Abstract
PURPOSE
Non–small-cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion (Exon20ins) mutations exhibits inherent resistance to approved tyrosine kinase inhibitors. Amivantamab, an EGFR-MET bispecific antibody with immune cell–directing activity, binds to each receptor's extracellular domain, bypassing resistance at the tyrosine kinase inhibitor binding site.
METHODS
CHRYSALIS is a phase I, open-label, dose-escalation, and dose-expansion study, which included a population with EGFR Exon20ins NSCLC. The primary end points were dose-limiting toxicity and overall response rate. We report findings from the postplatinum EGFR Exon20ins NSCLC population treated at the recommended phase II dose of 1,050 mg amivantamab (1,400 mg, ≥ 80 kg) given once weekly for the first 4 weeks and then once every 2 weeks starting at week 5.
RESULTS
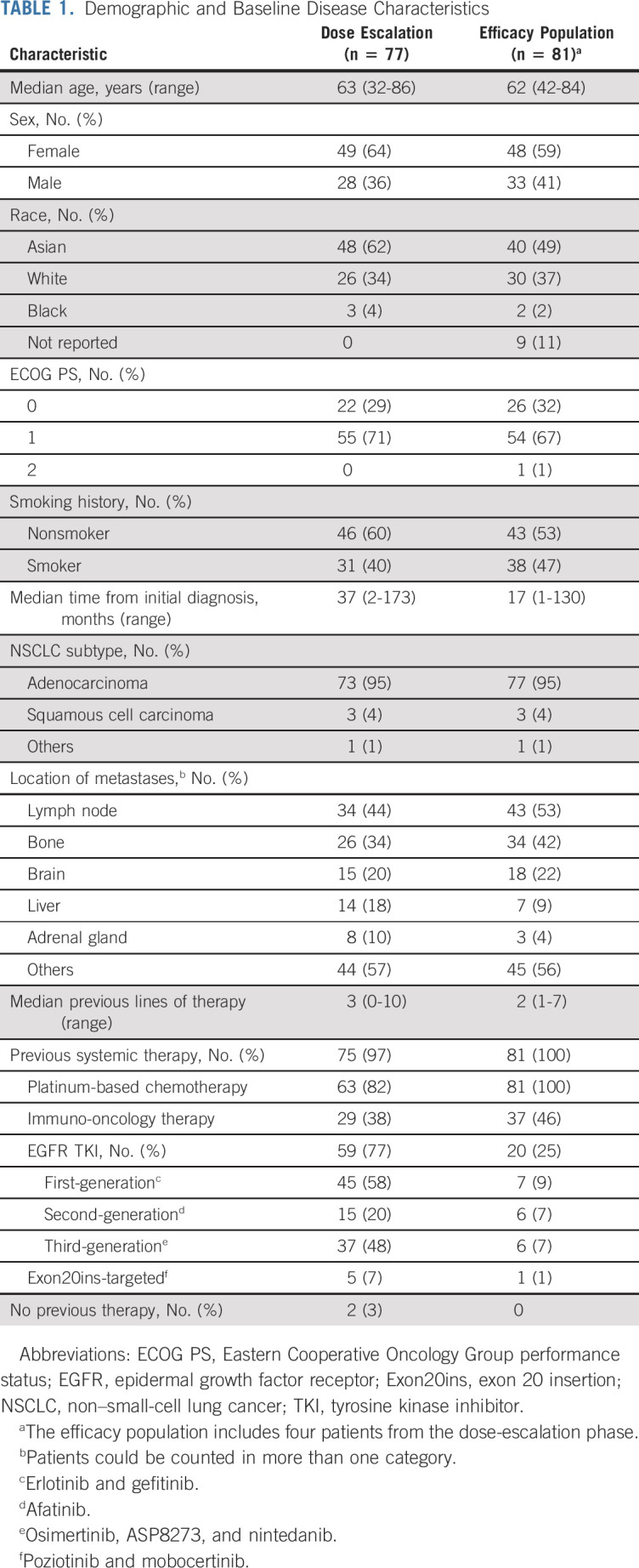
In the efficacy population (n = 81), the median age was 62 years (range, 42-84 years); 40 patients (49%) were Asian, and the median number of previous lines of therapy was two (range, 1-7). The overall response rate was 40% (95% CI, 29 to 51), including three complete responses, with a median duration of response of 11.1 months (95% CI, 6.9 to not reached). The median progression-free survival was 8.3 months (95% CI, 6.5 to 10.9). In the safety population (n = 114), the most common adverse events were rash in 98 patients (86%), infusion-related reactions in 75 (66%), and paronychia in 51 (45%). The most common grade 3-4 adverse events were hypokalemia in six patients (5%) and rash, pulmonary embolism, diarrhea, and neutropenia in four (4%) each. Treatment-related dose reductions and discontinuations were reported in 13% and 4% of patients, respectively.
CONCLUSION
Amivantamab, via its novel mechanism of action, yielded robust and durable responses with tolerable safety in patients with EGFR Exon20ins mutations after progression on platinum-based chemotherapy.
INTRODUCTION
Activating mutations in the epidermal growth factor receptor (EGFR) are a major oncogenic driver in non–small-cell lung cancer (NSCLC), with 85% of cases arising from an exon 19 deletion or exon 21 L858R point substitution.1-3 The third most frequently occurring mutations (≤ 12% of cases) are exon 20 insertion (Exon20ins) mutations, which are characterized by in-frame insertions and duplications near the C-helix of the EGFR kinase domain.4-8 Collectively, EGFR Exon20ins mutations are molecularly heterogeneous, with > 100 variants identified by next-generation sequencing (NGS).9
CONTEXT
Key Objective
To determine the recommended phase II dose of amivantamab, a novel epidermal growth factor receptor (EGFR)-MET bispecific antibody, and its antitumor activity in patients with EGFR exon 20 insertion (Exon20ins)–mutated non–small-cell lung cancer whose disease had progressed on platinum-based chemotherapy.
Knowledge Generated
To our knowledge, amivantamab is the first biologic therapy to demonstrate efficacy in patients with EGFR Exon20ins non–small-cell lung cancer after progression on standard-of-care platinum-based chemotherapy. Amivantamab exhibited a tolerable safety profile consistent with on-target inhibition of EGFR and MET pathways.
Relevance
We provide proof of concept that the EGFR can be effectively targeted through the extracellular domain for mutations that are resistant to EGFR tyrosine kinase inhibitors, including EGFR Exon20ins mutations, for which there are no approved therapies.
While similar to other EGFR mutations in biology and epidemiology,4,5 EGFR Exon20ins mutations are defined by an altered active site that sterically hinders tyrosine kinase inhibitor (TKI) binding, resulting in low response rates (0%-9%) with approved EGFR TKIs.10-14 As a result, the standard of care remains platinum-based chemotherapy, with an associated reduced median overall survival (OS) of 16 months, compared with 39 months in EGFR TKI–sensitive disease.12,15-20
Amivantamab (JNJ-61186372) is a fully human EGFR-MET bispecific antibody with immune cell–directing activity designed to engage two distinct driver pathways in NSCLC.21-23 By binding to each receptor's extracellular domain, amivantamab can inhibit ligand binding, promote receptor-antibody complex endocytosis and degradation, and induce Fc-dependent trogocytosis by macrophages and antibody-dependent cellular cytotoxicity by natural killer cells.21-23
CHRYSALIS, a first-in-human, phase I dose-escalation, and dose-expansion study (NCT02609776), evaluates the efficacy, safety, and pharmacokinetics of amivantamab in patients with advanced NSCLC. During the conduct of the study, amivantamab received Breakthrough Therapy Designation on the basis of the preliminary efficacy within the EGFR Exon20ins population, who had previous treatment with platinum-based chemotherapy and for whom limited treatment options were available. Here, we report the updated results from the postplatinum EGFR Exon20ins population.
METHODS
Patients
Eligible patients had confirmed metastatic or unresectable NSCLC and an Eastern Cooperative Oncology Group performance status ≤ 1 and had progressed on, were ineligible for, or declined standard-of-care therapy. Patients in dose expansion had measurable disease per RECIST version 1.1 and qualifying EGFR mutations or MET mutations or amplifications, as assessed by local testing or central NGS testing of circulating tumor DNA (ctDNA) or tumor tissue. Previous treatment with investigational EGFR Exon20ins–targeted TKIs was prohibited in the EGFR Exon20ins expansion cohort. Patients with untreated or active brain metastases were excluded; however, patients whose brain metastases were previously treated and asymptomatic at screening were eligible. Additional criteria are detailed in the Protocol (online only) and Data Supplement (online only).
