Abstract
T cell immunity is central for the control of viral infections. CoVac-1 is a peptide-based vaccine candidate, composed of SARS-CoV-2 T cell epitopes derived from various viral proteins1,2, combined with the Toll-like receptor 1/2 agonist XS15 emulsified in Montanide ISA51 VG, aiming to induce profound SARS-CoV-2 T cell immunity to combat COVID-19. Here we conducted a phase I open-label trial, recruiting 36 participants aged 18–80 years, who received a single subcutaneous CoVac-1 vaccination. The primary end point was safety analysed until day 56. Immunogenicity in terms of CoVac-1-induced T cell response was analysed as the main secondary end point until day 28 and in the follow-up until month 3. No serious adverse events and no grade 4 adverse events were observed. Expected local granuloma formation was observed in all study participants, whereas systemic reactogenicity was absent or mild. SARS-CoV-2-specific T cell responses targeting multiple vaccine peptides were induced in all study participants, mediated by multifunctional T helper 1 CD4+ and CD8+ T cells. CoVac-1-induced IFNγ T cell responses persisted in the follow-up analyses and surpassed those detected after SARS-CoV-2 infection as well as after vaccination with approved vaccines. Furthermore, vaccine-induced T cell responses were unaffected by current SARS-CoV-2 variants of concern. Together, CoVac-1 showed a favourable safety profile and induced broad, potent and variant of concern-independent T cell responses, supporting the presently ongoing evaluation in a phase II trial for patients with B cell or antibody deficiency.
Subject terms: Viral infection, Phase I trials, Peptide vaccines, SARS-CoV-2
A phase I open-label trial evaluating the immunogencity, reactogenicity and safety of a peptide-based SARS-CoV-2 vaccine candidate to induce SARS-CoV-2-specific T cell responses.
Main
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is linked to the death of millions of people3. As predominantly individuals with medical comorbidities are severely affected4, vaccines inducing long-lasting immunity, particularly in high-risk populations, are needed5–7.
CoVac-1 is a multi-peptide-based vaccine candidate designed to induce, upon a single vaccination, a broad and long-lasting SARS-CoV-2 T cell immunity resembling that acquired by natural infection, which is not affected by evolving viral variants of concern (VOCs). Thus, CoVac-1 is composed of multiple SARS-CoV-2 HLA-DR T cell epitopes derived from various viral proteins (spike, nucleocapsid, membrane, envelope and open reading frame 8 (ORF8)) that have been proven to be (1) frequently and HLA-independently recognized by T cells in convalescent individuals after COVID-19, (2) of pathophysiological relevance for T cell immunity to combat COVID-19, and (3) to mediate long-term immunity after infection1,2. CoVac-1 vaccine peptides are adjuvanted with the novel Toll-like receptor (TLR) 1/2 agonist XS15 emulsified in Montanide ISA51 VG, which endorse activation and maturation of antigen-presenting cells and prevent vaccine peptides from immediate degradation, enabling the induction of a potent T cell response8–10.
T cells have an important role for COVID-19 outcome and maintenance of SARS-CoV-2 immunity, even in the absence of humoral immune responses1,11–19. Thus, the induction of SARS-CoV-2 T cell immunity is a central goal for vaccine development and of particular importance for patients with congenital or acquired B cell deficiencies. The latter comprise patients with cancer or treatment-related immunoglobulin deficiency, who develop only limited humoral immunity after infection or vaccination and persist with a high risk for a severe course of COVID-1920–22.
Here we report the results of the open-label first-in-human phase I trial recruiting adults aged 18–80 years, to evaluate the safety, reactogenicity and immunogenicity of CoVac-1.
Participants
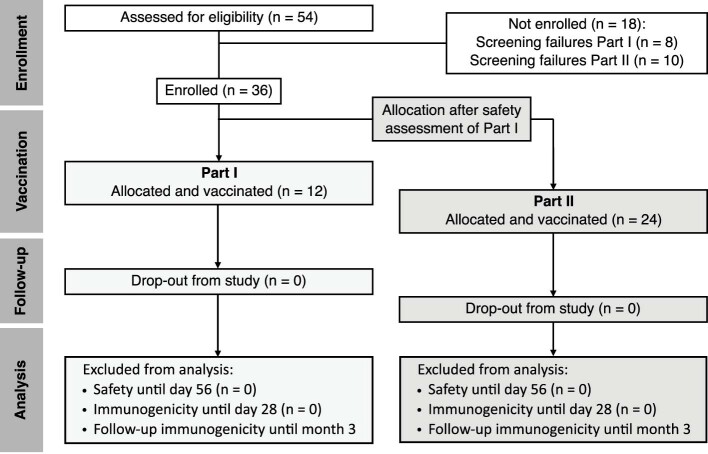
From 28 November 2020 to 15 January 2021, 12 healthy adults were enrolled in part I (age group 18–55 years), including sentinel dosing in the first participant. From 24 March 2021 to 1 April 2021, 24 adults were enrolled in part II (age group 56–80 years). Of part I and part II participants, 33% and 50%, respectively, were female participants. The median participant age was 38 (range 23–50) and 62 (range 56–70) years for part I and part II, respectively. All participants (pCoVs) received one dose of CoVac-1 on day 1 and were available for immunogenicity and safety analyses until day 28 (follow-up until month 3) and day 56, respectively (Extended Data Fig. 1). No major protocol violations occurred. Analyses of follow-up safety and long-term immunogenicity data (until month 6) are ongoing. Demographic and clinical characteristics of the participants are provided in Table 1.
Extended Data Fig. 1. Consort flow diagram of the trial.
The 18 participants who were not enrolled did not meet the inclusion criteria at screening. All 36 enrolled participants received one dose of the CoVac-1 vaccine. Safety oversight to proceed to part II was performed by an independent safety monitoring committee and approved by the Paul Ehrlich Institute and the local Ethics Committee after an interim safety and immunogenicity analysis of study participants included in part I on day 28 after vaccine administration. n, number.
Table 1.
Characteristics of participants
| Characteristics | All | Part I | Part II |
|---|---|---|---|
| Participants; n | 36 | 12 | 24 |
| Age; years | |||
| Median | 59.5 | 38.0 | 62.0 |
| Range | 23–70 | 23–50 | 56–70 |
| Mean (s.d.) | 54.8 (12.9) | 38.7 (8.2) | 62.8 (4.1) |
| Sex; n (%) | |||
| Female | 16 (44) | 4 (33) | 12 (50) |
| Male | 20 (56) | 8 (67) | 12 (50) |
| Ethnicity; n (%) | |||
| White | 36 (100) | 12 (100) | 24 (100) |
| Other | 0 (0) | 0 (0) | 0 (0) |
| Body mass indexa | |||
| Median | 24.4 | 24.9 | 24.4 |
| Range | 18.5–30.1 | 20.1–30.1 | 18.5–29.3 |
| Relevant pre-existing diseaseb; n (%) | |||
| Hypertension | 6 (16.7) | 0 (0) | 6 (25) |
| Previous malignant disease | 1 (2.8) | 0 (0) | 1 (4.2) |
| Mild psoriasis | 1 (2.8) | 0 (0) | 1 (4.2) |
aWeight in kg m−2; assessment was done at the time of screening.
bRelevant pre-existing disease includes conditions with increased risk of severe COVID-19 and with higher risk for CoVac-1 side effects.
