Abstract
In cattle, starting 4–5 days after estrus, preimplantation embryonic development occurs in the confinement of the uterine lumen. Cells in the endometrial epithelial layer control the molecular traffic to and from the lumen and, thereby determine luminal composition. Starting early postestrus, endometrial function is regulated by sex steroids, but the effects of progesterone on luminal cells transcription have not been measured in vivo. The first objective was to determine the extent to which progesterone controls transcription in luminal epithelial cells 4 days (D4) after estrus. The second objective was to discover luminal transcripts that predict pregnancy outcomes when the effect of progesterone is controlled. Endometrial luminal epithelial cells were collected from embryo transfer recipients on D4 using a cytological brush and their transcriptome was determined by RNASeq. Pregnancy by embryo transfer was measured on D30 (25 pregnant and 18 nonpregnant). Progesterone concentration on D4 was associated positively (n = 182) and negatively (n = 58) with gene expression. Progesterone-modulated transcription indicated an increase in oxidative phosphorylation, biosynthetic activity, and proliferation of epithelial cells. When these effects of progesterone were controlled, different genes affected positively (n = 22) and negatively (n = 292) odds of pregnancy. These set of genes indicated that a receptive uterine environment was characterized by the inhibition of phosphoinositide signaling and innate immune system responses. A panel of 25 genes predicted the pregnancy outcome with sensitivity and specificity ranging from 64%–96% and 44%–83%, respectively. In conclusion, in the early diestrus, both progesterone-dependent and progesterone-independent mechanisms regulate luminal epithelial transcription associated with pregnancy outcomes in cattle.
Keywords: cattle, fertility, gene regulation, progesterone, uterus
INTRODUCTION
In cattle, attachment of the conceptus and subsequent placentation start in the third week of gestation (1). Prior to attachment, embryonic development occurs in the confinement of the reproductive tract lumen, initially in the oviduct; moving to the uterus 4–5 days postestrus (2). Molecules that support embryo development in the uterus originate from the endometrial epithelium, both from endometrial glands and from the lining of the uterine lumen (3). Endometrial epithelial cells release molecules synthesized de novo to the uterine lumen. In addition, the epithelium also selectively transports molecules from different cellular and tissue origins to the uterine lumen. For example, essential amino acids are found in the uterine lumen and the corresponding transporters are expressed by endometrial cells (4). Thus, endometrial epithelial function determines the molecular milieu in which the embryo resides. It is expected that variations in the luminal molecular milieu can be decisive for the fate of the developing embryo. In fact, imposition of luminal washings, before embryo transfer, diminished embryo survival by more than 50% (5). Washings were conducted 4 or 7 days postestrus, which indicates that events occurring at very early stages of diestrus are already determinant for the pregnancy outcome. Indeed, using a model to generate contrasting states of uterine receptivity during early diestrus, comparisons confirmed the occurrence of different: 1) transcriptome profiles (6), 2) abundance of transcripts related to amino acids and metabolism (7), and 3) redox activity (8). Altogether, these reports support the notion that as early as the embryo arrives in the uterus, i.e., day 4 postestrus, specific luminal milieus may dictate the pregnancy outcome in cattle.
The overarching hypothesis is that there are molecular signatures in endometrial epithelial cells that are associated with pregnancy success or failure in cattle on day 4 postestrus, just prior to embryo transfer that occurs on day 7 postestrus. Most in vivo studies that attempted to discover molecular characteristics of the reproductive tract associated with receptivity to the embryo used endometrial biopsies as the source of tissue (9–12). Endometrial biopsies are usually 2–3 mm thick and contain variable proportions of the many cell types that constitute the endometrium (and even the myometrium), such as luminal epithelial, glandular epithelial, stromal, endothelial, lymphocytes, and other blood cells and smooth muscle (13). Transcriptomic analysis of complex tissues may not provide enough resolution to characterize cellular function, as the RNA from all cell types is extracted as a single pool. Indeed, in our previous study using biopsies to compare the endometrial transcriptome of cows that succeeded versus failed to maintain pregnancy, there was an enrichment in gene ontology terms associated with extracellular matrix remodeling, a process typically associated with stromal cells (9, 14). Due to the critical role played by the luminal epithelium as the gatekeeper of molecular access to the preimplantation embryo, it is critical to determine molecular functions associated with receptivity in that cellular compartment in vivo. This is challenging if endometrial biopsies are used as a tissue source because the relative proportion of endometrial epithelial cells is expected to be low (15). Furthermore, another limitation of previous studies is that endometrium was sampled during the estrous cycle preceding the cycle in which animals received embryo transfer (11, 12, 16, 17), thereby failing to capture cycle-specific molecular changes that influence pregnancy outcome. Collectively, there is a gap in knowledge regarding the regulation of endometrial luminal epithelial cell function during early diestrus in cattle. Here, we interrogated the endometrial luminal epithelium transcriptome of cows at day 4 postestrus and on the same cycle as the reproductive service, using a cytological brush. Collection by cytobrush is minimally invasive and allows harvesting of luminal samples highly enriched in luminal epithelial cells (15, 18).
The first objective was to determine the extent to which progesterone controls transcription of luminal epithelial cells 4 days postestrus. Progesterone concentration during the first week postestrus influences events that occur later in gestation and, ultimately, the pregnancy outcome (6, 8, 18–21). For example, progesterone concentrations measured 3 to 7 days postestrus are positively associated with conceptus elongation, which occurs 8 to 12 days later, and such effects are mediated through the endometrium (22, 23). However, it is expected that a portion of the effects of progesterone on the endometrial transcriptome does not necessarily affect receptivity to the embryo. The endometrial functions that are unrelated to progesterone, but support pregnancy, have been poorly explored. As a consequence, luminal genes that predict the pregnancy outcomes, and not just a response to progesterone, remain to be identified. Therefore, the second objective was to discover, on day 4 postestrus and on the same cycle as the embryo transfer, the luminal transcripts that predict pregnancy outcomes, when the effect of progesterone was controlled.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) from the University of Florida (UF) under the Protocol No. IACUC-202009814. Embryo transfer recipients were nonsuckled (n = 7) and suckled (n = 73) multiparous Bos indicus influenced cows (admixture of various proportions of Angus and Brahman genetics). Nonsuckled cows calved, but the calves died. Recipients had average body weight: 556.8 ± 9 kg (411–755 kg), age: 6.7 ± 0.3 yr (3.2–12.4), and days postpartum: 113 ± 9 days (24–271 d). All recipients conceived and calved yearly, and were therefore considered to be of proven fertility. Recipients were maintained under grazing conditions (Lolium), supplemented with hay, concentrate, and minerals to fulfill their maintenance requirements. Animals received water ad libitum. The experiment was conducted between April and July 2019.
Experimental Design
Estrous cycles were synchronized using the Select Synch + CIDR protocol [Protocols-for-Sire-Directories-2021-Final.pdf (beefrepro.org)]. On the day of CIDR removal, cows were fitted with an estrus detection patch (ESTROTECT, Rockway Inc., Spring Valley, WI). Estrous behavior was monitored once a day for 3 days. Animals were considered in estrus when 50% or more of the coating surface of the patch was removed. This was considered experimental day 0 (D0). Only animals displaying estrus remained in the study. Synchronizations were performed in two rounds with an interval of 49 days in between. A total of 53 and 60 recipient cows were synchronized in the first and second rounds, respectively. In the second round, 33 cows from the first round were resynchronized.
On D4, cows were submitted to the collection of uterine luminal samples using a cytological brush (Disposable cytology sampling brush 8”; Viamed Ltd, West Yorkshire, UK) according to Cardoso et al., (15) and blood samples were collected from coccygeal vessels. For logistical reasons, only 71 of the 84 eligible recipients were sampled. Eligibility was based on the presence of a corpus luteum (CL) on the day of embryo transfer (ET) occurring on D7. Prior to ET, cows received caudal epidural anesthesia [4 mL of 2% (w/v) lidocaine solution] (Vet One, Boise, ID). A single in vitro-produced embryo was deposited transcervically in the cranial third of the uterine horn ipsilateral to the CL-containing ovary. All ETs were performed by the same operator. Pregnancy per ET was determined by transrectal ultrasonography exam on D30 and was based on the presence or absence of an embryo with a heartbeat.
Sampling the Uterine Lumen
On D4 (i.e., 3 days prior ET), uterine luminal samples from the uterine body were collected transcervically from recipient cows using a cytobrush as described previously (15, 18). Briefly, the cytobrush was coupled to the tip of a conventional artificial insemination (AI) gun, covered by a disposable AI sheath, and protected by a sanitary plastic sheath. After epidural anesthesia, the apparatus was inserted via the cervix, and the cytological brush was exposed at the beginning of uterine body, right after the last cervical annular fold, and rotated to collect luminal epithelial cells (15). After retraction of the brush to the plastic sheath, the apparatus was withdrawn from the animal. Then, cytological brush was uncoupled from the apparatus, placed in a 2-mL DNase/RNase-free microcentrifuge tube containing 1 mL of sterile phosphate-buffered saline (Hyclone Cat. No. SH30256.01, South Logan, UT), and kept in a cooler containing chopped ice until further processing. Cells were recovered from brushes by vortex at maximum speed for 1 min and centrifugation at 400 g for 7 min. The resulting cell pellets were stored at −80°C for subsequent RNA extraction.
In Vitro Production of Embryos
Embryos were produced in vitro using oocytes from 18 Brahman donor cows and semen from four Brahman bulls. Breeding decisions were made by farm management. Ovum pick-up was performed as per standard technique, resulting in 692 oocytes collected. Cumulus oocyte complexes (COC) classified as suitable to culture were matured, fertilized, and cultured following a standardized protocol used in the laboratory (24). The in vitro-produced embryos were classified according to the stage of development and quality as described in the manual of the International Embryo Technology Society (25). Blastocyst or expanded blastocyst-stage embryos with a grade 1 or 2 quality score (n = 71, stage: 6.4 ± 0.7 and quality: 1.0 ± 0.2) were prepared and transported at 38.5°C to be transferred into the recipient cows. Ten frozen in vitro-produced Brahman embryos (stage: 5.3 ± 0.8 and quality: 1.2 ± 0.4) purchased from a commercial supplier were also transferred to recipients.
