Abstract
Obtaining correct amounts of essential elements, and avoiding toxic metals are key factors in dog health. Through analyzing major and trace elements in hair and blood of 50 healthy companion dogs using ICP-MS, we study their associations with dog characteristics and diet, hypothesizing that eating the same diet long-term results in strong correlations between hair and blood element concentrations, and that dog characteristics and diet affect element status. The correlation between hair and blood was significant for Hg (R = 0.601, p = 0.000) and Pb (R = 0.384, p = 0.010). The following associations were significant (p < 0.05): Dark hair had higher Ca and Mg compared to light hair. Females had higher hair Zn, blood Mn, and blood As compared to males. Blood Mn and Se increased, while blood Pb decreased with age. Raw diet fed dogs had higher hair Zn and Se compared to dry or mixed diet fed dogs, and lower blood Mn compared to dry diet fed dogs. Dry and mixed diet fed dogs had higher blood Cd compared to raw diet fed dogs. Mixed diet fed dogs had higher hair Ca and Mg compared to raw or dry diet fed dogs, and higher hair Pb compared to dry diet fed dogs. Wild game consumption was associated with higher blood Pb, and rice consumption with higher blood As. In conclusion, hair provides an alternative for assessing Hg and Pb exposure, and major and trace elements status is affected by hair color, sex, age, and diet.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11259-021-09854-8.
Keywords: Major elements, Trace elements, Toxic metals, Hair, Blood, Canine
Introduction
To maintain optimal health, dogs need to obtain the right amounts of major and trace elements, while avoiding exposure to harmful chemicals such as toxic metals. Recent studies have further emphasized the importance of element status in dog health by suggesting that trace elements play a role in the pathogenesis of several canine diseases (Vitale et al. 2019; Cedeño et al. 2020). Major elements, including calcium (Ca), magnesium (Mg), phosphorus (P), sodium (Na), and potassium (K), are needed by the body in larger quantities, whereas trace elements, such as iron (Fe), copper (Cu), zinc (Zn), manganese (Mn), selenium (Se), and chromium (Cr) are needed in smaller quantities. Major elements are involved in bone and teeth formation, nerve and muscle function, cell signaling, and acid-base balance, while trace elements act as co-factors in enzymes, and play important roles in antioxidant, hormone, and immune system functions (National Research Council 2006; Puertollano et al. 2011). Conversely, toxic metals, such as lead (Pb), mercury (Hg), cadmium (Cd), arsenic (As), aluminum (Al), and nickel (Ni) can disrupt neurological, reproductive, renal, and hematological systems. They also interfere with the absorption and metabolism of trace elements and increase oxidative stress (D’Souza et al. 2003).
There have been reports of dry dog foods providing either insufficient, excessive, or an inappropriate balance of major and trace elements such as Ca, P, Zn, Cu, Mn, and Se (Paulelli et al. 2018; Pereira et al. 2018; Kazimierska et al. 2020). Davies et al. (2017a) found that a majority of complete wet and dry pet foods sold in the UK were non-compliant according to current European guidelines. Meanwhile, raw food diets have been found to commonly be low in certain major and trace elements such as Ca, Zn, and Cu (Dillitzer et al. 2011). Excess Ca, or the presence of certain other dietary substances, such as phytate in grains, can also negatively impact the absorption of trace elements from the diet (National Research Council 2006). In addition, the absorption of elements can vary depending on elemental form, i.e. dry dog foods are commonly supplemented with inorganic elements that are less bioavailable compared to organic forms (Trevizan et al. 2013). Raw food diets for dogs contain organic elements from raw ingredients, such as meat, fish, bone, offal, eggs, and vegetables, and they are sometimes supplemented with additional organic, or inorganic elements (National Research Council 2006).
Several authors have also expressed concern about long-term exposure to toxic metals in certain dry dog foods (Davies et al. 2017a; De Nadai Fernandes et al. 2018; Kim et al. 2018; Paulelli et al. 2018; Rosendahl et al. 2020). Studies have shown higher concentrations of toxic metals in serum and liver in dogs that eat commercial diets compared to dogs that eat home-made diets (Lopez-Alonso et al. 2007; Tomza-Marciniak et al. 2012). Moreover, some specific dietary items such as wild game and rice have been associated with toxic metal exposure in dogs (Høgåsen et al. 2016; Rosendahl et al. 2020).
A dog’s major and trace element status can be assessed from various loci such as hair and blood. Hair analysis provides a reading of elemental deposition in the cells and interstitial spaces of the hair over a 2-3-month period, and thus gives a long-term assessment of element status or toxic metal exposure. On the other hand, blood analysis indicates the current intake of elements (Jenkins 1979; Ahmad et al. 2013). As blood is prone to fluctuations and homeostatic regulation, hair has been considered a more stable medium for reflecting dietary intake of elements in humans and other animals (Perry et al. 1976; Ghorbani et al. 2015; Kim et al. 2016). During the last decade the interest in hair analysis among canine researchers has increased and it has become evident that not only dietary intake, but also age, sex, hair color, physiological status, health status, living environment, laboratory washing procedures, and in some cases breed, may also affect hair element concentrations in dogs (Chyla and Zyrnicki 2000; Park et al. 2005; So et al. 2016; Davies et al. 2017b; Sgorlon et al. 2019; Chun et al. 2020). There is still a paucity of data on major and trace element, and toxic metal concentrations in hair (So et al. 2016; Davies et al. 2017b; Sgorlon et al. 2019) and blood (Panda et al. 2009; Viviano and Vanderwielen 2013; Sousa et al. 2013; Bahovschi et al. 2015; Ferreira et al. 2017; Langlois et al. 2017) of healthy dogs. Furthermore, research on the correlation between hair and blood element concentrations (Sousa et al. 2013), and the effect of common diet types, such as dry and raw food, on element status (Anturaniemi et al. 2020) is scarce.
The purpose of this study was to establish mean concentrations of major and trace elements in hair and blood of clinically healthy companion dogs to decide whether hair and blood element concentrations correlate with each other, and to assess the effect of age, sex, hair color, and diet on these elements. Our hypothesis was that major and trace elements correlate between hair and blood in dogs, since dogs are usually fed with the same diet for long periods of time. We also hypothesized that element status and toxic metal burden varies depending on which diet type the dog is eating, and that age, sex, and hair color affect element concentrations.
Materials and methods
Animals and study design
This was a non-controlled, cross-sectional study including a questionnaire and blood and hair samples from companion dogs living in their home-environment in two different regions in Finland, consisting of both urban, sub-urban, and rural environmental areas. Client-owned companion dogs (N = 50) were recruited via local dog groups on Facebook and then called for a visit to the Helsinki University small animal hospital or to a veterinary clinic in Pohjanmaa (Finland) for collection of hair and blood samples. Inclusion criteria were (i) dogs older than 1 year of age and over 10 kg of body weight, and (ii) dogs that had been eating their current diet for a minimum of six months. Exclusion criteria were (i) disease or therapy and (ii) pregnancy or lactation. After the visits, the dog owners filled in an online questionnaire about their dogs’ basic information, health status, and feeding. The health status of the dogs was determined by using owner-reported health information, a clinical examination, a complete blood count, and serum chemistry. The study included a large variety of breeds: thirteen mixed-breeds, four Nova Scotia Duck Tolling Retrievers, three Samoyeds, German Shepherds, and White Shepherds, two Gordon Setters, Kromfohrländers, Border Collies, and Australian Shepherds, and one Standard Poodle, Norwegian Elkhound, Boston Terrier, Czech Mountain Dog, Golden Retriever, Borzoi, Australian Kelpie, Whippet, Pumi, Cavalier King Charles Spaniel, Staffordshire Bull Terrier, Finnish Lapphund, Slovak Cuvac, French Bulldog, Bernese Mountain Dog, Cocker Spaniel, and Bullmastiff. The dogs were grouped into three diet groups based on what diet they had been eating for the previous six months: 80 % or more raw, 80 % or more dry, or mixed diet (Table 1). We define the raw diet as various types of unprocessed and unheated animal meat and by-products, which are high in animal protein and fat and low in carbohydrates. We define the dry diet as various commercially provided ultra-processed and often extruded kibbles, all of which are high in carbohydrates and lower in fat than raw diets. The protein in dry diets often originate from both animal and vegetal sources, and all dry diets have added micronutrients. Neither raw nor dry food nutrient composition or ingredient lists were collected for this study. Mixed diets consisted of dry, raw, home-cooked, and/or canned food. We define home-cooked food as foods cooked at home for either humans or dogs, hence also human food leftovers and/or snacks. The mixed diets (n=9) in this study had the following composition: first dog 60 % dry, 40 % raw; second dog 60 % dry, 25 % raw, 15 % home-cooked (added Zn); 3rd 60 % dry, 30 % raw, 10 % home-cooked (added Zn); 4th 70 % dry, 10 % raw, 10 % home-cooked, 10 % canned; 5th 45 % dry, 45 % raw, 10 % home-cooked; 6th 20 % dry, 10 % raw, 10 % home-cooked, 60 % canned; 7th 50 % dry, 50 % raw; 8th 70 % dry, 30 % home-cooked (added multivitamin); 9th 50 % dry, 50 % raw (added Zn, vitamin A and C, kelp, eggshells). The dogs were also grouped in relation to their consumption of wild game: never (n = 29), 1-6 times/year (n = 15), and monthly, weekly, or daily (n = 6); and of rice: never or 1-6 times/year (n = 22), and weekly or daily (n = 13). Dog owners’ smoking habits were included for Cd analyses. The study protocol was approved by the Animal Experiment Board in Finland (ELLA) (permit number: ESAVI/452/2020). All owners signed a written consent form.
Table 1.
