Disruptive technology. The term seems to provoke an unconscious sense of negativity and division because the word “disruptive” is often used to describe that which is unruly or troublesome. And yet, here we lay it together with technology, and create a phrase that in fact encapsulates and performs the opposite. While this term may seem infuriatingly familiar to those in business or finance, to the healthcare world, this term might be foreign or even unknown.
First defined in the mid 1990s by Clayton Christensen, a well-known business consultant, disruptive technology exploded and was quickly deemed one the most influential business ideas of the twenty-first century by the Economist [1]. This concept comprises technological innovation, of any and all types, with features or facets that disrupt the status quo: the established models, the conventional practices, the well-recognized and followed patterns. Yet these disruptions, while not always immediately welcomed, radically swept away the established to enact recognizably superior change. Top offenders found on the internet include artificial intelligence, high-speed travel, and robotics. The widespread arms of disruptive technology currently consume entities like e-commerce and ride-sharing while previously it elevated automobiles, electricity, and television. Clearly gaining weight and momentum in the business world, where does it stand within healthcare and surgery? It must hold a role and have laid a claim as it is a necessity for change, a necessity for accessibility, and a necessity for growth.
Almost simultaneously to when the term disruptive technology was coined, ERAS®, or enhanced recovery after surgery, took perioperative care by storm. Designed to improve quality of recovery and standardize care through multidisciplinary collaboration, ERAS® proved to have significant benefit across surgical specialties for both patients and healthcare systems. Its use quickly disrupted anecdotal practice or individualized treatment. However, similar to disruptive technologies, perhaps most akin to Sony’s early radios, ERAS® was initially used hesitantly and sparingly, only within the realm of colorectal surgery [2]. But as these principles showed considerable and clear benefit through compliance audits and big data sets (alas more disruptive technologies!), ERAS® extended beyond the initially described colorectal practices. Currently, there are over 30 published ERAS® guidelines, spanning 16 specialties including obstetrics and gynecology, cardiac surgery, and hepatobiliary. ERAS®, which we can consider a disruptive behavior that has evolved into a standard of care, has since spawned and/or spurred many disruptive technologies with its implementation. These have since led to significant breakthroughs and innovations in the following themes: (1) education; (2) prehabilitation and pre-operative care delivery; (3) intra- and post-operative care delivery; (4) patient experience; and (5) system’s building (Table 1). With many of these disruptive technologies spanning multiple of these themes, we will expand upon several to illustrate their impact and potential.
Table 1.
The disruptive technologies of ERAS®
| ERAS® disruptive technology | Benefit/disruption | Pertinent references |
|---|---|---|
| A: Education | ||
| 1. Virtual care | Video visits are non-inferior to in-person visits and offer convenience and accessibility | Harkey et al. (2021) [3] |
| 2. Virtual reality | HoloLens augmented reality aids visual learners through comprehensive practice and experience | Vavra et al. (2017) [4] |
| 3. Infographics | Infographics to improve patient and provider comprehension and recall of ERAS® pathway | Hughes et al. (2020) [5] |
| B: Prehabilitation and pre-operative care delivery | ||
| 4. Ride-sharing | Ride-sharing to decrease patient transportation barriers and improve healthcare access | Chaiyachati et al. (2018) [6] |
| 5. Amazon Pharmacy | Mail service prescription medication; pre- and post-operative treatment delivery (i.e., colon bundle, nutrition) to improve compliance and access | Schwab et al. (2019) [7] |
| 6. Prescriptive analytics | Use of data mining, predictive modeling and machine learning to risk-stratify and improve patient care | Pickens et al. (2019) [8] |
| 7. Prehabilitation smart device sensors | Smart sensors (i.e., Fitbits) to monitor at-home prehabilitation targets such as VO2 max to improve physical capacity prior to surgery | Baimas-George et al. (2020) [9] |
| C: Intra- and post-operative care delivery | ||
| 8. Closed loop anesthesia | Automated control system using objective patient feedback targets to improve intraoperative stability and outcomes | Brogi et al. (2017) [10] |
| 9. Automated continuous monitoring | Noninvasive monitoring systems to timely detect clinical problems through slight variations in physiologic parameters | Khanna et al. (2019) [11] |
| 10. HoloLens for pain control | Use of virtual/augmented reality offering alternatives to opioid medications | Spiegel et al. (2019) [12] |