Safety and reactogenicity
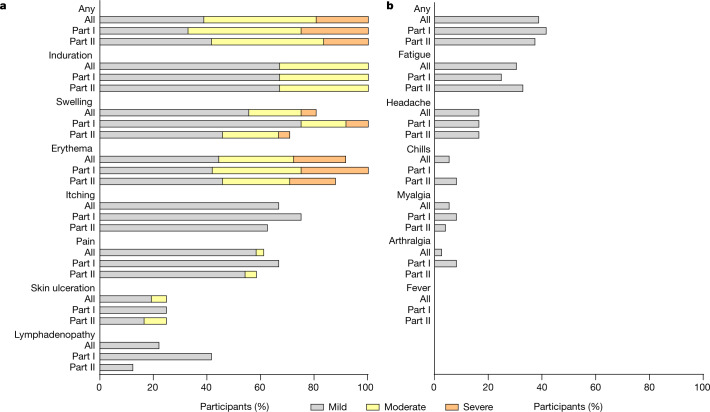
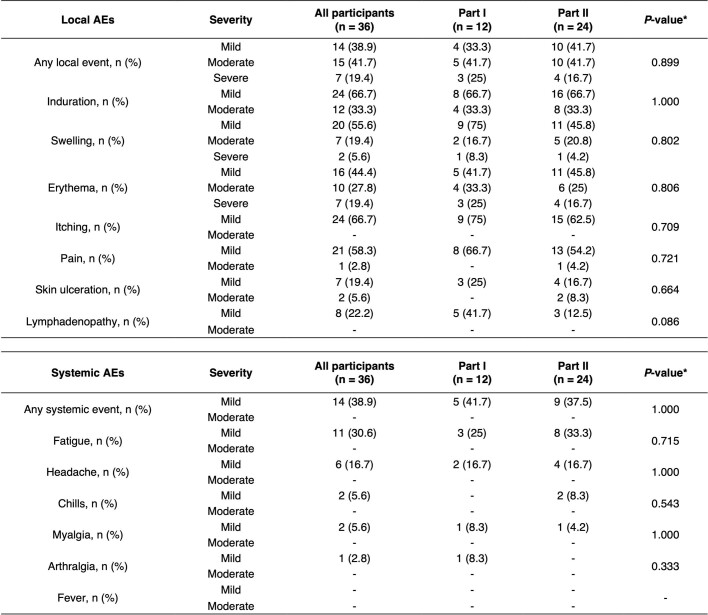
Data regarding solicited and unsolicited adverse events were available for all participants from diary cards (for 28 days after vaccination) and safety visits (until day 56). No participant discontinued the trial because of an adverse events. No serious adverse events and no grade 4 adverse events were reported. Reactogenicity in terms of solicited adverse events occurred in all participants (Fig. 1). Events were mild to moderate (grade 1–2) in 81% of participants. All participants showed expected formation of an induration (also called granuloma) at the injection site, which persisted beyond day 56. Severe adverse events (grade 3) comprised local erythema in 19%, accompanied by severe swelling in 6% of all participants. Grade 3 adverse events resolved within 2 days (median, range 1–7). Localized inguinal lymphadenopathy was reported by 22% of participants. Local skin ulceration at the vaccination site was reported by 25% of participants, with two participants in part II showing a grade 2 ulceration. Ulcerations in terms of small skin defects occurred between day 28 and day 56 and healed within 20 days (median, range 15–23) until day 56, none requiring any surgical intervention or drug treatment. No difference in local solicited adverse events was observed between part I and part II participants (Extended Data Table 1). No fever or other inflammatory systemic solicited adverse events were reported. Other systemic solicited adverse events occurred in 39% of all participants with no differences observed between part I and part II participants (Extended Data Table 1). All reported systemic solicited adverse events were mild, with transient fatigue being reported by 31% of participants.
Fig. 1. Local and systemic solicited adverse events.
a, b, Related local (a) and systemic (b) solicited adverse events within 56 days after vaccination. Severity was graded as mild (grade 1), moderate (grade 2) or severe (grade 3) based on the definition provided in Methods. Healthy adults 18–55 years of age were included in part I (n = 12), and participants 56–80 years of age were included in part II (n = 24). A detailed description of the data is presented in Extended Data Table 1.
Extended Data Table 1.
Local and systemic solicited AEs compared between part I and II
Related local and systemic solicited adverse events (AEs) assessed up to 56 days after vaccination. Severity was graded as mild (grade 1), moderate (grade 2), or severe (grade 3) based on the definition provided in the methods section. * P-values were calculated for the comparison of Part I and Part II using two-sided Fisher’s Exact test. n, number.
No clinically relevant changes in laboratory values were reported. In 31% of participants, acute phase reaction with elevated levels of C-reactive protein was observed.
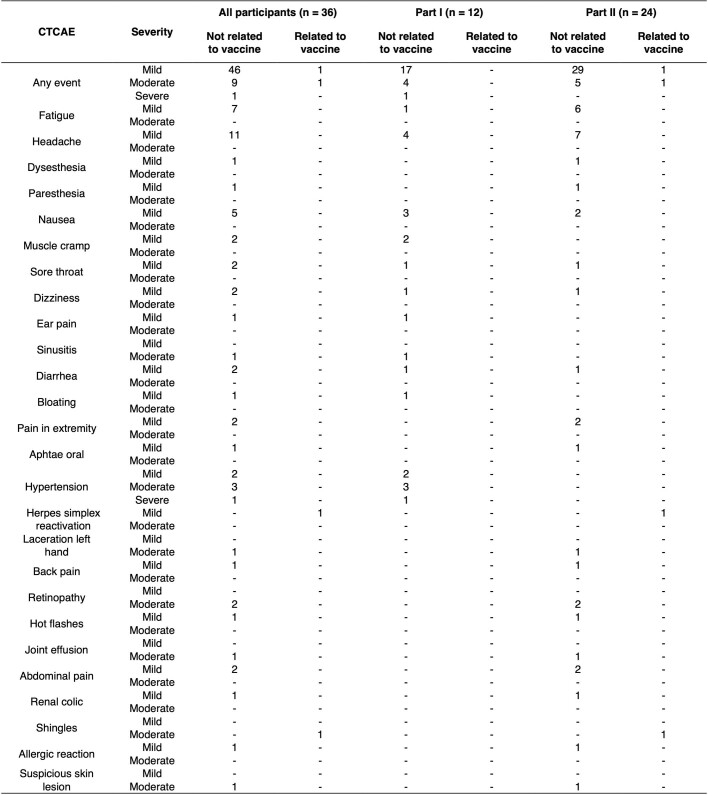
Fifty-eight unsolicited adverse events occurred that were predominantly mild (81%; Extended Data Table 2). Viral re-activations (varicella zoster and herpes simplex virus) were reported by two participants (grade 2 or lower) in part II of the trial.
Extended Data Table 2.
Unsolicited AEs classified according to CTCAE V5.0
Severity and relationship were judged by the investigator until day 56. AE, adverse event; CTCAE, common terminology criteria for adverse events; n, number.
Until day 56, no SARS-CoV-2 infection or immune-mediated medical condition was observed in any participant.
