Abstract
Objectives
Facial palsy is the most common manifestation of Lyme neuroborreliosis (LNB) in the United States. This study aimed to describe features of patients with early LNB presenting with facial palsy and to determine if corticosteroids in addition to antibiotic therapy was associated with unfavorable outcome.
Methods
Retrospective analysis of participants enrolled in clinical studies investigating Lyme disease (N = 486) identified 44 patients who had facial palsy from LNB. The House–Brackmann scale was used to quantify the facial nerve dysfunction.
Results
Most patients presented in the summer months. Erythema migrans, frequently associated with systemic symptoms, occurred in 29 patients. Thirteen patients presented with bilateral facial palsy, usually with sequential involvement. Fourteen patients had painful radiculopathy. Of the 38 patients treated with antibiotics before the resolution of the palsy who had complete follow‐up, 24 received both antibiotics and corticosteroids. Of these 38 patients, 34 recovered completely, 3 had nearly complete recovery, and 1 had moderate dysfunction. There were no differences between the treatment groups in achieving complete resolution of the palsy at 12 months or in time to complete recovery.
Interpretation
A history of rash compatible with erythema migrans or febrile illness in the weeks preceding the palsy are helpful clues pointing toward LNB and should be actively sought when evaluating patients with acute‐onset peripheral facial palsy, particularly bilateral facial palsy. Treatment with antibiotic therapy is highly effective and most patients will fully recover facial nerve function. Adjunctive corticosteroid therapy appears to not affect the speed of recovery or overall outcome in this retrospective observational study.
Introduction
Lyme disease, caused by Borreliella (Borrelia) burgdorferi and transmitted by the bite of the ticks of the Ixodes ricinus complex, is the most common tickborne illness in the United States (US) and Europe. 1 , 2 Borreliella burgdorferi enters the skin at the site of the tick bite, typically resulting in the erythema migrans (EM) skin lesion. From the inoculation site, the organism can disseminate and affect the skin, heart, joints, and nervous system. Borreliella burgdorferi causes most human disease in the US, while B. afzelli and B. garinii are the most common culprits in Europe. 3 Variations in the clinical manifestations of Lyme disease between the US and Europe are associated with differences in the Borreliella species causing the infection. 4
Lyme neuroborreliosis (LNB) occurs in about 15% of untreated patients in the US, 5 and it is the most frequent extracutaneous manifestation of Lyme disease in Europe. 6 , 7 LNB is divided between early and late manifestations (duration of signs and symptoms for more than 6 months), as well as between central and peripheral nervous system manifestations. 8 The most common manifestations of early (or acute) LNB are cranial neuropathy (particularly facial palsy), lymphocytic meningitis, and radiculoneuritis. Peripheral facial palsy is the most common presentation in the US, while adult patients with early LNB acquired in Europe (mostly associated with B. garinii infection) usually present with Bannwarth syndrome, a subacute meningoradiculoneuritis characterized by painful radiculitis and lymphocytic pleocytosis, sometimes accompanied by cranial nerve involvement (most often peripheral facial palsy). 7 , 9 , 10 , 11 , 12
Despite LNB being a relatively frequent manifestation of Lyme disease in the US, there have been few published studies describing the course and outcome in adults since the early descriptive series. 13 , 14 , 15 , 16 , 17 , 18 , 19 Moreover, two recent studies of US patients with facial palsy due to LNB raised concern that adjunctive corticosteroid treatment may lead to a worse outcome. 20 , 21 The aim of this study is to describe the clinical features, laboratory data, treatment, disease course, and outcome of a cohort of US patients with early LNB presenting with facial palsy; and to determine if treatment with corticosteroids in addition to antibiotic therapy was associated with unfavorable outcome when compared with only antibiotics therapy.
Methods
Patients
We performed a retrospective analysis of participants enrolled in clinical studies investigating Lyme disease (ClinicalTrials.gov identifiers NCT00028080 and NCT00001539) from 1997 to 2017. The studies were approved by the institutional review board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Written informed consent was obtained from all participants. NCT00028080 is a natural history study of patients with a recent diagnosis of Lyme disease. Patients are evaluated at the following approximate time points: baseline; 1, 3, 6, and 12 months; and yearly thereafter. NCT00001539 is a cohort study of patients with post‐treatment Lyme disease syndrome and include patients diagnosed with confirmed or probable Lyme disease, who have received recommended antibiotic therapy and have persistent or relapsing symptoms and/or signs for at least 6 months after therapy. Patients were identified through an electronic search using a search tool and data repository of medical records at the National Institutes of Health Clinical Center and intramural National Institute of Allergy and Infectious Diseases. All patients with a diagnosis of Lyme disease evaluated under the two clinical studies were included in the search. Patients were included in the study if they had a diagnosis of facial palsy associated with Lyme disease. All patients fulfilled the US Center for Diseases Control and Prevention 2017 Lyme Disease case definition. 22 Detailed information regarding the Lyme disease history, treatment, and outcome status were retrieved by medical record review. The House–Brackmann scale 23 was used to quantify the facial nerve dysfunction.