Study Design
CHRYSALIS is an ongoing, first-in-human, open-label, multicenter, two-part phase I study of amivantamab as monotherapy (Fig 1A) and in combination with other therapies in patients with advanced NSCLC. The current analysis presents the results of amivantamab monotherapy after platinum-based chemotherapy, in patients who harbored EGFR Exon20ins mutations. The results from the other populations are ongoing and will be reported separately (Fig 1B).
FIG 1.
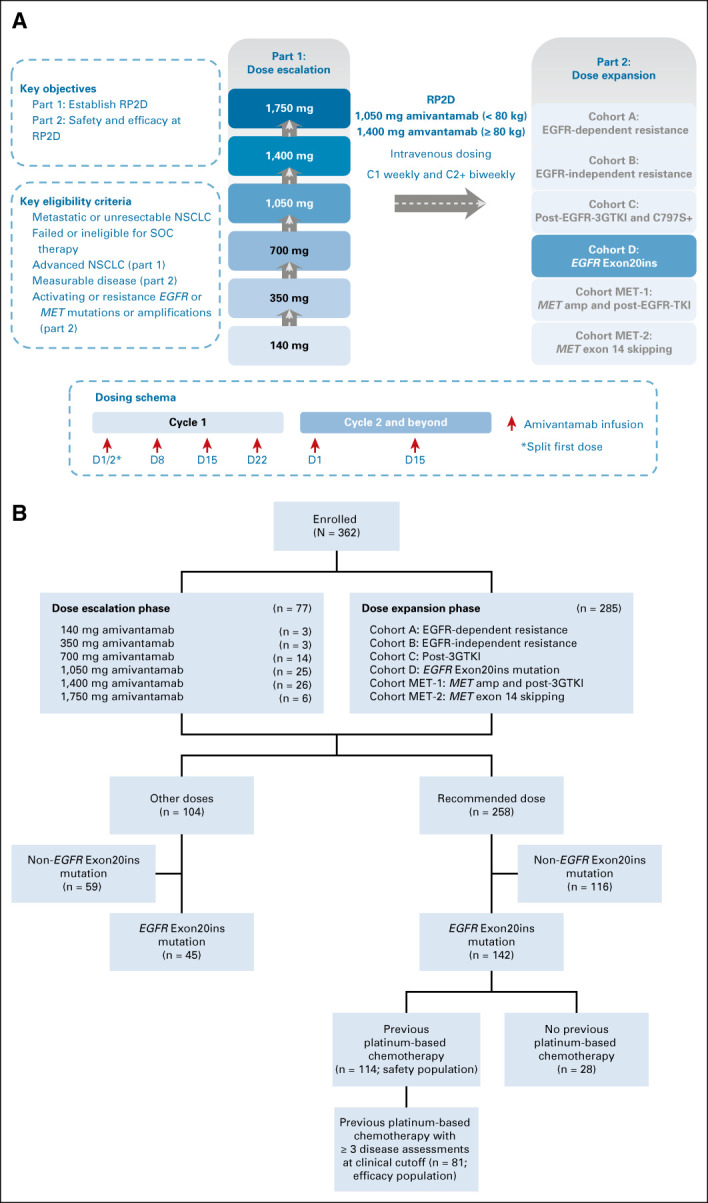
CHRYSALIS study design and patient disposition for amivantamab monotherapy. The CHRYSALIS study consisted of a dose-escalation and dose-expansion phase. (A) Patients with advanced NSCLC were enrolled in dose-escalation cohorts and patients were assigned to dose-expansion cohorts on the basis of EGFR and MET mutation status and previous therapy. (B) Patients were allocated to six different dose cohorts in the dose-escalation portion of the study. The safety population included all patients with EGFR Exon20ins NSCLC who had progressed on previous platinum-based chemotherapy and were treated at the RP2D (n = 114) by the data cutoff of June 8, 2020. At this clinical cutoff, the first 81 patients (four from dose escalation cohort, four from cohort A, and 73 from cohort D) met the criteria of having at least three scheduled disease assessments or discontinued, had disease progression, or died and were defined as the pivotal efficacy population. 3GTKI, third-generation tyrosine kinase inhibitor; amp, amplification; C, cycle; EGFR, epidermal growth factor receptor; Exon20ins, exon 20 insertion; NSCLC, non–small-cell lung cancer; RP2D, recommended phase II dose; SOC, standard of care; TKI, tyrosine kinase inhibitor.
The primary objective of dose escalation was to determine the maximum tolerated dose and recommended phase II dose (RP2D), and that of dose expansion was to evaluate the safety, tolerability, and antitumor activity of amivantamab at the RP2D. Primary end points for dose escalation and expansion were incidence of dose-limiting toxicity and overall response rate (ORR), respectively. Key secondary end points included duration of response (DOR), clinical benefit rate (CBR), progression-free survival (PFS), and OS.
A dose-escalation 3 + 3 design was used to assess amivantamab doses administered intravenously once weekly in the first 28-day cycle and every other week for subsequent cycles (Fig 1A). Additional enrollment (≤ 20 patients) in dose cohorts that were declared safe was allowed. For dose expansion, the RP2D was administered to cohorts assigned on the basis of qualifying EGFR and/or MET mutations or amplifications, and previous therapy.
Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent. Treatment beyond RECIST-defined disease progression was allowed in cases of continuous clinical benefit. To mitigate infusion-related reactions (IRRs), the first dose was split over two days and prophylactic premedication was required (Data Supplement). Management of rash was recommended per Protocol or in accordance with institutional guidelines (Data Supplement). The study was approved by an Independent Ethics Committee, and all patients provided written informed consent.
Study Assessments
Baseline imaging of thorax, abdomen, and pelvis was performed by computed tomography during screening. Response was assessed according to RECIST by the investigator at least every 6 weeks after the first amivantamab administration and confirmed by blinded independent central review (BICR). Baseline brain imaging by magnetic resonance imaging was performed for dose-expansion cohorts only. Monitoring for CNS disease was not mandatory and performed in accordance with local practice.
Patients with EGFR Exon20ins mutations were enrolled on the basis of local testing (tissue or ctDNA). Serum, plasma, and biopsy tissue were collected for pharmacokinetic, immunogenicity, or biomarker analyses. Guardant360 CDx (Guardant Health, Redwood City, CA) and Oncomine Dx Target Test (Thermo Fisher, Waltham, MA) companion diagnostics are being developed.
Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Statistical Analysis
An interim analysis was planned after dose-expansion cohorts enrolled ≥ 30 patients and had sufficient data to evaluate response (Data Supplement). For full expansion, assuming an ORR ≥ 35%, an enrollment of ≥ 60 patients was estimated to achieve a lower bound of 95% CI ≥ 12% (single-agent chemotherapy as the benchmark)24 with a one-sided alpha of .025. After receiving Breakthrough Therapy Designation for the population with previous platinum-based chemotherapy, in consultation with Health Authorities, a minimum of 80 patients was identified as a potential threshold, using similar assumptions, for a postplatinum-based chemotherapy comparison ORR of 23%.25
The safety population included patients with EGFR Exon20ins NSCLC who had progressed on platinum-based chemotherapy and were treated at the RP2D (n = 114) by the data cutoff of June 8, 2020. The pivotal efficacy population included the first 81 patients enrolled with EGFR Exon20ins NSCLC, after previous platinum-based chemotherapy (four patients from dose escalation and 77 from dose expansion [four from cohort A and 73 from cohort D]), who had at least three scheduled disease assessments or had discontinued, progressed, or died by the data cutoff of June 8, 2020 (Fig 1B). The efficacy data presented here reflect follow-up of this population through October 8, 2020, at which time all active responders in the efficacy population had ≥ 6 months of follow-up from the time of their first response.
ORR was calculated as the proportion of patients who achieved complete response (CR) or partial response (PR) as assessed by the investigator or BICR using RECIST. CBR was calculated as the proportion of patients achieving CR or PR or stable disease ≥ 11 weeks, corresponding to two disease assessments.
Data were summarized using descriptive statistics. Time-to-event end points were summarized using Kaplan-Meier estimates. No data imputation was applied for missing safety and efficacy evaluations. Additional statistical methods are provided in the Protocol.
RESULTS
Patients
Between May 27, 2016, and June 8, 2020, 362 patients were enrolled in the study. The initial dose escalation enrolled patients at two sites in South Korea and subsequently enrolled patients from sites in Japan and the United States to confirm the safety and pharmacokinetics of amivantamab, leading to a total enrollment of 77 patients. Across dose escalation and expansion, 258 patients were treated at the RP2D of 1,050 mg amivantamab (1,400 mg for patients ≥ 80 kg) given once weekly for the first 4 weeks and then once every 2 weeks starting at week 5. At the safety data cutoff of June 8, 2020, the median follow-up was 5.1 months (range, 0.2-29.3 months).