Immunogenicity
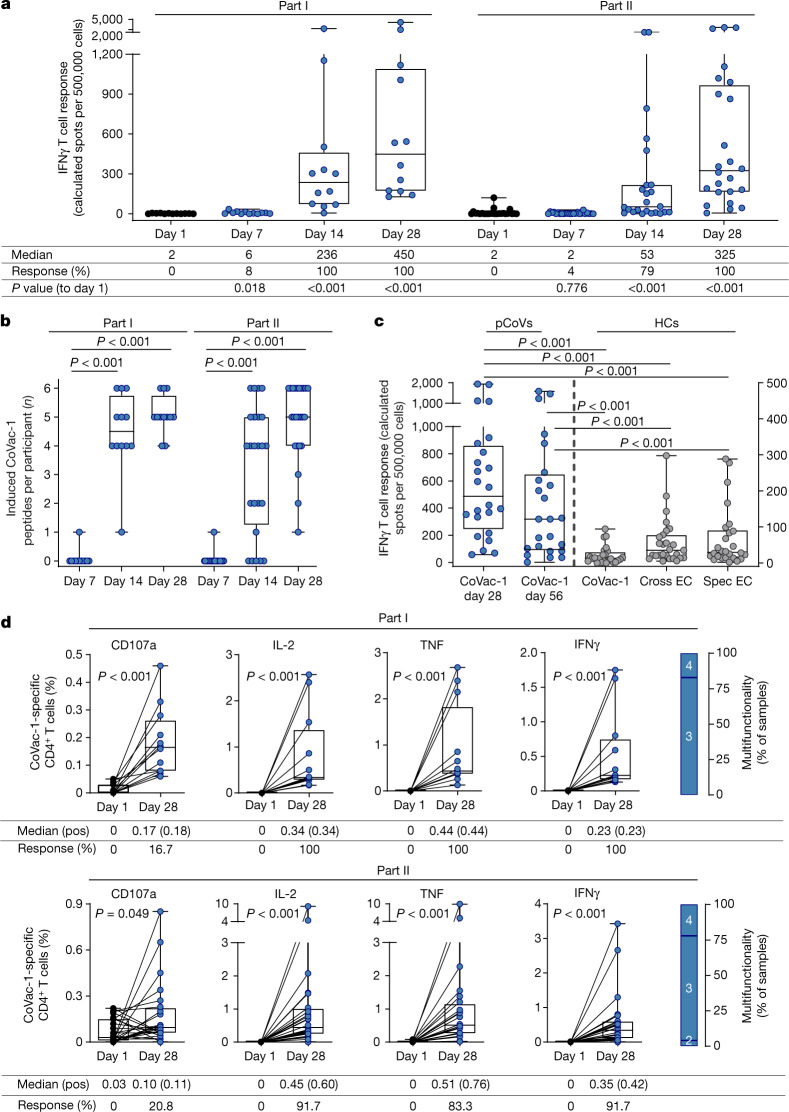
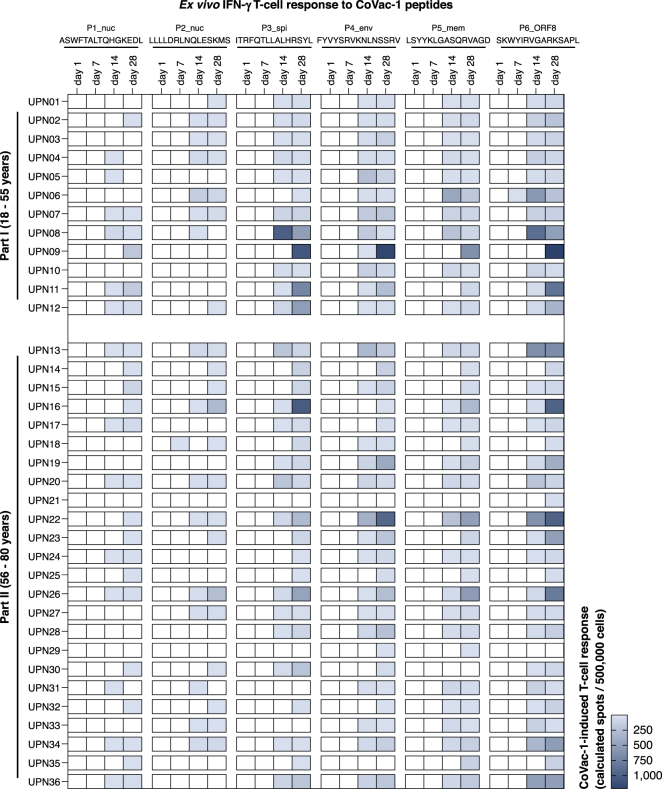
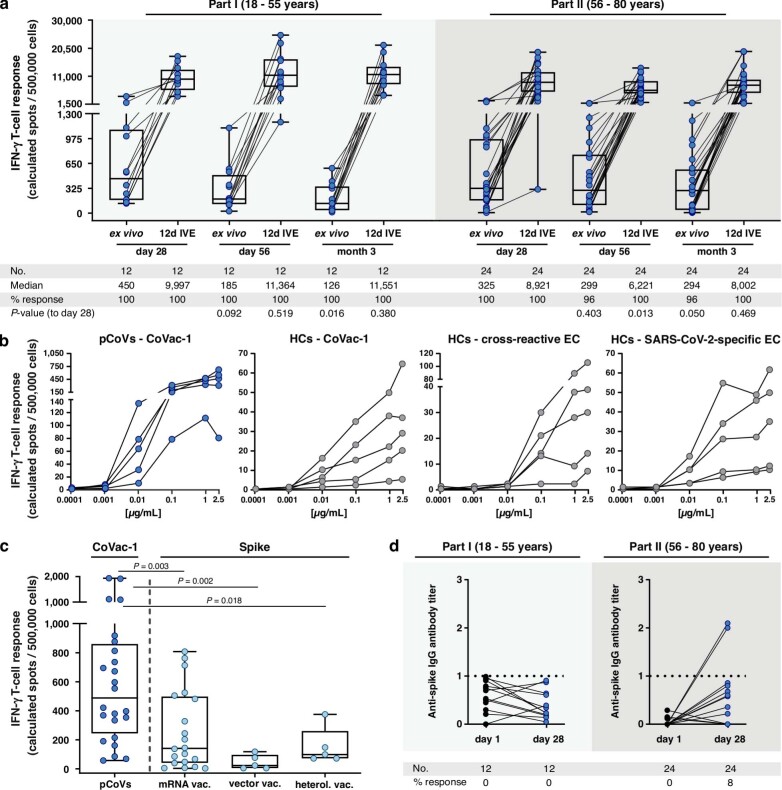
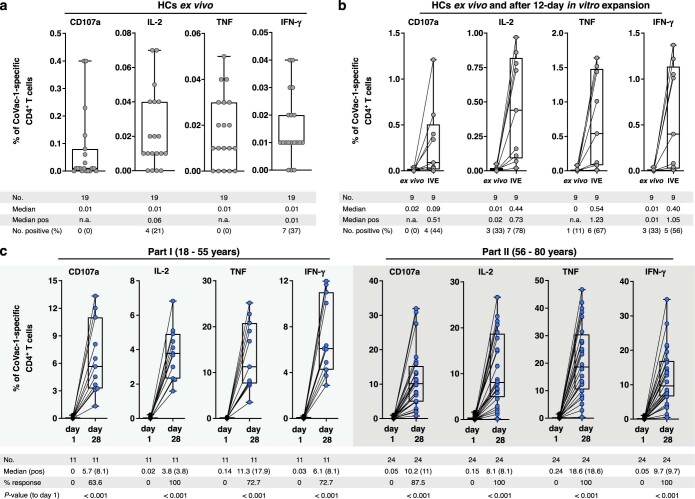
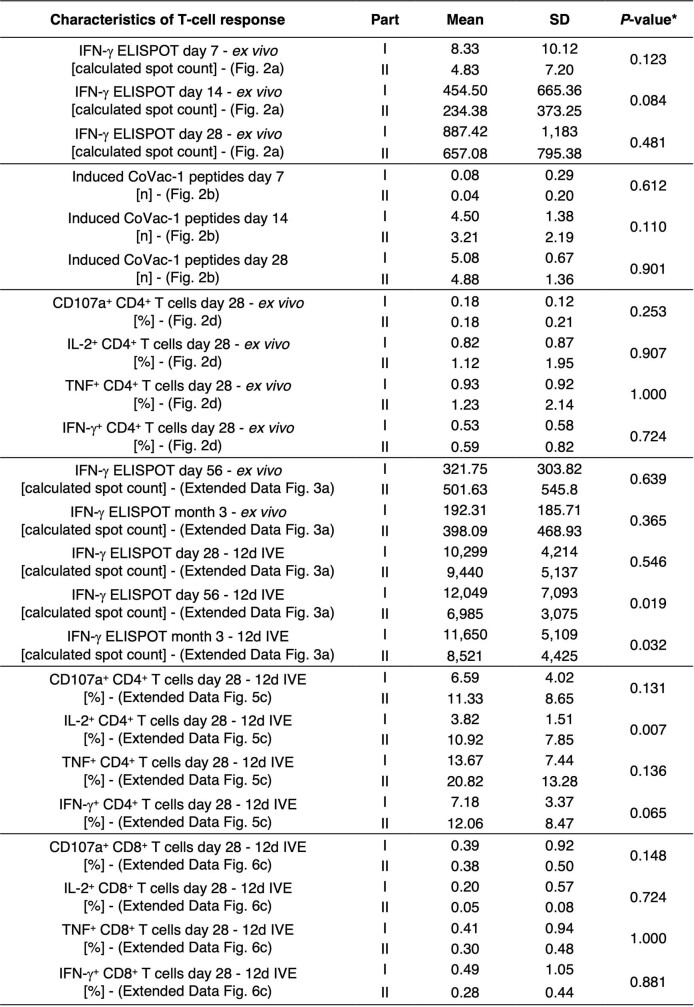
Immunogenicity of CoVac-1 was determined in terms of CD4+ and CD8+ T cell responses to the six SARS-CoV-2 HLA-DR vaccine T cell epitopes as well as to embedded HLA class I-binding peptides (Supplementary Table 1) using IFNγ enzyme-linked immunospot (ELISPOT) assays. T cell responses were assessed in all participants at baseline (day 1), on days 7, 14 and 28, as well as in the follow-up period on day 56 and month 3 after vaccination. None of the participants showed pre-existing SARS-CoV-2 T cell responses ex vivo at baseline. Vaccine-induced IFNγ T cell responses were observed in 100% of participants in part I and part II on day 28, showing a 200-fold or more and 100-fold or more increase (median calculated spot counts 2 (day 1) to 450 (day 28), and 2 (day 1) to 325 (day 28)) from baseline, respectively (Fig. 2a). Vaccine-induced T cell responses targeted multiple CoVac-1 peptides with a median 5 out of 6 peptides recognized by T cells of participants on day 28 (Fig. 2b, Extended Data Fig. 2). The CoVac-1 peptide P6_ORF8 derived from the ORF8 of SARS-CoV-2 showed most frequently induced T cell responses after vaccination (97%), followed by P5_mem and P4_env (both 94%), P3_spi (89%), P1_nuc (61%) and P2_nuc (58%; Extended Data Fig. 2). CoVac-1-induced T cell responses persisted in the follow-up analyses until month 3 in all participants. Intensity of IFNγ T cell response decreased ex vivo in part I participants over time, but equivalent expandability of CoVac-1-induced T cells was observed in both part I and part II participants, at month 3 compared with day 28 post-vaccination (Extended Data Fig. 3a). The intensity of CoVac-1-induced IFNγ T cell responses in participants of part I and part II at day 28 and day 56 (pCoVs (n = 24), median 488 and 319 calculated spot counts, respectively) was up to 39 times higher than T cell responses against CoVac-1 vaccine peptides (median 13), as well as to previously described SARS-CoV-2-specific (median 29) and cross-reactive (median 35) T cell epitopes1,2 in age-matched human convalescent individuals after COVID-19 collected 16–52 days after positive SARS-CoV-2 real-time PCR (Fig. 2c, Supplementary Table 2). Titration with decreasing peptide concentrations (2.5 µg ml−1 to 0.1 ng ml−1) revealed detection of CoVac-1 peptides by vaccine-induced T cells down to 1 ng ml−1 (10 ng ml−1 for 5 out of 5 pCoVs, 1 ng ml−1 for 3 out of 5 pCoVs). This was lower than the detection limits of SARS-CoV-2-specific T cells in human convalescent individuals for CoVac-1 vaccine peptides (10 ng ml−1 for 4 out of 5 human convalescent individuals, and 1 ng ml−1 for 0 out of 5 human convalescent individuals), SARS-CoV-2-specific (10 ng ml−1 for 5 out of 5 human convalescent individuals, and 1 ng ml−1 for 0 out of 5 human convalescent individuals) and cross-reactive T cell epitopes (10 ng ml−1 for 2 out of 5 human convalescent individuals, and 1 ng ml−1 for 0 out of 5 human convalescent individuals; Extended Data Fig. 3b). The intensity of CoVac-1-induced IFNγ T cell responses (pCoVs, median of 488 calculated spot counts) exceeded spike-specific T cell responses induced by mRNA-based (median 141), adenoviral vector-based (median 24) and heterologous (median 98) vaccination assessed 18–42 days after the second vaccination (Extended Data Fig. 3c, Supplementary Table 3).
Fig. 2. CoVac-1-induced T cell responses.
