Abstract
PURPOSE:
Insured patients with cancer face high treatment-related, out-of-pocket (OOP) costs and often cannot access financial assistance. We conducted a randomized, controlled trial of Bridge, a patient-facing app designed to identify eligible financial resources for patients. We hypothesized that patients using Bridge would experience greater OOP cost reduction than controls.
METHODS:
We enrolled patients with cancer who had OOP expenses from January 2018 to March 2019. We randomly assigned patients 1:1 to intervention (Bridge) versus control (financial assistance educational websites). Primary and secondary outcomes were self-reported OOP costs and subjective financial distress 3 months postenrollment. In post hoc analyses, we analyzed application for and receipt of financial assistance at 3 months postenrollment. We used chi-square, Mann-Whitney tests, and logistic regression to compare study arms.
RESULTS:
We enrolled 200 patients. The median age was 57 years (IQR, 47.0-63.0). Most patients had private insurance (71%), and the median household income was $62,000 in US dollars (USD) (IQR, $36,000-$100,000 [USD]). Substantial missing data precluded assessment of primary and secondary outcomes. In post hoc analyses, patients in the Bridge arm were more likely than controls to both apply for and receive financial assistance.
CONCLUSION:
We were unable to test our primary outcome because of excessive missing follow-up survey data. In exploratory post hoc analyses, patients who received a financial assistance app were more likely to apply for and receive financial assistance. Ultimately, our study highlights challenges faced in identifying measurable outcomes and retaining participants in a randomized, controlled trial of a mobile app to alleviate financial toxicity.
INTRODUCTION
In the United States, cancer remains one of the most expensive diseases to treat.1 Payers have increasingly transferred that cost burden to patients via cost-sharing mechanisms, placing patients at risk for developing financial toxicity.2,3 To help defray direct patient costs, a number of financial assistance programs exist that, typically, are sponsored by pharmaceutical manufacturers or charitable foundations and either cover the full cost of a drug or provide assistance with medication co-pays or other indirect expenses.4 Unfortunately, assistance programs often have unclear and stringent eligibility criteria, often withholding details on how financial need is documented, or employ complex application processes.4
More than 90% of cancer centers employ financial specialists and/or social workers to help patients navigate financial burdens.5,6 However, counselors frequently report being ill-equipped to screen for financial distress, thereby limiting aid for the most financially vulnerable patients.7 Together, these factors make it challenging for patients to benefit from financial assistance programs, exacerbating downstream cost-related harm, including treatment nonadherence and personal bankruptcy.8,9
Increasingly, mobile health technologies can promote effective shared decision making and better health outcomes.10-15 We created a mobile app (Bridge) through which patients can identify eligible financial assistance programs and initiate contact with financial counselors. A small study of the app examined usability and feasibility among a diverse panel of 30 insured patients with cancer (63% female, 23% non-White, and 23% employed). The results suggested that 27 of 30 (90%) patients found Bridge usable and 100% completed a full interaction with the app, demonstrating strong feasibility.16
Here, we describe the results and challenges of a randomized, controlled trial (RCT) of Bridge to connect patients to financial assistance programs. Our primary objective was to determine the impact of Bridge on reducing out-of-pocket (OOP) expenses. Our secondary objective was to assess whether Bridge would affect subjective financial distress and awareness of available financial resources. We hypothesized that Bridge users would experience reduced OOP costs and financial distress and improved awareness of assistance options. During the trial, we encountered many challenges highlighting the difficulty of conducting trials of financial toxicity interventions. In addition to reporting trial results, we provide detailed discussion of lessons learned to support success of future studies in this area.
METHODS
Overview and Patient Population
We enrolled English-speaking adults receiving treatment for any stage or diagnosis of cancer at Duke Cancer Center from January 2018 to March 2019 who self-reported treatment-related OOP expenses, access to a mobile device, and ≥ 6-month life expectancy as assessed by their oncologist. Eligible patients were approached in clinic or while receiving therapy in the treatment center. We excluded patients who were enrolled on another clinical trial, were expected to cease treatment in the next 6 months, or had Medicaid or no insurance. We later excluded patients with Medicare plus supplemental insurance because many of these patients were not eligible for financial assistance programs. When this eligibility criterion was changed, 22 such patients had been randomly assigned. All participants provided written informed consent, and this study was approved by the Duke University School of Medicine Institutional Review Board.
