Abstract
To investigate the significance of serological human T-cell lymphotropic virus type 1 (HLTV-1) Gag indeterminate Western blot (WB) patterns in the Caribbean, a 6-year (1993 to 1998) cross-sectional study was conducted with 37,724 blood donors from Guadeloupe (French West Indies), whose sera were routinely screened by enzyme immunoassay (EIA) for the presence of HTLV-1 and -2 antibodies. By using stringent WB criteria, 77 donors (0.20%) were confirmed HTLV-1 seropositive, whereas 150 (0.40%; P < 0.001) were considered HTLV seroindeterminate. Among them, 41.3% (62) exhibited a typical HTLV-1 Gag indeterminate profile (HGIP). Furthermore 76 (50.7%) out of the 150 HTLV-seroindeterminate subjects were sequentially retested, with a mean duration of follow-up of 18.3 months (range, 1 to 70 months). Of these, 55 (72.4%) were still EIA positive and maintained the same WB profile whereas the others became EIA negative. This follow-up survey included 33 persons with an HGIP. Twenty-three of them (69.7%) had profiles that did not evolve over time. Moreover, no case of HTLV-1 seroconversion could be documented over time by studying such sequential samples. HTLV-1 seroprevalence was characterized by an age-dependent curve, a uniform excess in females, a significant relation with hepatitis B core (HBc) antibodies, and a microcluster distribution along the Atlantic coast of Guadeloupe. In contrast, the persons with an HGIP were significantly younger, had a 1:1 sex ratio, did not present any association with HBc antibodies, and were not clustered along the Atlantic façade. These divergent epidemiological features, together with discordant serological screening test results for subjects with HGIP and with the lack of HTLV-1 proviral sequences detected by PCR in their peripheral blood mononuclear cell DNA, strongly suggest that an HGIP does not reflect true HTLV-1 infection. In regard to these data, healthy blood donors with HGIP should be reassured that they are unlikely to be infected with HTLV-1 or HTLV-2.
Human T-cell lymphotropic virus type 1 (HTLV-1) (27, 33) has been etiologically associated with both adult T-cell leukemia (43) and tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) (14). This retrovirus has a worldwide distribution (27) with foci of endemicity in the Caribbean (6, 12, 29, 30, 35, 36, 46), southeastern Japan (48), sub-Saharan Africa (11, 13, 26, 28), and areas of South America (37, 38) and the Middle East. HTLV-1 is transmitted between sexual partners and also from mother to child (mainly through prolonged breast feeding) and via blood (transfusion or needle sharing) (27, 48). Posttransfusional TSP/HAM cases seem to be more severe and to evolve faster than nonposttransfusional ones (27, 41, 48). Therefore, public health authorities of many countries have implemented routine screening for antibodies to HTLV-1 and -2 in blood banks (4, 5, 6, 8, 9, 10, 18, 22, 29, 32, 35, 36, 37, 38, 46, 48; S. L. Stramer, J. P. Brodsky, J. Trenbeath, L. Taylor, B. Peoples, and R. Y. Dodd, Abstr. 52nd Annu. Meet. Am. Assoc. Blood Banks, abstr. S483, 1999). This is the case in the French overseas territories including the West Indian island of Guadeloupe (an area where HTLV-1 is endemic [35, 36]), where blood bank screening for HTLV-1 and -2 became mandatory in January 1989 (8).
There are several diagnostic methods for the detection of HTLV-1 and -2 antibodies, including enzyme immunoassays (EIAs), the particle agglutination assay (PAA), immunofluorescence assays, Western blotting (WB), and the radioimmunoprecipitation assay (3, 4, 7, 10, 21, 24, 37, 45; Stramer et al., Abstr. 52nd Annu. Meet. Am. Assoc. Blood Banks). Repeatedly reactive samples are further tested by WB. Stringent HTLV WB criteria require that an HTLV-1-infected individual have an antibody response to the complete range of the core bands (p19, p24, and pr53), in addition to the respective recombinant glycoprotein (gd21) and to type-specific synthetic peptide MTA-1 (HTLV-1). However, especially in tropical areas, indeterminate HTLV serologic test results (i.e., WB patterns reactive to only part of the viral proteins) appear commonly, leading to difficulties in interpretation and counseling (2, 6, 11, 12, 13, 15, 16, 18, 19, 20, 23, 26, 28, 31, 37, 38, 44). Previous epidemiological studies, particularly in Cameroon (central Africa), have reported that indeterminate WB patterns (notably those exhibiting p19, p26, p28, p32, p36, and pr53, which have been termed the HTLV-1 Gag indeterminate profile [HGIP]) were not associated with true HTLV-1 infection (26, 28).
The main purposes of the present cross-sectional study, conducted among healthy blood donors from Guadeloupe, a tropical area of endemicity for HTLV-1, were (i) to assess the overall HTLV-indeterminate WB (and more specifically HGIP) seroprevalence and its temporal trend during a 6-year survey, (ii) to compare the main epidemiological determinants of HTLV-1-infected subjects (age, relationship of sex to positivity for hepatitis B core (HBc) antibodies, geographical origin) with those of the individuals exhibiting an HTLV-1-indeterminate WB, and (iii) to search for the presence of HTLV-1 in the peripheral blood mononuclear cell (PBMC) DNA of blood donors with an HGIP by WB by using PCR.
MATERIALS AND METHODS
Area.
Guadeloupe, an overseas French department of 1,705 km2, is located in the middle of the Lesser Antilles in the West Indies. The total population consists of 420,000 inhabitants, a large proportion (about 80%) being “black Creoles” with an African ancestry and a smaller proportion (about 15%) being “Indians” of Asian descent (Hindus).
Population studied.
From January 1993 to December 1998, 37,724 donors (48.1% male and 51.9% female) were recruited. All fulfilled the French criteria for blood donation: full consent, free donation (i.e., no financial incentives), and age 18 to 65 years. Seventy percent of the donors were between 18 and 39 years old. The total number of subjects was 34,525 when Guadeloupean blood donors who did not reside in Guadeloupe at the time of donation were not included.