| 11. Outcome situational awareness | An interactive software platform providing real-time individualized clinical, financial, and patient-reported outcomes for surgeons through automatic data population | Lyman et al. (2020) [13] |
| 12. Vertical compliance | Real-time variable ranking through electronic medical record to create individualized risk predictions based on ERAS® pathway compliance | Baimas-George et al. (2020) [14] |
| 13. Horizontal compliance | Longitudinal adherence of all patients to ERAS® index elements audited into data registries to establish outcome associations | Baimas-George et al. (2020) [15] |
| D: Patient experience | ||
| 14. Patient-reported outcomes | Mobile applications for real-time collection of clinical outcomes | Pickens et al. (2019) [16] |
| 15. Internet support groups | Global interest groups facilitating support and fostering community | Koball et al. (2017) [17] |
| 16. Voice assistants (i.e., Alexa) | Virtual assistants trained through artificial intelligence to triage patient questions and concerns | Sezgin et al. (2020) [18] |
| E: System’s building | ||
| 17. Team building simulation | Use of simulated scenarios to improve team building exercises and procedures | Zheng et al. (2008) [19] |
| 18. Cumulative sum analytics | Use of kinematic data to objectively evaluate surgical performance | Lyman et al. (2020) [20] |
| 19. Lean principles | Identification and elimination of unnecessary resource utilization in ERAS® pathways to decrease time, resources, and cost | Collar et al. (2012) [21] |
| 20. Time-driven activity-based costing (TDABC) pathways | Identification of cost drivers to accurately assess ERAS® finances and identify processes to target for quality improvement | Allin et al. (2021) [22] |
The first of these disruptive technologies discussed is the use of mobile applications for real-time collection of patient-reported outcomes, or PROs [16]. With compliance correlating to clinical outcomes within ERAS® pathways, assessing and tracking patient participation and experience can provide significant insight. Creation of a mobile application, accessible by any smartphone or tablet device, ensued and allowed patients enrolled in ERAS® pathways to offer feedback on perioperative care. Not only did this application also provide education and reminders for ERAS® pathway steps, but it surveyed patients for qualitative outcomes pre- and post-operatively. This disruptive technology creates a feasible avenue for real-time tracking and analysis with subsequent dynamic feedback for immediate improvements in perioperative care.
A similar offshoot followed PROs and was termed “vertical compliance” [14]. The ERAS® Interactive Audit System (EIAS) database maintains prospectively collected compliance data for patients in ERAS® centers across the world (hint—another use of disruptive technology). This data was utilized to create a model of tailored risk predictions based on individual ERAS® pathway adherence. This “vertical compliance” algorithm allows for real-time variable ranking through the electronic medical record such that pathway items can be categorized by effect on clinical outcomes. It further can identify and subsequently focus on patients who may be on paths to substandard outcomes.
With the arrival of 2020’s disruptive disaster, a disruptive technology that was just beginning to infiltrate surgical care was afforded rapid expansion and growth. Virtual care technology has been an exciting and evolving entity as it offers patients and providers significant convenience and accessibility. Prior to the COVID-19 pandemic, a randomized controlled trial demonstrated non-inferiority between virtual care video visits and in-person visits after simple laparoscopic acute care procedures [3]. With the arrival of COVID-19, society and the healthcare system have been dramatically altered, catalyzing swift acceptance and implementation of virtual care. Now virtual care visits are being utilized throughout all surgical disciplines, including pre-operative consultations and ERAS® education classes, daily hospital rounding visits, and post-operative assessments [23]. While this may have been rapidly implemented, it is sustainable, effective, eliminates social disparities, and is clearly here to stay.
Current systems make it not practical or even feasible for an individual surgeon to accurately track and monitor all of their own outcomes without significant support staff assistance. This need led to the development of “outcome situational awareness” (OSA) which is the examination and balance of the clinical and clerical responsibilities of a modern surgeon [13]. It is an interactive software platform that provides real-time individualized financial, patient-reported, and clinical outcomes for surgeons through automatic data population from medical records, administrative records, and interactive patient mobile applications to a centralized database. This platform allows for assessment and comparison of outcomes with peers and national standards. It additionally utilizes predictive analytics to alert to unexpected or negative trends before becoming clinically apparent and has been utilized for monitoring in real-time the benefits of ERAS® implementation.