In the efficacy population, the median age was 62 years (range, 42-84), 48 patients (59%) were women, 40 (49%) were Asian, and all had received previous platinum-based chemotherapy (Table 1). Eighteen patients (22%) had a history of treated brain lesions before receiving the first dose. The median number of previous lines of therapy was two (range, 1-7); 20 (25%) had previous treatment with EGFR TKIs, and 37 (46%) had previous immuno-oncology therapies. At the efficacy data cutoff of October 8, 2020, the median follow-up was 9.7 months (range, 1.1-29.3 months).
TABLE 1.
Demographic and Baseline Disease Characteristics
Pharmacokinetics, Pharmacodynamics, and Immunogenicity
No maximum tolerated dose had been identified through the maximum assessed dose of 1,750 mg; therefore, selection of the RP2D of 1,050 mg (1,400 mg for patients ≥ 80 kg) was based on safety, pharmacokinetic, and pharmacodynamic data. Amivantamab exhibited linear pharmacokinetics at 350-1,750 mg and nonlinear pharmacokinetics below 350 mg (Data Supplement). The mean nonspecific linear clearance of amivantamab was 0.36 L/d, with a mean half-life of 11.3 days, associated with linear elimination. The RP2D of 1,050 mg provided saturation of circulating serum EGFR and MET targets and coverage of the preclinically established target concentration of 168 μg/mL. Saturation of circulating targets started at 350 mg for EGFR and 140 mg for MET after a single dose, consistent with manifestation of on-target EGFR (rash) and MET (hypoalbuminemia and peripheral edema) toxicities (Data Supplement). Complete saturation of EGFR and MET circulating targets throughout the dosing period was achieved at ≥ 700 mg (Data Supplement). To reduce pharmacokinetic variability and exposure differences, two-tiered weight-based dosing was established using population pharmacokinetic analysis. The RP2D of 1,400 mg for patients ≥ 80 kg provided similar exposure to those < 80 kg at 1,050 mg (Data Supplement).
The incidence of antibodies to amivantamab was low. No evident impact of antibody titer levels on pharmacokinetic parameters, clinical activity, or safety of amivantamab was observed (Data Supplement).
Safety
The safety profile of the EGFR Exon20ins safety population and patients treated at the RP2D was consistent with on-target anti-EGFR and anti-MET activity (Table 2). The median treatment duration was 3.7 months for the safety population (range, 0.03-23.9 months) and patients treated at the RP2D (range, 0.03-29.7 months). Safety in the dose-escalation cohorts is presented in the Data Supplement.
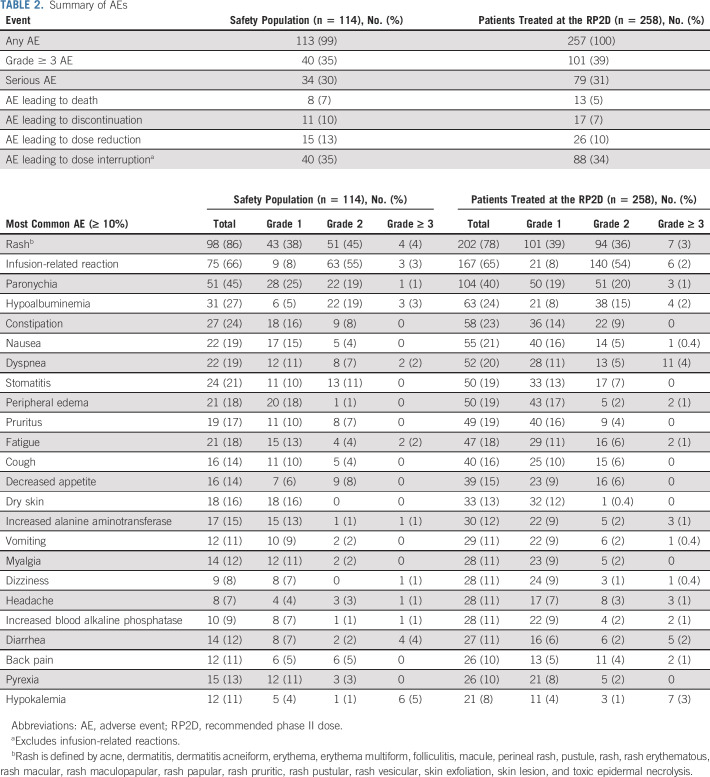
TABLE 2.
Summary of AEs
AEs associated with EGFR inhibition included rash (including dermatitis acneiform) in 98 patients (86%), paronychia in 51 (45%), stomatitis in 24 (21%), pruritus in 19 (17%), and diarrhea in 14 (12%). AEs associated with MET inhibition included hypoalbuminemia and peripheral edema in 31 (27%) and 21 (18%) patients, respectively. Interstitial lung disease (including pneumonitis) was reported in five patients (4%).
IRRs were commonly observed (75 [66%]), occurred almost exclusively on cycle 1, day 1 (93%) or day 2 (4%; first dose is split over two days), and rarely recurred with subsequent dosing (one event was reported after cycle 2 [0.09% of doses administered]; Data Supplement). Given the observed risk with the first exposure, amivantamab was initially administered at a reduced rate of 25 mL/h in the first 2 hours and increased to 50 mL/h for the remainder of the day 1 infusion of 350 mg. With this administration, the median time to first onset of IRR was 45 minutes and the majority of IRRs were grade 1-2; predisposing factors were not identified.
Grade ≥ 3 AEs were observed in 40 patients (35%; Table 2), with most frequent being hypokalemia in six (5%) and rash, pulmonary embolism, diarrhea, and neutropenia in four (4%) each. Treatment-related grade ≥ 3 AEs were reported in 18 patients (16%); most common included rash in four (4%) and IRR and neutropenia in three (3%) each. Serious AEs occurred in 34 patients (30%); pulmonary embolism and back pain were most frequently reported (3% each; Data Supplement). Treatment-related serious AEs were reported in 10 patients (9%) and included IRR and diarrhea (two patients each; 2%) and single reports each of cellulitis, infected dermal cyst, interstitial lung disease, pneumonitis, atrial flutter, rash, and toxic epidermal necrolysis.
Treatment-related dose reductions occurred in 15 patients (13%), with rash (11 [10%]) being most frequently reported. Five patients (4%) had treatment-related discontinuation: rash and IRR in two (1.8%) each and paronychia in one (1%). There were no treatment-related grade 5 events.
Efficacy
Tumor response.
In the efficacy population, three confirmed CRs and 29 PRs were observed, for an ORR of 40% (95% CI, 29 to 51) as assessed by BICR (Table 3). With 15 responders remaining on treatment at the data cutoff, the median DOR was 11.1 months (95% CI, 6.9 to not reached), with 75% of responses observed at the first disease assessment (Fig 2A and the Data Supplement). The CBR, which included an additional 28 patients with stable disease ≥ 11 weeks, was 74% (95% CI, 63 to 83). The investigator-assessed ORR of 36% (95% CI, 25 to 47) was consistent with the BICR (Data Supplement). Antitumor activity was observed across all prespecified and post hoc subpopulations (Fig 2B).
TABLE 3.
Response as Assessed by Blinded Independent Central Review
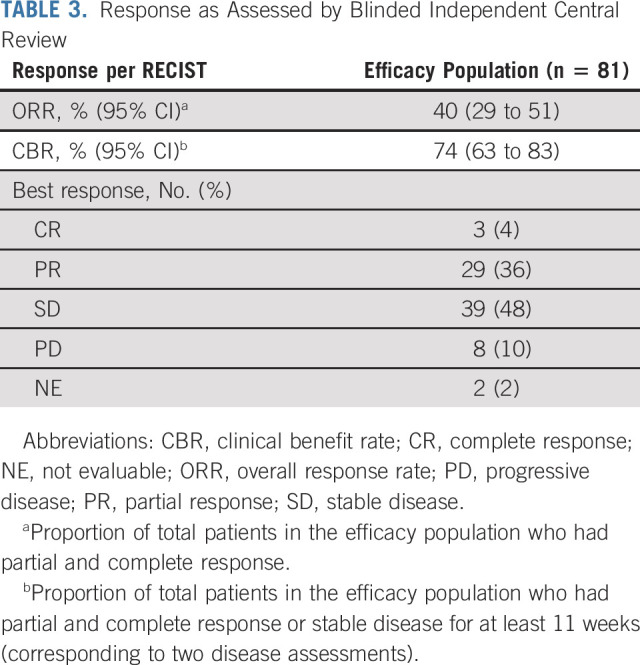
FIG 2.
