Abstract
Purpose
Our purpose was to assess the suitability of airway-implanted internal fiducial markers and an external surrogate of respiratory motion for motion management during radiation therapy of lung tumors.
Methods and Materials
We analyzed 4-dimensional computed tomography scans acquired during radiation therapy simulation for 28 patients with lung tumors who had anchored fiducial markers bronchoscopically implanted inside small airways in or near the tumor in a prospective trial. We used a linear mixed model to build population-based correlative models of tumor and surrogate motion. The first 24 of the 28 patients were used to build correlative models, and 4 of the 28 consecutive patients were excluded and used as an internal validation cohort. Of the 24 patients from the model building cohort, all were used for the models based on the internal fiducial. The external surrogate was completely visualized in 11 patients from the model building cohort, so only those were used for the models based on the external surrogate. Furthermore, we determined the predicted residual error sum of squares for our correlative models, which may serve as benchmarks for future research.
Results
The motion of the internal fiducials was significantly associated with the tumor motion in the anterior-posterior (P < .0001) and superior-inferior (SI) directions (P < .0001). We also observed a strong correlation of the external surrogate anterior-posterior motion to the tumor dominant SI motion (P < .0001). In the validation cohort, the internal fiducial SI motion was the only reliable predictor of lung tumor motion.
Conclusions
The internal fiducials appear to be more reliable predictors of lung tumor motion than the external surrogate. The suitability of such airway-implanted internal fiducial markers for advanced motion management techniques should be further investigated. Although the external surrogate seems to be less reliable, its wide availability and noninvasive application support its clinical utility, albeit the greater uncertainty will need to be compensated for.
Introduction
Precise delivery of high effective doses of therapeutic radiation with optimal avoidance of nearby normal organs at risk is the hallmark of modern radiation therapy (RT). Image guidance and respiratory-motion management are crucial for delivering conformal doses to moving targets because these techniques optimize the accuracy of target localization and may enable normal tissue sparing.1
In passive motion management methods, the planning target volumes are often defined by encompassing the entire range of tumor motion observed on 4-dimensional computed tomography (4D-CT), the so-called “internal target volume” approach,2 or by using a midventilation approach.3 Passive motion management methods often result in large target volumes, thus increasing normal tissue exposure to irradiation. In contrast, active motion management techniques, including breath-hold, gating, and tracking may allow for more accurate target localization and thus facilitate dose escalation and sparing of adjacent healthy tissues. In breath-hold techniques, a reproducible state of maximum breath-hold in inspiration (deep-inspiration breath-hold [DIBH]) or expiration (deep-expiration breath-hold) is used to immobilize the target.4 In respiratory gating, the breathing cycle is monitored, and the beam turned on only during a predefined interval.5 Real-time tumor tracking during RT holds great potential in increasing confidence in precisely targeting the true tumor position and may hypothetically eliminate the need for tumor-margins to account for motion. The motion management techniques can be accomplished by different signals of respiratory motion: (1) imaging of the tumor itself via, for example, fluoroscopy; (2) imaging of radiopaque internal fiducial markers implanted in or near the tumor; (3) inference of the tumor position from external surrogates of respiratory motion; and (4) nonradiographic tracking of an active or passive signaling device implanted in or near the tumor.6
Recently, a world-wide survey assessed the clinical practice of respiratory motion management, including gating in free-breathing or breath-hold and tracking, in 200 radiation oncology departments.7 External surrogates were the most commonly used signal for motion management (61%). However, there might be substantial error between external surrogates and internal tumor motion.8,9 Internal fiducial markers are thought to be more reliable for respiratory motion management. These may take the form of radiopaque fiducials that can be placed either percutaneously or using an intravascular coil method. Percutaneous placement of fiducial markers is an invasive procedure and may lead to complications such as pneumothorax10 and hemorrhage.11 Alternatively, fiducial markers may be deployed endobronchially using navigational bronchoscopy.12,13 An anchored internal fiducial that functions as an electromagnetic transponder has been developed and approved by the U.S. Food and Drug Administration for motion management in thoracic malignancies14; the anchored design prevents them from migrating within the airways.15,16 After the bronchoscopic implant of 3 such internal fiducials inside the small airways in or near the tumor, they can be tracked during RT using an electromagnetic array placed above the patient and used to predict the target position.17
The accuracy of predicting the target position based on external or internal surrogates is a key issue of motion management techniques.6 Quantifying the correlation of respiratory motion of surrogates and tumors and determining random and systematic errors may increase confidence in the delivered dose and facilitate their use for active motion management techniques, such as breath-hold, gating or tumor tracking, and the deployment of limited tumor margins to address respiratory motion.