Bridge
Bridge is an experimental mobile health application developed by Vivor, a healthcare technology company, with support from a Small Business Technology Transfer grant sponsored by the National Cancer Institute (5R42CA210699-03). It expands upon Vivor's commercially available financial assistance platform, which requires financial counselors to initiate the process, a requirement that presents obstacles to securing assistance in busy academic medical centers. Its design is based on the results of the aforementioned usability study and qualitative feedback. Following informed consent and working alongside Vivor's commercially available platform, Bridge extracts clinical information from the electronic health record (EHR) via a daily data transfer process. After receiving a text message prompt, patients supplement EHR data by entering household income information into Bridge, and the app identifies appropriate financial assistance programs. Patients are then prompted to contact financial counselors for further information and assistance with program enrollment. Financial counselors are responsible for following up with patients and enrolling them into assistance programs.
Study Design and Independent Variables
The sample size was calculated using standardized effect sizes because we did not find any studies identifying a clinically meaningful difference (in absolute dollars) in OOP costs. A sample size of 100 per arm was determined to provide 80% power to detect a moderate sized difference (Cohen's d = 0.4) comparing OOP costs between Bridge and control groups with two-sided type I error of 5%.17
We randomly assigned patients 1:1 to either intervention (Bridge) or control using REDCap.18 Intervention patients received text messages from Bridge and interacted with the app on their own devices. Control patients were directed to standard-of-care financial assistance, defined as widely accessible, existing websites that broadly inform patients with cancer on financial assistance without any personalized component.19 Patients received compensation of $20 in US dollars (USD) at 6 months from enrollment.
Demographic variables collected included age, sex, race or ethnicity, marital status, education level, employment status, and insurance status. Patients also provided household size and estimates of household income. Using household income and marital status, we created an additional variable for whether a participant lived above or below US poverty thresholds ($16,247 [USD] for two person household and $12,784 [USD] for one person based on 2018 Census data).20 Clinical and treatment-related variables included cancer diagnosis, stage, and treatment regimen.
Outcomes
Patients completed surveys at study enrollment before random assignment and at 1-, 3-, and 6-months postrandomization to assess changes in OOP costs (primary outcome), financial distress, and health-related quality of life (QOL) (secondary outcomes). The initial assessment was completed in clinic at enrollment. Patients were e-mailed follow-up assessments to complete at home and were called for reminders within a 2-week window of each time point.
We determined OOP costs by summing expenses and co-payments reported by patients on an 11-item questionnaire covering insurance premiums, pill chemotherapy, other prescription medications, clinical visits, procedures and other tests, medical equipment, travel, alternative therapies, over-the-counter medications, diet, and others. The primary outcome was OOP costs at 3 months. This time point was chosen to provide sufficient time for financial resources to take effect with minimal effect of attrition because of illness or death. Although we acknowledge that self-reported OOP costs have not been validated, the questionnaire used for OOP cost assessment has been used in previous cost-related studies,3 and large national studies have relied on self-report of OOP costs.21–23
Financial distress was assessed using the FACT-COST, a validated measure developed specifically to assess subjective financial distress in patients with cancer.24 The COST scale ranges from 0 to 44 based on 11 question items, and lower scores indicated greater financial toxicity.24 We queried patients to assess knowledge of financial resources specific to their care (eg, patient assistance programs, co-payment assistance programs, foundations, etc) by asking “How aware are you of available financial assistance resources for your cancer treatment?” with choices “Very unaware, Unaware, Neither aware nor unaware, Aware, and Very Aware.” Although not validated, this measure is specifically tailored for our site and study.
For completion of follow-up surveys, patients were contacted by phone up to twice. When requested, additional copies of surveys were mailed to patients. During data collection and while reminding patients of follow-up surveys, we determined a high degree of missing follow-up data (see details below in Results section), limiting adequate assessment of both primary and secondary outcomes. We therefore conducted a post hoc analysis with data from the EHR, an institutional pharmacy database, Bridge, and Financial Care Counselors to assess additional outcomes of individual application for and receipt of financial assistance. The absence of documented request for financial assistance was considered equivalent to not requesting assistance, and all patients except for one had ≥ 1 visit with an oncologist within 6 months of enrollment. This post hoc analysis included all patients in the study, regardless of whether their self-reported data were missing.
