinical relevance of CAFs, including the prognostic indications and therapeutic strategies, needs to be reevaluated and redefined based on the distinct roles of specific CAF subpopulations.
Use of single-cell analysis techniques.
We anticipate that modern technologies, such as scRNA-seq and other single-cell analysis techniques (such as single-cell whole-genome sequencing, single-cell genome and transcriptome sequencing, and single-cell methylome and transcriptome sequencing), will be utilized widely and routinely as new standards for the detailed taxonomy of distinct CAF subpopulations at the single-cell level. Numerous marker genes of CAFs, as previously identified using conventional methods, such as flow cytometry, immunohistochemistry and immunostaining, have been confirmed using contemporary single-cell analysis techniques, including αSMA, FAP, LOX and PDGFRα (Supplementary Table 1). Despite the unprecedented potential of scRNA-seq to decipher cellular heterogeneity, this technique has several limitations: (1) genes with low levels of expression are difficult to measure accurately owing to the limited sequencing depth; (2) transcript levels of given genes might not always reflect the actual protein levels owing to additional translational and posttranslational regulatory mechanisms; and (3) the cell-clustering algorithms, including the most commonly used non-linear dimensionality projections, namely t-distributed stochastic neighbour embedding (t-SNE) and uniform manifold approximation and projection (UMAP), will always yield subclusters according to artificially selected clustering parameters, even when no functionally or biologically meaningful subclusters exist147. Therefore, additional validation using complementary methodologies, including CyTOF, multiplex flow cytometry and/or multiplex immunostaining, is necessary for further investigations of the proposed CAF subpopulations. Functional validation using various in vitro and in vivo model systems is also required to establish whether the proposed CAF subpopulations are biologically relevant.
Unified CAF classification and taxonomy.
The current classification and taxonomy of CAF subpopulations lacks a unifying standardized system to reconcile the different (or even contradictory) observations made by various research groups, often in the same cancer type and using similar scRNA-seq or CyTOF techniques. Indeed, the subclustering and definition of CAF subpopulations is somewhat subjective and can therefore vary between observers. In this regard, a consensus on the major biomarkers and hierarchical clustering maps of CAF subpopulations based on scRNA-seq or CyTOF analysis needs to be developed in the coming years.
Subsequently, a standardized experimental system with specific, dedicated and reliable reagents and protocols needs to be developed for the identification, staining and/or labelling, sorting, cell culture, and functional assessment of CAF subpopulations both in vitro and in vivo, similar to those developed for research on immune cell populations148. This standardization will then enable precise definition and characterization of CAF subpopulations with specified functional roles and prognostic values, thereby facilitating the development of specific therapeutic interventions targeting certain CAF subpopulations and/or pathways in the future.
Functional validation of CAF subsets.
Current techniques, such as scRNA-seq and CyTOF, are focused on the identification of heterogeneous CAF subpopulations based on the expression profile of signature genes and proteins, respectively. These observations need to be complemented by functional studies in order to connect the gene and/or protein signatures of distinct CAF subpopulations with their precise functional roles in cancers. Further investigations of the functional roles of distinct CAF subpopulations require the utilization of sophisticated model systems, including new transgenic mouse models, organoids and patient-derived xenografts13,52. We expect such functional validation to be one of the key focus areas in the field of CAF research. The identification of novel biomarkers for fibroblasts and CAFs based on the new scRNA-seq datasets would facilitate subsequent investigations using specific Cre-recombinase drivers and lineage-tracing techniques to further elucidate the origins and functions of CAFs in the context of GEMMs. For example, using novel dual-recombinase GEMMs149,150, we were able to lineage trace CAFs and cancer cells, respectively, or genetically delete type I collagen specifically in αSMA+ myofibroblasts56, both in the context of autochthonous pancreatic cancer. In addition, a suite of Dre recombinase-based models has been developed for marking a range of cell types, including CAFs151, which provides additional methods to track and target CAFs. In this regard, we have generated Pdpn-tk and Pdpn-Cre mouse models (enabling cell depletion and genetic manipulation of Pdpn-lineage cells) to investigate tumour lymphangiogenesis152 and these GEMMs might also be useful in evaluating CAFs given that multiple studies have implicated PDPN as a CAF biomarker15,21,23,153.