In this in silico study, using 4D-CT data from a prospective trial, we tested the hypothesis that motion of bronchoscopically airway-implanted internal fiducial markers and/or a commonly used external surrogate for monitoring respiratory motion are well correlated with lung tumor respiratory motion during free-breathing. We then built population-based correlative models for predicting tumor displacement based on the displacement of the studied surrogates to assess the suitability of internal fiducials and the external surrogate for subsequent use at treatment to determine the tumor position of the day in the presence of interfraction variability.
A strong population-based correlation of surrogates with tumor position during free-breathing would increase confidence in the internal fiducials and warrant further investigation of their application for advanced motion management techniques such as tumor tracking or gating. An ongoing prospective trial (NCT No. NCT02434809; www.clinicaltrials.gov) is currently investigating gating based on such airway-implanted fiducials, which function as electromagnetic transponders, to reduce target position uncertainty in radiation treatment of lung cancer, while another ongoing trial (NCT No. NCT02111681; www.clinicaltrials.gov) is studying DIBH based on the same internal fiducials.
Methods and Materials
Patients
The cohort in this study consists of 28 patients with lung tumors in an institutional review board–approved prospective protocol (NCT No. NCT02111681; www.clinicaltrials.gov) who were treated with RT during DIBH that was monitored and gated by a system to track implanted electromagnetic transponders. As part of the protocol, the patients underwent a 4D-CT at simulation in uncoached free breathing, using a commercially available system for motion management that uses an external surrogate. This system tracks an infrared reflective gating block on the patient's anterior abdominal surface, slightly inferior to the xyphoid, as an external surrogate of respiratory motion.
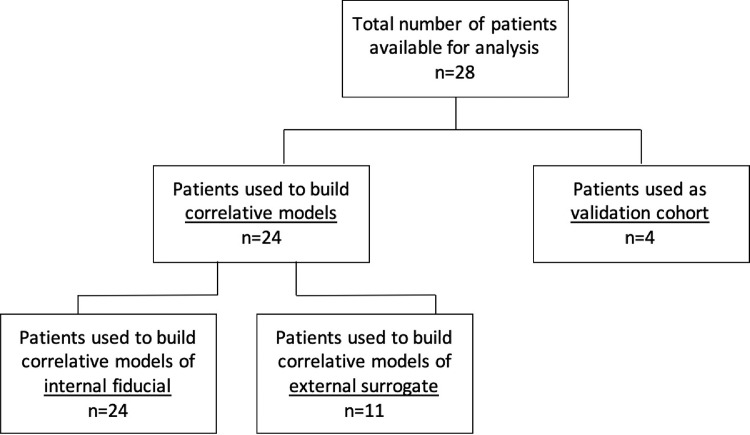
From the cohort of 28 patients, the first 24 treated in the study protocol were used to build correlative models predicting tumor displacement, while the consecutive 4 patients were used as an internal validation cohort to test the predictive power of our models (Fig. 1). All the measurements in this study were based on the 4D-CT scans. Because the external surrogate was completely imaged in the CT scans of only 11 of the 24 patients used to build the correlative models, the sample sizes of the different correlative models differ. The models for tumor and internal fiducial motion are based on 24 patients, whereas the models for tumor and external surrogate motion are based on 11 patients.
Fig. 1.
Study diagram indicating the distribution of patients in the correlative model building cohort and the internal validation cohort.
Technique and data acquisition
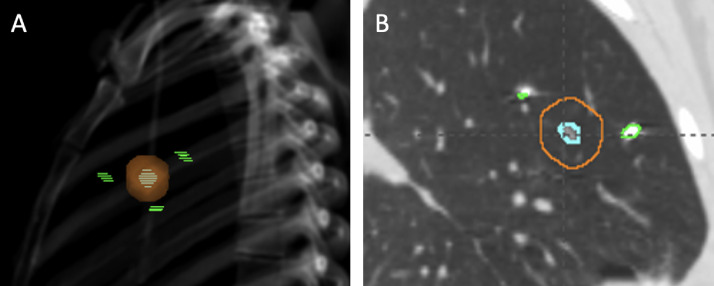
Three anchored transponders were bronchoscopically implanted by a pulmonologist, ideally in a triangular pattern in or near the tumor. After a minimum of 4 days post-transponder implantation, patients underwent CT simulation for RT planning, which included a free-breathing 4D-CT scan. Because the tracking system is not compatible with CT scanner geometry, breathing monitoring at simulation used the motion management system with the external surrogate that was described previously. Our present study is based on the analysis of the free-breathing 4D-CT scan. A gold-nickel-copper coil inside the transponders allows their radiographic visualization, and therefore for this study, we considered the transponders (hereafter referred to as “fiducials”) as passive, radiopaque fiducials (Fig. 2).