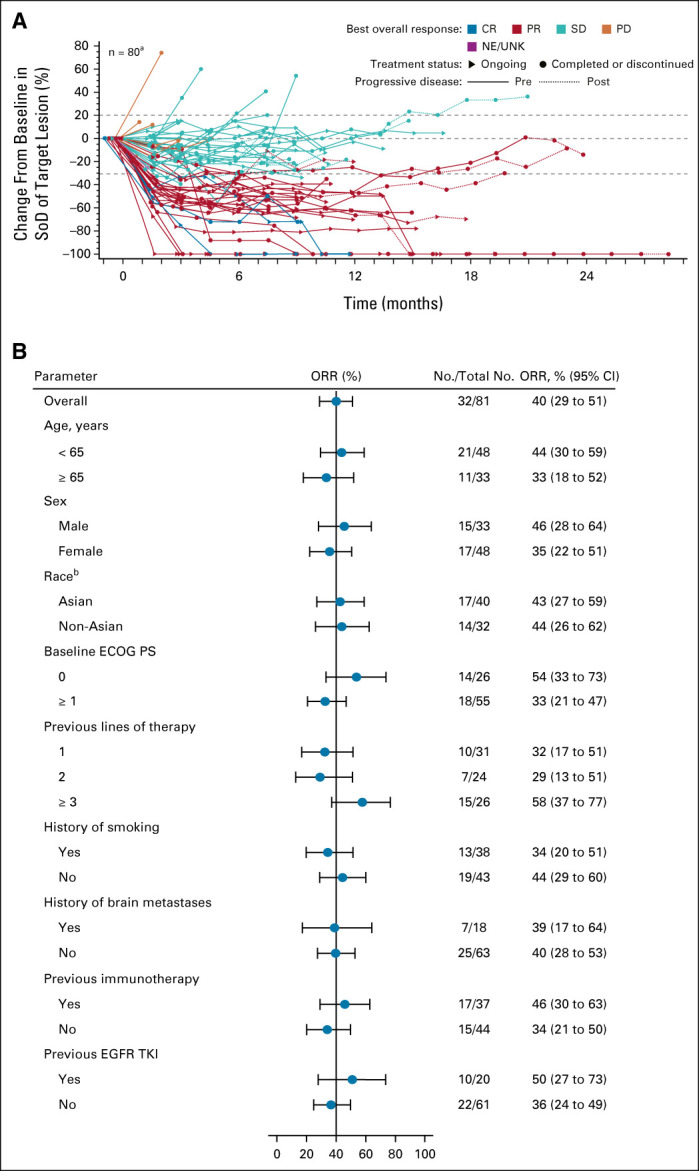
Tumor response over time and ORR by subgroups. (A) Spider plot of percent change from baseline in sum of target lesion diameters over time in the efficacy population (n = 81) as assessed by BICR. aOne patient discontinued before any disease assessment and is not included in the plot. Dotted lines at 20% and –30% indicate thresholds for PD and PR, respectively, as per RECIST, v1.1. (B) Results of prespecified (age, sex, race, baseline ECOG PS, history of smoking, and previous immunotherapy) and post hoc (history of brain metastases, previous lines of therapy, and previous EGFR TKI) subgroup analysis of ORR in the efficacy population on the basis of BICR. bDoes not include nine patients with race not reported and multiple race. BICR, blinded independent central review; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; SoD, sum of lesion diameters; TKI, tyrosine kinase inhibitor; UNK, unknown.
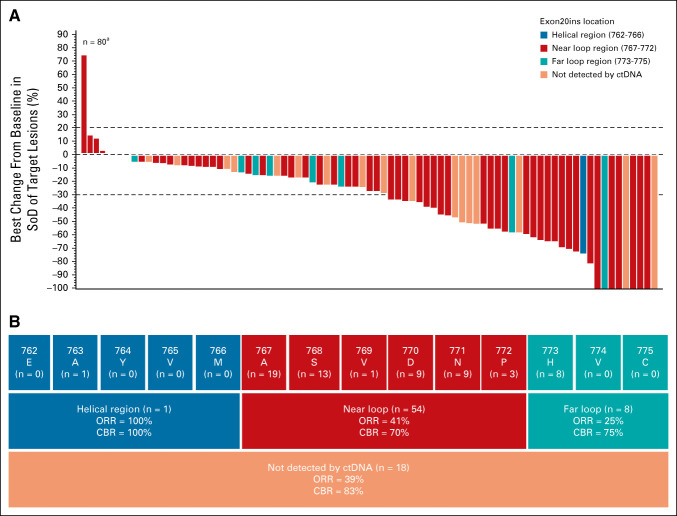
All 81 patients in the efficacy population had ctDNA or tumor samples submitted for central testing, of which 63 had detectable ctDNA, identifying 25 distinct Exon20ins variants. Antitumor responses were observed in patients who harbored insertions within the helical, near-loop, and far-loop regions of exon 20 (Figs 3A and 3B). Through central NGS testing, one patient was identified with MET amplification (copy number of 8); this patient had a PR.
FIG 3.
Tumor reduction and responses in the efficacy population. (A) Waterfall plot displaying best percent change from baseline in sum of target lesion diameters by location of EGFR Exon20ins (determined by Guardant360 testing) for patients in the efficacy population (n = 81) as assessed by BICR. aOne patient discontinued before any disease assessment and is not included in the plot. Dotted lines at 20% and –30% indicate thresholds for progressive disease and partial response, respectively, as per RECIST, v1.1. (B) Insertion regions of EGFR Exon20ins identified in the efficacy population and ORR as assessed by BICR for each key region of exon 20 (blue, red, and teal boxes). Site of EGFR Exon20in could not be identified by ctDNA analysis for 18 patients (dark orange). BICR, blinded independent central review; CBR, clinical benefit rate; ctDNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; Exon20ins, exon 20 insertion; ORR, overall response rate; SoD, sum of lesion diameters.
PFS and OS.
Progression or death occurred in 47 patients (58%); the median PFS was 8.3 months (95% CI, 6.5 to 10.9) by BICR and investigator (95% CI, 5.5 to 10.6) assessments. The median OS was 22.8 months (95% CI, 14.6 to not reached), although with 23 deaths, this end point remains immature.
DISCUSSION
Patients with EGFR Exon20ins NSCLC have among the poorest prognoses of patients with NSCLC. A recent real-world analysis demonstrated a 13% ORR across second-line treatments, with a median PFS of 3.5 months.26 Using a similar real-world data set, a median OS of 12.5 months was reported in the relapsed or refractory setting.15 Given amivantamab's unique mechanism of action and the unmet medical need associated with EGFR Exon20ins NSCLC, this population was among the initial populations selected for exploration of amivantamab activity.
The therapeutic challenge with EGFR Exon20ins–directed TKI therapy has been overcoming steric hindrance at the active site, while maintaining selectivity against the wild-type receptor to minimize toxicity. Two EGFR Exon20ins–directed TKIs, poziotinib and mobocertinib, have recently reported results. Among 115 patients with EGFR Exon20ins NSCLC, poziotinib demonstrated a 14.8% ORR. Rates of treatment-related grade ≥ 3 rash and diarrhea were 28% and 26%, respectively.27 Mobocertinib showed a 28% ORR in 114 patients with EGFR Exon20ins NSCLC who progressed on platinum-based chemotherapy. Grade ≥ 3 treatment-related AEs were reported in 46%. Treatment-related AEs of diarrhea in 90% of patients (21% grade 3-4) and rash in 45% were reported.28
The safety profile of amivantamab was consistent with expected on-target toxicities associated with inhibition of EGFR and MET. IRRs were frequently observed but were low grade, primarily limited to the first infusion, and rarely occurred with further dosing. The risk of IRR was mitigated by splitting the first dose over two days and through administration of prophylactic premedication (Data Supplement) and reduced initial infusion rates using diluent priming of tubing to ensure slow initial exposure to amivantamab. The incidence of severe toxicity and toxicity-related discontinuations were low despite a lack of selectivity against the wild-type EGFR, suggesting that the bispecific nature of amivantamab may affect the safety profile, potentially through altered target cell selectivity (eg, tumor cells).21,29,30
Early efficacy in this study identified clinically significant monotherapy activity of amivantamab in EGFR Exon20ins NSCLC in the chemotherapy-naive (n = 10) and chemotherapy-relapsed setting (n = 29). This experience led to Breakthrough Therapy Designation in both the United States and China for the latter population on the basis of the investigator-assessed ORR of 41%, the median DOR of 7 months, and the CBR of 72%.31 These preliminary data were confirmed in the current expanded population of 81 patients with increased follow-up, demonstrating a BICR-assessed ORR of 40%, a median DOR of 11.1 months, and a CBR of 74%. On the basis of these data, amivantamab is approved in the United States for the treatment of patients with EGFR Exon20ins NSCLC whose disease progressed on or after platinum-based chemotherapy.