Characteristics of the study population
| Signalment | Study population (N=50) | |
|---|---|---|
| Mean age (min-max), years | 4.5 (1.0-12.1) | |
| Sex, n (%) | Male | 25 (50) |
| Female | 25 (50) | |
| Hair color, n (%) | Lighta | 29 (58) |
| Darkb | 19 (38) | |
| Mixedc | 2 (4) | |
| Diet, n (%) | Rawd | 21 (42.9) |
| Dryd | 19 (38.8) | |
| Mixede | 9 (18.4) |
a70-100 % of hair sample was white, cream, red, or gray
b70-100 % of hair sample was black or brown
c50 % of hair sample was black and 50 % red (excluded from statistical analyses involving hair color)
d80 % or more of total diet for a minimum of six months
eMix of dry, raw, home-cooked and/or canned food for a minimum of six months (one dog had only eaten the current diet for 1.4 months and was therefore excluded)
Hair samples
Dog owners were asked not to wash their dogs with shampoo during the two weeks preceding the visit. The fur was carefully cleaned with A12t Dilutus 80 % ethanol disinfectant on a clean cotton gauze and then cut close to the skin using clean stainless-steel scissors. Hair samples were collected primarily from the neck area (n = 45), and in few cases from the chest (n = 2) or tail (n = 3) area. The distal part of the hair sample was discarded, leaving only the 3 cm of hair that had been closest to the skin. The amount of hair in one sample was approximately one tablespoon or 125 mg. Based on visual classification, each hair sample was classified as having either dark or light hair color. This did not however always correspond to the dog’s coat color as a whole. Hair samples were put in individual paper envelopes and stored in room temperature and were all sent in one batch to Analytical Research Laboratories, Inc. (Phoenix, USA) for analysis of Ca, Mg, P, Na, K, Fe, Cu, Mn, Zn, Se, Cr, Pb, Hg, Cd, As, Al, and Ni content. At the laboratory, 40 mg of unwashed hair was digested in PTFE reflux tubes containing a combination of nitric acid and perchloric acid in an open vessel and using a hot block/plate. After digestion, each sample was reconstituted to 2 ml using laboratory grade deionized water, and then analyzed with inductively coupled plasma mass spectrometry (Perkin Elmer ICP-MS nexION 2000B). To ensure the accuracy of the results, quality control procedures were implemented, including the use of known controls at the beginning, middle, and end of every batch of hair samples. Any reading that was out of the normally expected range led to retesting of the hair sample. The element concentrations were reported as µg/g.
Blood samples
Blood was collected from the cephalic vein into Vacuette 1 mL EDTA for complete blood count and 6 mL plain serum tubes for clinical chemistry. All samples were fasting samples. Complete blood cell counts were determined with the ADVIA 2120i Hematology System with multispecies software (Siemens Healthcare Diagnostics) and the cyanmethemoglobin method for hemoglobin measurements. For the biochemical analyses, the collected blood was allowed to clot and then centrifuged (2100× g, 15 min). Measurements were performed using a Konelab 30i chemistry analyzer (ThermoFisher Scientific). Analyses were performed immediately after collection, though in some cases (when samples were taken in Pohjanmaa), samples were sent by mail and analyzed the following day.
For multielemental analysis, blood was collected from the cephalic vein into 6 ml NH Trace Elements Sodium Heparin tubes and then divided into 1.5 ml Eppendorf tubes and stored in -20 °C until their analysis 6-12 months later. All samples were fasting samples. Analysis of whole blood Se, Zn, Cu, Mn, Fe, Cr, As, Cd, Hg, and Pb was performed at the Department of Environmental Sciences, Jožef Stefan Institute (Ljubljana, Slovenia). Altogether 0.3 g of whole blood samples was transferred into pre-cleaned teflon digestion vials. Samples were digested with 0.5 ml of 65 % nitric acid (suprapur) in a microwave system (ULTRAWAVE, Single Reaction Chamber Microwave Digestion System, MILESTONE, Italy) using the following protocol: (1) 20 min temperature rise to 240 °C, (2) kept 12 min at 240 °C and max 100 bar. Digested solutions were transferred into measuring tubes and diluted to 5 ml with Milli-Q water. Prepared solutions were measured by Triple Quadrupole Inductively coupled plasma Mass spectrometry (ICP-QQQ, Agilent 8800, California, USA). Isotopes monitored were: 52Cr, 55Mn, 57Fe, 63Cu, 66Zn, 75As, 78Se, 114Cd, 202Hg, and 208Pb. External calibration was used for quantification. Accuracy of results was checked by the use of two reference materials: Seronorm Whole blood Level 1 (lot: 1,702,821) and Level 2 (lot: 1,702,825). The quality control results are presented in Online Resource 1. Limits of detection for blood elements used were three times the standard deviation of several blank samples: 1.5 ng/g for Cr, 0.4 ng/g for Mn, 40 ng/g for Fe, 1.5 ng/g for Cu, 150 ng/g for Zn, 0.2 ng/g for As, 1 ng/g for Se, 0.02 ng/g for Cd, 0.04 ng/g for Hg and 0.4 ng/g for Pb. The study results are reported as ng/g.
Statistical analyses
SPSS software (version 25; IBM SPSS Statistics) was used for all statistical analyses. The normality of data was assessed using the Shapiro-Wilk test and a natural log- or square root transformation was applied on element variables that did not follow a normal distribution (16/17 hair elements, 6/10 blood elements). Concentrations below the limit of detection (LOD) were assigned a value of LOD divided by the square root of 2. Correlations between hair and blood elements were assessed using Pearson’s correlation. General linear models (GLM) were used to determine the effect of diet (raw, dry, or mixed), sex (male or female), hair color (light or dark), and age on hair and blood element concentrations. In addition, the effect of consuming Pb-shot game (never, 1-6 times per year, or monthly/weekly/daily) on hair and blood Pb concentration, and the effect of consuming rice (never or weekly/daily) on hair and blood As concentration was also assessed. Main effects of diet, sex, hair color, and age on element concentrations, as well as possible interactions (one by one) were tested, and non-significant terms were dropped from the final models. The assumption of error variance equality was assessed using Levene’s test and the Bonferroni correction was used for pairwise comparisons between diet groups. Blood Cd had a large number of <LOD values (21/46, 45.7 %) and hair As a large number of dogs (32/46, 69.6 %) showing the lowest value reported by the laboratory analysis results (0.01 µg/g). In these cases, the Mann Whitney U/Kruskal-Wallis ANOVA test was performed to assess differences between subgroups. Statistical significance was set at P < 0.05 in all analyses.
Results
Study population
Clinically healthy dogs (n = 50) were included in this study. The characteristics of the study population are presented in Table 1.
Hair and blood analyses
Mean hair element concentrations are presented in Table 2. The following extreme outliers (±3 times the interquartile range) were removed among hair elements and are mentioned in the discussion where appropriate: Ca (3560 and 2470 µg/g); Na (10,460 µg/g); K (730 and 730 µg/g); Fe (375 and 138 µg/g); Mn (10.35, 5.08, and 2.6 µg/g); Cr (2.25 µg/g); Pb (0.41 µg/g); Hg (0.55 and 0.27 µg/g); Cd (0.04, 0.02, 0.02, and 0.02 µg/g); Al (403.5 and 105.4 µg/g); As (0.22, 0.22, 0.15, and 0.14 µg/g); Ni (6.23, 1.67, and 1.39 µg/g).
Table 2.