Fig. 2.
Representative images of lung tumor and bronchoscopically implanted fiducials. (A) Digitally reconstructed radiograph with the tumor (cyan dashed) and the planning target volume (orange) surrounded by 3 fiducials (green dashed). (B) Computed tomography scan (sagittal plane) showing the tumor (cyan) and the planning target volume (orange) with 2 fiducials (green) on the same plane.
The 4D-CT was acquired with a slice thickness of 2.5 mm, with 512 × 512 voxels. The 4D-CT data set was reconstructed into separate 3D-CT scans at each of 10 distinct respiratory phases (0%-90%) providing 10 volumetric data sets for each patient that sampled the complete respiratory cycle. We used end-exhalation, designated as 50%, as our reference phase. End-inhalation was designated as 0%. The images were imported into the treatment planning system, where all contouring was performed.
The gross tumor volume (GTV), the internal fiducials, and the external surrogate, when completely imaged, were contoured on each 4D-CT phase. GTV delineation was supervised by a radiation oncologist. The coordinate system used to describe our data is X = left-right (LR), Y = anterior-posterior (AP), and Z = superior-inferior (SI). The centroid coordinates of each structure were determined using a commercially available treatment planning system.
Quantification of motion
The motion of the centroid of the GTV (hereafter “GTV”), the centroid of the triangle formed by the 3 internal fiducials (hereafter “internal fiducial”), and the centroid of the external surrogate (hereafter “external surrogate”) were quantified on the 4D-CT scans by the difference between its coordinates in a selected phase and the coordinates in phase 50% in the LR, AP, and SI direction. We define excursion of a structure as the absolute value of the maximum displacement between the structure's position at end-exhalation and end-inhalation (phase 0%) as observed on the 4D-CT scans.
Statistical analysis of tumor-surrogate correlation and correlative model development
The correlation of the internal fiducial or external surrogate motion, in relation to the GTV's motion, was examined using a linear mixed model. It models the linear relationship between the dependent variable (GTV motion) and independent variables (external surrogate or internal fiducials). The displacements relative to the position at end-exhalation for the AP and SI directions were modeled respectively.
The rationale behind using a linear mixed model is that all patients are assumed to share a common intercept and slope, called the “fixed effect,” while each patient is also assumed to have a certain degree of variation from such fixed effects, that is, individual random effects of intercept and slope. Under such assumptions, the linear mixed models can be represented as18
In this linear mixed model, j and i denote the j-th 4D-CT phase of the i-th patient for the GTV () and the predictor (ie, centroid of the external surrogate or of the internal fiducials) movements, respectively, in the Y- or Z-dimensions. Both the random intercept and the random slope are patient-specific. The random error is for the j-th phase of the 4D-CT of the i-th patient. Furthermore, we assume the usual normal distributions for and that and are jointly normal with covariance and In addition, we assume ’s are independent across i and ’s are independent for all i and j. Such assumptions are typically used in the theory of linear mixed model. To implement the linear mixed models, we used R 3.1.1 with packages nlme, lme4, and lmmfit (http://www.r-project.org/). The restricted maximum likelihood approach was applied to generate statistical significance level, with a P value <.05 considered as statistically significant. To preliminarily assess the performance of the model, we also provided the predicted residual sum of squares (PRESS), which were calculated using the R package lmmfit statistical software.18
We applied the correlative models to predict the tumor motion in the 4 patients who were excluded initially to serve as a validation cohort. As the position of both tumor and surrogates was known in these patients, the surrogate displacement could be used to calculate the point predictions and 95% prediction intervals of the GTV displacement. The actually measured GTV displacement could then be compared with the computed predictions. If the prediction works well, the calculated 95% prediction interval should cover at least 95% of the actual values.