Laboratory evaluation
Standard cerebrospinal fluid (CSF) analyses included cell counts, total protein, glucose, immunoglobulin (Ig) G, and albumin. Borreliella burgdorferi polymerase chain reaction (PCR) targeting the 16sRNA gene 24 was used to tests CSF samples. Measurement of intrathecal production of B. burgdorferi‐specific antibodies on paired CSF and serum specimens was performed at Stony Brook Lyme Disease laboratory and/or at Imugen. At Stony Brook, samples were analyzed by a polyvalent enzyme‐linked immunosorbent assay (ELISA) against B. burgdorferi whole cell sonicate. CSF and serum samples were diluted to equivalent IgG concentration and the ELISA optical density used to determine the B. burgdorferi CSF/serum antibody index (AI). At Imugen, serum and CSF samples were analyzed using antibody capture enzyme immunoassays (EIAs) for IgM, IgG, and IgA to B. burgdorferi. 25
Statistical analyses
Counts and percentages were used to summarize the associated manifestations of Lyme disease in patients with facial palsy. Comparisons between participants with unilateral and bilateral facial palsy and treatment, as well as between treatment and achievement of complete recovery were performed through Fisher's exact test. Comparisons in time to complete recovery were performed through a Cox proportional hazards model. Given there were participants with multiple measurements due to bilateral facial palsy, the standard error of the hazard ratio statistic and associated confidence intervals were estimated via bootstrap with 10,000 samples.
Results
Study cohort
From 486 patients, 44 were identified as having facial palsy associated with LNB. Most patients were adults, and the median age was 43 years. There were two teenagers. Of the 44 patients, 26 (59%) were male. Most patients acquired the infection in Maryland (30) and Virginia (8). Thirty‐two patients were evaluated under protocol NCT00028080. Most of these patients (22) were seen within the first 21 days of the facial palsy onset. One patient was seen 4 weeks after start of facial palsy, but within a week of starting antibiotic therapy. Three patients were seen at 5, 7, and 9 weeks from the start of facial palsy, but within 4 weeks of the completion of antibiotic therapy. Two other patients were first seen 3 months from the start of facial palsy for evaluation of new symptoms. Four patients were first evaluated after 6 months from the start of the facial palsy, of those two were evaluated for late LNB, one for Lyme arthritis and one for a second episode of Lyme disease. There were 12 patients with post‐treatment Lyme disease syndrome evaluated under NCT00001539. From these 12 patients, 5 were seen within the first year and 5 were seen in the 2nd year from the start of the facial palsy. Two patients had facial palsy 4 and 8 years before their evaluation.
Associated manifestations of Lyme disease
Only six patients had a history of a recognized tick bite. A history of EM was present in 29 patients (Table 1). A single EM was present in 12 patients, a single EM followed by multiple EM lesions occurred in 13 patients, while four patients presented with multiple EM without a recognized single EM lesion. A non‐specific febrile illness in the summer (a “summer flu”), characterized by fever, chills, headache, fatigue or malaise, neck and back pain or stiffness, myalgias, and arthralgias was the initial presentation of 15 patients without EM. The median time between the start of illness and the development of facial palsy was 19.5 days (range 9–64 days). Patients who presented with EM also frequently presented with systemic symptoms (16/29, 55%) as part of the initial presentation, with the most common symptoms being fever, headache, and fatigue. Of the 25 patients who presented with an initial single EM, the rash preceded the facial palsy by a median of 22 days, while patients without a history of EM had a median interval of 19.5 days between the start of illness and development of facial palsy. Most patients presented with their initial illness in June and developed facial palsy in July (Fig. 1).
Table 1.
Associated manifestations of Lyme disease in patients with facial palsy.
| No. (%) | |
|---|---|
| Presentation | |
| Erythema migrans (EM), all | 29 (66) |
| Single EM | 12 (27) |
| Single EM followed by multiple EM | 13 (30) |
| Multiple EM | 4 (9) |
| Non‐specific febrile illness with no recognized EM | 15 (34) |
| Other symptoms and signs | |
| Fatigue | 34 (77) |
| Fever and/or chills | 31 (70) |
| Headache | 28 (64) |
| Myalgias | 23 (52) |
| Arthralgias | 22 (50) |
| Neck pain or stiffness | 18 (41) |
| Radicular pain | 14 (32) |
Figure 1.
