Keywords: alcohol, circadian clock, ethanol, skeletal muscle
Abstract
Circadian rhythms are central to optimal physiological function, as disruption contributes to the development of several chronic diseases. Alcohol (EtOH) intoxication disrupts circadian rhythms within liver, brain, and intestines, but it is unknown whether alcohol also disrupts components of the core clock in skeletal muscle. Female C57BL/6Hsd mice were randomized to receive either saline (control) or alcohol (EtOH) (5 g/kg) via intraperitoneal injection at the start of the dark cycle [Zeitgeber time (ZT12)], and gastrocnemius was collected every 4 h from control and EtOH-treated mice for the next 48 h following isoflurane anesthetization. In addition, metyrapone was administered before alcohol intoxication in separate mice to determine whether the alcohol-induced increase in serum corticosterone contributed to circadian gene regulation. Finally, synchronized C2C12 myotubes were treated with alcohol (100 mM) to assess the influence of centrally or peripherally mediated effects of alcohol on the muscle clock. Alcohol significantly disrupted mRNA expression of Bmal1, Per1/2, and Cry1/2 in addition to perturbing the circadian pattern of clock-controlled genes, Myod1, Dbp, Tef, and Bhlhe40 (P < 0.05), in muscle. Alcohol increased serum corticosterone levels and glucocorticoid target gene, Redd1, in muscle. Metyrapone prevented the EtOH-mediated increase in serum corticosterone but did not normalize the EtOH-induced change in Per1, Cry1 and Cry2, and Myod1 mRNA expression. Core clock gene expression (Bmal, Per1/2, and Cry1/2) was not changed following 4, 8, or 12 h of alcohol treatment on synchronized C2C12 myotubes. Therefore, binge alcohol disrupted genes of the core molecular clock independently of elevated serum corticosterone or direct effects of EtOH on the muscle.
NEW & NOTEWORTHY Alcohol is a myotoxin that impairs skeletal muscle metabolism and function following either chronic consumption or acute binge drinking; however, mechanisms underlying alcohol-related myotoxicity have not been fully elucidated. Herein, we demonstrate that alcohol acutely interrupts oscillation of skeletal muscle core clock genes, and this is neither a direct effect of ethanol on the skeletal muscle, nor an effect of elevated serum corticosterone, a major clock regulator.
INTRODUCTION
Alcohol consumption is highly prevalent in the United States and across the world as both a social practice and, in the case of alcoholics, a disease. In humans, binge drinking is defined as alcohol consumption that elevates blood alcohol levels to ≥0.08 g/dL, which occurs after ∼5 or more drinks for men and 4 or more drinks for women within ∼2 h. In 2018, ∼26% of people in the United States 18 yr or older reported having participated in binge drinking in the last month, and in 2010, this pattern of drinking has been estimated to cost the United States almost $190 billion dollars (1). Although alcohol intake affects numerous organs, the impact of binge alcohol on skeletal muscle cannot be overlooked, as muscle represents ∼40%–45% of total body mass and contributes substantially to overall health and physical function (2). Despite progress in the field, our knowledge of the extent to which binge alcohol injures skeletal muscle remains incomplete.
To identify novel processes in the skeletal muscle affected by alcohol that may therefore contribute to alcoholic muscle injury, we used RNA sequencing. This approach identified significant changes to the expression of genes that comprise the core molecular clock. The molecular clock includes a positive arm, containing the heterodimer of the proteins Brain and Muscle Arnt Like-1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK), which transcribe various clock-controlled genes including Retinoid-related orphan receptor-α (Rorα) and Nuclear receptor subfamily 1 group D member 1 (Nr1d1 or Reverbα), which are nuclear receptors that can modify the transcriptional activity of the core clock (3). Other clock-controlled genes that exhibit a circadian expression pattern include D-box binding protein (Dbp), thyrotroph embryonic factor (Tef), Basic helix-loop-helix family member E40 (BhlhE40), and the transcription factor Myogenic Differentiation 1 (Myod1). The negative arm comprises the period proteins (PER1-3) and the cryptochrome proteins (CRY1-2) that feedback to repress BMAL1/CLOCK transcriptional activity (4, 5). These two arms function in a transcription-translation feedback loop to regulate the diurnal transcription of genes involved in numerous cellular processes (6). The core molecular clock has emerged as a major area of interest in relation to skeletal muscle health and disease, as disruption of the positive arm of the core molecular clock in skeletal muscle impairs several metabolic processes including glucose and lipid metabolism, as well as muscle force generating capacity (7–11). Therefore, disruption to the core molecular clock and subsequent downstream targets specifically within the skeletal muscle has the potential to negatively impact skeletal muscle function and whole body metabolic health.
Given the important role of the core clock in the regulation of skeletal muscle metabolism and function, the purpose of this work was to determine the extent to which acute binge alcohol intoxication disrupts the core molecular clock in skeletal muscle. We then sought to identify potential factors contributing to alcohol-induced disruption in the core clock. In all, this work further increases our understanding of the impact of binge alcohol on skeletal muscle.
METHODS
Experimental Design
Experiment 1: RNA sequencing to determine new gene programs modulated by acute alcohol intoxication in skeletal muscle.
RNA sequencing was performed on gastrocnemius (GAS) muscle samples with the plantaris left attached obtained from a previously published study in which mice were treated acutely with alcohol (12, 13).
Experiment 2: Assessing changes in core clock genes 0–24 h after acute alcohol intoxication.
Animals.
Female (n = 39) C57BL/6Hsd mice (15 wk old) were purchased from Envigo (Indianapolis, IN). Female mice were used as several sources have shown that females may experience more severe alcoholic injury and are at a higher risk for alcoholic liver disease (14–16). Upon arrival, all mice were individually housed in the Biomedical Research Facility (BRF) vivarium at Florida State University (FSU) for at least 3 wk before the start of the experiment. Mice were housed in a temperature-controlled (25°C) environment on a 12:12 light/dark cycle, where the lights came on at 12 AM and went off at 12 PM. Mice were given ad libitum access to water and chow (Lab Diet #5001; LabDiet, St. Louis, MO) throughout the entire experiment.
Mice were randomly assigned to one of three groups of equal body weight: baseline (n = 3), control (saline; n = 18), or alcohol (EtOH; n = 18). Mice in control and EtOH groups received an intraperitoneal (IP) injection of either 0.9% saline (control) or EtOH diluted in saline (5 g/kg body wt), at the start of the dark cycle [Zeitgeber time 12 (ZT12)], which corresponds to the active phase in the animals and the peak mRNA expression of the period genes and Cry2 (17). Intraperitoneal injection was the optimal method of delivery as it is faster to perform, allowing for animals to be injected within the narrow window of time needed for the circadian experiment, is not influenced by the contents of the stomach, and has a lower risk for health-related complications (e.g., esophagus puncture) compared with gavage. This dose of alcohol was used, as it has previously been shown to induce key features of alcoholic myopathy including decreased skeletal muscle protein synthesis and anabolic signaling (18). Furthermore, recent work from Pruett et al. (19) determined that a dose of 5 g/kg alcohol in mice is translatable to human consumption, as doses required to achieve a similar area under the curve of blood alcohol kinetics in mice are approximately twofold higher than in humans. As no mortality following intoxication was observed in the present experiments, this was not a lethal dose to the mice. Lastly, the feeding and activity patterns of the mice were not controlled for or measured during the experiment, as we did not want to interrupt the normal behavioral rhythms of the animals.
Baseline mice did not receive an injection and were euthanized at ZT12. Beginning at ZT16, three control and three EtOH mice were euthanized every 4 h for the next 20 h to complete a 24-h collection period. The light cycle of the housing room and the separate euthanasia space were maintained on the normal 12-h light/dark schedule: The rooms were dark from ZT12–ZT24 and light from ZT0–ZT12. When mice were euthanized from ZT12–ZT24, they were transported in a plastic lightproof container, while remaining in their home cage and were then anesthetized with isoflurane before euthanasia via cervical dislocation under red light. The lights were then turned on and the liver and gastrocnemius (GAS) were immediately excised, frozen in liquid nitrogen, and stored at −80°C until further analysis. All other muscles (i.e., plantaris and soleus) were removed from the GAS before freezing. Mice were singly housed to allow the investigator to quietly remove one animal at a time from the housing room for euthanasia to avoid disruption to the other animals and limit any interruption to their normal behaviors and avoid an induction of stress hormones.
Blood was collected from an additional cohort of animals (CON; n = 11; EtOH; n = 17) using an identical experimental protocol as just described. At 30 min, 4 h, 8 h, and 12 h postinjection, n = 2–3 control and n = 3–5 EtOH mice were euthanized. Upon collection, blood was either stored at room temperature for 30–60 min to allow for clotting and isolation of serum or transferred to an EDTA-treated tube, placed on ice and used for plasma isolation. Serum samples were centrifuged at 2,000 g for 15 min and samples were stored at −80°C. Plasma samples were centrifuged at 10,000 g for 10 min and stored at −80°C until blood alcohol concentrations (BACs) analysis. All procedures were approved by Animal Care and Use Committee at Florida State University and conform to American Veterinary Medical Association (AVMA) guidelines.
Experiment 3: Assessing changes in core clock genes 24–48 h after acute alcohol intoxication.
A follow-up experiment was conducted to extend our data points to include 24–48 h post alcohol intoxication. Identical methods, as described above for experiment 2, were used in female (n = 42) C57BL/6Hsd mice (13–15 wk old), purchased from Envigo (Indianapolis, IN). As above, mice were randomly assigned to control (CON; n = 21) or alcohol (EtOH; n = 21) groups that were matched for body weight and n = 3 mice per treatment per time point were euthanized for tissue collection over the 24-h experimental period.
Experiment 4: Effects of alcohol on expression of key components of the core clock in C2C12 myotubes in culture.
C2C12 myoblasts purchased from American Type Culture Collection (ATCC) (Manassas, VA) were cultured in Dulbecco’s modified eagle’s medium (DMEM) (VWR, #10–017-CV), with 10% fetal bovine serum (Atlas Biologicals, EF-0500-A) and 1% penicillin-streptomycin (Gibco, #15070-063). At ∼50%, confluency myoblasts were transferred to 12-well plates until reaching ∼90%–95% confluency, at which time medium was changed to DMEM containing 2% horse serum (Gibco, #16050130) for differentiation to myotubes for 3–4 days. On day 4, after differentiation, the core molecular clock within the myotubes was synchronized by treating with 1 µM dexamethasone (Sigma-Aldrich, #D1756) for 90 min. After the treatment, the myotubes were washed with 1X phosphate-buffered saline (PBS) and returned to differentiation media for 32 h to allow for full synchrony of the core clock in the myotubes (20). Myotubes were then treated with fresh media (CON) or fresh media containing 100 mM alcohol (EtOH), as this concentration has been shown to recapitulate features of alcoholic muscle disease (21, 22). Cells were harvested at baseline (BL) before the addition of new media, 4 h, 8 h, and 12 h following the addition of alcohol (or fresh control media). Cells were collected in TRI reagent (Zymo Research, Irvine, CA) and stored at −80°C until analysis. This experiment was repeated three times and the data are expressed as the mean of all three trials.
Experiment 5: Inhibition of corticosterone synthesis and the effects of alcohol intoxication.
Female C57BL/6Hsd mice (15 wk old), purchased from Envigo (Indianapolis, IN), were individually housed in the FSU BRF vivarium for at least 2 wk prior testing. Mice were then randomly assigned to one of three groups: control (CON; n = 5), alcohol (EtOH; n = 7), or alcohol + metyrapone (EtOH-MET; n = 7). Mice received a 100 mg/kg body wt ip injection of metyrapone (VWR, Cat. No. 101095-552) diluted in saline or saline alone (CON). Ninety minutes after metyrapone treatment, a 5 g/kg body wt ip injection of either saline (control) or EtOH was administered. All mice were euthanized 4 h later, as described above, and gastrocnemius muscles were collected and immediately frozen in liquid nitrogen and stored at −80°C. Blood was collected, as described above. A metryapone only group was also included in this experiment, but inclusion of this group did not alter the outcome variables or conclusions of the study; therefore, this group was not included for the sake of clarity. All procedures were approved by Animal Care and Use Committee at Florida State University and conform to AVMA guidelines.