Assay of Circulating Progesterone Concentrations
The blood samples were kept on ice before being centrifuged at 1,500 g for 15 min at 4°C. Plasma was stored at −20°C until assayed by liquid-liquid phase, double-antibody radioimmunoassay using assay reagents supplied by MpBio (Cat. No 07–170105; Santa Ana, CA) as validated previously (26). The minimum detectable progesterone concentration was 0.005 ng/tube. Interassay and intraassay estimates of coefficients of variation were <10%.
RNA Isolation
Total RNA was extracted from 66 samples selected randomly out of the 71 collected by cytobrush. The luminal epithelial cells from these samples were then suspended in 500 µL buffer RLT supplemented with β-mercaptoethanol at 1% (RNeasy Mini-Kit QIAGEN No. 74104, Germantown, MD) and disrupted by vortexing at maximum speed for 2 min. The resulting lysate was pipetted to QIAshredder spin column (QIAGEN No. 79654) placed in a 2-mL collection tube and centrifuged for 2 min at full speed for removal of cellular debris and mucus. After clearing, the filtrate was mixed with 500 µL of 70% ethanol and the entire content was loaded and processed in an RNeasy spin column (RNeasy Mini Kit) as per the manufacturer’s guidelines. Samples were subject to on-column DNase treatment using RNase-free DNase set (QIAGEN No. 79254). Total RNA was eluted with 30 µL of RNase-free water. Concentration and purity of total RNA were evaluated using spectrophotometry (NanoDrop 2000 Spectrophotometer, Thermo Fisher Scientific, Waltham, MA) by absorbance at 260 nm and the 260/280 nm ratios, respectively. Total RNA extracts were stored at –80°C.
From the 66 samples extracted, 14 rendered low RNA concentrations [14.4 ng/μl (3.9 to 36.5 ng/µL)] and were not used for sequencing. Out of the remaining 52 samples, 43 (n = 25 pregnant and n = 18 nonpregnant) rendered satisfactory amounts of total RNA (2,397.8 ± 203.1 ng; range 738.5–7,829.2 ng), with good quality (A260/280: 2.0 ± 0.05; range 1.9–2.1) and integrity based on Qubit 2.0 Fluorometer (RIN: 8.2 ± 0.8; range from 6.4 to 9.4) for RNASeq, according to the recommendations of the commercial company that performed the sequencing.
Library Generation and RNA Sequencing
RNA samples (n = 43) were sequenced by Novogene Corporation (https://en.novogene.com). Sequencing libraries were constructed using the RNA-NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA). The sequencing was performed using NovaSeq 6000 (Illumina, Sacramento, CA).
RNASeq Analysis: Quality Control, Mapping, and Gene Expression Estimation
The quality of the RNASeq reads was evaluated using the software FastQC (version 0.11.7, Babraham Bioinformatics, UK). Read trimming and adapter removal were performed using the software Trim Galore (version 0.4.4, Babraham Bioinformatics, UK) with the following parameters: –paired, –quality 20, –length 50, –clip_R1 15, –clip_R2 15, –three_prime_clip_R1 5, and –three_prime_clip_R2 5. After editing, paired-end reads were mapped to the ARS-UCD1.2 bovine reference genome using the software Hisat2 (v2.1.0) (27). The number of reads that mapped to each annotated gene in the bovine genome was estimated using htseq-count (v0.6.1p1) with the option intersection-nonempty (28).
Bioinformatics and Statistical Analyses
After removal of lowly expressed genes, i.e., genes with counts per million 0.5 in at least half of the samples, a total of 15,226 genes from 43 samples were analyzed. The analyses were performed using the R package edgeR (29). This R package combines 1) the use of the trimmed mean of M-values as normalization method, 2) an empirical Bayes approach for estimating tagwise negative binomial dispersion values, and finally, 3) quasi-likelihood negative binomial generalized log-linear models and quasi-likelihood tests for detecting differentially expressed genes.
Consistent with the objectives of this paper, the endometrial luminal epithelium transcriptome was interrogated by two complementary approaches: 1) to evaluate the effect of progesterone concentration on gene expression (Model 1) and 2) to evaluate the effect of gene expression on odds of pregnancy by controlling for the progesterone effects (Model 2).
Model 1.
Changes in expression of each gene (dependent variable) were measured in respect to changes in the concentration of progesterone (independent variable). The relationship between progesterone concentration and gene expression was evaluated by modeling the mean count (µ) for gene g in sample i as follows:
where β0 is a general intercept, block represents the round of trial (2 levels), P4i represents progesterone concentration in sample i, and oi is the sample-specific offset, defined here as the log-transformed library size. Note that βp4 represents the change in log-average expression level of gene g when progesterone concentration increases one unit (1 ng/mL). For each gene, the null hypothesis H0:βp4 = 0 was tested using a quasi-likelihood F test. Differentially expressed genes (DEGs) were determined using an FDR-adjusted P < 0.10. The DEGs altered by the progesterone concentration (DEG-P4) are represented as log-fold change. The log-change is the expression per unit of increase in progesterone concentration. Thus, a positive log-fold change means that there was a positive relationship between progesterone concentration and gene expression, if progesterone concentration increased, the expression of the gene also increased. On the other hand, a negative log-fold change indicated a negative relationship.
Model 2.
Changes in the odds of pregnancy (dependent variable) were associated with the expression of each gene (independent variable), after mathematically controlling for the concentrations of progesterone on D4. The impact that the expression level of gene g has on pregnancy outcome was evaluated using the following logistic regression:
where Pp represents the probability of pregnancy, block represents the round of trial (2 levels), P4i represents progesterone concentration in sample i, and log(gegi) represents the log-transform normalized gene expression level of gene g in sample i. Note that βGE represents the change in the log-odds when log-transformed normalized gene expression level increases one unit with the influence of progesterone level held constant. For each gene, the null hypothesis H0:βGE = 0 was tested using a likelihood ratio test. In this context, the likelihood ratio test detects genes whose expression levels affect the probability of pregnancy, adjusting simultaneously for differences in progesterone concentrations. Of note, we have taken the unique approach of using gene expression to predict a binary dependent variable (pregnant or nonpregnant), which has intrinsically low statistical power and DEGs were determined using unadjusted P values ≤ 0.05. An alternative, more statistically powerful, approach of considering the pregnancy outcome measured on D30 as the independent variable to explain gene expression measured on D4, is questionable. The magnitude and direction of the effect of gene expression (DEG-PREG) on the pregnancy outcome (i.e., odds of pregnancy) were estimated by a regression coefficient. Specifically, the regression coefficient should be interpreted as the change in log-odds of pregnancy per unit of increase in gene expression. A negative coefficient means that there was a negative relationship between gene expression and pregnancy success. For example, if the expression of the gene increases, then the odds of pregnancy decrease. On the other hand, a positive coefficient indicates a positive relationship. For the sake of completeness, we have also run a model without controlling for the concentrations of progesterone on D4.
Ingenuity pathway analyses of differentially expressed genes.
Pathway analyses were performed using Ingenuity Pathway Analysis (IPA, QIAGEN) for the transcriptomic data sets generated by analyzing the endometrial transcriptome according to models 1 and 2. The IPA platform successfully mapped 12,794 out of the 15,226 genes analyzed. The set of DEGs used in each analysis was determined based on FDR < 0.10, for model 1, and unadjusted P ≤ 0.05, for model 2. These cut-off values were compatible with the ideal set size for IPA core analysis, that is between 200 and 3,000 genes [User_Manual.pdf (qiagenbioinformatics.com)]. Emphasis was given to the significant canonical pathways and function annotations that were predicted to be increased or decreased based on a z-score ≥ 2.0 and ≤ −2.0, respectively. Furthermore, IPA was used to connect the data set molecules with predicted upstream regulators and downstream functions (i.e., regulator effects analysis) using the IPA algorithms (30). The predictions were deemed to be activated or inhibited based on the z-score criteria.
Multivariate analysis.
The analyses were run using the SIMCA software (Version 16.0; Umetrics company, Umea, Sweden) by entering the natural logarithm of expression values of DEGs altered by progesterone concentrations, or modulating odds of pregnancy. For the progesterone data set, the samples were combined in six groups, based on pregnancy status (P and NP) and range of progesterone concentration (P4; 0.27–0.51, 0.52–1.42, and 1.43–1.88 ng/mL), as follows: NP | P4: 0.27–0.51, P | P4: 0.27–0.51, NP | P4: 0.52–1.42, P | P4: 0.52–1.42, NP | P4: 1.43–1.88, and P | P4: 1.43–1.88. These ranges of progesterone concentration comprise low, intermediate, and high progesterone concentrations. Then, for each gene, the mean of each group was entered in the software, unit variance-scaled, and submitted to hierarchical clustering analysis, using Ward’s method, to evaluate how these six groups clustered. The means were used aiming to reduce variability within the different ranges of progesterone concentration. For the genes modulating odds of pregnancy, the DEGs were entered individually, i.e., for each sample, and a principal component analysis (PCA) analysis was run. Then, a supervised method (orthogonal partial least squares discriminant analysis; OPLS-DA) was applied to discover a set of genes that most contributed to the separation between pregnant and nonpregnant cows. This contribution was based on the parameter “variable importance in the projection” (VIP), which displays the overall importance of each variable (X) on all responses (Y) cumulatively overall components. Terms with VIP larger than 1 are the most relevant for explaining Y. The DEGs from model 2 were fitted to an OPLS-DA model and successfully generated VIP values. The top 25 genes according to VIP were submitted to Receiver Operating Characteristic (ROC) curve analysis using MedCalc software package (Version 19.6; MedCalc Software, Mariakerke, Belgium). The analysis returned the area under the curve (AUC) and the cutoff value based on the optimal criterion for calculation of specificity and sensitivity values of predictions. Heatmaps were generated using the ClustVis software (https://biit.cs.ut.ee/clustvis/).
Univariate analysis.
The effect of progesterone concentrations on pregnancy per ET was determined by logistic regression using the GLIMMIX procedure of SAS (version 9.4, SAS Institute Inc., Cary, NC) and a model fitted to binomial distribution. The independent variable, progesterone concentration, was sliced into ten-percentile classes and then included into the model as a covariate to estimate its effect on the odds of pregnancy. The linear, quadratic, and cubic effects of ten-percentile classes were tested in the model.