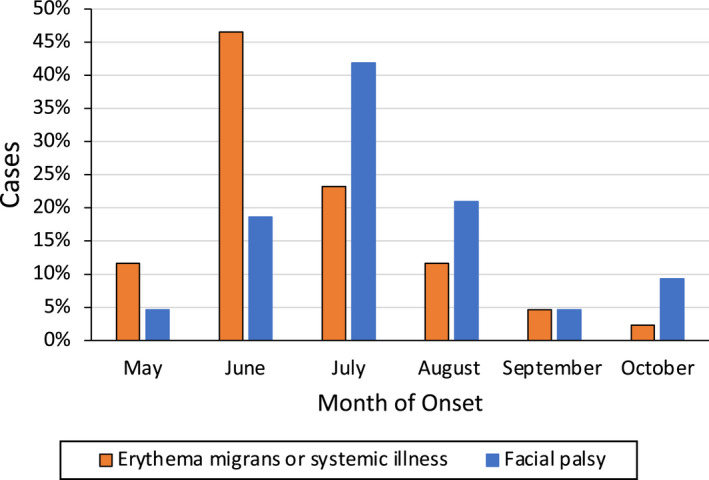
Months of onset of illness and facial palsy from Lyme disease.
Facial nerve palsy characteristics
The facial palsy was unilateral in 31 patients, with the right and left sides of the face being affected in similar proportions. Thirteen patients presented with bilateral facial palsy, with 10 presenting initially with unilateral involvement which was followed by palsy of the contralateral facial nerve a median of 6.5 days later (range 2–12 days). Interestingly, while unilateral facial palsy occurred equally in males and females, bilateral palsy occurred primarily in males (77% of patients with bilateral facial palsy). Bannwarth syndrome (subacute painful meningoradiculitis) occurred in 4 patients, while another 10 patients had a compatible history, but no cerebrospinal fluid evaluation was performed at the time.
Laboratory evaluation
Of the 39 patients who had results from serological evaluation at the time of the facial palsy available for review, all were positive by first‐tier test. Only 33 of these 39 patients had also immunoblot results available for review, and 31 of these 33 were positive following the US Center for Disease Control and Prevention two‐tier criteria. 26 The two other patients were positive by a first‐tier test and IgM immunoblot criteria but did not fulfill the IgG immunoblot criteria, with disease duration longer than 30 days (both had 4 signature IgG bands). Of the 23 patients who had CSF evaluation available, 17 had a white cell count above 5 cells/mm3, with a median of 28 cells (range 7–149), predominantly lymphocytes. CSF protein levels were above 45 mg/dL in fourteen patients, with a median value of 82 mg/dL (range 49–223). Borreliella burgdorferi PCR was negative in all 15 samples tested at the National Institutes of Health Clinical Center and 3 of 3 samples tested at outside commercial laboratories. Fifteen patients had intrathecal AI measured, with 5 tested only by antibody capture EIA, 1 tested only by ELISA, and 9 tested by both methods. Of the 14 samples tested by antibody capture EIA, 6 had positive AI (above 1.5), 6 had borderline AI results (1–1.5), and 2 were negative (AI < 1). Of the 10 samples tested by ELISA, the AI was positive in 1, borderline in 3, and negative in 6. One sample was tested only by ELISA and had a borderline AI. For the 9 samples tested by both methods, and considering positive and borderline results together, 4 results were concordant (3 positive and 1 negative in both tests) and 5 were discordant. All discordant results had positive or borderline results by antibody capture EIA and negative results by ELISA. At an AI cutoff >1.5 or ≥1, the antibody capture assay had a sensitivity of 43% (95% CI 19–70) and 86% (95% CI 56–98), respectively, while the ELISA method sensitivity was 10% (95% CI 0.6–49) and 33% (95% CI 9–69). Overall, of the 15 samples tested, 6 had AI index above 1.5 (40% sensitivity) and 13 had a borderline or positive result (87% sensitivity).
Treatment and outcome
Of the 44 patients, the palsy resolved before any therapy in two patients, and with corticosteroids alone in another patient. These patients were treated with antibiotic therapy after the resolution of the facial palsy. Of the 41 patients who were treated with antibiotics before the resolution of the palsy, 15 were treated with antibiotics alone and 26 received both antibiotics and corticosteroids. There was no relationship between having unilateral or bilateral palsies and receiving antibiotics or both antibiotics and corticosteroids (two‐tailed p = 0.43, Fisher's exact Test). Patients were most often treated with doxycycline and ceftriaxone, and the median duration of antibiotic therapy was 32 days. The median duration of steroid therapy was 9 days.