Results
Tumor volumes and range of tumor motion
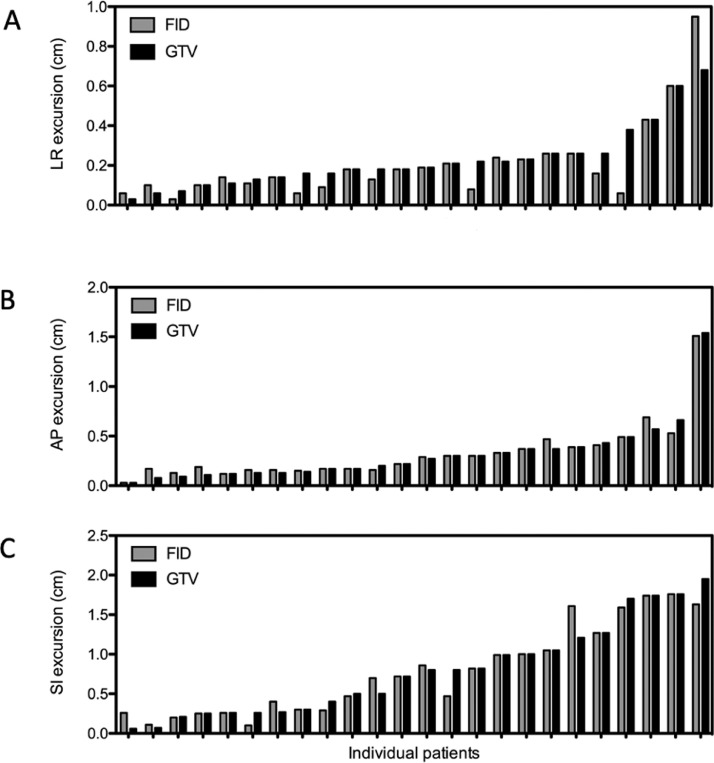
The patient characteristics are summarized in Table E1. The GTV motion was largest in the SI direction, with a mean excursion of 0.79 cm (Table 1). In the AP and LR directions, the mean excursions were 0.32 cm and 0.23 cm, respectively. The GTV excursions in the different directions are displayed in Figure 3. A more detailed summary of GTV and excursion of the individual patients can be found in Table E2. For the internal fiducials, the motion amplitude during the breathing cycle was similar to the tumors, with a mean excursion and range of 0.78 cm in the SI direction, 0.35 cm in the AP direction, and 0.20 cm in the LR direction. We only assessed the external surrogate motion on the 4D-CT scans in the AP direction as the associated motion management system would only capture motion in this dimension. The mean AP excursion of the external surrogate was 0.51 cm.
Table 1.
Maximum motion of structures in 3 directions
| Mean ± SD, cm (range) |
|||
|---|---|---|---|
| Structure | Left-right (X) | Anterior-posterior (Y) | Superior-inferior (Z) |
| GTV (n = 24) | 0.23 ± 0.15 (0.03-0.68) | 0.32 ± 0.31 (0.03-1.54) | 0.79 ± 0.58 (0.06-1.95) |
| Fiducial centroid (n = 24) | 0.20 ± 0.20 (0.03-0.95) | 0.35 ± 0.29 (0.08-1.51) | 0.78 ± .57 (0.1-2.0) |
| RPM block (n = 11) | 0.51 ± 0.28 (0.10-1.02) | ||
Abbreviations: GTV = gross tumor volume; RPM = real-time position management; SD = standard deviation.
The RPM block excursions were only measured in the anterior-posterior direction. Patients from the validation cohort (n = 4) were not included in this analysis.
Fig. 3.
Excursion (cm) of lung tumor GTV (black) and fiducial centroid (gray) between end-exhalation and end-inhalation for the 24 patients included in the correlative models, (A) in X (left-right) direction, (B) in Y (anterior-posterior) direction, and (C) in Z (superior-inferior) direction. Abbreviations: AP = anterior-posterior; FID = fiducial centroid; GTV = gross tumor volume; LR = left-right; SI = superior-inferior.
Correlation of tumor motion with internal fiducials and external surrogate
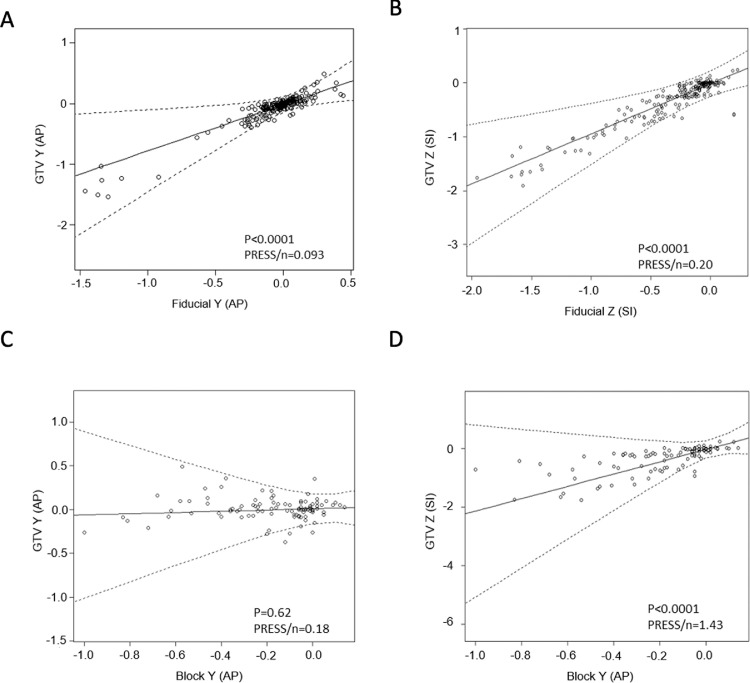
To investigate the correlation of the displacement between the GTV and the external surrogate or the internal fiducials, we used the linear mixed model. Figure 4 displays the graphs of the linear mixed models for the correlation of the GTV and the external surrogates or the internal fiducials, respectively, in the AP and SI dimensions. Each circle represents an individual patient's GTV displacement versus the displacement of the internal fiducials or the external surrogate, respectively.