Statistical Analysis
We calculated descriptive statistics to examine baseline features of the cohort by study arm. To test both our primary and secondary hypotheses, that Bridge will reduce both OOP costs and financial distress and increase knowledge of available resources compared with standard of care, we calculated the change of these outcomes from baseline to 3 months and compared by treatment arm using the Mann-Whitney test. To determine whether Bridge would increase the odds that patients apply for and receive assistance, we conducted a post hoc analysis and fit an adjusted logistic regression model with treatment arm as the predictor. Stepwise variable selection was used to select covariates to include in the models using an entry and stay threshold of 0.10. Candidate covariates were age, sex, marital status, race, complete OOP cost data, and poverty status. For applied for assistance, sex and complete OOP cost data were selected, whereas only complete OOP cost data were selected for receiving assistance. All analyses were done using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
Demographics
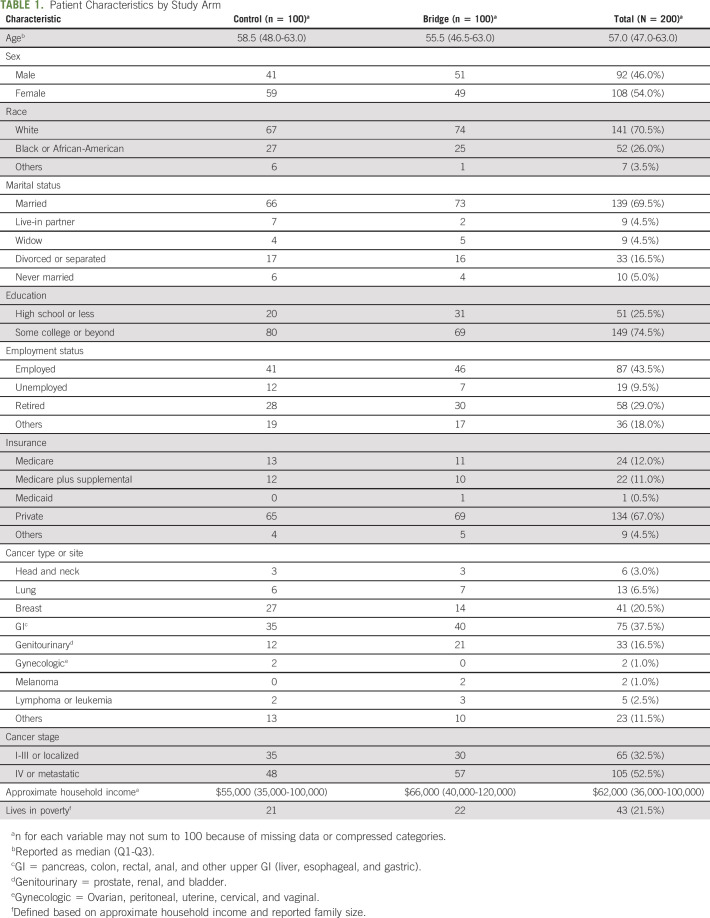
Of 1,792 patients screened, 993 patients were ineligible and 379 were deferred as they were preoccupied initially, and we could not assess later because of limited bandwidth to see all eligible patients. We approached 420 patients. Of those, 219 declined and one withdrew before random assignment, resulting in 200 participants (48% enrollment rate) (Appendix Fig A1, online only). The median age of the overall cohort was 57 years (IQR, 47.0-63.0). Most patients were White (71%), married (70%), and either employed full-time (44%) or retired (29%) and had education surpassing high school (75%). Most patients had private insurance (67%), and the median household income was $62,000 (USD) (IQR, $36,000-$100,000 [USD]). Based on income and reported household size, 22% of patients were living below US poverty thresholds. GI (38%) and breast (21%) were the most common cancer diagnoses. A slight majority of patients had stage IV or metastatic disease (53%) (Table 1).
TABLE 1.
