Abstract
Burkina Faso has one of the highest malaria burdens in sub-Saharan Africa despite the mass deployment of insecticide-treated nets (ITNs) and use of seasonal malaria chemoprevention (SMC) in children aged up to 5 years. Identification of risk factors for Plasmodium falciparum infection in rural Burkina Faso could help to identify and target malaria control measures. A cross-sectional survey of 1,199 children and adults was conducted during the peak malaria transmission season in the Cascades Region of south-west Burkina Faso in 2017. Logistic regression was used to identify risk factors for microscopically confirmed P. falciparum infection. A malaria transmission dynamic model was used to determine the impact on malaria cases averted of administering SMC to children aged 5–15 year old. P. falciparum prevalence was 32.8% in the study population. Children aged 5 to < 10 years old were at 3.74 times the odds (95% CI = 2.68–5.22, P < 0.001) and children aged 10 to 15 years old at 3.14 times the odds (95% CI = 1.20–8.21, P = 0.02) of P. falciparum infection compared to children aged less than 5 years old. Administration of SMC to children aged up to 10 years is predicted to avert an additional 57 malaria cases per 1000 population per year (9.4% reduction) and administration to children aged up to 15 years would avert an additional 89 malaria cases per 1000 population per year (14.6% reduction) in the Cascades Region, assuming current coverage of pyrethroid-piperonyl butoxide ITNs. Malaria infections were high in all age strata, although highest in children aged 5 to 15 years, despite roll out of core malaria control interventions. Given the burden of infection in school-age children, extension of the eligibility criteria for SMC could help reduce the burden of malaria in Burkina Faso and other countries in the region.
Subject terms: Risk factors, Epidemiology, Malaria
Introduction
Impressive reductions in malaria occurred throughout sub-Saharan Africa from 2000 to 20151. Progress, however, has not been geographically uniform. In many high-burden countries, parasite prevalence rates and mortality from malaria remain obstinately high despite roll out of key malaria control interventions including insecticide-treated nets (ITNs), use of seasonal malaria chemoprevention (SMC) in children aged less than five years, and diagnosis and treatment using artemisinin combination therapy (ACTs)2.
Burkina Faso has highly intense and seasonal malaria transmission and is one of the ten countries in the world with the highest burden of malaria3. It has been designated as a High Burden High Impact country by the World Health Organisation (WHO) with calls for a more aggressive approach to malaria control3. Burkina Faso has seen increases in malaria cases from 7.8 million cases in 20144 to 11.5 million cases in 2018 and 11.3 million cases in 2020, according to the National Malaria Control Programme (NMCP)5,6. Malaria remains the main cause of outpatient attendance at health centres in all age groups, and severe malaria was responsible for 69% of deaths in children aged 1 to 15 years in medical centres and hospitals in 20206. Increases in malaria cases are being observed despite national ITN distribution campaigns in 2010, 2013, 2016 and 2019 and introduction of seasonal malaria chemoprevention (SMC) in children aged 3 to 59 months since 2014. SMC (previously termed intermittent preventive treatment in children) requires the administration of the anti-malarial drugs sulfadoxine-pyrimethamine (SP) and amodiaquine (AQ) monthly for four months during the high transmission season to prevent malaria and is recommended by WHO across the Sahel, where transmission is highly seasonal7. Potential explanations for the stagnating progress in malaria control include low ITN usage or lack of ITN bioefficacy and durability8–11, a rise in the resistance of vectors to pyrethroid insecticides used to treat ITNs12–14, and an increase in outdoor vector biting14.
A cross-sectional survey was carried out in 2017 to determine the prevalence of P. falciparum infection in different age groups and identify risk factors in south-west Burkina Faso. These data were used to parameterise a mathematical model of malaria transmission to explore how expanding the age range for using SMC would impact the malaria burden in the study area.
Methods
Study design
A cross-sectional survey of P. falciparum prevalence and risk factors for infection was conducted in October and November 2017 shortly after the peak of malaria transmission.
Study site
The study was conducted in ten villages in the Banfora Health District, an area of Sudanian savannah in the Cascades region, south-west Burkina Faso (lying between 10°40’ to 10°04′13’’ north latitude and 5°01′21’’ to 4°46′18’’ west longitude) with a population of 392,498 in 2017 (Fig. 1) . The study site experiences intense seasonal malaria transmission with peaks during the rainy season, from May to November15, with most cases occurring in September16. Plasmodium falciparum accounts for 90% of cases15 and the main malaria vectors are Anopheles gambiae, An. coluzzii and, to a lesser extent, An. arabiensis14,17. The site has an entomological inoculation rate (EIR) of 80 infective bites per child during the transmission season8, with another study suggesting up to 52 infective bites per person per night during the peak transmission season in October18. The NMCP undertook an ITN universal coverage campaign (distribution of one ITN for two persons) in 2010, 2013 and 2016 using nets either treated with permethrin or deltamethrin. No additional nets were distributed by the study team. Indoor residual spraying was not conducted during the study or the preceding 12 months. In Burkina Faso since 2014, children aged 3 to 59 months receive SMC on four occasions during the transmission season using SP-AQ as per WHO recommendations7.
Figure 1.
Map of the 10 study villages: (A) location of Burkina Faso, (B) location of Cascades Region in Burkina Faso and (C) location of Banfora Health District and study villages in Cascades Region. The map was generated using QGIS 3.1619. Background layers were downloaded for OpenStreetMap20, villages were digitised by the authors using GPS coordinates collected in the field using a GARMIN eTREX 10 GPS.
Sampling design
A random sample of 10 villages were selected from a list of villages in the study area using a two-stage process. Five health centres in the study area were chosen with each health centre having a catchment radius of approximately 10 km. Two villages were randomly selected from each catchment area, giving a total of 10 villages, at least 3 km apart. Permission to enter the communities was sought from village leaders.
An age-stratified cross-sectional survey of both children and adults in three age groups (2 to < 10 years, 10 to < 30 years, ≥ 30 years) was conducted to determine the prevalence of P. falciparum. The survey aimed to sample 1,200 individuals, 400 from each of the three age strata. 150 study subjects (50 in each age strata) were randomly selected from the Health and Demographic Surveillance System (HDSS) lists of the 10 study villages and entered the screening process. Each study subject was selected from a different household. The first 40 individuals per age strata and per village who provided informed consent were enrolled. Participants were excluded if they were currently participating in a trial of a malaria vaccine or drug, under chemoprophylaxis (except for SMC) or currently participating in a related cohort study8.