Fig. 4.
Correlative linear mixed model for lung tumor (GTV) displacement and internal fiducials centroid (fiducial) or external surrogate centroid (block) (all quantities in cm). (A) Lung tumor displacement along the Y (AP) direction and fiducial displacement along the Y (AP) direction; n = 24. (B) Lung tumor displacement along the Z (SI) direction and fiducial displacement along the Z (SI) direction; n = 24. (C) Lung tumor displacement along the Y (AP) direction and external surrogate displacement along the Y (AP) direction; n = 11. (D) Lung tumor displacement along the Z (SI) direction and external surrogate displacement along the Y (AP) direction; n = 11. AP displacements are positive if the structure moves anterior relative to end-exhalation; SI displacements are positive if it moves superior relative to end-exhalation. Dots are data points, solid lines are best fits for prediction model, and dashed curves are 95% prediction intervals. Abbreviations: AP = anterior-posterior; block = centroid of gating block used as external surrogate; fiducial = fiducial centroid; GTV = gross tumor volume of the lung tumor; PRESS = predicted residual sum of squares; SI = superior-inferior.
The P values in Table E3 indicate whether the fixed slopes in the different correlative models are significantly different from zero, that is, whether the predictors are significantly associated with the GTV motion. The motion of the internal fiducials was significantly associated with the GTV motion in both AP and SI directions (P < .0001). Interestingly, the AP motion of the external surrogate was only associated with the SI motion of the GTV (P < .0001), the direction in which the respiratory target motion was largest, not with the AP motion of the GTV (P = .62).
The PRESS is a commonly used criterion to evaluate the performance of predictors. Smaller values of PRESS in general indicate a better prediction of GTV motion by the surrogate marker under structured modeling assumptions. The PRESS values of the different correlative models can be found in Table E3. Because the sets of predictors are not nested, ie, one model is not a submodel of another, and the sample sizes are different, as not all patients had the gating block completely imaged, one should not make inferences for directly comparing PRESS and drawing conclusions on a predictor's superiority using such values. Such PRESS values, however, may serve as benchmarks for future research and comparisons.
Correlative model of lung tumor motion
The linear mixed model was used to assess the correlation of GTV with the external surrogate and the internal fiducials, respectively. The linear mixed model accounts for (1) variability among patients by incorporating random intercept and slope and (2) variability within the same patient by specifying a random error term in the model.
Owing to the complexity of the computation of the prediction intervals, we supplied plots to facilitate the usage of our results (Fig. 4). The full set of the parameters used to calculate the point prediction of the GTV and the estimated standard error can be found in Table E3. In the Supplementary Material we also illustrate how to predict the GTV position based on the position of the fiducials and the set of parameters from Table E4 using the formula for the linear mixed model described by Laird and Ware19 or by using the plots in Figure 4.
We applied the correlative models to predict the tumor motion in the 4 patients who were excluded initially to serve as a validation cohort. If the correlative models reliably predict the GTV position from the displacement of the external surrogates and the internal fiducials, respectively, the calculated 95% prediction interval should cover at least 95% of the actual values. This held true for the correlative model of GTV SI and internal fiducial SI displacement (GTV Z vs FID Z), where 96% of the predicted GTV displacements fell within the 95% prediction interval (data not shown). The correlative model of GTV AP and internal fiducial AP displacement (GTV Y vs FID Y) correctly predicted 78% of the GTV displacements. The models of GTV AP and external surrogate AP displacement (GTV Y vs BLOCK Y) and GTV SI and external surrogate AP displacement (GTV Z vs BLOCK Y) correctly predicted 76% and 62%, respectively.