Hair element concentrations (µg/g) in clinically healthy dogs (N=50)
| Element concentrations from literature (So et al. 2016) | Group | n | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
|
Ca 588.00± 307.85 (mean ±SD) |
All dogs | 48 | 508.75 | 383.52 | 100 | 1950 |
| Raw | 19 | 327.37 | 219.67 | 130 | 790 | |
| Dry | 19 | 586.84 | 361.30 | 100 | 1310 | |
| Mixed | 9 | 730.00 | 560.40 | 260 | 1950 | |
|
Mg 124.00± 66.53 |
All dogs | 50 | 155.60 | 126.24 | 30 | 590 |
| Raw | 21 | 135.71 | 158.13 | 30 | 590 | |
| Dry | 19 | 158.42 | 88.71 | 30 | 330 | |
| Mixed | 9 | 197.78 | 119.56 | 80 | 460 | |
|
P 260.00± 37.71 |
All dogs | 50 | 311.80 | 61.90 | 210 | 490 |
| Raw | 21 | 306.19 | 75.73 | 210 | 490 | |
| Dry | 19 | 314.74 | 38.35 | 260 | 410 | |
| Mixed | 9 | 318.89 | 75.24 | 230 | 420 | |
|
Na 2854.00± 859.25 |
All dogs | 49 | 1793.88 | 1127.90 | 110 | 5130 |
| Raw | 20 | 1609.50 | 982.52 | 110 | 3570 | |
| Dry | 19 | 1911.05 | 1232.45 | 680 | 5130 | |
| Mixed | 9 | 1838.89 | 1289.69 | 240 | 3790 | |
|
K 142.00± 76.85 |
All dogs | 48 | 167.71 | 110.50 | 40 | 560 |
| Raw | 20 | 164.50 | 132.25 | 40 | 560 | |
| Dry | 19 | 162.11 | 97.62 | 60 | 490 | |
| Mixed | 8 | 187.50 | 97.06 | 40 | 340 | |
|
Fe 16.20± 4.94 |
All dogs | 48 | 33.48 | 17.50 | 13 | 98 |
| Raw | 20 | 31.20 | 15.02 | 13 | 66 | |
| Dry | 19 | 31.90 | 17.22 | 13 | 69 | |
| Mixed | 8 | 42.00 | 23.87 | 27 | 98 | |
|
Cu 8.80± 1.03 |
All dogs | 50 | 8.34 | 1.35 | 6 | 13 |
| Raw | 21 | 8.76 | 1.61 | 6 | 13 | |
| Dry | 19 | 7.95 | 0.91 | 7 | 10 | |
| Mixed | 9 | 8.33 | 1.32 | 6 | 10 | |
|
Mn 0.39± 0.17 |
All dogs | 47 | 0.69 | 0.44 | 0.17 | 2.31 |
| Raw | 20 | 0.72 | 0.47 | 0.17 | 1.72 | |
| Dry | 19 | 0.58 | 0.31 | 0.20 | 1.37 | |
| Mixed | 7 | 0.90 | 0.65 | 0.43 | 2.31 | |
|
Zn 136.00± 8.43 |
All dogs | 50 | 135.60 | 15.54 | 100 | 170 |
| Raw | 21 | 142.38 | 16.40 | 110 | 170 | |
| Dry | 19 | 131.58 | 12.14 | 110 | 160 | |
| Mixed | 9 | 130.00 | 15.81 | 100 | 150 | |
|
Se 1.01± 0.19 |
All dogs | 50 | 0.53 | 0.24 | 0.10 | 1.33 |
| Raw | 21 | 0.63 | 0.28 | 0.24 | 1.33 | |
| Dry | 19 | 0.45 | 0.21 | 0.10 | 0.95 | |
| Mixed | 9 | 0.45 | 0.15 | 0.21 | 0.74 | |
|
Cr 1.07± 0.20 |
All dogs | 49 | 0.88 | 0.25 | 0.42 | 1.73 |
| Raw | 20 | 0.87 | 0.27 | 0.42 | 1.53 | |
| Dry | 19 | 0.87 | 0.18 | 0.53 | 1.19 | |
| Mixed | 9 | 0.93 | 0.33 | 0.61 | 1.73 | |
|
Pb 0.06± 0.05 |
All dogs | 49 | 0.09 | 0.06 | 0.01 | 0.30 |
| Raw | 21 | 0.09 | 0.06 | 0.02 | 0.23 | |
| Dry | 19 | 0.07 | 0.07 | 0.01 | 0.30 | |
| Mixed | 8 | 0.12 | 0.04 | 0.07 | 0.21 | |
|
Hg 0.08± 0.03 |
All dogs | 48 | 0.08 | 0.04 | 0.01 | 0.21 |
| Raw | 21 | 0.08 | 0.05 | 0.01 | 0.21 | |
| Dry | 18 | 0.07 | 0.04 | 0.02 | 0.14 | |
| Mixed | 8 | 0.09 | 0.04 | 0.03 | 0.14 | |
|
Cda 0.020± 0.000 |
All dogs | 46 | 0.01 | 0 | 0.01 | 0.01 |
| Raw | 20 | 0.01 | 0 | 0.01 | 0.01 | |
| Dry | 17 | 0.01 | 0 | 0.01 | 0.01 | |
| Mixed | 8 | 0.01 | 0 | 0.01 | 0.01 | |
|
As 0.09± 0.07 |
All dogs | 46 | 0.02 | 0.02 | 0.01 | 0.09 |
| Raw | 21 | 0.02 | 0.02 | 0.01 | 0.09 | |
| Dry | 17 | 0.02 | 0.02 | 0.01 | 0.08 | |
| Mixed | 7 | 0.02 | 0.01 | 0.01 | 0.04 | |
|
Al 4.82± 3.27 |
All dogs | 48 | 21.42 | 12.93 | 6.70 | 63.30 |
| Raw | 21 | 21.56 | 12.02 | 6.70 | 50.60 | |
| Dry | 19 | 21.59 | 15.99 | 6.70 | 63.30 | |
| Mixed | 7 | 21.36 | 7.53 | 15.00 | 35.70 | |
|
Ni 0.10± 0.07 |
All dogs | 47 | 0.24 | 0.17 | 0.06 | 0.90 |
| Raw | 19 | 0.27 | 0.20 | 0.09 | 0.90 | |
| Dry | 19 | 0.19 | 0.12 | 0.06 | 0.47 | |
| Mixed | 8 | 0.29 | 0.14 | 0.09 | 0.57 |
Concentrations from literature (So et al. 2016) are reported under the chemical symbol
Extreme outliers excluded; n, number of dogs included for each element; mean, arithmetic mean; SD, standard deviation
aAll dogs showed the lowest value reported by the laboratory analysis results (0.01 µg/g)
Mean blood element concentrations are presented in Table 3. Three dogs had not fasted prior to blood sampling and were excluded. The following extreme outliers were removed among blood elements and are mentioned in the discussion where appropriate: Zn (8420 and 7160 ng/g); As (11.85 and 6.38 ng/g); Cd (0.13 ng/g); Hg (5.33 ng/g); Pb (77.39 and 48.78 ng/g).
Table 3.
Blood element concentrations (ng/g) in clinically healthy dogs (N=50)
| Element concentrations from literature if found | Group | n (<LOD) | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
|
Fe 116400± 6700 (mean± SE)a 551886.79± 16037.74 (mean± SD)b |
All dogs | 47 | 605382.98 | 62129.09 | 486000 | 742000 |
| Raw | 20 | 610400.00 | 67522.63 | 486000 | 742000 | |
| Dry | 18 | 607777.78 | 50774.65 | 508000 | 706000 | |
| Mixed | 8 | 587625.00 | 79260.76 | 503000 | 713000 | |
|
Cu 480± 40; 290-740 (mean± SE; range)a |
All dogs | 47 | 475.73 | 54.02 | 386.40 | 614.20 |
| Raw | 20 | 483.79 | 59.04 | 349.70 | 614.20 | |
| Dry | 18 | 475.58 | 52.31 | 386.40 | 565.20 | |
| Mixed | 8 | 461.48 | 48.68 | 413.40 | 531.70 | |
|
Mn 55.15; 46.29-64.28 (median; range)c |
All dogs | 47 | 30.84 | 9.78 | 14.50 | 59.50 |
| Raw | 20 | 27.52 | 8.19 | 14.50 | 46.70 | |
| Dry | 18 | 33.54 | 10.50 | 16.60 | 59.50 | |
| Mixed | 8 | 33.40 | 11.00 | 20.20 | 48.80 | |
|
Zn 4760± 170; 3690-5930 (mean± SE; range)a |
All dogs | 45 | 3685.56 | 542.69 | 2850 | 5630 |
| Raw | 20 | 3639.00 | 429.77 | 2850 | 4270 | |
| Dry | 17 | 3714.12 | 679.15 | 2930 | 5630 | |
| Mixed | 7 | 3765.71 | 570.58 | 3120 | 4820 | |
|
Se 320.75; 132.08-943.40 (median; range)d |
All dogs | 47 | 388.14 | 40.95 | 310.10 | 476.60 |
| Raw | 20 | 379.58 | 42.01 | 320.00 | 476.60 | |
| Dry | 18 | 398.22 | 40.04 | 310.10 | 457.90 | |
| Mixed | 8 | 389.37 | 42.45 | 341.70 | 453.60 | |
| Cr | All dogs | 46 (31) | 3.67 | 6.07 | 1.06 | 24.83 |
| Raw | 19 (16) | 3.03 | 5.86 | 1.06 | 24.83 | |
| Dry | 18 (11) | 4.17 | 6.31 | 1.06 | 23.49 | |
| Mixed | 8 (4) | 4.17 | 7.02 | 1.06 | 21.36 | |
|
Pb 1; 1-4 (median, IQR)e |
All dogs | 45 (2) | 3.50 | 2.72 | 0.28 | 12.37 |
| Raw | 18 (1) | 3.71 | 2.98 | 0.28 | 11.61 | |
| Dry | 18 (1) | 3.39 | 3.00 | 0.28 | 12.37 | |
| Mixed | 8 | 3.32 | 1.73 | 1.59 | 5.63 | |
|
Hg 0.16-12.38 (min-max)f |
All dogs | 46 | 0.53 | 0.35 | 0.12 | 1.61 |
| Raw | 20 | 0.52 | 0.25 | 0.20 | 0.90 | |
| Dry | 17 | 0.51 | 0.40 | 0.10 | 1.60 | |
| Mixed | 8 | 0.63 | 0.45 | 0.10 | 1.40 | |
| Cd | All dogs | 46 (21) | 0.03 | 0.01 | 0.01 | 0.06 |
| Raw | 20 (13) | 0.02 | 0.01 | 0.01 | 0.05 | |
| Dry | 17 (6) | 0.03 | 0.02 | 0.01 | 0.06 | |
| Mixed | 8 (2) | 0.03 | 0.01 | 0.01 | 0.05 | |
| As | All dogs | 45 | 1.15 | 1.09 | 0.04 | 4.30 |
| Raw | 20 | 0.92 | 0.85 | 0.04 | 3.53 | |
| Dry | 16 | 1.07 | 1.04 | 0.14 | 3.62 | |
| Mixed | 8 | 1.98 | 1.48 | 0.76 | 4.30 |
Concentrations from literature are reported under the chemical symbol if found (e.g., comparable analytical method, healthy dogs)
Extreme outliers and non-fasted cases excluded; n, number of dogs included for each element; LOD, limit of detection; mean, arithmetic mean; SD, standard deviation; IQR, interquartile range; SE, standard error
aPanda et al. 2009, has been converted to a comparable unit (µg/g to ng/g)
bBahovschi et al. 2015, has been converted to a comparable unit (mg/l to ng/g)
cFerreira et al. 2017
dViviano and Vanderwielen 2013, has been converted to a comparable unit (µg/ml to ng/g)
eLanglois et al. 2017
fSousa et al. 2013
When comparing hair and blood concentrations of individual elements, significant positive correlations were found only for Hg and Pb. All correlations are presented in Table 4.
Table 4.