P. falciparum infection survey
A finger-prick blood sample was taken from each participant. Two blood slides were prepared and a malaria rapid diagnostic test (RDT, SD BIOLINE Malaria Ag P.f/Pan screening test Abbott, Geonggi-do, Republic of Korea) performed for point-of-care diagnosis of those with fever (axillary temperature ≥ 37.5 °C) or history of fever in the past 48 h. Individuals with positive RDTs were offered treatment with artemether-lumefantrine (AL) according to national guidelines21. Thick blood films were stained with Giemsa and examined under 100-fold magnification by experienced microscopists centrally at Centre National de Recherche et de Formation sur le Paludisme (CNRFP) in Banfora. Parasite counts were recorded per high power field and 100 fields counted before a slide was declared negative. Each slide was read separately by two independent microscopists. Discrepancies in positive and negative reads and parasite counts differing by more than tenfold between the two reads were resolved by a third reader. The final result was average of the two-closer readings. Blood spots were also taken for polymerase chain reaction analysis but unfortunately due to a lack of amplification we did not obtain results.
Risk factor survey
At the same time as the infection survey, a questionnaire was administered to the study participant (or caregiver for children, as indicated) to gather information on demographics (age, gender, ethnicity, religion, education, occupation), use of malaria control measures (bednet use the previous night, use of topical or household insecticides e.g. insecticide aerosols, mosquito coils in the past week, receipt of SMC in previous month), house construction (roof material, whether the space between the wall and the roof, i.e. the eaves, were closed), presence of electricity or functioning fan, presence of animals within 5 m of the household, and travel history outside of the village in the previous two weeks. Information was also collected from the study participant or the head of household (as indicated) on asset ownership and household characteristics, following standard procedures used in the Burkina Faso Demographic and Health Survey (DHS)22.
Human landing catches were carried out inside houses during the 2017 transmission season (1st June to 17th December) in each of the 10 villages and are a subset of results previously published by Sanou et al14. Four randomly selected households were sampled on one night every month (total of 187 collections due to public holidays), with a different group of households selected the following month to maximise spatial coverage. Households selected for entomological sampling were not necessarily the households of participants in the cross- sectional survey. Volunteers were recruited from the villages and trained to collect mosquitoes landing on their legs between 19.00 h to 06.00 h. Mosquitoes were typed to species using established morphological keys. Phenotypic insecticide resistance was measured using WHO tube tests as per standard procedures23. Assays were performed with An. gambiae s.l. mosquitoes reared from larvae collected in seven study villages during the 2017 transmission season (because of limited availability of larval habitats in the other three villages during the period of the survey)14.
Sample size considerations
A random sample of 400 individuals from each of the three age groups (2 to < 10 years, 10 to < 30 years, ≥ 30 years) were selected from 10 villages giving a total sample size of 1,200 individuals. Assuming a true parasite prevalence ranging between 40 to 60% across the three age groups24, the study was able to measure the point prevalence of P. falciparum infection by microscopy with a 5% precision at the 95% confidence level25. No sample size calculation was performed for the mosquito collections and instead the number of households and trapping nights were determined based on logistical constraints.
Data management and statistical analysis
Data were collected on personal digital assistants (PDAs) programmed with an electronic data capture system, Kobo Collect (Version 1.4.8). Forms were piloted in the field prior to use and had drop-down boxes and consistency checks to avoid data entry errors. PDAs were uploaded by fieldworkers weekly to a central computer.
The primary outcome was P. falciparum infection confirmed by microscopy (any level of parasite density). Secondary outcomes were: (i) prevalence of symptomatic malaria defined as axillary temperature ≥ 37.5 °C (or history of fever within the previous 48 h) with microscopically confirmed P. falciparum infection and (ii) prevalence of high-density P. falciparum infection (> 5,000 parasites/µL) detected by microscopy.
Principal component analysis was used to calculate the socio-economic status (SES) factor score (based on asset ownership and household characteristics). SES factor scores were ranked and households divided into five equal wealth quintiles (1 being the poorest, through to 5, least poor). The EIR or estimated number of infectious bites per study participant during the transmission season was calculated using the formula EIR = Ma × S × d where Ma is the human biting rate, estimated from the arithmetic mean number of female An. gambiae s.l. caught per human landing catches across the six-month transmission season, where S is the proportion of female An. gambiae s.l. found to be sporozoite positive by village and d is the number of days in the transmission season.
Mean values were compared using a t-test and proportions compared using chi-squared tests. Parasite prevalence was estimated as the proportion of subjects infected divided by the number of subjects tested. Logistic regression was used to investigate the association between malaria infection and risk factors, adjusting for clustering by village. Univariate analysis was conducted followed by construction of a simple multivariate model in which every risk factor was included, irrespective of whether the variable was significant in the univariate model. All analyses were carried out using Stata 15 (Statacorp, Texas, USA).
Malaria transmission dynamic model
A widely used transmission dynamics mathematical model of malaria was used to investigate the impact of modifying SMC in the Cascades region of Burkina Faso. The individual-based stochastic model mechanistically captures transmission of P. falciparum malaria in humans and Anopheles mosquito vectors. All differential equations describing the dynamics of the infection in populations with malaria control interventions have been comprehensively reported in Griffin et al.26 and Winskill et al.27 whilst the model code is available from https://github.com/jamiegriffin/Malaria_simulation. This model captures the age distribution of infection in areas with different levels of endemicity28 and has been used to investigate the impact of SMC29. Here we use a version of the model calibrated for the region (Lambert et al., unpublished) which is parameterised with local epidemiological8 and entomological data14,18 and fitted to estimates of malaria prevalence (assessed by microscopy) across the Cascades region collated by the Malaria Atlas Project (https://malariaatlas.org/). The calibration process uses the method of maximum likelihood (assuming a beta-binomial model) to select a value of the mosquito-to-human density parameter (and corresponding over-dispersion parameter of the beta-binomial). It is used to predict the number of clinical cases per person in the whole population between 2019 and 2022 following the mass ITN campaign in 2019. Results are averaged over the three-year period (the time between ITN campaigns) as cases will depend on ITN use which drops following mass distribution. The type of nets distributed varied at the district level within the Cascades region. Here we assume that the population received either pyrethroid-only or pyrethroid-piperonyl butoxide (PBO) ITNs30. PBO is an insecticide synergist which inhibits the action of resistance-associated metabolic enzymes of the cytochrome P450 family and improves control of pyrethroid-resistant anopheline mosquitoes. The added advantage of pyrethroid-PBO ITNs over pyrethroid-only ITNs is estimated from a meta-analyses of experimental hut trial data31 and the level of resistance for the region estimated using discriminating dose bioassays14. The impact of changing SMC is assessed using the same method as outlined previously32, either halting SMC, maintaining the existing age range of 3–59 months or extending the upper age limit to 10 or 15 years. In all simulations with SMC, coverage is assumed to match previous years (81% of children receiving treatment each round)2.
