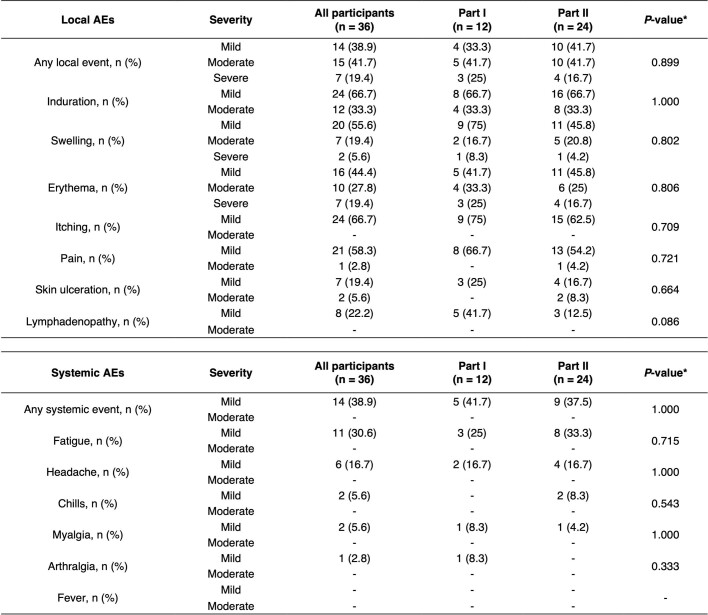
Extended Data Table 1.
Local and systemic solicited AEs compared between part I and II
Related local and systemic solicited adverse events (AEs) assessed up to 56 days after vaccination. Severity was graded as mild (grade 1), moderate (grade 2), or severe (grade 3) based on the definition provided in the methods section. * P-values were calculated for the comparison of Part I and Part II using two-sided Fisher’s Exact test. n, number.