Owing to the highly dynamic heterogeneity of CAFs and the lack of specific biomarkers, establishing clinically relevant experimental models that enable real-time tracking of CAFs remains challenging. Future functional studies will undoubtedly benefit from novel cell lineage-tracing and genetic manipulation (Cre-loxP) model systems, although these models are still limited by the paucity of CAF-specific biomarker genes that can serve as driver promoters for Cre-recombinase and fluorescence reporter genes. Specifically, commonly used types of lineage-tracing models include (1) a conventional Cre driver line (such as αSMA-Cre154) integrated with a fluorescence reporter allele; (2) an inducible Cre driver line (such as αSMA-CreERT2 (REF.155), Gli1-CreERT2 (REF.156), Grem1-CreERT157, Hoxb6-CreERT157 or Islr-CreERT2 (REFS157,158)) with a fluorescence reporter allele; (3) a fluorescence reporter gene driven by a promoter derived from a putative CAF biomarker gene, which enables real-time transcriptional tracking of the putative biomarker (such as αSMA-RFP40,58,159,160, Col1a1-DsRed/YFP/eGFP40,41, Gli1-eGFP156 or FSP1-GFP84,160); and (4) cell lineage-depleting models using a thymidine kinase54,58,123,160 or diphtheria toxin receptor54,99,123,161 system (such as αSMA-tk58 or FAP-DTR98,99). We expect that new biomarkers of CAFs identified using scRNA-seq and CyTOF techniques will facilitate the generation of new lineage-tracing models in the future. Intravital imaging has also been used to dynamically image CAFs in tumours94,162. Implementation of the aforementioned labelling systems combined with further development of high-throughput and non-invasive dynamic imaging techniques will elucidate how CAFs evolve during tumour progression.
Furthermore, connecting CAF heterogeneity with the clinical relevance and functional roles of the various CAF subpopulations in human cancers is also challenging, even with the utilization of transgenic mouse models, organoids and patient-derived xenografts. Currently, the speculated functional roles of the CAF subpopulations in human cancers are largely based on the expression profiles of gene clusters associated with certain cellular pathways, processes and/or activities. Discrepancies between observations made in experimental model systems and human cancers remain an obstacle to the clinical development of precise therapeutic strategies targeting certain CAF subtypes, although future research promises to be exciting and will probably help overcome such hurdles.
Conclusions
CAFs have long been investigated as an attractive therapeutic target; however, many therapeutic strategies targeting CAFs or associated stromal components have failed to improve clinical outcomes, underscoring the importance of dissecting the heterogeneous subpopulations and diverse functions of CAFs in a context-dependent manner. Modern techniques, such as scRNA-seq and CyTOF, have provided new opportunities to decipher the heterogeneity of CAFs on the basis of signature gene and protein expression profiles. Nevertheless, measures should be taken in future studies to systemically define the functional roles of CAFs and CAF subpopulations in relation to the transcriptional signatures of CAFs. Comprehensive characterization and functional validation of CAF subtypes in both preclinical and clinical studies might inform the development of novel diagnostic and therapeutic approaches predicated on CAFs.
Supplementary Material
Key points.
Cancer-associated fibroblasts (CAFs) are found in both primary and metastatic tumours; studies using modern cell sorting and sequencing technologies have provided exciting new insights into the potential therapeutic and prognostic value of CAFs.
In particular, studies using single-cell RNA sequencing and genetically engineered mouse models have begun to reveal the heterogeneity and functional roles of CAFs, which are dynamic and context dependent.
The precise functional roles of various CAF subtypes remain largely undefined, which requires future investigations integrating functional studies using multiple model systems with transcriptomic and/or proteomic data at single-cell resolution.
The identification and precise characterization of the tumour-promoting and tumour-restraining functions of different CAF populations might provide opportunities to develop novel diagnostic and therapeutic approaches.
Acknowledgements
The CAF-related work of the authors is supported by MD Anderson Cancer Center, and was supported by the Cancer Prevention and Research Institute of Texas (CPRIT) award RP150231. The NIH National Cancer Institute grant P01CA117969 supports ECM-related research in the Kalluri laboratory.
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41571-021-00546-5.