Pearson correlations between element concentrations in hair and blood
| Element | n | r | p-value |
|---|---|---|---|
| Fe | 46 | 0.083 | 0.585 |
| Cu | 47 | -0.049 | 0.745 |
| Mn | 45 | 0.269 | 0.074 |
| Zn | 45 | -0.204 | 0.179 |
| Se | 47 | -0.028 | 0.851 |
| Cr | 46 | 0.068 | 0.652 |
| Pb | 44 | 0.384** | 0.010 |
| Hg | 45 | 0.601*** | 0.000 |
| Cd | a | a | |
| As | 42 | 0.022 | 0.889 |
n, number of dogs included for each element; r, Pearson correlation coefficient
**Correlation is significant at the 0.01 level (2-tailed)
***Correlation is significant at the 0.001 level (2-tailed)
aCannot be computed because hair Cd is constant
Association of hair elements with dog characteristics and diet
The results of GLMs to assess the effect of sex, hair color, age, and diet on hair element concentrations are summarized in Table 5.
Table 5.
General linear models (GLMs) relating hair element concentrations with dog characteristics and diet
| Hair element | Fdf | p-value | |
|---|---|---|---|
| Ca | Model | 47.903,41 | 0.000 |
| Color | 110.821 | 0.000 | |
| Diet | 4.252 | 0.021 | |
| Mg | Model | 20.925,41 | 0.000 |
| Color | 83.841 | 0.000 | |
| Diet | 4.142 | 0.023 | |
| Sex * color | 5.121 | 0.029 | |
| Pa | Model | 4.618,38 | 0.001 |
| Sex | 9.271 | 0.004 | |
| Age * color | 5.401 | 0.026 | |
| Diet * color | 3.332 | 0.047 | |
| Na | Model | 4.963,43 | 0.005 |
| Age | 6.011 | 0.018 | |
| Age * color | 5.001 | 0.031 | |
| Ka | Model | 5.663,42 | 0.002 |
| Color | 4.811 | 0.034 | |
| Age | 5.331 | 0.026 | |
| Age * color | 11.561 | 0.001 | |
| Zn | Model | 5.483,45 | 0.003 |
| Sex | 8.561 | 0.005 | |
| Diet | 3.542 | 0.037 | |
| Se | Model | 4.728,38 | 0.000 |
| Diet | 8.732 | 0.001 | |
| Diet * color | 5.622 | 0.007 | |
| Diet * age | 5.952 | 0.006 | |
| Pb | Model | 3.672,45 | 0.033 |
| Diet | 3.672 | 0.033 | |
| Nib | Model | 2.884,40 | 0.035 |
| Diet * sex | 4.432 | 0.047 |
Only significant models and factors are shown; F, F-ratio; df, degrees of freedom
aThe model was significant, but the assumption of equality of error variances could not be met
bDue to the mixed diet group only having one male dog, this dog was removed from the analysis
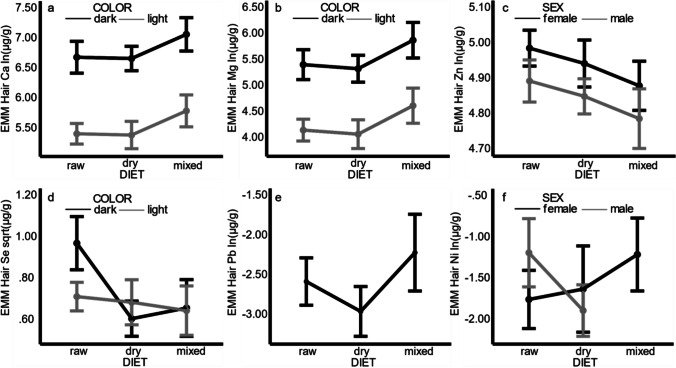
Hair Ca and Mg were significantly higher in dark-colored compared to light-colored dogs, and in dogs that ate mixed diets compared to raw (p = 0.045 for both Ca and Mg) or dry (p = 0.031 and 0.029, respectively) diets (Fig. 1a and b). Also, the effect of hair color on hair Mg concentrations was greater in males than in females, and hair Na increased with age in dark-colored (p = 0.031) dogs.
Fig. 1.
Effect of dog characteristics and diet on hair calcium (a), magnesium (b), zinc (c), selenium (d), lead (e), and nickel (f) concentrations in 50 healthy dogs. EMM, estimated marginal means. The error bars are the confidence intervals for the EMMs
Hair Zn was significantly higher in female compared to male dogs, and in dogs that ate raw diets compared to mixed diets (p = 0.036) (Fig. 1c). Among the raw diet fed dogs in our study, 11/21 received additional Zn supplements, two ate commercial raw diets with added Zn, and two received a multivitamin that may have contained Zn.
Hair Se was significantly higher in dogs that ate raw diets compared to dry (p = 0.001) or mixed diets (p = 0.005), especially in dark-colored dogs (Fig. 1d). Furthermore, dry diet fed dogs had higher hair Se with older age, mixed diet fed dogs had lower hair Se with older age, and raw diet fed dogs had similar hair Se concentrations at all ages. Of the raw fed dogs, only two received Se-supplemented diets, and two received a multivitamin that may have contained Se.
Hair Pb was significantly higher in dogs that ate mixed diets compared to dry diets (p = 0.039) (Fig. 1e). We could confirm that an extreme outlier for hair Pb among the dry diet fed dogs had an owner that worked at a metal recycling plant. According to the final model, male dogs that ate raw diets had higher hair Ni compared to those that ate dry diets, while female dogs that ate mixed diets had higher hair Ni compared to those that ate raw or dry diets (Fig. 1f).
For hair P and K, we found significant models, but the assumption of equality of error variances could not be met (Levene’s test: F = 2.51, p = 0.021 and F = 4.47, p = 0.04, respectively). Hair P was significantly higher in female compared to male dogs and increased with age in dark-colored dogs. Eating a raw diet had a different effect on hair P depending on the hair color: compared to dogs that ate dry or mixed diets, dark-colored dogs that ate raw diets had higher hair P, and light-colored dogs that ate raw diets had lower hair P. Hair K was higher in dark-colored dogs older than three years of age compared to light-colored dogs and increased with age in dark-colored dogs. For hair Fe, Cu, Mn, Cr, Hg, Cd, As, and Al, we did not find any significant models or factors. Original analysis results for hair element concentrations in individual dogs are presented in separate figures in Online Resource 2.
Association of blood elements with dog characteristics and diet
The results of GLMs to assess the effect of sex, age, and diet on element concentrations in blood are summarized in Table 6.
Table 6.
General linear models (GLMs) relating blood element concentrations with dog characteristics and diet
| Blood element | Fdf | p-value | |
|---|---|---|---|
| Mn | Model | 6.724,41 | 0.000 |
| Sex | 10.141 | 0.003 | |
| Age | 8.781 | 0.005 | |
| Diet | 4.312 | 0.020 | |
| Se | Model | 4.471,45 | 0.040 |
| Age | 4.471 | 0.040 | |
| Pb | Model | 6.642,42 | 0.003 |
| Age | 8.351 | 0.006 | |
| Pb-shot game | 4.911 | 0.032 | |
| As | Model | 9.962,29 | 0.001 |
| Sex | 13.651 | 0.001 | |
| Rice | 6.491 | 0.016 |
Only statistically significant models and factors are shown; F, F-ratio; df, degrees of freedom
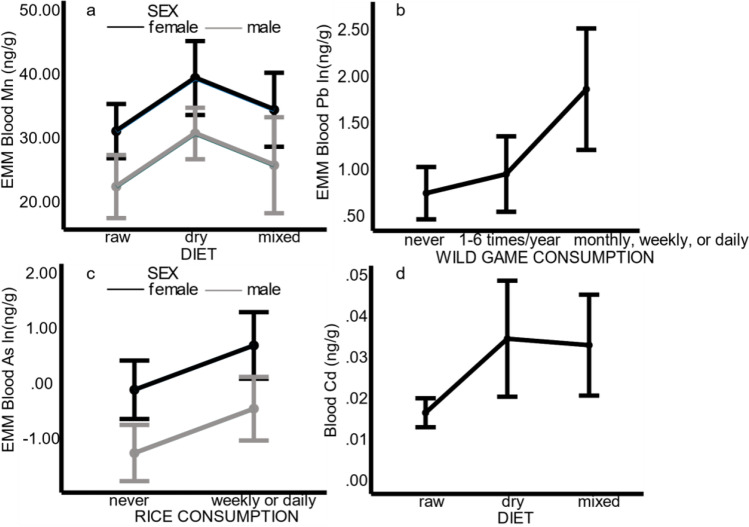
Blood Mn was significantly higher in female compared to male dogs and increased with age in both sexes. Moreover, blood Mn was significantly higher in dogs that ate dry compared to raw diets (p = 0.017) (Fig. 2a). Blood Se increased with age in all dogs.
Fig. 2.
Effect of dog characteristics and diet on blood manganese (a), lead (b), arsenic (c), and cadmium (d) concentrations in 50 healthy dogs. EMM, estimated marginal means. The error bars represent the confidence intervals for the EMMs
Blood Pb decreased with age and was significantly affected by consumption of wild game. Dogs that ate wild game monthly, weekly, or daily had significantly higher blood Pb compared to dogs that never ate wild game (p = 0.009). Dogs that consumed wild game only 1-6 times/year also had higher blood Pb concentrations than those that never consumed wild game, but this difference was not significant (p = 0.063) (Fig. 2b). The mean blood Pb concentration in dogs consuming wild game monthly, weekly, or daily, was 7.2 ng/g (SD 3.14; min 4.36; max 11.61 ng/g), while it was 3.54 ng/g (SD 2.15; min 0.28; max 8.04 ng/g) in those consuming wild game 1-6 times/year, and 2.80 ng/g (SD 2.40; min 0.28; max 12.37) in those that never consumed wild game. Finally, we could confirm that an extreme outlier (12.37 ng/g) among dogs that never consumed wild game, in fact ate a dry dog food containing game meat.