Ethical consideration
Adult study participants and the caregivers of children aged under 20 years provided informed consent to participate in the cross-sectional survey. In addition, minors aged 12 to 19 years were required to provide written assent before they could be enrolled in the study. All the consenting and assenting processes were conducted in the presence of an impartial witness if the participant was illiterate. Ethical approval for this study was provided by the Burkina Faso Health Research Ethics Committee (Deliberation No 2016–12-137), CNRFP Institutional Bioethics Committee (No2016/000007/MS/SG/CNRFP/CIB), Durham University’s Department of Biosciences Ethics Committee (SBBS/EC/MIRA) and Liverpool School of Tropical Medicine Ethical Committee (Protocol number: 16/047). The study was conducted in compliance with principles set out by the International Conference on Harmonization Good Clinical Practice, the Declaration of Helsinki and the regulatory requirements of Burkina Faso.
Results
Socio-demographic characteristics of the survey participants
A total of 1,199 individuals were surveyed, 399 (33.3%) were aged 2 to < 10 years, 397 (33.1%) aged 10 to < 30 years and 403 (33.6%) aged ≥ 30 years (Table 1). 55.0% of the study population were female (660/1199). The most common ethnic groups were Gouin and Turka (51.9%, 622/1199) and Karaboro (18.8%, 226/1199). The respondents were Muslim (35.4%, 784/1199), Animists (20.3%, 243/1199) and Christians (14.3%, 172/1199). Most study participants were illiterate (70.5%, 845/1199) and farmers (54.8%, 657/1199), whereas 43.5% (522/1199) of participants were not engaged in income generating work either because they were too young or because they were too old to work. 90.7% of study participants (1,087/1,199) reported sleeping under a bednet the previous night. 9.2% (110/1199) of the population reported using a topical repellent in the last week, while 6.2% (74/1199) reported using household insecticides (e.g. insecticide aerosols, mosquito coils). Only 1.8% (22/1199) of the study population reported travelling outside their village in the two weeks before the survey. The EIR in the study area was 188 bites per person over the transmission season ranging from 0 in Sitiena village to 336 in Dangouindougou village.
Table 1.
Characteristics of participants of the cross-sectional survey.
| Characteristic | Overall, N (%) N = 1199 | 2 to < 10 years, n = 399 | 10 to < 30 years, n = 397 | > 30 years, n = 403 |
|---|---|---|---|---|
| Age (mean / SD) | 23.7 (19.1) | 5.9 (2.3) | 18.0 (5.7) | 46.9 (12.8) |
| Female gender | 660 (55.0) | 191 (47.9) | 214 (53.9) | 255 (63.3) |
| Ethnicity | ||||
| Gouin/Turka | 622 (51.9) | 213 (53.4) | 197 (49.6) | 212 (52.6) |
| Karaboro | 226 (18.8) | 73 (18.3) | 72 (18.1) | 81 (20.1) |
| Other ethnic group | 351 (29.3) | 113 (28.3) | 128 (32.2) | 110 (27.3) |
| Religion | ||||
| Muslim | 784 (65.4) | 255 (63.9) | 270 (68.0) | 259 (64.3) |
| Christian | 172 (14.3) | 60 (15.0) | 58 (14.6) | 54 13.4) |
| Animist | 243 (20.3) | 84 (21.1) | 69 (17.4) | 90 (22.3) |
| Education | ||||
| Illiterate | 845 (70.5) | 298 (74.7) | 201 (50.6) | 346 (86.1) |
| Literate | 353 (29.5) | 101 (25.3) | 196 (49.4) | 56 (13.9) |
| Occupation | ||||
| Farming | 657 (54.8) | 40 (10.0) | 245 (61.7) | 372 (92.3) |
| Commercial and office workers | 20 (1.7) | 1 (0.3) | 6 (1.5) | 13 (3.2) |
| None or retired | 522 (43.5) | 358 (89.7) | 146 (36.8) | 18 (4.5) |
| Used any bednet the previous night | 1087 (90.7) | 376 (94.2) | 346 (87.2) | 365 (90.6) |
| History of travel in the previous 2 weeks | 22 (1.8) | 9 (2.3) | 5 (1.3) | 8 (2.0) |
| Received SMC in previous month | 169 (42.4) | |||
| Eave construction of the sleeping room of the subject* | ||||
| Closed | 789 (65.8) | 276 (69.2) | 273 (68.8) | 240 (59.6) |
| Open | 198 (16.5) | 41 (10.3) | 74 (18.6) | 83 (20.6) |
| Roof construction of the sleeping room of the subject* | ||||
| Metal | 689 (57.5) | 217 (54.4) | 229 (57.7) | 243 (60.3) |
| Non-metal (Thatch) | 298 (24.9) | 100 (25.1) | 118 (29.7) | 80 (19.9) |
| Sleeping room of subject has a functioning lightbulb installed* | 763 (63.6) | 274 (68.7) | 247 (62.2) | 242 (60.0) |
| Sleeping room of subject has a functioning fan | 74 (6.2) | 34 (8.5) | 16 (4.0) | 24 (6.0) |
| Animals tethered within 5 m of this part of the house* | 65 (5.4) | 27 (6.8) | 19 (4.8) | 19 (4.7) |
| Use of topical repellent in last week | 110 (9.2) | 38 (9.5) | 32 (8.1) | 40 (9.9) |
| Use of household insecticides in the last week* | 74 (6.2) | 22 (5.5) | 24 (6.0) | 28 (6.9) |
*Missing data.
