Abstract
Objective
This study aimed to compare effects of cerebral small‐vessel disease (cSVD) burden and cerebral artery stenosis (CAS) on acute ischemia in intracerebral hemorrhage (ICH) and their interaction with mean arterial pressure (MAP) change.
Methods
We recruited consecutive patients with acute primary ICH. Brain magnetic resonance imaging and angiography were performed to quantify diffusion‐weighted imaging (DWI) lesions, CAS, and cSVD markers, which were calculated for the total cSVD score. Multivariable regression models were adopted to explore their associations by DWI lesions size (<15 vs. ≥15 mm) and median MAP change stratification.
Results
Of 305 included patients (mean age 59.5 years, 67.9% males), 77 (25.2%) had DWI lesions (small, 79.2%; large, 20.8%) and 67 (22.0%) had moderate and severe CAS. In multivariable analysis, small DWI lesions were independently associated with higher total cSVD score (odds ratio [OR] 1.81, 95% confidence interval [CI] 1.36–2.41). and large DWI lesions were associated with more severe CAS (OR 2.51, 95% CI 1.17–5.38). This association was modified by MAP change (interaction p = 0.016), with stratified analysis showing an increased risk of large DWI lesions in severe CAS with greater MAP change (≥44 mmHg) (OR 3.48, 95% CI 1.13–10.74) but not with mild MAP change (<44 mmHg) (OR 1.21, 95% CI 0.20–7.34).
Interpretation
Total cSVD burden is associated with small DWI lesions, whereas the degree of CAS is associated with large DWI lesions, specifically with greater MAP change, suggesting that large‐artery atherosclerosis may be involved in ischemic brain injury, which is different from small‐vessel pathogenesis in ICH.
Introduction
Primary intracerebral hemorrhage (ICH) is the second leading cause of death and disability in cases of stroke worldwide. The frustrating outcomes in patients with ICH highlight the need to better explore its potentially pathophysiological mechanisms. The development of magnetic resonance imaging (MRI) technology has provided the most potent approach to understanding the etiology of hemorrhage. Diffusion‐weighted imaging (DWI) hyperintensities distant from the hematoma were considered to be acute ischemic lesions, 1 , 2 , 3 , 4 , 5 which were identified in 11–41% of patients with acute ICH. 2 , 4 , 6 , 7 , 8 They further aggravated brain injury. Most of these lesions were small, punctate, and asymptomatic, while patchy or medium to large lesions were often ignored. DWI lesions were not only associated with poor prognosis 6 , 7 , 9 , 10 but also increased the risk of recurrent stroke. 5 , 11 , 12 , 13 However, the underlying pathogenesis remains unclear. Most studies have associated DWI lesions with MRI markers of cerebral small‐vessel disease (cSVD) 6 , 14 , 15 , 16 , 17 ; some have associated them with blood pressure (BP) reduction, 2 , 7 , 18 while others have argued against any correlation with BP reduction. 9 , 19 , 20 , 21 However, to date, no study has combined magnetic resonance angiography (MRA) to evaluate DWI lesions in patients with ICH.
Hypertension and age are the most common risk factors for primary ICH. Long‐term hypertension not only causes microangiopathies, such as cerebral microbleeds (CMBs), white matter hyperintensities (WMHs), and lacunes, but also induces large‐artery atherosclerotic stenosis which may be subclinical before ICH. Large‐vessel pathologies also exist in patients with ICH. 22 , 23 However, few studies have comparatively analyzed the impact of small‐vessel disease and large‐artery atherosclerosis on patients with acute ICH. Whether the magnitude of BP change could influence this association needs to be further explored.
Therefore, we conducted a prospective study to explore the association of DWI lesions with total cSVD burden, degree of cerebral artery stenosis (CAS), and their interaction with mean arterial pressure (MAP) change, further elucidating the impact of microangiopathy and macroangiopathy on patients with acute ICH.
Methods
Study population
We performed a prospective observational study in patients with acute primary ICH from January 2017 to May 2021. Patients with acute ICH were consecutively enrolled from the stroke center of Zhengzhou People's Hospital. The inclusion criteria were: (1) age ≥ 18 years; (2) ICH diagnosed by CT scan; (3) admitted to hospital within 24 hours of symptom onset; and (4) MRI scan was taken at 28 days after admission. The exclusion criteria were as follows: (1) isolated intraventricular hemorrhage; (2) secondary causes of ICH, such as hemorrhagic infarct, cerebral venous thrombosis, aneurysm, arteriovenous malformations, moyamoya disease, or brain tumor hemorrhage; (3) patients with incomplete clinical or MRI data. The study was approved by the Ethics Committee of Zhengzhou People's Hospital and was in line with Helsinki guidelines. Informed consent was obtained from each patient or their legal surrogate.
Data collection
Patient data, including demographics, vascular risk factors (smoking, hypertension, diabetes mellitus, prior ischemic stroke, coronary artery disease, atrial fibrillation, and prior ICH), previous medication (antihypertensive drugs, antiplatelet drugs, anticoagulant drugs, statins), complications (deep venous thrombosis, pneumonia, stress ulcer), time to MRI scan, in‐hospital intravenous antihypertensive treatment, laboratory tests, National Institute of Health Stroke Scale (NIHSS) score, NIHSS change from admission to discharge, initial Glasgow Coma Scale (GCS) score, initial BP in the emergency department, and the highest and lowest BPs prior to MRI were collected. Delta MAP was calculated as the difference between the highest and lowest MAP prior to MRI. 2 , 7 The likely etiologies of ICH were determined to be hypertensive angiopathy, probable erebral amyloid angiopathy (CAA) according to classic Boston criteria, 24 and anticoagulant‐associated or undetermined cause.
Neuroimaging measurements
All patients underwent CT on admission and MRI on 1.5 or 3.0 T MR scanners within 28 days after admission. Imaging sequences included DWI with apparent diffusion coefficient (ADC) map, T1‐weighted, T2‐weighted, fluid‐attenuated inversion recovery (FLAIR), susceptibility‐weighted imaging (SWI), and MRA. Imaging parameters are shown in Data S1. DWI lesions were rated by a trained neurologist (L.T.) with >5 years' experience in neuroimaging review. CT, MRI, and MRA were assessed by two trained neuroradiologists (Y.S., P.Q.) blinded to the clinical information, and finally reached a consensus.