RNA sequencing.
RNA was extracted from the gastrocnemius-plantaris complex using the miRNeasy minikit (Qiagen), as per the manufacturer’s instructions. mRNA was isolated from the total RNA using an NEBNext Poly(A) mRNA Magnetic Isolation Module, as per instructions (New England Biolabs). The remainder of the sequencing procedures were performed at Florida State University at the Translational Science Laboratory. cDNA libraries were generated from the isolated mRNA using an NEBNext Ultra RNA library prep kit for Illumina (New England Biolabs) and eight nucleotide i7 and i5 dual-index primers (NEBNext multiplex oligos for Illumina, Dual Index Primers) were incorporated into each sample. The library construction was done according to the NEB manuals, modified for use with a Beckman Biomek 4000 at the FSU Biological Sciences core laboratory. Five libraries were generated per group (control or alcohol) for a total of 10 libraries that were multiplexed into one lane of the sequencing run on an Illumina NovaSeq 6000 S1 flowcell, with single end, 100 base reads. All RIN values were above 7.0. Initial quality control analysis of each sequenced library was performed using fastQC software (http://www.bioinformatics.babraham.ac.uk/projects/fastqc). The sequencing reads were further analyzed using RNA-Seq Alignment version 1.1.1 (Illumina BaseSpace application). The reads were aligned with Tophat 2 (23) to the mouse genome (UCSC mm10) using default parameters and counts for each gene were generated. Statistical analyses of the treatment groups were performed using DESeq2 to determine differentially expressed genes (24). The list of DEGs was uploaded into the publicly available Database for Annotation, Visualization and Integrated Discovery (DAVID) software (https://david.ncifcrf.gov/) and analyzed using functional category analysis. Gene expression data for this study is available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE183665.
RNA extraction, cDNA synthesis, and RT-PCR.
The entire GAS from experiments 2 and 3 were powdered in liquid nitrogen using a mortar and pestle. Of the powdered muscle, 20 mg was homogenized in 600 µL of TRI reagent (Zymo Research, Irvine, CA). Similarly, a portion of the liver was homogenized in 600 µL of TRI reagent. In C2C12 experiments, myotubes were harvested directly in TRI reagent. For all experiments, RNA was isolated as per manufacturer’s instructions using the Direct-zol RNA MiniPrep kit with on column DNase treatment (Cat. No. R2052; Zymo Research, Irvine, CA). cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit as per manufacturer’s instructions (Cat. No. 4368814; Applied Biosystems, Foster City, CA). Relative mRNA levels were determined by RT-PCR using the SYBR-green master mix (Cat. No. A25776; Applied Biosystems, Foster City, CA) on a QuantStudio3. The conditions for RT-PCR with SYBR Green included an initial 2 min at 50°C and 2 min at 95°C, followed by 40 cycles that included a 15-s denature step at 95°C, a 15-s annealing step at 55°C, and a 1-min extension step at 72°C within each cycle. A melt curve analysis was performed for each primer pair to ensure that a single product was efficiently amplified and the product sizes for each primer pair were verified via agarose gel electrophoresis before experimentation. All primer sequences used for Sybr green are listed in Table 1. Measurement of Thyrotrophic Embryonic Factor (Tef) (assay ID Mm00457513) and Basic Helix-Loop-Helix-Family Member E40 (Bhlhe40) (assay ID Mm00478593) were quantified using TaqMan predesigned primer probes (Applied Biosystems) and TaqMan fast advanced master mix (Cat. No. 4444557; Applied Biosystems, Foster City, CA). RT-PCR results were analyzed using the delta, delta CT method and referenced to the Ribosomal Protein Lateral Stalk Subunit P0 (Rplp0) gene or the 60S Ribosomal Protein L26 (Rpl26) gene, which did not significantly change with treatment or time.
Table 1.
Primer sequences
| Gene Symbol | Forward (5′-3′) | Reverse (5′-3′) | Amplicon Size, bp |
|---|---|---|---|
| Bmal1 | TGGAGGGACTCCAGACATT | TTGCTGCCTCATCGTTACTG | 173 |
| Per1 | GTCCCCTGGTCCTCTACACA | GCCCGAGATTCAATGAAGAG | 159 |
| Per2 | GCCACCCTGAAAAGGAAGT | GGTGAGGGACACCACACTCT | 184 |
| Per3 | GTCGAGAGGAGGTGCTGAAG | TCTGTCTTCACAGGCGACAC | 173 |
| Cry1 | TTCACTGCTACTGCCCTGTG | CACTTGGCAACCTTCTGGAT | 151 |
| Cry2 | GATGTGTTCCCAAGGCTGTT | GTGGTTTCTGCCCATTCAGT | 196 |
| Clock | GAGGTCGTCCTTCAGCAGTC | CGCTGCTCTAGCTGGTCTTT | 171 |
| Rev-Erbα | GCACCTGCCAACAGTCTA | GCTGAGAAAGGTCACGGAAG | 197 |
| Rorα | GGAAGAGTTTGTGTTCTATGCACC | TTCCATCTTCTCGGTGGTTC | 177 |
| Myod1 | ACCCAAGGTGGAGATCCTG | CATCATGCCATCAGAGCAGT | 200 |
| Dbp | TCTAGGGACACACCCAGTCC | TGGTTGAGGCTTCAGTTCCT | 159 |
| Rplpo | CAACCCAGCTCTGGAGAAAC | GTTCTGAGCTGGCACAGTGA | 169 |
| Redd1 | TGGTGCCCACCTTTCAGTTG | GTCAGGGACTGGCTGTAACC | 121 |
| Rpl26 | TGTTCGCGGACACTACAAAG | CGGTCCTTGTCCAGCTTTAG | 174 |
Western blotting and analysis.
Western blotting was performed, as previously described (25). Powdered GAS (∼40 mg) was homogenized using a glass-on-glass homogenization in 10 volumes of buffer (10 µL/mg of muscle) consisting of 50 mM HEPES (pH 7.4), 0.1% Triton-X 100, 4 mM EGTA, 10 mM EDTA, 15 mM Na4P2O7, 100 mM β-glycerophosphate, 25 mM NaF, 5 mM Na3VO4, and 10 µL/mL protease inhibitor cocktail (Cat. No. P8340, Sigma-Aldrich, St. Louis, MO). Muscle extracts were centrifuged for 10 min at 10,000 g at 4°C, and the supernatant fraction was quantified via the Bradford method (Cat. No. 5000006, Bio-Rad, Hercules, CA). All samples were diluted to the same concentration in 2× sample buffer. For each time point, an equal amount of protein and volume from each sample within a group (control or alcohol) was pooled together to determine the overall circadian expression patterns within that group at each time point, as previously described for large data sets (17, 26). Proteins were fractionated on 4%–20% Bio-Rad Criterion precast gels (Hercules, CA) and transferred to PVDF membranes. Ponceau-S staining was used to assess effective transfer and equal protein loading. Membranes were blocked (1 h) with 5% nonfat dried milk in Tris-buffered saline + 0.1% Tween 20 (Tris-buffered saline-Tween 20). Membranes were then incubated overnight at 4°C with an antibody against regulated in development and DNA damage 1 (REDD1) (1:500; 10638-1-AP, Protein Technologies, Rosemont, IL). We have previously validated the REDD1 antibody using REDD1 knockout mice (27). After incubation with the appropriate secondary antibody 1:10,000 (Cat. No. A120–101; Bethyl Laboratories; Montgomery, TX), the antigen-antibody complex was visualized by enhanced chemiluminescence using Clarity reagent (Bio-Rad, Hercules, CA) on a Bio-Rad ChemiDoc Touch imaging system. ImageJ software (National Institutes of Health, Bethesda, MD) was used to quantify all images, including Ponceau-S stain.
Blood alcohol concentrations.
The BAC was measured in plasma samples using the Analox System (AM-1) (Stourbridge, UK). Samples were read in duplicate and averaged.
Serum corticosterone concentrations.
Corticosterone concentration was measured in serum samples via ELISA, as per the manufacturer’s instructions (Cat. No. 501320; Cayman Chemical, Ann Arbor, MI). Standards and samples were loaded in duplicate and results were averaged within each sample.
Statistical Analysis
Experiments 2–5.
Experiments 2 and 3 were analyzed separately, as the samples were not harvested in tandem. Gene expression data for experiment 2 and 3 were first analyzed using one-way ANOVA (Time) to evaluate rhythmicity of the gene expression data, where P < 0.05 indicates differences across time points and presumably circadian rhythmicity. A sum-of-squares F test was then used to determine whether a single cosinor model fit both the control and alcohol groups, where a significant P value indicates differences in rhythmicity between the groups. Cosinor analysis was completed using the predefined 24-h period and the least squares method to fit the curve using the equation, Y = Baseline + Amplitude × cos(Frequency × X + Phaseshift) and compared control versus alcohol treatment. A significant P value of P < 0.05 indicates rejection of the null or zero-amplitude hypothesis with a 95% threshold and is indicative of a significant rhythm. A nonsignificant P value (P > 0.05) indicates that the null hypothesis is accepted and control and alcohol cosinor parameters are not different, meaning alcohol did not alter the circadian rhythm of that gene’s expression. The specific parameters of the cosinor curve were then determined, which included the MESOR or midline estimating statistic of rhythm for mRNA expression, the amplitude defined as half the differences between the peak and the trough of mRNA expression, and the acrophase corresponding to the peak of the rhythm in hours. If the estimate of the time of the acrophase fell outside of the experimental time frame the acrophase was adjusted to fit within the timing of the data input into the model. As mice were maintained on a 12-h light/dark cycle, their period length was fixed to 24 h. For each parameter (MESOR, amplitude, acrophase), the mean and standard error are reported along with the P value of the unpaired t test between control and alcohol-treated groups. For those genes in the alcohol-treated group that the null hypothesis was accepted (P > 0.05) indicating a lack of rhythmicity, no values for MESOR, amplitude, or acrophase were calculated. The corresponding figures are available in the supplementary data file and those figures are referred to in the text using the abbreviation SF. In addition to cosinor analysis, a two-way ANOVA (Time, EtOH) with Bonferroni post hoc comparisons was employed to determine differences between treatment groups for all genes presented. Serum corticosterone levels were analyzed using a two-way ANOVA (Time, EtOH) with Bonferroni post hoc analysis. In experiment 4, each result represents three biological replicates (n = 3 per group, per replicate). Data were analyzed via two-way ANOVA (Time, EtOH) for the detection of main effects and Bonferroni post hoc analysis was used to compare specific groups if a significant interaction was observed. Experiment 5 was analyzed using a one-way ANOVA with Tukey’s post hoc to assess gene expression. All data analysis was completed using GraphPad Prism (San Diego, CA). Significance was set at P < 0.05 for all analyses.
RESULTS
Binge Alcohol Alters the Expression of Core Clock Genes and Clock-Controlled Genes in Skeletal Muscle
RNA sequencing identified “Circadian Rhythm” as one of the 55 pathways altered by acute alcohol intoxication when all genes (1,683) with an unadjusted P value of less than 0.05 were analyzed (Supplemental Table S1; https://doi.org/10.6084/m9.figshare.14607657.v1). This pathway piqued our interest, as circadian rhythms are important in skeletal muscle metabolism and function and are disrupted by alcohol in several other tissues (7, 28–31). The specific genes identified included Clock, Per3, Cry1, Rora, F-box and leucine-rich repeat protein 3 (Fbxl3), basic helix-loop-helix family member E41 (Bhlhe41), and cullin1 (Cul1) (Table 2). To assess the extent to which acute alcohol intoxication alters the expression of the core clock genes in skeletal muscle, mice were administered saline (control) only or EtOH at the beginning of the dark cycle, and the expression of genes that encode components of the core clock and genes that are known to be under control of the core clock were assessed every 4 h for 48 h.