a–c, CoVac-1-induced T cell responses assessed ex vivo by IFNγ ELISPOT assays using peripheral blood mononuclear cells from study participants of part I (n = 12) and part II (n = 24) collected before vaccination (day 1) and at different time points after vaccination (days 7, 14, 28 and 56) or from human convalescent individuals (HCs). The intensity of T cell responses is depicted as cumulative calculated spot counts (mean spot count of technical replicates normalized to 500,000 cells minus the respective negative control) (a). The number of CoVac-1 T cell epitopes (n = 6) per participant that elicited a vaccine-induced T cell response (b). Intensities of CoVac-1-induced IFNγ T cell responses assessed ex vivo in part I and part II study participants (pCoVs; n = 24, day 28 and day 56, left y axis) compared with T cell responses detected in HCs (right y axis) against CoVac-1 vaccine peptides and previously published1,2 SARS-CoV-2-specific (spec) and cross-reactive (cross) T cell epitope compositions (ECs; CoVac-1 n = 24, cross EC n = 27, spec EC n = 26) (c). d, Frequencies of functional CoVac-1‐induced CD4+ T cells in study participants before vaccination (day 1) and at day 28 following vaccination using ex vivo intracellular cytokines (IFNγ, TNF and IL-2) and surface marker staining (CD107a). The right graph displays the proportion of samples revealing difunctional (2), trifunctional (3) or tetrafunctional (4) T cells. Pos, positive. In a–d, the box plots or combined box-line plots show the median with 25th or 75th percentiles, and minimum and maximum whiskers. In a, b, d, two-sided Wilcoxon signed-rank test was used; in c, two-sided Mann–Whitney U-test was used. Healthy adults 18–55 years of age were included in part I, and participants 56–80 years of age were included in part II. pos, positive.
Extended Data Fig. 2. Intensities of CoVac-1-induced T cell responses ex vivo assessed in IFNγ ELISPOT assays.
Heatmap of CoVac-1-induced T cell response intensities (calculated spots per 500,000 cells, color gradient blue) to single CoVac-1 peptides (nuc, nucleocapsid; spi, spike; env, envelope; mem, membrane; ORF, open reading frame) in ex vivo IFNγ ELISPOT assays using PBMCs from study participants (uniform participant number, UPN) of part I (n = 12) and part II (n = 24) before vaccination (day 1) and at different time points after vaccination (day 7, day 14, day 28).
Extended Data Fig. 3. Characterization of CoVac-1-induced immune responses.
(a) CoVac-1-induced long-term T cell responses assessed ex vivo or after 12-day in vitro expansion (IVE) in study participants of part I and II at day 56 and month 3 after vaccination (compared to day 28) using IFNγ ELISPOT assays. Intensity of T cell responses is depicted as calculated spot counts (mean spot count of technical replicates normalized to 500,000 cells minus the respective normalized negative control). (b) Peptide titration in ex vivo IFNγ ELISPOT assays using PBMCs from study participants (pCoVs, n = 5, day 28) or from human COVID-19 convalescent donors (HCs, n = 5) with decreasing peptide concentrations (2.5 µg mL-1 to 0.1 ng mL-1) of CoVac-1 (panel 1 and 2) or SARS-CoV-2 cross-reactive (panel 3) and SARS-CoV-2 specific (panel 4) epitope compositions (ECs). (c) Intensities of CoVac-1-induced IFNγ T cell responses assessed ex vivo in study participants of part I and part II (pCoVs, n = 24, day 28) compared to spike-specific T cell responses in healthy immunized donors after second vaccination with approved mRNA vaccines (n = 20), vector-based vaccines (n = 5), or heterologous vaccination (n = 5). (d) Anti-spike IgG antibody titers assessed on day 1 prior to vaccination and on day 28 after vaccination. Values < 0.1 were set to zero and values ≥ 1.0 were considered positive. (a, c) Box plots or combined box-line plots show median with 25th or 75th percentiles, and min/max whiskers. (a) two-sided Wilcoxon signed-rank test, (c) two-sided Mann-Whitney U-test. no, number.
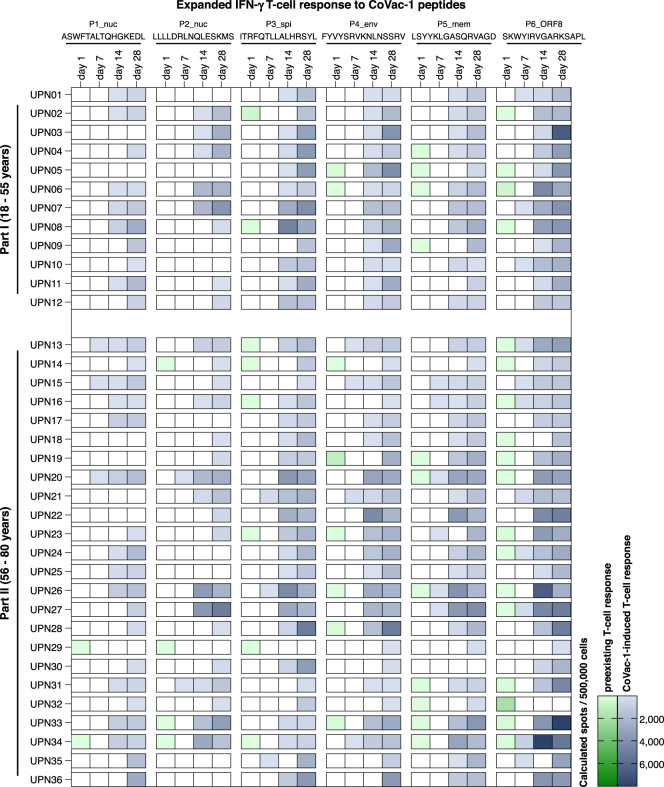
In vitro expansion of CoVac-1-specific T cells revealed pre-existing low-frequency T cell responses to single-vaccine peptides at baseline in 61% of participants that could be boosted at least twofold by CoVac-1, as observed on day 28 in all but one participant (Extended Data Fig. 4).