The outcome at 12 months was known for 38 patients. Two patients with unilateral facial palsy had <4 weeks of follow‐up. One patient with bilateral facial palsy had completely recovered on one side, and nearly complete recovery of the other side at his last assessment at 4 weeks. Of the 38 patients, 34 recovered completely and 3 had nearly complete recovery. Only one patient had moderate residual deficit, with a House–Brackmann score of 3 (Table 2). There were no differences in the treatment groups (antibiotics or antibiotics and corticosteroids) in achieving complete resolution of the palsy at 12 months (p = 1, Fisher's exact test). All patients with bilateral facial palsy had a full recovery.
Table 2.
Outcome of facial palsy from Lyme disease.
| Treatment | HB = 1 | HB = 2 or 3 |
|---|---|---|
| Antibiotic | 13 | 1 |
| Antibiotic and corticosteroid | 21 | 3 |
There were no differences in outcome at 12 months between the treatment groups (p = 1, Fisher's exact test). HB, House–Brackmann grade.
For 31 patients (9 patients with bilateral and 22 with unilateral facial palsy) and 39 palsies, we had detailed information regarding the interval from start to resolution. One patient with bilateral facial palsy had complete recovery of one side at 4 weeks but did not return for further follow‐up, and therefore only one side is included in the analysis. Of these 31 patients, 11 were treated with antibiotics alone, while 20 received both antibiotics and corticosteroids. Of the 38 paralyzes with House–Brackmann scores of 1 and 2 and documented time to recovery, the median interval to recovery was 2 months (range 0.5–6). We next examined if there was a significant difference noted in the time to complete recovery (House–Brackmann scores of 1) between patients who received corticosteroids plus antibiotics versus antibiotics alone using the proportional hazards model of Cox with variance estimates obtained via bootstrap (10,000 samples) to test the between groups difference. The participants who did not recover (House–Brackmann scores of 2 and 3) were censored at 12 months. There were no differences between the two treatment groups (corticosteroids plus antibiotics over antibiotics alone) regarding the time to recovery when analyzed from either the start of the facial palsy (hazard ratio 0.617, 95% confidence interval 0.259–1.677, p = 0.31) (Fig. 2A) or start of antibiotic therapy (hazard ratio 0.656, 95% confidence interval 0.294–1.644, p = 0.336) (Fig. 2B). Using the same strategy, we found the time to recovery from the start of facial palsy to be similar for patients with unilateral and bilateral involvement (bilateral over unilateral involvement hazard ratio 1.246, 95% confidence interval 0.647–2.424, p = 0.514) (Fig. 3).
Figure 2.
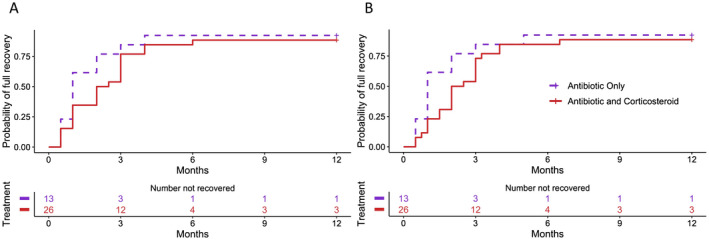
Time to recovery of facial palsy from Lyme neuroborreliosis by treatment group. (A) Time to recovery from start of facial palsy. (B) Time to recovery from start of antibiotic therapy. Patients with House–Brackmann scores of 1 were considered recovered. Participants who did not fully recovered (House−Brackmann score of 2 and 3) were censored at 12 months. Analysis using the Cox proportional hazards model showed no differences between the two groups.
Figure 3.
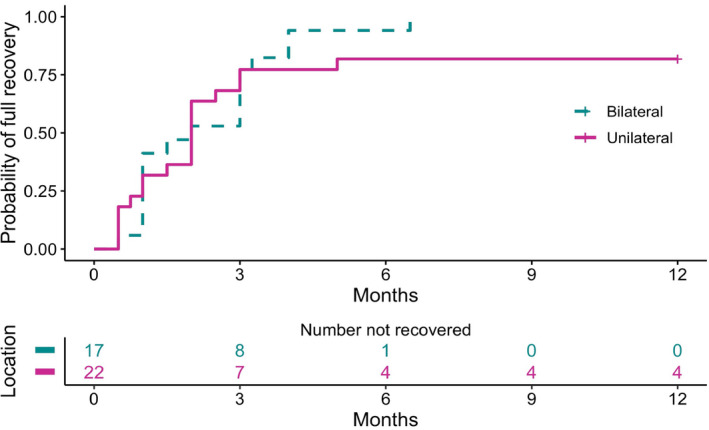
Time to recovery of patients with unilateral and bilateral facial palsy from Lyme neuroborreliosis. Time to recovery is shown from the start of facial palsy. Patients with House–Brackmann scores of 1 were considered recovered. Participants who did not fully recovered (House–Brackmann score of 2 and 3) were censored at 12 months. Analysis using the Cox proportional hazards model showed no differences between the two groups.