Blood As was significantly higher in female compared to male dogs. Dogs that ate rice weekly or daily had significantly higher blood As compared to dogs that did not eat rice (Fig. 2c). For blood Cd, we found a significant difference between subgroups of diet and dog-owners’ smoking habits (Kruskal-Wallis ANOVA p = 0.028). Post-hoc comparisons revealed that among dogs with non-smoking owners, blood Cd was significantly higher when fed dry (p = 0.003) or mixed diets (p = 0.007), when compared to raw diets (Fig. 2d), whereas there was no significant effect of diet among dogs with smoking owners. However, we did not observe any significant difference in blood Cd concentrations between dogs with smoking and non-smoking owners. No significant models or factors for blood Fe, Cu, Zn, Cr, and Hg were found. Original analysis results for blood element concentrations in individual dogs are presented in separate figures in Online Resource 3.
Discussion
To determine the correlation between hair and blood element concentrations, and to assess how these elements are affected by diet type and dog characteristics, mean element concentrations in hair and blood were established in healthy companion dogs.
In accordance with previous studies (Tsai et al. 2000; Chyla and Zyrnicki 2000), hair Ca and Mg concentrations were significantly higher in dark-colored compared to light-colored dogs, highlighting the importance of considering hair color in future research. Hair Ca and Mg concentrations were also affected by diet, being higher in mixed diet fed dogs. Hair Ca concentration may reflect dietary Ca content (Ghorbani et al. 2015; Kim et al. 2016), and as many of the mixed diet fed dogs ate complete dry foods mixed with bone-containing raw foods, the total Ca content of their diets was probably higher. However, other dietary factors such as vitamin D and Mg can also raise hair Ca concentrations (Jeruszka-Bielak and Brzozowska 2011). In fact, we removed two extreme outliers for hair Ca, and both dogs’ diets were supplemented with Mg.
In accordance with human studies (Tamburo et al. 2016; Zhu et al. 2018), hair Zn concentration was higher in female compared to male dogs. However, previous dog studies did not find any sex-associated difference in hair Zn (Davies et al. 2017b; Sgorlon et al. 2019). Hair Zn was also higher in raw compared to mixed diet fed dogs. This could be due to either differences in Zn content or in Zn absorption of the consumed diets. The absorption of Zn can be lowered by high dietary amounts of Ca or phytate. Phytate is a substance commonly found in dry dog food ingredients such as corn, wheat, rice, and soy, but rarely in raw, meat based diets (National Research Council 2006). Considering that the mixed diet fed dogs in our study ate diets consisting of 20-70 % dry food, and had higher hair Ca, it is possible that Ca and phytate affected Zn absorption in these dogs. Furthermore, several studies have found higher hair Zn in dogs that are fed diets with organic Zn, as it has better bioavailability compared to inorganic forms (Lowe et al. 1994; Trevizan et al. 2013). The raw fed dogs’ diets included organic Zn from raw ingredients such as red meat, liver, poultry, eggs, and fish (Cummings and Kovacic 2009), whereas dry dog foods commonly include inorganic forms of Zn (National Research Council 2006). The inorganic forms of Zn are also more sensitive to dietary Ca and phytate (Cummings and Kovacic 2009). According to a recent study, only 58.2 % of the total Zn content in dry dog foods was bioavailable to dogs (Gregório et al. 2020). Anyway, the content of Ca, phytate, and Zn in the dogs’ diets were not assessed in this study, and thus, further research is needed to explain the reason for higher hair Zn concentration in raw diet fed dogs. We could not see any difference in hair Zn between the raw diet fed dogs that received Zn supplements and those that did not (data not shown). This suggests that the organic forms of Zn in these meat-based diets is enough to meet the animal’s Zn requirement, since animal-based proteins and the protein-content in the diet is positively correlated with Zn intake (Sandstrom and Cederblad 1980; Sandstrom et al. 1980; Wapnir 2000). The effect of the diet type on the dietary requirements of Zn should be further studied to assure that the requirements are sufficient, and not too high, for dogs that get Zn mostly in organic form.
Blood Mn, which is considered a valid indicator of Mn status in animals (Clegg et al. 1986), was lower in raw compared to dry diet fed dogs. Foods rich in Mn include cereal grains, rice, legumes, nuts, seeds, spinach, seafood, and certain spices such as ginger; while most animal sources, with the exception of feathers (Abduljaleel et al. 2012), wool (Grace 1983), and tripe of grazing animals (Grace 1983; Grace et al. 2008), have very low Mn content (Martins et al. 2020). According to Dillitzer et al. (2011), 10-25 % of raw food diets contain very little Mn, and it is thus possible that some raw fed dogs in our study received too little Mn, especially if the diets did not include tripe or other Mn-rich food sources. In contrast, dry dog foods, which include both Mn-rich ingredients and supplemental Mn (National Research Council 2006), often contain an excess of Mn (Gagne et al. 2013; Pereira et al. 2018; Kazimierska et al. 2020), which could also have impacted on the observed difference in blood Mn concentration between raw and dry diet fed dogs in our study. Due to the lack of data on blood Mn concentrations in healthy dogs, it is difficult to draw conclusions from our results. In a study by Ferreira et al. (2017) healthy dogs fed a standardized dry food had a median blood Mn concentration that was two-fold the mean Mn concentration of the raw diet fed dogs in our study, however the Mn concentrations in their study were overall higher than in ours, so the results might not be comparable. Interestingly, the Mn content in the hair of mixed and raw diet fed dogs seemed to be higher than that of dry diet fed dogs in our study, although not significantly. Further research regarding the effect of diet on blood and hair Mn concentrations in healthy dogs should be conducted in the future. Until then, we recommend ensuring a sufficient Mn content in meat-based raw food diets. Blood Mn was also higher in female compared to male dogs, corresponding to what has been observed in humans (Baldwin et al. 1999; Clark et al. 2007), and it also increased with age, which was previously seen in dogs with non-hepatic illnesses, but not in healthy control dogs (Gow et al. 2010).
Hair Se concentration has been used to indicate dietary Se intake (Górski et al. 2018; Son et al. 2018) and Se status in animals (Christodoulopoulos et al. 2003; Davis et al. 2014). Raw fed dogs had higher hair Se compared to dry and mixed diet fed dogs, suggesting that raw food diets had a higher content or bioavailability of Se. All raw fed dogs were eating animal products of Finnish origin, which may have improved their Se status, considering that Se-fortified fertilizers used in Finland since 1985 have led to a considerably improved Se status of the Finnish population (Pietinen et al. 2010). Whole food ingredients such as meat, offal, fish, or eggs in raw food diets provide Se in organic form (National Research Council 2006), which is more bioavailable compared to the inorganic forms of Se (Moreda-Piñeiro et al. 2017) that are often added to dry dog foods (National Research Council 2006). For example, pigs fed a diet with organic Se had higher hair Se concentration compared to those being fed a similar diet with inorganic Se (Kim and Mahan 2001). Moreover, Se accessibility in pet foods can be negatively affected by heat-processing (Van Zelst et al. 2015). Interestingly, Se has been reported to have a role in the prevention of Ca oxalate calculi in dogs (Santhosh Kumar and Selvam 2003; Liu et al. 2015), as has the raw food diet (Dijcker et al. 2012). The positive effect of raw diets on hair Se concentration was more pronounced in dark-colored dogs, which is in accordance with findings by Kim & Mahan (2001) that dark-haired pigs retained more Se in their hair as dietary Se content increased. Christodoulopoulos et al. (2003) suggested that the phenomenon is caused by a higher content of melanin, containing sulfur amino acids that bind Se. Se increased with age in the blood of all dogs, and in the hair of dry diet fed dogs, but not in the hair of raw or mixed diet fed dogs. In horses, Brummer-Holder et al. (2020) found that blood Se increased with age, while hair Se instead tended to decreased with age, which is similar to what we observed in the raw and mixed diet fed dogs in our study.
Hg concentrations were positively correlated between hair and blood, which is in accordance to findings by Sousa et al. (2013) and Lieske et al. (2011). Pb concentrations were also positively correlated between hair and blood, which has previously been seen in cattle (Patra et al. 2007) and humans (Sanna et al. 2003), but not in dogs. This suggests that hair can be used as a surrogate for blood in assessing dogs’ exposure to Hg and Pb. An extreme outlier for Hg (blood Hg 10-fold and hair Hg 7-fold higher than means) was fed a dry dog food containing 18 % white deep-sea fish. As pet foods containing swordfish, shark, tuna, trout, pike, and bass are known sources of Hg exposure in dogs (Tegzes 2013), we believe that further studies should asses Hg concentrations in dogs that eat these types of fish-based dry foods on a daily basis.
Blood Pb concentration was significantly higher in dogs consuming wild game monthly, weekly, or daily. According to a report by Høgåsen et al. (2016), feeding trimmings of Pb-shot game represent a risk of Pb intoxication in dogs. Eating wild game has also been associated with elevated blood Pb levels in humans (Iqbal et al. 2009), but the interpretation of such studies are complicated by confounding factors such handling of ammunition and inhaling gunfire fumes (Fustinoni et al. 2017; Green and Pain 2019). Thus, dogs who are not prone to these factors, can act as valuable sentinels (Backer et al. 2001). Although the blood Pb concentrations in our study were far below what is considered indicative of Pb toxicosis in dogs (>300-350 ng/g), and no dogs showed signs of gastrointestinal or neurological symptoms associated with Pb toxicity, Pb has no beneficial biological function and the ideal blood Pb concentration is zero (Wismer 2013). Actually, even low-level Pb exposure has been associated with subclinical effects on immune system, organ function, and cognition (Langlois et al. 2017).
Overall, blood Pb concentration increased with age in our study, which is in agreement with previous reports of younger dogs showing higher blood Pb concentrations than older dogs (Lopez-Alonso et al. 2007; Langlois et al. 2017). Moreover, Pb concentrations were significantly higher in dogs eating mixed compared to dry diets, which could possibly be related to a lower nutrient content of these diets (e.g. Zn) leading to an increased absorption of Pb (Wismer 2013). According to Pedrinelli et al. (2017, 2019) micronutrient deficiency is commonly seen in home-cooked diets for dogs. Another possible explanation, considering that Pb accumulates in bone tissue (Fox 1987), is a higher intake of bone and bonemeal in mixed diet fed dogs. This theory is also supported by the higher hair Ca concentration in mixed diet fed dogs. Finally, we removed an extreme outlier for hair Pb, which was probably related to its owner working at a metal recycling plant. It is well known that family members with occupational exposure can bring Pb dust home on clothes and shoes, exposing children to Pb (UNICEF 2020).