The role of MET expression and/or activation is not well-defined in EGFR Exon20ins disease; therefore, the anti-MET activity of amivantamab may not play a large role in initial response in this population. Only one patient was identified with baseline MET amplification, and this patient achieved a confirmed PR, suggesting that when both driver pathways are active, amivantamab retains antitumor activity.32 Furthermore, it is possible that the anti-MET activity of amivantamab may contribute to response duration, by preventing emergence of tumor resistance through MET activation.
Amivantamab has demonstrated preliminary activity in EGFR TKI–resistant tumors driven by EGFR secondary mutations (T790M and/or C797S) or new MET amplification.32-34 Similarly, one patient from the present study, previously treated with poziotinib and with T790M resistance mutation, had a PR to amivantamab. The ability to inhibit EGFR-based and/or MET-based resistance mechanisms to EGFR TKIs was the basis for the bispecific strategy underlying amivantamab development, and this study suggests that both pathways need not be activated for initial amivantamab response.32,34
Limitations of this study were related to both the early phase of the study and the patient population under study. The analysis presented here does not include the full enrollment of the EGFR Exon20ins population, but represents a subset of patients without standard of care and with sufficient follow-up to support regulatory review. As such, it includes all postplatinum patients with EGFR Exon20ins enrolled on the CHRYSALIS study, through the clinical cutoff. An analysis of the entire EGFR Exon20ins population, including those without previous chemotherapy treatment, will be conducted after sufficient follow-up. As an exploratory phase I study that was not randomized and did not include a control arm, interpretation of the data must be made by historical comparison within the literature, or through the use of real-world evidence, to inform clinical outcomes in a population that has been excluded from most phase III EGFR-mutated NSCLC studies. Additionally, not all Exon20ins mutations were detectable by ctDNA analysis and tumor tissues were often not of sufficient quality or quantity, limiting genomic data available for central analysis. Finally, as patients with active or untreated brain metastases were excluded from the study, the activity of amivantamab in CNS disease will need to be explored in future studies.
In conclusion, amivantamab is the first bispecific antibody to demonstrate clinically meaningful efficacy in patients with EGFR Exon20ins NSCLC. Amivantamab has the potential to target other EGFR-driven and/or MET-driven tumors, as monotherapy or in combination, given its favorable safety profile.32-35 These combined approaches are under investigation in the CHRYSALIS study35 and in frontline and relapsed EGFR-mutated NSCLC (NCT04487080, NCT04538664, and NCT04077463). These early data suggest that specificity of an antibody-based strategy can be successfully broadened through a bispecific approach, while maintaining clinically significant activity in a population thought to be dependent on only one of the targeted driver proteins.
ACKNOWLEDGMENT
The authors would like to thank all the patients who participated in this study and their families and caregivers. The authors would also like to thank the physicians and nurses who cared for the patients and the staff at the clinical sites.
K.L.R. is currently at Cedars-Sinai Medical Center, Los Angeles, CA.
APPENDIX
TABLE.
List of Investigators
Keunchil Park
Consulting or Advisory Role: AstraZeneca, Lilly, Ono Pharmaceutical, Bristol Myers Squibb, MSD, Blueprint Medicines, Amgen, Merck KGaA, LOXO, AbbVie, Daiichi Sankyo, Boehringer Ingelheim, JNJ, Eisai, Puma Biotechnology
Speakers' Bureau: Boehringer Ingelheim
Research Funding: AstraZeneca, MSD Oncology
Eric B. Haura
Consulting or Advisory Role: Janssen Oncology, Revolution Medicines, Ellipses Pharma, Janssen Research & Development, Amgen
Research Funding: Janssen, Novartis, Revolution Medicines, AstraZeneca, Incyte, Genentech, Spectrum Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Protein-Protein Interactions as Biomarkers Patent
Natasha Leighl
Honoraria: Boehringer Ingelheim
Consulting or Advisory Role: Xcovery
Research Funding: Novartis, Roche Canada, Guardant Health, MSD, EMD Serono, Lilly
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bristol Myers Squibb, Nektar, GlaxoSmithKline, Roche, AstraZeneca
Paul Mitchell
Honoraria: MSD, AstraZeneca, Roche
Consulting or Advisory Role: Roche, Specialised Therapeutics, PUMA Biotechnology, AstraZeneca, Boehringer Ingelheim, BMS, MSD, Amgen
Catherine A. Shu
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Genentech/Roche
Research Funding: Celgene, Genentech/Roche, Janssen, MedImmune
Travel, Accommodations, Expenses: Genentech/Roche
Nicolas Girard
Employment: AstraZeneca
Consulting or Advisory Role: Roche, Lilly, Boehringer Ingelheim, AstraZeneca, Novartis, Pfizer, Bristol Myers Squibb, MSD, Takeda, GlaxoSmithKline, AbbVie, Pharmamar, Janssen, Sanofi
Research Funding: Roche, AstraZeneca, Boehringer Ingelheim
Travel, Accommodations, Expenses: Roche, AstraZeneca, Bristol Myers Squibb, MSD Oncology
Santiago Viteri
Consulting or Advisory Role: Roche, Bristol Myers Squibb, Janssen, Takeda, Reddy Pharma Iberia, Merck KGaA
Speakers' Bureau: Bristol Myers Squibb, Roche
Travel, Accommodations, Expenses: Roche, MSD, Merck KGaA
Ji-Youn Han
Honoraria: Roche, AstraZeneca, Bristol Myers Squibb, Takeda
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Bristol Myers Squibb, Lilly, Novartis, Takeda, Pfizer
Research Funding: Roche, Pfizer, Ono Pharmaceutical, Takeda
Chee Khoon Lee
Honoraria: AstraZeneca, Pfizer, Amgen, Takeda, Yuhan, Boehringer Ingelheim
Consulting or Advisory Role: Novartis, Boehringer Ingelheim, Takeda, AstraZeneca, Yuhan, Amgen
Research Funding: AstraZeneca, Roche, Merck KGaA
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
Alexander I. Spira
Stock and Other Ownership Interests: Lilly
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer
Consulting or Advisory Role: Array BioPharma, Incyte, Amgen, Novartis, AstraZeneca/MedImmune, Mirati Therapeutics, Gritstone Oncology, Jazz Pharmaceuticals, Merck, Bristol Myers Squibb
Research Funding: Roche, AstraZeneca, Boehringer Ingelheim, Astellas Pharma, MedImmune, Novartis, Newlink Genetics, Incyte, AbbVie, Ignyta, LAM Therapeutics, Trovagene, Takeda, Macrogenics, CytomX Therapeutics, LAM Therapeutics, Astex Pharmaceuticals, Bristol Myers Squibb, Loxo, Arch Therapeutics, Gritstone Oncology, Plexxikon, Amgen, Loxo, Daiichi Sankyo, ADC Therapeutics, Janssen Oncology, Mirati Therapeutics, Rubius Therapeutics