Discussion
Facial palsy is the most common manifestation of early LNB in the US, but there are few studies describing the clinical features and outcome in adult patients. Our study adds new evidence to the field and brings to attention important points for the recognition of early LNB in the US. Early diagnosis and treatment are important to prevent complications of the infection. One of the main issues is to distinguish facial palsy due to LNB from Bell's palsy, the most common cause of unilateral facial nerve palsy and thought to be associated with herpes simplex reactivation. As shown in our and other studies, 6 , 7 , 11 , 12 , 16 , 19 , 27 , 28 , 29 , 30 the history and clinical picture will often give clues pointing toward the diagnosis of LNB. Most cases occur in the summer months. Important points included a history or presence of a skin lesion compatible with EM, which occurred in 66% of our patients. Constitutional symptoms (particularly fatigue, headache, fever, chills, stiff neck, and arthralgias) were very common, present in 70% of the cases, and a substantial number of patients presented only with a “summer flu.” The median interval between the first symptoms or signs to the facial palsy was 19.5 days in our study, which is consistent with previous studies. 16 , 27 , 30 As with other manifestations of Lyme disease in the US, the majority of our patients did not recall a tick bite. These clues (exposure to ticks in an endemic area, but not necessarily a history of recognized tick bite, constitutional symptoms and/or skin lesions, and presentation in the summer months) in a patient presenting with facial palsy should bring early LNB to the forefront of the clinician's differential diagnosis.
Similar to an earlier study, 16 bilateral facial palsy accounted for 30% of the cases and predominantly occurred in males. Bilateral involvement usually occurred consecutively, with an interval of 6.5 days apart, analogous to the interval reported in a study from Sweden. 31 It has been reported that US adult patients with early LNB present less frequently with severe radicular pain. 19 Our study agrees with this assessment, with 32% of our patients presenting with radicular pain. In comparison, in a study of 194 adult patients diagnosed with LNB in Denmark from 2015 to 2017, 70% of the patients had radicular pain and 43% had facial nerve palsy. 7 Interestingly, the prevalence of radicular pain in our study was similar to studies assessing patients with LNB‐associated facial palsy in Slovenia 30 and Poland 32 ; radicular pain was present in 13 of 64 (20%) and 13 of 38 patients (34%), respectively.
Detection of B. burgdorferi DNA in CSF was negative in 18 of the 18 patients tested. These results are expected, as current assays for direct detection of B. burgdorferi have very low sensitivity in CSF samples and these assays are not recommended for the diagnosis of LNB in the US. 8 New methods and strategies that would increase sensitivity of direct assays are urgently needed. 33 Currently, the detection of intrathecally produced B. burgdorferi‐specific antibodies using the CSF/serum AI is recommended to diagnose LNB in both the US and Europe, 8 , 34 , 35 if CSF testing is performed. Most studies supporting the use of intrathecal AI index are from Europe, where the sensitivity of tests range from 42% to 91%. 7 , 11 , 12 , 29 , 30 , 32 , 36 The impression based on the few early US studies is that a positive intrathecal AI is less common in the US. 14 , 17 , 25 , 37 Our results, while evaluated in only 15 patients, shows an overall sensitivity of 40% when using a cutoff >1.5, increased to 87% when using a cutoff value ≥1.0. Although we compare the two assays in only 10 patients, the antibody capture assay was more sensitive than the ELISA methodology. Only 4 of 10 samples (40%) tested by ELISA had an intrathecal AI result of ≥1. Moreover, 5 samples that were negative by ELISA had intrathecal AI results ≥1.0 using the antibody capture assay. The difference in sensitivity between the two assays is likely to explain the lower sensitivity reported in a recent study of 19 LNB patients from the US. 38 This study, which used the ELISA assay to measure the intrathecal AI index, had 42% positivity using a cutoff value ≥1.0. These results highlight differences between test results based on methods, assays and interpretation 10 , 39 , 40 and the acute need for efforts to compare and standardize test methodologies. A major issue with all the current available methodologies for intrathecal AI testing in the US is the relatively large amount of CSF required for testing, which significantly limits the possibility of comparative studies.