Assessment of cSVD burden
The imaging markers of cSVD included WMHs, enlarged perivascular spaces (EPVS), CMBs, and lacunes, which were evaluated according to STRIVE. 25 The severity of WMHs was quantified using the Fazekas scale 26 for periventricular white matter hyperintensities (PWMHs) and deep white matter hyperintensities (DWMHs) (range, 0–3); EPVS were quantified in the basal ganglia (BG) and centrum semiovale (CSO) regions (0 = no EPVS; 1 = 1–10 EPVS; 2 = 11–20 EPVS; 3 = 21–40 EPVS; 4 ≥ 40 EPVS) 20 ; CMBs were rated on SWI sequences as rounded or circular small hypointense foci with a diameter of 2–5 mm; lacunes were rated on FLAIR sequences as small, ovoid, fluid‐filled cavities with a diameter of 3–15 mm. The cSVD burden was estimated by calculating the total cSVD score, where a point was awarded to each of the following (range, 0–4): (1) presence of lacunes; (2) presence of CMBs; (3) BG‐EPVS >10; and (4) PWMHs with Fazekas score of 3 and/or DWMHs with Fazekas score of 2–3.
Assessment of CAS
The degree of CAS was evaluated on MRA according to WASID 27 criteria including the intracranial segment of the internal carotid artery (ICA), A1 and A2 segment of anterior cerebral artery (ACA), M1 and M2 segment of middle cerebral artery (MCA), P1 and P2 segment of posterior cerebral artery (PCA), vertebral artery, and basilar artery (BA). Patients were divided into three groups: mild stenosis group (<50%), moderate stenosis group (50–69%), and severe stenosis group (≥70%).
Assessment of DWI lesions
DWI lesions were defined as high‐signal intensity lesions on DWI accompanied by low‐signal intensity on ADC maps. DWI with high‐signal intensity lesions in close (<10 mm) to the hematoma were excluded. 7 The size, number, location, laterality relative to hematoma, and artery territories distribution of the DWI lesions were recorded. Small DWI lesions were defined as the maximum diameter of lesions <15 mm, and large DWI lesions were defined as the maximum diameter of lesions ≥15 mm.
Statistical analysis
Continuous variables with normal distributions were presented as means with standard deviations and were compared using the Student's t‐test. Variables with non‐normal distributions were presented as medians with interquartile ranges (IQR) and were compared using the Mann–Whitney U‐test. Categorical variables were described as numbers with percentages and were analyzed using χ 2 or Fisher's exact test. Patients were stratified by the size of DWI lesions (<15 vs. ≥15 mm) and median MAP change (<44 vs. ≥44 mmHg), respectively. The associations between DWI lesions and total cSVD burden, degree of CAS, and the interaction with MAP fluctuation were investigated using multivariable logistic regression and stratified analysis after adjusting for clinically relevant variables and statically significant covariates (univariate variables with value of p < 0.10). The result was presented as odds ratio (OR) and 95% confidence interval (CI). We examined the multicollinearity and interaction effects of the covariates. Two‐tailed p < 0.05 were considered statistically significant. All analyses were performed using SPSS (version 25.0; IBM Statistics, Armonk, NY, USA).
Results
Clinical and imaging characteristics
A total of 305 patients with acute ICH were finally included in this study. The flowchart of patients selection is presented in Figure 1. The mean age of these patients was 59.5 ± 12.6 years and 67.9% were male. Of the 305 patients, 111 (36.4%) patients were given intravenous antihypertensive treatment in‐hospital; of them, urapidil was administered in 80 cases (72.1%), nicardipine in 25 cases (22.5%), nimodipine in 10 cases (9.0%), and nitroprusside in 3 cases (2.7%). Table 1 shows the clinical and imaging characteristics of all patients and subgroups without DWI lesions, with small DWI lesions, and with large DWI lesions. Compared to patients without DWI lesions, patients with small DWI lesions were more likely to have greater MAP change (48 vs. 43 mmHg, p = 0.019), higher initial systolic blood pressure (SBP) (174 vs. 166 mmHg, p = 0.017), higher hemoglobin A1c (5.8% vs. 5.5%, p = 0.025), higher rates of deep venous thrombosis (11.5% vs. 3.5%, p = 0.030), and higher total cSVD score (3 vs. 1, p < 0.001). Each marker of cSVD was significantly different in patients with small DWI lesions versus without DWI lesions, but the distribution of moderate to severe CAS did not differ. Patients with large DWI lesions were more likely to have a history of ischemic stroke (50.0% vs. 23.7%, p = 0.041), greater MAP change (52 vs. 43 mmHg, p = 0.008), higher initial SBP (185 vs. 166 mmHg, p = 0.018), higher rates of deep venous thrombosis (18.8% vs. 3.5%, p = 0.028), and more moderate to severe stenosis of ICA (56.3% vs. 3.1%, p < 0.001), MCA (50.0% vs. 10.1%, p < 0.001), and PCA (25.0% vs. 7.5%, p = 0.041) than patients without DWI lesions. No significant differences were observed in each marker of cSVD and total cSVD score in patients with large DWI lesions versus without DWI lesions.
Figure 1.
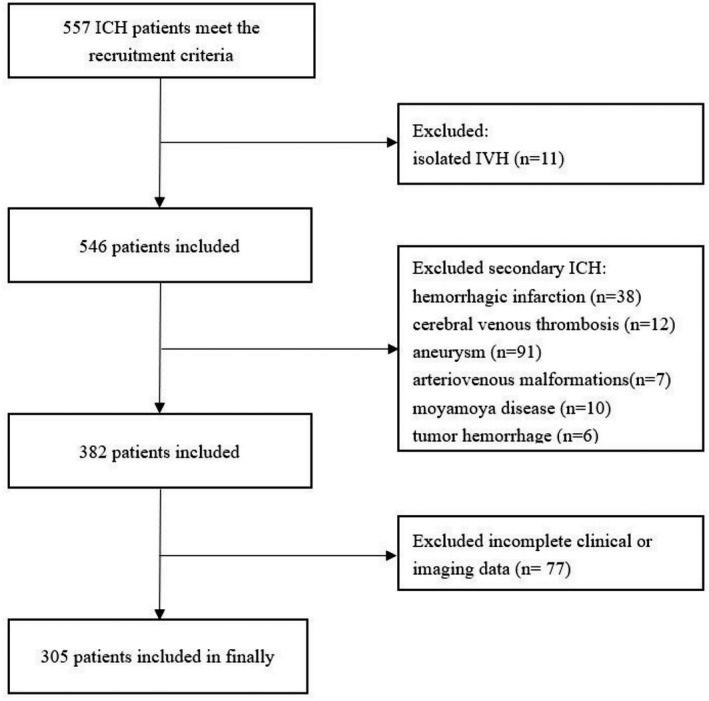
Flowchart of patients selection.
Table 1.