HTLV serological assays and WB classification criteria.
All serum samples were screened for HTLV-1 and -2 using HTLV-1 whole-virus enzyme-linked immunosorbent assays (HTLV-1 1.0 and 2.0 EIA; Abbott, North Chicago, Ill.) according to the manufacturer's instructions. Samples were considered reactive if the optical density ratio was equal to or greater than 0.8 (grey zone of 20%). All specimens that were twice repeatedly reactive (RR) in EIA were further evaluated with confirmatory WB. Two different WB assays were performed during two studied periods. In the first period (January 1993 to December 1995), we used HTLV-1 WB version 2.3 (WB2.3; Genelabs Diagnostic Biotechnology, Singapore, Republic of Singapore). This kit contains viral lysates, recombinant protein r21e, derived from the transmembrane proteins of both HTLV-1 and HTLV-2 and type-specific synthetic peptides derived from the external glycoprotein of HTLV-1 (MTA-1 or rgp46-I) and HTLV-2 (K-55 or rgp46-II). However, as previously reported, this WB gives some false-positive results. A retrospective investigation was hence performed by retesting each frozen serum stored at −80°C and exhibiting an indeterminate WB2.3 profile with the WB2.4 (Genelabs Diagnostic Biotechnology). In the second period (January 1996 to December 1998), we used WB2.4 as the confirmatory test. Finally, when sufficient RR frozen sera were available, additional retrospective screening testing was performed with two assays: a new-generation EIA that uses recombinant proteins and selected peptides as HTLV antigens (EIA HTLV-1/II; Genelabs Diagnostic Biotechnology) and a viral-lysate-coated PAA (Serodia HTLV-1; Fujirebio, Tokyo, Japan).
Seropositivity was interpreted according to the stringent criteria issued by the HTLV European Research Network (40). A WB was scored as HTLV-1-positive only if bands for the gag proteins p19 and p24 and the env proteins gd21 and MTA-1 were present. It was scored as HTLV-2 positive if p24, gd21, and K-55 bands were identified and was HTLV positive but untypeable if p24, p19, and gd21 were observed. It was considered indeterminate if any other band patterns were present. Negative samples were those that did not exhibit any band.
Statistical analysis.
For the calculation of specificity, the number of true negatives was taken as the numerator whereas the total of true negatives plus false positives was taken as the denominator. True negatives were defined as samples that were Abbott EIA negative. False positives were defined as samples that were EIA positive (RR in Abbott EIA) and WB either negative or indeterminate. Variables including age, gender, HBc antibodies, and geographical origin were investigated and compared for HTLV-1-positive, HTLV-1-indeterminate, and HTLV-1-negative donors. Data were compared using χ2, trend χ2, and Fisher's exact tests; P values computed at the 0.05 level were reported as measures of statistical significance. All statistical analyses were performed using Epi Info (Centers for Disease Control and Prevention, Atlanta, Ga.), version 6.02b, software.
HTLV-1 molecular analysis.
DNA was extracted from PBMCs using a commercial DNA kit (QIAamp DNA blood minikit; Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. PCR was carried out as previously described (26). Briefly, each reaction tube contained 1 μg of DNA, 0.2 mM deoxynucleoside triphosphate mixture (Boehringer GmbH, Mannheim, Germany), 5 μl of 10× reaction buffer (Perkin-Elmer Cetus, Norwalk, Conn.), 0.25 μM (each) oligonucleotide primer (Pharmacia, Piscataway, N.J.), 2.5 mM MgCl2 (Perkin-Elmer Cetus), and 2.5 U of Taq DNA polymerase (Perkin-Elmer Cetus) in a total volume of 50 μl. The sequences of HTLV-1- and -2-specific primers and appropriate probes were as previously described (26). The primers and probes were as follows: pol region, primers SK110 and SK111 amplifying both HTLV-1 and HTLV-2 and probes SK112 for HTLV-1 and SK188 for HTLV-2; gag region, HTLV-1-specific primers GAG1 and GAG2 and probe GAGS (17); tax region, primers KKPX1 and KKPX2 amplifying both HTLV-1 and HTLV-2 and probes KKPXs (HTLV-1 specific) and SK45 (HTLV-1 and HTLV-2) (25). Housekeeping gene β-globin was studied to ensure that all extracted DNAs were amplifiable using primers PCO4 and GH20 (26).
HTLV-1 molecular analysis was performed for 43 subjects: 24 HTLV-1-seropositive healthy donors, 1 HTLV-1-seronegative donor, and 18 donors exhibiting an HTLV-1-indeterminate WB pattern.
RESULTS
HTLV-1 serological results.
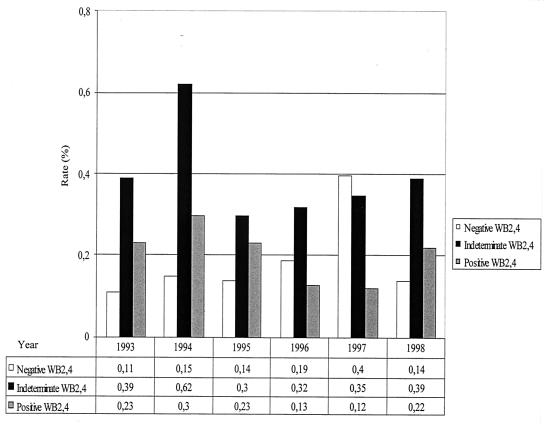
Out of the 37,724 enrolled blood donors, 297 (0.79%) were RR in Abbott EIA. Of these, 77 (25.9%) were HTLV-1 seropositive by WB2.4, yielding an overall 0.20% seroprevalence (95% confidence interval [CI], 0.16 to 0.26%), and 150 (50.5%) had an HTLV-1-seroindeterminate WB2.4 pattern. This leads to an overall 0.40% prevalence of HTLV-1-seroindeterminate donors (CI, 0.34 to 0.47%), significantly higher than that of HTLV-1-positive donors (P < 0.0001). Finally, 70 samples (23.6%) were WB2.4 seronegative. As shown in Fig. 1, the annual prevalence rates for HTLV-1-seroindeterminate results, obtained with WB2.4, were relatively stable during the studied period (except for a 0.62% peak, of unknown origin, in 1994). They were also steadily and significantly higher than the HTLV-1-positive rates, especially for 1994, 1996, and 1997 (P = 0.005, 0.02, and 0.008, respectively).