Dong-Wan Kim
Research Funding: Alpha Biopharma, AstraZeneca/MedImmune, Hanmi, Janssen, Merus, Mirati Therapeutics, MSD, Novartis, Ono Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, Yuhan, Boehringer Ingelheim, Amgen, Daiichi Sankyo, Chong Kun Dang Pharmaceutical, BridgeBio Pharma, GlaxoSmithKline
Travel, Accommodations, Expenses: Daiichi Sankyo, Amgen
Ki Hyeong Lee
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Pfizer, Lilly
Rachel E. Sanborn
Honoraria: AstraZeneca, Amgen
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, EMD Serono, Blueprint Medicines, Daiichi Sankyo/Lilly, Janssen Oncology, Macrogenics
Research Funding: Bristol Myers Squibb, MedImmune, Merck, AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
José Trigo
Consulting or Advisory Role: Takeda, Boehringer Ingelheim, Bristol Myers Squibb, Merck Serono
Speakers' Bureau: MSD Oncology, AstraZeneca, Merck Serono, Roche
Travel, Accommodations, Expenses: MSD Oncology, Bristol Myers Squibb, AstraZeneca
Koichi Goto
Honoraria: Guardant Health, Janssen, Daiichi Sankyo Co Ltd, Amgen, Eisai, Kyowa Kirin Co Ltd, Bristol Myers Squibb, AstraZeneca Japan, Pfizer, Chugai Pharma, Taiho Pharmaceutical, Ono Pharmaceutical, Novartis, Lilly Japan, Boehringer Ingelheim, Life Technologies, MSD K.K, Nippon Kayaku, Takeda, Otsuka
Consulting or Advisory Role: Otsuka
Research Funding: Medical & Biological Laboratories Co Ltd, Kyowa Kirin Co Ltd, Amgen Astellas BioPharma, Kissei Pharmaceutical, Merck, Merus, NEC Corporation, Spectrum Pharmaceuticals, Shanghai HaiHe Pharmaceutical, MSD K.K, AstraZeneca Japan, Taiho Pharmaceutical, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Sumitomo Dainippon Pharma Co Ltd, Takeda, Astellas Pharma, Eisai, Lilly Japan, Pfizer, Bristol Myers Squibb, Ignyta, Life Technologies, Janssen, Xcoo, Loxo, Sysmex, Amgen
James Chih-Hsin Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, Takeda, Lilly, Pfizer
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Clovis Oncology, Lilly, MSD Oncology, Merck Serono, Celgene, Bayer, Pfizer, Ono Pharmaceutical, Bristol Myers Squibb, Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Takeda Oncology, Amgen, Incyte, Merck Serono, Jenssen, GSK, Takeda, Daiichi Sankyo, Novartis
Travel, Accommodations, Expenses: Pfizer
Ramaswamy Govindan
Honoraria: Genentech/AbbVie, AbbVie, Geneplus-Beijing Institute
Consulting or Advisory Role: Genentech/Roche, AbbVie, AstraZeneca/MedImmune, Pfizer, Bristol Myers Squibb, Nektar, Jounce Therapeutics, F. Hoffmann La Roche AG, Janssen, Amgen, Achilles Therapeutics
Joshua M. Bauml
Consulting or Advisory Role: Bristol Myers Squibb, Merck, AstraZeneca, Genentech, Celgene, Boehringer Ingelheim, Guardant Health, Takeda, Novartis, Janssen, Ayala Pharmaceuticals, Regeneron, Inivata, Novartis, Foundation Medicine
Research Funding: Merck, Carevive Systems, Novartis, Incyte, Bayer, Janssen, AstraZeneca, Takeda, Amgen, Pfizer, Mirati Therapeutics
Pilar Garrido
Consulting or Advisory Role: Roche Pharma AG, AstraZeneca, MSD Oncology, Bristol Myers Squibb, Takeda, Lilly, Pfizer, Novartis/Pfizer, Boehringer Ingelheim, AbbVie, Amgen, Bayer, Gebro Pharma, Nordic Group, Boehringer Ingelheim, Janssen Biotech, Janssen Oncology, GlaxoSmithKline
Speakers' Bureau: Roche Pharma AG, Takeda, AstraZeneca, MSD Oncology, BMS, Pfizer, Novartis, Boehringer Ingelheim, Nordic Group, Janssen, Boehringer Ingelheim, Janssen Oncology
Travel, Accommodations, Expenses: AstraZeneca, Roche, Bristol Myers Squibb
Other Relationship: Janssen Oncology, Novartis
Matthew G. Krebs
Honoraria: Roche
Consulting or Advisory Role: Roche, Achilles Therapeutics, Janssen, Seattle Genetics, OM Pharma, Bayer
Speakers' Bureau: Roche, Janssen
Research Funding: Roche
Travel, Accommodations, Expenses: AstraZeneca, BerGenBio, Immutep
Karen L. Reckamp
Consulting or Advisory Role: Amgen, Tesaro, Boehringer Ingelheim, Takeda, AstraZeneca, Seattle Genetics, Calithera Biosciences, Genentech, Blueprint Medicines, Daiichi Sankyo/Lilly, EMD Serono, Janssen Oncology, Lilly, Merck KGaA
Research Funding: Bristol Myers Squibb, Pfizer, ARIAD, Xcovery, Adaptimmune, Genentech/Roche, Boehringer Ingelheim, AbbVie, ACEA Biosciences, Loxo, GlaxoSmithKline, Guardant Health, Janssen Oncology, Seattle Genetics, Zeno Pharmaceuticals, Calithera Biosciences, Elevation Oncology, Daiichi Sankyo/Astra Zeneca
John Xie
Employment: JNJ
Stock and Other Ownership Interests: JNJ
Joshua C. Curtin
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Nahor Haddish-Berhane
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Amy Roshak
Employment: Janssen Pharmaceutical Company of Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Dawn Millington
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Janssen Research & Development
Patricia Lorenzini
Employment: Janssen Pharmaceuticals of Johnson & Johnson
Meena Thayu
Employment: Janssen Oncology
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Other Relationship: Janssen Oncology
Roland E. Knoblauch
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Travel, Accommodations, Expenses: Johnson & Johnson
Byoung Chul Cho
Leadership: Gencurix Inc, Interpark Bio Convergence Corp
Stock and Other Ownership Interests: TheraCanVac Inc, Gencurix Inc, Bridgebio Therapeutics, KANAPH Therapeutic Inc, Cyrus Therapeutics, Interpark Bio Convergence Corp
Consulting or Advisory Role: Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol Myers Squibb, Yuhan, Pfizer, Janssen, Takeda, MSD, Ono Pharmaceutical, Eli Lilly, Medpacto, Blueprint Medicines, KANAPH Therapeutic Inc, Brigebio Therapeutics, Cyrus Therapeutics, Guardant Health, Oscotec Inc
Research Funding: Novartis, Bayer, AstraZeneca, MOGAM Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, MSD, AbbVie, Medpacto, GIInnovation, Eli Lilly, Blueprint Medicines, Interpark Bio Convergence Corp
Patents, Royalties, Other Intellectual Property: Champions Oncology
Other Relationship: DAAN Biotherapeutics
No other potential conflicts of interest were reported.
See accompanying article on page 3403
PRIOR PRESENTATION
Presented at the ASCO 2020 Annual Meeting, virtual, May 29-31, 2020, and updated data were presented at the International Association for the Study of Lung Cancer World Conference on Lung Cancer (WCLC) 2020 Annual Meeting, virtual, January 28-31, 2021.
SUPPORT
This study was funded by Janssen R&D LLC M.G.K. acknowledges support from National Institute for Health Research (NIHR) Manchester Biomedical Research Centre and NIHR Manchester Clinical Research Facility at The Christie and Manchester Experimental Cancer Medicine Centre (Manchester, United Kingdom). Medical writing assistance was funded by Janssen Global Services LLC and provided by Tracy T. Cao, PhD (Janssen Global Services LLC).