RESULTS
Recipients
During the 3-day period of estrous behavior monitoring, 84 out 113 cows were in estrus (74.3%). Of the cows detected in estrus, 81 received an embryo and 46 became pregnant, resulting in a pregnancy per ET of 56.7% (46/81). Pregnancy per ET did not differ (P > 0.10) among cows receiving a frozen [50.0%, (5/10)] or fresh [57.7%, (41/71)] embryo, neither among experimental rounds [round 1: 45.2% (14/31) and round 2: 64.0% (32/50)]. The samples submitted to RNA sequencing (n = 43) were homogeneously distributed among rounds (11 and 14 pregnant and 10 and 8 nonpregnant for rounds 1 and 2, respectively). There was also no difference (P > 0.10) in days postpartum, age, or body weight according to pregnancy status.
RNA-Seq
Forty-three endometrial cytobrush samples (n = 18 nonpregnant and n = 25 pregnant) were submitted to RNA sequencing, producing on average 23.7 million reads per sample (ranges = 18.4 to 31.8 million). Sequencing data can be accessed through NCBI GEO with accession number GSE171577. Approximately 70% of the total reads were uniquely mapped to genes annotated in the latest bovine reference genome (ARS-UCD1.2).
Model 1
Overview of transcriptome response.
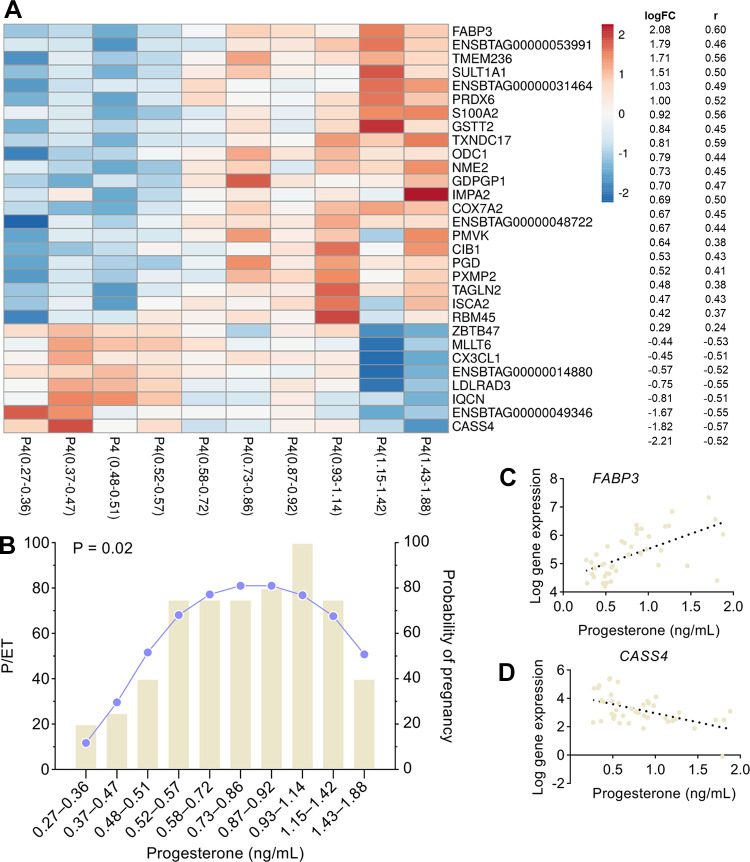
Model 1 determined the relationship between the circulating concentrations of progesterone and changes on the expression of each gene in the endometrial luminal epithelium. In the Supplemental Table S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.17071997.v1, all genes are represented according to the log-fold change, log counts per million (measure of expression level of each gene), P value, and FDR. Progesterone concentration on D4 was associated with the upregulation of 182 genes and the downregulation of 58 genes (FDR < 0.10), for a total of 240 DEG-P4. These DEG-P4 represent ∼1.6% of the 15,226 expressed genes in endometrial epithelial cells. The gene expression of the top 30 DEG-P4, based on P-value, is depicted in Fig. 1A according to the ten-percentile of progesterone concentrations. There was a quadratic relationship (P = 0.02) between progesterone concentration and pregnancy per ET (Fig. 1B). The relationship between progesterone concentration and the expression of the most positively and negatively correlated individual genes, based on the r value, is represented in Fig. 1, C and D, respectively.
Figure 1.
Relationship between plasma progesterone (P4) concentrations on day 4 after estrus and A: the expression of the top 30 most significant DEGs at the luminal epithelium, according to the ten-percentile classes of progesterone concentrations; B: the pregnancy per embryo transfer according to the ten-percentile classes of progesterone concentrations; C and D: the expression of the two DEGs with the greatest positive and negative correlation coefficient (r). DEGs, differentially expressed genes.
Canonical pathways.
Analysis of DEG-P4 returned 37 significant canonical categories (P ≤ 0.05 or −log10 P value ≥ 1.3), as listed in the Supplemental Table S2, from which the most significant are represented in Fig. 2A. Oxidative phosphorylation, mitochondrial dysfunction, sirtuin signaling pathway, and polyamine regulation in cancer colon were the most significant pathways. The three first pathways include 60 DEG-P4, representing 25% of the total DEG-P4, and they share 13 common DEG-P4 such as: ATP synthase (ATP5MC1), NADH ubiquinone oxireductases (e.g., NDUFA1, NDUFA13, NDUFA6, NDUFB4, and NDUFS4), succinate dehydrogenase complex flavoprotein subunit A (SDHA), and ubiquinol-cytochrome c reductases (UQCRFS1). The transcription of all genes in these three pathways was positively associated with progesterone concentration, which indicates that progesterone stimulates metabolism and energy production via oxidative phosphorylation in the endometrial epithelial cells. Furthermore, in the pathway, polyamine regulation in cancer colon, progesterone was associated positively with the key gene, ornithine decarboxylase 1 (ODC1). This gene encodes a rate-limiting enzyme involved in polyamine biosynthesis, important in a variety of functions, such as cell proliferation.
Figure 2.
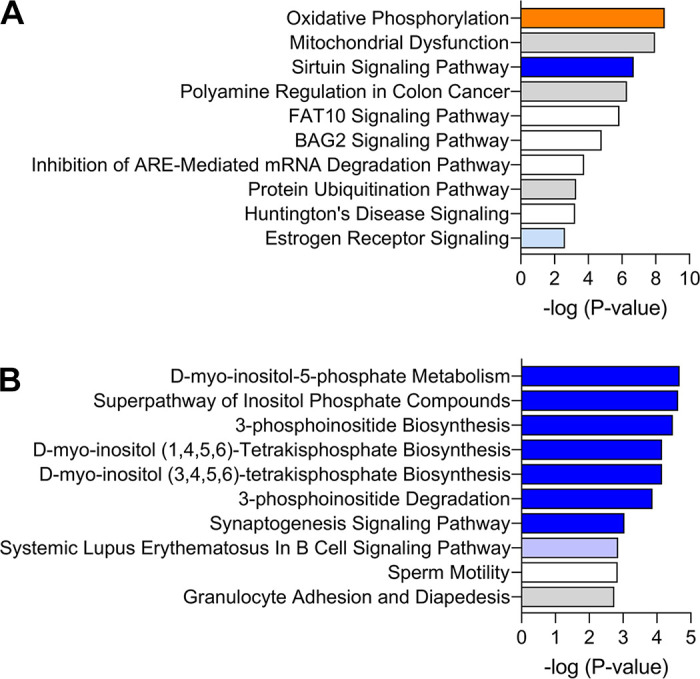
Top 10 enriched canonical pathways in the analysis of: A: progesterone concentrations and B: pregnancy outcomes. The orange and blue-colored bars indicate predicted pathway activation or predicted inhibition, respectively, according to the z-score. White bars are those with a z-score at or very close to 0. Gray bars indicate pathways where no prediction can be made.
Functional annotation.
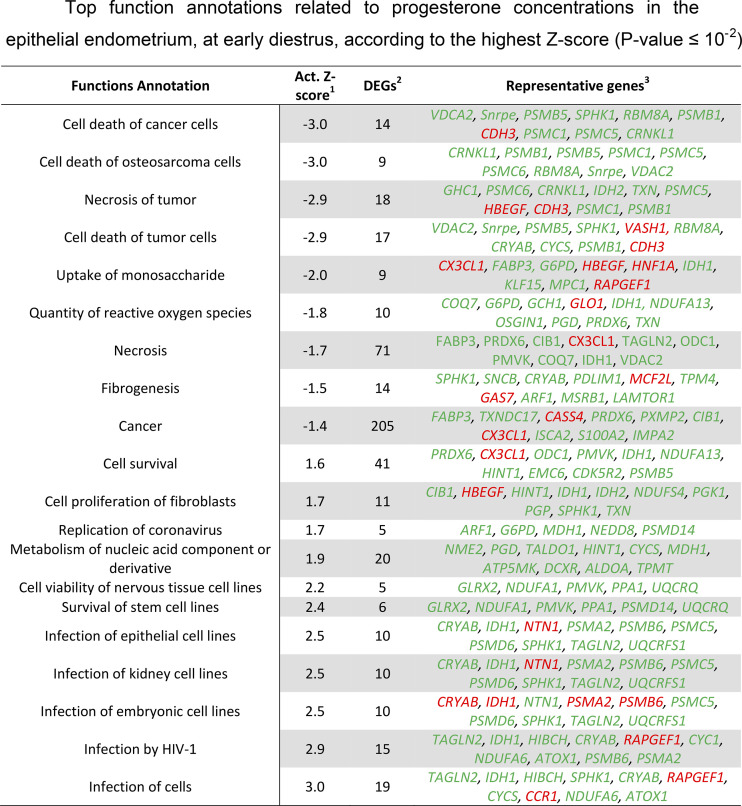
This analysis returned 500 significant (P < 0.05) functional annotations classified within 75 biofunction categories (Supplemental Table S2). Among these functions, only 11 were predicted to be activated (z-score > |2|), of which four were decreased and seven increased. In Fig. 3, the top 20 functions are represented, based on the greatest z-score. The results from Fig. 3 indicate inhibition of functions related to cell death due to apoptosis or necrosis and stimulation of cell proliferation and survival. Thus, elevation of progesterone concentration at early diestrus seems to stimulate proliferation and metabolic activity in endometrial epithelial cells.