Discussion
In this study, we have invoked the statistical tool of linear mixed models for a population-based correlation of airway-implanted internal fiducials, external surrogates, and lung tumor respiratory motion. This is because individual patients may present different slopes and intercepts within the linear model, as confirmed by the parameter estimates (see Table E3 for the estimates of random elements) and individual scatter plots (figure not shown). Incorporating such random elements takes into account patient heterogeneity and generates more realistic predicted values of motion. Alternatively, our method can be seen as regarding each patient as a cluster and then considering within-cluster correlations. The population-based correlative models we built should not be used to predict the tumor position in any individual patient or influence individual internal target volume margins. For such purposes, patient individual data likely predict the motion range and errors more accurately. Population-based correlative modeling studies of internal markers and external surrogates, respectively, with lung tumor motion might rather serve as benchmarks to compare the reliability of different respiratory surrogates for complex active motion management techniques, such as breath-hold, gating, or tracking during RT. Future work should focus on validating the predictive models of respiratory tumor motion in larger independent patient cohorts. We have therefore provided indices of predictive power, PRESS, to allow for further comparisons of our predictive models and techniques.
External respiratory surrogates are still the most commonly used signals for motion management during RT.7 Respiratory motion management based on external markers only has been shown to be potentially misleading due to the possible discrepancy between external markers and true internal target motion.8,9 Although Chi et al20 demonstrated a strong correlation between abdominal motion and the SI tumor motion during the respiratory cycle, supporting the utilization of external surrogates for gated RT when the marker block used in widely available systems is placed adequately and consistently near the abdominal region to reduce errors, the external surrogate should be used with caution. However, for lung lesions with large respiratory motion amplitudes of 7 mm and more, significant day-to-day motion variability makes the pretreatment 4D-CT unreliable.21 Predicting the tumor position based on an external surrogate will therefore likely be inaccurate. Consequently, active management strategies for real-time tumor tracking, using frequent radiographic imaging with positional adjustment or electromagnetic transponders, should be applied during RT.22, 23, 24 Although we observed a strong association between the external surrogate AP motion and the tumor SI motion in our study, our correlative models failed to reliably predict tumor motion based on external surrogate displacement in the validation cohort. The lung tumor and fiducial motion patterns observed in our study are in line with previously published studies. Sarudis et al25 also showed that lung tumor motion is generally larger in the SI direction. Besides that, the authors found that motion amplitude in the SI direction increases for tumors located in the middle and lower parts of the lungs. To address this in our cohort, we compared the excursion of lower and upper lobe tumors using nonpaired Mann-Whitney tests for nonparametric data. However, no significant differences in the excursion for lower and upper lobe tumors were observed (AP direction, P = .7; SI direction, P = .8), while the mean excursion of lower lobe lesions was larger in all dimensions. Schmitt et al17 found comparable intrafraction motion of bronchoscopically implanted internal surrogates in patients with lower and upper lobe tumors. The population-based approach used in our study supports the notion that an external surrogate alone cannot be confidently used to predict a lung tumor's motion. However, their wide availability and noninvasive application still support their clinical utility, although the greater uncertainty will need to be compensated for with larger tumor margins, resulting in higher radiation exposure to organs at risk.
In our study, the internal airway-implanted markers appear to be more reliable predictors of lung tumor motion during free-beathing than the external surrogates. The suitability of airway-implanted internal fiducials for complex motion management techniques, including breath-hold, gating, and tracking, should thus be further investigated in large prospective trials. A recent study by Steiner et al26 found that 4D-CT under-predicts lung tumor motion during RT compared with the real-time imaging of the internal fiducials that were also applied in our study, using their function as electromagnetic beacons. Therefore, future studies should also focus on building correlative models based on fiducial motion as detected by the real-time imaging system. An ongoing prospective trial (NCT No. NCT02434809; www.clinicaltrials.gov) is currently investigating gating based on airway-implanted electromagnetic markers to reduce target position uncertainty in radiation treatment of lung cancer, while another ongoing trial (NCT No. NCT02111681; www.clinicaltrials.gov) is studying DIBH based on the same airway-implanted markers. Those studies should also validate the population-based correlative models to estimate the predictive power of different surrogates of respiratory motion. Additionally, although there are numerous applications of radiographic tracking of passive fiducials on a commercial robotic RT system,7 current methods of monitoring passive fiducials on conventional gantry-based linear accelerators are limited.24 For the fiducials in this study, active electromagnetic tracking by using their functionality as resonant circuits might allow for more consistent and reliable detection of their position at treatment and thus improve upon the simulation-based prediction of the target motion to assure accurate beam delivery. The ability to detect variations of the tumor motion amplitude and shifts/drifts from the baseline position in real-time during a treatment session could enable couch adjustments to move the tumor back into position. Ideally, such couch adjustments would be computer controlled.27
A number of limitations should be taken into account when interpreting the results of our study. The manual delineation of fiducials and external surrogate on 4D-CT scans might have introduced an element of uncertainty and potential error to our measurements. We do, however, consider the effect of the delineation of external surrogate and internal fiducials as minor, given their known and easily distinguishable shape on CT scans. Because our study was conducted on a single 4D-CT scan for each patient (ie, over a period of 1-2 minutes), variabilities of the motion amplitude and shifts/drifts of the baseline respiratory motion that would occur between fractions and within the delivery time of a single treatment have not been accounted for. Additionally, breathing variability during a common fraction of RT is likely greater than during the respiratory cycle captured on a single 4D-CT at simulation. Thus, the motion data from simulation that was used for correlative modeling might be less representative of actual respiratory motion during RT. As our validation cohort consisted of only 4 patients, the effect of outliers is large, which might explain why tumor SI motion was only reliably predicted based on the model using fiducial SI displacement.