Patient Characteristics by Study Arm
Baseline Financial Assessment
The median FACT-COST financial distress score was 24 in the control arm and 23 in the Bridge arm. Of the 200 patients, 186 (93%) provided a numerical estimate for their OOP costs at baseline. The median monthly OOP cost was $1,110 (USD) (IQR, $413-$2,195 [USD]) for Bridge patients and $775 (IQR, $280-$2,421 [USD]) for control patients. Payment for insurance premiums constituted the largest amount of OOP expenses for the entire cohort (median, $325 [USD]: IQR, $80-$684 [USD]), followed by expenses for travel to appointments (median, $130 [USD], IQR, $40-$450 [USD]). The median COST score was 23.1 (IQR, 15.4-31.9) for Bridge patients and 23.7 (IQR, 17.6-33.6) for control patients.
Effect on OOP Costs and Financial Distress
Considerable missingness limited meaningful assessment of primary and secondary outcomes. OOP cost data were available at both baseline and 3 months for 55 of 200 (27.5%) patients, and COST scores were reported for 64 of 200 (32.0%) patients at both time points (Table 2). We noted several factors associated with missing outcomes. Patients randomly assigned to control (83%) were more likely to have missing OOP costs versus those randomly assigned to Bridge (62%) (P < .01). In addition, African-Americans were more likely than Whites to have missing OOP costs (87% missing v 66% for Whites, P = .01), and those living in poverty (86%) were more likely to have missing OOP costs than patients not living in poverty (69%) (P = .03). For COST score, similar results for missingness were seen regarding study arm (80% of controls missing v 56% of Bridge, P < .01). Age also differed for missing COST scores (median age for missing at either time point 58.0, IQR, 48.0-64, v median for complete data 52.0, IQR, 45.0-61.5, P = .04).
TABLE 2.
Missingness of OOP Costs or Financial Distress Score at Either Baseline or 3 Months
Utilization of Financial Assistance
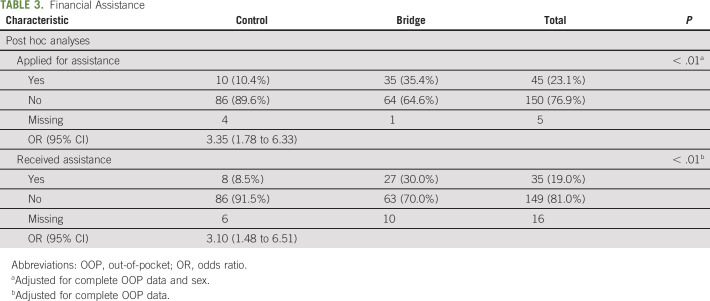
We retrieved data on application for and receipt of financial assistance from electronic records for 96% of control patients and for 99% of Bridge patients (Table 3). The results of our adjusted, exploratory post hoc analysis suggested that patients in the Bridge arm had increased odds of applying for financial assistance compared with those in the control arm (35% of Bridge v 10% control; odds ratio, 3.35; 95% CI, 1.78 to 6.33; P < .01). Bridge patients also had increased odds of receiving financial assistance compared with control patients (30% Bridge v 9% control; odds ratio, 3.10; 95% CI, 1.48 to 6.51; P < .01).
TABLE 3.
Financial Assistance
DISCUSSION
We were unable to directly assess the effect of Bridge on OOP costs because of high trial attrition and missing follow-up survey data. Given this substantial limitation, we conducted a post hoc analysis to help frame future studies on this topic. However, we focus this discussion primarily on the limitations of this study and lessons learned for future investigations.
The primary limitation to our study was missing follow-up data. This problem is well-described in cancer clinical trials, particularly those studying palliative and/or QOL interventions.25–29 Several studies note attrition rates ranging from 34% to 80%, although most have attrition rates ranging from approximately 30%-50%.28,30,31 Our study had a considerably higher attrition rate with missing follow-up data from 73% of participants. The most likely reason for missing data in our study was that follow-up was conducted by phone rather than in person. We also did not compensate patients for each survey, which might have affected their completion. Several other common reasons might explain these attrition rates, including consent information that focuses on right to withdraw without explaining the value of retention, study length, and physical decline and/or emotional distress.27,29,32 We also found that African American patients were more likely to be missing follow-up data, a finding that has been previously described.27,33 Notably, control patients were more likely to have missing follow-up data, suggesting that Bridge might have better engaged participants with respect to monitoring their treatment-related costs. Furthermore, control patients might have not seen the utility in continuing to provide follow-up information if they were not actively connected to any assistance. Finally, we acknowledge that failure to complete follow-up surveys may itself be an indicator of worsened financial or QOL-related distress or disease progression, particularly for those living below the poverty threshold.
