Abstract
In this study, we have looked for an optimum media glucose concentration and compared glucose consumption in three vascular cell types, endothelial cells (ECs), vascular smooth muscle cells (VSMCs), and adventitial fibroblasts (AFs) with or without angiotensin II (AngII) stimulation. In a subconfluent 6-well experiment in 1 mL DMEM with a standard low (100 mg/dL), a standard high (450 mg/dL), or a mixed middle (275 mg/dL) glucose concentration, steady and significant glucose consumption was observed in all cell types. After 48-h incubation, media that contained low glucose was reduced to almost 0 mg/dL, media that contained high glucose remained significantly higher at ∼275 mg/dL, and media that contained middle glucose remained closer to physiological range. AngII treatment enhanced glucose consumption in AFs and VSMCs but not in ECs. Enhanced extracellular acidification rate by AngII was also observed in AFs. In AFs, AngII induction of target proteins at 48 h varied depending on the glucose concentration used. In low glucose media, induction of glucose regulatory protein 78 or hexokinase II was highest, whereas induction of VCAM-1 was lowest. Utilization of specific inhibitors further suggests essential roles of angiotensin II type-1 receptor and glycolysis in AngII-induced fibroblast activation. Overall, this study demonstrates a high risk of hypo- or hyperglycemic conditions when standard low or high glucose media is used with vascular cells. Moreover, these conditions may significantly alter experimental outcomes. Media glucose concentration should be monitored during any culture experiments and utilization of middle glucose media is recommended for all vascular cell types.
Keywords: fibroblasts, angiotensin II, endothelial cells, glycolysis, vascular smooth muscle cells
INTRODUCTION
Glucose is one of the main nutrients the body uses to produce and store energy. Accordingly, circulating glucose levels are tightly controlled by changes in supply and or demand. A pancreatic hormone, insulin, is a master regulator of circulating glucose. Conditions such as diabetes or insulinoma disrupt this control resulting in hyperglycemia or hypoglycemia, which can be life-threatening. However, insulin-independent glucose uptake is also a critical mechanism of glucose utilization in the body as a whole and in cultured cells (1, 2). The vascular system is composed primarily of endothelial cell (EC) monolayer, vascular smooth muscle cell (VSMC) medial layers, and fibroblasts forming adventitia and these cells are at the forefront of exposure to circulating glucose. Both in vitro and in vivo experiments have demonstrated critical regulatory roles of glucose utilization such as glycolysis in these cell types for their physiological and pathophysiological responses. Hypoxia-induced enhancement in EC glycolysis is required for angiogenesis, whereas hyper- and hypoglycemia are causes for endothelial dysfunction (3, 4). Enhancement of VSMC glycolysis has been implicated in atherosclerosis (5) and high glucose media elicits dedifferentiation of VSMC (6). Glycolysis is also involved in myofibroblast formation and pathological fibrosis (7). Angiotensin II (AngII) is an important mediator of essential hypertension and causes a myriad of signaling responses in these cell types (8) including insulin-independent glucose uptake in VSMCs (9).
Although the information above suggests that any experimental outcomes in these cell types are potentially influenced by extracellular glucose availability and its cellular metabolism, there is a lack of attention paid to the supply and utilization of glucose in many cultured experiments. Surveillance of core vascular-related journals for the past 5 yr demonstrated that less than 20% of published articles declared the media glucose concentration used (69/394 = 17.51%, Supplemental Table S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.16906825.v1) highlighting the lack of rigor regarding this critical condition. In human cell culture media, glucose concentration was mostly unspecified by the suppliers. Moreover, optimal media glucose concentration to perform cell response studies such as with AngII stimulation remains obscure. Accordingly, the present studies were designed to seek ideal media glucose concentration(s) for three vascular cell types with or without AngII stimulation with emphasis on changes in glucose consumption. By using three distinct glucose concentrations in DMEM, we identified ideal glucose media for stimulation experiments in these cell types. The basal and stimulated glucose consumption appears highest in VSMCs and adventitial fibroblasts (AFs), respectively. Not surprisingly, distinct AngII effects were observed in induction of ER stress and inflammatory responses in the fibroblasts. We further tested the roles of Ang II type 1 (AT1) receptor and glycolysis in fibroblast stress protein induction in response to AngII in the ideal glucose concentration.
MATERIALS AND METHODS
Journal Survey
We applied a search method to identify the manuscripts utilizing 1) target cell types of VSMC, EC, and fibroblasts, 2) media types, and 3) if glucose concentration was specified in the manuscript. If more than two target cell types and/or media were used, we prioritize DMEM media with glucose information over other media types. Ovid MEDLINE searches were performed on American Journal of Physiology Cell Physiology and core cardiovascular journals (Circulation, Circulation Research, Hypertension, Arteriosclerosis Thrombosis and Vascular Biology, American Journal of Physiology Heart and Circulatory Physiology) published between 2016 and 2020. The search term(s) in the combination were 1) vascular smooth muscle cells.mp, 2) endothelial cell mp., or Endothelial Cells/, and 3) fibroblast.mp., or Fibroblasts/. To better estimate the most recent trends in the field, the search was performed with maximum 50 hits from the most recent years, going back no further than 5 yr.
Animal
All animal procedures were performed with prior approval of the Temple University Institutional Animal Care and Use Committee (No. 4625) and in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. To obtain rat thoracic aortas, 12-wk-old male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were euthanatized with CO2 overdose and subsequent exsanguination and bilateral thoracotomy. Thoracic aorta was then collected by cutting the aorta above the level of the diaphragm.
Cell Culture and Treatment
VSMCs were isolated from rat aortas by explant method as previously described (10, 11) and cultured in DMEM supplemented with 100 mg/dL “D”-glucose with 1 mM sodium pyruvate (10–014-CV; Corning, Glendale, AZ), 100 IU penicillin, 100 μg/mL streptomycin, and 10% FBS supplementation (970685-085; VWR International, Radnor, PA). Subcultured VSMCs were supplemented with growth media twice a week and passaged every week. Both freshly isolated and frozen stocked cells from two independent rats at passage 4–5 were used for experiments. We confirmed smooth muscle α actin expression in rat VSMC (Supplemental Fig. S1A).
Primary aortic ECs (RA-6052 Frozen; Cell Biologics, Chicago, IL) and primary AF (RA-6075 Frozen; Cell Biologics, Chicago, IL) derived from 6 to 8-wk-male Sprague-Dawley rats were cultured in 100 mg/dL d-glucose DMEM with 1 mmol/L sodium pyruvate (10–014-CV; Corning, Glendale, AZ), 100 IU penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (FBS) supplementation (970685-085; VWR International, Radnor, PA). Subcultured EC (single lot) and AF (two independent lots) were supplemented with growth media twice a week and passaged every week. Cells from passages 3–9 were used for experiments. In addition, we have used primary cultured and nonstocked AF explanted from 12-wk-male Sprague-Dawley rat thoracic aortas by the modified explant method (10, 11). For the AF explant culture, aorta sediments are placed on culture plate with adventitia side to the bottom plastic (instead of the endothelial cell-denuded side for VSMC). We confirmed a fibroblast marker, S100A4 expression in rat AF (Supplemental Fig. S1B).
Approximately 80%–90% confluent cells on six-well culture plates (353046; Corning, Glendale, AZ) were serum-starved for 48 h in 1 mL DMEM to avoid over confluency at the end point of experiments. For serum starvation and subsequent incubation with experimental treatments, DMEM with three distinct glucose concentrations [100 mg/dL low glucose (LG)-DMEM, 450 mg/dL high glucose (HG)-DMEM (10–013-CV; Corning, Glendale, AZ), and 275 mg/dL mixed middle glucose (MG)-DMEM (1:1 = LG-DMEM/HG-DMEM)] were used. After starvation, cells were incubated with fresh 1 mL DMEM 1 h before stimulation with 100 nM AngII (A9525; Sigma-Aldrich, St. Louis, MO) or vehicle (1 μL H2O) for up to 48 h. For the 48-h stimulations, the AngII stimulation was repeated at the 24-h time point. Rat recombinant platelet-derived growth factor-BB 25 ng/mL (PDGF-BB, 520-BB; R&D Systems, Minneapolis, MN) was used for AF 24–48-h stimulation without a repeated addition. For pharmacological intervention, cells were incubated with fresh DMEM for 1 h, then pretreated with indicated drugs or vehicle controls for 30 min before stimulation with AngII. 2-Deoxy-d glucose 2DG (21916; Chem Impex, Wood Dale, IL) and an AT1 receptor antagonist Olmesartan (HY-17004; MedChemExpress, Monmouth Junction, NJ) were solubilized in MG-DMEM using DMSO with final dilution of 1/1,000.
Human coronary artery VSMCs (FC-0031; Lifeline Cell Technology, Frederick, MD) were subcultured in the growth media (LL-0014; Lifeline Cell Technology, Frederick, MD) and induced quiescence with incubation in 1% FCS supplemented basal media (LM-0002; Lifeline Cell Technology, Frederick, MD) for 24 h. The cells were then incubated with fresh 1% FCS containing media for 24–48 h with or without AngII stimulation for glucose measurements. Human dermal lymphatic EC (H-6064L; Cell Biologics, Chicago, IL) were subcultured in the growth media (H1168; Cell Biologics, Chicago, IL) and induced quiescence with incubation in 1% FCS supplemented basal media (H1168b; Cell Biologics, Chicago, IL) for 24 h. The cells were then incubated with fresh 1% FCS containing basal media for 24–48 h with or without AngII stimulation for glucose measurements.
Immunofluorescent Staining
Rat primary aortic VSMC and AF were cultured on glass coverslips until 70%–75% confluency, followed by serum deprivation for a period of 48 h in LG-DMEM. Cells were fixed with 4% paraformaldehyde solution and then washed three times with PBS. Cells were made permeable through incubation with 0.5% Triton X-PBS for 30 min at 4°C and blocked for 1 h at room temperature with 5% BSA in PBS. Cells were incubated with an antibody against a smooth muscle marker, α-smooth muscle actin αSMA (NBP2-33006; Novus biologicals, Centennial CO) at 1 µg/mL or an antibody against a fibroblast marker, S100A4 (bs-3759R, Bioss, Woburn MA) at 1 µg/mL overnight at 4°C. Cells were then washed with PBS three times and incubated with goat anti-Rabbit IgG Alexa Fluor 488 (A11034; Thermo Fisher Scientific, Waltham MA) at 1/1,000 dilution for 1 h in the dark. After washing three times with PBS, cells were mounted on glass slides with Prolong Gold Antifade Mountant with DAPI (P36931; Thermo Fisher Scientific, Waltham MA). Minus primary controls were included for all stains to ensure specificity. Cells were imaged at ×40 magnification on EVOS M5000 microscope (Thermo Fisher Scientific, Waltham MA).