Conclusion
The external surrogates seem to be a less reliable predictor of lung tumor motion during free-breathing than the internal fiducials. However, wide availability and noninvasive application of external surrogates still support their clinical utility, although the greater uncertainty will need to be compensated for with larger tumor margins, resulting in higher radiation exposure to organs at risk. It is important to note that intra- and interfraction changes of the internal-external relation, which have not been taken into account in our study, may further limit the reliability of the external surrogates, unless combined with additional imaging. For those less-expected changes, real-time monitoring of internal markers would likely provide an additional advantage over the external surrogates.
Footnotes
Sources of support: This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748 and by a research grant to the Memorial Sloan Kettering Cancer Center, the sponsor of the research, from Varian Medical Systems. The collaborator was not involved in study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Disclosures: Dr Rimner reports grants, personal fees, and nonfinancial support from Varian Medical Systems during the conduct of the study, grants from Boehringer Ingelheim, grants from Pfizer, grants and personal fees from AstraZeneca, grants and personal fees from Merck, personal fees from Cybrexa, nonfinancial support from Philips/Elekta, personal fees from MoreHealth, and personal fees from ResearchToPractice outside the submitted work. Dr Chawla, Mr Czmielewski, Mrs Dick-Godfrey, Mrs Gelb, Dr Lee, Dr Li, Dr Lovelock, Dr Sidiqi, Dr Wang, Dr Willman, Dr Yorke, and Dr Zhang report grants and nonfinancial support from Varian Medical Systems during the conduct of the study. Dr Wu reports grants and nonfinancial support from Varian Medical Systems during the conduct of the study, grants and nonfinancial support from CivaTech Oncology, Inc, personal fees from AstraZeneca, and nonfinancial support from AlphaTau Medical outside the submitted work.
Data sharing statement: Research data are not available at this time.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2021.100885.
Appendix. Supplementary materials
References
- 1.Caillet V, Booth JT, Keall P. IGRT and motion management during lung SBRT delivery. Physica Medica. 2017;44:113–122. doi: 10.1016/j.ejmp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Shih HA, Jiang SB, Aljarrah KM, Doppke KP, Choi NC. Internal target volume determined with expansion margins beyond composite gross tumor volume in three-dimensional conformal radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:613–622. doi: 10.1016/j.ijrobp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Wolthaus JWH, Schneider C, Sonke JJ, et al. Mid-ventilation CT scan construction from four-dimensional respiration-correlated CT scans for radiotherapy planning of lung cancer patients. Int J Radiat Oncol Biol Phys. 2006;65:1560–1571. doi: 10.1016/j.ijrobp.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Mageras GS, Yorke E. Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation. Treatment. 2004;14:65–75. doi: 10.1053/j.semradonc.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Berson AM, Emery R, Rodriguez L, et al. Clinical experience using respiratory gated radiation therapy: Comparison of free-breathing and breath-hold techniques. Int J Radiat Oncol Biol Phys. 2004;60:419–426. doi: 10.1016/j.ijrobp.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33:3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 7.Anastasi G, Bertholet J, Poulsen P, et al. Patterns of practice for adaptive and real-time radiation therapy (POP-ART RT) part I: Intra-fraction breathing motion management. Radiother Oncol. 2020;153:79–87. doi: 10.1016/j.radonc.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunjan S, Starkschall G, Prado K, Dong L, Balter P. Lack of correlation between external fiducial positions and internal tumor positions during breath-hold CT. Int J Radiat Oncol Biol Phys. 2010;76:1586–1591. doi: 10.1016/j.ijrobp.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Li R, Mok E, Han B, Koong A, Xing L. Evaluation of the geometric accuracy of surrogate-based gated VMAT using intrafraction kilovoltage x-ray images. Med Phys. 2012;39:2686–2693. doi: 10.1118/1.4704729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kothary N, Heit JJ, Louie JD, et al. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Intervent Radiol. 2009;20:235–239. doi: 10.1016/j.jvir.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Yousefi S, Collins BT, Reichner CA, et al. Complications of thoracic computed tomography-guided fiducial placement for the purpose of stereotactic body radiation therapy. Clin Lung Cancer. 2007;8:252–256. doi: 10.3816/CLC.2007.n.002. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder C, Hejal R, Linden PA. Coil spring fiducial markers placed safely using navigation bronchoscopy in inoperable patients allows accurate delivery of CyberKnife stereotactic radiosurgery. J Thoracic Cardiovasc Surg. 2010;140:1137–1142. doi: 10.1016/j.jtcvs.2010.07.085. [DOI] [PubMed] [Google Scholar]
- 13.Harley DP, Krimsky WS, Sarkar S, Highfield D, Aygun C, Gurses B. Fiducial marker placement using endobronchial ultrasound and navigational bronchoscopy for stereotactic radiosurgery: An alternative strategy. Annal Thorac Surg. 2010;89:368–374. doi: 10.1016/j.athoracsur.2009.09.048. [DOI] [PubMed] [Google Scholar]
- 14.WCG FDAnews. Varian announces 510(k) clearance for anchored beacon transponder. Available at: https://www.fdanews.com/articles/186510-varian-announces-510k-clearance-for-anchored-beacon-transponder. Accessed January 1, 2021.
- 15.Bolliger CT, Koegelenberg CFN, Von Groote-Bidlingmaier F, et al. First report of implantation of anchored electromagnetic fiducials in human lung cancers for real-time tumor localization and tracking during radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:S578–S579. [Google Scholar]
- 16.Osmond J, Lupica G, Harris E, Zin H, Allinson N, Evans P. High-speed tracking of moving markers during radiotherapy using a CMOS active pixel sensor. Radiat Oncol Biol. 2011;81:S762–S763. [Google Scholar]
- 17.Schmitt D, Nill S, Roeder F, Gompelmann D, Herth F, Oelfke U. Motion monitoring during a course of lung radiotherapy with anchored electromagnetic transponders: Quantification of inter- and intrafraction motion and variability of relative transponder positions. Strahlentherapie und Onkologie. 2017;193:840–847. doi: 10.1007/s00066-017-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Weiss RE, Jennrich RI, Wenger NS. PRESS model selection in repeated measures data. Comput Stat Data Anal. 1999;30:169–184. [Google Scholar]
- 19.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 20.Chi PCM, Balter P, Luo D, Mohan R, Pan T. Relation of external surface to internal tumor motion studied with cine CT. Med Phys. 2006;33:3116–3123. doi: 10.1118/1.2241993. [DOI] [PubMed] [Google Scholar]
- 21.Dhont J, Vandemeulebroucke J, Burghelea M, et al. The long- and short-term variability of breathing induced tumor motion in lung and liver over the course of a radiotherapy treatment. Radiother Oncol. 2017;126:339–346. doi: 10.1016/j.radonc.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Nuyttens JJ, Prévost JB, Praag J, et al. Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: Marker placement and early results. Acta Oncologica. 2006;45:961–965. doi: 10.1080/02841860600902205. [DOI] [PubMed] [Google Scholar]
- 23.van der Voort van Zyp NC, Prévost JB, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: Clinical outcome. Radiother Oncol. 2009;91:296–300. doi: 10.1016/j.radonc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Vinogradskiy Y, Goodman KA, Schefter T, Miften M, Jones BL. The clinical and dosimetric impact of real-time target tracking in pancreatic SBRT. Int J Radiat Oncol Biol Phys. 2019;103:268–275. doi: 10.1016/j.ijrobp.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarudis S, Karlsson Hauer A, Nyman J, Bäck A. Systematic evaluation of lung tumor motion using four-dimensional computed tomography. Acta Oncologica. 2017;56:525–530. doi: 10.1080/0284186X.2016.1274049. [DOI] [PubMed] [Google Scholar]
- 26.Steiner E, Shieh CC, Caillet V, et al. Both four-dimensional computed tomography and four-dimensional cone beam computed tomography under-predict lung target motion during radiotherapy. Radiother Oncol. 2019;135:65–73. doi: 10.1016/j.radonc.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 27.McNamara J, Lovelock D, Yorke E, Goodman K, Rimner A, Mageras G. Correcting drift in target position during radiotherapy via computer‐controlled couch adjustments on a C‐Arm Linac. Medical Physics. 2011;38:3858. doi: 10.1118/1.4802736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.