Clinical and imaging characteristics of ICH patients.
| Variables | All | Without DWI lesions | With DWI lesions | p‐value | ||
|---|---|---|---|---|---|---|
| Small | Large | p small | p large | |||
| N (%) | 305 (100) | 228 (74.8) | 61 (20.0) | 16 (5.2) | ||
| Demographic | ||||||
| Age, (years), mean (SD) | 59.5 (12.6) | 58.9 (12.8) | 61.3 (12.1) | 61.8 (12.1) | 0.175 | 0.382 |
| Male, n (%) | 207 (67.9) | 156 (68.4) | 39 (63.9) | 12 (75.0) | 0.506 | 0.785 |
| Vascular risk factors, n (%) | ||||||
| Hypertension | 243 (79.7) | 178 (78.1) | 51 (83.6) | 14 (87.5) | 0.344 | 0.566 |
| Diabete mellitus | 84 (27.5) | 56 (24.6) | 23 (37.7) | 5 (31.3) | 0.041 | 0.765 |
| Coronary artery disease | 54 (17.7) | 37 (16.2) | 13 (21.3) | 4 (25.0) | 0.351 | 0.575 |
| Atrial fibrillation | 9 (3.0) | 8 (3.5) | 1 (1.6) | 0 (0) | 0.740 | 0.972 |
| Prior ischemic stroke | 83 (27.2) | 54 (23.7) | 21 (34.4) | 8 (50.0) | 0.089 | 0.041 |
| Prior ICH | 52 (17.0) | 38 (16.7) | 10 (16.4) | 4 (25.0) | 0.959 | 0.609 |
| Smoking, current or quit <5 years | 108 (35.4) | 78 (34.2) | 22 (36.1) | 8 (50.0) | 0.787 | 0.201 |
| Body mass index, (kg/m2), median (IQR) | 25.2 (22.9–28.1) | 24.9 (22.7–28.0) | 25.4 (23.2–29.3) | 27.0 (22.9–29.4) | 0.315 | 0.218 |
| Previous medication, n (%) | ||||||
| Antihypertensive drugs | 164 (53.8) | 117 (51.3) | 38 (62.3) | 9 (56.3) | 0.127 | 0.703 |
| Antiplatelet drugs | 56 (18.4) | 36 (15.8) | 15 (24.6) | 5 (31.3) | 0.109 | 0.210 |
| Oral anticoagulants | 5 (1.6) | 2 (0.9) | 2 (3.3) | 1 (6.3) | 0.197 | 0.185 |
| Statins | 47 (15.4) | 30 (13.2) | 13 (21.3) | 4 (25.0) | 0.112 | 0.343 |
| Complication, n (%) | ||||||
| Deep venous thrombosis | 18 (5.9) | 8 (3.5) | 7 (11.5) | 3 (18.8) | 0.030 | 0.028 |
| Pneumonia | 89 (29.2) | 58 (25.4) | 23 (37.7) | 8 (50.0) | 0.058 | 0.065 |
| Stress ulcer | 20 (6.6) | 16 (7.0) | 2 (3.3) | 2 (12.5) | 0.438 | 0.752 |
| Clinical features | ||||||
| NIHSS at admission, median (IQR) | 5 (3–12) | 5 (3–11) | 4 (2–13) | 8 (3–13) | 0.632 | 0.397 |
| NIHSS change from admission to discharge, median (IQR) | 2 (1–5) | 3 (1–6) | 2 (0–5) | 1 (−1–2) | 0.091 | 0.001 |
| Initial GCS, median (IQR) | 15 (14–15) | 15 (14–15) | 15 (14–15) | 15 (14–15) | 0.680 | 0.547 |
| Time to MRI, (day), median (IQR) | 11 (6–16) | 11 (6–16) | 12 (6–16) | 8 (6–12) | 0.411 | 0.230 |
| Initial SBP, (mmHg), median (IQR) | 168 (153–186) | 166 (152–185) | 174 (164–187) | 185 (161–197) | 0.017 | 0.018 |
| Initial DBP, (mmHg), median (IQR) | 100 (88–110) | 97 (85–112) | 101 (95–110) | 100 (96–113) | 0.173 | 0.106 |
| Delta MAP, (mmHg), median (IQR) | 44 (35–54) | 43 (35–52) | 48 (38–62) | 52 (41–64) | 0.019 | 0.008 |
| Intravenous antihypertensive treatment in‐hospital, n (%) | 111 (36.4) | 83 (36.4) | 19 (31.1) | 9 (56.3) | 0.445 | 0.113 |
| Presumed etiology of ICH, n (%) | 0.163 | 0.136 | ||||
| Hypertensive angiopathy | 210 (68.9) | 153 (67.1) | 44 (72.1) | 13 (81.3) | ||
| Cerebral amyloid angiopathy | 36 (11.8) | 26 (11.4) | 10 (16.4) | 0 (0) | ||
| Anticoagulation or undetermined cause | 59 (19.3) | 49 (21.5) | 7 (11.5) | 3 (18.8) | ||
| Laboratory tests | ||||||
| WBC, (×109/L), median (IQR) | 7.4 (5.8–9.4) | 7.4 (5.8–9.1) | 7.5 (5.8–10.2) | 8.2 (6.0–10.0) | 0.593 | 0.558 |
| Neutrophil, ×109/L, median (IQR) | 5.3 (3.9–7.1) | 5.3 (3.8–6.7) | 5.5 (3.9–7.3) | 6.0 (3.2–7.9) | 0.490 | 0.654 |
| Triglyceride, (mmol/L), median (IQR) | 1.3 (0.8–1.9) | 1.3 (0.8–1.7) | 1.2 (0.8–2.3) | 1.2 (0.8–1.7) | 0.734 | 0.904 |
| Total cholesterol (mmol/L), median (IQR) | 4.4 (3.6–5.0) | 4.3 (3.6–4.9) | 4.5 (3.5–5.3) | 4.7 (3.7–5.6) | 0.226 | 0.186 |
| LDL‐C, (mmol/L), mean (SD) | 2.6 (1.0) | 2.5 (1.0) | 2.6 (0.9) | 2.9 (1.0) | 0.472 | 0.206 |
| Homocysteine, (μmol/L), median (IQR) | 14.6 (11.8–18.6) | 14.6 (11.5–19.2) | 14.6 (12.7–17.7) | 13.5 (10.6–14.9) | 0.643 | 0.374 |
| HbA1C (%), median (IQR) | 5.6 (5.2–6.0) | 5.5 (5.1–5.9) | 5.8 (5.2–6.8) | 5.6 (5.4–6.0) | 0.025 | 0.176 |
| Fasting blood glucose (mmol/L), median (IQR) | 6.3 (5.2–7.5) | 6.0 (5.1–7.5) | 6.5 (5.3–7.8) | 6.8 (5.9–7.6) | 0.178 | 0.084 |
| Fibrinogen, (g/L), median (IQR) | 2.5 (2.1–3.1) | 2.5 (2.1–3.0) | 2.6 (2.1–3.2) | 2.5 (2.3–3.7) | 0.369 | 0.285 |
| CT findings | ||||||
| Location of hematoma, n (%) | ||||||
| Deep | 219 (71.8) | 167 (73.2) | 39 (63.9) | 13 (81.3) | 0.153 | 0.682 |