Table 2.
Genes within the circadian rhythm pathway changed by alcohol
| Gene | Fold Change | Unadjusted P Value |
|---|---|---|
| Fbxl3 | 0.74 ± 0.13 | 0.008 |
| Rora | 0.7 ± 0.1 | 0.02 |
| bhlhe41 | 1.74 ± 0.70 | 0.0006 |
| Clock | 0.84 ± 0.05 | 0.03 |
| Cry1 | 0.74 ± 0.15 | 0.004 |
| Cul1 | 0.84 ± 0.11 | 0.01 |
| Per3 | 1.56 ± 0.53 | 0.004 |
Genes within the circadian rhythm pathway identified to be different by DAVID analysis following RNA sequencing of control and acute alcohol treated skeletal muscle of mice (experiment 1).
When looking specifically at the effects of alcohol (via 2-way ANOVA), the mRNA expression of core clock genes was disrupted by alcohol up through 48-h post intoxication including a main effect of alcohol on the mRNA expression of Clock, Bmal1, Per1, Per2, Cry1, Cry2, but not Per3 (Fig. 1, A–G). Similarly, several clock-controlled genes, including Rorα, Rev-erbα, Myod1, Dbp, Tef, and Bhlhe40, were also changed by alcohol over time, as indicated by significant main effects from 0 to 48 h after intoxication (Fig. 2, A–F). Of note, blood alcohol concentrations returned to baseline values (i.e., 0 mmol/L) by the 12-h time point after peaking at 165 ± 10.1 mmol/L 30-min post intoxication. Therefore, any differences observed at 12 h and thereafter represent the lasting effects of the binge episode and not a direct effect of the presence of alcohol itself.
Figure 1.
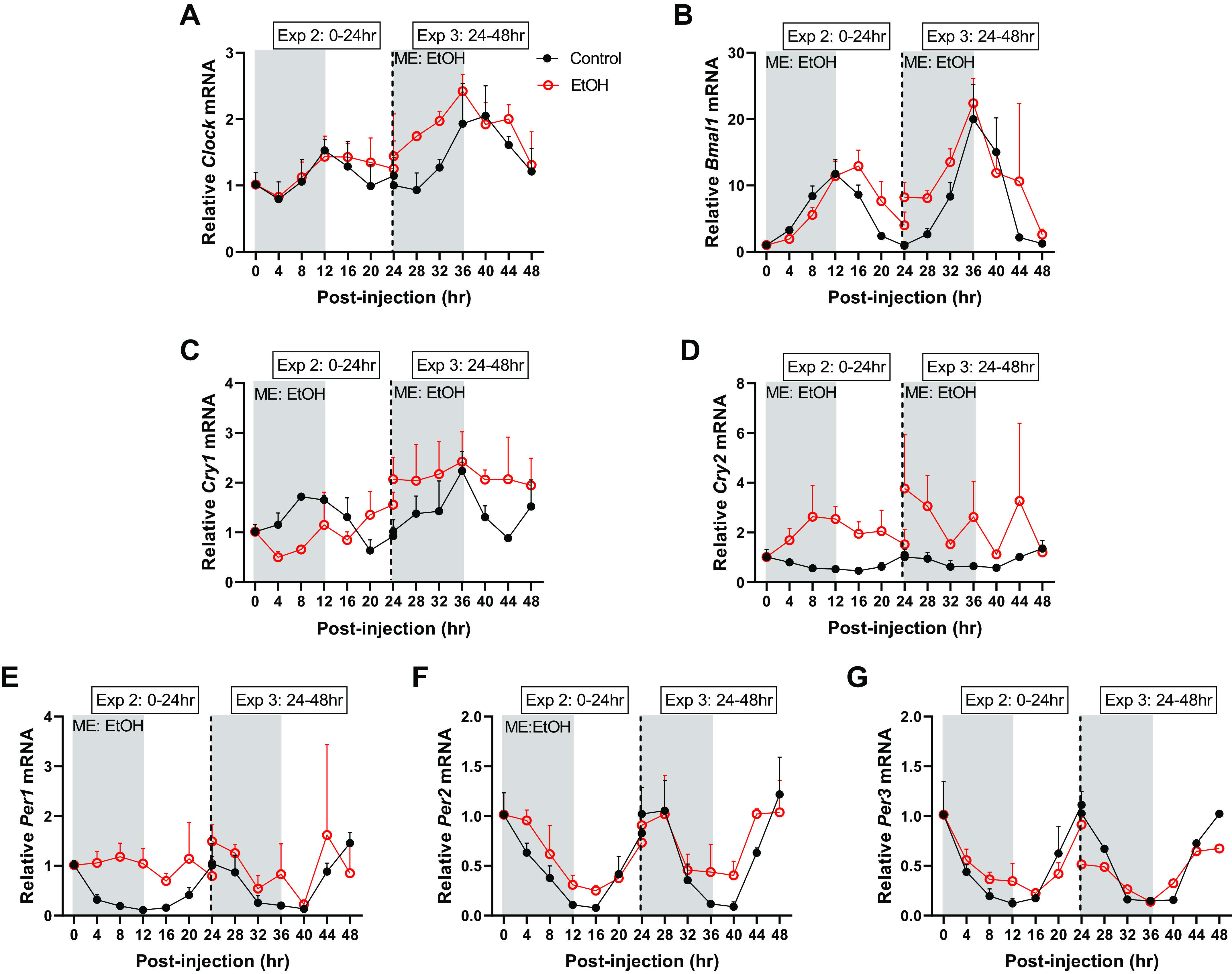
The effects of acute alcohol intoxication on mRNA expression of core clock components, Clock (A), Bmal1 (B), Cry1 (C), Cry2 (D), Per1 (E), Per2 (F), and Per3 (G) from experiment 2 (0–24 h) and experiment 3 (24–48 h), for gastrocnemius. The vertical dotted line represents the division of the two experiments, and gray boxes depict the dark cycle, whereas white boxes depict the light cycle. The 0-h time point is the time of injection and baseline tissue collection (n = 3). The black spheres represent control (n = 3), and the open red spheres represent EtOH (n = 3). Data are presented as means ± SD for each time point. Main effects and interactions were determined by two-way ANOVA (Time, EtOH) where P ≤ 0.05, and the main effect of alcohol is displayed on the graph as ME: EtOH.
Figure 2.
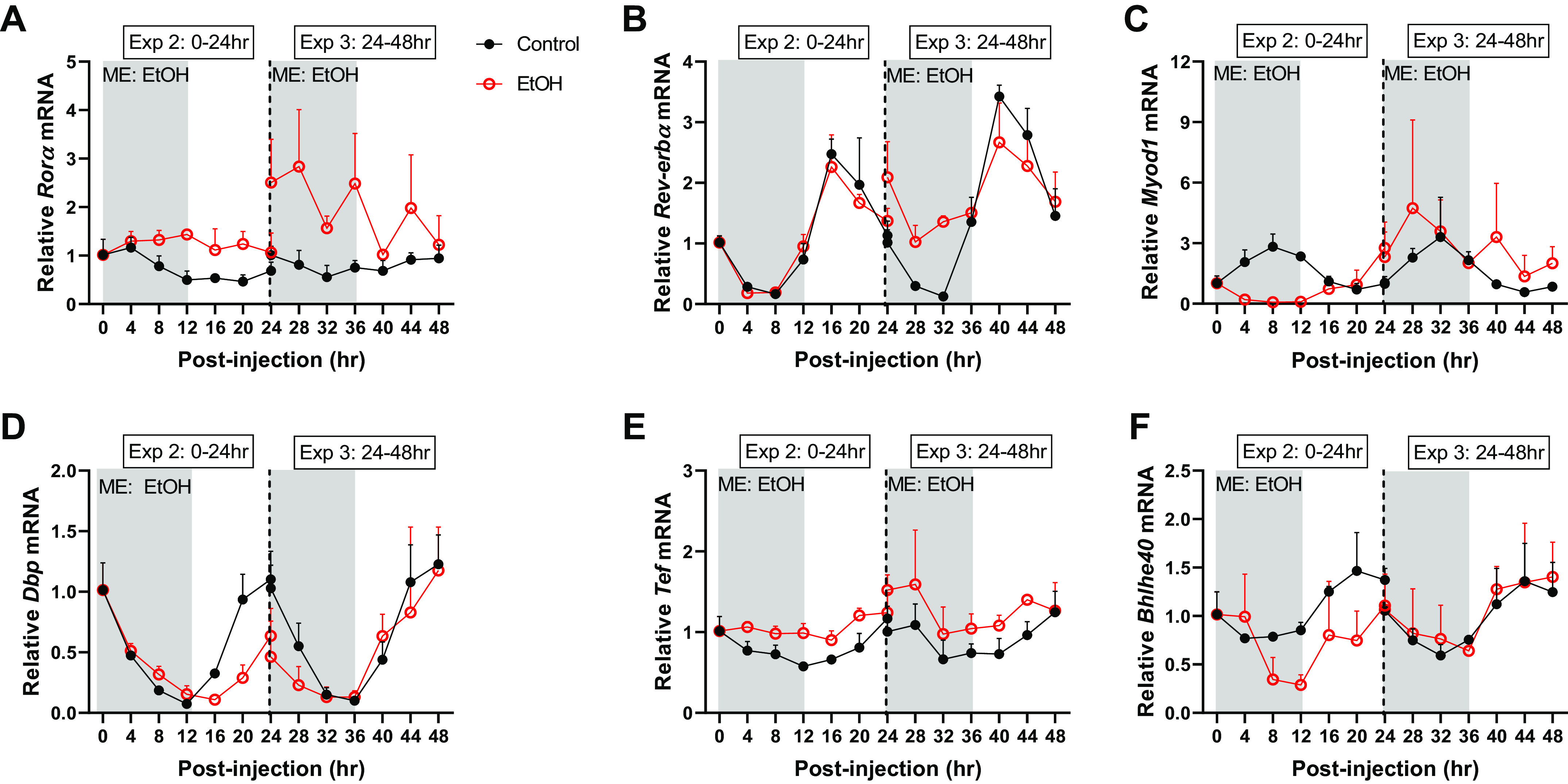
The effects of acute alcohol intoxication on mRNA expression of CCGs, Rorα (A), Rev-erbα (B), Myod1 (C), Dbp (D), Tef (E), and Bhlhe40 (F) in experiment 2 (0–24 h) and experiment 3 (24–48 h). The vertical dotted line represents the division of the two experiments, and gray boxes depict the dark cycle, whereas white boxes depict the light cycle. The 0-hr time point is the time of injection and baseline tissue collection (n = 3). The black spheres represent control (n = 3), and the open red spheres represent EtOH (n = 3). Data are presented as means ± SD for each time point. Main effects and interactions were determined by two-way ANOVA (Time, EtOH) where P ≤ 0.05 and the effect of alcohol is displayed on the graph as ME: EtOH.
Binge Alcohol Disrupts Gene Oscillation and Oscillatory Amplitude of Core Clock Genes and Clock-Controlled Genes in Skeletal Muscle
One-way ANOVA was performed to assess rhythmic mRNA expression patterns within each treatment group (0–24 h or 24–48 h) (Table 3). From 0–24 h post alcohol treatment, the mRNA expression of each of the core clock and clock-controlled genes measured in the control group were deemed to have a rhythmic expression pattern except for Clock (Table 3). Alcohol intoxication abolished circadian oscillation for Per1, Cry2, Rorα, Tef, and Bhlhe40 from 0 to 24 h (Table 3). At 24–48 h, the mRNA expression of all genes in the control group again exhibited rhythmicity, except for Rorα (Table 3). Alcohol intoxication continued to disrupt oscillation of Clock, Per1, Per3, Cry1, Cry2, Myod1, Tef, and Bhlhe40 at 24–48 h (Table 3). Comparison of rhythmicity between control and alcohol groups using Cosinor analysis showed an effect of alcohol on the rhythmic expression of Bmal1, Per1, Per2, Cry1, Cry2, Rorα, Myod1, Dbp, Tef, and Bhlhe40 in experiment 2 (0–24 h) and for Bmal1, Per2, Per3, Cry1, Cry2, Rorα, Reverbα, and Tef in experiment 3 (24–48 h) (Table 3).