Measurement of Media Glucose
GE100 blood glucose monitoring system (GE/Bionime, Taichung City, Taiwan) was used to assess media glucose concentration. Media glucose concentration was measured with standard curve (Supplemental Fig. S2A) which was made with 450 mg/dL HG-DMEM, 275 mg/dL MG-DMEM or 187.5 mg/dL glucose DMEM (3:1 = LG-DMEM/HG-DMEM), and 100 mg/dL LG-DMEM. To enhance the dynamic range of media glucose measurements, experimental samples were mixed with equal volume of LG-DMEM before the measurements.
Immunoblotting
AF on six-well plates were lysed in 1× SDS lysis buffer, heated at 95°C for 5 min, ∼50 μg proteins were loaded, and the samples were subjected to SDS-PAGE gel electrophoresis as previously described (10, 11). Briefly, 10% acrylamide/bisacrylamide resolving gels were used and transferred onto 0.45 µm nitrocellulose membranes overnight at 30 V. Membranes were blocked for 1 h in 5% nonfat dry milk in TBS. Protein detection was performed using primary antibodies for 78 kDa glucose-regulated protein GRP78 (70 ng/mL, 11587-1-AP; Proteintech, Rosemont, IL), vascular cell adhesion molecule-1 (VCAM1; 47.3 ng/mL, ab134047; Abcam, Cambridge, UK), hexokinase II HK2 (50 ng/mL, A01389; Boster Biological Technology, Pleasanton, CA), pyruvate kinase 2 PKM2 (60 ng/mL, 15822-1-AP; Proteintech, Rosemont, IL), GAPDH (100 ng/mL, MAB374; Sigma-Aldrich, St. Louis, MO), β-actin (6.1 ng/mL, 4970; Cell Signaling Technology, Danvers, MA), c-Fos (20 ng/mL sc-52; Santa Cruz Biotechnology, Dallas, TX), and early growth response-1 Egr1 (1/10,000, 4153; Cell Signaling Technology, Danvers, MA) incubated in TBS-T (0.01% Tween 20) overnight at 4°C, rocking. Secondary antibody incubation (100 ng/mL, IRDye 800CW Goat anti-Rabbit IgG 926–32211; LI-COR, Lincoln, NE, IRDye 680RD Goat anti-Rabbit IgG 926–68071; LI-COR, Lincoln, NE, IRDye 800CW Goat anti-Mouse IgG 926–32210; LI-COR, Lincoln, NE, and IRDye 680RD Goat anti-Mouse IgG 926–68070; LI-COR, Lincoln, NE) followed for ∼1 h at room temperature with subsequent detection and analyses with the LI-COR Odyssey system and the Image Studio software (LI-COR, Lincoln, NE). For normalization, we have used β-actin expression and GAPDH expression. For comparison, protein expression in MG-DMEM without a stimulation was set as 1 because it is the closest to physiological glucose levels.
Antibody Validation
αSMA; The manufacturer validated this antibody with positive and negative immunoblotting data with cardiac differentiated and undifferentiated cell lines. S100A4; The manufacture validated this antibody with positive immunostaining in various tissues. GRP78; We have validated this antibody with adenoviral transduction of GRP78 cDNA (12, 13). VCAM1; The manufacturer validated this antibody using a VCAM1 knockout cell line and the control cell line. Using this antibody, we have shown the induction of VCAM1 by tumor necrosis factor-α in rat aortic EC (14) and by AngII in VSMC (15). HK2; The manufacturer validated this antibody using tissues and cell lines known to express HK2. PKM2; The manufacturer validated this antibody using siRNA against PKM2. GAPDH; The manufacturer validated this antibody using several cell lines. β-actin; The manufacturer validated this antibody using fusion proteins and tissues expressing β-actin. c-Fos; Although this antibody has been discontinued, the manufacturer listed 565 citations using this antibody. We have also shown induction of c-Fos by AngII in rat VSMC using this antibody (16).
Evaluation of Glycolysis
A Seahorse XF96 extracellular flux analyzer was employed to measure extracellular acidification rates (ECARs) using the glycolytic stress test kit (Seahorse Biosciences, North Billerica, MA). AF (0.15 × 105 cell/well) were seeded on Seahorse plate (101085-004; Seahorse Biosciences, North Billerica, MA) and serum starved in 0.1 mL MG-DMEM for 48 h. For ECAR evaluation, upon replacement of Seahorse DMEM media with l-glutamine, cells were immediately stimulated with 100 nM AngII or vehicle (H2O). Nonglycolytic acidification was measured for 18 min, then 10 mM (180 mg/dL) glucose was injected to measure basal glycolysis for 26 min, followed by 3 µM oligomycin to inhibit mitochondrial ATP production and reveal maximal glycolytic capacity for 26 min, and finally 50 mM 2DG was injected to completely inhibit glycolysis. After the experiment, protein concentration was measured and ECARs were analyzed using the Wave software.
Statistical analysis.
Data are presented as means ± SE (standard error). Comparisons were performed via one-way ANOVA with the post hoc Tukey’s method for multiple groups using Prism software (GraphPad, San Diego, CA). Differences were considered statistically significant at P < 0.05.
RESULTS
Journal Survey for Utilization of DMEM with Glucose Information
DMEM with relatively low (100 mg/dL) or high (450 mg/dL) glucose supplementation is one of the most frequently used media for culturing common vascular cell types such as those obtained from experimental animals. Among the target journals, we have sampled 412 articles using fibroblasts, VSMCs and ECs of which 40% used DMEM (Fig. 1A and Supplemental Table S1). DMEM utilization was more than 60% in fibroblast and VSMC articles, whereas it was 24% in EC articles (Fig. 1, B–D). One potential reason for these differences is higher ratio of human cell articles with EC. In all articles using DMEM, only 30% of articles declared the glucose concentration (Fig. 1E). Among the articles declared, strikingly high utilization of high glucose DMEM was observed with fibroblasts (∼80%) and VSMC (∼60%). In contrast only 25% of EC article used high glucose DMEM (Fig. 1, F–H). However, there is a clear lack of our knowledge if low or high glucose media is ideal for EC, VSMC, or fibroblasts to perform physiologically relevant experiments in culture.
Figure 1.
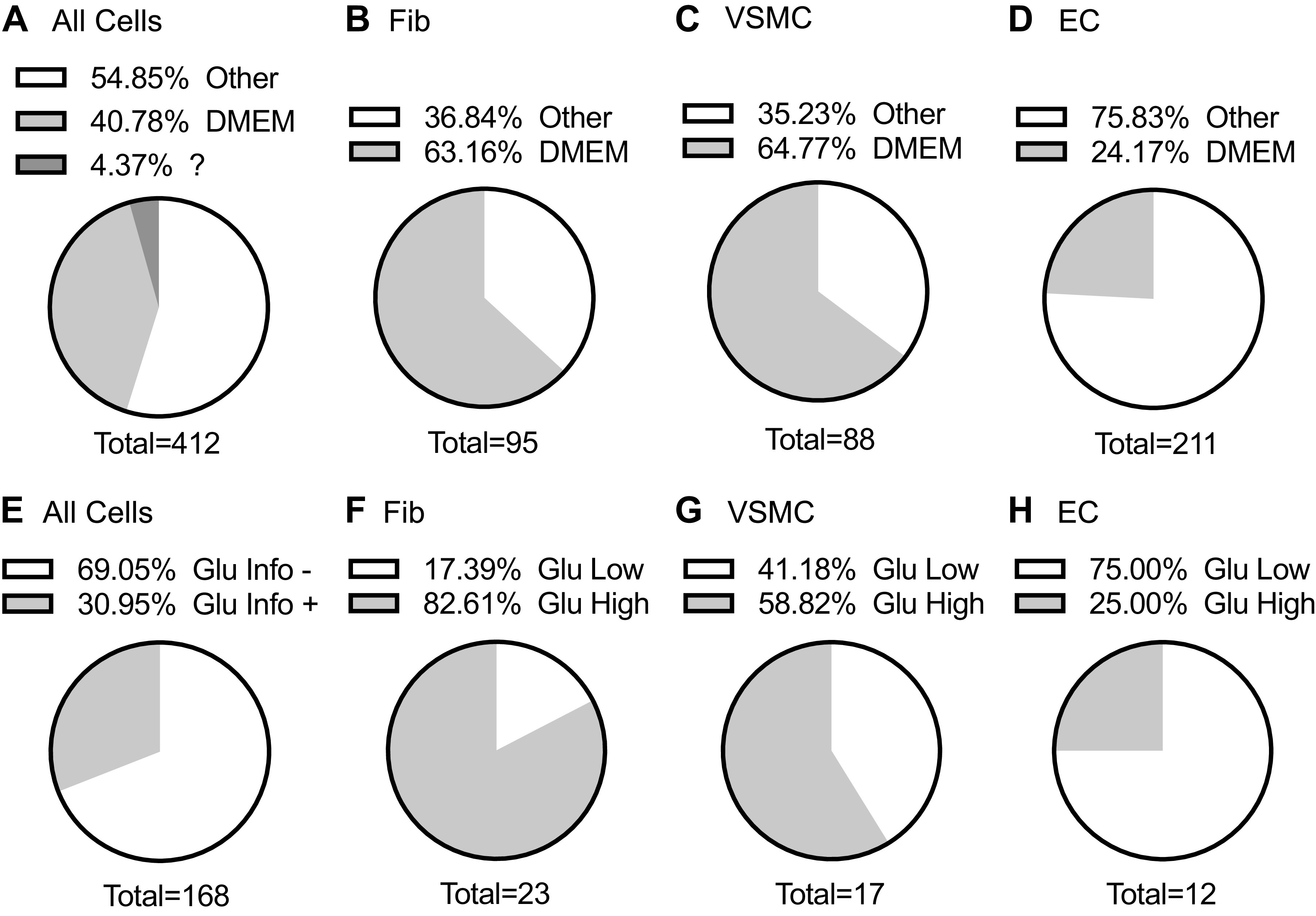
Journal survey for utilization of DMEM with glucose information. Among the target journals, we have sampled 412 articles. The percentages of DMEM articles reporting usage of DMEM for all cells (A), fibroblasts (B), VSMC (C), and EC (D) were indicated. The percentages of articles with declared DMEM glucose concentrations for all cells (E), fibroblasts (F), VSMC (G), and EC (H) were indicated. EC, endothelial cell; Fib, fibroblast cell; Glu High, high glucose medium (450 mg/dL); Glu Info, glucose information; Glu Low, low glucose medium (100 mg/dL); VSMC, vascular smooth muscle cell.