References
- 1.Hanahan D & Coussens LM Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 3, 422–433 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Madhavan S & Nagarajan S GRP78 and next generation cancer hallmarks: an underexplored molecular target in cancer chemoprevention research. Biochimie 175, 69–76 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Pietras K & Ostman A Hallmarks of cancer: interactions with the tumor stroma. Exp. Cell Res 316, 1324–1331 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Quail DF & Joyce JA Microenvironmental regulation of tumor progression and metastasis. Nat. Med 19, 1423–1437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronnov-Jessen L, Petersen OW & Bissell MJ Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol. Rev 76, 69–125 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Schulz M, Salamero-Boix A, Niesel K, Alekseeva T & Sevenich L Microenvironmental regulation of tumor progression and therapeutic response in brain metastasis. Front. Immunol 10, 1713 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vong S & Kalluri R The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer 2, 1139–1145 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coussens LM & Werb Z Inflammation and cancer. Nature 420, 860–867 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review on the connection between inflammation and cancer.
- 11.Kalluri R The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Ohlund D, Elyada E & Tuveson D Fibroblast heterogeneity in the cancer wound. J. Exp. Med 211, 1503–1523 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review on CAFs and their putative heterogeneity.
- 13.Sahai E et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; Article providing a consensus framework of CAF biology.
- 14.Bernard V et al. Single-cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin. Cancer Res 25, 2194–2205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elyada E et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosein AN et al. Cellular heterogeneity during mouse pancreatic ductal adenocarcinoma progression at single-cell resolution. JCI Insight 5, e129212 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng J et al. Single-cell RNA-seq highlights intratumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 29, 725–738 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez CX et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 10, 232–253 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Li H et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet 49, 708–718 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Zhang L et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181, 442–459.e29 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Puram SV et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624.e24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartoschek M et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun 9, 5150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman G et al. Cancer-associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4+ and PDPN+ CAFs to clinical outcome. Nat. Cancer 1, 692–708 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieffer Y et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 10, 1330–1351 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Costa A et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33, 463–479.e10 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Lambrechts D et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med 24, 1277–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Davidson S et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 31, 107628 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z et al. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun 11, 5077 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virchow R Die cellularpathologie in ihrer begründung auf physiologische und pathologische gewebelehre. Zwanzig vorlesungen gehalten während der monate februar, märz und april 1858 im Pathologischen institute zu Berlin 440 (A. Hirschwald, 1858). [Google Scholar]
- 30.Tarin D & Croft CB Ultrastructural features of wound healing in mouse skin. J. Anat 105, 189–190 (1969). [PubMed] [Google Scholar]
- 31.Arina A et al. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl Acad. Sci. USA 113, 7551–7556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronnov-Jessen L & Petersen OW Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab. Invest 68, 696–707 (1993). [PubMed] [Google Scholar]
- 33.Orimo A et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Driskell RR et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachem MG et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115, 421–432 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Apte MV et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 44, 534–541 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin C, Evason KJ, Asahina K & Stainier DY Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Invest 123, 1902–1910 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karnoub AE et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Mishra PJ et al. Carcinoma-associated fibroblastlike differentiation of human mesenchymal stem cells. Cancer Res. 68, 4331–4339 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quante M et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19, 257–272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raz Y et al. Bone marrow-derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J. Exp. Med 215, 3075–3093 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeisberg EM, Potenta S, Xie L, Zeisberg M & Kalluri R Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 67, 10123–10128 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Zeisberg EM et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med 13, 952–961 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Bochet L et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 73, 5657–5668 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Dvorak HF Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med 315, 1650–1659 (1986). [DOI] [PubMed] [Google Scholar]; A comprehensive review on the connection between cancer and wound healing.
- 46.Ueba T et al. Transcriptional regulation of basic fibroblast growth factor gene by p53 in human glioblastoma and hepatocellular carcinoma cells. Proc. Natl Acad. Sci. USA 91, 9009–9013 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mills LD et al. Loss of the transcription factor GLI1 identifies a signaling network in the tumor microenvironment mediating KRAS oncogene-induced transformation. J. Biol. Chem 288, 11786–11794 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox TR & Erler JT Fibrosis and cancer: partners in crime or opposing forces? Trends Cancer 2, 279–282 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Schafer M & Werner S Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol 9, 628–638 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Sugimoto H, Mundel TM, Kieran MW & Kalluri R Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther 5, 1640–1646 (2006). [DOI] [PubMed] [Google Scholar]; Early study revealing the putative heterogeneity of CAFs.