Blood As concentration was higher in dogs that were consuming rice daily or weekly compared to dogs that were never consuming rice, however, we did not see any association between rice consumption and hair As concentrations, as we did in our previous study (Rosendahl et al. 2020). The two extreme outliers for blood As were not consuming rice frequently, but both ate fish-based dry foods, which have been associated with higher levels of As compared to other dry foods (Davies et al. 2017a). Unfortunately, due to small sample size, fish consumption could not be assessed in this study, but we recommend that future studies include it as a confounding factor.
In non-smoking people, the main source of Cd exposure is food (European Food Safety Authority 2012). Among dogs with non-smoking owners, blood Cd concentrations were higher in dogs that were fed dry or mixed, compared to raw diets. Tomza-Marciniak et al. (2012) also found that dogs eating commercial or mixed diets had higher serum Cd compared to those that only ate home-made diets. Wheat, rice, and potatoes, all common staple ingredients in dry dog foods, generally contain more Cd than meat, egg, and dairy products (Genchi et al. 2020). Other possible sources of Cd in commercial dog foods are Ca and Zn supplements (National Research Council 2005). Anyway, the observed Cd concentrations in our study were not indicative of toxicity, and the Cd concentration in commercially available dry dog foods (Duran et al. 2010; Abd-Elhakim et al. 2016; Davies et al. 2017b) appears to be well below the legal maximum (European commission 2013). However, even low-level exposure to Cd has been associated with increased oxidative stress (Lovásová et al. 2013) and negative effects on bone health (Åkesson et al. 2014).
Male dogs that ate raw diets had higher hair Ni compared to male dogs that ate dry diets, while female dogs that ate mixed diets had higher hair Ni compared to female dogs that ate raw or dry diets. Due to lack of previous research on hair Ni concentrations in dogs, these findings are difficult to explain and require further research.
Our study has some limitations. The small sample size may have interfered with identifying significant relationships from the data. In addition, to avoid too small sub-groups in statistical models, the number of factors that could be assessed was limited. One excluded factor was the dogs’ living environment, which according to our preliminary data inspection did not have a significant effect on hair and blood element concentrations. We find it more likely that living close to mining or industrialized areas may have affected hair and blood element concentrations in some dogs, and this should be assessed in future studies. We cannot exclude the risk for external contamination of hair samples. According to Chun et al. (2020), acetone-based washing procedures are unsuitable when measuring elements in dogs’ hair because they can alter hair element concentrations, and thus, we instead cleaned the hair with alcohol prior to cutting the hair sample. Furthermore, as we used recently grown hair cut closest to the dogs’ skin, the impact of different hair growth patterns in different breeds of dogs could have affected our results. Another limitation to our study was the broadness of the three diet categories. However, data regarding these broad diet categories may serve as a good base for future studies that may use more specific diets with known macronutrient and ingredient composition, including information regarding the geographical origin of the raw materials. This would further help address and explain the possible role that differing processing methods may have had on the study outcome, especially between raw versus dry food diets. Our study was also limited by the uncontrolled environment and heterogeneity of our study population. Historically studies like this would have been performed using laboratory dogs in a calory cage setting. However, these types of studies are outdated. Besides being unethical, they do not properly mirror the diet and environment of companion dogs and hence do not offer information regarding companion dogs’ exposure to major and trace elements in their natural habitat. Finally, we chose to use whole blood in our study, even though trace elements have previously been assessed in dogs’ serum (Vitale et al. 2019; Cedeño et al. 2020). There are several reasons for this. First, when studying the correlation between hair and blood Hg concentrations, we wanted to be able to compare our results with those by Sousa et al. (2013), who used hair and whole blood to measure Hg in dogs. Second, our study included Pb, which is, according to existing literature, measured from whole blood in both humans (Iqbal et al. 2009) and dogs (Wismer 2013). Third, in the case of certain elements, such as Se, serum is considered to reflect a more short-term dietary intake, whereas whole blood reflects a more long-term intake (Thomson 2004), which we considered more suitable for our study aim. Finally, by using whole blood we minimized the risk of hemolysis of red blood cells interfering with results of intracellular trace elements such as Mn and Fe (Laur et al. 2020).
Nutritional status have been considered a fifth vital sign that should be assessed alongside temperature, pulse, respiration, and pain in the standard physical examination for small animals (Freeman et al. 2011). In this study we presented basic mean values for hair and blood element concentrations in healthy dogs. We further reinforced the evidence that hair can be used as a surrogate for blood in monitoring dogs’ exposure to Hg and Pb. Based on our results, we could also conclude that when compared to dry diets, raw diets do not seem to be associated with lower major or trace element concentrations in dogs, except for in the case of Mn. These findings should be considered in the future, since the recommended allowances for dogs are made mainly for the dry food industry. Since our study dogs were considered healthy, there is no reason to suspect nutrient deficiencies in any of them. Further research needs be conducted to study if there should be different kinds of requirement limits for nutrients in different feeding types. Our findings also suggest that wild game should not be fed frequently to dogs, due to risk for elevated blood Pb concentrations. The significance of lower hair Se and Zn status in dry and mixed diet fed dogs and lower hair Mn status in raw diet fed dogs to dogs’ health requires further research. Finally, we concluded that hair color, age, and sex may affect some hair and blood element concentrations, and therefore these factors need to be considered in future studies. For example, when measuring hair Ca and Mg concentrations in a dog with multiple colors in its coat, we suggest mixing hair of different colors to get a more accurate element status for that dog. Given the scarcity of data on hair and blood element concentrations in dogs, our results are difficult to interpret, and future studies with larger sample size are required to validate our results.
Supplementary Information
(PDF 39 kb)
(PDF 156 kb)
(PDF 137 kb)
Acknowledgements
The funders of the study were Victoriastiftelsen, the Swedish Cultural Foundation in Finland, and Svensk-Österbottniska Samfundet. Their support is gratefully acknowledged. We would also like to express our gratitude to Emilia Brännback, as well as Merja Ranta and Lilja Jääskeläinen at the Central Laboratory of the Department of Equine and Small Animal Medicine, for their assistance in hair and blood sample collection and analyses. Finally, we thank Ingvar Bergdahl for fruitful discussions about the toxic metal lead.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Sarah Rosendahl, Johanna Anturaniemi, Kristiina A. Vuori, and Anna Hielm-Björkman. The first draft of the manuscript was written by Sarah Rosendahl and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. The research was supported by grants from Victoriastiftelsen, the Swedish Cultural Foundation in Finland, and Svensk-Österbottniska Samfundet.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study protocol was approved by the Animal Experiment Board in Finland (ELLA) (permit number: ESAVI/452/2020).
Consent to participate
Written informed consent was obtained from the dog-owners.
Consent for publication
Dog-owners signed informed consent regarding publishing their dogs’ data.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/13/2022
Missing Open Access funding information has been added in the Funding Note.