Glucose Consumption by Vascular Cells with or without AngII Stimulation
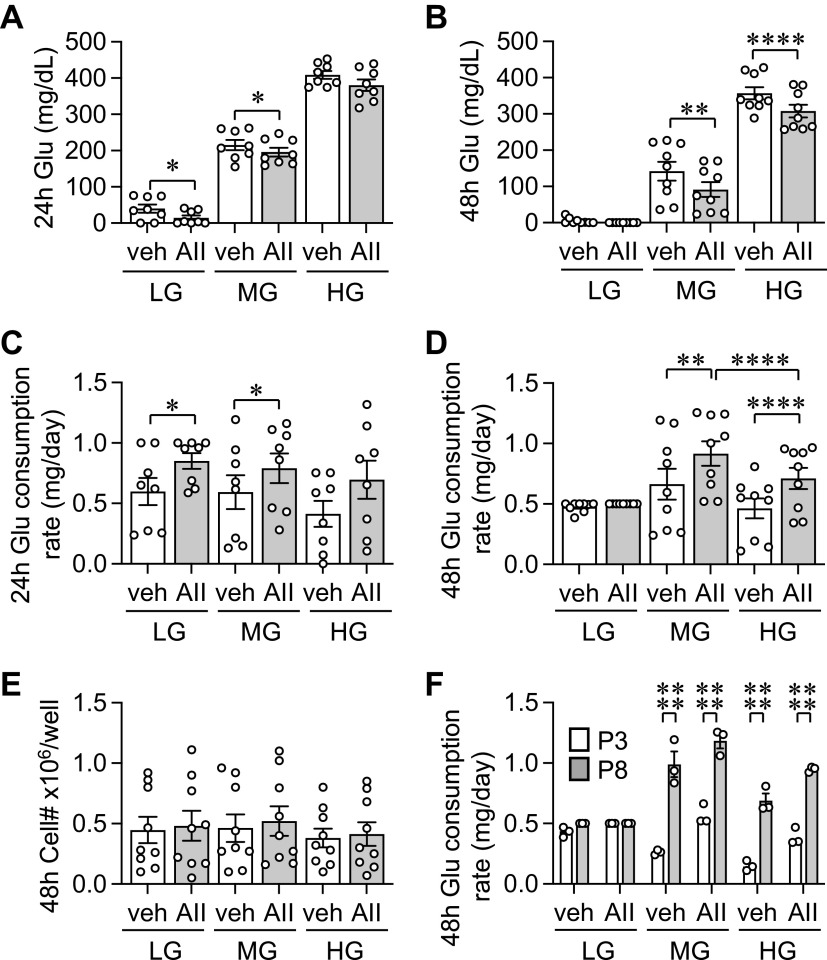
Conditioned media glucose concentrations were monitored at 24- and 48-h time points with AngII or vehicle treatment in AF cultured in LG-, MG-, or HG-DMEM (Fig. 2, A and B). AF consumed media glucose under all tested conditions. In LG-DMEM at 24 h, media glucose concentrations were very low in the control basal condition. At 48 h, media glucose concentrations reached almost 0 under stimulated and unstimulated conditions. In AF, enhanced glucose consumption rate with AngII stimulation was seen in all passages tested and statistically significant in LG- and MG-DMEM at 24 h and MG- and HG-DMEM at 48 h. The glucose consumption rate was highest in MG-DMEM with 48-h AngII stimulation (Fig. 2, C and D). No significant change in cell numbers was observed regardless of the glucose concentration or AngII stimulation at 48 h (Fig. 2E). In addition, the media glucose concentration did not affect the basal glucose consumption rate at 24 or 48 h. We also observed higher passage cells such as passage 8 consistently increased their glucose consumption rates compared with lower passage 3 cells at 48 h in MG and HG media (Fig. 2F). We are unable to conclude if the same holds true in LG media because glucose was fully consumed regardless of the passage.
Figure 2.
Glucose consumption of adventitial fibroblasts. The cultured adventitial fibroblasts (AFs) from frozen stock were serum starved for 48 h with LG-DMEM. Conditioned media glucose concentrations were monitored at 24- and 48-h time points with 100 nM AngII (AII) or vehicle (veh: 0.1% H2O final) treatment in LG-, MG-, or HG-DMEM. Glucose concentration at 24-h (A) and 48-h (B) and glucose consumption rate at 24-h (C) and 48-h (D) were determined (n = 9). Cell numbers were assessed after cells were detached with trypsin (E) (n = 9). Comparison of glucose consumption rates between low and high passages (F) (n = 3). One-way ANOVA with post hoc Tukey’s test showed *P < 0.05, **P < 0.01 and ****P < 0.0001. Data represent means ± SE from independent experiments as indicated. HG, high glucose; LG, low glucose; MG, middle glucose; P, passage.
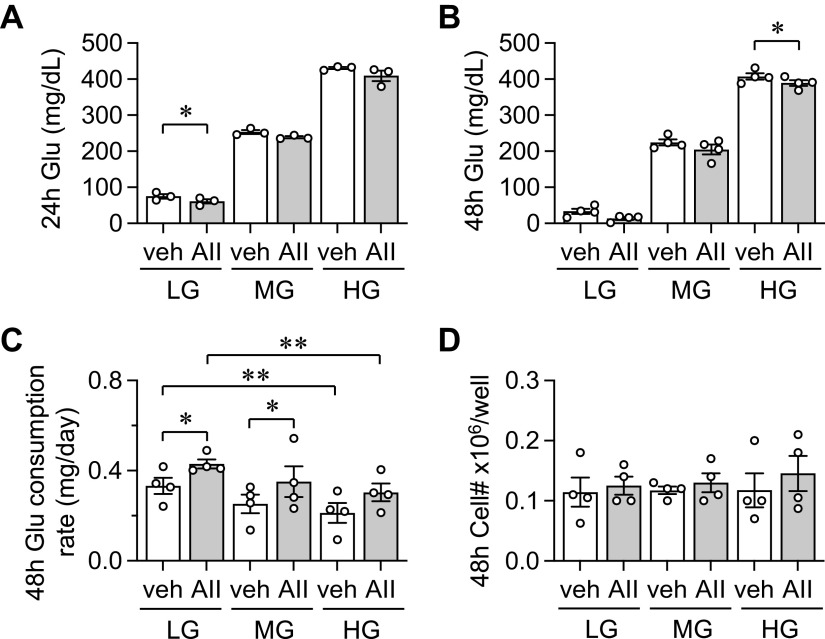
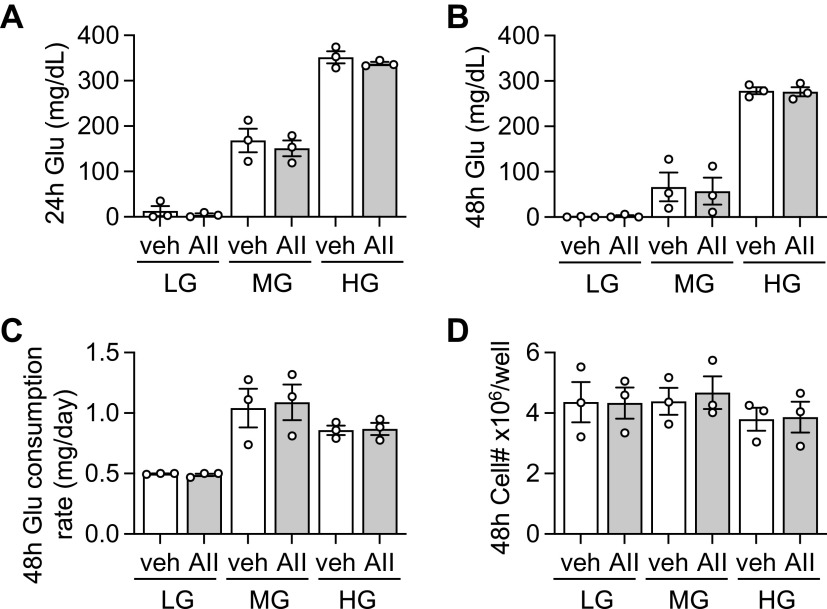
Although n numbers were limited, we have assessed media glucose consumption in VSMC and EC as these cells are markedly responsive to AngII stimulation (8) and known to be highly reliant on glycolysis for their functions (3, 5). In VSMC, AngII treatment further reduced media glucose concentration, which was significant at 24 h in LG-DMEM and 48 h in HG-DMEM. Media glucose levels became hypoglycemic at 24-h with stimulation and very low at 48-h in LG-DMEM with or without stimulation (Fig. 3, A and B). AngII also significantly enhanced glucose consumption rate in LG- and MG-DMEM. In addition, VSMC consumed less glucose in HG-DMEM than LG-DMEM regardless of the stimulation (Fig. 3C). No change was observed in cell numbers at all conditions (Fig. 3D). In EC no enhancement of glucose consumption was observed with AngII stimulation. EC completely consumed media glucose at 24-h in LG-DMEM. No change was observed in glucose consumption rate or cell numbers at all conditions (Fig. 4, A–D).
Figure 3.
Glucose consumption of VSMC. Conditioned media glucose concentrations were monitored at 24- and 48-h time points with AngII (AII) or vehicle (veh: 0.1% H2O final) treatment in VSMC cultured in LG-, MG-, or HG-DMEM. The cultured VSMCs were serum starved for 48 h in LG-DMEM. Glucose concentration at 24-h (A) (n = 3) and 48-h (B) (n = 4) and glucose consumption rate at 48-h (C) were determined (n = 4). Cell numbers were assessed after cells were detached with trypsin (D) (n = 4). One-way ANOVA with post hoc Tukey’s test showed *P < 0.05 and **P < 0.01. Data represent means ± SE from independent experiments as indicated. AII, angiotensin II; HG, high glucose; LG, low glucose; MG, middle glucose; VSMC, vascular smooth muscle cell.
Figure 4.
Glucose consumption of EC. Conditioned media glucose concentrations were monitored at 24- and 48-h time points with AngII (AII) or vehicle (veh: 0.1% H2O final) treatment in EC cultured in LG-, MG-, or HG-DMEM. The cultured VSMCs were serum starved for 48 h in LG-DMEM. Glucose concentration at 24 h (A) and 48 h (B) and glucose consumption rate at 48 h (C) were determined. Cell numbers were assessed after cells were detached with trypsin (D). One-way ANOVA with post hoc Tukey’s test showed no statistical significance between the relevant groups. Data represent means ± SE from three independent experiments. AII, angiotensin II; EC, EC, endothelial cell; HG, high glucose; LG, low glucose; MG, middle glucose.
Among the three cell types, AF and VSMC consumed ∼8–9 times more glucose than EC when normalized with cell number in MG-DMEM at a baseline condition, even though glucose consumption rates per well in these cells are relatively comparable (Supplemental Fig. S2, B and C). The difference in glucose consumed per cell is due to the much smaller size of EC compared with AF or VSMC and therefore glucose consumption rate per cell is greater for the larger AF or VSMC.
To enhance relevance of our rat VSMC and EC experiments, we have examined glucose consumption by human coronary VSMC and human dermal lymphatic EC with or without AngII stimulation. Human VSMC and human lymphatic EC consumed media glucose more than rat VSMC and rat EC, respectively. Human VSMC and lymphatic EC showed no response to AngII (Supplemental Fig. S3, A–D). We also used a distinct ligand, PDGF, in primary cultured rat aortic AF, based on the prior report using human dermal fibroblasts (17). PDGF stimulated glucose consumption more than AngII in the AF with MG media and was associated with a trend of increased cell proliferation (Supplemental Fig. S4, A–C).