Malariometric characteristics of the survey participants
Overall P. falciparum prevalence detected by microscopy was 32.8% (393/1199) in the study area (Table 2). Prevalence of P. falciparum was highest in children aged 5 to < 10 years old (53.7%) and 10 to < 15 years old (56.7%) compared to other age strata, with these two age groups responsible for 55.0% of the parasite burden in the population (216/393 P. falciparum positive participants) (Fig. 2). Overall, 3.3% (40/1199) of individuals had high density P. falciparum infections (> 5,000 parasites/µL), with prevalence highest in children aged 2 to < 5 years old (9.0%, 14/155) and 5 to < 10 years old (9.0%, 22/244) (Table 2). None of those aged > 30 years had high density P. falciparum infections. Geometric mean density of P. falciparum parasites was highest in children aged 2 to < 5 years at 3333.9 (1924.2–5776.3). Overall, the P. falciparum gametocyte prevalence was 4.8% (57/1199) with slightly higher prevalence among children < 15 years than in older age groups. Only a few individuals harboured other Plasmodium species with 0.8% (9/1199) infected with P. malariae and 1.7% (20/1199) with mixed P. falciparum and P. malariae infection.
Table 2.
Parasitological characteristics of cross-sectional survey participants.
| Variable | Overall N = 1199 | 2 to < 5 years N = 155 | 5 to < 10 years N = 244 | 10 to < 15 years N = 150 | 15 to < 20 years N = 117 | > 20 years N = 533 | |
|---|---|---|---|---|---|---|---|
| Plasmodium infection | |||||||
| P. falciparum | n (%) | 393 (32.8) | 36 (23.2) | 131 (53.7) | 85 (56.7) | 44 (37.6) | 97 (18.2) |
| P. malariae | n (%) | 9 (0.8) | 0 | 7 (2.9) | 1 (0.7) | 0 | 1 (0.2) |
| Mixed infection (P. falciparum + P. malariae) | n (%) | 20 (1.7) | 2 (1.3) | 12 (4.9) | 4 (2.7) | 1 (0.9) | 1 (0.2) |
| P. ovale | n (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| P. vivax | n(%) | 0 | 0 | 0 | 0 | 0 | 0 |
| P. falciparum high density (> 5,000 parasites/µL) prevalence, detected by microscopy | n (%) | 40 (3.3)* | 14 (9.0) | 22 (9.0) | 4 (2.7) | 0 | 0 |
| Geometric mean of P. falciparum density per μL | (95%CI) | 573.5 (491.0–669.7) | 3333.9 (1924.2–5776.3) | 882.0 (682.7–1139.5) | 507.9 (385.9–668.4) | 294.9 (200.6–433.6) | 234.3 (182.6–300.6) |
| Gametocyte prevalence P.f (any density) | n (%) | 57 (4.8) | 12 (7.7) | 17 (7.0) | 10 (6.7) | 3 (2.6) | 15 (2.8) |
| Geometric mean P.f gametocytes density per μL | (95% CI) | 31.3 (22.8–43.0) | 66.1 (25.1–174.3) | 40.2 (21.1–76.6) | 20.6 (10.1–41.9) | 35.4 (11.6–108.4) | 16.7 (12.0–23.3) |
|
Fever (body temperature ≥ 37.5 °C or history of fever in the previous 48 h) |
n (%) | 80 (6.7) | 8 (5.2) | 14 (5.7) | 13 (8.7) | 5 (4.3) | 40 (7.5) |
| Prevalence of symptomatic P. falciparum malaria (fever + microscopy positive) | n (%) | 32 (2.7) | 3 (1.9) | 9 (3.7) | 9 (6.0) | 3 (2.6) | 8 (1.5) |
| Prevalence of asymptomatic P. falciparum infection | n (%) | 361 (30.1) | 33 (21.3) | 122 (50.0) | 76 (50.7) | 41 (35.0) | 89 (16.7) |
*2/40 were mixed P.f/P.m infections.
Figure 2.
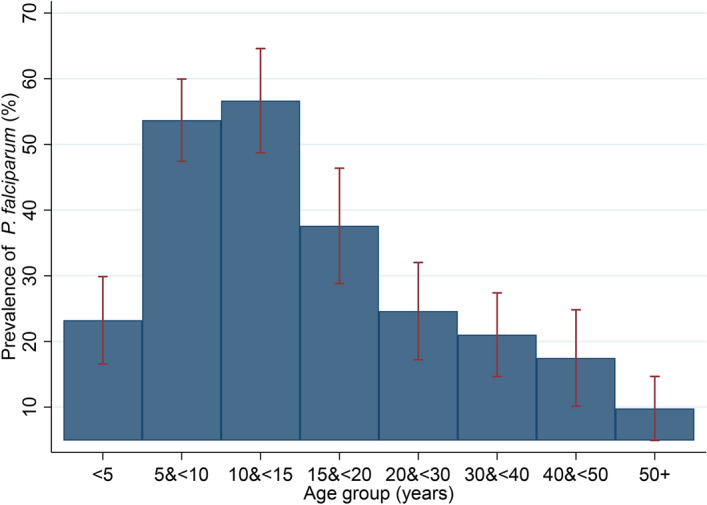
Prevalence of P. falciparum infection by age group in study population. Error bars are 95% confidence intervals.
Prevalence of symptomatic P. falciparum malaria (those with fever or reported fever within 48 h with microscopically confirmed parasitaemia) was 2.7% (32/1199) in the study population. Asymptomatic P. falciparum infection was found in 30.1% (361/1199) of all study participants, and was highest in children aged 5 to < 10 years (50.0%, 122/244) and 10 to < 15 years old (50.7% 76/150).
Risk factors for P. falciparum infection in survey participants
In the multivariate analysis, children aged 5 to < 10 years old were at 3.74 times the odds (95% CI = 2.68–5.22, P < 0.001) and those aged 10 to 15 years old at 3.14 times the odds (95% CI = 1.20–8.21, P = 0.02) of P. falciparum infection compared to children aged less than 5 years old (Table 3). Reporting sleeping under an ITN the previous night was associated with 0.47 times the odds of P. falciparum infection (95% CI = 0.31–0.71, P < 0.001), compared to those that did not report using an ITN. There was evidence of reduced odds of P. falciparum infection among individuals that reported travel in the previous fortnight (OR = 0.51, 95% CI = 0.30–0.87, P = 0.01) and individuals sleeping in a room with electric fan (OR = 0.39, 95% CI = 0.18–0.81, P = 0.01), although numbers of those reporting travel in the past 2 weeks and sleeping with a fan were relatively few (4/1199 and 74/1199, respectively). No association was found between risk of P. falciparum infection and gender, ethnicity, education, occupation, religion, socio-economic status, eave status, roof material, presence of an electric light in the sleeping room, use of topical repellent or household insecticides, having animals tethered within 5 m of the house, EIR and % mortality in a WHO tube test against 0.05% deltamethrin.