FIG. 1.
Temporal trends of HTLV-1-positive, -indeterminate, and -negative results obtained from WB2.4 among Guadeloupean blood donors from 1993 to 1998.
As shown in Table 1 and Fig. 2, careful examination of the 150 HTLV-indeterminate WB2.4 profiles allowed the identification of several different profile categories. The HGIP, exhibiting reactivities to p19, p26, p28, p32, p36, and pr53, but lacking both p24 and Env bands, was the most frequent (62 of 150 or 41.3%). Other indeterminate WB2.4 profiles were identified, including those with isolated bands such as p24 (n = 40), p19 (n = 12), or gd21 (n = 10). Furthermore, 20 specimens displayed reactivity to only one gag protein (p19 or p24) plus one env-encoded glycoprotein (i.e., gd21), either associated or not with MTA-1. Among these 20 specimens, the most common WB profile (n = 14) exhibited reactivities to gd21 and p19 associated with faint p26, p28, p32, p36, pr53 bands but lacking both p24 and MTA-1 reactivities. This WB profile was closely related to HGIP, except for the presence of the gd21 reactivity.
TABLE 1.
Indeterminate HTLV-1 WB2.4 profiles in samples from Guadeloupean blood donors from 1993 to 1998
| WB pattern | n | Frequency (%) | Results of supplementary serologic tests (no. positive/no. tested [%])
|
|
|---|---|---|---|---|
| PAA | Recombinant EIA | |||
| p19 + p26 + p28 + p32 + p36 + pr53 (HGIP) | 62 | 41.3 | 5/55 (9.1) | 6/55 (10.9) |
| p24 alone | 40 | 26.7 | 0/36 (0) | 4/36 (11.1) |
| gd21 + p19a | 14 | 9.3 | 4/10 (40.0) | 2/10 (20.0) |
| p19 alone | 12 | 8.0 | 2/10 (20.0) | 1/10 (10.0) |
| gd21 alone | 10 | 6.7 | 0/8 (0.0) | 2/8 (25.0) |
| gd21 + p24 | 3 | 2.0 | 0/3 (0.0) | 1/3 (33.3) |
| gd21 + p19 + MTA-1 | 3 | 2.0 | 1/1 (100.0) | 1/1 (100.0) |
| p24 + p28 | 2 | 1.3 | 0/2 (0.0) | 1/2 (50.0) |
| Othersb | 4 | 2.7 | 0/4 (0.0) | 1/4 (25.0) |
| Total | 150 | 12/129 (9.3) | 19/129 (14.7) | |
gd21 and p19 are associated with faint p26, p28, p32, p36, and pr53 bands.
One each for K-55 alone, p24 plus pr53, p28 plus pr53, and p24 plus p28 plus pr53.
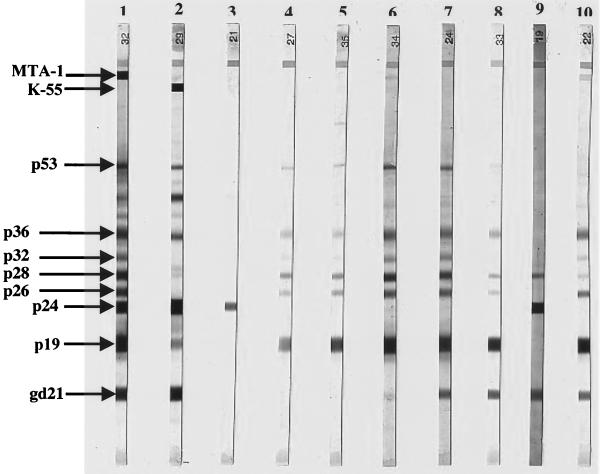
FIG. 2.
WB analysis using WB2.4 from Genelabs Diagnostic Biotechnology. Lane 1, HTLV-1-positive control; lane 2, HTLV-2-positive control; lane 3, serum from one Guadeloupean blood donor with an isolated p24 band; lanes 4 and 5, sera from two Guadeloupean blood donors with an HGIP; lane 6, serum from one Guadeloupean blood donor exhibiting gd21 (weak), p19, p26, p28, p32, p36, and pr53 bands but without reactivities to both p24 and env-encoded glycoprotein MTA-1; lanes 7 and 8, sera from three Guadeloupean blood donors exhibiting gd21, p19, p26, p28, p32, p36, and pr53 bands but without reactivities to both p24 and MTA-1; lane 9, serum from one Guadeloupean blood donor exhibiting reactivities to gd21, p24, and p28, but without reactivities to both p19 and MTA-1; lane 10, serum from one Guadeloupean blood donor with a nearly complete WB profile but lacking p24 reactivity. This subject was positive in PCR.
Among the 150 HTLV-indeterminate WB sera, 129 could be retested by complementary tests and the majority were scored negative when screened by PAA or recombinant EIA, leading an enhanced overall specificity of 90.7 or 85.3%, respectively (Table 1).
Considering the first 3-year screening period (1993 to 1995), the use of WB2.3 and WB2.4 gave similar percentages of indeterminate results, 0.47% (93 of 19,797) for WB2.3 and 0.43% (86 of 19,797; P = 0.60) for WB2.4, with strictly identical values for overall specificity (99.4%). HGIP again was the most-often-encountered pattern (39 of 93 or 41.9% with WB2.3) (data not shown).