Regarding the ideal glucose concentration for vascular cell culture, LG-media experiments have a clear risk for hypoglycemic condition especially those with growth factor stimulation or high passage cells. Frequent media exchange with glucose monitoring seems necessary when using LG-DMEM. However, HG-DMEM is also not an ideal media for all cell types because media glucose concentrations were at hyperglycemia range (>275 mg/dL) in all cell types even after 48-h incubation.
Simulation on changes in glucose concentrations was made based on the average glucose consumption rates in rat vascular cells (AF 0.66 mg/day, VSMC 0.25 mg/day, and EC 1.04 mg/day) at confluency on six-well plates (9.6 cm2) with 2 mL as well as for standard 10 cm round dish with 10 mL, 12 well with 1 mL, or 96 well with 0.1 mL (Fig. 5). Based on our experimental observation, we recommend 1:1 to 3:1 LG/HG-mixed DMEM media experiments for vascular cells especially with an agonist stimulation to avoid hypo or hyper-glycemic stress during the experiments. Extreme high or low media glucose concentrations are highly likely to influence experimental outcomes in these cells.
Figure 5.
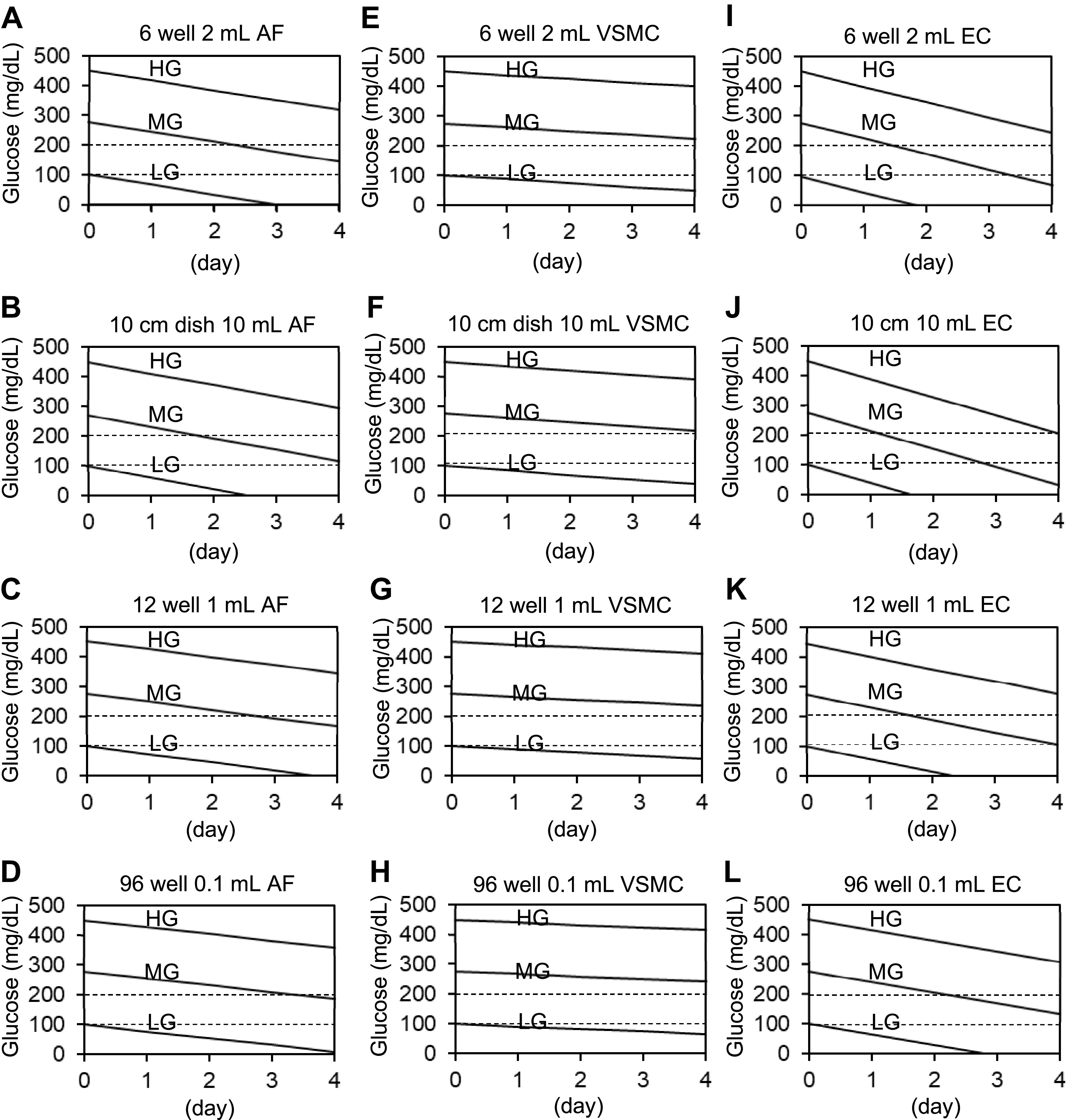
Simulation on changes in glucose concentrations in vascular cell types. Simulation on changes in glucose concentrations with three types of media (LG 100 mg/dL, MG 275 mg/dL, or HG 450 mg/dL) was made based on the average glucose consumption rates (AF 0.66 mg/day, VSMC 0.25 mg/day, and EC 1.04 mg/day) at confluency on 6-well plates (9.6 cm2). Simulation of 6-well plates with 2 mL of media [AF (A), VSMC (E), and EC (I)]. Simulation of 10 cm dish with 10 mL of AF (B), VSMC (F), and EC (J) with three types of medium. Simulation of 12-well plates with 1 mL of AF (C), VSMC (G), and EC (K) with three types of medium. Simulation of 96-well plates with 0.1 mL of AF (D), VSMC (H), and EC (L) with three types of medium. AF, adventitial fibroblasts; EC, endothelial cell; HG, high glucose; LG, low glucose; MG, middle glucose; VSMC, vascular smooth muscle cell.
Changes in Basal and Stimulated Protein Expression by Availability of Extracellular Glucose
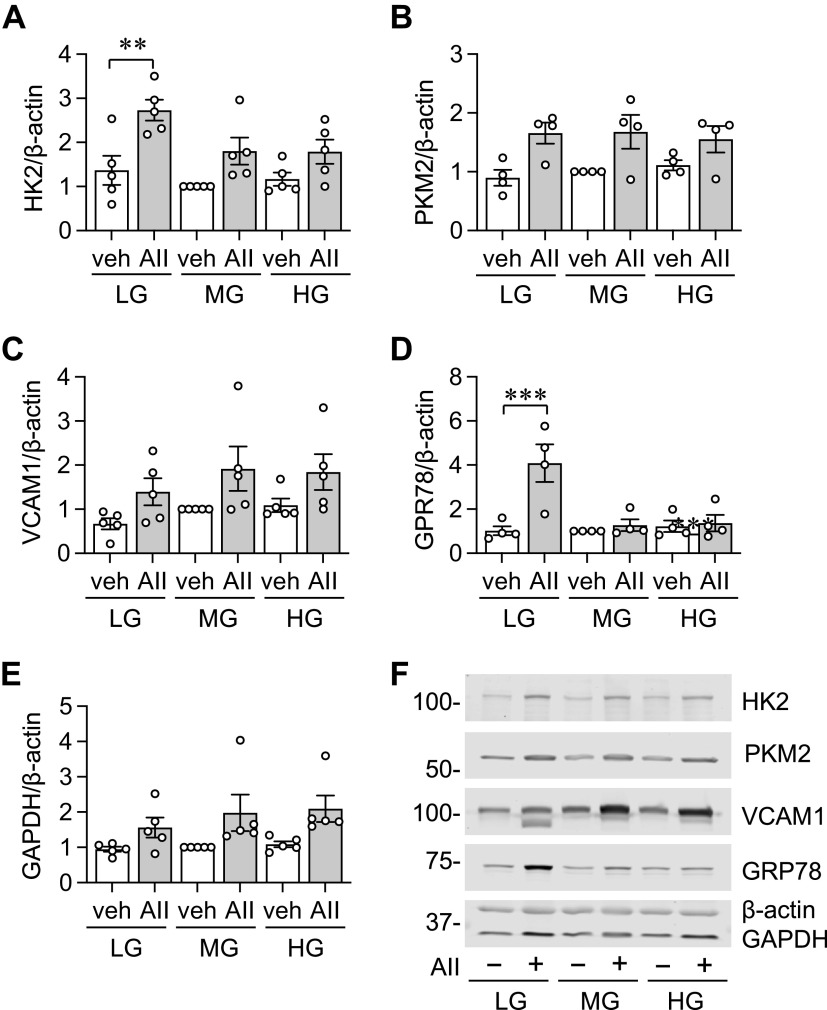
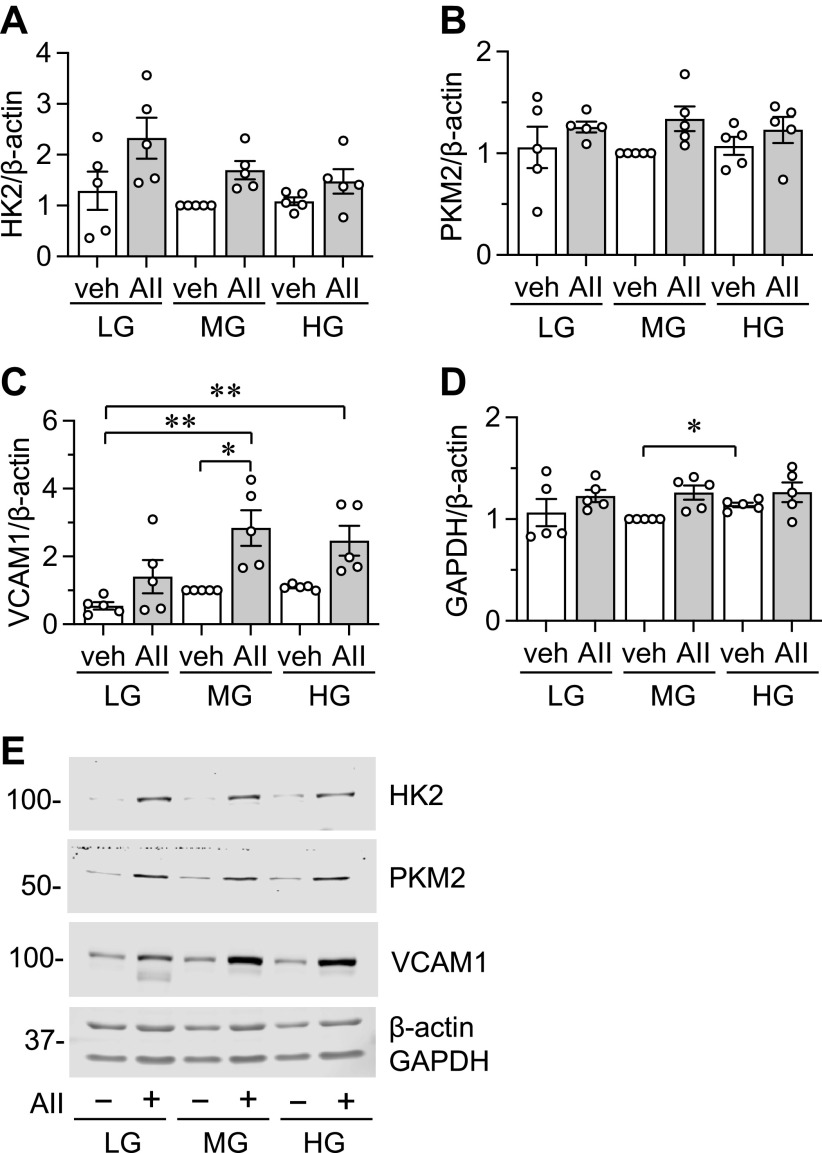
Since AF significantly consumes glucose and the consumption was enhanced by AngII, we have investigated if extracellular glucose availability affects basal and AngII stimulated protein expression in AF under the above media conditions. VCAM-1 and GRP78 were selected due to the inducibility by AngII and as a marker of inflammation and ER stress (12, 15), respectively. HK2 and PKM2 were included to assess a change in glycolysis. We also included two housekeeping proteins, GAPDH and β-actin. To be consistent but also more translational, experiments were set up in two ways regarding initial serum starvation. Initial serum starvation was performed in LG media followed by AngII stimulation in LG, MG, or HG media (Fig. 6 and Supplemental Fig. S5); or initial serum starvation was performed in the same glucose level media that was used for stimulation (Fig. 7 and Supplemental Fig. S6). For comparison, protein expression in MG-DMEM without AngII stimulation was set as 1 because it is the closest to physiological glucose levels.