Although limited in our ability to assess Bridge's impact on primary outcomes, our post hoc analysis suggests Bridge users had increased odds of applying for and receiving financial assistance. Of note, most patients in both study arms who completed assistance applications received assistance (80% in control and 77% in Bridge). Bridge users, however, were more likely to apply than control patients. Together, these data suggest that the main barrier to receipt of financial assistance may be lack of awareness of programs. Still, application for and receipt of assistance among Bridge users was low at approximately 35%, suggesting that lack of knowledge and stringent eligibility criteria for financial assistance programs remain challenges for patients. However, this post hoc analysis is retrospective and therefore subject to bias, particularly with regard to differential documentation of financial assistance application and receipt between Bridge and control groups.
Although our app focuses on connecting patients to financial assistance programs, the use of these programs is a topic of considerable debate. By reducing cost-sharing (ie, the patient's OOP responsibility for health care, including co-insurance, co-pays, and deductibles), these assistance programs may have several downstream effects that perpetuate high drug costs and limit access: 4 First, decreased cost-sharing may remove the financial disincentive for the use of expensive drugs when generic alternatives are available or when expensive therapy has questionable benefit.34,35 Second, the cumbersome application process may exacerbate inequalities in access for low-income individuals or those with low health literacy.4,36 Yet, for patients who may otherwise be without means to afford treatment, financial assistance programs have utility while we seek broader policy solutions. However, further research is needed to determine the efficacy of Bridge in this regard.
Our study faced other limitations aside from high trial attrition because of follow-up via phone rather than in person. First, although Vivor's assistance program database is comprehensive, not all programs were included. Second, some patients might have experienced treatment changes during the study period. Since many assistance programs are drug-specific, these changes might have affected eligibility and the timely receipt of assistance. Third, we designed our eligibility criteria to increase representativeness of study participants and generalizability of results, including only requiring a “yes” to the question, “Have you incurred OOP costs?” However, based on timing of treatment initiation and billing, otherwise eligible patients might have not yet incurred any costs and self-selected out of our study. Fourth, although all patients except one had ≥ 1 oncology visit after enrollment, we did not abstract the number of follow-up encounters or the effect on follow-up survey completion. Finally, our post hoc analysis was retrospective, creating the possibility of bias introduced by differential documentation of outcomes between groups.
Our study highlights key challenges in RCTs of mobile applications to connect patients to financial resources. First, financial outcome selection is critical. Although self-report of OOP expenses has been previously used in large national studies,3,20–22 such measures are subject to recall bias. Other measures might have better served as our primary outcome, including our post hoc outcome of documented application for and receipt of financial assistance, which does not rely on patient self-report. Second, complete OOP cost data were observed to confound the relationship between Bridge and post hoc outcomes, suggesting that follow-up attrition is meaningful regarding application for and receipt of financial aid. Although we adjusted for complete OOP costs, incorporation of claims or hospital billing data may be useful to obtain OOP cost data even in settings of high patient attrition to minimize this confounding factor. Third, future financial toxicity intervention studies should pay particular attention to limiting missing data, including selecting patients with better performance status and conducting follow-up assessments in person.29 This also includes potentially contacting patients for follow-up more than twice. Patients with declining health status during the study period would also be better served with this follow-up approach. Finally, investigators should acknowledge the sensitive nature of questions arising in financially focused studies and understand those questions might affect longitudinal study participation. As mentioned, conducting follow-up assessments in person may help mitigate this concern, and future studies should take into consideration the number of follow-up visits occurring after enrollment as a potential confounding factor.
In conclusion, reducing financial toxicity in patients with cancer is essential given its well-described detriment to well-being and treatment adherence. Our study highlights challenges faced in identifying outcomes and retaining participants in an RCT of a mobile application to alleviate financial toxicity. Future research should identify means to overcome these methodological challenges when designing financial toxicity intervention studies.