Table 3.
Risk factors for P. falciparum infection in study participants.
| Risk factors | P. falciparum prevalence n/N (%) | Univariate analysisa | Multivariate analysisb | ||
|---|---|---|---|---|---|
| Crude OR (95%CI) | P-value | Adjusted OR (95%CI) | P-value | ||
| Age (years) | |||||
| 2 to < 5 | 36/155 (23.2) | 1 | - | 1 | |
| 5 to < 10 | 131/244 (53.7) | 3.83 (2.48–5.91) | < 0.001 | 3.74 (2.68–5.22) | < 0.001 |
| 10 to < 15 | 85/150 (56.7) | 4.32 (2.19–8.53) | < 0.001 | 3.14 (1.20–8.21) | 0.02 |
| 15 to < 20 | 44/117 (37.6) | 1.99 (1.17–3.40) | 0.01 | 1.31 (0.81–2.12) | 0.28 |
| ≥ 20 | 97/533 (18.2) | 0.74 (0.51–1.07) | 0.11 | 0.45 (0.26–0.75) | 0.002 |
| Gender | |||||
| Male | 199/539 (36.9) | 1 | 1 | ||
| Female | 194/660 (29.4) | 0.71 (0.58–0.87) | 0.001 | 0.79 (0.56–1.10) | 0.16 |
| Ethnicity | |||||
| Gouin and Turka | 214/622 (34.4) | 1 | 1 | ||
| Karaboro | 65/226 (28.8) | 0.77 (0.57–1.04) | 0.09 | 1.02 (0.69–1.51) | 0.91 |
| Other ethnic groups | 114/351 (32.5) | 0.92 (0.63–1.34) | 0.66 | 0.95 (0.57–1.57) | 0.85 |
| Education | |||||
| Illiterate | 232/845 (27.5) | 1 | 1 | ||
| Literate | 161/353 (45.6) | 2.22 (1.92–2.56) | < 0.001 | 1.15 (0.93–1.44) | 0.20 |
| Occupation | |||||
| None / retired | 217/522 (41.6) | 1 | 1 | ||
| Farmer / pastoral sector | 171/657 (26.0) | 0.49 (0.38–0.65) | < 0.001 | 1.42 (0.94–2.17) | 0.10 |
| Commercial and Office worker | 5/20 (25.0) | 0.47 (0.18–1.25) | 0.13 | 1.16 (0.30–4.52) | 0.83 |
| Religion | |||||
| Muslim | 253/784 (32.3) | 1 | 1 | ||
| Christian | 56/172 (32.6) | 1.01 (0.61–1.67) | 0.96 | 0.78 (0.50–1.22) | 0.37 |
| Animist | 84/243 (34.6) | 1.11 (0.86–1.44) | 0.43 | 0.88 (0.64–1.21) | 0.44 |
| Socio-Economic Status quintile | |||||
| Quintile 1 (lowest) | 79/237 (33.3) | 1 | 1 | ||
| Quintile 2 (low) | 81/238 (34.0) | 0.96 (0.91–1.02) | 0.21 | 0.99 (0.89–1.09) | 0.82 |
| Quintile 3 (middle) | 84/239 (35.1) | ||||
|
Quintile 4 (high) |
72/235 (30.6) | ||||
|
Quintile 5 (highest) |
73/237 (30.8) | ||||
| History of travel in the previous 2 weeks | |||||
| No | 389/1177 (33.1) | 1 | 1 | ||
| Yes | 4/22 (18.2) | 0.45 (0.22–0.94) | 0.03 | 0.51 (0.30–0.87) | 0.01 |
| Slept under a bednet the previous night | |||||
| No | 44/111 (39.6) | 1 | 1 | ||
| Yes | 348/1087 (32.0) | 0.72 (0.47–1.10) | 0.13 | 0.47 (0.31–0.71) | < 0.001 |
| Eave construction of the sleeping room of the subject | |||||
| Closed | 258/789 (32.7) | 1 | 1 | ||
| Open | 59/198 (29.8) | 0.87 (0.52–1.45) | 0.60 | 1.55 (0.99–2.41) | 0.05 |
| Roof construction of the sleeping room of the subject | |||||
| Metal | 229/689 (33.2) | 1 | 1 | ||
| Thatch/mud | 88/298 (29.5) | 0.84 (0.62–1.13) | 0.26 | 1.10 (0.61–1.96) | 0.76 |
| Sleeping room of subject has a functioning lightbulb installed | |||||
| No | 67/223 (30.0) | 1 | 1 | ||
| Yes | 250/763 (32.8) | 1.13 (0.89–1.45) | 0.31 | 1.23 (0.78–1.93) | 0.37 |
| Sleeping room of subject has a functioning fan | |||||
| No | 301/913 (33.0) | 1 | 1 | ||
| Yes | 16/74 (21.6) | 0.56 (0.38–0.82) | 0.003 | 0.39 (0.18–0.81) | 0.01 |
| Animals tethered within 5 m of the house | |||||
| No | 298/922 (32.3) | 1 | 1 | ||
| Yes | 19/65 (29.2) | 0.86 (0.44–1.68) | 0.67 | 1.12 (0.54–2.33) | 0.76 |
| Use of topical repellent in last week | |||||
| None | 355/1089 (32.6) | 1 | 1 | ||
| Yes | 38/110 (34.5) | 1.09 (0.77–1.55) | 0.62 | 0.95 (0.42–2.12) | 0.90 |
| Use of household insecticides in the last week | |||||
| None | 290/913 (31.8) | 1 | 1 | ||
| Yes | 27/74 (36.5) | 1.23 (0.99–1.54) | 0.07 | 1.68 (0.79–3.61) | 0.18 |
| EIR (village-level) | 1.00 (1.00–1.00) | 0.03 | 1.00 (0.99–1.00) | 0.76 | |
| % mortality in WHO tube test against 0.05% deltamethrin | 1.65 (1.21–2.25) | 0.002 | 2.10 (0.82–5.37) | 0.12 | |
aAdjusted for clustering by village.
bAdjusted for clustering by village and all other factors in model.