Figure 6.
Protein expression in adventitial fibroblasts (AFs) with LG-DMEM starvation and AngII or vehicle stimulation under distinct media glucose conditions for 48 h. The cultured rat aortic AFs from frozen stock were serum starved for 48 h in LG-DMEM. AFs were stimulated with 100 nM AngII (AII) or vehicle (veh) for 48 h as indicated and immunoblotting was performed [ n = 5 (A), n = 4 (B), n = 5 (C), n = 4 (D), n = 5 (E)]. F: signal intensities were used to calculate the expression ratio of each protein to β-actin as indicated. For comparison, protein expression in MG-DMEM without AngII stimulation was set as 1 due to the closest physiological glucose fluctuation. One-way ANOVA with post hoc Tukey’s test showed **P <0.01 and ***P < 0.001. Data represent means ± SE from independent experiments as indicated. AII, angiotensin II; HG, high glucose; LG, low glucose; MG, middle glucose.
Figure 7.
Protein expression in adventitial fibroblasts (Afs) under each medium condition with serum starvation media corresponding to stimulation media. The cultured rat aortic AFs from frozen stock were serum starved for 48 h in LG-, MG-, or HG-DMEM as indicated. AFs were stimulated with 100 nM AngII (AII) or vehicle (veh) in LG-, MG-, or HG-DMEM for 48 h and immunoblotting was performed (A–D). E: signal intensities were used to calculate the expression ratio of each protein to β-actin as indicated. For comparison, protein expression in MG-DMEM without AngII stimulation was set as 1 because it is the closest to physiological glucose levels. One-way ANOVA with post hoc Tukey’s test showed *P < 0.05 and **P < 0.01. Data represent means ± SE from five independent experiments as indicated. AF, adventitial fibroblasts; AII, angiotensin II; HG, high glucose; LG, low glucose; MG, middle glucose.
With LG starvation and β-actin or GAPDH normalization, AngII increased HK2 in LG media. With β-actin normalization, no significant change in PKM2 expression was observed although there was a trend of AngII-dependent induction. With LG starvation and GAPDH normalization, AngII enhanced PKM2 expression in MG media. With β-actin normalization, no significant change in VCAM-1 expression was observed although there was a trend of AngII-dependent induction. With GAPDH normalization, AngII in MG or HG media increased VCAM-1 expression compared with LG media alone suggesting a synergy between higher glucose concentration and AngII for VCAM-1 induction. We also reproducibly observed induction of nonglycosylated form of VCAM-1 with AngII stimulation, but only in LG media. With LG starvation and β-actin or GAPDH normalization, AngII increased GRP78 expression only in LG media. In addition, regardless of media glucose concentration, there was a trend of increase in GAPDH with AngII stimulation and β-actin normalization (Fig. 6 and Supplemental Fig. S5).
With serum starvation media corresponding to stimulation media, AngII increased HK2 with GAPDH normalization in LG media. Only a trend of PKM2 induction by AngII was observed with β-actin normalization in MG-DMEM. AngII enhanced VCAM-1 induction in MG media with β-actin or GAPDH normalization. AngII plus MG or HG media also increased VCAM-1 expression compared with LG media alone. In addition, moderate increase in GAPDH was observed when HG-DMEM and MG-DMEM with no stimulation were compared (Fig. 7 and Supplemental Fig. S6).
We have also tested if distinct media glucose concentration affects acute gene induction by AngII at 1 h in AF. AngII significantly stimulated induction of c-Fos regardless of the glucose concentration with β-actin normalization. AngII significantly stimulated induction of c-Fos in LG- and MG-DMEM with GAPDH normalization. In addition, there is a trend of inverse relationship between the induction of c-Fos or Egr1 and media glucose concentration (Supplemental Fig. S7, A–E). Overall, these data suggest that AngII preferentially induces HK2 and GRP78 with low glucose media, and PKM2 and VCAM-1 with middle glucose media. VCAM-1 induction by AngII is also enhanced with middle to high glucose media. However, no clear preference was seen in AngII-induced c-Fos induction. In addition, β-actin normalization is recommended over GAPDH normalization when metabolic alterations are expected or observed in the experiments.
AT1 Receptor and Glycolysis Drive Glucose Consumption and VCAM-1 Induction in AngII-Stimulated AF
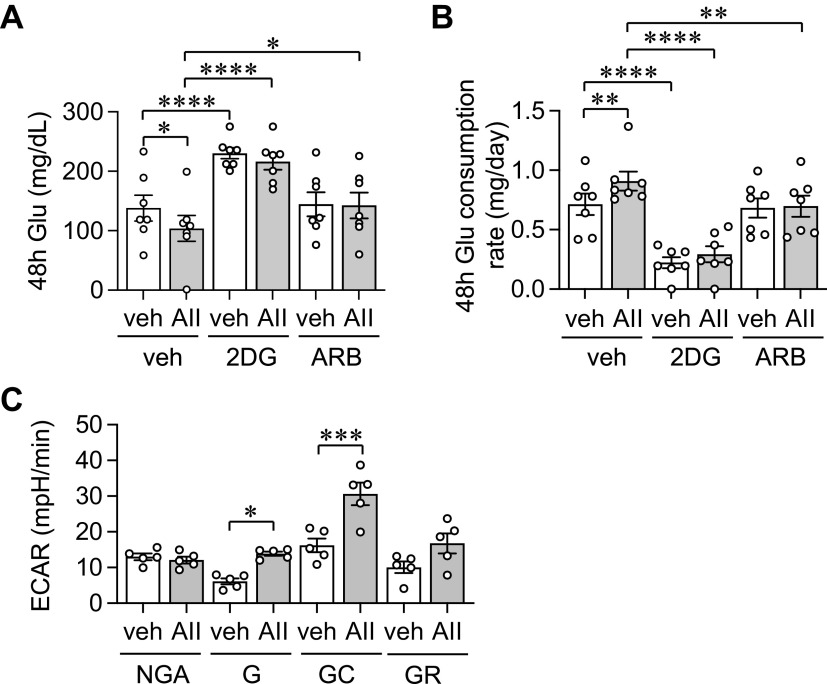
Fibroblast AT1 receptor has been implicated in cardiovascular remodeling (18) and glycolysis appears critical for tissue fibrosis (7). Accordingly, the involvements of AT1 receptor and glycolysis were examined in AngII-enhanced glucose consumption in AF. AT1 receptor antagonist and a glycolysis inhibitor 2DG attenuated AngII-induced glucose consumption in AF (Fig. 8, A and B and Supplemental Fig. S8). In addition, we have examined extracellular acidification rate by a glycolysis stress test with Agilent Seahorse XF analyzer to evaluate basal glycolysis and glycolytic capacity in AF with or without AngII stimulation. AngII significantly stimulated both basal glycolysis and glycolytic capacity in AF (Fig. 8C). These data indicate that glycolysis is a driving force of enhanced glucose consumption via AT1 receptor activation in AF.
Figure 8.
Role of AT1 receptor and glycolysis in glucose consumption in AFs with AngII stimulation. Conditioned media glucose concentrations were monitored at 48-h time points with AngII (AII) or vehicle (veh: 0.1% H2O final) treatment in purchased AFs pretreated with vehicle (veh: 0.1% DMSO final), 1 mM 2DG, or 10 μM Olmesartan (ARB) in MG-DMEM (A). Upon 48-h incubation, glucose consumption rates were determined (B). ECARs were determined with 100 nM AngII (AII) or vehicle (veh: H2O) treatment with a glycolysis stress test kit (C). One-way ANOVA with post hoc Tukey’s test showed *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Data represent means ± SE from independent experiments (n = 5, A and B) and 5 replicates (C) as indicated. AF, adventitial fibroblast; ARB, AngII receptor blocker; AT1, receptor angiotensin II type-1 receptor; ECAR, extracellular acidification rate; G, glycolysis; GC, glycolytic capacity; GR, glycolytic reserve; NGA, nonglycolytic acidification.