Table 3.
Analysis of circadian gene expression in the gastrocnemius of control and alcohol-treated mice
| Muscle | One-Way ANOVA Results |
Cosinar Results |
||||
|---|---|---|---|---|---|---|
| 0–24 h |
24–48 h |
0–24 h |
24–48 h |
|||
|
P Value |
P Value |
P Value |
P Value |
|||
| Gene | Control | EtOH | Control | EtOH | Con vs. EtOH | |
| Clock | 0.12 | 0.13 | 0.0004 | 0.09 | 0.59 | 0.05 |
| Bmal1 | <0.0001 | <0.0001 | <0.0001 | 0.01 | <0.0001 | 0.03 |
| Per1 | <0.0001 | 0.61 | <0.0001 | 0.33 | <0.0001 | 0.40 |
| Per2 | <0.0001 | <0.0001 | <0.0001 | 0.01 | 0.02 | 0.03 |
| Per3 | <0.0001 | 0.0001 | 0.0002 | 0.27 | 0.20 | 0.03 |
| Cry1 | 0.0002 | 0.02 | 0.02 | 0.97 | <0.0001 | 0.001 |
| Cry2 | 0.002 | 0.14 | 0.004 | 0.30 | <0.0001 | 0.01 |
| Reverbα | <0.0001 | <0.0001 | <0.0001 | 0.01 | 0.89 | <0.0001 |
| Rorα | 0.004 | 0.61 | 0.23 | 0.12 | <0.0001 | <0.0001 |
| Myod1 | <0.0001 | 0.001 | 0.01 | 0.52 | <0.0001 | 0.11 |
| Dbp | <0.0001 | <0.0001 | <0.0001 | 0.01 | 0.0001 | 0.12 |
| Tef | 0.001 | 0.10 | 0.03 | 0.21 | <0.0001 | 0.003 |
| Bhlhe40 | 0.001 | 0.06 | 0.05 | 0.14 | 0.001 | 0.93 |
Analysis of circadian gene expression in the gastrocnemius of control and alcohol-treated mice. One-way ANOVAs were used to evaluate changes in gene expression overtime representative of circadian rhythmicity. A sum-of-squares F test was used to determine whether a single cosinor model fit both control and EtOH-treated groups to further indicate differences in rhythmicity between the two groups. Values are P values from control and alcohol-treated female mice at the time points indicated, with bold values indicating significance.
When looking specifically at the circadian properties of core clock gene expression (via cosinor analysis), several genes were disrupted by alcohol. Alcohol intoxication did not alter Clock expression until 24–48 h after intoxication, at which point an increase in MESOR, or the overall average expression of Clock, was detected along with a shift in acrophase, or the time of peak Clock expression (Tables 4–5 and Fig. 1A, Supplemental Figs. S1A and S3A; https://doi.org/10.6084/m9.figshare.14607615.v1 and https://doi.org/10.6084/m9.figshare.14607627.v1). A main effect of alcohol on Bmal1 expression coincided with increased MESOR values 0–48 h (Tables 4–5 and Fig. 1B). Furthermore, the acrophase of Bmal1 was shifted to the right at 0–24 h, before shifting leftward in alcohol-treated muscle from 24 to 48 h (Fig. 1B and Tables 4–5, Supplemental Figs. S1B and S3B). Amplitude, which compares half the difference between the peak and the trough of mRNA expression of control and alcohol groups, was also reduced for Bmal1 from 0 to 24 h following alcohol intoxication (Tables 4–5, Supplemental Figs. S1B and S3B). In the negative arm of the clock, Cry1 and Cry2 were altered by alcohol intoxication (Fig. 1, C–D). Despite MESOR being decreased in Cry1 and increased in Cry2 0–24 h after intoxication, there were no changes in the amplitude of either gene (Table 4). Furthermore, a phase delay was detected from 0 to 24 h in alcohol-treated mice for both Cry1 and Cry2, whereas an alcohol-induced disruption in their rhythmicity at 24–48 h precluded the determination of these characteristics (MESOR, amplitude, Acrophase) at later time points (Tables 4–5). Alcohol also led to a loss of rhythmicity from 0 to 24 h in Per1, whereas the MESOR and acrophase of Per2 were increased by alcohol at 0–24 h (Table 4, Supplemental Fig. S1, E–F). Rhythmicity of Per1 was restored by 24–48 h, although its amplitude was reduced and the acrophase occurred earlier (Table 5, Supplemental Fig. S3E). Likewise, the amplitude of Per2 was also decreased during this recovery period (Table 5). Alcohol led to a shift in the acrophase of Per3 (0–24 h and 24–48 h) in addition to a decrease in its amplitude (0–24 h and 24–48 h) (Tables 4 and 5, Fig. 1E, Supplemental Fig. 1G). Therefore, alcohol intoxication disrupted the oscillation of the mRNA expression of core clock genes up to 48 h after intoxication.
Table 4.
Circadian characteristics of gastrocnemius 0–24 h after alcohol intoxication
| Muscle |
0–24 h |
Control |
EtOH |
|||
|---|---|---|---|---|---|---|
| Gene | Measure | Average | Means ± SE | Average | Means ± SE | P Value |
| Clock | MESOR (mRNA expression) | 1.15 | 0.06 | 1.22 | 0.06 | 0.38 |
| Amplitude (mRNA expression) | 0.24 | 0.09 | 0.28 | 0.09 | 0.73 | |
| Acrophase, h | 24.58 | 0.40 | 24.11 | 0.29 | 0.35 | |
| Bmal1 | MESOR | 5.95 | 0.25 | 6.99 | 0.43 | 0.04 |
| Amplitude | 5.36 | 0.33 | −5.84 | 0.60 | <0.0001 | |
| Acrophase | 25.17 | 0.07 | 27.57 | 0.10 | <0.0001 | |
| Per1 | MESOR | 0.40 | 0.05 | |||
| Amplitude | 0.42 | 0.06 | ||||
| Acrophase | 34.60 | 0.16 | ||||
| Per2 | MESOR | 0.44 | 0.03 | 0.57 | 0.04 | 0.01 |
| Amplitude | −0.43 | 0.05 | −0.39 | 0.05 | 0.59 | |
| Acrophase | 12.21 | 0.12 | 35.78 | 0.14 | <0.0001 | |
| Per3 | MESOR | 0.46 | 0.05 | 0.51 | 0.05 | 0.50 |
| Amplitude | 0.48 | 0.06 | −0.33 | 0.06 | <0.0001 | |
| Acrophase | 34.66 | 0.15 | 12.33 | 0.21 | <0.0001 | |
| Cry1 | MESOR | 1.26 | 0.05 | 0.99 | 0.09 | 0.01 |
| Amplitude | 0.48 | 0.07 | 0.34 | 0.13 | 0.34 | |
| Acrophase | 19.44 | 0.15 | 35.67 | 0.37 | <0.0001 | |
| Cry2 | MESOR | 0.69 | 0.04 | 2.01 | 0.15 | <0.0001 |
| Amplitude | 0.29 | 0.05 | 0.61 | 0.20 | 0.13 | |
| Acrophase | 16.55 | 0.21 | 19.00 | 0.37 | <0.0001 | |
| Reverbα | MESOR | 1.11 | 0.08 | 1.09 | 0.07 | 0.88 |
| Amplitude | −1.15 | 0.13 | −1.03 | 0.10 | 0.44 | |
| Acrophase | 32.97 | 0.10 | 24.09 | 0.09 | <0.0001 | |
| Rorα | MESOR | 0.71 | 0.05 | |||
| Amplitude | 0.32 | 0.07 | ||||
| Acrophase | 14.69 | 0.22 | ||||
| MyoD | MESOR | 1.67 | 0.08 | 0.72 | 0.18 | <0.0001 |
| Amplitude | 1.10 | 0.11 | 0.89 | 0.25 | 0.45 | |
| Acrophase | 19.80 | 0.10 | 35.03 | 0.31 | <0.0001 | |
| Dbp | MESOR | 0.52 | 0.03 | 0.39 | 0.04 | 0.02 |
| Amplitude | 0.52 | 0.04 | −0.35 | 0.06 | <0.0001 | |
| Acrophase | 34.90 | 0.09 | 12.20 | 0.19 | <0.0001 | |
| Tef | MESOR | 0.79 | 0.03 | 1.04 | 0.03 | <0.0001 |
| Amplitude | 0.23 | 0.04 | 0.11 | 0.05 | 0.57 | |
| Acrophase | 28.24 | 0.21 | 34.73 | 0.49 | <0.0001 | |
| Bhlhe40 | MESOR | 1.05 | 0.04 | 0.71 | 0.07 | 0.0003 |
| Amplitude | −0.37 | 0.06 | 0.36 | 0.10 | <0.0001 | |
| Acrophase | 32.58 | 0.16 | 34.73 | 0.31 | <0.0001 | |
Circadian characteristics including the MESOR, amplitude, and acrophase 0–24 h after alcohol treatment (experiment 2). For each parameter (MESOR, amplitude, and acrophase), the mean and standard error are reported along with the P value of the unpaired t test between control and alcohol-treated groups (P < 0.05). For those genes in the alcohol-treated group that the null hypothesis was rejected indicating a lack of rhythmicity, no values for MESOR, amplitude, or acrophase were calculated. Bold values indicate significance.
Table 5.
Circadian characteristics of gastrocnemius 24–48 h after alcohol intoxication
| Muscle |
24–48 h |
Control |
EtOH |
|||
|---|---|---|---|---|---|---|
| Gene | Measure | Average | Means ± SE | Average | Means ± SE | P Value |
| Clock | MESOR (mRNA expression) | 1.46 | 0.11 | 1.79 | 0.11 | 0.04 |
| Amplitude (mRNA expression) | 0.26 | 0.16 | −0.22 | 0.17 | 0.05 | |
| Acrophase, h | 54.65 | 0.55 | 36.13 | 0.69 | <0.0001 | |
| Bmal1 | MESOR | 8.47 | 0.77 | 12.01 | 1.17 | 0.02 |
| Amplitude | −9.05 | 1.03 | −6.75 | 1.55 | 0.22 | |
| Acrophase | 58.36 | 0.13 | 47.09 | 0.26 | <0.0001 | |
| Per1 | MESOR | 0.61 | 0.06 | 0.92 | 0.17 | 0.10 |
| Amplitude | 0.59 | 0.08 | −0.41 | 0.23 | 0.0002 | |
| Acrophase | 59.64 | 0.15 | 56.58 | 0.64 | <0.0001 | |
| Per2 | MESOR | 0.56 | 0.05 | 0.71 | 0.05 | 0.06 |
| Amplitude | 0.58 | 0.06 | 0.35 | 0.07 | 0.02 | |
| Acrophase | 59.34 | 0.12 | 59.65 | 0.23 | 0.25 | |
| Per3 | MESOR | 0.49 | 0.05 | 0.40 | 0.06 | 0.26 |
| Amplitude | 0.49 | 0.06 | 0.24 | 0.08 | 0.02 | |
| Acrophase | 58.59 | 0.14 | 59.96 | 0.37 | 0.01 | |
| Cry1 | MESOR | 1.45 | 0.10 | |||
| Amplitude | -0.39 | 0.14 | ||||
| Acrophase | 47.60 | 0.39 | ||||
| Cry2 | MESOR | 0.84 | 0.05 | |||
| Amplitude | 0.32 | 0.07 | ||||
| Acrophase | 59.72 | 0.24 | ||||
| Reverbα | MESOR | 1.52 | 0.09 | 1.80 | 0.10 | 0.04 |
| Amplitude | −1.68 | 0.13 | −0.74 | 0.15 | 0.14 | |
| Acrophase | 51.93 | 0.07 | 51.80 | 0.18 | 0.51 | |
| Rorα | MESOR | 0.79 | 0.05 | 1.93 | 0.22 | <0.0001 |
| Amplitude | 0.18 | 0.06 | 0.42 | 0.32 | 0.46 | |
| Acrophase | 58.94 | 0.39 | 39.53 | 0.69 | <0.0001 | |
| MyoD | MESOR | 1.69 | 0.16 | |||
| Amplitude | 1.35 | 0.23 | ||||
| Acrophase | 45.04 | 0.16 | ||||
| Dbp | MESOR | 0.58 | 0.04 | 0.47 | 0.08 | 0.25 |
| Amplitude | 0.57 | 0.06 | 0.44 | 0.12 | 0.30 | |
| Acrophase | 36.11 | 0.12 | 36.51 | 0.26 | 0.17 | |
| Tef | MESOR | 0.89 | 0.05 | 1.24 | 0.07 | 0.0002 |
| Amplitude | 0.24 | 0.06 | 0.25 | 0.10 | 0.95 | |
| Acrophase | 59.62 | 0.29 | 40.73 | 0.44 | <0.0001 | |
| Bhlhe40 | MESOR | 0.96 | 0.06 | 1.02 | 0.08 | 0.55 |
| Amplitude | 0.38 | 0.09 | 0.37 | 0.12 | 0.94 | |
| Acrophase | 54.52 | 0.21 | 36.64 | 0.30 | 0.005 | |
Circadian characteristics including the MESOR, amplitude, and acrophase for experiment 3 (24–48 h). For each parameter (MESOR, amplitude, acrophase), the mean and standard error are reported along with the P value of the unpaired t test between control and alcohol-treated groups (P < 0.05). For those genes in the alcohol-treated group that the null hypothesis was rejected indicating a lack of rhythmicity, no values for MESOR, amplitude, or acrophase were calculated. Bold values indicate significance.