Among the 150 HTLV-seroindeterminate subjects, 76 (50.7%) were sequentially retested, for a mean duration of follow-up of 18.3 months (range, 1 to 70 months). Of these, 55 (72.4%) maintained the same WB profile whereas the others became EIA negative. This follow-up survey included 33 HGIP persons, of whom 23 (69.7%) had a profile that did not evolve during time. Moreover, no case of HTLV-1 seroconversion over time could be documented with such sequential samples.
Comparative epidemiological features of HTLV-1-seropositive, HGIP, and HTLV-1-seronegative donors.
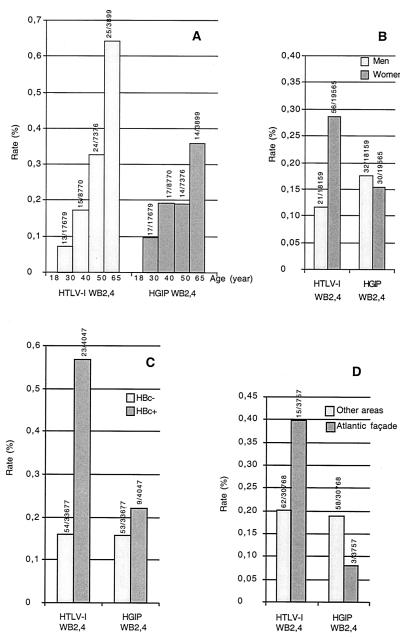
HGIP was chosen because it represented the most common and homogenous indeterminate WB profile. According to the epidemiological determinants, striking differences between HTLV-1-positive persons and those having an HGIP were detected. HTLV-1-positive blood donors showed increasing seropositivity rates with age (trend P < 0.0001) and were significantly older (≥40 years) than HGIP donors (P = 0.03) (Fig. 3A). Moreover, HTLV-1 seroprevalence was overrepresented among females (0.29% versus 0.12% for males; P < 0.001), significantly differing in this respect from HGIP seroprevalence (P = 0.003) (Fig. 3B). Indeed, HGIP subjects were equally balanced between males (0.18%) and females (0.15%), similar to HTLV-seronegative donors (P = 0.58) (Fig. 3B). Furthermore, HTLV-1-seropositive donors were significantly more likely to be positive (0.57%) than negative (0.16%) (P < 0.001) for HBc antibodies. This significantly differentiated them from HGIP donors (P = 0.03) (Fig. 3C). By contrast, the percentages of HGIP persons positive (0.22%) and negative (0.16%) for HBc antibodies were similar to those for HTLV-seronegative donors (P = 0.33) (Fig. 3C). Finally, the HTLV-1 seroprevalence was clearly greater along the Atlantic façade of Guadeloupe (0.40%), an area of microendemicity, than in other areas (0.20%) (P = 0.016), which was not the case for HGIP persons (P = 0.01) (Fig. 3D). By contrast, no significant difference in this geographic determinant between HGIP and HTLV-1-seronegative donors (0.08% for Atlantic façade versus 0.19% for other areas; P = 0.13) could be detected.
FIG. 3.
(A) Seroprevalences of HTLV-1 and HGIP according to age among Guadeloupean blood donors (P = 0.03 between HTLV-1-positive and HGIP donors by WB2.4 [1993 to 1998]). (B) Seroprevalences of HTLV-1 and HGIP according to gender among Guadeloupean blood donors (P = 0.003 between HTLV-1-positive and HGIP donors by WB2.4 [1993 to 1998]). (C) Seroprevalences of HTLV-1 and HGIP according to HBc antibody positivity among Guadeloupean blood donors (P = 0.03 between HTLV-1-positive and HGIP donors by WB2.4 [1993 to 1998]). (D) Seroprevalences of HTLV-1 and HGIP according to HBc antibody positivity among Guadeloupean blood donors (P = 0.01 between HTLV-1-positive and HGIP donors by WB2.4 [1993 to 1998]).
Detection of HTLV-1 DNA sequences in the PBMCs by PCR.
All the 43 studied DNA samples gave a positive result with primers that amplify the β-globin gene. A positive PCR signal was clearly detected in the PBMC DNA of 22 out of 24 HTLV-1-seropositive specimens with all the primer pairs, as well as in the HTLV-1- and HTLV-2-positive control DNAs. However, for two HTLV-1-seropositive specimens having an optical density ratio by EIA of >15 and exhibiting a peculiar WB profile with strong env protein (gd21 and MTA-1) but very weak gag protein (p19 and p24) antibody reactivities, PCR results were negative. Furthermore, new PBMC DNAs were extracted for these two persons and retested by PCR for HTLV-1, but the results remained negative. No signal could be detected in the PBMC DNAs of 17 HTLV-1-seroindeterminate subjects, including 13 persons with an HGIP, 3 subjects with a gd21-plus-p19 pattern, and 1 person with an isolated p24 band. No signal was also obtained from the DNA of the HTLV-1- and -2-seronegative specimen as well as for the control DNA-free tube. A sample (6802) exhibiting a faint gd21- and p19-positive MTA-1 pattern but lacking p24 gave positive PCR results only with primer pairs amplifying the gag and tax genes. To avoid a possible lack of PCR sensitivity, we performed a nested PCR (one for the tax gene and the other for the long terminal repeat region) as previously described (26) for the few samples with discordant results. Only the three HTLV-1-positive controls and sample 6802 gave HTLV-1-positive results.