Notably, all of our patients were seropositive for B. burgdorferi antibodies when tested by a first‐tier test, and the majority was also positive by the standard two‐tier testing criteria. 26 This shows that the current two‐tier algorithm performs relatively well in this situation. However, due to the relatively long turnaround time for these tests, a point‐of‐care test based on the modified or alternative two‐EIA approach would be particularly helpful in patients with manifestations of early disseminated Lyme borreliosis, like our patients with facial palsy, as well as patients with carditis, and children with Lyme arthritis. 41
Overall, we have shown that the outcome of facial palsy in patients with early LNB is excellent, with most patients having complete (88%) or almost complete (9.5%) recovery. Only one patient had a more severe residual deficit, with moderate dysfunction. These results are similar to previous reports, 16 , 18 but differ from two recent studies, where corticosteroids, in addition to antibiotic therapy, could have a deleterious effect in palsy from Lyme disease. 20 , 21 This is a serious concern, as most cases of peripheral facial nerve palsy in adults are classified as idiopathic (Bell's palsy) and randomized trials have shown a significant benefit from treating idiopathic facial nerve palsy with short course of corticosteroids treatment, with the aim of reducing inflammation and limiting nerve damage. A short course of corticosteroid treatment (with or without antiviral treatment), initiated within 72 h after symptom onset is the recommended treatment for idiopathic facial palsy in adults. 42 In our cohort, we found no evidence that corticosteroids had a harmful effect, and there were no significant differences between patients who were treated with antibiotics alone or who had received antibiotics and corticosteroids in achieving complete recovery, or in the time to recovery from facial palsy. Similar results were seen in a prospective open trial of adjunctive corticosteroids in LNB peripheral facial palsy treated with doxycycline, which showed no change in outcome. 43
Our study has several limitations. The retrospective design is subject to bias with the potential for missing data as well as subjective recall bias from the patient. The timing of recovery was estimated based on chart review, and while most patients were followed prospectively, more frequent assessments until the resolution of the palsy would lead to more precise estimates of the recovery rate. While observational data can support inferences regarding treatment outcomes of different interventions, the effect of the addition of corticosteroids to antibiotic treatment of LNB peripheral facial palsy would ideally be studied in a randomized placebo‐controlled trial. Unfortunately, such a clinical trial would be logistically difficult to perform at least in the US, due to the relatively small number of cases presenting to most centers, requiring a large research network. We estimate that for a superiority trial designed to detect a hazard ratio of 0.656 for the group receiving antibiotic when compared with the group receiving both antibiotic and corticosteroids and 90% of participants achieving full recovery (as seen in our study), at the 5% significance level with 80% power, using a 1:1 randomization, would require 198 participants.
In summary, peripheral facial palsy is a frequent manifestation of early LNB in the US and LNB should be included in the differential of patients presenting with acute‐onset peripheral facial palsy. There are many clues from the illness history and the physical examination that are helpful in the diagnosis of LNB and should be actively sought when evaluating patients with peripheral facial palsy. LNB should always be included in the differential diagnosis of patients who present with bilateral facial nerve palsy, with either simultaneous or sequential nerve involvement. Treatment with antibiotic therapy is highly effective and most patients will recover facial nerve function fully, usually within 2 months. Adjunctive corticosteroid therapy does not appear to affect the speed of recovery or overall outcome. Most patients with LNB peripheral facial palsy are seropositive for B. burgdorferi antibody by EIA at presentation; a point of care test based on the modified two‐EIA approach would be a most welcome addition to the diagnostic armamentarium of early LNB patients and patients presenting with other manifestations of early disseminated Lyme disease.
Conflict of Interest
AM has a patent US 8,926,989; and is an unpaid Scientific Advisor to the Global Lyme Alliance and to the American Lyme Disease Foundation. All other authors: no disclosures relevant to the manuscript.
Authors' Contributions
A. Marques designed the study, contributed to data acquisition, data analysis and interpretation, and drafting of the manuscript. G. Okpali and K. Liepshutz contributed to data acquisition. A. M. Ortega‐Villa performed, and interpreted the data analysis. All authors assisted in revision of the manuscript and approved the final version for submission.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (A. Marques, G. Okpali, and A. M. Ortega‐Villa) and by the National Cancer Institute, National Institutes of Health Contract No. HHSN261200800001E (K. Liepshutz). Funding sources had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Funding Information
This research was supported by National Cancer Institute (grant/award number: HHSN261200800001E) and Division of Intramural Research, National Institute of Allergy and Infectious Diseases (grant/award number: AI000695).
Funding Statement
This work was funded by Division of Intramural Research, National Institute of Allergy and Infectious Diseases grant AI000695; National Cancer Institute grant HHSN261200800001E.