With regards to clock-controlled genes, alcohol intoxication increased Rorα from 0 to 24 h leading to a loss of rhythmicity (Table 4, Supplemental Fig. S2A; https://doi.org/10.6084/m9.figshare.14607624.v1). From 24–48 h, the MESOR remained elevated after prior alcohol intoxication and a phase advance occurred as indicated by an earlier peak in oscillation of Rorα (Table 5, Supplemental Fig. S4A; https://doi.org/10.6084/m9.figshare.14607651.v1). Similarly, the acrophase of Reverbα occurred earlier immediately following alcohol intoxication (Table 4, Supplemental Fig. S2B), whereas at 24–48 h after intoxication, the MESOR or the overall average expression was increased (Supplemental Fig. S4B and Table 5). The mRNA expression of Myod1 was also dysregulated by alcohol with changes in MESOR and acrophase from 0 to 24 h and a loss of rhythmicity at 24–48 h (Tables 4–5, Supplemental Fig. S3C and Fig. S4C). Alcohol reduced the amplitude and MESOR of Dbp and induced an acute phase delay in Dbp expression, which was only apparent from 0 to 24 h (Tables 4 and 5, Supplemental Fig. S4D). From 0 to 48 h, alcohol intoxication increased the overall average expression of Tef (i.e., MESOR), as well as led to significant changes in its acrophase (Tables 4 and 5, Supplemental Fig. S2E and S4E). The greatest alcohol-induced disruptions in Bhlhe40 were observed from 0 to 24 h during which the MESOR was reduced, amplitude increased, and acrophase delayed (Table 4, Supplemental Fig. S2F and S4F), while from 24–48 h only the acrophase remained different (Table 5, Supplemental Fig. S4F). Thus, acute alcohol intoxication alters the diurnal expression of genes that are known to be under the control of the core clock.
Binge Alcohol Differentially Affects Liver Expression of Core Clock Genes Compared with Skeletal Muscle
Next, we assessed whether changes to the expression of clock-related genes in the skeletal muscle corresponded to similar changes in the liver. Interestingly, acute alcohol decreased the mRNA expression of Bmal1, Per2, and Cry1, with no effect on Clock, Per1, or Cry2 at 0–24 h after intoxication (Fig. 3, A–F). One-way ANOVA and Cosinar analysis indicated that although alcohol alters all genes expression over the 24-h period, Clock and Cry2 mRNA expression in the liver was not circadian (Fig. 3G). Alcohol also altered the amplitude of Clock, Bmal1, and Per2; the MESOR of Bmal1, Per2, and Cry1; and the acrophase of Clock and Per2 in the liver (Fig. 3H). Therefore, alcohol-induced changes in core clock genes differ across tissues.
Figure 3.
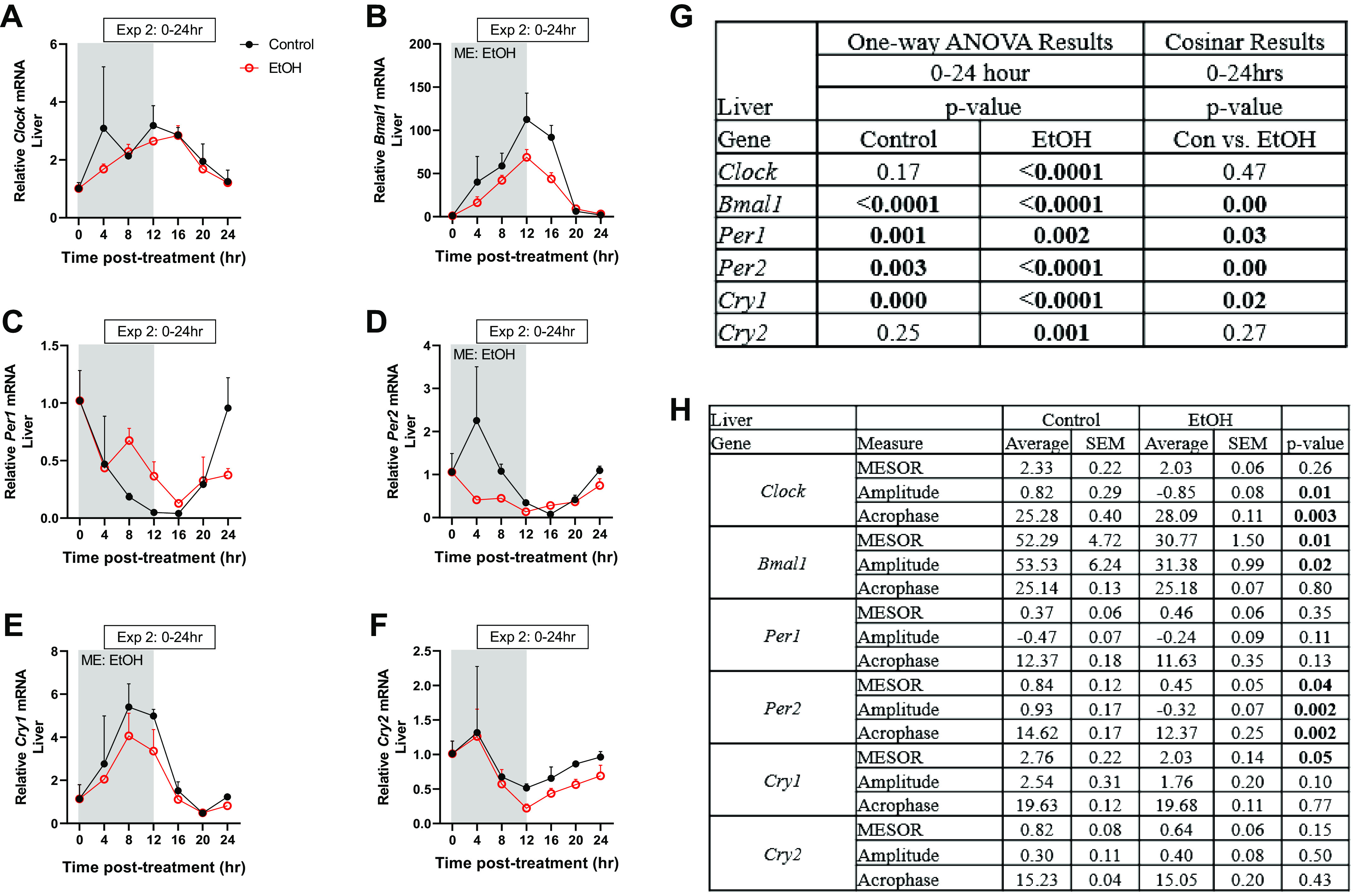
The effects of acute alcohol intoxication on the mRNA expression of core clock genes Clock (A), Bmal1 (B), Per1 (C), Per2 (D), Cry1 (E), and Cry2 (F) in liver samples from experiment 2 (0–24 h), as well as one-way ANOVA (G) and cosinor analysis (H). The 0-h time point is the time of injection and baseline tissue collection (n = 3). The black spheres represent control (n = 3), and the open red spheres represent EtOH- (n = 3) treated mice. Data are presented as means ± SD for each time point. Main effects and interactions were determined by two-way ANOVA (Time, EtOH) where P ≤ 0.05 and the main effect of alcohol is displayed on the graph as ME: EtOH. Bold values indicate significance.
Alcohol Is Not Sufficient to Alter the Expression of Core Clock Genes in Myotubes In Vitro
An in vitro system was then employed to determine whether the changes in core clock genes expression in skeletal muscle were mediated directly by alcohol. The mRNA expression of core clock genes Bmal1, Per1, Cry1, and Cry2 and the clock-controlled gene Rorα were largely unaffected by alcohol treatment (Fig. 4, A–E). In contrast to what was observed in vivo, acute alcohol significantly increased Myod1 mRNA expression in vitro (Fig. 4F). Similarly, no changes in the mRNA expression of Bmal1, Per1, Cry1, or Cry2 were observed following treatment with 200 mM EtOH, a dose of alcohol that exceeded in vivo BAC levels (data not shown). Therefore, these in vitro data suggest that alcohol may not be directly disrupting expression of core clock genes in skeletal muscle.
Figure 4.
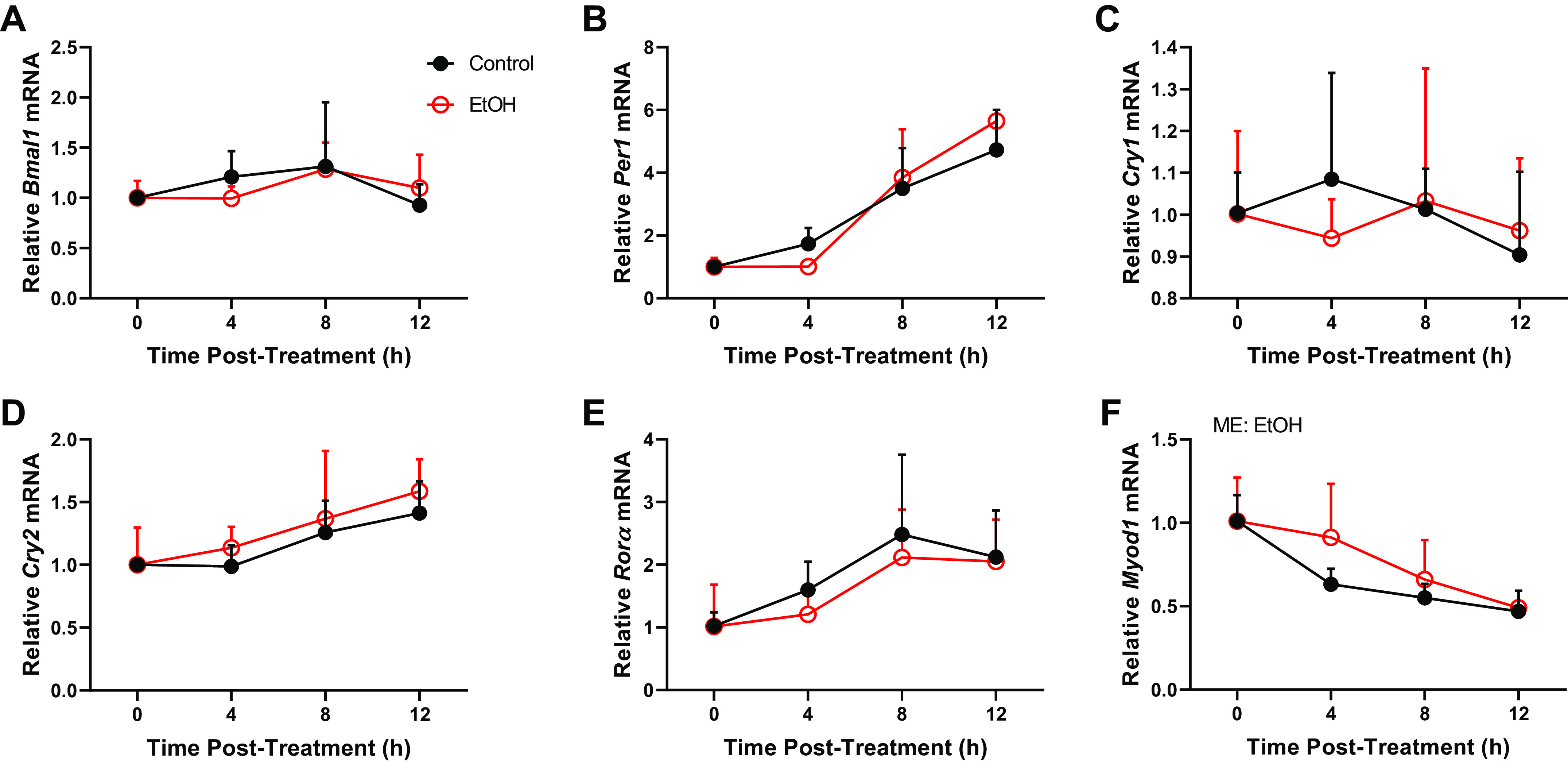
The effects of ethanol exposure (100 mM) on C2C12 myotubes on the mRNA expression of core clock components and CCGs, Bmal1 (A), Per1 (B), Cry1 (C), Cry2 (D), Rorα (E), and Myod1 (F) from experiment 4 (0–12 h) presented as means ± SD (P ≤ 0.05). Main effects and interactions were determined by two-way ANOVA (Time, EtOH) where P ≤ 0.05 and the effect of alcohol is displayed on the graph as ME: EtOH.