Appendix
FIG A1.
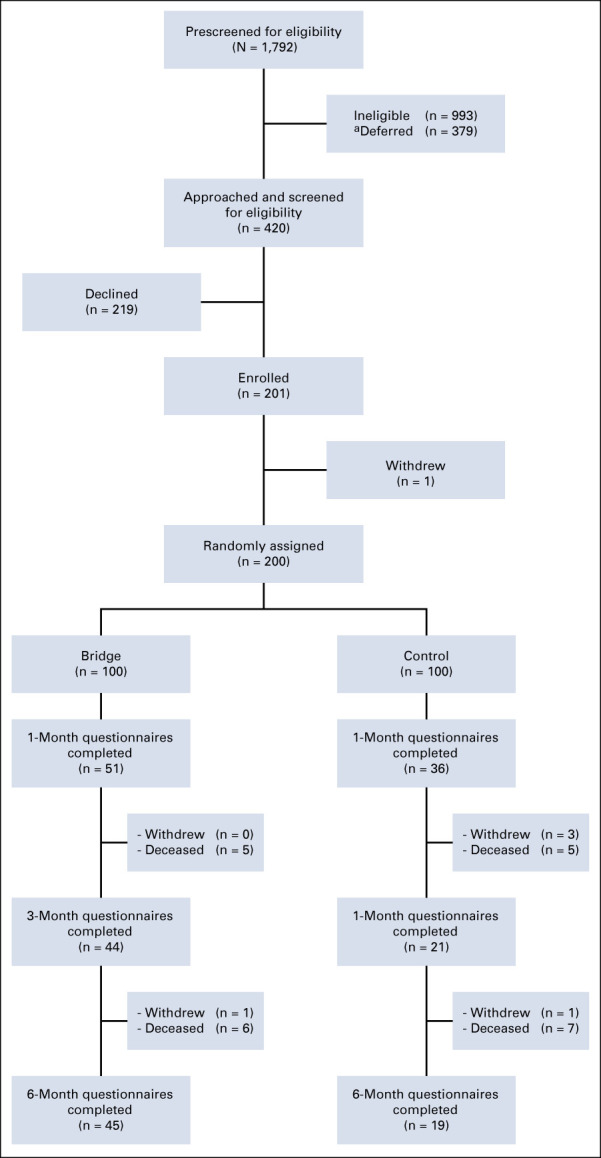
CONSORT diagram. aRefers to patients who were initially preoccupied (ie, sleeping) and unable to be seen later because of limited bandwidth to approach all eligible patients.
Jonathan Nicolla
Employment: Prepped Health
Leadership: Prepped Health, Acclivity Health
Stock and Other Ownership Interests: Prepped Health
Consulting or Advisory Role: Acclivity Health
Patents, Royalties, Other Intellectual Property: Receive royalties from Duke University for which I am listed on Inventor Disclosure Forms filed within the university for the creation of mobile health applications
Jesse D. Troy
Honoraria: Gamida Cell, Synthetic Biologics
Consulting or Advisory Role: The EMMES Corporation, AegisCN, Cohortias, Gamida Cell
Research Funding: Seattle Genetics, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: 16/493,754 (pending), 16/477,110, 2019-549537 (pending), 10-2019-7029841 (pending), PCT/US2018/022174, PCT/US2018/013623,62/470,431, Royalties from SinoCell Technologies, Inc
Other Relationship: Community Data Roundtable
Anthony D. Sung
Consulting or Advisory Role: Celltrion
Research Funding: Novartis, Cellective, Merck, Seres Therapeutics, Enterome
Ben Gagosian
Employment: Vivor LLC
Stock and Other Ownership Interests: Vivor LLC
Ian Manners
Employment: Vivor
Leadership: Vivor
Stock and Other Ownership Interests: Vivor
S. Yousuf Zafar
Employment: Shattuck Labs
Stock and Other Ownership Interests: Shattuck Labs
Consulting or Advisory Role: AIM Specialty Health, McKesson, RTI Health Solutions, Discern Health, WIRB-Copernicus Group
Research Funding: AstraZeneca
(OPTIONAL) Uncompensated Relationships: Vivor, Family Reach Foundation
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in Poster at the ASCO Virtual Annual Meeting, May 29, 2020.