DISCUSSION
HTLV-1- and -2-seroindeterminate WB patterns are prevalent worldwide, with rates fluctuating considerably according to countries. The present 0.4% seroindeterminate rate found in Guadeloupe (French West Indies) appeared comparable to those previously documented for blood donors in other West Indian or South American countries, such as Martinique (0.50%) (6) and Brazil (0.63%) (38). This rate also appeared clearly higher than those found among donors from areas where infection is not endemic, such as metropolitan France (0.0033 per thousand) (8) and the United States (0.035%) (22, 24, 32), but much lower than the rates reached in Cameroon (11% among a rural population) (28) and in Congo (3% among pregnant women) (42). This high frequency of indeterminate results clearly emphasizes the difficulty in assessing the real HTLV-1 seroprevalence, especially in tropical areas where indeterminate WB patterns peak and lead to misclassification. As a consequence, many earlier studies, particularly those performed in Africa but also in the Caribbean, have probably overestimated the HTLV-1 seroprevalences (2, 4, 6, 11, 12, 13, 15, 19, 20, 31).
In our study, the seroindeterminate rates obtained with WB2.3 and WB2.4 did not significantly differ and thereby did not change the overall specificity for serologic confirmation of HTLV-1 infection. Indeed, even if the highly sensitive and specific gd21-based WB2.4 assay eliminated the majority of false-positive transmembrane protein-related results, a significant number of specimens still reacted to gag proteins and were always categorized, by use of WB2.4, as HTLV indeterminate. Finally, indeterminate results due to reactivity to bands other than gd21 are still observed, so that an appropriate confirmatory test remains of major concern (32; Stramer et al., Abstr. 52nd Annu. Meet. Am. Assoc. Blood Banks). By contrast, our survey showed that, when these indeterminate samples were tested by additional recombinant EIA and by PAA, most of them were found HTLV negative. Similar data have been recently described in the United States, where a significant proportion of false-positive HTLV-1 and -2 results are obtained among blood donors, reflecting a weak specificity of the HTLV-1 and -2 screening. The use of a dual EIA algorithm for HTLV-1 and -2 among blood donors is now required in the United States in an attempt to reduce the number of expansive and nonconclusive WB tests. This algorithm process has been evaluated on a large scale and approved by the Food and Drug Administration (Stramer et al., Abstr. 52nd Annu. Meet. Am. Assoc. Blood Banks).
Although our survey disclosed several different indeterminate WB patterns, the leading one among local blood donors was the HGIP, previously described in Cameroon (26, 28), other African countries (11, 42, 44), and Melanesia (20, 31). Among all the HTLV-indeterminate patterns, the frequency of HGIP in Guadeloupe was particularly high regardless of the WB version used (more than 40% for both WB2.3 and WB2.4). The reason for and significance of this peculiar blot pattern prevalence remain unclear. Several hypotheses have been put forward. One is the possibility of cross-reactivity to epitopes present on bacteria or parasites (notably Plasmodium falciparum) (15, 26, 34). However, Guadeloupe has no specific bacterial environment and malaria was eradicated about 50 years ago. Further, none of our 62 blood donors with an HGIP had traveled in areas where malaria is endemic. Another hypothesis may link the indeterminate reactivity to an immune response to closely related endogenous retroviruses (1) or to exogenous simian T-lymphotropic viruses (44), the latter being unlikely in our series, as recently described for the United States by Busch et al. (5).
With regard to epidemiology, our subjects exhibiting HGIP were significantly younger than those confirmed HTLV-1 seropositive. Furthermore, the HGIP prevalence was related neither to gender nor to HBc antibodies and did not cluster in the Atlantic façade of Guadeloupe, which is the area of highest HTLV-1 prevalence. Finally, the epidemiological profile of individuals with HGIP appeared close to those of HTLV-1-seronegative individuals and markedly contrasted with those of HTLV-1-seropositive individuals. Such contrast confirms the initial data obtained by Mauclère et al. in Cameroon (28) but extends these findings to the Caribbean area, strongly suggesting that such an indeterminate Gag WB pattern does not appear to reflect true HTLV-1 infection. This statement was confirmed by the use of PCR. Indeed, this technique did not detect HTLV-1 sequences in the PBMC of 13 tested HGIP persons. It must be pointed out that the majority of previous studies, performed in various areas, also failed to detect HTLV-1 proviral sequences, even by the use of highly conserved HTLV-1 and HTLV-2 primers on fresh or cultured PBMCs of those individuals presenting an HTLV indeterminate WB pattern (15, 18, 22, 26, 31, 44). However, a recent report has described the amplification of an HTLV-1 tax sequence from patients with neurological disease exhibiting an HGIP WB reactivity. This suggests that this seroindeterminate WB pattern might be associated in some rare cases with defective HTLV-1 strains or with a novel retrovirus having homology with HTLV-1, or finally with slowly replicating HTLV-1 (39, 47). In addition, it seems unlikely that the HGIP may represent a delayed or slow seroconversion, because most of our followed-up subjects did not show any evolution of their WB profile over time and because the minority who did became EIA negative. However, we noticed that some of the latter retained an HGIP, but with a significantly decreased response to the Gag bands, likely reflecting a lower level of antigenic stimulation. Finally, in our study, all seroindeterminate patterns do not correspond to an HTLV-1 seroconversion, contrary to a recent study carried out in Martinique, where 3 of 49 HTLV-seroindeterminate donors were reported as being HTLV-1 seroconverters (6).
In conclusion, our data confirm that the stringent criteria for WB positivity proposed by the HTLV European Research Network (40) must be accurately carried out, especially for samples originating from tropical areas. These criteria state that, to be considered HTLV-1 positive, WB-tested sera must react with at least two native gag proteins, p19 and p24, in addition to two recombinant env glycoproteins, gd21 and MTA-1. However, special attention must be paid to low-intensity signals: indeed, two “HTLV-1-seropositive” specimens in our study exhibiting a peculiar pattern with strong env protein reactivities but very weak gag protein reactivities were PCR negative. In such rare cases of faintly positive samples, it seems necessary to perform PCR in order to distinguish between true and false HTLV-1 seropositivity. Conversely, one indeterminate sample in our study with the gd21+ p19+ p24− profile along with MTA-1 reactivity was PCR positive. Similar results have been obtained in metropolitan France, where two indeterminate samples with the same pattern were also PCR positive (10). On the basis of these data, and by analogy with HTLV-2 seropositivity criteria, which required only three bands (i.e., gd21, p24, and K-55), we propose that HTLV-1 seropositivity should be based on the presence of at least the three reactivities gd21, p19, and MTA-1, even if p24 is lacking. By contrast, when both MTA-1 and p24, or the env protein reactivities (such as HGIP) are lacking, our survey failed to detect, by PCR, evidence of HTLV-1 provirus in all cases. Healthy blood donors with such HGIP test results should be reassured that they are unlikely to be infected with HTLV-1 or HTLV-2.