| Lobar | 70 (23.0) | 51 (22.4) | 17 (27.9) | 2 (12.5) | 0.368 | 0.541 |
| Infratentorial | 21 (6.9) | 13 (5.7) | 7 (11.5) | 1 (6.2) | 0.196 | 0.928 |
| Intraventricular extension | 73 (23.9) | 52 (22.8) | 17 (27.9) | 4 (25.0) | 0.410 | 0.766 |
| Subarachnoid extension | 17 (5.6) | 10 (4.4) | 7 (11.5) | 0 (0) | 0.074 | 0.839 |
| Initial hematoma volume, (mL), median (IQR) | 7.8 (3.6–16.0) | 7.2 (3.5–16.9) | 8.0 (5.0–15.0) | 10.4 (1.0–11.9) | 0.316 | 0.537 |
| MRI findings | ||||||
| WMHs, Fazekas score, median (IQR) | 2 (1–3) | 2 (1–3) | 3 (2–4) | 3 (2–4) | <0.001 | 0.135 |
| PWMHs, Fazekas score, median (IQR) | 1 (1–2) | 1 (0–1) | 2 (1–2) | 1 (1–2) | <0.001 | 0.168 |
| DWMHs, Fazekas score, median (IQR) | 1 (1–2) | 1 (1–1) | 1 (1–2) | 1 (1–2) | <0.001 | 0.153 |
| Moderate to severe WMHs, n (%) | 91 (29.8) | 56 (24.6) | 29 (47.5) | 6 (37.5) | <0.001 | 0.394 |
| BG‐EPVS, median (IQR) | 1 (1–2) | 1 (1–2) | 2 (1–2) | 1 (1–2) | <0.001 | 0.423 |
| CSO‐EPVS, median (IQR) | 1 (1–2) | 1 (1–2) | 2 (1–3) | 1 (1–2) | <0.001 | 0.531 |
| BG‐EPVS >10, n (%) | 104 (34.1) | 64 (28.1) | 34 (55.7) | 6 (37.5) | <0.001 | 0.603 |
| CSO‐EPVS >10, n (%) | 111 (36.4) | 72 (31.6) | 32 (52.5) | 7 (43.8) | <0.001 | 0.315 |
| Presence of CMBs, n (%) | 207 (67.9) | 144 (63.2) | 52 (85.2) | 11 (68.8) | 0.001 | 0.653 |
| Number of CMBs, median (IQR) | 2 (0–7) | 2 (0–5) | 4 (1–8) | 3 (0–11) | <0.001 | 0.290 |
| Deep CMBs, median (IQR) | 2 (0–4) | 1 (0–3) | 2 (1–6) | 3 (0–7) | <0.001 | 0.114 |
| Lobar CMBs, median (IQR) | 0 (0–2) | 0 (0–1) | 0 (0–3) | 0 (0–2) | 0.092 | 0.745 |
| Presence of lacunes, n (%) | 141 (46.2) | 94 (41.2) | 40 (65.6) | 7 (43.8) | 0.001 | 0.843 |
| Number of lacunes, median (IQR) | 0 (0–2) | 0 (0–1) | 1 (0–2.5) | 0 (0–2) | <0.001 | 0.759 |
| Total cSVD score, median (IQR) | 2 (1–3) | 1 (0–3) | 3 (1–4) | 2 (1–3) | <0.001 | 0.364 |
| MRA findings | ||||||
| Distribution of moderate to severe cerebral artery stenosis, n (%) | ||||||
| Internal carotid artery | 20 (6.6) | 7 (3.1) | 4 (6.6) | 9 (56.3) | 0.375 | <0.001 |
| Anterior cerebral artery | 12 (3.9) | 9 (3.9) | 2 (3.3) | 1 (6.3) | 0.805 | 0.499 |
| Middle cerebral artery | 37 (12.1) | 23 (10.1) | 6 (9.8) | 8 (50.0) | 0.954 | <0.001 |
| Posterior cerebral artery | 28 (9.2) | 17 (7.5) | 7 (11.5) | 4 (25.0) | 0.312 | 0.041 |
| Vertebral artery | 10 (3.3) | 5 (2.2) | 4 (6.6) | 1 (6.3) | 0.184 | 0.337 |
| Basilar artery | 4 (1.3) | 1 (0.4) | 2 (3.3) | 1 (6.3) | 0.114 | 0.127 |
p small value: patients with small DWI lesions versus without DWI lesions; p large value: patients with large DWI lesions versus without DWI lesions. ICH, intracerebral hemorrhage; DWI, diffusion‐weighted imaging; SBP, systolic blood pressure; DBP, diastolic blood pressure; IQR, interquartile range; NIHSS, National Institute of Health Stroke Scale; GCS, Glasgow Coma Scale; MAP, mean arterial pressures; WBC, white blood cell count; LDL‐C, low‐density lipoprotein cholesterol; HbA1C, hemoglobin A1c; CT, computed tomography; MRI, magnetic resonance imaging; MRA, magnetic resonance angiography; DWMHs, deep white matter hyperintensities; PWMHs, periventricular white matter hyperintensities; BG‐EPVS, basal ganglia enlarged perivascular spaces; CSO‐EPVS, centrum semiovale enlarged perivascular spaces; CMBs, cerebral microbleeds; cSVD, cerebral small‐vessel disease.
Characteristics of DWI lesions
Among 305 patients, 25.2% (77/305) patients presented with DWI lesions (Imaging examples Fig. 2). Of these, 79.2% (61/77) had small DWI lesions, 20.8% (16/77) had large DWI lesions. The median diameter of small DWI lesions was 3 (IQR 2–7) mm, 88.5% were asymptomatic infarction, and the median diameter of large DWI lesions was 21 (IQR 16–38) mm, all of which were symptomatic infarction. Overall, 12.5% (38/305) had multiple lesions which were mostly small DWI lesions (84.2%) (punctate, round, or ovoid) with an increasing trend in proportion with the increasing number of DWI lesions (Fig. 3A). Small DWI lesions were mainly located in the cortical and subcortical regions of lobes (65%) and scattered in multiple vascular territories (66.7%). Large DWI lesions were mostly single, mainly located in deep structures (56.3%), contralateral to the hematoma (68.8%), and in moderately to severely stenosed arterial territories (77.8%) (Fig. 3B–D).
