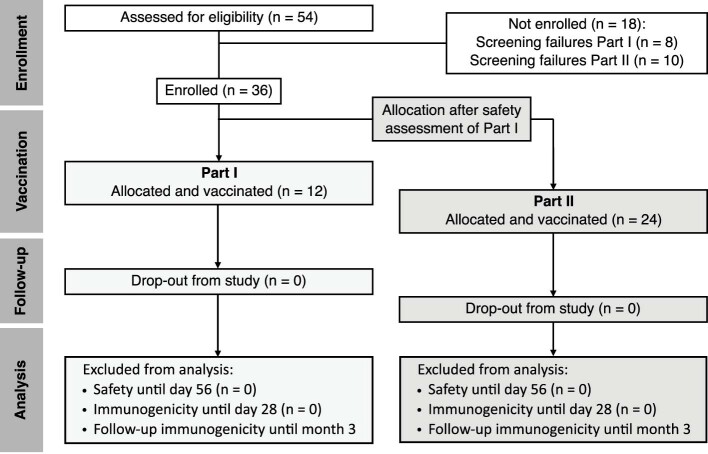
Extended Data Fig. 1. Consort flow diagram of the trial.
The 18 participants who were not enrolled did not meet the inclusion criteria at screening. All 36 enrolled participants received one dose of the CoVac-1 vaccine. Safety oversight to proceed to part II was performed by an independent safety monitoring committee and approved by the Paul Ehrlich Institute and the local Ethics Committee after an interim safety and immunogenicity analysis of study participants included in part I on day 28 after vaccine administration. n, number.