Glucocorticoids Contribute to the Alcohol-Induced Gene Expression Profile in Skeletal Muscle, but Do Not Affect Expression of Core Clock Genes
Glucocorticoids are known to mediate control of the skeletal muscle core molecular clock in vivo. Specifically, the glucocorticoid receptor binds to the Per1 promoter within 1 h of exposure to the hormone, and glucocorticoids are sufficient to increase Per1 mRNA in skeletal muscle within 2 h of exposure (32–34). Acute alcohol intoxication increases circulating glucocorticoids, including corticosterone in rodents (35). Consistent with this, serum corticosterone levels were substantially elevated through 8 h post intoxication (Fig. 5A), coinciding with the increase in Per1 mRNA (i.e., Fig. 1E). Along these lines, the mRNA expression of the putative glucocorticoid target gene Redd1 was increased at these time points (0–8 h) and beyond (Fig. 5B). The increase in Redd1 mRNA coincided with an increase in the corresponding protein pattern (Fig. 5, C–D). Consistent with a lack of effect of alcohol itself on the changes in expression of these genes, treatment of C2C12 myotubes with alcohol was also not sufficient to increase Redd1 mRNA expression (Fig. 6E).
Figure 5.
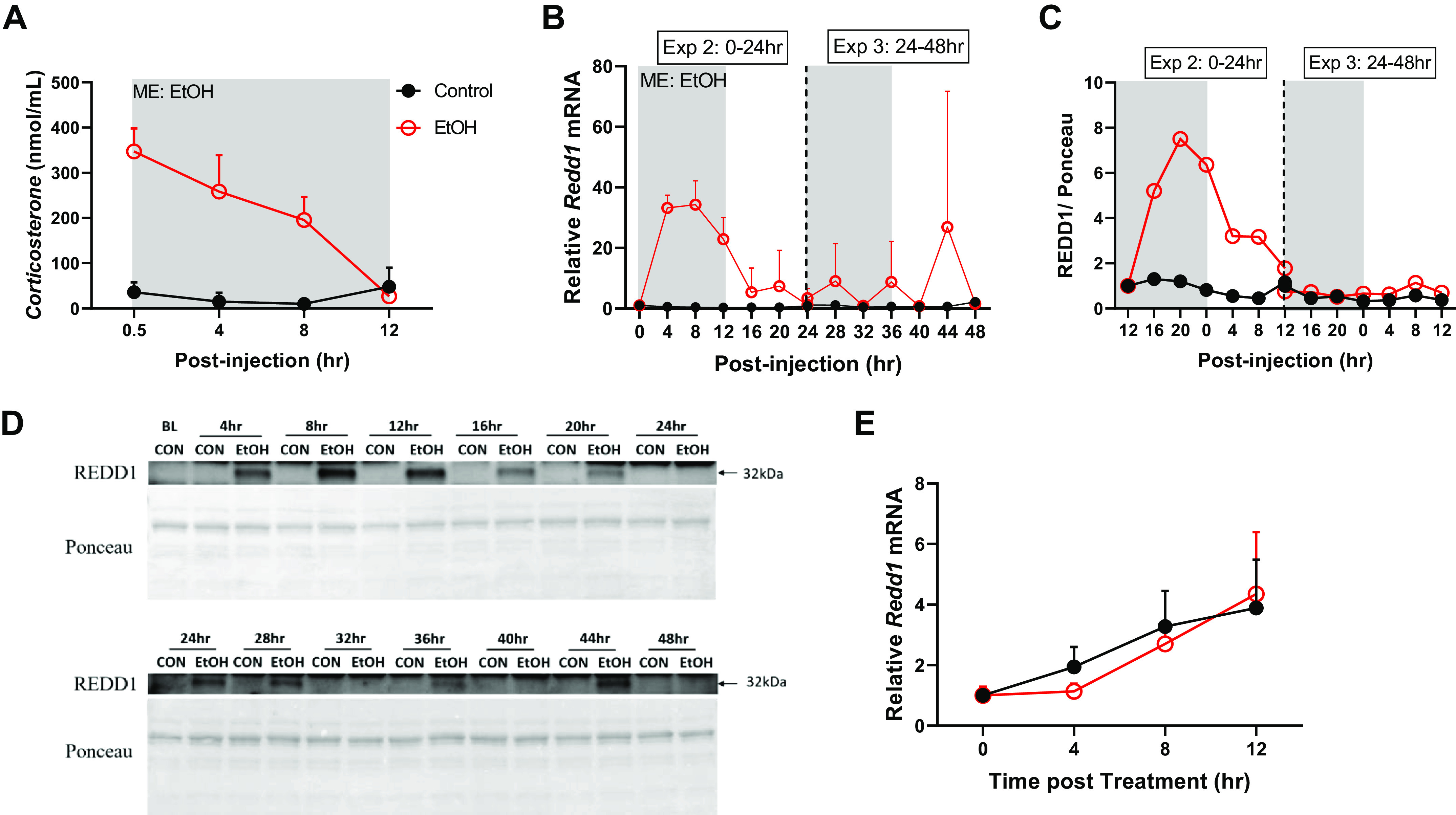
The effects of acute alcohol intoxication on serum corticosterone (A) and the mRNA expression of Redd1 (B) from experiment 2 (0–24 h), experiment 3 (24–48 h), and experiment 4 (0–12 h). In B and C, the vertical dotted line represents the division of the two experiments and gray boxes depict the dark cycle, whereas white boxes depict the light cycle. The 0-h time point is the time of injection and baseline tissue collection (n = 3). The black spheres represent control (n = 3), and the open red spheres represent EtOH (n = 3). C: REDD1 protein over the 48-h period with the corresponding Western blot images shown in D. E: the mRNA expression of Redd1 from experiment 4 in C2C12 myotubes treated with alcohol (100 mM) for 12 h (n = 3/replicate; 3 replicates were performed). A two-way ANOVA (Time, EtOH) was used to determine effects of alcohol listed. Main effects and interactions were determined by two-way ANOVA (Time, EtOH) where P ≤ 0.05 and the effect of alcohol is displayed on the graph as ME: EtOH. All data are presented as means ± SD.
Figure 6.
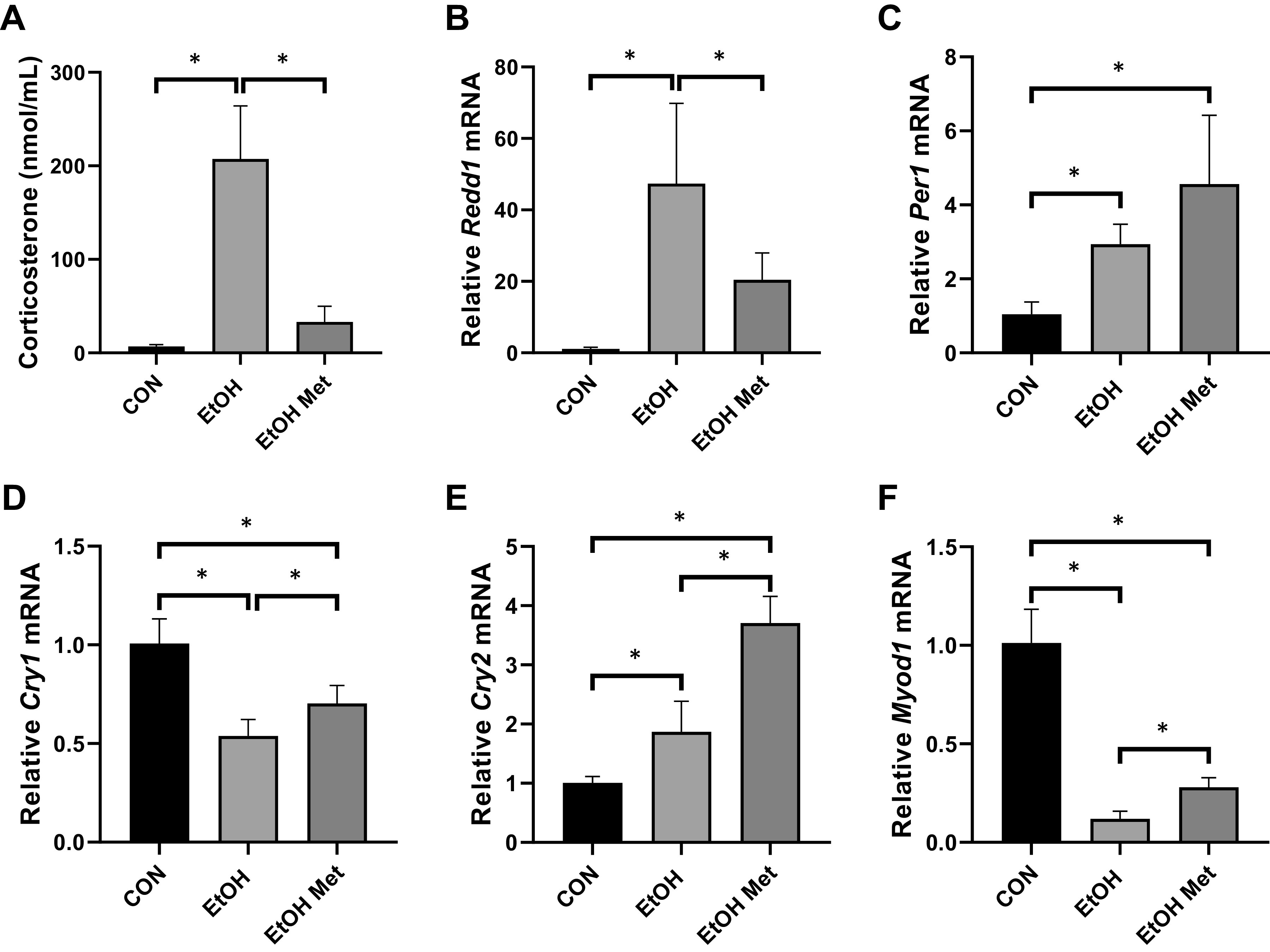
The effects of metyrapone on alcohol-induced increases in serum corticosterone levels (A) and mRNA expression of Redd1 (B), Per1 (C), Cry1 (D), Cry2 (E), and Myod1 (F) from experiment 5 where mice were treated with either saline or metyrapone followed by either saline or alcohol 2 h later. All data are presented as means ± SD (*P ≤ 0.05) and were analyzed by a one-way ANOVA.