CLINICAL TRIAL INFORMATION
NCT02609776 (CHRYSALIS)
DATA SHARING STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
AUTHOR CONTRIBUTIONS
Conception and design: Keunchil Park, Nicolas Girard, Santiago Viteri, Joshua K. Sabari, José Trigo, Jong-Seok Lee, John Xie, Roland E. Knoblauch, Byoung Chul Cho
Provision of study materials or patients: Keunchil Park, Natasha B. Leighl, Paul Mitchell, Santiago Viteri, Ji-Youn Han, Sang-We Kim, Alexander I. Spira, Dong-Wan Kim, Ki Hyeong Lee, Rachel E. Sanborn, José Trigo, Koichi Goto, James Chih-Hsin Yang, Ramaswamy Govindan, Joshua M. Bauml, Pilar Garrido, Matthew G. Krebs, Karen L. Reckamp, John Xie
Collection and assembly of data: Keunchil Park, Eric B. Haura, Natasha B. Leighl, Catherine A. Shu, Nicolas Girard, Santiago Viteri, Ji-Youn Han, Sang-We Kim, Chee Khoon Lee, Joshua K. Sabari, Alexander I. Spira, Tsung-Ying Yang, Ki Hyeong Lee, Rachel E. Sanborn, José Trigo, Koichi Goto, Jong-Seok Lee, James Chih-Hsin Yang, Ramaswamy Govindan, Joshua M. Bauml, Matthew G. Krebs, Karen L. Reckamp, John Xie, Dawn Millington, Patricia Lorenzini, Meena Thayu, Roland E. Knoblauch, Byoung Chul Cho
Data analysis and interpretation: Keunchil Park, Eric B. Haura, Natasha B. Leighl, Paul Mitchell, Catherine A. Shu, Nicolas Girard, Santiago Viteri, Ji-Youn Han, Sang-We Kim, Chee Khoon Lee, Joshua K. Sabari, Alexander I. Spira, Tsung-Ying Yang, Dong-Wan Kim, Ki Hyeong Lee, Rachel E. Sanborn, José Trigo, Koichi Goto, James Chih-Hsin Yang, Ramaswamy Govindan, Joshua M. Bauml, Pilar Garrido, Matthew G. Krebs, Karen L. Reckamp, John Xie, Joshua C. Curtin, Nahor Haddish-Berhane, Amy Roshak, Dawn Millington, Patricia Lorenzini, Meena Thayu, Roland E. Knoblauch, Byoung Chul Cho
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Amivantamab in EGFR Exon 20 Insertion–Mutated Non–Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Keunchil Park
Consulting or Advisory Role: AstraZeneca, Lilly, Ono Pharmaceutical, Bristol Myers Squibb, MSD, Blueprint Medicines, Amgen, Merck KGaA, LOXO, AbbVie, Daiichi Sankyo, Boehringer Ingelheim, JNJ, Eisai, Puma Biotechnology
Speakers' Bureau: Boehringer Ingelheim
Research Funding: AstraZeneca, MSD Oncology
Eric B. Haura
Consulting or Advisory Role: Janssen Oncology, Revolution Medicines, Ellipses Pharma, Janssen Research & Development, Amgen
Research Funding: Janssen, Novartis, Revolution Medicines, AstraZeneca, Incyte, Genentech, Spectrum Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Protein-Protein Interactions as Biomarkers Patent
Natasha Leighl
Honoraria: Boehringer Ingelheim
Consulting or Advisory Role: Xcovery
Research Funding: Novartis, Roche Canada, Guardant Health, MSD, EMD Serono, Lilly
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bristol Myers Squibb, Nektar, GlaxoSmithKline, Roche, AstraZeneca
Paul Mitchell
Honoraria: MSD, AstraZeneca, Roche
Consulting or Advisory Role: Roche, Specialised Therapeutics, PUMA Biotechnology, AstraZeneca, Boehringer Ingelheim, BMS, MSD, Amgen
Catherine A. Shu
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Genentech/Roche
Research Funding: Celgene, Genentech/Roche, Janssen, MedImmune
Travel, Accommodations, Expenses: Genentech/Roche
Nicolas Girard
Employment: AstraZeneca
Consulting or Advisory Role: Roche, Lilly, Boehringer Ingelheim, AstraZeneca, Novartis, Pfizer, Bristol Myers Squibb, MSD, Takeda, GlaxoSmithKline, AbbVie, Pharmamar, Janssen, Sanofi
Research Funding: Roche, AstraZeneca, Boehringer Ingelheim
Travel, Accommodations, Expenses: Roche, AstraZeneca, Bristol Myers Squibb, MSD Oncology
Santiago Viteri
Consulting or Advisory Role: Roche, Bristol Myers Squibb, Janssen, Takeda, Reddy Pharma Iberia, Merck KGaA
Speakers' Bureau: Bristol Myers Squibb, Roche
Travel, Accommodations, Expenses: Roche, MSD, Merck KGaA
Ji-Youn Han
Honoraria: Roche, AstraZeneca, Bristol Myers Squibb, Takeda
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Bristol Myers Squibb, Lilly, Novartis, Takeda, Pfizer
Research Funding: Roche, Pfizer, Ono Pharmaceutical, Takeda
Chee Khoon Lee
Honoraria: AstraZeneca, Pfizer, Amgen, Takeda, Yuhan, Boehringer Ingelheim
Consulting or Advisory Role: Novartis, Boehringer Ingelheim, Takeda, AstraZeneca, Yuhan, Amgen
Research Funding: AstraZeneca, Roche, Merck KGaA
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
Alexander I. Spira
Stock and Other Ownership Interests: Lilly
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer
Consulting or Advisory Role: Array BioPharma, Incyte, Amgen, Novartis, AstraZeneca/MedImmune, Mirati Therapeutics, Gritstone Oncology, Jazz Pharmaceuticals, Merck, Bristol Myers Squibb
Research Funding: Roche, AstraZeneca, Boehringer Ingelheim, Astellas Pharma, MedImmune, Novartis, Newlink Genetics, Incyte, AbbVie, Ignyta, LAM Therapeutics, Trovagene, Takeda, Macrogenics, CytomX Therapeutics, LAM Therapeutics, Astex Pharmaceuticals, Bristol Myers Squibb, Loxo, Arch Therapeutics, Gritstone Oncology, Plexxikon, Amgen, Loxo, Daiichi Sankyo, ADC Therapeutics, Janssen Oncology, Mirati Therapeutics, Rubius Therapeutics
Dong-Wan Kim
Research Funding: Alpha Biopharma, AstraZeneca/MedImmune, Hanmi, Janssen, Merus, Mirati Therapeutics, MSD, Novartis, Ono Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, Yuhan, Boehringer Ingelheim, Amgen, Daiichi Sankyo, Chong Kun Dang Pharmaceutical, BridgeBio Pharma, GlaxoSmithKline
Travel, Accommodations, Expenses: Daiichi Sankyo, Amgen
Ki Hyeong Lee
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Pfizer, Lilly
Rachel E. Sanborn
Honoraria: AstraZeneca, Amgen
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, EMD Serono, Blueprint Medicines, Daiichi Sankyo/Lilly, Janssen Oncology, Macrogenics
Research Funding: Bristol Myers Squibb, MedImmune, Merck, AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
José Trigo
Consulting or Advisory Role: Takeda, Boehringer Ingelheim, Bristol Myers Squibb, Merck Serono
Speakers' Bureau: MSD Oncology, AstraZeneca, Merck Serono, Roche
Travel, Accommodations, Expenses: MSD Oncology, Bristol Myers Squibb, AstraZeneca
Koichi Goto
Honoraria: Guardant Health, Janssen, Daiichi Sankyo Co Ltd, Amgen, Eisai, Kyowa Kirin Co Ltd, Bristol Myers Squibb, AstraZeneca Japan, Pfizer, Chugai Pharma, Taiho Pharmaceutical, Ono Pharmaceutical, Novartis, Lilly Japan, Boehringer Ingelheim, Life Technologies, MSD K.K, Nippon Kayaku, Takeda, Otsuka
Consulting or Advisory Role: Otsuka
Research Funding: Medical & Biological Laboratories Co Ltd, Kyowa Kirin Co Ltd, Amgen Astellas BioPharma, Kissei Pharmaceutical, Merck, Merus, NEC Corporation, Spectrum Pharmaceuticals, Shanghai HaiHe Pharmaceutical, MSD K.K, AstraZeneca Japan, Taiho Pharmaceutical, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Sumitomo Dainippon Pharma Co Ltd, Takeda, Astellas Pharma, Eisai, Lilly Japan, Pfizer, Bristol Myers Squibb, Ignyta, Life Technologies, Janssen, Xcoo, Loxo, Sysmex, Amgen
James Chih-Hsin Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, Takeda, Lilly, Pfizer
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Clovis Oncology, Lilly, MSD Oncology, Merck Serono, Celgene, Bayer, Pfizer, Ono Pharmaceutical, Bristol Myers Squibb, Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Takeda Oncology, Amgen, Incyte, Merck Serono, Jenssen, GSK, Takeda, Daiichi Sankyo, Novartis
Travel, Accommodations, Expenses: Pfizer
Ramaswamy Govindan
Honoraria: Genentech/AbbVie, AbbVie, Geneplus-Beijing Institute
Consulting or Advisory Role: Genentech/Roche, AbbVie, AstraZeneca/MedImmune, Pfizer, Bristol Myers Squibb, Nektar, Jounce Therapeutics, F. Hoffmann La Roche AG, Janssen, Amgen, Achilles Therapeutics
Joshua M. Bauml
Consulting or Advisory Role: Bristol Myers Squibb, Merck, AstraZeneca, Genentech, Celgene, Boehringer Ingelheim, Guardant Health, Takeda, Novartis, Janssen, Ayala Pharmaceuticals, Regeneron, Inivata, Novartis, Foundation Medicine
Research Funding: Merck, Carevive Systems, Novartis, Incyte, Bayer, Janssen, AstraZeneca, Takeda, Amgen, Pfizer, Mirati Therapeutics
Pilar Garrido
Consulting or Advisory Role: Roche Pharma AG, AstraZeneca, MSD Oncology, Bristol Myers Squibb, Takeda, Lilly, Pfizer, Novartis/Pfizer, Boehringer Ingelheim, AbbVie, Amgen, Bayer, Gebro Pharma, Nordic Group, Boehringer Ingelheim, Janssen Biotech, Janssen Oncology, GlaxoSmithKline
Speakers' Bureau: Roche Pharma AG, Takeda, AstraZeneca, MSD Oncology, BMS, Pfizer, Novartis, Boehringer Ingelheim, Nordic Group, Janssen, Boehringer Ingelheim, Janssen Oncology
Travel, Accommodations, Expenses: AstraZeneca, Roche, Bristol Myers Squibb
Other Relationship: Janssen Oncology, Novartis
Matthew G. Krebs
Honoraria: Roche
Consulting or Advisory Role: Roche, Achilles Therapeutics, Janssen, Seattle Genetics, OM Pharma, Bayer
Speakers' Bureau: Roche, Janssen
Research Funding: Roche
Travel, Accommodations, Expenses: AstraZeneca, BerGenBio, Immutep
Karen L. Reckamp