Potential impact of extending the age eligibility for SMC
The transmission modelling indicates that at 91% usage of pyrethroid ITNs, administration of SMC to children aged under 5 years reduces all-age clinical malaria case incidence by 14.7% from 789 cases per 1000 people/year to 673 cases/1000 people/year in the Cascades Region (assuming 81% coverage of SMC) (Fig. 3). Extending the age eligibility to children under 10 years old would reduce clinical malaria case incidence by a further 10% (to 606 cases/1000 people/year). Extending SMC eligibility to children under 15 years old would reduce case incidence by 14.6% to 575 cases/1000 people/year. Assuming coverage of pyrethroid-PBO nets, administration of SMC to children under 10 years old would reduce clinical malaria case incidence by 9.4% to 552 cases/1000 people/year, while administration to under 15 years old would reduce case incidence by 14.6% to 520 cases/1000 people/year.
Figure 3.
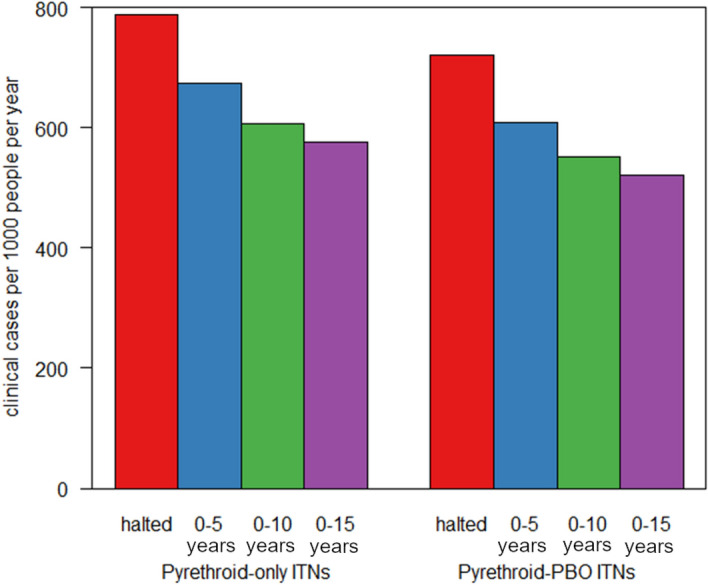
Clinical incidence of malaria predicted by either halting or extending the age range of the annual SMC campaign. Model predictions for the average number of clinical cases per year per 1000 people if SMC was halted (red bar), continued implementation across the existing target age (0–5 years, blue bar) or extended to 0–10 years old (green bar) or 0–15 years (purple bar). Incidence is averaged over the whole population (all ages) over a three-year period following the change in policy to reflect the regularity of mass ITN campaigns. ITNs are assumed to be either pyrethroid-only ITNs (left bars) or pyrethroid- piperonyl butoxide (PBO) ITNs (right bars).
Discussion
Symptomatic malaria was rare in the cross-sectional survey and 91.9% of P. falciparum infections were asymptomatic which presents a challenge for malaria control since these individuals will not seek care and therefore infections will not be cleared33. We show a high burden of P. falciparum infection of 32.8% across all age groups at the end of the malaria transmission season in 2017 despite ITN universal coverage campaigns in 2010, 2013 and 2016 and malaria case management with ACTs. P. falciparum prevalence was 23.2% in children under 5 years old. P. falciparum prevalence was, however, highest in children aged 5 to < 10 years old (53.7%) and 10 to < 15 years old (56.7%) suggesting these ages are an important reservoir of infection in the community. While we used post-hoc age strata in this analysis, the large sample size was sufficient to identify significant differences between the under 5 years old, 5–10 years old and 10–15 years old children.
Administration of SMC to school age children aged 5 to 15 years could have a substantial impact on clinical malaria incidence in Burkina Faso. We show that against a background of pyrethroid-PBO ITNs, expanding the age group for SMC to 5–10 years old could avert an additional 57 cases/1000 people and to 10–15 years old could avert an additional 89 cases/1000 people/year in the Cascades Region of Burkina Faso. This is comparable to the additional 64 cases/1000 people/year predicted to be averted by switching from pyrethroid ITNs (673 cases/1000 people/year) to pyrethroid-PBO ITNs (609 cases/1000 people/year), assuming continued SMC administration to under 5 years old in the Cascades Region of Burkina Faso. The impact of expanding eligibility for SMC will depend on the type of ITN deployed in a region. Here we model the impact of SMC against a background of standard pyrethroid ITNs and pyrethroid-PBO ITNs. Further second generation ITNs are being rolled out, including for example, chlorfenapyr and alpha-cypermethrin ITNs. We lack field data to model the impact of chlorfenapyr and alpha-cypermethrin ITNs and so the models presented should be refined once the effectiveness of these ITNs is better understood.
Intermittent preventive treatment of malaria in school children (aged 5 to 15 years) has been trialled extensively across sub-Saharan Africa in both seasonal and perennial transmission settings with different treatment regimens (e.g. once, termly, monthly). A systematic review of intermittent preventive treatment of malaria in school age children found a 73% reduction in P. falciparum prevalence, 60% reduction in clinical malaria and 23% reduction in anaemia using study level meta-analysis of 13 studies conducted in West, Central and East Africa34. Individual participant data meta-analysis identified a marginal effect of intermittent preventive treatment in children aged 10–15 years on cognitive test scores, although no difference was found in all ages34. The review found intermittent preventive treatment to be well tolerated and acceptable to communities. School-aged children are significant reservoirs of human-to-mosquito transmission35–37. There is some evidence that administration of intermittent preventive treatment to school-age children can confer a community-level benefit to those not receiving intermittent preventive treatment. A clinical trial evaluating administration of SMC to children aged up to 10 years in Senegal showed a 26% reduction in malaria incidence in adults and children too old to receive SMC38. Our transmission dynamic models predict a 14.6% reduction in malaria incidence in all ages, which is probably a reflection of the higher transmission in our study setting compared to Senegal.