Extended Data Fig. 4. Intensities of CoVac-1-induced T cell responses assessed in IFNγ ELISPOT assays after 12-day in vitro expansion.
Heatmap of preexisting (color gradient green) or CoVac-1-induced (color gradient blue) T cell response intensities (calculated spots per 500,000 cells) to single CoVac-1 peptides (nuc, nucleocapsid; spi, spike; env, envelope; mem, membrane; ORF, open reading frame) in IFNγ ELISPOT assays after 12-day in vitro expansion of PBMCs from study participants (uniform participant number, UPN) of part I (n = 12) and part II (n = 24) before vaccination (day 1) and at different time points after vaccination (day 7, day 14, day 28).
CoVac-1-induced CD4+ T cells displayed a multifunctional T helper 1 (TH1) phenotype with positivity for IFNγ, tumour necrosis factor (TNF), interleukin-2 (IL-2) and CD107a (Fig. 2d). The magnitude of CoVac-1-induced CD4+ T cell responses did not differ between part I and part II participants and was up to 40 times higher than SARS-CoV-2-specific CD4+ T cell responses of human convalescent individuals (0.42% versus 0.01% (median positive samples) CoVac-1-specific IFNγ+CD4+ T cells in part II participants versus human convalescent individuals, respectively; Fig. 2d, Extended Data Fig. 5a). The frequency of functional CD4+ T cells was increased up to 40-fold after in vitro expansion (17.9% versus 0.44% (median positive samples) of CoVac-1-specific TNF+CD4+ T cells in part I participants), reaching up to 15 times higher levels than expanded CoVac-1-specific T cells of human convalescent individuals (18.6% versus 1.23% (median positive samples) CoVac-1-specific TNF+CD4+ T cells in part II participants versus human convalescent individuals, respectively), indicating potent expandability of CoVac-1-induced T cells upon SARS-CoV-2 exposure (Extended Data Fig. 5b, c).
Extended Data Fig. 5. CoVac-1-induced CD4+ T cell responses in human COVID-19 convalescents and study participants.
(a–c) Frequencies of CoVac-1‐specific CD4+ T cells in (a) human convalescent samples (HCs) after SARS-CoV-2 infection analyzed ex vivo (n = 19) and (b) after 12-day in vitro expansion (n = 9), and (c) in study participants of part I (n = 11) and part II (n = 24) after 12-day in vitro expansion of PBMCs collected prior to vaccination (day 1) or on day 28 following vaccine administration. Functionality of CD4+ T cells was assessed for upregulation of the degranulation marker CD107a and production of the T helper 1 (Th1) cytokines (IFNγ, TNF, and IL 2). (a–c) Box plots or combined box-line plots display median with 25th or 75th percentiles, and min/max whiskers, two-sided Wilcoxon signed-rank test, n.a., not applicable; no, number; pos, positive.
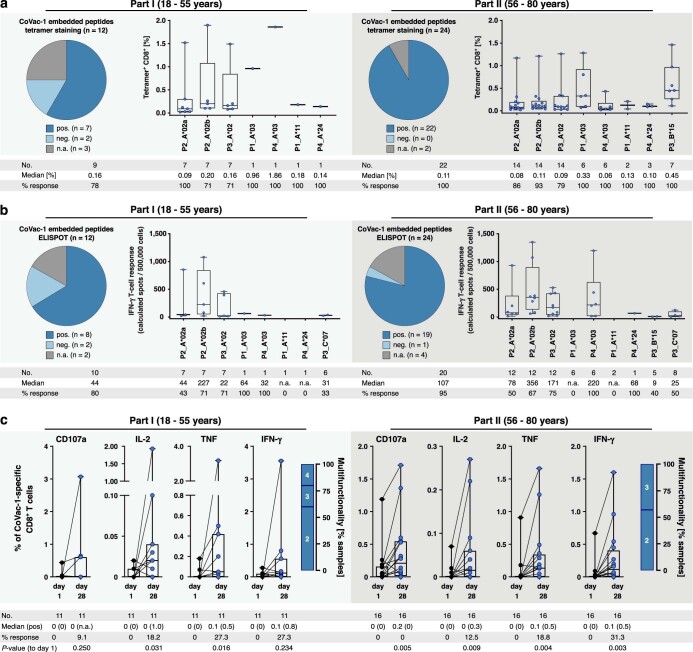
Vaccine-induced CD8+ T cell responses, identified after in vitro expansion by tetramer staining and IFNγ ELISPOT assay with HLA-matched, CoVac-1-embedded, HLA class I peptides (Supplementary Table 1) were detected in 78% and 80% of participants in part I and 100% and 95% of participants in part II with matching HLA allotypes, respectively (Extended Data Fig. 6a, b). CoVac-1-induced CD8+ T cells showed a polyfunctional phenotype reflected by IFNγ, TNF, IL-2 and CD107a production or expression (Extended Data Fig. 6c).
Extended Data Fig. 6. CoVac-1-induced CD8+ T cell responses to HLA class I-restricted CoVac-1-embedded peptides and CoVac-1 peptides.
T cell responses to HLA class I-restricted SARS-CoV-2 peptides embedded within the CoVac-1 vaccine peptides (matching the HLA allotype of the respective participant) were assessed by (a) tetramer staining and (b) IFNγ ELISPOT assays after in vitro expansion of PBMCs from study participants (part I and II) obtained on day 28 after vaccination. Pie charts display number of samples with (a) specific T cells or (b) IFNγ T cell responses to CoVac-1-embedded peptides (pos, positive; neg, negative; n.a., not assessed). Dots represent frequencies of peptide-specific T cells shown for individual donors with detected T cell responses only. (c) Frequencies of functional CoVac-1‐induced CD4+ T cells in study participants prior to vaccination (day 1) and at day 28 following vaccination using intracellular cytokine (IFNγ, TNF, and IL-2) and surface marker staining (CD107a). The right graph displays the proportion of samples revealing difunctional (2), trifunctional (3), or tetrafunctional (4) T cell responses. (a–c) Box plots or combined box-line plots show median with 25th or 75th percentiles, and min/max whiskers, two-sided Wilcoxon signed-rank test. no, number; pos, positive.
No relevant differences were observed for immunogenicity parameters between part I and part II participants except for the frequency of IL-2+ CoVac-1-specific CD4+ T cells following 12-day in vitro expansion at day 28, which was increased in part II participants, and for the expandability of CoVac-1-induced T cells at the follow-up time points (day 56 and month 3), which was decreased in part II compared with part I participants (Extended Data Table 3).
Extended Data Table 3.
Comparison of immunogenicity between part I and part II
* P-values were calculated for the comparison of Part I and Part II using two-sided Mann-Whitney U-test. 12d IVE, 12-day in vitro expansion; n, number; SD, standard deviation.
In addition to T cell responses, the induction of low-concentration SARS-CoV-2 anti-spike IgG antibodies could be observed in two participants on day 28 (Extended Data Fig. 3d).