Treatment with metyrapone affectively blocked the induction of serum corticosterone by alcohol intoxication and this coincided with a substantial blunting of the alcohol-mediated increase in Redd1 mRNA (Fig. 6, A and B). However, treatment with metyrapone before alcohol intoxication did not prevent the alcohol-mediated changes in the expression of core clock genes Per1, Cry1, and Cry2 (Fig. 6, C–E), nor did it prevent the alcohol-mediated decrease in expression of Myod1 (Fig. 6F).
DISCUSSION
Alcohol interferes with circadian rhythmicity across several tissues (e.g., liver, gut, and brain) (28, 29, 36), although there is a paucity of information on its effect on the skeletal muscle. Herein, we show that acute binge alcohol alters the circadian expression patterns of multiple genes of the skeletal muscle core clock, as well as those that are clock controlled. In addition, we show that alcohol-induced changes to the core clock gene expression are not conserved between organ systems (i.e., muscle to liver). The changes to muscle core clock genes do not appear to be mediated directly by alcohol, at least in vitro, nor do they appear to be regulated by the potent circadian regulator, corticosterone, despite its role in the induction of other alcohol-sensitive genes. Therefore, this is the first report to show an alcohol-induced disruption in the normal expression of genes within the core clock and those under circadian control within the skeletal muscle, and that this occurs in a manner independent of a direct effect of alcohol and corticosterone.
Although the impact of alcohol on skeletal muscle health varies by dose and duration, it is known to reliably disrupt skeletal muscle physiology and metabolism, with higher levels of intoxication causing the greatest effects (37). Presently, we showed that a single alcohol binge significantly disrupted the circadian rhythmicity of several genes within the skeletal muscle with some alterations persisting for at least 2 days. As the experiment was concluded after 48 h, we can only speculate when rhythmicity would eventually be restored within the muscle. However, the long-term consequences of repetitive binge drinking on muscle circadian clock disruption could induce deleterious metabolic and functional changes in the skeletal muscle, similar to models of genetic circadian disruption (e.g., Bmal1 knockout) (9, 38). Moreover, chronic circadian disruption could represent one mechanism through which chronic alcohol use disorder leads to myopathy; however, no data presently exist describing the effects of core clock disruption on alcoholic myopathy. Finally, as the core clock serves as a transcriptional regulator, this temporary alcohol-induced interruption in the normal cyclic expression of core clock genes likely influences the expression of many other pathways implicated in skeletal muscle health that are negatively impacted by alcohol consumption.
The importance of maintaining normal rhythmicity and circadian function within the skeletal muscle is exemplified in models of Bmal1 and Clock disruption in which skeletal muscle metabolism and function are impaired (9, 39). Presently, a single dose of ethanol markedly disrupted several core clock genes including the Periods (Per) and Cryptochromes (Cry). Under normal circumstances, gene transcription of Per and Cry, is controlled by the CLOCK:BMAL1 heterodimer via DNA binding in the nucleus. Currently, however, rhythmicity of Per1, Cry1, and Cry2 is lost and Cry2 exhibits a phase delay (i.e., rightward shift), but whether this is a result of alcohol’s effect on the CLOCK:BMAL1 heterodimer remains unknown. The overall induction of transcription of the Per and Cry genes could indicate that the CLOCK:BMAL1 heterodimer is remaining bound to the E-box regions of the DNA and is transcriptionally active. Alternatively, a lack of translation and reduced accumulation of PER:CRY due to alcohol’s well-described suppressive effects on protein synthesis in the muscle (40) could disrupt the autoregulatory feedback loop in which PER:CRY inhibit CLOCK:BMAL1 transcriptional activity. This alternative would be consistent with the half-life of PER2 (∼200 min), which may also contribute to the lack of repression by the PER:CRY heterodimer (41), potentially resulting in the continued stimulation of not only Per and Cry but also Clock and Bmal1, which was observed 16–36 h after intoxication. The phase delay in Clock and Bmal1 (0–24 h) may have contributed to the similarly observed delay in Dbp, as its transcription is highly dependent on these core clock components (42, 43). Overall, alcohol intoxication significantly changed the rhythmic expression patterns of the core clock genes and their downstream transcription factors, which included elevation of their overall average expression (i.e., MESOR), and shifting of their peak (i.e., acrophase), with many of these effects lasting for more than 24 h beyond the clearance of alcohol from the blood.
Presently, we showed that the presence of the alcohol itself, at least in an in vitro system, was not responsible for the skeletal muscle-specific changes in core clock and clock-controlled gene expression following alcohol intoxication indicating other alcohol-induced responses and signals produced by peripheral and central tissues were likely contributing. For example, alcohol reaches the master circadian regulator, the suprachiasmatic nucleus (SCN), within 20–40 min of intoxication (via intraperitoneal injection), where it can impair both photic (i.e., light) and nonphotic phase resetting and presumably entrainment (44). However, the speed of transmission of this inhibitory effect from the SCN to the skeletal muscle remains unknown and is not a likely explanation for the current findings as the first light cue the animals would have been exposed to would have occurred 12 h after alcohol intoxication and several core clock genes showed disruption before that time point. The importance of changes in neuronal signaling and neuromuscular transmission during alcohol intoxication in relation to molecular perturbations within the skeletal muscle, remains largely unknown especially in the context of circadian signaling (45). Furthermore, whether neuromuscular effects of alcohol on the muscle clock would persist following the clearance of alcohol, as they did in our model, is also unknown. Therefore, due to the immediacy of the response to alcohol intoxication in relation to the modulation of clock gene expression within the muscle (i.e., within 4 h), and the lasting effects on rhythmic gene expression, we believe it is more likely that a blood-borne factor or alcohol metabolite is causing the disruption in genes of the core clock.
Glucocorticoids represent a nonphotic circadian entrainment cue for peripheral tissues, as their release is influenced by the SCN through regulation of corticotropin-releasing hormone and one of the most prominent glucocorticoids, cortisol (in humans)/corticosterone (in mice), which reliably oscillates over the 24-h cycle. In adrenalectomized rats, acute methylprednisolone treatment initiated oscillatory patterns of Bmal1b, Per2, Bhlhb3, Dbp, Reverbα, and Reverbβ in the gastrocnemius suggesting their rhythmic expression patterns rely heavily on corticosterone concentrations (32). Based on these data and knowledge that intoxicating levels of alcohol stimulate the hypothalamic-pituitary-adrenal axis leading to the release of corticosterone in rodents (46), it was unexpected that blocking the alcohol-induced increase in corticosterone was not sufficient to prevent the alterations in the skeletal muscle core clock genes. As metyrapone was used to inhibit adrenal 11-β-hydroxylase activity and block the synthesis of corticosterone from 11-deoxycorticosterone (47, 48), it is possible that an increase in a corticosterone synthesis pathway intermediate could have caused the observed response to alcohol. Alternatively, the suppression of corticosterone production by metyrapone could have increased adrenocorticotropic hormone (ACTH), due to decreased negative feedback from corticosterone, or an increase in neural stimulations of paraventricular nuclei in the hypothalamus (49–51). The effects of ACTH, independent of the normal downstream stimulation of glucocorticoid release, on genes included in the skeletal muscle core clock are unknown. However, ACTH treatment to adrenals removed from mPer2::Luc mice caused a phase delay in vitro indicating the potential for ACTH to regulate the core clock (52), although whether this would translate to the skeletal muscle remains speculative at this point. Finally, neither catecholamines nor sympathetic activity were measured so we cannot exclude the possibility that alcohol influenced these factors or other types of intraorgan cross talk in its control of the skeletal muscle clock. Overall, our experiments revealed that glucocorticoids do not serve as the intermediaries causing the alcohol-induced effects on the core clock in the skeletal muscle, though they do contribute to the alcohol-induced change to the genetic landscape.
Alcohol is widely recognized as a myotoxin affecting several aspects of skeletal muscle physiology, which over time could lead to myopathy. The core clock genes within the skeletal muscle appear to be uniquely sensitive to the effects of alcohol intoxication when compared with the expression of similar core clock genes in the liver following intoxication. Presently, alcohol intoxication suppressed liver circadian expression of Bmal1, Per2, and Cry1 while having no significant effect on the mRNA expression of Clock, Per1, or Cry2. These data are similar to previously published work showing that acute alcohol (3.5 g/kg) administered at the start of the light cycle also led to significant decreases in the mRNA expression of Cry1 and no change in Clock and Per1 expression; but differs in that we observed significant main effects of alcohol on Arntl, and Per2, while they did not (29). Importantly, our data indicate that the effects of alcohol are tissue specific and that either the tissues respond differently to circulating factors and/or alcohol metabolites, or that different mechanisms are responsible for the alcohol-induced clock disruption within each of these metabolic tissues. Similarly, previous work has shown a markedly different response to fasting between the liver and skeletal muscle in the circadian pattern of clock gene expression, despite similar exposure to circulating factors (53). Also of note, the fasting-induced changes within the skeletal muscle differ from the presently observed alcohol-induced effects, indicating that the response to alcohol is unlikely to be caused by a cessation or reduction in food intake and/or cage activity following the brief period of unconsciousness caused by the alcohol intoxication. An interesting avenue of future work includes determination of the metabolic status of these animals in relation to the core clock disruption, as this could influence the circadian clock and vice versa.
Presently, we show that the skeletal muscle core clock and the expression of several clock-controlled genes are perturbed by acute alcohol intoxication. These findings will need to be tested across a range of doses and drinking schemes to determine whether there is a threshold of effect and whether there are long-term implications of this acute disruption. Furthermore, the timing of intoxication and route of administration (IP vs. oral) may also influence outcome measures including blood alcohol concentrations and gene expression patterns. For example, in humans, consumption of alcohol in the morning leads to higher peak blood alcohol concentrations due to time of day differences in alcohol metabolizing enzymes (54), whereas gavage directly into the stomach can also slow the rise in BAC (55). Alternatively, the expression pattern of the core clock genes would be different if alcohol was given at the end of the dark period [i.e., Bmal1 and Cry1 would be at their peak and the period genes (1–3), their lowest levels of expression], and thus the relationship between alcohol’s effects and normal diurnal variations in gene expression would likely vary from those presently observed. Over time, repetitive drinking at the same time of the day each day could lead to skeletal muscle clock reentrainment similar to that observed following scheduled exercise (56). However, the physiological and health implications of an alcohol-induced clock perturbation remain to be determined and this type of repetitive binge drinking should be experimentally tested to see whether it does in fact lead to sustained disruptions of circadian rhythms that could ultimately cause metabolic dysfunction, similar to that observed in shift workers (57). Overall, this work represents an important step in the continued discovery of the multifaceted actions alcohol has on skeletal muscle.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.14607657.v1.
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.14607615.v1.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.14607624.v1.
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.14607627.v1.
Supplemental Fig. S4: https://doi.org/10.6084/m9.figshare.14607651.v1.
GRANTS
The work is supported by National Institutes of Health (NIH) National Institute on Alcohol Abuse and Alcoholism Grant AA11290 (to C.H.L.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.H.L., B.S.G., and J.L.S. conceived and designed research; A.L.T., J.A.L., M.L.R., C.V., B.S.G., and J.L.S. performed experiments; A.L.T., J.A.L., C.A.W., C.V., and J.L.S., analyzed data; A.L.T., J.A.L., M.L.R., C.A.W., K.A.E., C.L., B.S.G., and J.L.S. interpreted results of experiments; A.L.T., J.A.L., and J.L.S. prepared figures; A.L.T., C.A.W., and J.L.S. drafted manuscript; A.L.T., J.A.L., M.L.R., C.A.W., K.A.E., C.L., C.H.L., C.V., B.S.G., and J.L.S. edited and revised manuscript; A.L.T., J.A.L., M.L.R., C.A.W., K.A.E., C.L., C.H.L., C.V., B.S.G., and J.L.S. approved final version of manuscript.