Figure 3.
Top function annotations related to progesterone concentrations in the epithelial endometrium, at early diestrus, according to the highest z-score (P value ≤ 10−2). 1: activation z-score (negative and positive z-scores predict decrease and increase of function annotations, respectively). Functions with z-score ≥ |2| are predicted to be activated. 2: differentially expressed genes (DEGs). 3: top 10 DEGs among the function annotations based on P-value. Genes in red and green are related negatively and positively with progesterone concentrations.
Regulator effects.
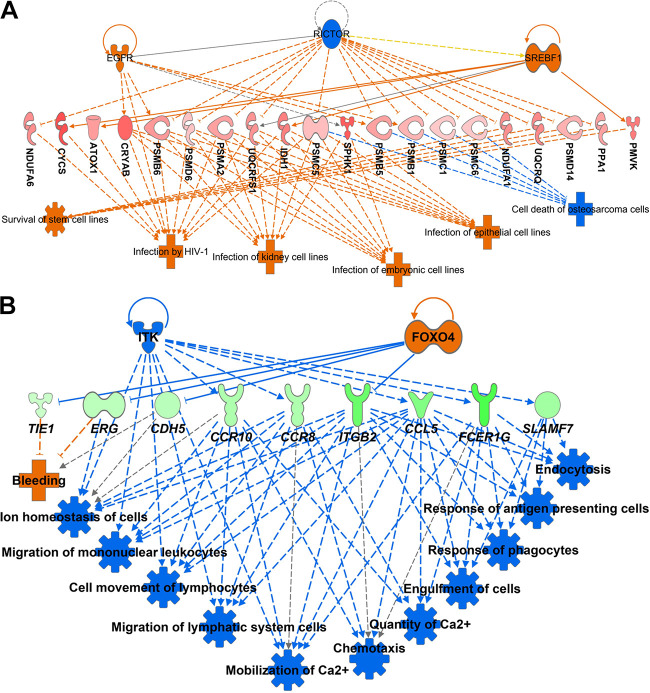
The regulator effects algorithm in IPA connects upstream regulators, data set molecules, and downstream functions (i.e., annotation functions) affected in the data set. This approach generates hypotheses that can explain how the activation or inhibition of an upstream regulator affects the downstream target molecular expression and the impact of such expression on functions with predicted activity (absolute z-score ≥ |2.0|). The analysis returned two networks and identified four associated upstream regulators (Supplemental Table S3). The regulators were epidermal growth factor receptor (EGFR), RPTOR independent companion of MTOR complex 2 (RICTOR), sterol regulatory element-binding transcription factor 1 (Srebp), and nuclear receptor subfamily 4 group member 1 (NR4A1). The network with the greatest score is represented in Fig. 4A. In this network, there was an increased activity of functions related to infection by HIV-1, infection of kidney cell lines, infection of embryonic cells lines, infection of epithelial cell lines, and survival of stem cell lines. These functions are indicative of processes related to cellular proliferation and metabolic activity. The other function annotation, represented in Fig. 4A, was cell death of osteosarcoma cells, with a decreased activity. Collectively, the state of these functions corroborates with the idea that progesterone concentration supports cellular survival at the superficial endometrium.
Figure 4.
Downstream annotation functions associated with plasma progesterone concentrations on day 4 after estrus (A) and pregnancy outcomes (B). Representation of upstream regulators, target genes in the dataset, and the consequent downstream effects. Upstream regulators and downstream effects colored in orange and blue, represents increased and decreased predicted activity, respectively. DEGs colored in red, and green denote a positive and negative relationship, respectively, with progesterone concentrations or odds of pregnancy. Color intensity indicates the degree of up or downregulation in the data set. Lines colored in orange and blue represents a relationship that leads to activation or inhibition, respectively. Gray and yellow lines indicate that the effect could not be predicted, or there was an inconsistency with the state of the downstream molecule, respectively. Solid and dashed lines imply direct and indirect relationships, respectively. The pointed and blunted arrowheads represent activation and inhibitory relationships, respectively. Legends for the shape of molecules and functions is at the IPA website (https://qiagen.secure.force.com/KnowledgeBase/articles/Basic_Technical_Q_A/Legend). DEGs, differentially expressed genes; IPA, Ingenuity Pathway Analysis.
Model 2
Overview of transcriptome response.
Model 2 determined the effect of the expression of each gene in the endometrial luminal epithelium on odds of pregnancy, after adjusting for influence of progesterone concentration. In Supplemental Table S1, all the genes according to the regression coefficient and P value are represented. There were 25 and 292 genes related positively and negatively with odds of pregnancy (unadjusted P ≤ 0.05), respectively, for a total of 317 DEG-PREG. These DEG-PREG represent ∼2% of the 15,226 expressed genes in the endometrium epithelial cells. There were only four DEG-PREG (ADHC1, ZNF687, PTGER1, and GAS7) in common between model 1 and model 2, indicating that the predictors were independent of progesterone concentrations. This is to be expected because the analysis of pregnancy effects was conducted using progesterone as a covariate. The top 25 genes, based on contribution to the pregnancy success, are listed in Table 1. A complementary analysis, i.e., disregarding the adjustment for the influence of progesterone concentration in the model (Supplemental Table S4), resulted in 18 and 501 genes related positively and negatively with odds of pregnancy (unadjusted P ≤ 0.05). Among these 519 DEG-PREG, there were 290 DEG-PREG in common with model 2 and only 10 DEG-PREG in common with model 1. These 519 DEG-PREG did not change the interpretation of the results from IPA or multivariate analyses obtained with model 2 (Supplemental Table S4). Hence, throughout the manuscript, we only focused on results obtained with model 2, as described previously.
Table 1.
Biomarker study using the top 25 genes for grouping cows according to pregnancy outcomes
| Genes (x) | Statistical Analysis |
Biomarker Study |
|||||
|---|---|---|---|---|---|---|---|
| P Value | Estimate | Odds of Pregnancy (y) | Cutoff | Sensitivity, % | Specificity, % | AUC | |
| CARMIL2 | 1.70E-04 | −4.41 | ≤ 4.23 | 80 | 55.6 | 0.75 | |
| (59.3–93.2) | (30.8–78.5) | (0.59–0.87) | |||||
| SCARNA2 | 4.80E-04 | −2.89 | ≤ 5.14 | 80 | 83.3 | 0.82 | |
| (59.3–93.2) | (58.6–96.4) | (0.67–0.92) | |||||
| HLX | 2.10E-03 | −2.50 | ≤ 3.61 | 72 | 77.8 | 0.76 | |
| (50.6–87.9) | (52.4–93.6) | (0.61–0.88) | |||||
| ENSBTAG00000053696 | 2.20E-03 | −2.88 | ≤ 6.25 | 76 | 66.7 | 0.75 | |
| (54.9–90.6) | (41.0–86.7) | (0.60–0.87) | |||||
| CSF2RA | 3.30E-03 | −3.93 | ≤ 5.68 | 88 | 55.6 | 0.76 | |
| (68.8–97.5) | (30.8–78.5) | (0.61–0.88) | |||||
| ZFHX2 | 3.60E-03 | −3.67 | ≤ 6.46 | 96 | 50 | 0.75 | |
| (79.6–99.9) | (26.0–74.0) | (0.60–0.87) | |||||
| PHF21A | 4.00E-03 | −4.64 | ≤ 6.24 | 76 | 72.2 | 0.78 | |
| (54.9–90.6) | (46.5–90.3) | (0.63–0.89) | |||||
| MRTFA | 4.10E-03 | −4.86 | ≤ 6.70 | 68 | 77.8 | 0.73 | |
| (46.5–85.1) | (52.4–93.6) | (0.58–0.86) | |||||
| RASGRP2 | 4.90E-03 | −1.55 | ≤ 3.75 | 88 | 44.4 | 0.72 | |
| (68.8–97.5) | (21.5–69.2) | (0.57–0.85) | |||||
| LRCH4 | 5.00E-03 | −5.61 | ≤ 7.00 | 68 | 77.8 | 0.68 | |
| (46.5–85.1) | (52.4–93.6) | (0.53–0.82) | |||||
| PALD1 | 6.90E-03 | −2.86 | ≤ 5.12 | 64 | 77.8 | 0.72 | |
| (42.5–82.0) | (52.4–93.6) | (0.57–0.85) | |||||
| ENSBTAG00000051433 | 7.10E-03 | −1.23 | ≤ 2.49 | 88 | 61.1 | 0.76 | |
| (68.8–97.5) | (35.7–82.7) | (0.61–0.88) | |||||
| PWWP2B | 7.60E-03 | −3.77 | ≤ 6.00 | 64 | 77.8 | 0.73 | |
| (42.5–82.0) | (52.4–93.6) | (0.58–0.86) | |||||
| CLIP3 | 8.10E-03 | −2.59 | ≤ 5.83 | 72 | 55.6 | 0.71 | |
| (50.6–87.9) | (30.8–78.5) | (0.55–0.84) | |||||
| TTC28 | 1.10E-02 | −2.56 | ≤ 6.30 | 84 | 50 | 0.69 | |
| (63.9–95.5) | (26.0–74.0) | (0.53–0.83) | |||||
| NUMA1 | 1.20E-02 | −3.48 | ≤ 9.34 | 80 | 50 | 0.71 | |
| (59.3–93.2) | (26.0–74.0) | (0.55–0.84) | |||||
| BICRA | 1.20E-02 | −3.57 | ≤ 6.38 | 88 | 66.7 | 0.75 | |
| (68.8–97.5) | (41.0–86.7) | (0.60–0.87) | |||||
| POU6F1 | 1.30E-02 | −2.08 | ≤ 4.01 | 76 | 61.1 | 0.72 | |
| (54.9–90.6) | (35.7–82.7) | (0.56–0.84) | |||||
| KLF7 | 1.40E-02 | −2.71 | ≤ 4.48 | 84 | 61.1 | 0.73 | |
| (63.9–95.5) | (35.7–82.7) | (0.57–0.86) | |||||
| KDM6B | 1.50E-02 | −3.88 | ≤ 7.73 | 80 | 55.6 | 0.71 | |
| (59.3–93.2) | (30.8–78.5) | (0.56–0.84) | |||||
| NFIX | 1.60E-02 | −2.53 | ≤ 7.47 | 84 | 61.1 | 0.73 | |
| (63.9–95.5) | (35.7–82.7) | (0.57–0.85) | |||||
| ATN1 | 1.60E-02 | −4.81 | ≤ 8.54 | 68 | 83.3 | 0.72 | |
| (46.5–85.1) | (58.6–96.4) | (0.56–0.84) | |||||
| TNRC18 | 1.80E-02 | −3.82 | ≤ 6.79 | 68 | 77.8 | 0.72 | |
| (46.5–85.1) | (52.4–93.6) | (0.57–0.85) | |||||
| KMT2D | 2.20E-02 | −2.02 | ≤ 7.94 | 76 | 66.7 | 0.72 | |
| (54.9–90.6) | (41.0–86.7) | (0.56–0.85) | |||||
| AHDC1 | 3.60E-02 | −2.87 | ≤ 6.79 | 88 | 55.6 | 0.69 | |
| (68.8–97.5) | (30.8–78.5) | (0.53–0.83) | |||||
AUC, area under the curve.