The potential benefit of expanding the age group eligible for SMC in Burkina Faso needs to be weighed against its potential risks, including development of drug resistance and/or the risk of hindering acquisition or maintenance of immunity. Cost implications and feasibility would also need to be considered. Use of SP-AQ for SMC in Burkina Faso avoids the first line treatment AL thus minimising potential harmful impacts should resistance develop. While theoretical models predict an ‘immunological deficit’ leading to higher burden in older children39, evidence from studies of intermittent preventive treatment in infants and children under 5 years old does not indicate this40,41. As malaria declines, the burden of malaria shifts to older children28,42,43 and so identification of interventions to protect these groups should be a priority. While expanding SMC and changing to pyrethroid-PBO ITNs will avert a considerable number of clinical cases, moving towards elimination in high transmission areas will require sustained coverage of existing interventions and development of additional interventions.
So far, Senegal is the only country in the Sahel that is administering SMC to children aged up to 10 years with clinical trials showing a high protective efficacy against malaria38,44,45. Delivery of SMC to children under 10 years old in Senegal is conducted by community health workers who conduct visits house-to-house. Interestingly, Bâ et al. found that while increasing the age group almost doubled the target population, it only increased the number of households to visit by 13%45. Whether community-based delivery would be feasible in Burkina Faso needs to be considered. School-based SMC delivery is another option46,47, although schools are often closed during the rainy season which can limit use of this delivery route during the peak transmission season47.
As well as age, this study identified several other important risk factors for malaria. Study participants who reported sleeping under an ITN the previous night had a 53% lower odds of P. falciparum infection after adjusting for all other risk factors. This suggests that bednets continue to provide personal protection in the study area despite high levels of insecticide resistance13,48. We also found evidence of an association between use of a fan and reduced P. falciparum infection, although sleeping in a room with an electric fan was not common in the study population (6.2%). As well as making sleeping under an ITN more comfortable, if sufficiently powerful fans will also discourage landing and feeding of malaria mosquitoes.
Conclusion
P. falciparum infection in the Cascades Region of Banfora in Burkina Faso remains high despite universal coverage with ITNs and access to diagnosis and treatment. P. falciparum infection burden was concentrated in the 5–15 years old children and was predominantly asymptomatic. Additional interventions are needed to target this population group. Transmission dynamic modelling suggests that, even against a background of pyrethroid-PBO ITNs, administration of SMC to school age children may be able to substantially reduce malaria burden in south-west Burkina Faso and other countries in the region. Further research should be conducted to determine the feasibility, effectiveness and cost-effectiveness of SMC administered to 5–15 year old children in Burkina Faso and other countries in the Sahel.
Acknowledgements
The authors wish to thank the CNRFP staff, community members, opinion leaders, the community health workers, research assistants, field supervisors and workers whose cooperation and help made this study possible.
Author contributions
Conceived and designed the study: S.W.L., A.L.W., A.T., N.F.S., H.R. Conducted field and laboratory work: J.B.Y., A.T., A.O., Z.A.O. A.D., A.T., M.L., I.S., A.S. Conducted data analysis: J.B.Y., A.L.W., S.W.L., A.T., A.O., E.A. Conducted transmission dynamic modelling: B.L., T.C.. Drafted manuscript: A.W., J.B.Y., T.C. Contributed to and approved the final manuscript: J.B.Y., A.L.W., S.W.L., A.T., A.O., B.L., Z.A.O., A.D., A.T., M.L., I.S., A.S., E.W., E.A., N.F.S., H.R., T.C. All authors read and approved the final manuscript.
Funding
This project was supported by the Wellcome Trust (Wellcome Trust Collaborative Award “Improving the efficacy of malaria prevention in an insecticide resistant Africa (MiRA)” to the Liverpool School of Tropical Medicine grant agreement number 200222/Z/15/Z). SWL and ALW are supported by the Global Challenges Research Fund and Biotechnology and Biological Sciences Research Council (BB/R00532X/1) award to the BOVA Network (Building Out Vector borne diseases in sub-Saharan Africa). AT and JB received support from the UK MRC and the UK DFID (#MR/R010161/1) under the MRC/DFID Concordat agreement and as part of the EDCTP2 programme supported by the European Union. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Malaria Report 2020. WHO; 2020. [Google Scholar]
- 3.WHO & RBM Partnership to End Malaria . High Burden to High Impact: A Targeted Malaria Response. World Health Organization; 2019. [Google Scholar]
- 4.Ministère de la Santé Burkina Faso. Annuaire statistique 2014. (Ministère de la Santé Burkina Faso, Ouagadougou, 2015).
- 5.Ministère de la Santé Burkina Faso. Annuaire statistique 2018 (Ministère de la Santé Burkina Faso, Ouagadougou, 2019).
- 6.Ministère de la Santé Burkina Faso. Annuaire Statistique 2020. (Ministère de la Santé Burkina Faso, Ouagadougou, 2020).
- 7.WHO. WHO Policy Recommendation: Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. (World Health Organization, Geneva, 2012).
- 8.Yaro JB, et al. A cohort study to identify risk factors for Plasmodium falciparum infection in Burkinabe children: implications for other high burden high impact countries. Malar J. 2020;19:109. doi: 10.1186/s12936-020-03443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt S, et al. Coverage and system efficiencies of insecticide-treated nets in Africa from 2000 to 2017. eLife. 2015 doi: 10.7554/eLife.09672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karl S, Katusele M, Freeman TW, Moore SJ. Quality control of long-lasting insecticidal nets: are we neglecting It? Trends Parasitol. 2021;37:610–621. doi: 10.1016/j.pt.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay SW, Thomas MB, Kleinschmidt I. Threats to the effectiveness of insecticide-treated bednets for malaria control: thinking beyond insecticide resistance. Lancet Glob. Health. 2021;3:109. doi: 10.1016/S2214-109X(21)00216-3. [DOI] [PubMed] [Google Scholar]
- 12.Toé KH, et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness in Burkina Faso. Emerg. Infect. Dis. 2014;20:1691–1696. doi: 10.3201/eid2010.140619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes A, Lissenden N, Viana M, Toé KH, Ranson H. Anopheles gambiae populations from Burkina Faso show minimal delayed mortality after exposure to insecticide-treated nets. Parasit. Vectors. 2020;13:17. doi: 10.1186/s13071-019-3872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanou A, et al. Insecticide resistance and behavioural adaptation as a response to long-lasting insecticidal net deployment in malaria vectors in the Cascades region of Burkina Faso. Sci. Rep. 2021;11:17569. doi: 10.1038/s41598-021-96759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiono AB, et al. Malaria incidence in children in South-West Burkina Faso: comparison of active and passive case detection methods. PLoS ONE. 2014;9:e86936. doi: 10.1371/journal.pone.0086936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiono AB, et al. Dynamics of malaria transmission and susceptibility to clinical malaria episodes following treatment of Plasmodium falciparum asymptomatic carriers: results of a cluster-randomized study of community-wide screening and treatment, and a parallel entomology study. BMC Infect. Dis. 2013;13:535. doi: 10.1186/1471-2334-13-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanou A, et al. Evaluation of mosquito electrocuting traps as a safe alternative to the human landing catch for measuring human exposure to malaria vectors in Burkina Faso. Malar J. 2019;18:386. doi: 10.1186/s12936-019-3030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guglielmo F, et al. Quantifying individual variability in exposure risk to mosquito bites in the Cascades region, Burkina Faso. Malar J. 2021;20:44. doi: 10.1186/s12936-020-03538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.QGIS Geographic Information System http://www.qgis.org (QGIS Association, 2021).