Figure 2.
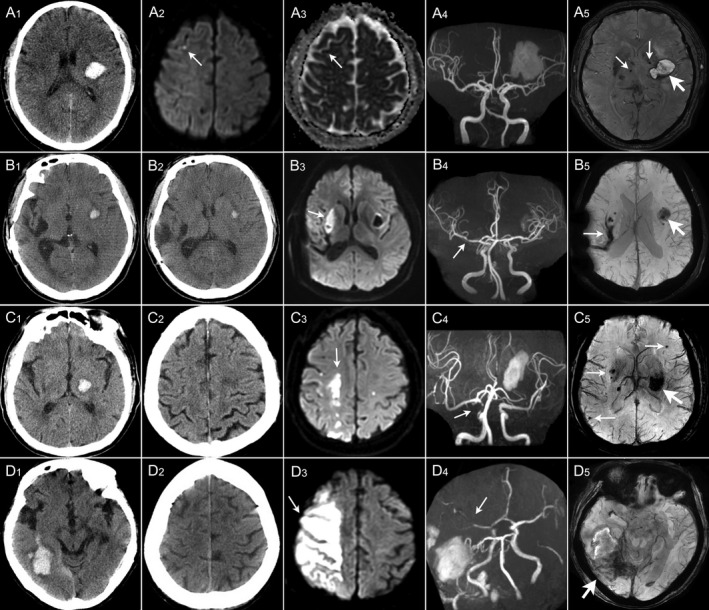
Imaging examples of large and small DWI lesions in four patients with acute intracerebral hemorrhage. (A) In a 69‐year‐old man with left BG hemorrhage (A1), diffusion‐weighted imaging (DWI) sequence (A2) shows a small DWI hyperintensity lesion (thin arrow) in the right frontal lobe with corresponding low signal intensity on ADC sequence (A3) and magnetic resonance angiography (MRA) (A4) shows no stenosis in the intracranial artery; susceptibility‐weighted imaging (SWI) (A5) shows a hematoma (thick arrow) in left BG and CMBs in bilateral BG (thin arrow). (B) In a 43‐year‐old man with left putamen hemorrhage (B1), DWI sequence (B3) shows a large DWI hyperintensity lesion (thin arrow) in the deep branch territory of right middle cerebral artery (MCA) (right BG) which was normal on CT (B2) at admission and MRA (B4) shows mild stenosis (thin arrow) in M1 segment of right MCA; SWI (B5) shows a hematoma (thick arrow) in left putamen and an old hemorrhagic cavity in right BG (thin arrow). (C) In a 70‐year‐old man with left thalamus hemorrhage (C1), DWI sequence (C3) shows borderzone infarct (thin arrow) in borderzone territories of MCA and ACA which was normal on CT (C2) at admission and MRA (C4) shows moderate stenosis in the terminal of right internal carotid artery (thin arrow); SWI (C5) shows a hematoma in left BG (thick arrow) and multiple CMBs (thin arrows). (D) In a 68‐year‐old woman with right temporal lobe hemorrhage with subarachnoid extension (D1). DWI sequence (D3) shows a large DWI hyperintensity lesion (thin arrow) in right MCA territories (right frontal and parietal lobes) which was normal on CT (D2) at admission and MRA (D4) shows severe stenosis to occlusion in M2 segment of right MCA (thin arrow); SWI (D5) shows a hematoma in right temporal and occipital lobes (thick arrow).
Figure 3.
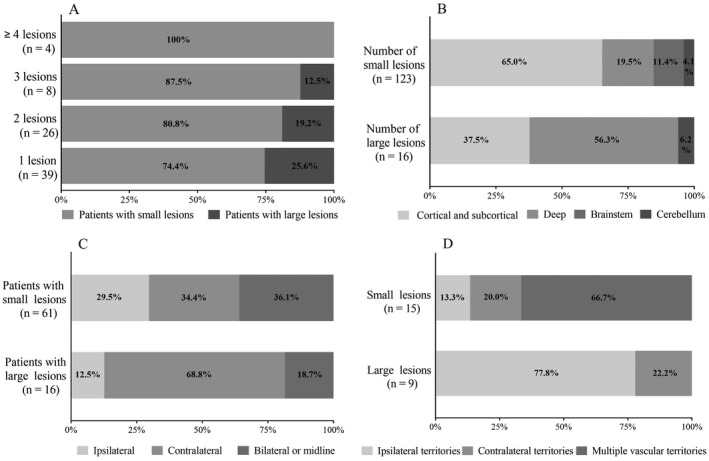
Number, size, location, and distribution of DWI lesions. (A) Number and size of DWI lesions; (B) location of DWI lesions; (C) laterality relative to hematoma; (D) territory distribution in moderate to severe artery stenosis. DWI, diffusion‐weighted imaging.
Association between cSVD burden and different sizes of DWI lesions
The distribution of total cSVD burden was significantly different among the groups (p = 0.001, Fig. 4A). Patients with small DWI lesions had a higher total cSVD burden than those without DWI lesions (p < 0.001). The cSVD burden was positively correlated with the number of small DWI lesions (r = 0.221, p < 0.001). In multivariable analyses, a high cSVD burden was independently associated with small DWI lesions (OR 1.81, 95% CI 1.36–2.41, p < 0.001) (Fig. 5) but not with large DWI lesions (OR 0.83, 95% CI 0.47–1.43, p = 0.490) (Fig. 6).
Figure 4.
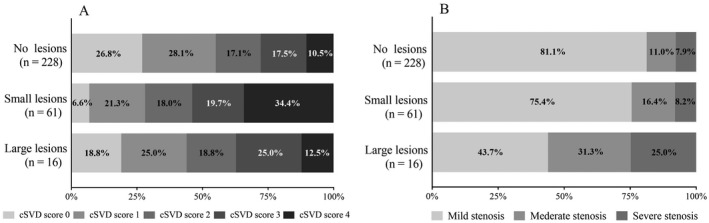
(A) Distribution of total cSVD burden. (B) Distribution of cerebral artery stenosis. cSVD, cerebral small‐vessel disease.
Figure 5.