SUPPORT
Supported by National Cancer Institute grant 5R42CA210699-03.
AUTHOR CONTRIBUTIONS
Conception and design: Aaron M. Tarnasky, Fred A. P. Friedman, Steven Wolf, Ben Gagosian, Kathryn I. Pollak, Ian Manners, S. Yousuf Zafar
Collection and assembly of data: Aaron M. Tarnasky, George N. Tran, Jonathan Nicolla, Fred A. P. Friedman, Steven Wolf, Anthony D. Sung, Kanan Shah, Jakob Oury, Jillian C. Thompson, Kathryn I. Pollak, S. Yousuf Zafar
Data analysis and interpretation: Aaron M. Tarnasky, George N. Tran, Fred A. P. Friedman, Steven Wolf, Jesse D. Troy, Anthony D. Sung, Jakob Oury, Kathryn I. Pollak, S. Yousuf Zafar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Mobile Application to Identify Cancer Treatment–Related Financial Assistance: Results of a Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jonathan Nicolla
Employment: Prepped Health
Leadership: Prepped Health, Acclivity Health
Stock and Other Ownership Interests: Prepped Health
Consulting or Advisory Role: Acclivity Health
Patents, Royalties, Other Intellectual Property: Receive royalties from Duke University for which I am listed on Inventor Disclosure Forms filed within the university for the creation of mobile health applications
Jesse D. Troy
Honoraria: Gamida Cell, Synthetic Biologics
Consulting or Advisory Role: The EMMES Corporation, AegisCN, Cohortias, Gamida Cell
Research Funding: Seattle Genetics, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: 16/493,754 (pending), 16/477,110, 2019-549537 (pending), 10-2019-7029841 (pending), PCT/US2018/022174, PCT/US2018/013623,62/470,431, Royalties from SinoCell Technologies, Inc
Other Relationship: Community Data Roundtable
Anthony D. Sung
Consulting or Advisory Role: Celltrion
Research Funding: Novartis, Cellective, Merck, Seres Therapeutics, Enterome
Ben Gagosian
Employment: Vivor LLC
Stock and Other Ownership Interests: Vivor LLC
Ian Manners
Employment: Vivor
Leadership: Vivor
Stock and Other Ownership Interests: Vivor
S. Yousuf Zafar
Employment: Shattuck Labs
Stock and Other Ownership Interests: Shattuck Labs
Consulting or Advisory Role: AIM Specialty Health, McKesson, RTI Health Solutions, Discern Health, WIRB-Copernicus Group
Research Funding: AstraZeneca
(OPTIONAL) Uncompensated Relationships: Vivor, Family Reach Foundation
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mariotto AB, Yabroff KR, Shao Y, et al. : Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 103:117-128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppercorn J: Financial toxicity and societal costs of cancer care: Distinct problems require distinct solutions. Oncologist 22:123-125, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zafar SY, Peppercorn JM, Schrag D, et al. : The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. Oncologist 18:381-390, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zafar SY, Peppercorn JM: Patient financial assistance programs: A path to affordability or a barrier to accessible cancer care? J Clin Oncol 35:2113-2116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Association of Community Cancer Centers : 2014 Trends Survey. Rockville, MD, Association of Community Cancer Centers, 2014 [Google Scholar]
- 6.Khera N, Sugalski J, Krause D, et al. : Current practices for screening and management of financial distress at NCCN member institutions. J Natl Compr Canc Netw 18:825-831, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Smith SK, Nicolla J, Zafar SY: Bridging the gap between financial distress and available resources for patients with cancer: A qualitative study. J Oncol Pract 10:e368-e372, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey S, Blough D, Kirchhoff A, et al. : Washington state cancer patients found to be at greater risk for bankruptcy than people without A cancer diagnosis. Health Aff 32:1143-1152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dusetzina SB, Winn AN, Abel GA, et al. : Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol 32:306-311, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Greene J, Hibbard JH, Sacks R, et al. : When patient activation levels change, health outcomes and costs change, too. Health Aff (Millwood) 34:431-437, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Maria Adela G, Ronen R, David WB, et al. : Information Technology for Patient Empowerment in Healthcare. Berlin, Boston, De Gruyter, 2015 [Google Scholar]