If we can use technology to assess surgical outcomes, what about using it to analyze and predict disease-specific or treatment-specific patient outcomes? Deep learning through artificial intelligence can be “trained” to identify and/or predict disease or response to treatment that is more sensitive than the human eye. Demonstrated to be efficient in predicting pathologic tumor response to neoadjuvant chemotherapy in pancreatic adenocarcinoma and in identifying the malignant potential of pancreatic cystic neoplasms, it has also been used with ERAS® pathways to triage patient care and assess wounds for infection risk [24, 25]. One can bet this disruptive approach is only going to continue to infiltrate and expand across healthcare systems.
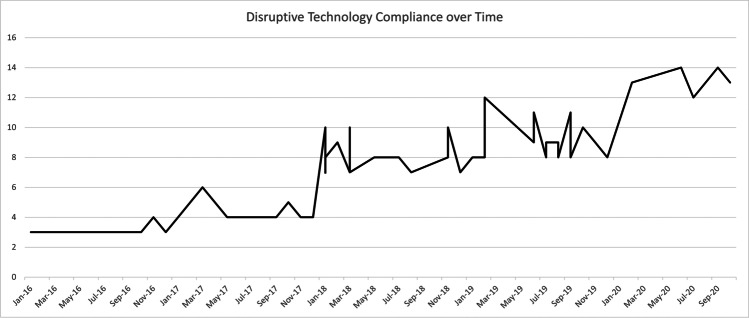
Other disruptive technologies encountered in conjunction with ERAS® include but are not limited to simulation for team building, cumulative sum analytics (CUSUM), and prescriptive analytics [8, 20, 26]. While each of these and the presented disruptive technologies begin through, yes, disruption, and perhaps, yes, significant frustration, each offers benefit and improvement if given the chance. We assessed Carolinas Medical Center of Atrium Health’s experience with disruptive technologies to establish an adoption benchmark. Their hepatobiliary surgical department follows ERAS® pathways for eleven procedures including open and robotic pancreaticoduodenectomy, open, robotic, and laparoscopic left pancreatectomy, and open, robotic, and laparoscopic major or minor hepatectomy. In order to identify the clinical penetrance of disruptive technology, we analyzed one of these procedures, robotic left pancreatectomy (RLP), assuming uniform permeation across all eleven hepatobiliary procedures as the same clinical team of nurses, providers, and educators exist throughout. After obtaining IRB approval, we identified 53 RLP patients between 2016 and 2020, and assessed adoption of 20 disruptive technologies (Fig. 1). While there was generalized improvement over time, in close examination of each technology, compliance adoption varies based on implementation requirements. Implementation can be either system-based, provider-based, or both provider- and patient-based (Fig. 2). Healthcare or hospital system–based technologies result in immediate implementation across all patients (Fig. 2A) whereas provider-based technologies are gradually adopted as an understanding needs to be established and then applied (Fig. 2B). Strategies that are both provider- and patient-based behave more erratically as compliance and adoption require patient dedication and investment outside of system and provider factors (Fig. 2C).
Fig. 1.
Implementation of 20 disruptive technologies in patients undergoing robotic left pancreatectomy between 2016 and 2020
Fig. 2.
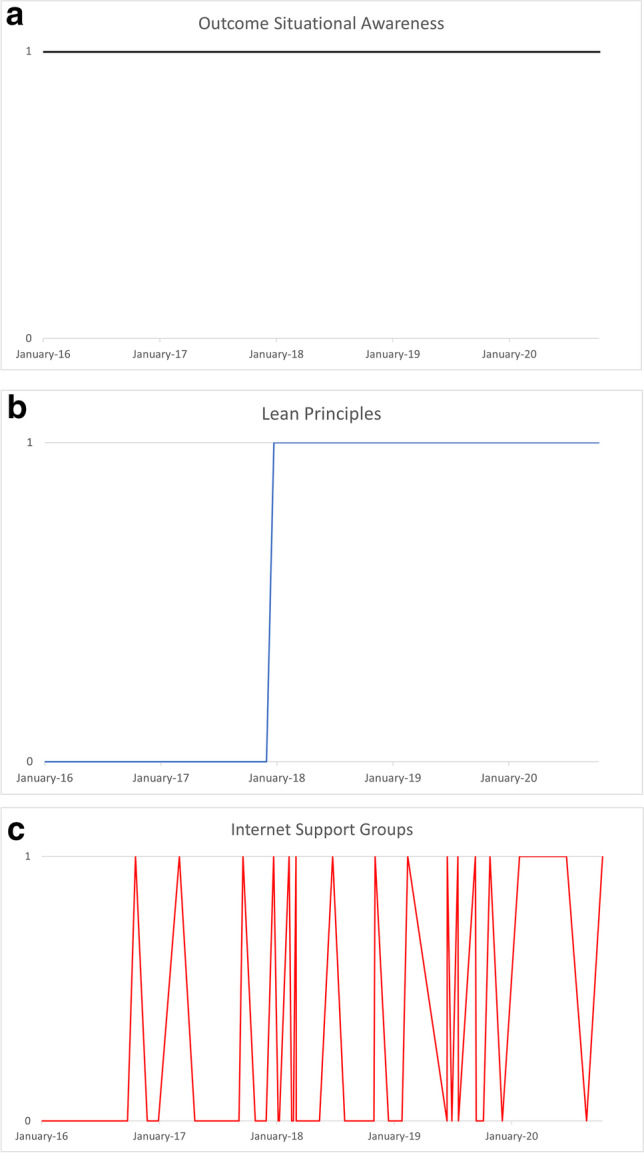
Different implementation strategies of disruptive technology over time. A System-based implementation of outcome situational awareness; B provider-based implementation of lean principles; C provider- and patient-based implementation of internet support groups
We know technology can be often daunting and sometimes overwhelming; however, it can drive growth and growth can drive improvement. So, while your armamentarium may hold the steps to a pancreaticoduodenectomy, allow it to also hold the steps for ERAS® implementation and its disruptive offspring (Table 1). We are in a new era where technology is limitless and if we embrace its extraordinary achievements, we can continue to improve for ourselves, for our patients, and for our healthcare systems.