ACKNOWLEDGMENTS
We thank Renaud Mahieux for critical review of this manuscript.
We thank the Agence Nationale de Recherche contre le SIDA for financial support.
REFERENCES
- 1.Banki K, Maceda J, Hurley E, Ablonczy E, Mattson D H, Szegedy L, Hung C, Perl A. Human T-cell lymphotropic virus (HTLV)-related endogenous sequence, HRES-1, encodes a 28-kDa protein: a possible autoantigen for HTLV-I gag-reactive autoantibodies. Proc Natl Acad Sci USA. 1992;89:1939–1943. doi: 10.1073/pnas.89.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonis J, Preux P M, Nzisabira L, Letenneur L, Muhirwa G, Buzingo T, Kamuragiye A, Preux C, Ngoga E, Dumas M, Denis F. HTLV-I in Burundi (East Africa): lack of reactivity to the HTLV-I immunodominant envelope epitope. J Acquir Immune Defic Syndr. 1994;7:1099–1100. [PubMed] [Google Scholar]
- 3.Brodine S K, Kaime E M, Roberts C, Turnicky R P, Lal R B. Simultaneous confirmation and differentiation of human T-lymphotropic virus types I and II infection by modified Western blot containing recombinant envelope glycoproteins. Transfusion. 1993;33:925–929. doi: 10.1046/j.1537-2995.1993.331194082384.x. [DOI] [PubMed] [Google Scholar]
- 4.Busch M P, Laycock M, Kleinman S H, Wages J W, Jr, Calabro M, Kaplan J E, Khabbaz R F, Hollingsworth C G. Accuracy of supplementary serologic testing for human T-lymphotropic virus types I and II in US blood donors. Retrovirus Epidemiology Donor Study. Blood. 1994;83:1143–1148. [PubMed] [Google Scholar]
- 5.Busch M P, Switzer W M, Murphy E L, Thomson R, Heneine W. Absence of evidence of infection with divergent primate T-lymphotropic viruses in United States blood donors who have seroindeterminate HTLV test results. Transfusion. 2000;40:443–449. doi: 10.1046/j.1537-2995.2000.40040443.x. [DOI] [PubMed] [Google Scholar]
- 6.Cesaire R, Bera O, Maier H, Lezin A, Martial J, Ouka M, Kerob-Bauchet B, Ould Amar A K, Vernant J C. Seroindeterminate patterns and seroconversions to human T-lymphotropic virus type I positivity in blood donors from Martinique, French West Indies. Transfusion. 1999;39:1145–1149. doi: 10.1046/j.1537-2995.1999.39101145.x. [DOI] [PubMed] [Google Scholar]
- 7.Cossen C, Hagens S, Fukuchi R, Forghani B, Gallo D, Ascher M. Comparison of six commercial human T-cell lymphotropic virus type I (HTLV-I) enzyme immunoassay kits for detection of antibody to HTLV-I and -II. J Clin Microbiol. 1992;30:724–725. doi: 10.1128/jcm.30.3.724-725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courouce A M, Pillonel J, Lemaire J M, Maniez M, Brunet J B. Seroepidemiology of HTLV-I/II in universal screening of blood donations in France. AIDS. 1993;7:841–847. doi: 10.1097/00002030-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Cowan E P, Nemo G J, Williams A E, Alexander R K, Vallejo A, Hewlett I K, Lal R B, Dezzutti C S, Gallahan D, George K, Pancake B A, Zucker-Franklin D, McCurdy P R, Tabor E. Absence of human T-lymphotropic virus type I tax sequences in a population of normal blood donors in the Baltimore, MD/Washington, DC, area: results from a multicenter study. Transfusion. 1999;39:904–909. doi: 10.1046/j.1537-2995.1999.39080904.x. [DOI] [PubMed] [Google Scholar]
- 10.Defer C, Coste J, Descamps F, Voisin S, Lemaire J M, Maniez M, Couroucé A M the Retrovirus Study Group of The French Society of Blood Transfusion. Contribution of polymerase chain reaction and radioimmunoprecipitation assay in the confirmation of human T-lymphotropic virus infection in French blood donors. Transfusion. 1995;35:596–600. doi: 10.1046/j.1537-2995.1995.35795357884.x. [DOI] [PubMed] [Google Scholar]
- 11.Delaporte E, Peeters M, Durand J P, Dupont A, Schrijvers D, Bedjabaga L, Honore C, Ossari S, Trebucq A, Josse R, Merlin M. Seroepidemiological survey of HTLV-I infection among randomized populations of western central African countries. J Acquir Immune Defic Syndr. 1989;2:410–413. [PubMed] [Google Scholar]
- 12.de The G, Gessain A, Gazzolo L, Robert-Guroff M, Najberg G, Calender A, Peti M, Brubaker G, Bensliman A, Fabry F, et al. Comparative seroepidemiology of HTLV-I and HTLV-III in the French West Indies and some African countries. Cancer Res. 1985;45:4633s–4636s. [PubMed] [Google Scholar]
- 13.Dumas M, Houinato D, Verdier M, Zohoun T, Josse R, Bonis J, Zohoun I, Massougbodji A, Denis F. Seroepidemiology of human T-cell lymphotropic virus type I/II in Benin (West Africa) AIDS Res Hum Retroviruses. 1991;7:447–451. doi: 10.1089/aid.1991.7.447. [DOI] [PubMed] [Google Scholar]
- 14.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 15.Gessain A, Mahieux R, de Thé G. HTLV-I “indeterminate” Western blot patterns observed in sera from tropical regions: the situation revisited. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:316–318. [PubMed] [Google Scholar]