- 51.Kanzaki R & Pietras K Heterogeneity of cancer-associated fibroblasts: opportunities for precision medicine. Cancer Sci. 111, 2708–2717 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biffi G & Tuveson DA Diversity and biology of cancer-associated fibroblasts. Physiol. Rev 101, 147–176 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen S et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat. Cell Biol 23, 87–98 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Affo S et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 39, 866–882.e11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y et al. Single-cell multiomics sequencing reveals prevalent genomic alterations in tumor stromal cells of human colorectal cancer. Cancer Cell 38, 818–828.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Chen Y et al. Type I collagen deletion in αSMA+. myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 39, 548–565.e6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; Study showing that deletion of type I collagen in αSMA-expressing stromal cells exacerbates pancreatic cancer.
- 57.Wu SZ et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 39, e104063 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozdemir BC et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohlund D et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med 214, 579–596 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Study revealing that two distinct populations of CAFs exist in pancreatic cancer.
- 60.Monteran L & Erez N The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front. Immunol 10, 1835 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Umetsu DT, Katzen D, Jabara HH & Geha RS Antigen presentation by human dermal fibroblasts: activation of resting T lymphocytes. J. Immunol 136, 440–445 (1986). [PubMed] [Google Scholar]
- 62.Kundig TM et al. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science 268, 1343–1347 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Lakins MA, Ghorani E, Munir H, Martins CP & Shields JD Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T cells to protect tumour cells. Nat. Commun 9, 948 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nitta T et al. Fibroblasts as a source of self-antigens for central immune tolerance. Nat. Immunol 21, 1172–1180 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Givel AM et al. miR200-regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat. Commun 9, 1056 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pelon F et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun 11, 404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su S et al. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172, 841–856.e16 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Ligorio M et al. Stromal microenvironment shapes the intratumoral architecture of pancreatic cancer. Cell 178, 160–175.e27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z et al. Tumor microenvironment-derived nrg1 promotes antiandrogen resistance in prostate cancer. Cancer Cell 38, 279–296.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Francescone R et al. Netrin G1 promotes pancreatic tumorigenesis through cancer-associated fibroblast-driven nutritional support and immunosuppression. Cancer Discov. 11, 446–479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korc M Pancreatic cancer-associated stroma production. Am. J. Surg 194, S84–S86 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahadevan D & Von Hoff DD Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol. Cancer Ther 6, 1186–1197 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Feig C et al. The pancreas cancer microenvironment. Clin. Cancer Res 18, 4266–4276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whittle MC & Hingorani SR Fibroblasts in pancreatic ductal adenocarcinoma: biological mechanisms and therapeutic targets. Gastroenterology 156, 2085–2096 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Biffi G et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9, 282–301 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi Y et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 569, 131–135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cortot AB et al. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res. 73, 834–843 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pein M et al. Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs. Nat. Commun 11, 1494 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ershaid N et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat. Commun 10, 4375 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas DA & Massague J TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Fridlender ZG et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mariathasan S et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tauriello DVF et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018). [DOI] [PubMed] [Google Scholar]
- 84.O’Connell JT et al. VEGF-A and Tenascin-C produced by S100A4+stromal cells are important for metastatic colonization. Proc. Natl Acad. Sci. USA 108, 16002–16007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tape CJ et al. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 165, 1818 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhowmick NA et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Boire A et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120, 303–313 (2005). [DOI] [PubMed] [Google Scholar]
- 88.Sternlicht MD et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 98, 137–146 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar V et al. Cancer-associated fibroblasts neutralize the antitumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 32, 654–668.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mhaidly R & Mechta-Grigoriou F Fibroblast heterogeneity in tumor micro-environment:role in immunosuppression and new therapies. Semin. Immunol 48, 101417 (2020). [DOI] [PubMed] [Google Scholar]
- 91.Fidler IJ et al. Modulation of tumor cell response to chemotherapy by the organ environment. Cancer Metastasis Rev. 13, 209–222 (1994). [DOI] [PubMed] [Google Scholar]
- 92.Farmer P et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat. Med 15, 68–74 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Meads MB, Gatenby RA & Dalton WS Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat. Rev. Cancer 9, 665–674 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Vennin C et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun 10, 3637 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heldin CH, Rubin K, Pietras K & Ostman A High interstitial fluid pressure - an obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Shimoda M et al. Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat. Cell Biol 16, 889–901 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Chauhan VP et al. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc. Natl Acad. Sci. USA 116, 10674–10680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kraman M et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330, 827–830 (2010). [DOI] [PubMed] [Google Scholar]; Study showing that depletion of FAP-expressing stromal cells restores antitumour immunity.