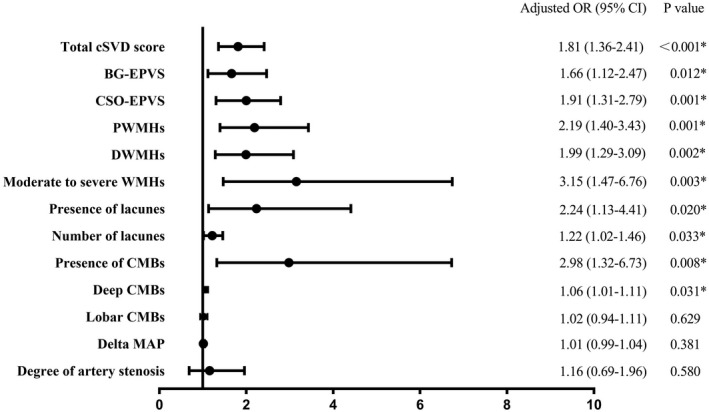
Multivariate analysis for the association of small DWI lesions with cSVD and CAS. Adjusted for age, sex, body mass index, hypertension, diatetes mellitus, ischemic stroke, smoking, initial systolic blood pressure, initial hematoma volume, subarachnoid extension, pneumonia, initial National Institute of Health Stroke Scale, hemoglobin A1c, delta MAP, and CAS. DWI, diffusion‐weighted imaging; cSVD, cerebral small‐vessel disease; CAS, cerebral artery stenosis; MAP, mean arterial pressure.
Figure 6.
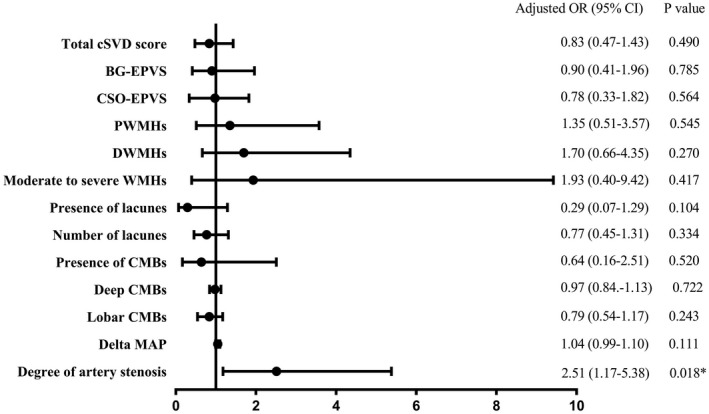
Multivariate analysis for the association of large DWI lesions with cSVD and CAS. Adjusted for age, sex, body mass index, hypertension, diatetes mellitus, ischemic stroke, smoking, initial systolic blood pressure, initial hematoma volume, pneumonia, initial National Institute of Health Stroke Scale, fasting blood; delta MAP, and CAS. DWI, diffusion‐weighted imaging; cSVD, cerebral small‐vessel disease; CAS, cerebral artery stenosis; MAP, mean arterial pressure.
Association between the degree of CAS and different sizes of DWI lesions
Moderate to severe CAS was observed in 22.0% (67/305) patients. The degree of CAS was significantly different among the groups (p = 0.029, Fig. 4B). Compared to patients without DWI lesions, patients with large DWI lesions had more severe CAS (p = 0.006), especially in the ICA and MCA. Multivariable analyses showed that CAS was independently associated with large DWI lesions (OR 2.51, 95% CI 1.17–5.38, p = 0.018) (Fig. 6) but not with small DWI lesions (OR 1.16, 95% CI 0.69–1.96, p = 0.580) (Fig. 5).
When stratified analysis (Table 2) by median MAP change (<44 vs. ≥44 mmHg) was performed, more severe CAS was associated with increased risk of large DWI lesions with greater MAP change (OR 3.48, 95% CI 1.13–10.74, p = 0.030) but not with mild MAP change (OR 1.26, 95% CI 0.32–4.93, p = 0.737). This association between CAS and large DWI lesions was modified by MAP change (interaction term: OR 2.75, 95% CI 1.21–6.26, p = 0.016). While the association between total cSVD burden and small DWI lesions was not modified by MAP change (interaction term: OR: 0.91, 95% CI 0.59–1.40, p = 0.665).
Table 2.
Association of DWI lesions with cSVD burden and degree of CAS stratified according to median MAP change.
| Units | MAP change <44 mmHg | MAP change ≥44 mmHg | ||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p‐value | Adjusted OR (95% CI) | p‐value | |||
| Small DWI lesions | ||||||
| Total cSVD burden | Per 1 score increase | Model 1 | 1.706 (1.152–2.527) | 0.008 | 1.654 (1.207–2.267) | 0.002 |
| Model 2 | 2.023 (1.231–3.323) | 0.005 | 1.515 (1.030–2.229) | 0.035 | ||
| Degree of CAS | Per 1 grade stenosis increase | Model 1 | 1.762 (0.958–3.241) | 0.069 | 0.643 (0.273–1.512) | 0.311 |
| Model 2 | 1.853 (0.868–3.958) | 0.111 | 0.447 (0.160–1.252) | 0.126 | ||
| Large DWI lesions | ||||||
| Total cSVD burden | Per 1 score increase | Model 1 | 0.788 (0.290–2.143) | 0.641 | 1.295 (0.719–2.330) | 0.389 |
| Model 2 | 0.743 (0.203–2.719) | 0.653 | 0.840 (0.384–1.840) | 0.664 | ||
| Degree of CAS | Per 1 grade stenosis increase | Model 1 | 1.584 (0.503–4.898) | 0.432 | 3.938 (1.614–9.606) | 0.003 |
| Model 2 | 1.263 (0.324–4.926) | 0.737 | 3.478 (1.127–10.736) | 0.030 | ||
Multivariate logistic regression analysis for the association of small DWI lesions with cSVD and CAS: Model 1: adjusted for age and sex. Model 2: as for model 1 plus initial hematoma volume, initial National Institutes of Health Stroke Scale, initial systolic blood pressure, ischemic stroke, diatetes mellitus, subarachnoid extension, pneumonia, hemoglobin A1c. Multivariate logistic regression analysis for the association of large DWI lesions with cSVD and CAS: Model 1: adjusted for age and sex. Model 2: as for model 1 plus initial hematoma volume, initial National Institutes of Health Stroke Scale, initial systolic blood pressure, ischemic stroke, pneumonia, fasting blood glucose. DWI, diffusion‐weighted imaging; cSVD, cerebral small vessel disease; CAS, cerebral artery stenosis; MAP, mean arterial pressures.