Impact of SARS-CoV-2 variants on CoVac-1
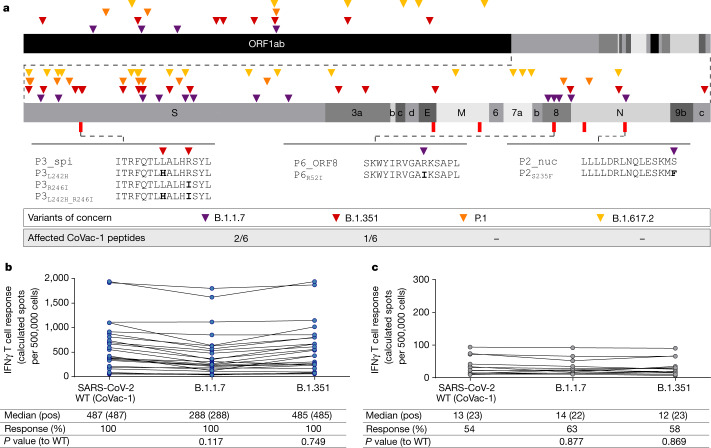
The impact of SARS-CoV-2 VOCs declared by the World Health Organization as of 1 October 2021 (B.1.1.7 (also known as Alpha), B.1.351 (also known as Beta), P.1 (also known as Gamma) and B.1.617.2 (also known as Delta)) on CoVac-1 was analysed comparing CoVac-1 peptides with the corresponding mutated regions of the respective source proteins described for each VOC (Supplementary Table 4). The sequences of 50% of vaccine peptides were not affected by any variant-defining or associated mutation23–26 (Supplementary Table 4). None of the mutations of P.1 and B.1.617.2 affect CoVac-1 vaccine peptides. Variant B.1.1.7 comprises two mutations affecting P2_nuc and P6_ORF8 with a single amino acid change, respectively. Two mutations of B.1.351 affect P3_spi with either one or two amino acid changes (Fig. 3a).
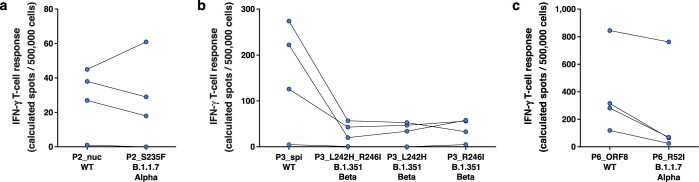
Fig. 3. Role of SARS-CoV-2 variants of concern on CoVac-1 peptides and immunogenicity.
a, Colour-coded mutations described for variants of concern are shown together with corresponding affected CoVac-1 peptides. b, c, Intensities of T cell responses (calculated spot counts) to CoVac-1 peptides as well as to the corresponding peptide pools comprising the CoVac-1-affecting mutations of B.1.1.7 and B.1.351 were assessed ex vivo by IFNγ ELISPOT assays using peripheral blood mononuclear cells from study participants of part I (n = 12) and part II (n = 24) collected on day 28 after vaccination (pCoVs) (b) or from HCs (c). Two-sided Mann–Whitney U-test was used.
T cell responses to peptide pools comprising the B.1.1.7 and B.1.351 mutated peptides P2_nuc, P3_spi and P6_ORF8 were detectable in 100% of part I and part II participants with CoVac-1-induced T cell responses to P2_nuc, P3_spi and P6_ORF8 wild-type (WT) peptides (Fig. 3b). Although the intensity of T cell responses to single-peptide variants (P3_spi and P6_ORF8) was reduced compared with WT peptides, the intensity of CoVac-1-induced T cell responses targeting the variant peptide pools was unaffected and at least 10-fold higher than T cell responses to WT and variant peptide pools observed in human convalescent individuals (median calculated spot counts 288 pCoVs B.1.1.7, 485 pCoVs B1.351, 13 WT human convalescent individuals, 14 B1.1.7 human convalescent individuals and 12 B.1.351 human convalescent individuals; Fig. 3c, Extended Data Fig. 7).
Extended Data Fig. 7. Vaccine-induced IFNγ T cell response to CoVac-1 peptides affected by mutations of SARS-CoV-2 variants of concern (VOC).
CoVac-1-induced T cell response to the single wild-type (WT) CoVac-1 peptides (P2_nuc (nucleocapsid), P3_spi (spike), P6_ORF8 (open reading frame 8)) in comparison to corresponding peptides comprising mutations of B.1.1.7-Alpha and B.1.351-Beta VOC were assessed by ex vivo IFNγ ELISPOT assay for (a) P2_nuc, (b) P3_spi, and (c) P6_ORF8 using PBMCs from study participants (n = 4) collected on day 28 after CoVac-1 vaccination.
Discussion
Our phase I trial shows that the CoVac-1 vaccine candidate has a favourable safety profile and induces potent T cell responses after a single vaccination. Local granuloma formation was observed in all study participants representing an expected and intended local reaction after Montanide-based vaccination9,27, which enables continuous local stimulation of SARS-CoV-2-specific T cells required for induction of long-lasting T cell responses without systemic inflammation. Follow-up data until month 3 after vaccination showed persistence of T cell responses, which is in line with previous experience with XS15-adjuvanted peptide vaccinations8 and data from SARS-CoV-1 convalescent individuals, where T cell immunity persisted for up to 17 years16. CoVac-1-induced TH1 CD4+ T cell responses were complemented by multifunctional CD8+ T cells, counteracting the theoretical risk of vaccine-associated enhanced respiratory disease, which has been associated with a TH2-driven immune response28. The phenotype of CoVac-1-induced T cells resembles that acquired upon natural infection1,2,11,16, but with higher magnitude than the SARS-CoV-2 T cell responses in human convalescent individuals as well as than spike-specific T cell responses induced by mRNA-based, vector-based and heterologous vaccination5–7,29, substantiating the profound T cell immunity induced by CoVac-1. This is further supported by the high diversity of CoVac-1-induced T cells that target multiple vaccine peptides from different viral proteins, which is central for effective anti-viral defence1,30–32. These broad T cell responses induced by CoVac-1 remain unaffected by current SARS-CoV-2 VOCs, which were associated with loss of neutralizing antibody capacity in convalescent individuals after COVID-19 and after vaccination33–35.
In single participants, despite negative results in sequential SARS-CoV-2 PCRs, induction of SARS-CoV-2 anti-spike IgG antibodies was documented after vaccination. This might be due to CoVac-1-induced profound CD4+ T cell responses, which not only stimulate B cells upon virus encounter but also may boost pre-existing cross-reactive SARS-CoV-2 antibodies, which were reported in 3–15% of unexposed individuals36.
T cell-mediated immunity and in particular CD4+ T cells are indispensable for the generation of protective antibody responses, reinforcement of CD8+ T cell responses37–39 as well as direct killing of virus-infected cells40,41. The relevance of anti-viral T cell responses during acute infection and for long-term immunity has also been proven specifically for SARS-CoV-21,2,13–19. Moreover, cases of asymptomatic SARS-CoV-2 exposure, as well as reports from patients with congenital B cell deficiency document cellular immune responses without seroconversion, providing evidence for T cell immunity in disease control even in the absence of neutralizing antibodies14,42. Accordingly, CoVac-1 may well serve as a (complementary) vaccine to induce T cell immunity, particularly in elderly and immunocompromised individuals with impaired ability to mount sufficient immune responses after SARS-CoV-2 vaccination with currently approved vaccines20,21.
Limitations of our trial include the small sample size, low ethnic diversity, as well as the non-equivalent time points of sample collection in the comparison of vaccine-induced and infection-induced SARS-CoV-2 T cells.
In conclusion, the safety and immunogenicity results of this trial indicate that CoVac-1 is a promising multi-peptide vaccine candidate for induction of profound SARS-CoV-2 T cell immunity, which builds the basis for a presently ongoing phase II study evaluating CoVac-1 in patients with congenital or acquired B cell defects, including patients with cancer after B cell-depleting therapy and disease-related immunoglobulin deficiency (NCT04954469).
Methods
Trial design and oversight
The phase I trial (ClinicalTrials.gov identifier: NCT04546841) was designed by and conducted at the Clinical Collaboration Unit (CCU) Translational Immunology, University Hospital Tübingen, Germany. Men as well as nonpregnant women aged 18–55 years, without any relevant pre-existing conditions and adults aged 56–80 years with stable medical conditions were included in part I and part II of the study, respectively. A detailed description of the inclusion and exclusion criteria can be found in the Supplementary Information. Health status was based on medical history and clinical laboratory values, vital signs and physical examination at screening. Participants with a proven history of SARS-CoV-2 infection (real-time PCR or antibody test) were excluded. Before enrolment, all participants provided their written informed consent. As a safety measure, sentinel dosing of the first participant treated in part I was conducted with a follow-up period of 28 days after vaccination followed by a sponsor safety assessment before proceeding with the vaccination of further study participants. Safety assessment of the sentinel dosing participant is described in detail in the Supplementary Information. The trial was open-label (no blinding) without a control arm (no randomization).