Authors’ contribution
Maria Baimas-George – concept, design of work, data acquisition and analysis, drafted manuscript, critical revision.
Nicolas Demartines – concept, design of work, critical revision.
Dionisios Vrochides– concept, design of work, data acquisition, critical revision.
Declarations
Ethical approval
Not required for this manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schumpeter (2020) Clayton Christensen’s insights will outlive him. The Economist. https://www.economist.com/business/2020/01/30/clayton-christensens-insights-will-outlive-him
- 2.Bardram L, Funch-Jensen P, Jensen P, Crawford ME, Kehlet H. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet. 1995;345:763–764. doi: 10.1016/S0140-6736(95)90643-6. [DOI] [PubMed] [Google Scholar]
- 3.Harkey K, Kaiser N, Zhao J, et al. Postdischarge virtual visits for low-risk surgeries: a randomized noninferiority clinical trial. JAMA Surg. 2021;156(3):221–228. doi: 10.1001/jamasurg.2020.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vávra P, Roman J, Zonča P, et al. Recent development of augmented reality in surgery: a review. J Healthc Eng. 2017;2017:4574172. doi: 10.1155/2017/4574172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes AJ, McQuail P, Keogh P, Synnott K. Infographics improve comprehension and recall at the orthopaedic journal club. J Surg Educ. 2020;78(4):1345–1349. doi: 10.1016/j.jsurg.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Chaiyachati KH, Hubbard RA, Yeager A, et al. Association of rideshare-based transportation services and missed primary care appointments: a clinical trial. JAMA Intern Med. 2018;178:383–9. doi: 10.1001/jamainternmed.2017.8336. [DOI] [PubMed] [Google Scholar]
- 7.Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A Retrospective Database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25:332–40. doi: 10.18553/jmcp.2019.25.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickens RC, King L, Barrier M, et al. Clinically meaningful laboratory protocols reduce hospital charges based on institutional and ACS-NSQIP® risk calculators in hepatopancreatobiliary surgery. Am Surg. 2019;85:883–894. doi: 10.1177/000313481908500843. [DOI] [PubMed] [Google Scholar]
- 9.Baimas-George M, Watson M, Elhage S, Parala-Metz A, Vrochides D, Davis BR. Prehabilitation in frail surgical patients: a systematic review. World J Surg. 2020;44(11):3668–3678. doi: 10.1007/s00268-020-05658-0. [DOI] [PubMed] [Google Scholar]
- 10.Brogi E, Cyr S, Kazan R, Giunta F, Hemmerling TM. Clinical performance and safety of closed-loop systems: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2017;124:446–55. doi: 10.1213/ANE.0000000000001372. [DOI] [PubMed] [Google Scholar]
- 11.Khanna AK, Hoppe P, Saugel B. Automated continuous noninvasive ward monitoring: future directions and challenges. Crit Care. 2019;23:194. doi: 10.1186/s13054-019-2485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiegel B, Fuller G, Lopez M et al (2019) Virtual reality for management of pain in hospitalized patients: a randomized comparative effectiveness trial. PLoS One 14:e0219115 [DOI] [PMC free article] [PubMed]
- 13.Lyman WB, Passeri M, Murphy K, et al. The next step in surgical quality improvement: outcome situational awareness. Can J Surg. 2020;63:E120–E122. doi: 10.1503/cjs.000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baimas-George M, Cochran A, Watson M, et al. Vertical compliance: a novel method of reporting patient specific ERAS compliance for real-time risk assessment. Int J Med Inform. 2020;141:104194. doi: 10.1016/j.ijmedinf.2020.104194. [DOI] [PubMed] [Google Scholar]