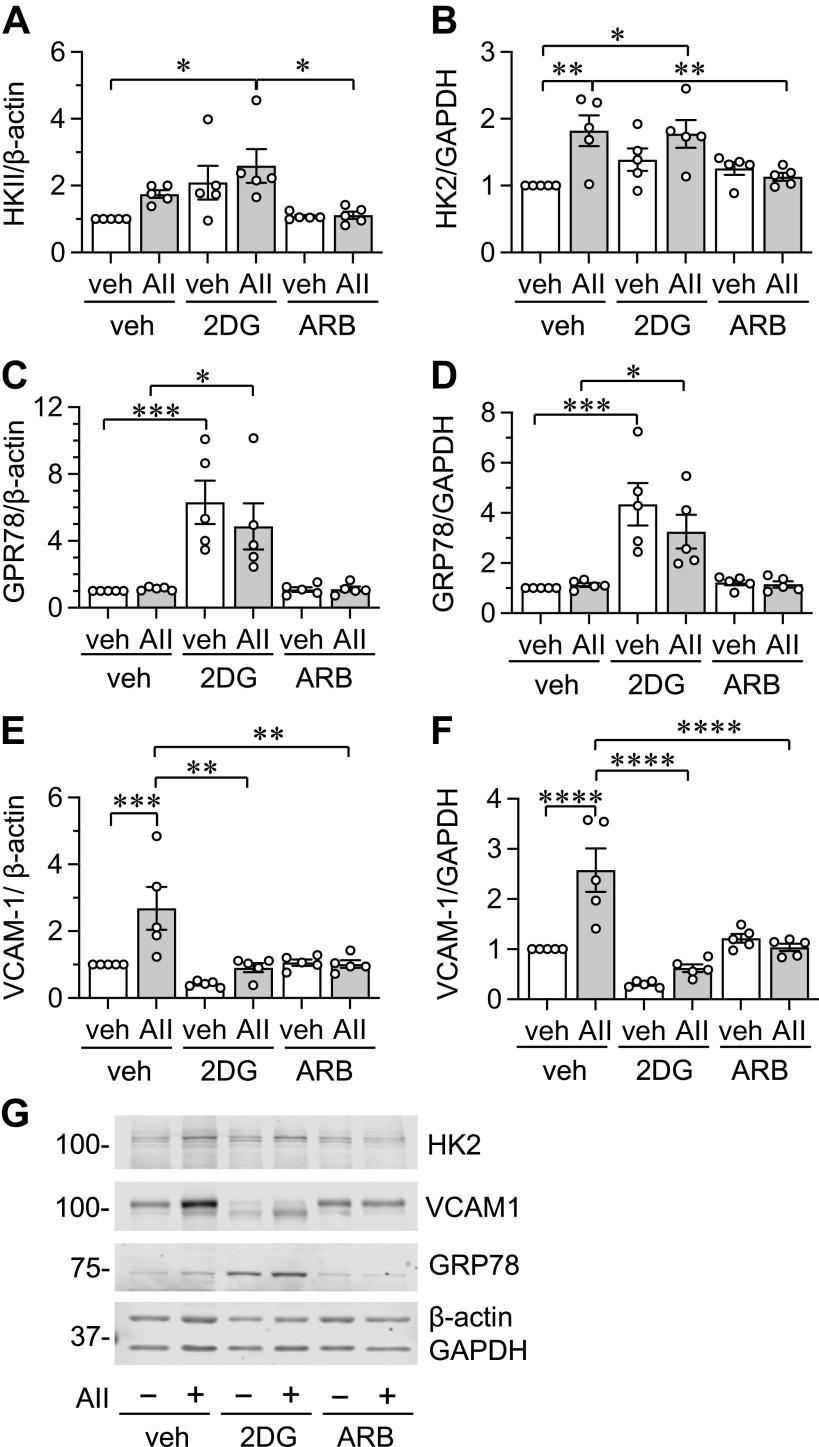
The involvement of AT1 receptor and glycolysis was further examined in AngII-induced protein induction in AF incubated in MG-DMEM. An AT1 receptor antagonist attenuated AngII-induced HK2 induction normalized with GAPDH. In addition, significant HK2 expression normalized with β-actin was observed in 2DG plus AngII condition (Fig. 9, A and B). As expected, inhibition of glucose utilization by 2DG significantly increased GRP78 regardless of the AngII stimulation (Fig. 9, C and D). 2DG and AT1 receptor antagonist significantly prevented AngII-induced VCAM-1 induction (Fig. 9, E–G), suggesting a specific involvement of glycolysis in AngII-induced inflammatory responses in AF.
Figure 9.
Role of AT1 receptor and glycolysis in protein expression in AFs with AngII stimulation. The involvements of AT1 receptor and glycolysis were examined in AngII-induced protein induction in cultured AF from frozen stock. A–G: the cultured rat aortic AFs were serum starved for 48 h in MG-DMEM. The AFs pretreated with vehicle (veh), 10 mM 2DG, or AngII receptor blocker (ARB) 10 μM for 30 min were stimulated with 100 nM Ang II (AII) or veh (H2O) for 48 h. Signal intensities were used to calculate the expression ratio of each protein to β-actin or GAPDH as indicated. For comparison, protein expression in MG-DMEM without AngII stimulation was set as 1 due to the closest physiological glucose fluctuation. One-way ANOVA with post hoc Tukey’s test showed *P < 0.05, **P < 0.01, ***P < 0.001 and ****P <0.0001. Data represent means ± SE from five independent experiments as indicated. AF, adventitial fibroblast; AT1 receptor angiotensin II type-1 receptor; MG, middle glucose.
DISCUSSION
This study demonstrated that three major vascular cell types (EC, VSMC, and AF) significantly consume glucose under serum-starved conditions regardless of the given extracellular glucose concentration. Our data indicate that there is a high risk of hypoglycemic condition when these (and probably any) cells are cultured with so-called normal (euglycemic) glucose media such as DMEM with relatively low 100 mg/dL glucose. The risk seems especially high in smaller cell types such as EC grown to confluence because the increased cell number per media volume reduces the glucose concentrations rather quickly, even though cellular glucose requirements for EC are less than the larger VSMC and AF. Moreover, AngII and other growth promoting factors for given cell types may further stimulate glucose consumption and enhance hypoglycemic condition, which hampers proper interpretation of the experimental outcomes. According to our simulation, we advocate the usage of mixed glucose media with 187.5 (3:1 = LG/HG such as for rat VSMC)–275 (1:1 = LG/HG such as for rat AF and EC) mg/dL over 100 mg/dL glucose media when cells will be serum starved and incubated for 24 h or longer. For cell types that need a specialized media where glucose concentration is unknown or for cell cultured in any media with low glucose concentration, glucose concentration should be frequently monitored, and media glucose should be adjusted to an ideal level before the experiments. All cell types used in this study did not require high glucose media for cell growth. We do not recommend the usage of high glucose media as cells will be exposed to extremely high glucose condition, which may affect the outcomes. Accordingly, the frequently utilized normal versus high glucose protocol to simulate human diabetic conditions may need reconsideration for vascular cells as normal glucose culture longer than 24 h likely produces hypoglycemic stress, whereas standard high glucose media are too high to simulate human diabetic conditions. In addition, our survey of core journals clearly demonstrated a lack of scientific rigor in describing important media glucose information. We advocate the publishers and editors to mandate inclusion of glucose information or the exact catalog number of the media used for submission to improve scientific rigor, reproducibility, and communication.
In cultured aortic EC, we did not observe AngII-induced enhancement in glucose consumption. This is not due to a lack of AT1 receptor in this cell type since we have previously observed several AngII-induced responses in LG-DMEM including mitochondrial fission, senescence, and enhanced leucocyte adhesion which were considered to be AT1 receptor-dependent (19). AngII-induced EC inflammatory responses may not require stimulated glycolysis and are likely mediated via mitochondrial oxidative stress (8).
In VSMC, we found that AngII enhanced glucose consumption regardless of the media glucose concentration. These findings are consistent with a past publication in rat aortic VSMC in which AngII stimulated glucose uptake and expression of glucose transporter 1 (GLUT1) (9). VSMC glycolysis such as via PKM2 appears critical for VSMC phenotype switching and arterial hyperplasia (20). Moreover, smooth muscle specific GLUT1 transgenic mice demonstrated enhanced atherosclerosis lesion formation via enhanced glycolysis (21). The mice also showed medial hypertrophy in response to vascular injury (22). In contrast, suppression of aortic GLUT4 expression was observed in mice with AngII infusion (23). Interestingly, although AngII-induced hypertension was unaltered in smooth muscle specific GLUT4 transgenic mice, exaggerated vascular contractile responses and endothelial dysfunction were attenuated (24). GLUT4 and GLUT1 seem to mediate basal and stimulated glucose uptake in VSMC (9, 23). However, further research is desired to determine the physiological and pathophysiological roles of GLUT regulation and enhanced glycolysis via AngII. In this study, VSMCs obtained by the explant method were used. The explant VSMCs represent a synthetic phenotype demonstrating reduction in contractile proteins compared with VSMCs obtained by an enzymatic method (25, 26). We have confirmed that our explant VSMCs maintain smooth muscle α actin expression. Nonetheless, study expansion is desired to include younger passage VSMCs obtained by an enzymatic method.
In fibroblasts, glucose supply is a critical determinant of collagen secretion (27) and metabolic shift toward glycolysis appears essential for proliferative and inflammatory fibroblast activation (28). Our data extended these past findings in an in vitro activation model of AF by a relevant hypertensive hormone, AngII. As we previously observed in VSMC (12), AngII enhanced expression of GRP78, a master regulator of ER stress, in AF only in LG-DMEM. Although GRP78 was originally identified in chicken fibroblasts as a protein induced by glucose starvation (29), gradual depletion of glucose alone was insufficient to induce GRP78 in AF in the present study. However, GRP78 was significantly induced in AF when glucose utilization was completely prevented in the presence of 2DG in our study. This indicates that GRP78 induction with AngII is a direct reflection of ER stress as seen in VSMCs (13). Appearance of nonglycosylated form of VCAM-1 only in LG-DMEM with AngII stimulation further suggests specific enhancement of ER stress in this condition (30).
Recently, the contribution of HK2 induction and subsequent glycolysis in profibrotic action of transforming growth factor-β has been demonstrated in a model of pulmonary hypertension (31). However, a potentially protective role to attenuate cardiac myocyte hypertrophy was also demonstrated when HK2 was overexpressed with AngII stimulation, which was explained by the pentose phosphate pathway blocking oxidative stress (32). We observed here that HK2 was preferentially induced by AngII in low glucose condition. Under glucose deprivation, HK2 promotes autophagy by interacting with mammalian target of rapamycin (33). It is therefore reasonable to speculate a protective induction of HK2 by AngII in AF under ER stress.
In the present studies conducted on AF, PKM2 was significantly induced by AngII only in MG-DMEM where AngII also induced the highest glucose consumption. Conditional deletion (34) and utilization of selective inhibitors (35, 36) demonstrated that PKM2-mediated glycolysis is an essential mediator of fibroblast activation and fibrosis. Moreover, by using mouse embryonic fibroblasts stimulated by AngII or transforming growth factor-β, a requirement of enhanced glycolysis for myofibroblast differentiation has been demonstrated (37). It is therefore interesting to explore the role PKM2 potentially plays in fibroblast phenotype modulation in hypertension.
Raising the ambient glucose concentration has been shown to lead to induction of the immediate early genes including c-Fos and Egr-1 in several cell types (38–42). In VSMC, AngII and HG media had additive effects to activate activator protein-1 (43). However, little is known if AngII induction of immediate early genes is affected by distinct steady state glucose concentration. We observed a preferential trend of c-Fos induction in LG media over HG media in AF. HK2 gene promoter includes c-Fos responsible activator protein-1 site. AngII-induced c-Fos induction in LG DMEM may contribute to higher HK2 induction by AngII in LG DMEM.