- 99.Feig C et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; Study reporting the immune-evasive functions of FAP-expressing CAFs mediated through CXCL12.
- 100.Yang X et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 76, 4124–4135 (2016). [DOI] [PubMed] [Google Scholar]
- 101.Sousa CM et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parker SJ et al. Selective alanine transporter utilization creates a targetable metabolic niche in pancreatic cancer. Cancer Discov. 10, 1018–1037 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dalin S et al. Deoxycytidine release from pancreatic stellate cells promotes gemcitabine resistance. Cancer Res. 79, 5723–5733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Helms E, Onate MK & Sherman MH Fibroblast heterogeneity in the pancreatic tumor microenvironment. Cancer Discov. 10, 648–656 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bertero T et al. Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab. 29, 124–140.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Auciello FR et al. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov. 9, 617–627 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang CH et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olivares O et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat. Commun 8, 16031 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; References 101–108 report studies showing the regulatory functions of CAFs on cancer metabolism.
- 109.Kahlert C & Kalluri R Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med 91, 431–437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luga V & Wrana JL Tumor-stroma interaction: revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res. 73, 6843–6847 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Hu Y et al. Fibroblast-derived exosomes contribute to chemoresistance through priming cancer stem cells in colorectal cancer. PLoS One 10, e0125625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Richards KE et al. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 36, 1770–1778 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao H et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 5, e10250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mueller MM & Fusenig NE Friends or foes — bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 4, 839–849 (2004). [DOI] [PubMed] [Google Scholar]
- 115.Neesse A, Algul H, Tuveson DA & Gress TM Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64, 1476–1484 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Laklai H et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med 22, 497–505 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Study demonstrating that pancreatic cancer genotypes can influence stromal extracellular matrix content, architecture and stiffness.
- 117.Rhim AD et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Provenzano PP et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; References 117 and 118 report that cancer cell-derived Hedgehog proteins act on stromal cells and αSMA-expressing stromal cells to restrain pancreatic cancer progression.
- 119.Olive KP et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; Study indicating that targeting of the tumour stroma through Hedgehog signalling in pancreatic cancer might improve the delivery of chemotherapeutics.
- 120.Lee JJ et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc. Natl Acad. Sci. USA 111, E3091–E3100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Torphy RJ et al. Stromal content is correlated with tissue site, contrast retention, and survival in pancreatic adenocarcinoma. JCO Precis. Oncol 10.1200/PO.17.00121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shin K et al. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell 26, 521–533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bhattacharjee S et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J. Clin. Invest 131, e146987 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang H et al. Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J. Clin. Invest 130, 4704–4709 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Armstrong T et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin. Cancer Res 10, 7427–7437 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Bachem MG et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128, 907–921 (2005). [DOI] [PubMed] [Google Scholar]
- 127.Levental KR et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Egeblad M, Rasch MG & Weaver VM Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol 22, 697–706 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Catenacci DV et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J. Clin. Oncol 33, 4284–4292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ko AH et al. A phase I study of FOLFIRINOX Plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas 45, 370–375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Benson AB III et al. A phase II randomized, double-blind, placebo-controlled study of simtuzumab or placebo in combination with gemcitabine for the first-line treatment of pancreatic adenocarcinoma. Oncologist 22, 241–e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hecht JR et al. A phase II, randomized, double-blind, placebo-controlled study of simtuzumab in combination with FOLFIRI for the second-line treatment of metastatic KRAS mutant colorectal adenocarcinoma. Oncologist 22, 243–e23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramanathan RK et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J. Clin. Oncol 37, 1062–1069 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Van Cutsem E et al. Randomized phase III trial of pegvorhyaluronidase Alfa with Nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J. Clin. Oncol 38, 3185–3194 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uhlen M et al. A pathology atlas of the human cancer transcriptome. Science 357, eaan2507 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Moinfar F et al. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 60, 2562–2566 (2000). [PubMed] [Google Scholar]