- 15.Baimas-George M, Cochran A, Tezber K, et al. A 2-year experience with enhanced recovery after surgery: evaluation of compliance and outcomes in pancreatic surgery. J Nurs Care Qual. 2020;36(2):E24–E28. doi: 10.1097/NCQ.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 16.Pickens R, Cochran A, Tezber K, et al. Using a mobile application for real-time collection of patient-reported outcomes in hepatopancreatobiliary surgery within an ERAS® pathway. Am Surg. 2019;85:909–917. doi: 10.1177/000313481908500847. [DOI] [PubMed] [Google Scholar]
- 17.Koball AM, Jester DJ, Domoff SE, Kallies KJ, Grothe KB, Kothari SN. Examination of bariatric surgery Facebook support groups: a content analysis. Surg Obes Relat Dis. 2017;13:1369–75. doi: 10.1016/j.soard.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Sezgin E, Huang Y, Ramtekkar U, Lin S. Readiness for voice assistants to support healthcare deliveryduring a health crisis and pandemic. NPJ Digit Med. 2020;3:122. doi: 10.1038/s41746-020-00332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng B, Denk PM, Martinec DV, Gatta P, Whiteford MH, Swanström LL. Building an efficient surgical team using a bench model simulation: construct validity of the Legacy Inanimate System for Endoscopic Team Training (LISETT) Surg Endosc. 2008;22:930–7. doi: 10.1007/s00464-007-9524-1. [DOI] [PubMed] [Google Scholar]
- 20.Lyman WB, Passeri MJ, Murphy K, et al. An objective approach to evaluate novice robotic surgeons using a combination of kinematics and stepwise cumulative sum (CUSUM) analyses. Surg Endosc. 2020;35(6):2765–2772. doi: 10.1007/s00464-020-07708-z. [DOI] [PubMed] [Google Scholar]
- 21.Collar RM, Shuman AG, Feiner S, et al. Lean management in academic surgery. J Am Coll Surg. 2012;214:928–36. doi: 10.1016/j.jamcollsurg.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Etges A, Stefani LPC, Vrochides D, Nabi J, Polanczyk CA, Urman RD. A standardized framework for evaluating surgical enhanced recovery pathways: a recommendations statement from the tdabc in health-care consortium. J Health Econ Outcomes Res. 2021;8:116–24. doi: 10.36469/jheor.2021.24590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eller MB, Drummond J, Pate K, et al. The impact of patient focused pre-operative education on patient readiness for surgery and length of stay in complex abdominal surgery: the preoperative learning and readiness in surgery (POLaRiS) program. Clinical Nutrition ESPEN. 2018;25:194–195. doi: 10.1016/j.clnesp.2018.03.087. [DOI] [Google Scholar]
- 24.Watson MD, Baimas-George MR, Murphy KJ, et al. Pure and hybrid deep learning models can predict pathologic tumor response to neoadjuvant therapy in pancreatic adenocarcinoma: a pilot study. Am Surg. 2020;87(12):1901–1909. doi: 10.1177/0003134820982557. [DOI] [PubMed] [Google Scholar]
- 25.Watson MD, Lyman WB, Passeri MJ, et al. Use of artificial intelligence deep learning to determine the malignant potential of pancreatic cystic neoplasms with preoperative computed tomography imaging. Am Surg. 2020;87(4):602–607. doi: 10.1177/0003134820953779. [DOI] [PubMed] [Google Scholar]
- 26.Baimas-George M, Watson M, Murphy KJ, et al. Robotic pancreaticoduodenectomy may offer improved oncologic outcomes over open surgery: a propensity-matched single-institution study. Surg Endosc. 2020;34(8):3644–3649. doi: 10.1007/s00464-020-07564-x. [DOI] [PubMed] [Google Scholar]