References
- 1. Kugeler KJ, Schwartz AM, Delorey MJ, Mead PS, Hinckley AF. Estimating the frequency of lyme disease diagnoses, United States, 2010‐2018. Emerg Infect Dis. 2021;27(2):616‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stanek G, Strle F. Lyme borreliosis‐from tick bite to diagnosis and treatment. FEMS Microbiol Rev. 2018;42(3):233‐258. [DOI] [PubMed] [Google Scholar]
- 3. Steere AC, Strle F, Wormser GP, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marques AR, Strle F, Wormser GP. Comparison of Lyme disease in the United States and Europe. Emerg Infect Dis. 2021;27(8):2017‐2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CDC . Lyme disease—relative frequency of clinical features among confirmed cases‐United States, 2008‐2019. 2021 [updated 05/03/2021]. Accessed June 24, 2021. https://www.cdc.gov/lyme/stats/graphs.html
- 6. Andreasen AM, Dehlendorff PB, Knudtzen FC, Bodker R, Kjaer LJ, Skarphedinsson S. Spatial and temporal patterns of Lyme neuroborreliosis on Funen, Denmark from 1995‐2014. Sci Rep. 2020;10(1):7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nordberg CL, Bodilsen J, Knudtzen FC, et al. Lyme neuroborreliosis in adults: a nationwide prospective cohort study. Ticks Tick Borne Dis. 2020;11(4):101411. [DOI] [PubMed] [Google Scholar]
- 8. Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America, American Academy of Neurology, and American College of Rheumatology: 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Neurology. 2021;96(6):262‐273. [DOI] [PubMed] [Google Scholar]
- 9. Coipan EC, Jahfari S, Fonville M, et al. Imbalanced presence of Borrelia burgdorferi s.l. multilocus sequence types in clinical manifestations of Lyme borreliosis. Infect Genet Evol. 2016;42:66‐76. [DOI] [PubMed] [Google Scholar]
- 10. Marques AR. Lyme neuroborreliosis. Continuum (Minneap Minn). 2015;21(6 Neuroinfectious Disease):1729‐1744. [DOI] [PubMed] [Google Scholar]
- 11. Ogrinc K, Lusa L, Lotric‐Furlan S, et al. Course and outcome of early European Lyme neuroborreliosis (Bannwarth syndrome): clinical and laboratory findings. Clin Infect Dis. 2016;63(3):346‐353. [DOI] [PubMed] [Google Scholar]
- 12. Knudtzen FC, Andersen NS, Jensen TG, Skarphedinsson S. Characteristics and clinical outcome of lyme neuroborreliosis in a high endemic area, 1995‐2014: a retrospective cohort study in Denmark. Clin Infect Dis. 2017;65(9):1489‐1495. [DOI] [PubMed] [Google Scholar]
- 13. Halperin J, Luft BJ, Volkman DJ, Dattwyler RJ. Lyme neuroborreliosis. Peripheral nervous system manifestations. Brain. 1990;113(Pt 4):1207‐1221. [DOI] [PubMed] [Google Scholar]
- 14. Halperin JJ, Golightly M. Lyme borreliosis in Bell's palsy. Long Island Neuroborreliosis Collaborative Study Group. Neurology. 1992;42(7):1268‐1270. [DOI] [PubMed] [Google Scholar]
- 15. Steere AC, Pachner AR, Malawista SE. Neurologic abnormalities of Lyme disease: successful treatment with high‐dose intravenous penicillin. Ann Intern Med. 1983;99(6):767‐772. [DOI] [PubMed] [Google Scholar]
- 16. Clark JR, Carlson RD, Sasaki CT, Pachner AR, Steere AC. Facial paralysis in Lyme disease. Laryngoscope. 1985;95(11):1341‐1345. [PubMed] [Google Scholar]
- 17. Smouha EE, Coyle PK, Shukri S. Facial nerve palsy in Lyme disease: evaluation of clinical diagnostic criteria. Am J Otol. 1997;18(2):257‐261. [PubMed] [Google Scholar]
- 18. Halperin JJ. Facial nerve palsy associated with Lyme disease. Muscle Nerve. 2003;28(4):516‐517. [DOI] [PubMed] [Google Scholar]
- 19. Pachner AR, Steere AC. The triad of neurologic manifestations of Lyme disease: meningitis, cranial neuritis, and radiculoneuritis. Neurology. 1985;35(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 20. Wormser GP, McKenna D, Scavarda C, Karmen C. Outcome of facial palsy from Lyme disease in prospectively followed patients who had received corticosteroids. Diagn Microbiol Infect Dis. 2018;91(4):336‐338. [DOI] [PubMed] [Google Scholar]
- 21. Jowett N, Gaudin RA, Banks CA, Hadlock TA. Steroid use in Lyme disease‐associated facial palsy is associated with worse long‐term outcomes. Laryngoscope. 2017;127(6):1451‐1458. [DOI] [PubMed] [Google Scholar]
- 22. CDC . Lyme disease (Borrelia burgdorferi) 2017 case definition. 2017. [updated 04/16/2021]. Accessed June 24, 2021. https://ndc.services.cdc.gov/case‐definitions/lyme‐disease‐2017/