Canonical pathways.
Analysis of DEG-PREG that affected odds of pregnancy returned 80 significant (P ≤ 0.05 or −log10 P value ≥ 1.3) canonical categories (Supplemental Table S2), of which the most significant are represented in Fig. 2B. There was an intimate functional association among the first six pathway categories, which were d-myo-inositol-5 phospate-metabolism (9 DEGs), superpathway of inositol phosphate compounds (10 DEGs), 3-phosphoinositide biosynthesis (9 DEGs), d-myo-inositol (1, 4–6)-tetrakisphospate biosynthesis (8 DEGs), d-myo-inositol (3–6)-tetrakisphospate biosynthesis (8 DEGs), and 3-phosphoinositide degradation (8 DEGs). They all shared eight common genes, PALD1 (phosphatase domain containing paladin 1), PDCD1 (programmed cell death 1), PPP1R16B (protein phosphatase 1 regulatory subunit 16B), PTPN23 (protein tyrosine phosphatase nonreceptor type 23), PTPN6 (protein tyrosine phosphatase nonreceptor type 6), PTPN7 (protein tyrosine phosphatase non-receptor type 7), PTPRH (protein tyrosine phosphatase receptor type H), and SYNJ1 (synaptojanin 1). In each case, transcript abundance was negatively associated with the odds of pregnancy. These pathways are associated with cellular function control, cell signaling, and lipid metabolism. Here, inhibition of such pathways was related to uterine receptivity.
Functional annotation.
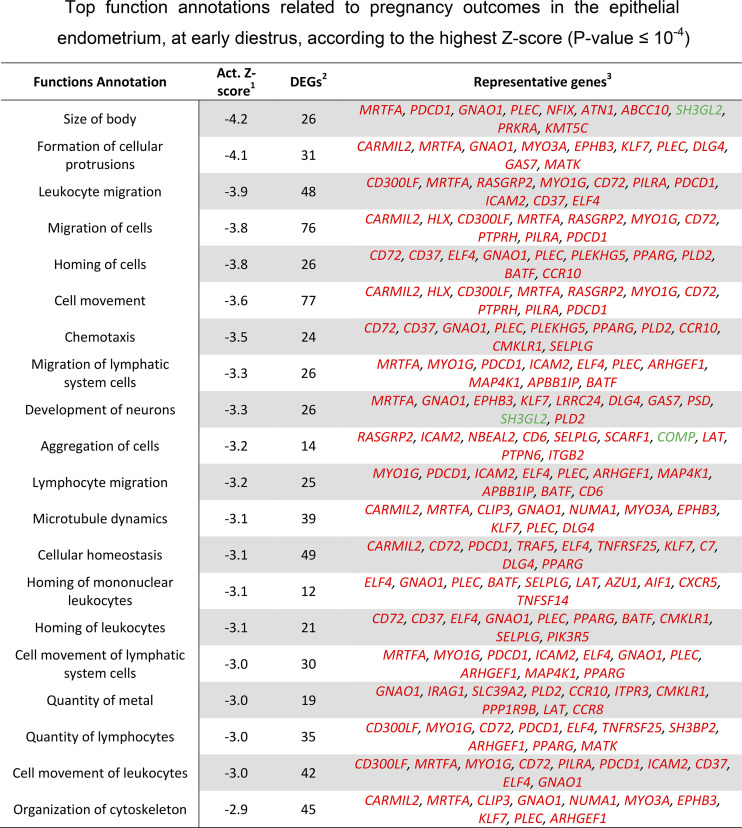
This analysis returned 500 significant (P < 0.05) functional annotations classified within 61 biofunction categories (Supplemental Table S2). Among these functions, 105 were predicted to be activated (z-score > |2|), of which 101 were decreased and four increased. In Fig. 5, the top 20 functions are represented, based on the greatest z-score. Majority of the inhibited functions, represented in Fig. 5, were associated with the innate immune system such as leukocyte migration, lymphocyte migration, quantity of lymphocytes, chemotaxis, and homing of leukocytes. Thus, there was a collective indication of a dampened activity of the innate immune system that was associated with uterine receptivity.
Figure 5.
Top function annotations related to pregnancy outcomes in the epithelial endometrium, at early diestrus, according to the highest z-score (P value ≤ 10−4). 1: activation z-score (negative and positive z-scores predict decrease and increase of function annotations, respectively). Functions with z-score ≥ |2| are predicted to be activated. 2: differentially expressed genes (DEGs). 3: top 10 DEGs among the function annotations based on P value. Genes in red and green are related negatively and positively with the pregnancy outcome.
Regulator effects.
To limit the number of the networks to the most relevant, we used a P value ≤ 0.01 and z-score ≥ |2.0| as cutoffs. We also considered only genes, RNAs, proteins, and endogenous chemicals to mammalian as possible upstream regulators. The analysis returned 20 networks (Supplemental Table S3) and the network with greatest consistent score (i.e., a measure of how causally consistent and densely connected a network is) was linked with downstream functions related to innate immune system (Fig. 4B). This network was mobilized toward a decreased activity of all depicted immune functions, thereby reinforcing the relevance of immune system modulation for uterine receptivity. Regulators were classified as transcription regulators (ERG, FOXO4, and STAT4), complexes (IgE and CD3), a kinase (ITK), a peptidase (USP22), a cytokine (IL4), and the mammalian endogenous chemical cholecalciferol.
Multivariate Component Analysis
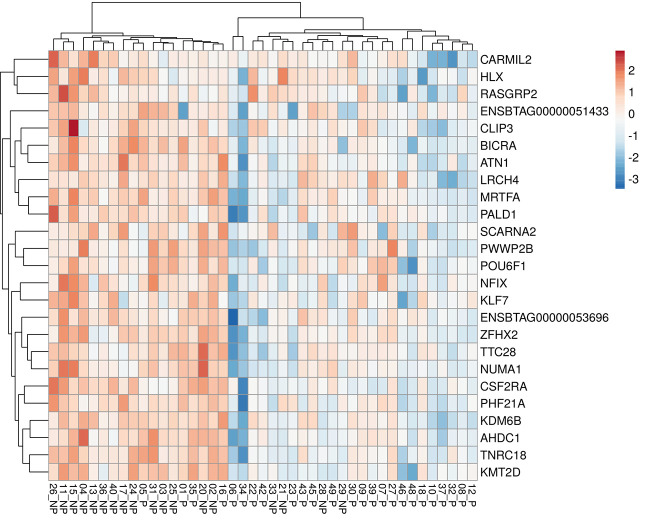
The hierarchical clustering analysis considering the six groups based on ranges of progesterone concentration and pregnancy outcomes (Fig. 6A) indicated that the gene expression profile for DEG-P4 between P and NP within the ranges of progesterone concentration, 0.27–0.51 ng/mL or 0.52–1.42 ng/mL, were similar. Although within the range of high progesterone concentration (1.43–1.88 ng/mL), the P and NP pair split apart, suggesting that, in this range, the gene expression profile for DEG-P4 between P and NP was different. Among the pregnant recipients, the majority of them, 19 out of 25 (76%), were in the range of progesterone concentration between 0.52 and 1.42 ng/mL, whereas most of the nonpregnant recipients, 10 out of 18 (55%) were in the range of progesterone concentration between 0.27 and 0.51 ng/mL. Although progesterone concentrations play such an important role in defining a receptive uterine environment, the average progesterone concentration between pregnant and nonpregnant recipients was not different (Fig. 6B). According to Fig. 6B, samples from nonpregnant recipients seemed to be grouped at the progesterone concentration extremes, especially at the lowermost. Moreover, in the PCA analyses, the DEG-PREG obtained in model 2 resulted in a separation between pregnant and nonpregnant recipients (Fig. 7A) that was similar to the DEG-PREG obtained when the adjustment for influence of progesterone concentration was not applied in the model (Fig. 7B). Both PCA models rendered quite similar R2X and Q2(X) cumulative sum of squares, respectively, 0.597 and 0.428, for the DEG-PREG from model 2, and, 0.645 and 0.498, for the DEG-PREG from model disregarding the influence of progesterone. As depicted in Fig. 7A, the R2X of first component explained 0.359 of variability between P and NP groups, whereas in Fig. 7B, it explained 0.407 of variability between them. For both PCA models, there was overlap between pregnant and nonpregnant recipients. Most noticeably, nonpregnant recipients tended to cluster [78% (14/18)] between coordinates 0 and 20 in Fig. 7, A and B. The OPLS-DA supervised analysis considering DEG-PREG obtained in model 2, resulted in 105 out of the 317 genes with a VIP >1 (Supplemental Table S5). The top 25 genes, based on VIP, were associated negatively with the odds of pregnancy (Table 1 and Fig. 8). This set of genes allowed a clear discrimination between pregnant and nonpregnant recipient cows (Fig. 8). Most of the pregnant cows clustered to the right side of the heatmap (21 out of 25) and nonpregnant cows to the left side (14 out of 18). Predictive ability for each of these 25 genes was evaluated by ROC curve analyses (Table 1). Sensitivity and specificity ranged from moderate to high (64.0 to 96.0 and 44.4 to 83.3, respectively).
Figure 6.