Discussion
In this study, we demonstrated that a high burden of cSVD was independently associated with the presence of small DWI lesions in acute primary ICH, regardless of MAP change. Severe CAS was independently associated with the presence of large DWI lesions. This association was modified by MAP change, with stratified analysis showing an increased risk of large DWI lesions in severe CAS with greater MAP change but not with mild MAP change. The greater MAP change drove most of the association. Previous studies have reported that DWI lesions were linked to small‐vessel arteriopathy 5 , 6 , 15 , 17 or intensive BP reduction. 2 , 7 , 18 However, they may be insufficient to explain the DWI lesions of different sizes, frequencies, and vascular territories in patients with ICH. MRA data on whether these lesions were linked to a specific stenosed artery territory is still lacking. We found that large DWI lesions were mostly single, mainly located in deep structures, contralateral to the hematoma, and in moderately to severely stenosed artery territory. Whereas small lesions were more likely to be multiple, mainly located in the cortical and subcortical regions, and scattered in multiple vascular territories. Moreover, the number of small lesions was positively correlated with the total cSVD burden. These findings confirmed that large‐artery atherosclerosis was also involved in ischemic brain injury, differing from the small‐vessel pathogenic mechanisms in ICH.
The precise pathophysiological mechanisms of DWI lesions in acute ICH are not yet well understood. Most studies have reported that ischemic lesions were related to CMB, 5 , 6 , 7 , 15 WMHs, 6 , 7 , 15 , 16 , 17 and CSO‐EPVS, 15 , 20 but few reported a relationship to lacunes. Our analysis showed that all MRI markers of cSVD were associated with small DWI lesions adjustment for other established confounding factors. This may be due, in part, to the lack of stratified analysis based on lesion size, because they have different pathogenesis. A single imaging marker of cSVD to estimate the overall impact of small‐vessel pathology is limited. Therefore, an MRI‐based cSVD score was proposed to capture the overall burden of small vessel disease. 28 We found the total cSVD score was better at revealing the association between the cSVD burden and small DWI lesions. Small DWI lesions may be a manifestation of active vasculopathy and coexist with ICH or present secondarily after ICH. A longitudinal study 2 observed that 35% of patients had DWI lesions at baseline, and 22% had new DWI lesions at 1 month that had not been present at baseline, while another longitudinal study 29 indicated that 50% had new DWI lesions at 1 week. Patients with microangiopathy had reduced cerebral autoregulation. Whether there is an interaction between cSVD and MAP change on ICH has not been previously studied. We found no significant interaction between them.
ICH and ischemic stroke share common atherosclerotic risk factors such as hypertension and advancing age. Little attention has been paid to the effect of large‐artery atherosclerosis on ICH. In our cohort, 22.0% of patients had moderate to severe CAS. Among them, ICA and MCA stenosis were especially associated with large DWI lesions. Some of the large lesions were borderzone infarctions, indicating the potential mechanism of hypoperfusion. Interestingly, when the hematoma was contralateral to the hemisphere with severe CAS, infarcts were mostly distributed in the severely stenotic artery territory; however, when the hemorrhage was ipsilateral to severe CAS, the infarcts were mostly located in the contralateral territory. The most likely explanation is the possible involvement of an artery steal blood mechanism. In addition, local thrombosis and artery‐to‐artery embolism may occur more frequently in patients with multiple atherosclerotic stenoses during the stress state of ICH, hemodynamic compromise, intracranial hypertension decreasing cerebral perfusion pressure, dehydration drugs resulting in reduced blood volume, and proinflammatory cascades. 30 Reports regarding the relationship between BP lowering and DWI lesions remain conflicting. In our study, MAP lowering alone was not associated with ischemia, which differed from the views of those supporting BP reduction. 2 , 7 , 18 Only in the setting of severe vasculopathy, aggressive change of BP was more likely to induce brain ischemic injury. There was a significant interaction of MAP change and severe underlying large‐artery atherosclerosis on ICH. The hypothesis that patients with large artery atherosclerotic changes are at greater risk of developing ischemic lesions after BP lowering should be tested in future randomized trials. Intensive BP lowering cannot improve overall outcomes without considering individual differences. 31 , 32 , 33 , 34 , 35 , 36
Certain limitations of this study warrant consideration. First, selection bias was unavoidable owing to the lack of MRI in patients with severe hemorrhage. Second, MRI could not be performed at the same time points because of the different severities of the ICH patients. Third, extracranial vessels were not assessed because patients with acute ICH cannot cooperate with long‐time MRI. Finally, the cross‐sectional study could not inform the direction of the association of DWI lesions with cSVD burden and CAS. Further longitudinal MRI studies should clarify whether these lesions are coexisting or secondary, and change in trends over time. However, this study has strengths. This study combining MRI and MRA for the first time compared the contribution of small‐vessel disease burden and large‐artery atherosclerotic burden to DWI lesions, and the interaction with MAP change in patients with acute ICH. In addition, the size, number, location relative to hematoma, and artery watershed distribution of the DWI lesions were also fully analyzed.
Conclusions
This study confirms that a high cSVD burden is independently associated with small DWI lesions, while severe CAS is independently associated with large DWI lesions, specifically with greater MAP change. Long‐term hypertensive vasculopathy may be a prevailing mechanism. These findings provide convincing evidence that ischemic brain injury in acute ICH is not only associated with greater microangiopathy burden but also with large‐artery atherosclerosis burden. It has important implications for developing optimal interventions for individualized BP control targets for subjects with different degrees of brain atherosclerosis to prevent further ischemic injury.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author Contributions
Ailing Zhang, Mengyang Ren, and Wenjing Deng participated in the conception and design of the study and manuscript drafting. Meijing Xi, Long Tian, Zhuoya Han, Weiping Zang, and Hao Hu participated in the acquisition and analysis of data. Bin Zhang, Ling Cui, Peihong Qi, and Yingjie Shang contributed to data collection, analysis, and manuscript revision. All authors substantially contributed to this manuscript and approved the final version.
Supporting information
Data S1. Imaging parameters of MRI and MRA.
Acknowledgments
This work was supported by grants from the Medical Scientific and Technology Research Foundation of Henan Province (2018020826).
Funding Information
This work was supported by grants from the Medical Scientific and Technology Research Foundation of Henan Province (2018020826).
Funding Statement
This work was funded by Medical Scientific and Technology Research Foundation of Henan Province grant 2018020826.