References
- Abd-Elhakim YM, El Sharkawy NI, Moustafa GG. An investigation of selected chemical contaminants in commercial pet foods in Egypt. J Vet Diagn Invest. 2016;28:70–75. doi: 10.1177/1040638715624733. [DOI] [PubMed] [Google Scholar]
- Abduljaleel SA, Shuhaiml-Othman M, Babji A. Assessment of trace metals contents in chicken (Gallus gallus domesticus) and quail (Coturnix coturnix japonica) tissues from selangor (Malaysia) J Environ Sci Technol. 2012;5:441–451. doi: 10.3923/jest.2012.441.451. [DOI] [Google Scholar]
- Ahmad G, Kuhi HD, Mohit A. A review Hair tissue analysis: an analytical method for determining essential elements, toxic elements, hormones and drug use and abuse. Intl Res J Appl Basic Sci. 2013;4:3675–3688. [Google Scholar]
- Åkesson A, Barregard L, Bergdahl IA, et al. Non-renal effects and the risk assessment of environmental cadmium exposure. Environ Health Perspect. 2014;122:431–438. doi: 10.1289/ehp.1307110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anturaniemi J, Zaldívar-López S, Moore R, et al. The effect of a raw vs dry diet on serum biochemical, hematologic, blood iron, B12, and folate levels in Staffordshire Bull Terriers. Vet Clin Pathol. 2020;49:258–269. doi: 10.1111/vcp.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer LC, Grindem CB, Corbett WT, et al. Pet dogs as sentinels for environmental contamination. Sci Total Environ. 2001;274:161–169. doi: 10.1016/S0048-9697(01)00740-9. [DOI] [PubMed] [Google Scholar]
- Bahovschi V, Zamboni CB, Silva LFFL, et al. Differences in iron concentration in whole blood of animal models using NAA. J Phys Conf Ser. 2015;630:012004. doi: 10.1088/1742-6596/630/1/012004. [DOI] [Google Scholar]
- Baldwin M, Mergler D, Larribe F, et al. Bioindicator and exposure data for a population based study of manganese. Neurotoxicology. 1999;20:343–354. [PubMed] [Google Scholar]
- Brummer-Holder M, Cassill BD, Hayes SH. Interrelationships between age and trace element concentration in horse mane hair and whole blood. J Equine Vet Sci. 2020;87:102922. doi: 10.1016/j.jevs.2020.102922. [DOI] [PubMed] [Google Scholar]
- Cedeño Y, Miranda M, Orjales I, et al. Serum concentrations of essential trace and toxic elements in healthy and disease-affected dogs. Animals. 2020;10:1–13. doi: 10.3390/ani10061052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulopoulos G, Roubies N, Karatzias H, Papasteriadis A. Selenium concentration in blood and hair of holstein dairy cows. Biol Trace Elem Res. 2003;91:145–150. doi: 10.1385/BTER:91:2:145. [DOI] [PubMed] [Google Scholar]
- Chun JL, Bang HT, Ji SY, et al. Comparison of sample preparation procedures of inductively coupled plasma to measure elements in dog’s hair. J Anim Sci Technol. 2020;62:58–63. doi: 10.5187/JAST.2020.62.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyla MA, Zyrnicki W. Determination of metal concentrations in animal hair by the ICP method: Comparison of various washing procedures. Biol Trace Elem Res. 2000;75:187–194. doi: 10.1385/BTER:75:1-3:187. [DOI] [PubMed] [Google Scholar]
- Clark NA, Teschke K, Rideout K, Copes R. Trace element levels in adults from the west coast of Canada and associations with age, gender, diet, activities, and levels of other trace elements. Chemosphere. 2007;70:155–164. doi: 10.1016/j.chemosphere.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Clegg MS, Lonnerdal B, Hurley LS, Keen CL. Analysis of whole blood manganese by flameless atomic absorption spectrophotometry and its use as an indicator of manganese status in animals. Anal Biochem. 1986;157:12–18. doi: 10.1016/0003-2697(86)90189-2. [DOI] [PubMed] [Google Scholar]
- Cummings JE, Kovacic JP. The ubiquitous role of zinc in health and disease. J Vet Emerg Crit Care. 2009;19:215–240. doi: 10.1111/j.1476-4431.2009.00418.x. [DOI] [PubMed] [Google Scholar]
- D’Souza HS, Menezes G, Venkatesh T. Role of essential trace minerals on the absorption of heavy metals with special reference to lead. Indian J Clin Biochem. 2003;18:154–160. doi: 10.1007/BF02867382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M, Alborough R, Jones L, et al. Mineral analysis of complete dog and cat foods in the UK and compliance with European guidelines. Sci Rep. 2017;7:17107. doi: 10.1038/s41598-017-17159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M, West J, Williams C, Gardner DS. Mineral status in canine medial coronoid process disease: A cohort study using analysis of hair by mass spectrometry. Vet Rec. 2017;180:448. doi: 10.1136/vr.103953. [DOI] [PubMed] [Google Scholar]
- Davis TZ, Stegelmeier BL, Hall JO. Analysis in horse hair as a means of evaluating selenium toxicoses and long-term exposures. J Agric Food Chem. 2014;62:7393–7397. doi: 10.1021/jf500861p. [DOI] [PubMed] [Google Scholar]
- De Nadai Fernandes EA, Elias C, Bacchi MA, Bode P. Trace element measurement for assessment of dog food safety. Environ Sci Pollut Res Int. 2018;25:2045–2050. doi: 10.1007/s11356-017-8541-4. [DOI] [PubMed] [Google Scholar]
- Dijcker JC, Hagen-Plantinga EA, Everts H, et al. Dietary and animal-related factors associated with the rate of urinary oxalate and calcium excretion in dogs and cats. Vet Rec. 2012;171:46. doi: 10.1136/vr.100293. [DOI] [PubMed] [Google Scholar]
- Dillitzer N, Becker N, Kienzle E. Intake of minerals, trace elements and vitamins in bone and raw food rations in adult dogs. Br J Nutr. 2011;106:S53–S56. doi: 10.1017/S0007114511002765. [DOI] [PubMed] [Google Scholar]
- Duran A, Tuzen M, Soylak M. Trace element concentrations of some pet foods commercially available in Turkey. Food Chem Toxicol. 2010;48:2833–2837. doi: 10.1016/j.fct.2010.07.014. [DOI] [PubMed] [Google Scholar]
- European commission Comission Regulation (EU) No 1275/2013 of 6 December 2013 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for arsenic, cadmium, lead, nitrites, volatile mustard oil and harmful botanical imp. Off J Eur Union. 2013;328:86–92. [Google Scholar]
- European Food Safety Authority (2012) Cadmium dietary exposure in the European population. EFSA J 10:2551 [DOI] [PMC free article] [PubMed]
- Ferreira MDF, Aylor AEA, Mellanby RJ, et al. Investigation of manganese homeostasis in dogs with anaemia and chronic enteropathy. Open Vet J. 2017;7:360–366. doi: 10.4314/ovj.v7i4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MR. Assessment of cadmium, lead and vanadium status of large animals as related to the human food chain. J Anim Sci. 1987;65:1744–1752. doi: 10.2527/jas1987.6561744x. [DOI] [PubMed] [Google Scholar]
- Freeman L, Becvarova I, Cave N, et al. WSAVA Nutritional assessment guidelines. J Small Anim Pract. 2011;52:385–396. doi: 10.1111/j.1748-5827.2011.01079.x. [DOI] [PubMed] [Google Scholar]
- Fustinoni S, Sucato S, Consonni D, et al. Blood lead levels following consumption of game meat in Italy. Environ Res. 2017;155:36–41. doi: 10.1016/j.envres.2017.01.041. [DOI] [PubMed] [Google Scholar]
- Gagne JW, Wakshlag JJ, Center SA, et al. Evaluation of calcium, phosphorus, and selected trace mineral status in commercially available dry foods formulated for dogs. J Am Vet Med Assoc. 2013;243:658–666. doi: 10.2460/javma.243.5.658. [DOI] [PubMed] [Google Scholar]
- Genchi G, Sinicropi MS, Lauria G, et al. The effects of cadmium toxicity. Int J Environ Res Public Health. 2020;17:3782. doi: 10.3390/IJERPH17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani A, Mohit A, Darmani Kuhi H. Effects of dietary mineral intake on hair and serum mineral contents of horses. J Equine Vet Sci. 2015;35:295–300. doi: 10.1016/j.jevs.2015.01.018. [DOI] [Google Scholar]
- Górski K, Kondracki S, Saba L. Selenium concentration in soil, and in the feed and hair coat of Polish Holstein-Friesian cows administered a mineral mixture. Indian J Anim Sci. 2018;88:1207–1210. [Google Scholar]
- Gow AG, Marques AI, Yool DA, et al. Whole blood manganese concentrations in dogs with congenital portosystemic shunts. J Vet Intern Med. 2010;24:90–96. doi: 10.1111/j.1939-1676.2009.0408.x. [DOI] [PubMed] [Google Scholar]
- Grace ND. Amounts and distribution of mineral elements associated with fleece-free empty body weight gains in the grazing sheep. New Zeal J Agric Res. 1983;26:59–70. doi: 10.1080/00288233.1983.10420952. [DOI] [Google Scholar]
- Grace ND, Castillo-Alcala F, Wilson PR. Amounts and distribution of mineral elements associated with liveweight gains of grazing red deer (Cervus elaphus) New Zeal J Agric Res. 2008;51:439–449. doi: 10.1080/00288230809510473. [DOI] [Google Scholar]
- Green RE, Pain DJ. Risks to human health from ammunition-derived lead in Europe. Ambio. 2019;48:954–968. doi: 10.1007/s13280-019-01194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregório BJR, Pereira AM, Fernandes SR, et al. Flow-based dynamic approach to assess bioaccessible zinc in dry dog food samples. Molecules. 2020;25:1333. doi: 10.3390/molecules25061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høgåsen HR, Ørnsrud R, Knutsen HK, Bernhoft A. Lead intoxication in dogs: risk assessment of feeding dogs trimmings of lead-shot game. BMC Vet Res. 2016;12:1–8. doi: 10.1186/S12917-016-0771-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Blumenthal W, Kennedy C, et al. Hunting with lead: association between blood lead levels and wild game consumption. Environ Res. 2009;109:952–959. doi: 10.1016/j.envres.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Jenkins DW (1979) Toxic trace metals in mammalian hair and nails. EPA/600/4-79/049 (NTIS PB80103997)
- Jeruszka-Bielak M, Brzozowska A. Relationship between nutritional habits and hair calcium levels in young women. Biol Trace Elem Res. 2011;144:63–76. doi: 10.1007/s12011-011-9030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazimierska K, Biel W, Witkowicz R. Mineral composition of cereal and cereal-free dry dog foods versus nutritional guidelines. Molecules. 2020;25:5173. doi: 10.3390/molecules25215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-T, Loftus JP, Mann S, Wakshlag JJ. Evaluation of arsenic, cadmium, lead and mercury contamination in over-the-counter available dry dog foods with different animal ingredients (red meat, poultry, and fish) Front Vet Sci. 2018;5:264. doi: 10.3389/fvets.2018.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Lee JY, Yang HR. Nutrient intakes and hair mineral contents of young children. Pediatr Gastroenterol Hepatol Nutr. 2016;19:123–129. doi: 10.5223/pghn.2016.19.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Mahan DC. Effect of dietary selenium source, level, and pig hair color on various selenium indices. J Anim Sci. 2001;79:949–955. doi: 10.2527/2001.794949x. [DOI] [PubMed] [Google Scholar]