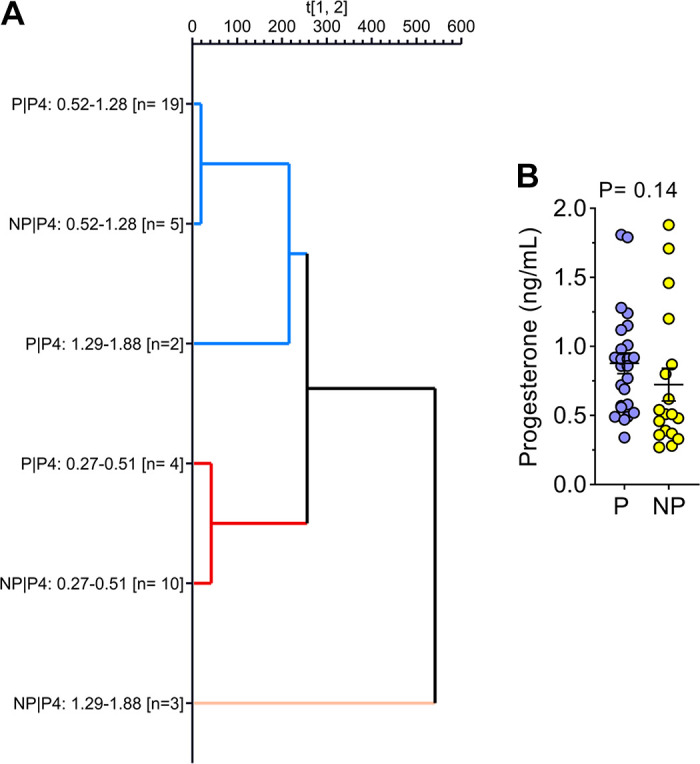
Hierarchical clustering analysis according to six predefined groups (A) and plasma progesterone concentration on day 4 postestrus according to the pregnancy outcome (B). The six groups represented on hierarchical clustering analysis graph were generated based on ranges of progesterone concentration (0.27–0.51, 0.52–1.42, and 1.43–1.88 ng/mL) and pregnancy outcomes (P: pregnant; NP: nonpregnant) after ET. The genes modulated by the plasma progesterone concentration on day 4 after estrus (240 DEGs; FDR < 0.10) were used in the hierarchical clustering analysis.
Figure 7.
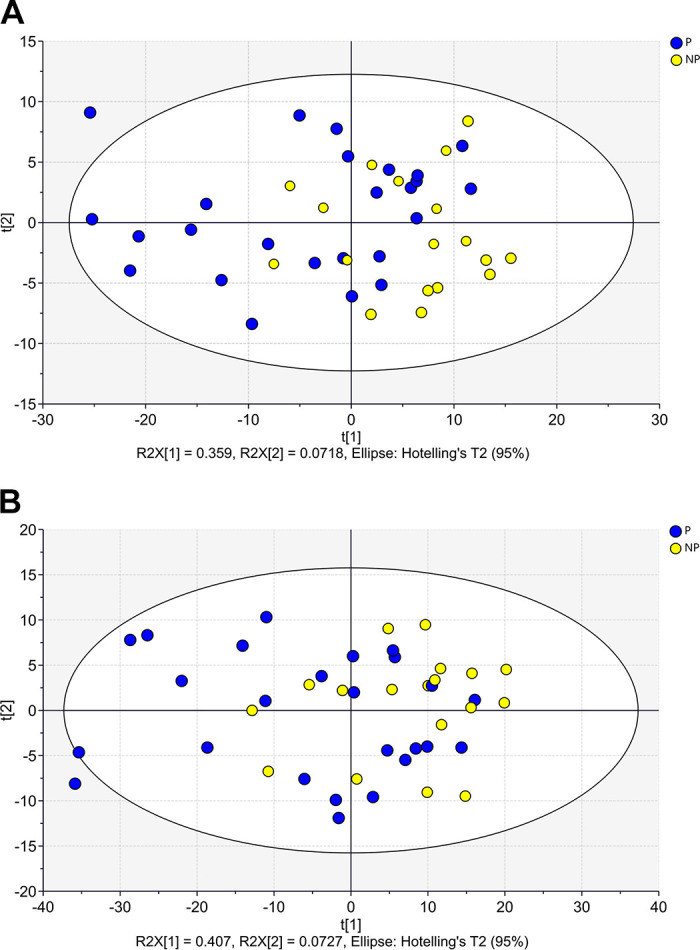
Principal component analysis (PCA) score plots considering: 317 DEGs modulating odds of pregnancy, after adjusting for influence of progesterone concentration in the model (A); and 519 DEGs modulating odds of pregnancy, when the influence of progesterone concentration was not adjusted in the model (B). DEG, differentially expressed genes.
Figure 8.
Heatmap depicting the 25 genes with the greatest variable importance in the projection (VIP) for separation of samples in the OPLS-DA analysis considering the 317 genes affecting odds of pregnancy (unadjusted P value ≤ 0.05), when progesterone concentration was adjusted in the model. Samples (P: pregnant and NP: nonpregnant) and genes were clustered applying Manhattan and complete-linkage method for distance and clustering measurements.
DISCUSSION
In cattle, the biochemical composition of the uterine lumen during the preimplantation window determines the pregnancy outcomes. Because the biochemical composition of the uterine lumen is largely dictated by the function of endometrial epithelial cells, we hypothesized that there are molecular signatures in endometrial epithelial cells that are associated with pregnancy success or failure. Here, for the first time, we used a minimally invasive method (i.e., cytobrush) to collect luminal epithelial cells in recipient cows before embryo transfer and to subsequently interrogate their transcriptome based on the circulating concentrations of progesterone and the pregnancy outcome. Moreover, we collected samples only 3 days before embryo transfer, versus other studies that collected samples from the estrous cycle preceding the pregnancy cycle (11, 12, 16, 17). To our knowledge, this is the first report to document the distinct characteristics of luminal epithelial function related to pregnancy outcome measured just before embryo arrival in the uterus. Our main finding was that progesterone concentrations are associated with proliferative and metabolic activities in the superficial endometrium. In addition, after we adjusted for the influence of progesterone, uterine receptivity was defined by dampening the activity of the innate immune system and signaling by phosphoinositide-dependent pathways. Finally, we identified a panel of 25 potential candidate genes for predicting pregnancy outcomes.
The circulating concentration of progesterone regulated the luminal epithelial function, influencing pregnancy outcome. There was a range of progesterone concentrations (0.52–1.42 ng/mL) in which most of the pregnant recipients were clustered [76% (19/25). Progesterone concentrations >1.42 ng/mL and specially <0.52 ng/mL resulted in poor pregnancy rates. Here, the progesterone concentration played a stimulatory role on the luminal epithelium transcriptome, stimulating 76% of the DEG-P4. These DEGs were fundamentally associated with energy production and metabolism, according to the canonical pathway analysis (Fig. 2A). In fact, there was a positive relationship between progesterone concentration and the expression of genes associated with the five protein-lipid enzyme complexes (Complexes I-V) located in the mitochondrial inner membrane: complex I (NADH: ubiquinone oxidoreductase), complex II (succinate: ubiquinone oxidoreductase), complex III (ubiquinol: ferrocytochrome c oxidoreductase), complex IV (cytochrome c oxidase), and complex V (ATP synthase). These findings are consistent with the fact that sex steroids can regulate mitochondrial activity (31). In cattle, animals classified as fertile or presenting elevated progesterone concentrations during early- to mid-diestrus (days 4 to 16 postestrus) showed upregulation of genes associated with oxidation/reduction, cellular protein metabolic process, and ATPase activity (6, 32–34). In accordance with the indication of increased production of ATP, functional annotation analysis (Fig. 3) indicated a number of functions associated with cell proliferation and inhibition of cell death (Fig. 4A). In the same sense, Forde et al. (33) observed that progesterone supplementation at early diestrus altered pattern of expression of genes involved in metabolic processes. In a previous study, we also observed a biosynthetic and metabolically active endometrial phenotype, during early diestrus, when animals were exposed to greater estradiol and progesterone concentrations during pre- and postovulatory phase, respectively (6). Such endocrine milieu also favored a transcriptional profile compatible with endometrial proliferation and inhibition of apoptosis, as confirmed in Arai et al. (35). One critical gene involved in the modulation of cell growth and proliferation is the fatty acid binding protein 3 or FABP3 (36, 37). This gene had the greatest regression coefficient (r = 0.60) with progesterone concentration (Fig. 1A) and it was involved in the following inhibited functions: uptake of monosaccharide, necrosis, and cancer (Fig. 3). In the endometrium, this gene had already been associated with the uptake, intracellular metabolism, and transport of long-chain fatty acids for the formation of histotroph (33). Overall, during early diestrus, progesterone affected expression of genes at the luminal epithelium that was associated with stimulus of biosynthesis, metabolism, proliferation, and prevention of apoptosis of epithelial cells. Mitochondria seem to be central in such progesterone-driven responses.
The second portion of the present study interestingly indicated that even when nonadjusting for the progesterone effect into the model, very few genes altered by progesterone concentration did explain pregnancy outcomes. Even though progesterone does play a crucial role in setting uterine receptivity, there was still a proportion of recipients that was able to become pregnant within the low and high range of progesterone concentrations. This observation implies that progesterone-independent mechanisms in the luminal epithelium are also necessary for maintenance of pregnancy. Our model 2, i.e., adjusted for the progesterone effects, allowed us to explore this aspect. In contrast with model 1, 92% of the DEG-PREG were negatively related to the odds of pregnancy, suggesting that the expression of genes contributing to the establishment of pregnancy was dampened. Such DEGs were mainly associated with the metabolism, biosynthesis, and degradation of myo-inositol, according to the canonical pathway analysis (Fig. 2B). Myo-inositol or inositol, and its biochemical derivatives, function as intracellular signal transducers from a series of regulators, including hormones, neurotransmitters, growth factors, and serum (38). In the Fig. 2B, for example, derivatives from 3-phosphoinositide biosynthesis pathway are involved in growth factor-dependent mitogenesis, membrane ruffling and glucose uptake, cell proliferation, oncogenic transformation, cell survival, cell migration, and intracellular protein trafficking (39). In addition, the gene inositol 1,4,5-trisphosphate receptor type 3 (ITPR3) associated negatively with odds of pregnancy, was present in the sperm motility pathway (Fig. 2B) and on quantity of metal function (Fig. 5). This gene encodes a receptor for IP3 [d-myo-inositol(1, 4, 5)-trisphosphate], which mediates the second messenger action of IP3 through the release of intracellular calcium, regulating cellular reactions that require free calcium, such as signal transduction, permeability of ion channels, control of enzyme activity, and apoptosis (38). One expected consequence for reduced signaling by inositol compounds is maintenance of epithelial cells integrity, because they are important for establishment of cell junctions, polarity, and migration (40, 41). Intracellular accumulation of inositol derivatives, such as phosphatidylinositol(3–5)-trisphosphate, for example, is implicated in cell motility, cytoskeletal regulation, and in chemotaxis process (40). Because luminal epithelial cells play a role as gatekeepers, alterations in balance of myo-inositol compounds intracellularly may alter the cell integrity, affecting the uterine luminal milieu and thereby embryonic development. Altogether, our findings support the idea that receptive luminal epithelium displays a dampened response to signaling involving inositol, and this may favor the integrity of the luminal barrier.