Consulting or Advisory Role: Amgen, Tesaro, Boehringer Ingelheim, Takeda, AstraZeneca, Seattle Genetics, Calithera Biosciences, Genentech, Blueprint Medicines, Daiichi Sankyo/Lilly, EMD Serono, Janssen Oncology, Lilly, Merck KGaA
Research Funding: Bristol Myers Squibb, Pfizer, ARIAD, Xcovery, Adaptimmune, Genentech/Roche, Boehringer Ingelheim, AbbVie, ACEA Biosciences, Loxo, GlaxoSmithKline, Guardant Health, Janssen Oncology, Seattle Genetics, Zeno Pharmaceuticals, Calithera Biosciences, Elevation Oncology, Daiichi Sankyo/Astra Zeneca
John Xie
Employment: JNJ
Stock and Other Ownership Interests: JNJ
Joshua C. Curtin
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Nahor Haddish-Berhane
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Amy Roshak
Employment: Janssen Pharmaceutical Company of Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Dawn Millington
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Janssen Research & Development
Patricia Lorenzini
Employment: Janssen Pharmaceuticals of Johnson & Johnson
Meena Thayu
Employment: Janssen Oncology
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Other Relationship: Janssen Oncology
Roland E. Knoblauch
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Travel, Accommodations, Expenses: Johnson & Johnson
Byoung Chul Cho
Leadership: Gencurix Inc, Interpark Bio Convergence Corp
Stock and Other Ownership Interests: TheraCanVac Inc, Gencurix Inc, Bridgebio Therapeutics, KANAPH Therapeutic Inc, Cyrus Therapeutics, Interpark Bio Convergence Corp
Consulting or Advisory Role: Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol Myers Squibb, Yuhan, Pfizer, Janssen, Takeda, MSD, Ono Pharmaceutical, Eli Lilly, Medpacto, Blueprint Medicines, KANAPH Therapeutic Inc, Brigebio Therapeutics, Cyrus Therapeutics, Guardant Health, Oscotec Inc
Research Funding: Novartis, Bayer, AstraZeneca, MOGAM Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, MSD, AbbVie, Medpacto, GIInnovation, Eli Lilly, Blueprint Medicines, Interpark Bio Convergence Corp
Patents, Royalties, Other Intellectual Property: Champions Oncology
Other Relationship: DAAN Biotherapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lynch TJ, Bell DW, Sordella R, et al. : Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129-2139, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. : EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 304:1497-1500, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. : EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 101:13306-13311, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcila ME, Nafa K, Chaft JE, et al. : EGFR exon 20 insertion mutations in lung adenocarcinomas: Prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 12:220-229, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxnard GR, Lo PC, Nishino M, et al. : Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 8:179-184, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riess JW, Gandara DR, Frampton GM, et al. : Diverse EGFR exon 20 insertions and co-occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J Thorac Oncol 13:1560-1568, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Petri ET, Halmos B, et al. : Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol 26:1742-1751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shigematsu H, Lin L, Takahashi T, et al. : Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97:339-346, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bauml JM, Viteri S, Minchom A, et al. : FP07.12 underdiagnosis of EGFR exon 20 insertion mutation variants: Estimates from NGS-based real-world datasets. J Thorac Oncol 16:S208-S209, 2021 [Google Scholar]
- 10.Kate S, Chougule A, Joshi A, et al. : Outcome of uncommon EGFR mutation positive newly diagnosed advanced non-small cell lung cancer patients: A single center retrospective analysis. Lung Cancer (Auckl) 10:1-10, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu JY, Yu CJ, Shih JY: Effectiveness of treatments for advanced non-small-cell lung cancer with exon 20 insertion epidermal growth factor receptor mutations. Clin Lung Cancer 20:e620-e630, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Yang JC, Sequist LV, Geater SL, et al. : Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-lung 2, LUX-lung 3, and LUX-lung 6. Lancet Oncol 16:830-838, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Kim TM, Ock C-Y, Kim M, et al. : Phase II study of osimertinib in NSCLC patients with EGFR exon 20 insertion mutation: A multicenter trial of the Korean Cancer Study Group (LU17-19). Ann Oncol 30:V628, 2019 [Google Scholar]
- 14.Robichaux JP, Elamin YY, Tan Z, et al. : Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 24:638-646, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dersarkissian M, Bhak R, Lin H, et al. : Real-world treatment patterns and survival in non-small cell lung cancer patients with EGFR exon 20 insertion mutations. J Thorac Oncol 14:S681, 2019 [Google Scholar]
- 16.Ramalingam SS, Vansteenkiste J, Planchard D, et al. : Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41-50, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Beau-Faller M, Prim N, Ruppert AM, et al. : Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: A multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 25:126-131, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naidoo J, Sima CS, Rodriguez K, et al. : Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: Clinical outcomes and response to erlotinib. Cancer 121:3212-3220, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasuda H, Park E, Yun CH, et al. : Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 5:216ra177, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CK, Davies L, Wu YL, et al. : Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: Individual patient data meta-analysis of overall survival. J Natl Cancer Inst 109, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Moores SL, Chiu ML, Bushey BS, et al. : A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res 76:3942-3953, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Vijayaraghavan S, Lipfert L, Chevalier K, et al. : Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol Cancer Ther 19:2044-2056, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Yun J, Lee SH, Kim SY, et al. : Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov 10:1194-1209, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Hanna N, Shepherd FA, Fossella FV, et al. : Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589-1597, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Garon EB, Ciuleanu TE, Arrieta O, et al. : Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 384:665-673, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Horn L, Lin HM, Padda SK, et al. : Indirect comparison of TAK-788 vs real-world data outcomes in refractory non-small cell lung cancer (NSCLC) with EGFR exon 20 insertions. J Clin Oncol 38:9580, 2020 [Google Scholar]
- 27.Le X, Goldman JW, Clarke JM, et al. : Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. J Clin Oncol 38, 2020. (abstr 9514) [Google Scholar]
- 28.Zhou C, Ramalingam S, Li B, et al. : OA04.03 mobocertinib in NSCLC with EGFR exon 20 insertions: Results from EXCLAIM and pooled platinum-pretreated patient populations. J Thorac Oncol 16:S108, 2021 [Google Scholar]
- 29.Huang S, van Duijnhoven SMJ, Sijts A, et al. : Bispecific antibodies targeting dual tumor-associated antigens in cancer therapy. J Cancer Res Clin Oncol 146:3111-3122, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarantow SW, Bushey BS, Pardinas JR, et al. : Impact of cell-surface antigen expression on target engagement and function of an epidermal growth factor receptor x c-MET bispecific antibody. J Biol Chem 290:24689-24704, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park K, John T, Kim S-W, et al. : Amivantamab (JNJ-61186372), an anti-EGFR-MET bispecific antibody, in patients with EGFR exon 20 insertion (exon20ins)-mutated non-small cell lung cancer (NSCLC). J Clin Oncol 38, 2020. (abstr 9512) [Google Scholar]
- 32.Haura EB, Cho BC, Lee JS, et al. : JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC). J Clin Oncol 37, 2019. (abstr 9009) [Google Scholar]
- 33.Cho BC, Lee J-S, Han J-Y, et al. : JNJ-61186372 (JNJ-372), an EGFR-cMET bispecific antibody, in advanced non-small cell lung cancer (NSCLC): An update on phase 1 results. Ann Oncol 29, 2018. (abstr VIII542) [Google Scholar]
- 34.Park K, Ahn M-J, Lee S-H, et al. : A first-in-human phase 1 trial of the EGFR-cMET bispecific antibody JNJ-61186372 in patients with advanced non-small cell lung cancer (NSCLC). J Thorac Oncol 13:S344-S345, 2018 [Google Scholar]
- 35.Cho BC, Lee KH, Cho EK, et al. : 1258O Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in combination with lazertinib, a 3rd-generation tyrosine kinase inhibitor (TKI), in advanced EGFR NSCLC. Ann Oncol 31:S813, 2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.