- Langlois DK, Kaneene JB, Yuzbasiyan-Gurkan V, et al. Investigation of blood lead concentrations in dogs living in flint, Michigan. J Am Vet Med Assoc. 2017;251:912–921. doi: 10.2460/javma.251.8.912. [DOI] [PubMed] [Google Scholar]
- Laur N, Kinscherf R, Pomytkin K, et al. ICP-MS trace element analysis in serum and whole blood. PLoS One. 2020;15:e0233357. doi: 10.1371/JOURNAL.PONE.0233357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske CL, Moses SK, Castellini JM, et al. Toxicokinetics of mercury in blood compartments and hair of fish-fed sled dogs. Acta Vet Scand. 2011;53:66. doi: 10.1186/1751-0147-53-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xu H, Zhong W, et al. Organic selenium alleviated the formation of ethylene glycol-induced calcium oxalate renal calculi by improving Osteopontin expression and antioxidant capability in dogs. Biol Trace Elem Res. 2015;168:392–400. doi: 10.1007/s12011-015-0373-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Alonso M, Miranda M, Garcia-Partida P et al (2007) Use of dogs as indicators of metal exposure in rural and urban habitats in NW Spain. Sci Total Environ 372:668–675 [DOI] [PubMed]
- Lovásová E, Rácz O, Cimboláková I, et al. Effects of chronic low-dose cadmium exposure on selected biochemical and antioxidant parameters in rats. J Toxicol Environ Health A. 2013;76:1033–1038. doi: 10.1080/15287394.2013.828249. [DOI] [PubMed] [Google Scholar]
- Lowe JA, Wiseman J, Cole DJA. Zinc source influences zinc retention in hair and hair growth in the dog. J Nutr. 1994;124:2575S–2576S. doi: 10.1093/jn/124.suppl_12.2575S. [DOI] [PubMed] [Google Scholar]
- Martins AC, Krum BN, Queirós L, et al. Manganese in the diet: bioaccessibility, adequate intake, and neurotoxicological effects. J Agric Food Chem. 2020;68:12893–12903. doi: 10.1021/acs.jafc.0c00641. [DOI] [PubMed] [Google Scholar]
- Moreda-Piñeiro J, Moreda-Piñeiro A, Bermejo-Barrera P. In vivo and in vitro testing for selenium and selenium compounds bioavailability assessment in foodstuff. Crit Rev Food Sci Nutr. 2017;57:805–833. doi: 10.1080/10408398.2014.934437. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Dogs and Cats. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- National Research Council . Mineral Tolerance of Animals: Second Revised Edition. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- Panda D, Patra RC, Nandi S, Swarup D. Oxidative stress indices in gastroenteritis in dogs with canine parvoviral infection. Res Vet Sci. 2009;86:36–42. doi: 10.1016/j.rvsc.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Lee MH, Kim SK. Studies on Cd, Pb, Hg and Cr values in dog hairs from urban Korea. Asian-Australasian J Anim Sci. 2005;18:1135–1140. doi: 10.5713/ajas.2005.1135. [DOI] [Google Scholar]
- Patra RC, Swarup D, Naresh R, et al. Tail hair as an indicator of environmental exposure of cows to lead and cadmium in different industrial areas. Ecotoxicol Environ Saf. 2007;66:127–131. doi: 10.1016/j.ecoenv.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Paulelli ACC, Martins AC, de Paula ES, et al. Risk assessment of 22 chemical elements in dry and canned pet foods. J Consum Prot Food Saf. 2018;13:359–365. doi: 10.1007/s00003-018-1178-5. [DOI] [Google Scholar]
- Pedrinelli V, Gomes MDOS, Carciofi AC. Analysis of recipes of home-prepared diets for dogs and cats published in Portuguese. J Nutr Sci. 2017;6:1–5. doi: 10.1017/jns.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrinelli V, Zafalon RVA, Rodrigues RBA, et al. Concentrations of macronutrients, minerals and heavy metals in home-prepared diets for adult dogs and cats. Sci Rep. 2019;9:13058. doi: 10.1038/s41598-019-49087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AM, Pinto E, Matos E, et al. Mineral composition of dry dog foods: impact on nutrition and potential toxicity. J Agric Food Chem. 2018;66:7822–7830. doi: 10.1021/acs.jafc.8b02552. [DOI] [PubMed] [Google Scholar]
- Perry TW, Beeson WM, Smith WH, Mohler MT. Effect of supplemental selenium of performance and deposit of selenium in blood and hair of finishing beef cattle. J Anim Sci. 1976;42:192–195. doi: 10.2527/jas1976.421192x. [DOI] [PubMed] [Google Scholar]
- Pietinen P, Männistö S, Valsta LM, Sarlio-Lähteenkorva S. Nutrition policy in Finland. Public Health Nutr. 2010;13:901–906. doi: 10.1017/S1368980010001072. [DOI] [PubMed] [Google Scholar]
- Puertollano MA, Puertollano E, Alvarez de Cienfuegos G, de Pablo MA. Dietary antioxidants: immunity and host defense. Curr Top Med Chem. 2011;11:1752–1766. doi: 10.2174/156802611796235107. [DOI] [PubMed] [Google Scholar]
- Rosendahl S, Anturaniemi J, Hielm-Bjorkman A. Hair arsenic level in rice-based diet-fed Staffordshire bull terriers. Vet Rec. 2020;186:e15. doi: 10.1136/vr.105493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom B, Arvidsson B, Cederblad A, Bjorn-Rasmussen E. Zinc absorption from composite meals. I. The significance of wheat extraction rate, zinc, calcium, and protein content in meals based on bread. Am J Clin Nutr. 1980;33:739–745. doi: 10.1093/ajcn/33.4.739. [DOI] [PubMed] [Google Scholar]
- Sandstrom B, Cederblad A. Zinc absorption from composite meals. II. Influence of the main protein source. Am J Clin Nutr. 1980;33:1778–1783. doi: 10.1093/ajcn/33.8.1778. [DOI] [PubMed] [Google Scholar]
- Sanna E, Liguori A, Palmas L, et al. Blood and hair lead levels in boys and girls living in two Sardinian towns at different risks of lead pollution. Ecotoxicol Environ Saf. 2003;55:293–299. doi: 10.1016/S0147-6513(02)00072-6. [DOI] [PubMed] [Google Scholar]
- Santhosh Kumar M, Selvam R. Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. J Nutr Biochem. 2003;14:306–313. doi: 10.1016/S0955-2863(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Sgorlon S, Mattiello A, Ronutti L, et al. Concentration of elements in the hair of growing and adult dogs. Ital J Anim Sci. 2019;18:1126–1134. doi: 10.1080/1828051X.2019.1621687. [DOI] [Google Scholar]
- So KM, Lee Y, Bok JD, et al. Analysis of ionomic profiles of canine hairs exposed to lipopolysaccharide (LPS)-induced stress. Biol Trace Elem Res. 2016;172:364–371. doi: 10.1007/s12011-015-0611-1. [DOI] [PubMed] [Google Scholar]
- Son AR, Jeong J, Park KR, et al. Effects of graded concentrations of supplemental selenium on selenium concentrations in tissues and prediction equations for estimating dietary selenium intake in pigs. PeerJ. 2018;6:e5791. doi: 10.7717/PEERJ.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AC, Teixeira IS, Marques B, et al. Mercury, pets’ and hair: baseline survey of a priority environmental pollutant using a noninvasive matrix in man’s best friend. Ecotoxicology. 2013;22:1435–1442. doi: 10.1007/s10646-013-1130-5. [DOI] [PubMed] [Google Scholar]
- Tamburo E, Varrica D, Dongarrà G. Gender as a key factor in trace metal and metalloid content of human scalp hair. A multi-site study. Sci Total Environ. 2016;573:996–1002. doi: 10.1016/j.scitotenv.2016.08.178. [DOI] [PubMed] [Google Scholar]
- Tegzes JH. Chapter 57 - Mercury. In: Peterson ME, Talcott PA, editors. Small Animal Toxicology. 3. Saint Louis: W.B. Saunders; 2013. pp. 629–634. [Google Scholar]
- Thomson CD. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr. 2004;58:391–402. doi: 10.1038/sj.ejcn.1601800. [DOI] [PubMed] [Google Scholar]
- Tomza-Marciniak A, Pilarczyk B, Bakowska M, et al. Lead, cadmium and other metals in serum of pet dogs from an urban area of NW Poland. Biol Trace Elem Res. 2012;149:345–351. doi: 10.1007/s12011-012-9433-6. [DOI] [PubMed] [Google Scholar]
- Trevizan L, Fischer MM, Rodenbusch CR, et al. Effects of diets containing organic and inorganic zinc sources on hair characteristics, zinc concentration in blood and hair, and the immune response of dogs. Acta Sci Vet. 2013;41:1154. [Google Scholar]
- Tsai YY, Wang CT, Chang WT, et al. Concentrations of potassium, sodium, magnesium, calcium, copper, zinc, manganese and iron in black and gray hairs in Taiwan. J Heal Sci. 2000;46:46–48. doi: 10.1248/jhs.46.46. [DOI] [Google Scholar]
- UNICEF (2020) The toxic truth. In: UNICEF. https://www.unicef.org/reports/toxic-truth-childrens-exposure-to-lead-pollution-2020. Accessed 26 Feb 2021
- Van Zelst M, Hesta M, Alexander LG, et al. In vitro selenium accessibility in pet foods is affected by diet composition and type. Br J Nutr. 2015;133:1888–1894. doi: 10.1017/S0007114515001324. [DOI] [PubMed] [Google Scholar]
- Vitale S, Hague DW, Foss K, et al. Comparison of serum trace nutrient concentrations in epileptics compared to healthy dogs. Front Vet Sci. 2019 doi: 10.3389/fvets.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviano KR, Vanderwielen B. Effect of n-acetylcysteine supplementation on intracellular glutathione, urine isoprostanes, clinical score, and survival in hospitalized ill dogs. J Vet Intern Med. 2013;27:250–258. doi: 10.1111/jvim.12048. [DOI] [PubMed] [Google Scholar]
- Wapnir RA. Zinc deficiency, malnutrition and the gastrointestinal tract. J Nutr. 2000;130:1388S–1392S. doi: 10.1093/jn/130.5.1388s. [DOI] [PubMed] [Google Scholar]
- Wismer T. Chapter 53 - Lead. In: Peterson ME, Talcott PA, editors. Small animal toxicology. 3. Saint Louis: W.B. Saunders; 2013. pp. 609–615. [Google Scholar]
- Zhu Y, Wang Y, Meng F, et al. Distribution of metal and metalloid elements in human scalp hair in Taiyuan, China. Ecotoxicol Environ Saf. 2018;148:538–545. doi: 10.1016/j.ecoenv.2017.10.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 39 kb)
(PDF 156 kb)
(PDF 137 kb)
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.