The trial was funded by the Ministry of Science, Research and the Arts Baden-Württemberg, Germany. The trial was approved by the Ethics Committee, University Tübingen (537/2020AMG1) and the Paul Ehrlich Institute and performed in accordance with the International Council for Harmonization Good Clinical Practice guidelines.
Safety assessment to proceed to part II was performed by an independent data safety monitoring board (DSMB).
Trial vaccine and adjuvant
CoVac-1, developed and produced by the Good Manufacturing Practices (GMP) Peptide Laboratory of the Department of Immunology, University Tübingen, is a peptide-based vaccine comprising six HLA-DR-restricted SARS-CoV-2 peptides (Supplementary Table 1) derived from various SARS-CoV-2 proteins (spike, nucleocapsid, membrane, envelope and ORF8) and the adjuvant lipopeptide synthetic TLR1/2 ligand XS158 (manufactured by Bachem AG) emulsified in Montanide ISA51 VG9 (manufactured by Seppic). CoVac-1 peptides represent dominant SARS-CoV-2 T cell epitopes (peptide-specific T cell responses detected in more than 50% and up to 100% of convalescent individuals after SARS-CoV-2 infection) validated in human convalescent individuals after SARS-CoV-2 infection to mediate long-term immunity1,2. CoVac-1 peptides were predicted and validated to bind to multiple HLA-DR molecules (promiscuous binding)1, which is important to enable HLA-independent induction of T cell responses by CoVac-11,2,43.
CoVac-1 HLA-DR T cell epitopes contain embedded HLA class I sequences for induction of both CD4+ and CD8+ T cell responses (Supplementary Table 1). CoVac-1 peptides were selected from viral non-surface proteins and their subunits or—in case of the spike protein-derived T cell epitope P3_spi—from buried (or hidden) amino acid sequences, which are not accessible for antibodies in their conformational state. The linear 15-amino acid peptides are characterized by a free N-terminal amino group and a free C-terminal carboxy group. All amino acid residues are in the l-configuration and were not chemically modified at any position. Synthetic peptides were manufactured by established solid-phase peptide synthesis procedures using Fmoc chemistry44,45.
The novel adjuvant XS15 hydrochloride is a water-soluble synthetic linear, nine amino acid peptide with a palmitoylated N terminus (Pam3Cys-GDPKHPKSF)8. Acting as a TLR1/2 ligand, XS15 strongly activates antigen-presenting cells8 and enables the induction of strong ex vivo CD8+ and TH1 CD4+ responses to viral peptides, including SARS-CoV-2 T cell epitopes, in preliminary in vivo analyses in a human volunteer upon a single subcutaneous injection of XS15 mixed to uncoupled viral peptides in a water-in-oil emulsion with Montanide ISA51 VG8,10. To our knowledge, this is the first report of the adjuvant XS15 being used in a human clinical trial. Montanide ISA51 VG is a mixture of a highly purified mineral oil (Drakeol 6VR) and a surfactant (mannide monooleate). When mixed with an aqueous phase in a 50:50 ratio, it forms a water-in-oil emulsion. Such a Montanide-based water-in-oil emulsion has been used as vaccine adjuvant in multiple clinical trials9,27, to build a depot at the vaccination site, thereby preventing vaccine peptides from immediate degradation and thus enhancing the immune response.
CoVac-1 peptides (250 µg per peptide) and XS15 (50 µg) are prepared as a water–oil emulsion 1:1 with Montanide ISA51 VG to yield an injectable volume of 500 µl. Each participant received one subcutaneous injection of the CoVac-1 vaccine in the lower abdomen on day 1.
The dosage of CoVac-1 vaccine peptides was determined based on results from various clinical trials evaluating peptide vaccines44,46–51 (including dose-finding studies for viral T cell epitopes), which showed significantly stronger immune responses to 250–500 µg versus 100 μg peptide dose, without significantly higher immune responses in the 1,000 µg versus 500 µg dose group47. Similar T cell responses were induced with 250 µg and 500 µg peptide doses. Regarding safety, even doses up to 30 mg per peptide did not raise any concerns48. On the basis of these data, the dose of 250 μg per peptide was used for CoVac-1 vaccine peptides.
The dosage of the TLR1/2 agonist XS15 was determined based on in vitro analyses of immune cell activation by TLR1/2. In these assays, 10 µg ml−1 XS15 was shown to be the most efficient dose for the stimulation of immune cells. Considering the formation of a granuloma after subcutaneous injection of XS15 emulsified in Montanide ISA51 VG, which leads to a size-dependent decrease in XS15 concentration8, 50 µg XS15 was selected to achieve the desired dosage of 10 µg ml−1 at the vaccination site. In a toxicity study in mice, 50 µg XS15 in Montanide ISA51 VG, applied subcutaneously, did not reveal any local or systemic toxicity beyond the long known and expected local toxicity of Montanide9,27. For a more detailed description of the dosage rationale for the vaccine peptides and the adjuvant, please refer to the Supplementary Information.
Safety assessment
Primary safety outcomes reflect the nature, frequency and severity of solicited adverse events until day 56 after vaccination. The documentation was facilitated by use of a volunteer diary (for 28 days after vaccination) and graded by the investigators according to a modified Common Terminology Criteria for Adverse Events (CTCAE) V5.0 grading scale (Supplementary Table 5). In addition, the number and percentage of participants with unsolicited events until day 56 were reported (documented according to CTCAE V5.0). Safety assessment included clinically significant changes in laboratory values (haematology and blood chemistry), serious adverse events, and adverse events of special interest, which included desired induration (granuloma) formation, SARS-CoV-2 infection, COVID-19 manifestations and immune-mediated medical conditions (Supplementary Tables 6, 7).
Immunogenicity assessment
Secondary outcome was the induction of CoVac-1-specific T cell responses to at least one of the CoVac-1 vaccine peptides evaluated on day 7, day 14 and day 28 by the IFNγ ELISPOT assay ex vivo and after in vitro T cell expansion (baseline day 1, before vaccination). Follow-up analyses of CoVac-1-induced T cell responses were performed on day 56 and month 3 after vaccination. The 12-day in vitro expansion of peptide-specific T cells was performed to enable detection of low-frequent, vaccine-induced and pre-existing SARS-CoV-2-specific T cells, as well as to prove the expandability of CoVac-1-induced T cells, which is of central importance for potent T cell response upon SARS-CoV-2 exposure. In this regard, the characterization of vaccine-induced CD8+ T cells was performed after 12-day in vitro expansion, due to the low frequency of peptide-specific CD8+ T cells observed ex vivo (Supplementary Table 8). PBMCs were pulsed with CoVac-1 peptides (5 μg ml−1 per peptide) and cultured for 12 days adding 20 U ml−1 IL-2 (Novartis) on days 3, 5 and 7. For IFNγ ELISPOT (ex vivo or after in vitro expansion), cells were stimulated with 1 µg ml−1 of HLA class I or 2.5 μg ml−1 of HLA-DR peptides and analysed in technical replicates. T cell responses were considered positive if the mean spot count was threefold or more higher than the mean spot count of the negative control and defined as CoVac-1-induced if the mean spot count post-vaccination was twofold or more higher than the respective spot count on day 1. CoVac-1-induced T cell responses were further characterized using tetramer (5 µg ml−1), cell-surface marker and intracellular cytokine staining. For intracellular cytokine staining, cells were stimulated with 10 µg ml−1 per peptide. The gating strategy applied for the analyses of flow cytometry-acquired data is provided in Supplementary Fig. 1. Immunogenicity results were compared with human convalescent individuals with PCR-confirmed SARS-CoV-2 infection and healthy volunteers vaccinated with an approved mRNA-based or vector-based vaccine or received heterologous vaccination (Supplementary Tables 2, 3). All assays were conducted in a blinded manner and are described in detail in the Supplementary Information.
Statistical analysis
The sample size calculation (36 participants) of the trial was based on the assumption that incidence of serious adverse events associated with administration of CoVac-1 does not exceed a predetermined rate of 5%. Safety data are displayed by counting the respective adverse event that has occurred at least once in a patient. The highest grading of this adverse event is indicated. Data are displayed as mean ± s.d., box plots as median with 25% or 75% quantiles and minimum and maximum whiskers. Continuous data were tested for distribution, and individual groups were tested by use of Fisher’s exact test, unpaired Mann–Whitney U-test or paired Wilcoxon signed-rank test, all performed as two-sided tests. No adjustment for multiple testing was done. Details regarding the statistical analysis plan and sample size calculation are provided in the Supplementary Information and the protocol.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-021-04232-5.