Genes associated with immune function were downregulated in endometrial luminal epithelial cells of cows that remained pregnant after embryo transfer. These genes were involved in the migration of mononuclear lymphocytes, chemotaxis, and response of antigen-presenting cells, among others (Figs. 4B and 5). These functions predicted to be decreased, suggested an overall suppression of innate immune response. The innate immune system is well-known as the first line of defense, recognizing foreign structures that are not normally found in the host (42, 43). Enrichment analyses indicate that a receptive uterine environment includes inhibition of important innate functions at the endometrial epithelium including recruitment, migration, and phagocytosis by different immune cell population. For instance, expression of genes related to chemokine signaling, depicted in Fig. 4B, such as CCL5 (C-C motif chemokine ligand 5), CCR8, and CCR10 (C-C motif chemokine receptors 8 and 10, respectively) was negatively associated with the odds of pregnancy. Chemokines are particularly important for coordination of cell migration and cell positioning in the immune system (44). Evidence in human, indicate that, at least in part, normal pregnancy is characterized by the differential expression and secretion of chemokines, inducing selective trafficking of leukocyte subsets to the maternal-fetal interface during early pregnancy (45). Other studies comparing the endometrial gene expression between fertile and subfertile cows, at days 6–7 of estrous cycle reported only mild differences in gene expression involving the immune system (12, 16, 17). A possible explanation for such lack of a robust immune effect may be due to the nature of the sample collection method used in those studies (i.e., biopsy). Specifically, uterine biopsies contain many other cell types and are expected to be enriched in stromal cells.
The relevance of the immune system at early diestrus for establishment of pregnancy, however, remains largely obscure. This is because it is only in response to the secretion of increasing amount of IFN-τ by the elongating conceptus, during days 14–21 of pregnancy, that significant changes on maternal immune response are verified in the endometrium (46–48). At this period, an exacerbated maternal immune response is detrimental to the establishment of pregnancy. On the other hand, in the same sense of our results, Beltman et al. (49) observed that, on day 7 of pregnancy, the abundance of proinflammatory genes in endometrial biopsies from heifers yielding viable embryos was lower than those yielding retarded embryos. Thus, imbalances in uterine immune function at early stages of pregnancy can be also detrimental to embryonic development, impairing conception. According to our findings, such imbalances occur at the superficial endometrium. Thus, consistent with the notion that receptivity may require dampening of specific uterine functions, activity of pathways associated with innate immune responses was attenuated in the superficial endometrium of cows that remained pregnant.
There is a transcriptomic signature written in luminal epithelial cells that predicts pregnancy outcomes. Here, we described the receptive transcriptome of luminal endometrial epithelial cells in the same cycle as the pregnancy, after controlling for the effects of progesterone. PCA analysis showed the distribution of animals according to the pregnancy outcome. Only four out 18 nonpregnant animals clustered to the left side of the PCA plot (Fig. 7A), where most pregnant animals were identified. These four recipients may have received less competent embryos, that did not survive despite a receptive uterus. Alternatively, they could represent pregnancy losses that occurred between days 4 and 30 for reasons not dependent on the day 4 luminal epithelial transcriptome. The presence of pregnant cows on the right side of the PCA ellipse (10 out of 25) is less clear, but we speculate that they represent a luminal condition that changed over time to one that was favorable to embryo development. When we used the top 25 genes that most contributed to the separation of animals by pregnancy outcome in the OPLS-DA analysis, there was a clearer discrimination between pregnant and nonpregnant recipient cows (Fig. 8). These genes represent promising candidates for future predicting studies. Indeed, ROC curve analysis indicated that these genes presented specificity and sensitivity values that were moderate to high (44.4 to 96.0%; Table1). A positive practical implication is that the abundance of this set of candidate genes may be evaluated on day 4, which is before embryo transfer on day 7, and may direct embryo recipient selection based on a transcript abundance signature.
One limitation of sampling the uterine body is that it is physically distant from the uterine-tubal junction, where embryo arrival from the oviduct will take place. Thus, it is plausible that patterns of gene expression will differ according to the region (50). However, the practical advantage of collecting a cytobrush sample is that it is relevant for applied studies and practical applications that require the study of luminal epithelial function in vivo. Future studies using tissues obtained postmortem should be directed to the validation of targets along the uterine horn. A second limitation is that there is a 26-day time interval between the sample collection and the phenotype measured. Thus, subsequent events during the gestation, unrelated to the D4 transcriptome, may affect the pregnancy outcome.
In conclusion, circulating concentrations of progesterone were associated with a pattern of expression of the DEG-P4 in the luminal epithelium, that indicated stimulation of cellular proliferation, metabolism, biosynthesis, and survival, at early diestrus. Mitochondrial responses were central to such cellular processes. However, uterine receptivity was predominantly marked by a number of DEGs that were distinct from those found associated with progesterone concentrations. The pattern of expression of these DEG-PREG in the luminal epithelium evidenced that uterine receptivity was determined by a reduction in activity of the innate immune system and signaling by phosphoinositide-dependent pathways. Collectively, the present study establishes not only the importance of progesterone on regulating the uterine function, but also that there are critical progesterone-independent features that can impact the pregnancy outcome.
SUPPLEMENTAL DATA
Supplemental Tables S1–S5: https://doi.org/10.6084/m9.figshare.17071997.v1.
GRANTS
This research was supported by the Florida Cattleman’s Association, the National Institute of Child Health and Human Development (Grant HD088352), and funding for E. Estrada-Cortés by CONACYT-México.
DISCLOSURES
P.J.H. is co-owner of Cooley Biotech LLC, the manufacturer of BBH7, which is a medium used for embryo culture. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.M., F.P., and M.B. conceived and designed research; T.M., M.S., F.A.C.C.S., and E.E.-C. performed experiments; T.M., M.S., and F.P. analyzed data; T.M., F.P., and M.B. interpreted results of experiments; T.M. prepared figures; T.M. drafted manuscript; T.M., M.S., E.E.-C., P.J.H., F.P., and M.B. edited and revised manuscript; T.M., M.S., F.A.C.C.S., E.E.-C., P.J.H., F.P., and M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are thankful to the UF Beef Unit, especially Danny Driver and the crew, for supporting our work with animals. We also thank Zoetis, for donating hormones used in this study, Dr. Jeremy Block, Vintage Veterinary, for performing ovum pick-up procedures, Dr. Joel Carter, Southern Cattle Co., for transferring the embryos, and Ky G. Pohler, Texas A&M, for running the progesterone assays.