- 12.Singh K, Drouin K, Newmark LP, et al. : Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff 35:2310-2318, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Rowland SP, Fitzgerald JE, Holme T, et al. : What is the clinical value of mHealth for patients? NPJ Digital Med 3:4, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han M, Lee E: Effectiveness of mobile health application use to improve health behavior changes: A systematic review of randomized controlled trials. Healthc Inform Res 24:207-226, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbard JH, Greene J, Overton V: Patients with lower activation associated with higher costs; delivery systems should know their patients' “scores”. Health Aff 32:216-222, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Zafar Y, Manners I, Nicolla J, et al. : Bridging the financial assistance gap: A pilot study of a patient-facing app to identify drug financial assistance programs. J Clin Oncol 35:e18296, 2017 [Google Scholar]
- 17.Cohen J: Statistical power analysis. Curr Dir Psychol Sci 1:98-101, 1992 [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377-381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Society of Clinical Oncology : Navigating Cancer Care: Financial Considerations. Alexandria, VA, American Society of Clinical Oncology, 2020 [Google Scholar]
- 20.Poverty Thresholds. Washington, DC, US Census Bureau, 2020 [Google Scholar]
- 21.Weir DR: Health and Retirement, Study. Ann Arbor, MI, University of Michigan Institute for Social Research, 2020 [Google Scholar]
- 22.Agency for Healthcare Research and Quality: Medical Expenditure Panel Survey : Your Experiences With Cancer. Rockville, MD, Agency for Healthcare Research and Quality, 2018 [Google Scholar]
- 23.Ekwueme DU, Zhao J, Rim SH, et al. : Annual out-of-pocket expenditures and financial hardship among cancer survivors aged 18-64 years—United States, 2011-2016. MMWR Morb Mortal Wkly Rep 68:494-499, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Souza JA, Yap BJ, Hlubocky FJ, et al. : The development of a financial toxicity patient-reported outcome in cancer: The COST measure. Cancer 120:3245-3253, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Neumark DE, Stommel M, Given CW, et al. : Research design and subject characteristics predicting nonparticipation in a panel survey of older families with cancer. Nurs Res 50:363-368, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Baquet CR, Commiskey P, Daniel Mullins C, et al. : Recruitment and participation in clinical trials: Socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev 30:24-33, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiqi AE, Sikorskii A, Given CW, et al. : Early participant attrition from clinical trials: Role of trial design and logistics. Clin Trials 5:328-335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinck GC, van den Bos GA, Kleijnen J, et al. : Methodologic issues in effectiveness research on palliative cancer care: A systematic review. J Clin Oncol 15:1697-1707, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Hui D, Glitza I, Chisholm G, et al. : Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer 119:1098-1105, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldervoll L, Loge J, Paltiel H, et al. : Are palliative cancer patients willing and able to participate in a physical exercise program? Palliat Support Care 3:281-287, 2006 [DOI] [PubMed] [Google Scholar]
- 31.McMillan SC, Weitzner MA: Methodologic issues in collecting data from debilitated patients with cancer near the end of life. Oncol Nurs Forum 30:123-129, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Kearney A, Rosala-Hallas A, Bacon N, et al. : Reducing attrition within clinical trials: The communication of retention and withdrawal within patient information leaflets. PLoS One 13:e0204886, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osann K, Wenzel L, Dogan A, et al. : Recruitment and retention results for a population-based cervical cancer biobehavioral clinical trial. Gynecol Oncol 121:558-564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ubel PA, Bach PB: Copay assistance for expensive drugs: A helping hand that raises costs. Ann Intern Med 165:878-879, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Ross JS, Kesselheim AS: Prescription-drug coupons—No such thing as a free lunch. N Engl J Med 369:1188-1189, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Zafar SY, Peppercorn J, Asabere A, et al. : Transparency of industry-sponsored oncology patient financial assistance programs using a patient-centered approach. J Oncol Pract 13:e240-e248, 2017 [DOI] [PubMed] [Google Scholar]