Adventitial inflammation has recently been implicated in hypertension pathology (43). In this study, VCAM-1 was induced by AngII preferentially at higher media glucose concentration. AngII plus high glucose condition has been shown to enhance certain signaling mechanisms such as AP-1, which is a key regulator of AngII-induced VCAM-1 induction (44, 45). In mice with AngII infusion, nuclear factor-κB also mediates AngII-induced VCAM-1 induction (46). Glycolysis inhibition at the level of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 attenuated nuclear factor-κB activation in endothelial cells (47) and is protective against atherosclerosis in apolipoprotein E deficient mice (48). It is therefore likely that extracellular high glucose and AngII synergistically promote vascular inflammatory responses via enhanced glycolysis.
Media glucose concentration is known to affect cellular metabolic activities including OCR. In cultured EC, HG media reduced OCR (49, 50). This cell response known as the Crabtree effect has been shown to be mediated by the p90 ribosomal S6 kinase in H9c2 cells. AngII is known to activate this kinase among many other protein kinases (8). However, we have observed that AngII increased OCR in VSMC with LG DMEM (15). It is therefore interesting to further explore how HG, AngII, or the combination modulates OCR in distinct vascular cell types.
Similar to AngII, we showed that PDGF stimulated glucose consumption in AF. It should be noted that PDGF was shown to induce glucose uptake and glycolysis in VSMC, which was associated with HK2 induction. Inhibition of glycolysis further attenuated PDGF-induced VSMC migration (51). Moreover, PDGF-induced VSMC DNA synthesis was enhanced in HG DMEM compared with LG DMEM (52). Therefore, it would be interesting to extend our VSMC experiments with PDGF stimulation.
In addition, our protein expression experiments focused on a limited number of targets, thus may not represent overall gene/protein expression profile under the distinct media glucose with or without AngII stimulation. However, there are several prior studies exploring such profiling with gene ontology analyses. For example, in line with our AngII plus high glucose condition in AF, EC transcriptome analysis with HG or LG media condition demonstrated that HG induced enhanced expression of inflammatory cytokines and intercellular adhesion molecule-1 (53). In VSMCs with AngII stimulation, a number of proinflammatory and profibrotic genes were upregulated (54). Moreover, proteomic analysis of AngII-induced upregulated gene ontology terms includes gluconeogenesis with upregulation of HK2 and PKM2 as we observed in fibroblasts (55). It is interesting to further expand our research in future to include proteomics experiments such as secretome analysis in vascular cells.
Most of our experiments were limited to vascular cells from male rats. A prior study reported that no sex difference was observed for basal or insulin-stimulated glucose uptake in cultured human skeletal muscle cells (56). In another study utilizing preadipocytes from human obese donors with Seahorse stress analysis, no sex difference was observed in ECAR glycolysis response under low or high glucose culture media. However, female cells showed higher mitochondrial oxygen consumption ratio, which was significantly attenuated by high glucose media (57). Based on these limited observations, no major sex differences are expected regarding glucose consumption in vascular cells. It is still possible that certain vascular cell responses may show sex difference such as mitochondrial response to high glucose media.
In conclusion, by using three distinct vascular cell types, this study demonstrated the important role media glucose plays in experimental outcomes. Low glucose media with AngII stimulation leads to enhancement of ER stress most likely due to a lack of sufficient glycolytic ATP supply. In contrast, high glucose media significantly enhances inflammatory VCAM-1 induction by AngII. Appropriate adjustment of extracellular glucose is therefore desired to set the baseline/control of culture experiments to be more physiologically relevant.
SUPPLEMENTAL DATA
Supplemental Table S1 and Supplemental Figs. S1–S8: https://doi.org/10.6084/m9.figshare.16906825.v1
GRANTS
This work was supported by the National Institutes of Health Grants RO1HL128324 (to S.E. and V.R.), RO1DK111042 (to R.S. and S.E.), and RO1NS109382 (to S.E. and T.H.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.T., K.O., and S.E. conceived and designed research; K.T., K.O., R.K., N.S., S.M.C., K.E., K.J.E., T.K., C.B.C., A.M.P., and A.K.S.P. performed experiments; K.T., K.O., S.M.C., K.J.E., and S.E. analyzed data; K.T., K.O., and S.E. interpreted results of experiments; K.T. and S.E. prepared figures; K.T., K.J.E., and S.E. drafted manuscript; K.J.E. edited and revised manuscript; K.T., K.O., R.K., N.S., S.M.C., K.E., K.J.E., T.K., C.B.C., A.M.P., A.K.S.P., M.V.A., R.S., V.R., T.H., and S.E. approved final version of manuscript.
REFERENCES
- 1.Ahrén B, Pacini G. Glucose effectiveness: lessons from studies on insulin-independent glucose clearance in mice. J Diabetes Investig 12: 675–685, 2021. doi: 10.1111/jdi.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Lei L, Liu D, Jovin I, Russell R, Johnson RS, Di Lorenzo A, Giordano FJ. Normal glucose uptake in the brain and heart requires an endothelial cell-specific HIF-1α-dependent function. Proc Natl Acad Sci USA 109: 17478–17483, 2012. doi: 10.1073/pnas.1209281109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P. Endothelial cell metabolism. Physiol Rev 98: 3–58, 2018. doi: 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, Gutterman DD, Widlansky ME. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 32: 712–720, 2012. doi: 10.1161/ATVBAHA.111.227389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J, Yang Y, Cheng A, Xu G, He F. Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Heart Circ Physiol 319: H613–H631, 2020. doi: 10.1152/ajpheart.00220.2020. [DOI] [PubMed] [Google Scholar]
- 6.Xi G, Shen X, Wai C, White MF, Clemmons DR. Hyperglycemia induces vascular smooth muscle cell dedifferentiation by suppressing insulin receptor substrate-1-mediated p53/KLF4 complex stabilization. J Biol Chem 294: 2407–2421, 2019. doi: 10.1074/jbc.RA118.005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibb AA, Lazaropoulos MP, Elrod JW. Myofibroblasts and fibrosis: mitochondrial and metabolic control of cellular differentiation. Circ Res 127: 427–447, 2020. doi: 10.1161/CIRCRESAHA.120.316958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 98: 1627–1738, 2018. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn LA, McCumbee WD. Regulation of glucose transport by angiotensin II and glucose in cultured vascular smooth muscle cells. J Cell Physiol 177: 94–102, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem 273: 8890–8896, 1998. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 11.Elliott KJ, Eguchi S. In vitro analysis of hypertensive signal transduction: kinase activation, kinase manipulation, and physiologic outputs. Methods Mol Biol 1527: 201–211, 2017. doi: 10.1007/978-1-4939-6625-7_16. [DOI] [PubMed] [Google Scholar]
- 12.Takayanagi T, Kawai T, Forrester SJ, Obama T, Tsuji T, Fukuda Y, Elliott KJ, Tilley DG, Davisson RL, Park JY, Eguchi S. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension 65: 1349–1355, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicalese S, Okuno K, Elliott KJ, Kawai T, Scalia R, Rizzo V, Eguchi S. 78 kDa glucose-regulated protein attenuates protein aggregation and monocyte adhesion induced by angiotensin II in vascular cells. Int J Mol Sci 21: 4980, 2020. doi: 10.3390/ijms21144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrester SJ, Preston KJ, Cooper HA, Boyer MJ, Escoto KM, Poltronetti AJ, Elliott KJ, Kuroda R, Miyao M, Sesaki H, Akiyama T, Kimura Y, Rizzo V, Scalia R, Eguchi S. Mitochondrial fission mediates endothelial inflammation. Hypertension 76: 267–276, 2020. doi: 10.1161/HYPERTENSIONAHA.120.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper HA, Cicalese S, Preston KJ, Kawai T, Okuno K, Choi ET, Kasahara S, Uchida HA, Otaka N, Scalia R, Rizzo V, Eguchi S. Targeting mitochondrial fission as a potential therapeutic for abdominal aortic aneurysm. Cardiovasc Res 117: 971–982, 2021. doi: 10.1093/cvr/cvaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eguchi S, Iwasaki H, Hirata Y, Frank GD, Motley ED, Yamakawa T, Numaguchi K, Inagami T. Epidermal growth factor receptor is indispensable for c-Fos expression and protein synthesis by angiotensin II. Eur J Pharmacol 376: 203–206, 1999. doi: 10.1016/s0014-2999(99)00357-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Wang Y, Shi Z, Liu J, Sun P, Hou X, Zhang J, Zhao S, Zhou BP, Mi J. Metabolic reprogramming of cancer-associated fibroblasts by IDH3α downregulation. Cell Rep 10: 1335–1348, 2015. doi: 10.1016/j.celrep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Poduri A, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Cassis LA, Daugherty A. Fibroblast angiotensin II type 1a receptors contribute to angiotensin ii-induced medial hyperplasia in the ascending aorta. Arterioscler Thromb Vasc Biol 35: 1995–2002, 2015. doi: 10.1161/ATVBAHA.115.305995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyao M, Cicalese S, Cooper HA, Eguchi S. Endoplasmic reticulum stress and mitochondrial biogenesis are potential therapeutic targets for abdominal aortic aneurysm. Clin Sci (Lond) 133: 2023–2028, 2019. doi: 10.1042/CS20190648. [DOI] [PubMed] [Google Scholar]
- 20.Jain M, Dhanesha N, Doddapattar P, Nayak MK, Guo L, Cornelissen A, Lentz SR, Finn AV, Chauhan AK. Smooth muscle cell-specific PKM2 (pyruvate kinase muscle 2) promotes smooth muscle cell phenotypic switching and neointimal hyperplasia. Arterioscler Thromb Vasc Biol 41: 1724–1737, 2021. doi: 10.1161/ATVBAHA.121.316021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wall VZ, Barnhart S, Kanter JE, Kramer F, Shimizu-Albergine M, Adhikari N, Wight TN, Hall JL, Bornfeldt KE. Smooth muscle glucose metabolism promotes monocyte recruitment and atherosclerosis in a mouse model of metabolic syndrome. JCI Insight 3: e96544, 2018. doi: 10.1172/jci.insight.96544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adhikari N, Basi DL, Carlson M, Mariash A, Hong Z, Lehman U, Mullegama S, Weir EK, Hall JL. Increase in GLUT1 in smooth muscle alters vascular contractility and increases inflammation in response to vascular injury. Arterioscler Thromb Vasc Biol 31: 86–94, 2011. doi: 10.1161/ATVBAHA.110.215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JL, Loberg RD, Duquaine D, Zhang H, Deo BK, Ardanaz N, Coyle J, Atkins KB, Schin M, Charron MJ, Kumagai AK, Pagano PJ, Brosius FC 3rd.. GLUT4 facilitative glucose transporter specifically and differentially contributes to agonist-induced vascular reactivity in mouse aorta. Arterioscler Thromb Vasc Biol 25: 1596–1602, 2005. doi: 10.1161/01.ATV.0000170137.41079.ab. [DOI] [PubMed] [Google Scholar]