- 16.Hayes C G, Burans J P, Oberst R B. Antibodies to human T lymphotropic virus type I in a population from the Philippines: evidence for cross-reactivity with Plasmodium falciparum. J Infect Dis. 1991;163:257–262. doi: 10.1093/infdis/163.2.257. [DOI] [PubMed] [Google Scholar]
- 17.Kawase K, Katamine S, Moriuchi R, Miyamoto T, Kubota K, Igarashi H, Doi H, Tsuji Y, Yamabe T, Hino S. Maternal transmission of HTLV-I other than through breast milk: discrepancy between the polymerase chain reaction positivity of cord blood samples for HTLV-I and the subsequent seropositivity of individuals. Jpn J Cancer Res. 1992;83:968–977. doi: 10.1111/j.1349-7006.1992.tb02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khabbaz R F, Heneine W, Grindon A, Hartley T M, Shulman G, Kaplan J. Indeterminate HTLV serologic results in U.S. blood donors: are they due to HTLV-I or HTLV-II? J Acquir Immune Defic Syndr. 1992;5:400–404. [PubMed] [Google Scholar]
- 19.Lal R, Lipka J J, Foung S K H, Hadlock K G, Reyes G R, Carney W P. Human T lymphotropic virus type I/II in Lake Lindu Valley, central Sulawesi, Indonesia. J Acquir Immune Defic Syndr. 1993;9:1067–1068. [PubMed] [Google Scholar]
- 20.Lal R, Rudolph D L, Nerurkar V, Yanagihara R. Humoral responses to the immunodominant gag and env epitopes of Human T-Lymphotropic virus type I among Melanesians. Vir Immunol. 1992;4:265–72. doi: 10.1089/vim.1992.5.265. [DOI] [PubMed] [Google Scholar]
- 21.Lal R B, Brodine S, Kazura J, Mbidde-Katonga E, Yanagihara R, Roberts C. Sensitivity and specificity of a recombinant transmembrane glycoprotein (rgp21)-spiked Western immunoblot for serological confirmation of human T-cell lymphotropic virus type I and type II infections. J Clin Microbiol. 1992;30:296–299. doi: 10.1128/jcm.30.2.296-299.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lal R B, Rudoph D L, Coligan J E, Brodine S K, Roberts C R. Failure to detect evidence of human T-lymphotropic virus (HTLV) type I and type II in blood donors with isolated gag antibodies to HTLV-I/II. Blood. 1992;80:544–550. [PubMed] [Google Scholar]
- 23.Lipka J J, Young K K Y, Kwok S Y, Reyes G R, Sninsky J J, Foung S K H. Significance of human T-lymphotropic virus type I indeterminant serological findings among healthy individuals. Vox Sang. 1991;61:171–176. doi: 10.1111/j.1423-0410.1991.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Shah M, Stramer S L, Chen W, Weiblen B J, Murphy E L. Sensitivity and specificity of human T-lymphotropic virus (HTLV) types I and II polymerase chain reaction and several serologic assays in screening a population with a high prevalence of HTLV-II. Transfusion. 1999;39:1185–1193. doi: 10.1046/j.1537-2995.1999.39111185.x. [DOI] [PubMed] [Google Scholar]
- 25.Mahieux R, Pecon-Slattery J, Gessain A. Molecular characterization and phylogenetic analyses of a new, highly divergent simian T-cell lymphotropic virus type 1 (STLV-1marc1) in Macaca arctoides. J Virol. 1997;71:6253–6258. doi: 10.1128/jvi.71.8.6253-6258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahieux R, Horal P, Mauclère P, Mercereau-Puijalon O, Guillotte M, Meertens L, Murphy E, Gessain A. Human T-cell lymphotropic virus type-1 Gag indeterminate Western blot patterns in central Africa: relationship to Plasmodium falciparum infection. J Clin Microbiol. 2000;38:4049–4057. doi: 10.1128/jcm.38.11.4049-4057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manns A, Hisada M, La Grenade L. Human T-lymphotropic virus type I infection. Lancet. 1999;353:1951–1958. doi: 10.1016/s0140-6736(98)09460-4. [DOI] [PubMed] [Google Scholar]
- 28.Mauclère P, Le Hesran J Y, Mahieux R, Salla R, Mfoupouendoun J, Abada E T, Millan J, de The G, Gessain A. Demographic, ethnic, and geographic differences between human T cell lymphotropic virus (HTLV) type I-seropositive carriers and persons with HTLV-I gag-indeterminate Western blots in central Africa. J Infect Dis. 1997;176:505–509. doi: 10.1086/514071. [DOI] [PubMed] [Google Scholar]
- 29.Montplaisir N, Valette L, Dezaphy Y, Neisson-Vernant C. Blood transfusion and HTLV1 infection in Martinique. In: Roman G C, Vernant J C, Osame M, editors. HTLV-I and the nervous system. New York, N.Y: Liss; 1989. pp. 533–539. [Google Scholar]
- 30.Murphy E L, Figueroa J P, Gibbs W N, Holding-Cobham M, Cranston B, Malley K, Bodner A J, Alexander S S, Blattner W A. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I. Demographic determinants. Am J Epidemiol. 1991;133:1114–1124. doi: 10.1093/oxfordjournals.aje.a115824. [DOI] [PubMed] [Google Scholar]
- 31.Nerurkar V R, Miller M A, Leon-Monzon M E, Ajdukiewicz A B, Jenkins C L, Sanders R C, Godec M S, Garruto R M, Yanagihara R. Failure to isolate human T cell lymphotropic virus type I and to detect variant-specific genomic sequences by polymerase chain reaction in Melanesians with indeterminate Western immunoblot. J Gen Virol. 1992;73:1805–1810. doi: 10.1099/0022-1317-73-7-1805. [DOI] [PubMed] [Google Scholar]