- 20.OpenStreetMap contributors. OpenStreetMap https://www.openstreetmap.org (2021).
- 21.Ministere de la Sante Burkina Faso. Directives nationales pour la prise en charge du paludisme dans les formations sanitaires du Burkina Faso. (2014).
- 22.Institut National de la Statistique et de la Démographie, Programme National de Lutte contre le Paludisme & ICF International. Enquête sur les Indicateurs du Paludisme (EIPBF) 2014 (2015).
- 23.WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes - second edition. (World Health Organization, Geneva, 2016).
- 24.Institut National de la Statistique et de la Démographie (INSD), Programme d’Appui au Développement Sanitaire (PADS), Programme National de Lutte contre le Paludisme (PNLP) & ICF. Enquête sur les indicateurs du paludisme au Burkina Faso 2017–2018. (ICF, Rockville, Maryland, USA, 2018).
- 25.Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2. Blackwell; 2003. [Google Scholar]
- 26.Griffin JT, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7:e1000324. doi: 10.1371/journal.pmed.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winskill P, Slater HC, Griffin JT, Ghani AC, Walker PGT. The US President's Malaria Initiative, Plasmodium falciparum transmission and mortality: a modelling study. PLoS Med. 2017;14:e1002448. doi: 10.1371/journal.pmed.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin JT, Ferguson NM, Ghani AC. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat. Commun. 2014;5:3136. doi: 10.1038/ncomms4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cairns ME, et al. Seasonality in malaria transmission: implications for case-management with long-acting artemisinin combination therapy in sub-Saharan Africa. Malar J. 2015;14:321. doi: 10.1186/s12936-015-0839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nash RK, et al. Systematic review of the entomological impact of insecticide-treated nets evaluated using experimental hut trials in Africa. CRPVBD. 2021;1:100047. doi: 10.1016/j.crpvbd.2021.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Churcher TS, Lissenden N, Griffin JT, Worrall E, Ranson H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife. 2016;4:e16090. doi: 10.7554/eLife.16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherrard-Smith E, et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat. Med. 2020;26:1411–1416. doi: 10.1038/s41591-020-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert. Rev. Anti. Infect. Ther. 2013;11:623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 34.Cohee LM, et al. Preventive malaria treatment among school-aged children in sub-Saharan Africa: a systematic review and meta-analyses. Lancet Global Health. 2020;8:e1499–e1511. doi: 10.1016/S2214-109X(20)30325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andolina C, et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect. Dis. 2021 doi: 10.1016/s1473-3099(21)00072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coalson JE, et al. Simulation models predict that school-age children are responsible for most human-to-mosquito Plasmodium falciparum transmission in southern Malawi. Malar J. 2018;17:147. doi: 10.1186/s12936-018-2295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonçalves BP, et al. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat. Commun. 2017;8:1133. doi: 10.1038/s41467-017-01270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cissé B, et al. Effectiveness of seasonal malaria chemoprevention in children under ten years of age in Senegal: a stepped-wedge cluster-randomised trial. PLoS Med. 2016;13:e1002175. doi: 10.1371/journal.pmed.1002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pemberton-Ross P, Smith TA, Hodel EM, Kay K, Penny MA. Age-shifting in malaria incidence as a result of induced immunological deficit: a simulation study. Malar J. 2015;14:287. doi: 10.1186/s12936-015-0805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grobusch MP, et al. No rebound of morbidity following intermittent preventive sulfadoxine-pyrimethamine treatment of malaria in infants in Gabon. J. Infect. Dis. 2009;200:1658–1661. doi: 10.1086/647990. [DOI] [PubMed] [Google Scholar]
- 41.Geerligs PD, Brabin BJ, Eggelte TA. Analysis of the effects of malaria chemoprophylaxis in children on haematological responses, morbidity and mortality. Bull. World Health Organ. 2003;81:205–216. [PMC free article] [PubMed] [Google Scholar]
- 42.Ceesay SJ, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet. 2008;372:1545–1554. doi: 10.1016/s0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Meara WP, et al. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–1562. doi: 10.1016/s0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Druetz T, et al. Impact evaluation of seasonal malaria chemoprevention under routine program implementation: a quasi-experimental study in Burkina Faso. Am. J. Trop. Med. Hyg. 2018;98:524–533. doi: 10.4269/ajtmh.17-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bâ E-H, et al. Implementation, coverage and equity of large-scale door-to-door delivery of Seasonal Malaria Chemoprevention (SMC) to children under 10 in Senegal. Sci. Rep. 2018;8:5489. doi: 10.1038/s41598-018-23878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke SE, et al. Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:127–138. doi: 10.1016/S0140-6736(08)61034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke SE, et al. Impact of a malaria intervention package in schools on Plasmodium infection, anaemia and cognitive function in schoolchildren in Mali: a pragmatic cluster-randomised trial. BMJ Glob. Health. 2017;2:e000182. doi: 10.1136/bmjgh-2016-000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toé KH, et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect. Dis. 2014;20:1691–1696. doi: 10.3201/eid2010.140619. [DOI] [PMC free article] [PubMed] [Google Scholar]