References
- 1. Ter Telgte A, Scherlek AA, Reijmer YD, et al. Histopathology of diffusion‐weighted imaging‐positive lesions in cerebral amyloid angiopathy. Acta Neuropathol. 2020;139:799‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menon RS, Burgess RE, Wing JJ, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71:199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee EJ, Kang DW, Warach S. Silent new brain lesions: innocent bystander or guilty party? J Stroke. 2016;18:38‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gregoire SM, Charidimou A, Gadapa N, et al. Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross‐sectional magnetic resonance imaging study. Brain. 2011;134:2376‐2386. [DOI] [PubMed] [Google Scholar]
- 5. Kang DW, Han MK, Kim HJ, et al. New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology. 2012;79:848‐855. [DOI] [PubMed] [Google Scholar]
- 6. Murthy SB, Cho SM, Gupta A, et al. A pooled analysis of diffusion‐weighted imaging lesions in patients with acute intracerebral hemorrhage. JAMA Neurol. 2020;77:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kidwell CS, Rosand J, Norato G, et al. Ischemic lesions, blood pressure dysregulation, and poor outcomes in intracerebral hemorrhage. Neurology. 2017;88:782‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai YH, Lee MH, Weng HH, et al. Fate of diffusion restricted lesions in acute intracerebral hemorrhage. PLoS One. 2014;9:e105970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garg RK, Khan J, Dawe RJ, et al. The influence of diffusion weighted imaging lesions on outcomes in patients with acute spontaneous intracerebral hemorrhage. Neurocrit Care. 2020;33:552‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivera‐Lara L, Murthy SB, Nekoovaght‐Tak S, et al. Influence of bleeding pattern on ischemic lesions after spontaneous hypertensive intracerebral hemorrhage with intraventricular hemorrhage. Neurocrit Care. 2018;29:180‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murthy SB, Diaz I, Wu X, et al. Risk of arterial ischemic events after intracerebral hemorrhage. Stroke. 2020;51:137‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murthy SB, Zhang C, Gupta A, et al. Diffusion‐weighted imaging lesions after intracerebral hemorrhage and risk of stroke: a MISTIE III and ATACH‐2 analysis. Stroke. 2021;52:595‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiegertjes K, Dinsmore L, Drever J, et al. Diffusion‐weighted imaging lesions and risk of recurrent stroke after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2021. doi: [DOI] [PubMed] [Google Scholar]
- 14. Ye XH, Gao T, Xu XH, et al. Factors associated with remote diffusion‐weighted imaging lesions in spontaneous intracerebral hemorrhage. Front Neurol. 2018;9:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu XH, Ye XH, Li JW, et al. Association between remote diffusion‐weighted imaging lesions and cerebral small vessel disease in primary intracerebral hemorrhage. Eur J Neurol. 2019;26:961‐968. [DOI] [PubMed] [Google Scholar]
- 16. Revel‐Mouroz P, Viguier A, Cazzola V, et al. Acute ischaemic lesions are associated with cortical superficial siderosis in spontaneous intracerebral hemorrhage. Eur J Neurol. 2019;26:660‐666. [DOI] [PubMed] [Google Scholar]
- 17. Auriel E, Westover MB, Bianchi MT, et al. Estimating total cerebral microinfarct burden from diffusion‐weighted imaging. Stroke. 2015;46:2129‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Zhang B, Lou M. The relation between acute intracerebral hemorrhage and diffusion‐weighted imaging lesions: a meta‐analysis. J Thromb Thrombolysis. 2021. doi: [DOI] [PubMed] [Google Scholar]
- 19. Gioia LC, Kate M, Choi V, et al. Ischemia in intracerebral hemorrhage is associated with leukoaraiosis and hematoma volume, not blood pressure reduction. Stroke. 2015;46:1541‐1547. [DOI] [PubMed] [Google Scholar]
- 20. Wu B, Yao X, Lei C, et al. Enlarged perivascular spaces and small diffusion‐weighted lesions in intracerebral hemorrhage. Neurology. 2015;85:2045‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butcher KS, Jeerakathil T, Hill M, et al. The intracerebral hemorrhage acutely decreasing arterial pressure trial. Stroke. 2013;44:620‐626. [DOI] [PubMed] [Google Scholar]
- 22. Zhang CY, Huang SR, Wang SY, et al. Clinical study of intracranial and extracranial atherosclerotic stenosis in spontaneous intracerebral hemorrhage patients. J Stroke Cerebrovasc Dis. 2018;27:286‐290. [DOI] [PubMed] [Google Scholar]
- 23. Boulouis G, Charidimou A, Auriel E, et al. Intracranial atherosclerosis and cerebral small vessel disease in intracerebral hemorrhage patients. J Neurol Sci. 2016;369:324‐329. [DOI] [PubMed] [Google Scholar]
- 24. Knudsen KA, Rosand J, Karluk D, et al. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537‐539. [DOI] [PubMed] [Google Scholar]
- 25. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fazekas F, Barkhof F, Wahlund LO, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002;13(suppl 2):31‐36. [DOI] [PubMed] [Google Scholar]
- 27. Chimowitz MI, Lynn MJ, Howlett‐Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305‐1316. [DOI] [PubMed] [Google Scholar]
- 28. Staals J, Makin SD, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small‐vessel disease burden. Neurology. 2014;83:1228‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garg RK, Alberawi M, Ouyang B, et al. Timing of diffusion weighted imaging lesions in spontaneous intracerebral hemorrhage. J Neurol Sci. 2021;425:117434. [DOI] [PubMed] [Google Scholar]
- 30. Shtaya A, Bridges LR, Esiri MM, et al. Rapid neuroinflammatory changes in human acute intracerebral hemorrhage. Ann Clin Transl Neurol. 2019;6:1465‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qureshi AI, Palesch YY, Foster LD, et al. Blood pressure‐attained analysis of ATACH 2 trial. Stroke. 2018;49:1412‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qureshi AI, Palesch YY, Barsan WG, et al. Intensive blood‐pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qureshi AI, Huang W, Lobanova I, et al. Outcomes of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage and excessively high initial systolic blood pressure: post hoc analysis of a randomized clinical trial. JAMA Neurol. 2020;77:1355‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leasure AC, Qureshi AI, Murthy SB, et al. Association of intensive blood pressure reduction with risk of hematoma expansion in patients with deep intracerebral hemorrhage. JAMA Neurol. 2019;76:949‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boulouis G, Morotti A, Goldstein JN, et al. Intensive blood pressure lowering in patients with acute intracerebral haemorrhage: clinical outcomes and haemorrhage expansion. Systematic review and meta‐analysis of randomised trials. J Neurol Neurosurg Psychiatry. 2017;88:339‐345. [DOI] [PubMed] [Google Scholar]
- 36. Sandset EC, Fischer U. Intensive blood pressure lowering provides no additional benefits and results in more adverse events. Evid Based Med. 2017;22:102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Imaging parameters of MRI and MRA.