- 32.Ownby H E, Korelitz J J, Busch M P, Williams A E, Kleinman S H, Gilcher R O, Nourjah P the Retrovirus Epidemiology Donor Study. Loss of volunteer blood donors because of unconfirmed enzyme immunoassay screening results. Transfusion. 1997;37:199–205. doi: 10.1046/j.1537-2995.1997.37297203524.x. [DOI] [PubMed] [Google Scholar]
- 33.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter K R, Aguiar J, Richards A, Sandjaya B, Ignatias H, Hadiputranto H, Ridley R G, Takacs B, Wignall F S, Hoffman S L, Hayes C G. Immune response against the Exp-1 protein of Plasmodium falciparum results in antibodies that cross-react with human T-cell lymphotropic virus type 1 proteins. Clin Diagn Lab Immunol. 1998;5:721–724. doi: 10.1128/cdli.5.5.721-724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouet F, Foucher C, Rabier M, Gawronski I, Taverne D, Chancerel B, Casman O, Strobel M. Human T-lymphotropic virus type I (HTLV-I) among blood donors from Guadeloupe: donation, demographic, and biological characteristics. Transfusion. 1999;39:639–644. doi: 10.1046/j.1537-2995.1999.39060639.x. [DOI] [PubMed] [Google Scholar]
- 36.Rouet F, Rabier R, Foucher C, Chancerel B, Agis F, Strobel M. Geographical clustering of human T-cell lymphotropic virus type I in Guadeloupe, an endemic Caribbean area. Int J Cancer. 1999;81:330–334. doi: 10.1002/(sici)1097-0215(19990505)81:3<330::aid-ijc3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Sabino E C, Zrein M, Taborda C P, Otani M M, Ribeiro-Dos-Santos G, Saez-Alquezar A. Evaluation of the INNO-LIA HTLV I/II assay for confirmation of human T-cell leukemia virus-reactive sera in blood bank donations. J Clin Microbiol. 1999;37:1324–1328. doi: 10.1128/jcm.37.5.1324-1328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segurado A A, Malaque C M, Sumita L M, Pannuti C S, Lal R B. Laboratory characterization of human T cell lymphotropic virus types 1 (HTLV-1) and 2 (HTLV-2) infections in blood donors from Sao Paulo, Brazil. Am J Trop Med Hyg. 1997;57:142–148. doi: 10.4269/ajtmh.1997.57.142. [DOI] [PubMed] [Google Scholar]
- 39.Soldan S S, Graf M D, Waziri A, Flerlage A N, Robinson S M, Kawanishi T, Leist T P, Lehky T J, Levin M C, Jacobson S. HTLV-I/II seroindeterminate Western blot reactivity in a cohort of patients with neurological disease. J Infect Dis. 1999;180:685–694. doi: 10.1086/314923. [DOI] [PubMed] [Google Scholar]
- 40.Taylor G P. The epidemiology of HTLV-I in Europe. J Acquir Immune Defic Syndr. 1996;13(Suppl. 1):S8–S14. doi: 10.1097/00042560-199600001-00003. [DOI] [PubMed] [Google Scholar]
- 41.Touze E, Gessain A, Lyon-Caen O, Gout O. Tropical spastic paraparesis/HTLV-I-associated myelopathy in Europe and in Africa: clinical and epidemiologic aspects. J Acquir Immune Defic Syndr. 1996;13(Suppl. 1):S38–S45. doi: 10.1097/00042560-199600001-00008. [DOI] [PubMed] [Google Scholar]
- 42.Tuppin P, Makuwa M, Guerma T, Bazabana M M, Loukaka J C, Jeannel D, M'Pele P, de The G. Low HTLV-I/II seroprevalence in pregnant women in Congo and a geographic cluster of an HTLV-like indeterminate Western blot pattern. J Acquir Immune Defic Syndr. 1996;11:105–107. doi: 10.1097/00042560-199601010-00014. [DOI] [PubMed] [Google Scholar]
- 43.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 44.Vandamme A M, Van Laethem K, Liu H F, Van Brussel M, Delaporte E, de Castro Costa C M, Fleischer C, Taylor G, Bertazzoni U, Desmyter J, Goubau P. Use of a generic polymerase chain reaction assay detecting human T-lymphotropic virus (HTLV) types I, II and divergent simian strains in the evaluation of individuals with indeterminate HTLV serology. J Med Virol. 1997;52:1–7. [PubMed] [Google Scholar]
- 45.Varma M, Rudolph D L, Knuchel M, Switzer W M, Hadlock K G, Velligan M, Chan L, Foung S K, Lal R B. Enhanced specificity of truncated transmembrane protein for serologic confirmation of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2 infections by Western blot (immunoblot) assay containing recombinant envelope glycoproteins. J Clin Microbiol. 1995;33:3239–3244. doi: 10.1128/jcm.33.12.3239-3244.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wattel E, Mariotti M, Agis F, Gordien E, Prou O, Courouce A M, Rouger P, Wain-Hobson S, Chen I S, Lefrere J J. Human T lymphotropic virus (HTLV) type I and II DNA amplification in HTLV-I/II-seropositive blood donors of the French West Indies. J Infect Dis. 1992;165:369–372. doi: 10.1093/infdis/165.2.369. [DOI] [PubMed] [Google Scholar]
- 47.Waziri A, Soldan S S, Graf M D, Nagle J, Jacobson S. Characterization and sequencing of prototypic human T-lymphotropic virus type 1 (HTLV-1) from an HTLV-1/2 seroindeterminate patient. J Virol. 2000;74:2178–2185. doi: 10.1128/jvi.74.5.2178-2185.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi K. Human T-lymphotropic virus type I in Japan. Lancet. 1994;343:213–216. doi: 10.1016/s0140-6736(94)90994-6. [DOI] [PubMed] [Google Scholar]