Supplementary information
The Supplementary Information contains: Supplementary Methods, Supplementary Study Results, Supplementary Tables 1–8 and Supplementary Fig. 1.
This file contains the redacted study protocol and statistical analysis plan.
Source data
Acknowledgements
We thank all of the participants of this trial and the members of the data safety monitoring board (P. Brossart, Bonn; H. Schild, Mainz; C. Wendtner, Munich); the technical and clinical staff of the CCU Translational Immunology, Department of Internal Medicine, University Hospital Tübingen; the team of the ‘Wirkstoffpeptid Labor’, Department of Immunology, Tübingen; the pharmacy of the University Hospital Tübingen; the data management team at the Institute for Clinical Epidemiology and Applied Biometry; the ‘Zentrum für Klinische Studien’ (particularly M. Walker) at the University Hospital Tübingen for support and coordination; and EMC Microcollections GmbH for provision of XS15. This work was supported by the Ministry of Science, Research and the Arts Baden-Württemberg, Germany (Sonderfördermassnahme COVID-19, TÜ17), Bundesministerium für Bildung und Forschung (BMBF, FKZ:01KI20130), the Robert Bosch Stiftung, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, grant WA 4608/1-2), the Deutsche Forschungsgemeinschaft under Germany’s Excellence Strategy (grant EXC2180-390900677), the German Cancer Consortium (DKTK), the Wilhelm Sander Stiftung (grant 2016.177.2), the José Carreras Leukämie-Stiftung (grant DJCLS 05 R/2017), the Fortüne Program of the University of Tübingen (Fortüne number 2451-0-0) and the Ernst-Jung-Preis awarded to H.-G.R.
Extended data figures and tables
Author contributions
J.S.H., H.-G.R., H.R.S. and J.S.W. were involved in the design of the overall study and strategy. C.M., H.-G.R., I.F. and M.W.L. provided feedback on the study design. T.B., C.T., A.N., Y.M., M.Roerden, J.B., J.Rieth and M.W. performed the immunogenicity analyses. J.S.H., M.M., J.Reusch, S.J., L.A., L.M.W., S.H., A.P., J.K., E.J., R.K. and J.S.W. conducted patient data and sample collection, as well as medical evaluation and analysis. J.S.H., M.M., J.Reusch, S.J., H.R.S. and J.S.W. collected data as study investigators. C.M. and I.F. developed the statistical design and oversaw the data analysis. M.D. and M.Richter conducted GMP production of CoVac-1. J.S.H., T.B., C.T., M.W.L., H.R.S. and J.S.W. drafted the manuscript. All authors supported the review of the manuscript.
Peer review information
Nature thanks Antonio Bertoletti and Julie Ledgerwood for their contribution to the peer review of this work. Peer review reports are available.
Data availability
Data supporting the findings of this study including the study protocol and the statistical analysis plan are supplied as source data with this paper. Further data, including de-identified participant data, are available after final completion of the trial report and are shared according to data sharing guidelines on reasonable request to the corresponding author (J.S.W.).
Competing interests
The University Hospital Tübingen is in the process of applying for a patent application (EP 20 169 047.6) covering the SARS-CoV-2 T cell epitopes included in CoVac-1 that lists A.N., T.B., H.-G.R. and J.S.W as inventors. EMC Microcollections GmbH is in the process of applying for a patent application (DE102016005550.2) covering the adjuvant XS15 included in CoVac-1 that lists H.-G.R. as an inventor. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jonas S. Heitmann, Tatjana Bilich, Claudia Tandler
Extended data
is available for this paper at 10.1038/s41586-021-04232-5.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-021-04232-5.
References
- 1.Nelde A, et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021;22:74–85. doi: 10.1038/s41590-020-00808-x. [DOI] [PubMed] [Google Scholar]
- 2.Bilich T, et al. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci. Transl. Med. 2021;13:eabf7517. doi: 10.1126/scitranslmed.abf7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Weekly epidemiological update on COVID-19. WHOhttps://www.who.int/docs/default-source/coronaviruse/situation-reports/20201012-weekly-epi-update-9.pdf (2021).
- 4.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramasamy MN, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rammensee HG, et al. A new synthetic toll-like receptor 1/2 ligand is an efficient adjuvant for peptide vaccination in a human volunteer. J. Immunother. Cancer. 2019;7:307. doi: 10.1186/s40425-019-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev. Vaccines. 2002;1:111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 10.Rammensee HG, et al. Designing a SARS-CoV-2 T-cell-inducing vaccine for high-risk patient groups. Vaccines. 2021;9:428. doi: 10.3390/vaccines9050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodda LB, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169–183.e17. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long QX, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 13.Tan AT, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34:108728. doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soresina A, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020;31:565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dan JM, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bert N, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 17.Grifoni A, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun J, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 19.Mateus J, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herishanu Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monin L, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee LY, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faria NR, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372:815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrew Rambaut et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. Virologicalhttps://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 (2020).
- 25.Mlcochova P, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tegally H, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 27.van Doorn E, Liu H, Huckriede A, Hak E. Safety and tolerability evaluation of the use of Montanide ISA51 as vaccine adjuvant: a systematic review. Hum. Vaccin. Immunother. 2016;12:159–169. doi: 10.1080/21645515.2015.1071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020;5:1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298:1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 31.Kiepiela P, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 32.Bilich T, et al. Preexisting and post-COVID-19 immune responses to SARS-CoV-2 in cancer patients. Cancer Discov. 2021;11:1982–1995. doi: 10.1158/2159-8290.CD-21-0191. [DOI] [PubMed] [Google Scholar]
- 33.Emary KRW, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu K, et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N. Engl. J. Med. 2021;384:1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng KW, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370:1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 38.Carvalho LH, et al. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 39.Kemball CC, et al. The antiviral CD8+ T cell response is differentially dependent on CD4+ T cell help over the course of persistent infection. J. Immunol. 2007;179:1113–1121. doi: 10.4049/jimmunol.179.2.1113. [DOI] [PubMed] [Google Scholar]
- 40.van de Berg PJ, van Leeuwen EM, ten Berge IJ, van Lier R. Cytotoxic human CD4+ T cells. Curr. Opin. Immunol. 2008;20:339–343. doi: 10.1016/j.coi.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji M, Romero P, Nussenzweig RS, Zavala F. CD4+ cytolytic T cell clone confers protection against murine malaria. J. Exp. Med. 1990;172:1353–1357. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallais F, et al. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg. Infect. Dis. 2021;27:113–121. doi: 10.3201/eid2701.203611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarke A, et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021;2:100204. doi: 10.1016/j.xcrm.2021.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilf N, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 45.Platten M, et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature. 2021;592:463–468. doi: 10.1038/s41586-021-03363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rini BI, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17:1599–1611. doi: 10.1016/S1470-2045(16)30408-9. [DOI] [PubMed] [Google Scholar]
- 47.Kran AM, et al. HLA- and dose-dependent immunogenicity of a peptide-based HIV-1 immunotherapy candidate (Vacc-4x) Aids. 2004;18:1875–1883. doi: 10.1097/00002030-200409240-00003. [DOI] [PubMed] [Google Scholar]
- 48.Sato Y, et al. Immunological evaluation of peptide vaccination for patients with gastric cancer based on pre-existing cellular response to peptide. Cancer Sci. 2003;94:802–808. doi: 10.1111/j.1349-7006.2003.tb01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noguchi M, et al. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003;57:80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 50.Atsmon J, et al. Safety and immunogenicity of multimeric-001-a novel universal influenza vaccine. J. Clin. Immunol. 2012;32:595–603. doi: 10.1007/s10875-011-9632-5. [DOI] [PubMed] [Google Scholar]
- 51.Feyerabend S, et al. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate. 2009;69:917–927. doi: 10.1002/pros.20941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Supplementary Information contains: Supplementary Methods, Supplementary Study Results, Supplementary Tables 1–8 and Supplementary Fig. 1.
This file contains the redacted study protocol and statistical analysis plan.
Data Availability Statement
Data supporting the findings of this study including the study protocol and the statistical analysis plan are supplied as source data with this paper. Further data, including de-identified participant data, are available after final completion of the trial report and are shared according to data sharing guidelines on reasonable request to the corresponding author (J.S.W.).