- 137.Kurose K et al. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat. Genet 32, 355–357 (2002). [DOI] [PubMed] [Google Scholar]
- 138.Patocs A et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N. Engl. J. Med 357, 2543–2551 (2007). [DOI] [PubMed] [Google Scholar]
- 139.McAndrews KM, Chen Y & Kalluri R Stromal cells exhibit prevalent genetic aberrations in colorectal cancer. Cancer Cell 38, 774–775 (2020). [DOI] [PubMed] [Google Scholar]
- 140.Bassez A et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat. Med 27, 820–832 (2021). [DOI] [PubMed] [Google Scholar]
- 141.Zafar H, Wang Y, Nakhleh L, Navin N & Chen K Monovar: single-nucleotide variant detection in single cells. Nat. Methods 13, 505–507 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lodato MA et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science 359, 555–559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nam AS et al. Somatic mutations and cell identity linked by Genotyping of Transcriptomes. Nature 571, 355–360 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang L et al. Single-cell whole-genome sequencing reveals the functional landscape of somatic mutations in B lymphocytes across the human lifespan. Proc. Natl Acad. Sci. USA 116, 9014–9019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Froeling FE et al. Retinoic acidinduced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology 141, 1486–1497 (2011). [DOI] [PubMed] [Google Scholar]
- 146.Sherman MH et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; Study demonstrating that treatment with vitamin D can reprogramme CAFs to a quiescent state and enhance response to chemotherapy in models of pancreatic cancer.
- 147.Kiselev VY, Andrews TS & Hemberg M Challenges in unsupervised clustering of single-cell RNA-seq data. Nat. Rev. Genet 20, 273–282 (2019). [DOI] [PubMed] [Google Scholar]; A comprehensive review on the challenges and caveats of single-cell RNA-sequencing data analysis
- 148.Waldman AD, Fritz JM & Lenardo MJ A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol 20, 651–668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schonhuber N et al. A next-generation dual-recombinase system for time-and host-specific targeting of pancreatic cancer. Nat. Med 20, 1340–1347 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen Y et al. Dual reporter genetic mouse models of pancreatic cancer identify an epithelial-to-mesenchymal transition-independent metastasis program. EMBO Mol. Med 10, e9085 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Han X et al. A suite of new Dre recombinase drivers markedly expands the ability to perform intersectional genetic targeting. Cell Stem Cell 28, 1160–1176.e7 (2021). [DOI] [PubMed] [Google Scholar]
- 152.Chen Y et al. Podoplanin+tumor lymphatics are rate limiting for breast cancer metastasis. PLoS Biol. 16, e2005907 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoshino A et al. Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: podoplanin in fibroblast functions for tumor progression. Cancer Res. 71, 4769–4779 (2011). [DOI] [PubMed] [Google Scholar]
- 154.LeBleu VS et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med 19, 1047–1053 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wendling O, Bornert JM, Chambon P & Metzger D Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis 47, 14–18 (2009). [DOI] [PubMed] [Google Scholar]
- 156.Garcia PE et al. Differential contribution of pancreatic fibroblast subsets to the pancreatic cancer stroma. Cell Mol. Gastroenterol. Hepatol 10, 581–599 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kobayashi H et al. The balance of stromal BMP signaling mediated by GREM1 and ISLR drives colorectal carcinogenesis. Gastroenterology 160, 1224–1239.e30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mizutani Y et al. Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res. 79, 5367–5381 (2019). [DOI] [PubMed] [Google Scholar]
- 159.LeBleu VS et al. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat. Med 19, 227–231 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Becker LM et al. Epigenetic reprogramming of cancer-associated fibroblasts deregulates glucose metabolism and facilitates progression of breast cancer. Cell Rep. 31, 107701 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mascharak S et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372, eaba2374 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hirata E et al. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell 27, 574–588 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lazard D et al. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc. Natl Acad. Sci. USA 90, 999–1003 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