- 24.Atkins KB, Seki Y, Saha J, Eichinger F, Charron MJ, Brosius FC. Maintenance of GLUT4 expression in smooth muscle prevents hypertension-induced changes in vascular reactivity. Physiol Rep 3: e12299, 2015. doi: 10.14814/phy2.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirschenlohr HL, Metcalfe JC, Weissberg PL, Grainger DJ. Proliferation of human aortic vascular smooth muscle cells in culture is modulated by active TGF-β. Cardiovasc Res 29: 848–855, 1995. [PubMed] [Google Scholar]
- 26.Campbell JH, Campbell GR. Smooth muscle phenotypic modulation – a personal experience. Arterioscler Thromb Vasc Biol 32: 1784–1789, 2012. doi: 10.1161/ATVBAHA.111.243212. [DOI] [PubMed] [Google Scholar]
- 27.Cechowska-Pasko M, Surazyński A, Bańkowski E. The effect of glucose deprivation on collagen synthesis in fibroblast cultures. Mol Cell Biochem 327: 211–218, 2009. doi: 10.1007/s11010-009-0059-8. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Riddle S, Zhang H, D'Alessandro A, Flockton A, Serkova NJ, Hansen KC, Moldovan R, McKeon BA, Frid M, Kumar S, Li H, Liu H, Caánovas A, Medrano JF, Thomas MG, Iloska D, Plecitá-Hlavatá L, Ježek P, Pullamsetti S, Fini MA, El Kasmi KC, Zhang Q, Stenmark KR. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation 134: 1105–1121, 2016. [Erratum in Circulation 139: e1, 2019]. doi: 10.1161/CIRCULATIONAHA.116.023171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AS, Delegeane A, Scharff D. Highly conserved glucose-regulated protein in hamster and chicken cells: preliminary characterization of its cDNA clone. Proc Natl Acad Sci USA 78: 4922–4925, 1981. doi: 10.1073/pnas.78.8.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Korennykh AV, Behrman SL, Walter P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc Natl Acad Sci USA 107: 16113–16118, 2010. doi: 10.1073/pnas.1010580107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin X, Choudhury M, Kang JH, Schaefbauer KJ, Jung MY, Andrianifahanana M, Hernandez DM, Leof EB. Hexokinase 2 couples glycolysis with the profibrotic actions of TGF-β. Sci Signal 12: eaax4067, 2019. doi: 10.1126/scisignal.aax4067. [DOI] [PubMed] [Google Scholar]
- 32.McCommis KS, Douglas DL, Krenz M, Baines CP. Cardiac-specific hexokinase 2 overexpression attenuates hypertrophy by increasing pentose phosphate pathway flux. J Am Heart Assoc 2: e000355, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts DJ, Tan-Sah VP, Ding EY, Smith JM, Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell 53: 521–533, 2014. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye Y, Xu L, Ding H, Wang X, Luo J, Zhang Y, Zen K, Fang Y, Dai C, Wang Y, Zhou Y, Jiang L, Yang J. Pyruvate kinase M2 mediates fibroblast proliferation to promote tubular epithelial cell survival in acute kidney injury. FASEB J 35: e21706, 2021. doi: 10.1096/fj.202100040R. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D'Alessandro A, Tauber J, Riddle S, Kumar S, Flockton A, McKeon BA, Frid MG, Reisz JA, Caruso P, El Kasmi KC, Ježek P, Morrell NW, Hu CJ, Stenmark KR. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation 136: 2468–2485, 2017. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan Q, Luo J, Zen K, Yang J. Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am J Physiol Renal Physiol 313: F561–F575, 2017. doi: 10.1152/ajprenal.00036.2017. [DOI] [PubMed] [Google Scholar]
- 37.Lombardi AA, Gibb AA, Arif E, Kolmetzky DW, Tomar D, Luongo TS, Jadiya P, Murray EK, Lorkiewicz PK, Hajnóczky G, Murphy E, Arany ZP, Kelly DP, Margulies KB, Hill BG, Elrod JW. Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation. Nat Commun 10: 4509, 2019. doi: 10.1038/s41467-019-12103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh FN. High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-β. Kidney Int 42: 647–656, 1992. doi: 10.1038/ki.1992.330. [DOI] [PubMed] [Google Scholar]
- 39.Kreisberg JI, Radnik RA, Ayo SH, Garoni J, Saikumar P. High glucose elevates c-fos and c-jun transcripts and proteins in mesangial cell cultures. Kidney Int 46: 105–112, 1994. doi: 10.1038/ki.1994.249. [DOI] [PubMed] [Google Scholar]
- 40.Han DC, Isono M, Hoffman BB, Ziyadeh FN. High glucose stimulates proliferation and collagen type I synthesis in renal cortical fibroblasts: mediation by autocrine activation of TGF-β. J Am Soc Nephrol 10: 1891–1899, 1999. doi: 10.1681/ASN.V1091891. [DOI] [PubMed] [Google Scholar]
- 41.Min W, Bin ZW, Quan ZB, Hui ZJ, Sheng FG. The signal transduction pathway of PKC/NF-κB/c-fos may be involved in the influence of high glucose on the cardiomyocytes of neonatal rats. Cardiovasc Diabetol 8: 8, 2009. doi: 10.1186/1475-2840-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vedantham S, Thiagarajan D, Ananthakrishnan R, Wang L, Rosario R, Zou YS, Goldberg I, Yan SF, Schmidt AM, Ramasamy R. Aldose reductase drives hyperacetylation of Egr-1 in hyperglycemia and consequent upregulation of proinflammatory and prothrombotic signals. Diabetes 63: 761–774, 2014. doi: 10.2337/db13-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meijles DN, Pagano PJ. Nox and inflammation in the vascular adventitia. Hypertension 67: 14–19, 2016. doi: 10.1161/HYPERTENSIONAHA.115.03622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Natarajan R, Scott S, Bai W, Yerneni KK, Nadler J. Angiotensin II signaling in vascular smooth muscle cells under high glucose conditions. Hypertension 33: 378–384, 1999. doi: 10.1161/01.hyp.33.1.378. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad M, Theofanidis P, Medford RM. Role of activating protein-1 in the regulation of the vascular cell adhesion molecule-1 gene expression by tumor necrosis factor-α. J Biol Chem 273: 4616–4621, 1998. doi: 10.1074/jbc.273.8.4616. [DOI] [PubMed] [Google Scholar]
- 46.Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell-specific NF-κB suppression attenuates hypertension-induced renal damage. Circ Res 101: 268–276, 2007. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 47.Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, Chaturvedi P, Hol J, Thienpont B, Teuwen LA, Schoors S, Boeckx B, Vriens J, Kuchnio A, Veys K, Cruys B, Finotto L, Treps L, Stav-Noraas TE, Bifari F, Stapor P, Decimo I, Kampen K, De Bock K, Haraldsen G, Schoonjans L, Rabelink T, Eelen G, Ghesquière B, Rehman J, Lambrechts D, Malik AB, Dewerchin M, Carmeliet P. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30: 968–985, 2016. doi: 10.1016/j.ccell.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perrotta P, Van der Veken B, Van Der Veken P, Pintelon I, Roosens L, Adriaenssens E, Timmerman V, Guns PJ, De Meyer GRY, Martinet W. Partial inhibition of glycolysis reduces atherogenesis independent of intraplaque neovascularization in mice. Arterioscler Thromb Vasc Biol 40: 1168–1181, 2020. doi: 10.1161/ATVBAHA.119.313692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sweet IR, Gilbert M, Maloney E, Hockenbery DM, Schwartz MW, Kim F. Endothelial inflammation induced by excess glucose is associated with cytosolic glucose 6-phosphate but not increased mitochondrial respiration. Diabetologia 52: 921–931, 2009. doi: 10.1007/s00125-009-1272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim D, Sankaramoorthy A, Roy S. Downregulation of Drp1 and Fis1 inhibits mitochondrial fission and prevents high glucose-induced apoptosis in retinal endothelial cells. Cells 9: 1662, 2020. doi: 10.3390/cells9071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heiss EH, Schachner D, Donati M, Grojer CS, Dirsch VM. Increased aerobic glycolysis is important for the motility of activated VSMC and inhibited by indirubin-3'-monoxime. Vascul Pharmacol 83: 47–56, 2016. doi: 10.1016/j.vph.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Little PJ, Allen TJ, Hashimura K, Nigro J, Farrelly CA, Dilley RJ. High glucose potentiates mitogenic responses of cultured ovine coronary smooth muscle cells to platelet derived growth factor and transforming growth factor-β1. Diabetes Res Clin Pract 59: 93–101, 2003. doi: 10.1016/s0168-8227(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 53.Yasuda H, Iwata Y, Nakajima S, Furuichi K, Miyake T, Sakai N, Kitajima S, Toyama T, Shinozaki Y, Sagara A, Miyagawa T, Hara A, Shimizu M, Kamikawa Y, Sato K, Oshima M, Yoneda-Nakagawa S, Kaneko S, Wada T. Erythropoietin signal protected human umbilical vein endothelial cells from high glucose-induced injury. Nephrology (Carlton) 24: 767–774, 2019. doi: 10.1111/nep.13518. [DOI] [PubMed] [Google Scholar]
- 54.Leung A, Trac C, Jin W, Lanting L, Akbany A, Sætrom P, Schones DE, Natarajan R. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res 113: 266–278, 2013. doi: 10.1161/CIRCRESAHA.112.300849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.García-Marqués F, Trevisan-Herraz M, Martínez-Martínez S, Camafeita E, Jorge I, Lopez JA, Méndez-Barbero N, Méndez-Ferrer S, Del Pozo MA, Ibáñez B, Andrés V, Sánchez-Madrid F, Redondo JM, Bonzon-Kulichenko E, Vázquez J. A novel systems-biology algorithm for the analysis of coordinated protein responses using quantitative proteomics. Mol Cell Proteomics 15: 1740–1760, 2016. doi: 10.1074/mcp.M115.055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rune A, Salehzadeh F, Szekeres F, Kühn I, Osler ME, Al-Khalili L. Evidence against a sexual dimorphism in glucose and fatty acid metabolism in skeletal muscle cultures from age-matched men and post-menopausal women. Acta Physiol (Oxf) 197: 207–215, 2009. doi: 10.1111/j.1748-1716.2009.02010.x. [DOI] [PubMed] [Google Scholar]
- 57.Keuper M, Berti L, Raedle B, Sachs S, Böhm A, Fritsche L, Fritsche A, Haring HU, Hrabě de Angelis M, Jastroch M, Hofmann SM, Staiger H. Preadipocytes of obese humans display gender-specific bioenergetic responses to glucose and insulin. Mol Metab 20: 28–37, 2019. doi: 10.1016/j.molmet.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 and Supplemental Figs. S1–S8: https://doi.org/10.6084/m9.figshare.16906825.v1