- 23. Vrabec JT, Backous DD, Djalilian HR, et al. Facial nerve grading system 2.0. Otolaryngol Head Neck Surg. 2009;140(4):445‐450. [DOI] [PubMed] [Google Scholar]
- 24. Marques AR, Weir SC, Fahle GA, Fischer SH. Lack of evidence of Borrelia involvement in Alzheimer's disease. J Infect Dis. 2000;182(3):1006‐1007. [DOI] [PubMed] [Google Scholar]
- 25. Steere AC, Berardi VP, Weeks KE, Logigian EL, Ackermann R. Evaluation of the intrathecal antibody response to Borrelia burgdorferi as a diagnostic test for Lyme neuroborreliosis. J Infect Dis. 1990;161(6):1203‐1209. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease C, Prevention . Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44(31):590‐591. [PubMed] [Google Scholar]
- 27. Peltomaa M, McHugh G, Steere AC. The VlsE (IR6) peptide ELISA in the serodiagnosis of lyme facial paralysis. Otol Neurotol. 2004;25(5):838‐841. [DOI] [PubMed] [Google Scholar]
- 28. Bremell D, Hagberg L. Clinical characteristics and cerebrospinal fluid parameters in patients with peripheral facial palsy caused by Lyme neuroborreliosis compared with facial palsy of unknown origin (Bell's palsy). BMC Infect Dis. 2011;10(11):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bierman SM, van Kooten B, Vermeeren YM, et al. Incidence and characteristics of Lyme neuroborreliosis in adult patients with facial palsy in an endemic area in The Netherlands. Epidemiol Infect. 2019;147:e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rojko T, Bogovic P, Lotric‐Furlan S, et al. Borrelia burgdorferi sensu lato infection in patients with peripheral facial palsy. Ticks Tick Borne Dis. 2019;10(2):398‐406. [DOI] [PubMed] [Google Scholar]
- 31. Dotevall L, Hagberg L. Successful oral doxycycline treatment of Lyme disease‐associated facial palsy and meningitis. Clin Infect Dis. 1999;28(3):569‐574. [DOI] [PubMed] [Google Scholar]
- 32. Moniuszko‐Malinowska A, Guziejko K, Czarnowska A, et al. Assessment of anti‐HSV antibodies in patients with facial palsy in the course of neuroborreliosis. Int J Clin Pract. 2021;75(3):e13749. [DOI] [PubMed] [Google Scholar]
- 33. Schutzer SE, Body BA, Boyle J, et al. Direct diagnostic tests for Lyme disease. Clin Infect Dis. 2019;68(6):1052‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rauer S, Kastenbauer S, Hofmann H, et al. Guidelines for diagnosis and treatment in neurology ‐ Lyme neuroborreliosis. Ger Med Sci. 2020;18:Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Figoni J, Chirouze C, Hansmann Y, et al. Lyme borreliosis and other tick‐borne diseases. Guidelines from the French Scientific Societies (I): prevention, epidemiology, diagnosis. Med Mal Infect. 2019;49(5):318‐334. [DOI] [PubMed] [Google Scholar]
- 36. Djukic M, Schmidt‐Samoa C, Lange P, et al. Cerebrospinal fluid findings in adults with acute Lyme neuroborreliosis. J Neurol. 2012;259(4):630‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med. 1990;323(21):1438‐1444. [DOI] [PubMed] [Google Scholar]
- 38. Eckman EA, Clausen DM, Herdt AR, Pacheco‐Quinto J, Halperin JJ. Specificity and diagnostic utility of cerebrospinal fluid CXCL13 in Lyme neuroborreliosis. Clin Infect Dis. 2021;72(10):1719‐1726. [DOI] [PubMed] [Google Scholar]
- 39. Theel ES, Aguero‐Rosenfeld ME, Pritt B, Adem PV, Wormser GP. Limitations and confusing aspects of diagnostic testing for neurologic Lyme disease in the United States. J Clin Microbiol. 2019;57(1):e01406‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wutte N, Archelos J, Crowe BA, et al. Laboratory diagnosis of Lyme neuroborreliosis is influenced by the test used: comparison of two ELISAs, immunoblot and CXCL13 testing. J Neurol Sci. 2014;347(1–2):96‐103. [DOI] [PubMed] [Google Scholar]
- 41. Marques AR. Revisiting the Lyme disease serodiagnostic algorithm: the momentum gathers. J Clin Microbiol. 2018;56(8):e00749‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gronseth GS, Paduga R, American Academy of N . Evidence‐based guideline update: steroids and antivirals for Bell palsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2012;79(22):2209‐2213. [DOI] [PubMed] [Google Scholar]
- 43. Avellan S, Bremell D. Adjunctive corticosteroids for Lyme neuroborreliosis peripheral facial palsy – a prospective study with historical controls. Clin Infect Dis. 2021;73(7):1211‐1215. [DOI] [PubMed] [Google Scholar]