REFERENCES
- 1.King GJ, Atkinson BA, Robertson HA. Development of the intercaruncular areas during early gestation and establishment of the bovine placenta. J Reprod Fertil 61: 469–474, 1981. doi: 10.1530/jrf.0.0610469. [DOI] [PubMed] [Google Scholar]
- 2.Hackett AJ, Durnford R, Mapletoft RJ, Marcus GJ. Location and status of embryos in the genital tract of superovulated cows 4 to 6 days after insemination. Theriogenology 40: 1147–1153, 1993. doi: 10.1016/0093-691X(93)90285-D. [DOI] [Google Scholar]
- 3.Spencer TE, Johnson GA, Bazer FW, Burghardt RC, Palmarini M. Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod Fertil Dev 19: 65–78, 2007. doi: 10.1071/RD06102. [DOI] [PubMed] [Google Scholar]
- 4.Forde N, Simintiras CA, Sturmey R, Mamo S, Kelly AK, Spencer TE, Bazer FW, Lonergan P. Amino acids in the uterine luminal fluid reflects the temporal changes in transporter expression in the endometrium and conceptus during early pregnancy in cattle. PLoS One 9: e100010, 2014. doi: 10.1371/journal.pone.0100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martins T, Pugliesi G, Sponchiado M, Gonella-Diaza AM, Ojeda-Rojas OA, Rodriguez FD, Ramos RS, Basso AC, Binelli M. Perturbations in the uterine luminal fluid composition are detrimental to pregnancy establishment in cattle. J Anim Sci Biotechnol 9: 70, 2017. doi: 10.1186/s40104-018-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesquita FS, Ramos RS, Pugliesi G, Andrade SCS, Van Hoeck V, Langbeen A, Oliveira ML, Gonella-Diaza AM, Gasparin G, Fukumasu H, Pulz LH, Membrive CM, Coutinho LL, Binelli M. The receptive endometrial transcriptomic signature indicates an earlier shift from proliferation to metabolism at early diestrus in the cow. Biol Reprod 93: 52, 2015. doi: 10.1095/biolreprod.115.129031. [DOI] [PubMed] [Google Scholar]
- 7.França MR, Silva MIS, Pugliesi G, Van Hoeck V, Binelli M. Evidence of endometrial amino acid metabolism and transport modulation by peri-ovulatory endocrine profiles driving uterine receptivity. J Anim Sci Biotechnol 8: 54, 2017. doi: 10.1186/s40104-017-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos RS, Oliveira ML, Izaguirry AP, Vargas LM, Soares MB, Mesquita FS, Santos FW, Binelli M. The periovulatory endocrine milieu affects the uterine redox environment in beef cows. Reprod Biol Endocrinol 13: 39, 2015. doi: 10.1186/s12958-015-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binelli M, Scolari SC, Pugliesi G, Van Hoeck V, Gonella-Diaza AM, Andrade SCS, Gasparin GR, Coutinho LL. The transcriptome signature of the receptive bovine uterus determined at early gestation. PLoS One 10: e0122874, 2015. doi: 10.1371/journal.pone.0122874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sá Filho MF, Gonella-Diaza AM, Sponchiado M, Mendanha MF, Pugliesi G, Ramos RS, Andrade SCS, Gasparin G, Coutinho LL, Goissis MD, Mesquita FS, Baruselli PS, Binelli M. Impact of hormonal modulation at proestrus on ovarian responses and uterine gene expression of suckled anestrous beef cows. J Anim Sci Biotechnol 8: 79, 2017. doi: 10.1186/s40104-017-0211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabaglino MB, Kadarmideen HN. Machine learning approach to integrated endometrial transcriptomic datasets reveals biomarkers predicting uterine receptivity in cattle at seven days after estrous. Sci Rep 10: 16981, 2020. doi: 10.1038/s41598-020-72988-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzoni G, Pedersen HS, Rabaglino MB, Hyttel P, Callesen H, Kadarmideen HN. Characterization of the endometrial transcriptome in early diestrus influencing pregnancy status in dairy cattle after transfer of in vitro-produced embryos. Physiol Genomics 52: 269–279, 2020. doi: 10.1152/physiolgenomics.00027.2020. [DOI] [PubMed] [Google Scholar]
- 13.Maclean A, Kamal A, Adishesh M, Alnafakh R, Tempest N, Hapangama DK. Human uterine biopsy: research value and common pitfalls. Int J Reprod Med 2020: 9275360–7., 2020. doi: 10.1155/2020/9275360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scolari SC, Pugliesi G, de Francisco Strefezzi R, Andrade SC, Coutinho LL, Binelli M. Dynamic remodeling of endometrial extracellular matrix regulates embryo receptivity in cattle. Reproduction 153: 49–61, 2017. doi: 10.1530/rep-16-0237. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso B, Oliveira ML, Pugliesi G, Batista EOS, Binelli M. Cytobrush: a tool for sequential evaluation of gene expression in bovine endometrium. Reprod Domest Anim 52: 1153–1157, 2017. doi: 10.1111/rda.13037. [DOI] [PubMed] [Google Scholar]
- 16.Salilew-Wondim D, Hölker M, Rings F, Ghanem N, Ulas-Cinar M, Peippo J, Tholen E, Looft C, Schellander K, Tesfaye D. Bovine pretransfer endometrium and embryo transcriptome fingerprints as predictors of pregnancy success after embryo transfer. Physiol Genomics 42: 201–218, 2010. doi: 10.1152/physiolgenomics.00047.2010. [DOI] [PubMed] [Google Scholar]
- 17.Killeen AP, Morris DG, Kenny DA, Mullen MP, Diskin MG, Waters SM. Global gene expression in endometrium of high and low fertility heifers during the mid-luteal phase of the estrous cycle. BMC Genomics 15: 234, 2014. doi: 10.1186/1471-2164-15-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista EOS, Cardoso BO, Oliveira ML, Cuadros FDC, Mello BP, Sponchiado M, Monteiro BM, Pugliesi G, Binelli M. Supplemental progesterone induces temporal changes in luteal development and endometrial transcription in beef cattle. Domest Anim Endocrinol 68: 126–134, 2019. doi: 10.1016/j.domaniend.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Simintiras CA, Sánchez JM, Mcdonald M, Martins T, Binelli M, Lonergan P. Biochemical characterization of progesterone-induced alterations in bovine uterine fluid amino acid and carbohydrate composition during the conceptus elongation window†. Biol Reprod 100: 672–685, 2019. doi: 10.1093/biolre/ioy234. [DOI] [PubMed] [Google Scholar]
- 20.Frade MC, Frade C, Cordeiro MB, de Sá Filho MF, Mesquita FS, Nogueira GP, Binelli M, Membrive CMB. Manifestation of estrous behavior and subsequent progesterone concentration at timed-embryo transfer in cattle are positively associated with pregnancy success of recipients. Anim Reprod Sci 151: 85–90, 2014. doi: 10.1016/j.anireprosci.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Pugliesi G, Santos FB, Lopes E, Nogueira É, Maio JRG, Binelli M. Improved fertility in suckled beef cows ovulating large follicles or supplemented with long-acting progesterone after timed-AI. Theriogenology 85: 1239–1248, 2016. doi: 10.1016/j.theriogenology.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Clemente M, De La Fuente J, Fair T, Al Naib A, Gutierrez-Adan A, Roche JF, Rizos D, Lonergan P. Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction 138: 507–517, 2009. doi: 10.1530/REP-09-0152. [DOI] [PubMed] [Google Scholar]
- 23.Carter F, Forde N, Duffy P, Wade M, Fair T, Crowe MA, Evans ACO, Kenny DA, Roche JF, Lonergan P. Effect of increasing progesterone concentration from day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod Fertil Dev 20: 368–375, 2008. doi: 10.1071/RD07204. [DOI] [PubMed] [Google Scholar]
- 24.Tríbulo P, Rivera RM, Ortega-Obando MS, Jannaman EA, Hansen PJ. Production and culture of the bovine embryo. Methods Mol Biol 2006: 115–129, 2019. doi: 10.1007/978-1-4939-9566-0_8. [DOI] [PubMed] [Google Scholar]
- 25.Robertson I, Nelson RE. Manual of the International Embryo Transfer Society, edited by Stringfellow DA, Seidel SE.. International Embryo Transfer Society: Champaign, IL. p. 103–116, 1998. [Google Scholar]
- 26.Pohler KG, Pereira MHC, Lopes FR, Lawrence JC, Keisler DH, Smith MF, Vasconcelos JLM, Green JA. Circulating concentrations of bovine pregnancy-associated glycoproteins and late embryonic mortality in lactating dairy herds. J Dairy Sci 99: 1584–1594, 2016. doi: 10.3168/jds.2015-10192. [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12: 357–360, 2015. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169, 2015. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson MD, Mccarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30: 523–530, 2014. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaignard P, Liere P, Thérond P, Schumacher M, Slama A, Guennoun R. Role of sex hormones on brain mitochondrial function with special reference to aging and neurodegenerative diseases. Front Aging Neurosci 9: 406, 2017. doi: 10.3389/fnagi.2017.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minten MA, Bilby TR, Bruno RGS, Allen CC, Madsen CA, Wang Z, Sawyer JE, Tibary A, Neibergs HL, Geary TW, Bauersachs S, Spencer TE. Effects of fertility on gene expression and function of the bovine endometrium. PLoS One 8: e69444, 2013. doi: 10.1371/journal.pone.0069444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forde N, Carter F, Fair T, Crowe MA, Evans ACO, Spencer TE, Bazer FW, McBride R, Boland MP, O’Gaora P, Lonergan P, Roche JF. Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol Reprod 81: 784–794, 2009. doi: 10.1095/biolreprod.108.074336. [DOI] [PubMed] [Google Scholar]
- 34.Forde N, Mehta JP, Minten M, Crowe MA, Roche JF, Spencer TE, Lonergan P. Effects of low progesterone on the endometrial transcriptome in cattle. Biol Reprod 87: 1–24., 2012. doi: 10.1095/biolreprod.112.103424. [DOI] [PubMed] [Google Scholar]
- 35.Arai M, Yoshioka S, Tasaki Y, Okuda K. Remodeling of bovine endometrium throughout the estrous cycle. Anim Reprod Sci 142: 1–9, 2013. doi: 10.1016/j.anireprosci.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Bauersachs S, Ulbrich SE, Reichenbach H-D, Reichenbach M, Büttner M, Meyer HHD, Spencer TE, Minten M, Sax G, Winter G, Wolf E. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod 86: 46, 2012. doi: 10.1095/biolreprod.111.094771. [DOI] [PubMed] [Google Scholar]
- 37.Spencer TE, Forde N, Lonergan P. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. J Dairy Sci 99: 5941–5950, 2015. doi: 10.3168/jds.2015-10070. [DOI] [PubMed] [Google Scholar]
- 38.Michell RH. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta 415: 81–147, 1975. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- 39.Kapeller R, Cantley LC. Phosphatidylinositol 3-kinase. Bioessays 16: 565–576, 1994. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- 40.Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci 121: 551–559, 2008. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skwarek LC, Boulianne GL. Great expectations for PIP: phosphoinositides as regulators of signaling during development and disease. Dev Cell 16: 12–20, 2009. doi: 10.1016/j.devcel.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Sheldon IM, Bromfield JJ. Innate immunity in the human endometrium and ovary. Am J Reprod Immunol 66: 63–71, 2011. doi: 10.1111/j.1600-0897.2011.01034.x. [DOI] [PubMed] [Google Scholar]
- 43.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev 206: 306–335, 2005. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 44.Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol 7: a016303, 2015. doi: 10.1101/cshperspect.a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du MR, Wang SC, Li DJ. The integrative roles of chemokines at the maternal – fetal interface in early pregnancy. Cell Mol Immunol 11: 438–448, 2014. doi: 10.1038/cmi.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ott TL. Symposium review: immunological detection of the bovine conceptus during early pregnancy. J Dairy Sci 102: 3766–3777, 2019. doi: 10.3168/jds.2018-15668. [DOI] [PubMed] [Google Scholar]
- 47.Fair T. The contribution of the maternal immune system to the establishment of pregnancy in cattle. Front Immunol 6: 7, 2015. doi: 10.3389/fimmu.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen PJ. The immunology of early pregnancy in farm animals. Reprod Domest Anim 46: 18–30, 2011. doi: 10.1111/j.1439-0531.2011.01850.x. [DOI] [PubMed] [Google Scholar]
- 49.Beltman ME, Forde N, Lonergan P, Crowe PA. Altered endometrial immune gene expression in beef heifers with retarded embryos. Reprod Fertil Dev 25: 966–970, 2013. doi: 10.1071/RD12232. [DOI] [PubMed] [Google Scholar]
- 50.Sponchiado M, Gomes NS, Fontes PK, Martins T, Del Collado M, Pastore ADA, Pugliesi G, Nogueira MFG, Binelli M. Pre-hatching embryo-dependent and -independent programming of endometrial function in cattle. PLoS One 12: e0175954, 2017. doi: 10.1371/journal.pone.0175954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1–S5: https://doi.org/10.6084/m9.figshare.17071997.v1.