REFERENCES
- 1.Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009-2011. Prev Chronic Dis 11: E206, 2014. doi: 10.5888/pcd11.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasarathy J, McCullough AJ, Dasarathy S. Sarcopenia in alcoholic liver disease: clinical and molecular advances. Alcohol Clin Exp Res 41: 1419–1431, 2017. doi: 10.1111/acer.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solt LA, Kojetin DJ, Burris TP. The REV-ERBs and RORs: molecular links between circadian rhythms and lipid homeostasis. Future Med Chem 3: 623–638, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193–205, 1999. doi: 10.1016/S0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 5.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science 288: 1013–1019, 2000. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 6.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 330: 1349–1354, 2010. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31: 86–95, 2007. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder EA, Harfmann BD, Zhang X, Srikuea R, England JH, Hodge BA, Wen Y, Riley LA, Yu Q, Christie A, Smith JD, Seward T, Wolf Horrell EM, Mula J, Peterson CA, Butterfield TA, Esser KA. Intrinsic muscle clock is necessary for musculoskeletal health. J Physiol 593: 5387–5404, 2015. doi: 10.1113/JP271436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA 107: 19090–19095, 2010. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle 6: 12, 2016. doi: 10.1186/s13395-016-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2: e377, 2004. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mekheal M, Steiner JL, Lang CH. Acute alcohol prevents the refeeding-induced decrease in autophagy but does not alter the increased protein synthetic response in heart. Alcohol 73: 79–88, 2018. doi: 10.1016/j.alcohol.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiner JL, Lang CH. Ethanol acutely antagonizes the refeeding-induced increase in mTOR-dependent protein synthesis and decrease in autophagy in skeletal muscle. Mol Cell Biochem 456: 41–51, 2019. doi: 10.1007/s11010-018-3488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker U, Deis A, Sørensen TI, Grønbaek M, Borch-Johnsen K, Müller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 23: 1025–1029, 1996. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 15.Fulham MA, Mandrekar P. Sexual dimorphism in alcohol induced adipose inflammation relates to liver injury. PLoS One 11: e0164225, 2016. doi: 10.1371/journal.pone.0164225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato N, Lindros KO, Baraona E, Ikejima K, Mezey E, Järveläinen HA, Ramchandani VA. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 25: 40S–45S, 2001. doi: 10.1097/00000374-200105051-00007. [DOI] [PubMed] [Google Scholar]
- 17.Rossetti ML, Esser KA, Lee C, Tomko RJ Jr, Eroshkin AM, Gordon BS. Disruptions to the limb muscle core molecular clock coincide with changes in mitochondrial quality control following androgen depletion. Am J Physiol Endocrinol Physiol 317: E631–E645, 2019. doi: 10.1152/ajpendo.00177.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang CH, Lynch CJ, Vary TC. Alcohol-induced IGF-I resistance is ameliorated in mice deficient for mitochondrial branched-chain aminotransferase. J Nutr 140: 932–938, 2010. doi: 10.3945/jn.109.120501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruett S, Tan W, Howell GE 3rd, Nanduri B. Dosage scaling of alcohol in binge exposure models in mice: an empirical assessment of the relationship between dose, alcohol exposure, and peak blood concentrations in humans and mice. Alcohol 89: 9–17, 2020. doi: 10.1016/j.alcohol.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemler D, Wolff CA, Esser KA. Time-of-day dependent effects of contractile activity on the phase of the skeletal muscle clock. J Physiol 598: 3631–3644, 2020. doi: 10.1113/JP279779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh SS, Kumar A, Welch N, Sekar J, Mishra S, Bellar A, Gangadhariah M, Attaway A, Al Khafaji HA, Wu X, Pathak V, Agrawal V, McMullen MR, Hornberger TA, Nagy LE, Davuluri G, Dasarathy S. Multiomics-identified intervention to restore ethanol-induced dysregulated proteostasis and secondary sarcopenia in alcoholic liver disease. Cell Physiol Biochem 55: 91–116, 2021. doi: 10.33594/000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Naga Prasad SV, Dasarathy S. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy 10: 677–690, 2014. doi: 10.4161/auto.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111, 2009. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steiner JL, Fukuda DH, Rossetti ML, Hoffman JR, Gordon BS. Castration alters protein balance after high-frequency muscle contraction. J Appl Physiol (1985) 122: 264–272, 2017. doi: 10.1152/japplphysiol.00740.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, Unluturk U, Li X, Kong X, Hyde AL, Gangl MR, Mair WB, Lee CH. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab 22: 709–720, 2015. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steiner JL, Crowell KT, Kimball SR, Lang CH. Disruption of REDD1 gene ameliorates sepsis-induced decrease in mTORC1 signaling but has divergent effects on proteolytic signaling in skeletal muscle. Am J Physiol Endocrinol Physiol 309: E981–E994, 2015. doi: 10.1152/ajpendo.00264.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW, Keshavarzian A. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS One 8: e67102, 2013. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaucher J, Kinouchi K, Ceglia N, Montellier E, Peleg S, Greco CM, Schmidt A, Forne I, Masri S, Baldi P, Imhof A, Sassone-Corsi P. Distinct metabolic adaptation of liver circadian pathways to acute and chronic patterns of alcohol intake. Proc Natl Acad Sci USA 116: 25250–25259, 2019. doi: 10.1073/pnas.1911189116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forsyth CB, Voigt RM, Burgess HJ, Swanson GR, Keshavarzian A. Circadian rhythms, alcohol and gut interactions. Alcohol 49: 389–398, 2015. doi: 10.1016/j.alcohol.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filiano AN, Millender-Swain T, Johnson R Jr, Young ME, Gamble KL, Bailey SM. Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PLoS One 8: e71684, 2013. doi: 10.1371/journal.pone.0071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almon RR, Yang E, Lai W, Androulakis IP, Ghimbovschi S, Hoffman EP, Jusko WJ, Dubois DC. Relationships between circadian rhythms and modulation of gene expression by glucocorticoids in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 295: R1031–R1047, 2008. doi: 10.1152/ajpregu.90399.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saracino PG, Rossetti ML, Steiner JL, Gordon BS. Hormonal regulation of core clock gene expression in skeletal muscle following acute aerobic exercise. Biochem Biophys Res Commun 508: 871–876, 2019. doi: 10.1016/j.bbrc.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Kuo T, Lew MJ, Mayba O, Harris CA, Speed TP, Wang J-C. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc Natl Acad Sci USA 109: 11160–11165, 2012. doi: 10.1073/pnas.1111334109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res 17: 854–859, 1993. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang T, Yang P, Zhan Y, Xia L, Hua Z, Zhang J. Deletion of circadian gene Per1 alleviates acute ethanol-induced hepatotoxicity in mice. Toxicology 314: 193–201, 2013. doi: 10.1016/j.tox.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Urbano-Márquez A, Fernández-Solà J. Effects of alcohol on skeletal and cardiac muscle. Muscle Nerve 30: 689–707, 2004. doi: 10.1002/mus.20168. [DOI] [PubMed] [Google Scholar]
- 38.Steiner JL, Lang CH. Dysregulation of skeletal muscle protein metabolism by alcohol. Am J Physiol Endocrinol Physiol 308: E699–E712, 2015. doi: 10.1152/ajpendo.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodge BA, Zhang X, Gutierrez-Monreal MA, Cao Y, Hammers DW, Yao Z, Wolff CA, Du P, Kemler D, Judge AR, Esser KA. MYOD1 functions as a clock amplifier as well as a critical co-factor for downstream circadian gene expression in muscle. elife 8: e43017, 2019. doi: 10.7554/eLife.43017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner JL, Lang CH. Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol (1985) 117: 1170–1179, 2014. doi: 10.1152/japplphysiol.00180.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamiya H, Ogawa S, Ouchi Y, Akishita M. Rigid cooperation of Per1 and Per2 proteins. Sci Rep 6: 32769, 2016. doi: 10.1038/srep32769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stratmann M, Suter DM, Molina N, Naef F, Schibler U. Circadian Dbp transcription relies on highly dynamic BMAL1-CLOCK interaction with E boxes and requires the proteasome. Mol Cell 48: 277–287, 2012. doi: 10.1016/j.molcel.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev 14: 679–689, 2000. doi: 10.1101/gad.14.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol 296: R411–R418, 2009. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed TE. Acute effects of ethanol in vivo on neuromuscular transmission. Pharmacol Biochem Behav 13: 811–815, 1980. doi: 10.1016/0091-3057(80)90212-9. [DOI] [PubMed] [Google Scholar]
- 46.Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res 726: 1–10, 1996. doi: 10.1016/0006-8993(96)00301-0. [DOI] [PubMed] [Google Scholar]
- 47.Strashimirov D, Bohus B. Effect of 2-methyl-1,2-bis-3-pyridl-1-propanone (SU-4885) on adrenocortical secretion in normal and hypophysectomized rats. Steroids 7: 171–180, 1966. doi: 10.1016/0039-128X(66)90024-9. [DOI] [PubMed] [Google Scholar]
- 48.Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology 21: 681–693, 1996. doi: 10.1016/S0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- 49.Deuschle M, Lecei O, Stalla GK, Landgraf R, Hamann B, Lederbogen F, Uhr M, Luppa P, Maras A, Colla M, Heuser I. Steroid synthesis inhibition with ketoconazole and its effect upon the regulation of the hypothalamus-pituitary-adrenal system in healthy humans. Neuropsychopharmacology 28: 379–383, 2003. doi: 10.1038/sj.npp.1300044. [DOI] [PubMed] [Google Scholar]
- 50.Van Vugt DA, Piercy J, Farley AE, Reid RL, Rivest S. Luteinizing hormone secretion and corticotropin-releasing factor gene expression in the paraventricular nucleus of rhesus monkeys following cortisol synthesis inhibition. Endocrinology 138: 2249–2258, 1997. doi: 10.1210/endo.138.6.5171. [DOI] [PubMed] [Google Scholar]
- 51.Rotllant D, Ons S, Carrasco J, Armario A. Evidence that metyrapone can act as a stressor: effect on pituitary-adrenal hormones, plasma glucose and brain c-fos induction. Eur J Neurosci 16: 693–700, 2002. doi: 10.1046/j.1460-9568.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 52.Yoder JM, Brandeland M, Engeland WC. Phase-dependent resetting of the adrenal clock by ACTH in vitro. Am J Physiol Regul Integr Comp Physiol 306: R387–R393, 2014. doi: 10.1152/ajpregu.00519.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinouchi K, Magnan C, Ceglia N, Liu Y, Cervantes M, Pastore N, Huynh T, Ballabio A, Baldi P, Masri S, Sassone-Corsi P. Fasting imparts a switch to alternative daily pathways in liver and muscle. Cell Rep 25: 3299–3314.e6, 2018. doi: 10.1016/j.celrep.2018.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reinberg A, Clench J, Aymard N, Galliot M, Bourdon R, Gervais P, Abulker C, Dupont J. Circadian variations of the effects of ethanol and of blood ethanol values in the healthy adult man. Chronopharmacological study. J Physiol (Paris) 70: 435–456, 1975. [PubMed] [Google Scholar]
- 55.Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol 17: 185–194, 1999. doi: 10.1016/S0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 56.Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc 44: 1663–1670, 2012. doi: 10.1249/MSS.0b013e318255cf4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swanson GR, Gorenz A, Shaikh M, Desai V, Kaminsky T, Van Den Berg J, Murphy T, Raeisi S, Fogg L, Vitaterna MH, Forsyth C, Turek F, Burgess HJ, Keshavarzian A. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am J Physiol Gastrointest Liver Physiol 311: G192–G201, 2016. doi: 10.1152/ajpgi.00087.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


