Abstract
Objective:
Literature on older-age bipolar disorder (OABD) is limited. This first-ever analysis of the Global Aging & Geriatric Experiments in Bipolar Disorder Database (GAGE-BD) investigated associations among age, BD symptoms, comorbidity, and functioning.
Methods:
This analysis used harmonized, baseline, cross-sectional data from 19 international studies (N = 1377). Standardized measures included the Young Mania Rating Scale (YMRS), Hamilton Depression Rating Scale (HAM-D), Montgomery-Asberg Depression Rating Scale (MADRS), and Global Assessment of Functioning (GAF).
Results:
Mean sample age was 60.8 years (standard deviation [SD] 12.2 years), 55% female, 72% BD I. Mood symptom severity was low: mean total YMRS score of 4.3 (SD 5.4) and moderate to severe depression in only 22%. Controlled for sample effects, both manic and depressive symptom severity appeared lower among older individuals (p’s < 0.0001). The negative relationship between older age and symptom severity was similar across sexes but was stronger among those with lower education levels. GAF was mildly impaired (mean = 62.0, SD = 13.3) and somatic burden was high (mean = 2.42, SD = 1.97). Comorbidity burden was not associated with GAF. However, higher depressive (p < 0.0001) and manic (p < 0.0001) symptoms were associated with lower GAF, most strongly among older individuals.
Conclusions:
Findings suggest an attenuation of BD symptoms in OABD, despite extensive somatic burden. Depressive symptom severity was strongly associated with worse functioning in older individuals, underscoring the need for effective treatments of BD depression in older people. This international collaboration lays a path toward a better understanding of aging in BD.
Keywords: aging, bipolar disorder, depression, functioning, mania, medical burden
Introduction
Between now and 2030, the number of people over 60 years of age will increase 3.5 times more rapidly than the general population.1 Roughly 25% of those with bipolar disorder (BD) are 60 years or older,2 and this proportion is likely to increase in tandem with changes in global demographics. Unfortunately, there is a dearth of research about the aging process in BD.3,4 The Global Aging & Geriatric Experiments in Bipolar Disorder Database (GAGE-BD) project, recently sponsored by the International Society for Bipolar Disorders (ISBD), pools archival research and clinical datasets to provide an evidence platform with potential to advance knowledge on older age BD (OABD).5 Key goals of investigators collaborating on the GAGE-BD project are to examine patterns of BD mood trajectory with age and to characterize the associations between BD symptoms, somatic comorbidities and functional status.
One crucial question, inadequately addressed in the literature, is how BD symptoms may change over the second half of life. Some data suggest that the prevalence of depressive episodes in OABD may increase,6 while other reports note that relapse leading to psychiatric hospitalization decreases over time,7 suggesting a possibly attenuated course of manic symptoms with aging. There are longitudinal studies of bipolar disorder, although follow-up is generally less than 5 years, and patients above age 50, and particularly patients above age 60 are seldom found.8 The Collaborative Depression Study (CDS), a long-term prospective study of patients with depression, tracked mood symptom-level changes over a long-term mean follow-up of 17.5 years.9 The CDS found that people with unipolar depression diagnosis and subsyndromal hypomanic symptoms, had a higher risk of later converting to bipolar disorder.9 In an analysis of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD), 78.5% of older patients (aged 60+, n=193) vs. 66.8% younger patients (aged <60, n=2249) achieved remission status over longitudinal follow-up, suggesting a more attenuated symptom profile.10
The US National Institute of Mental Health (NIMH) Collaborative Program on the Psychobiology of Depression (CDS) followed a large cohort of individuals with mood disorders for over 2 decades with at least annual assessment intervals.11 The oldest of three age cohorts in a 20-year follow-up had a mean age at intake of 50.3 years and a mean age at follow-up of 70.0 years compared to a youngest cohort with mean age of 24.2 (3.4) at intake and 44.2 (3.4) at follow-up and a middle cohort with mean age 36.0 (4.1) at intake and 56.0 (4.1) at follow-up. The oldest cohort spent somewhat less time in depressive and manic/hypomanic episodes than did those in the younger age groupings, but there was little change over the 20 years of follow-up in these measures.
In addition to gaps in evidence on BD symptom evolution, it is also not clear how somatic burden may be associated with BD symptoms or how symptoms and somatic burden may in turn be associated with functional impairment. Patients with OABD have an average of three to four comorbid somatic conditions, including metabolic syndrome in up to half of individuals, hypertension (45–69%), diabetes mellitus (18–31%), cardiovascular disease (9–49%), respiratory illness (4–15%), arthritis (16–21%), endocrine abnormalities (17–22%), as well as atopic diseases such as allergic rhinitis and asthma (6–20%).12,13 A report by Gildengers and colleagues found that although older adults with BD or major depressive disorder have comparable overall somatic burden, individuals with BD have more endocrine/metabolic and respiratory illnesses.14
There are a number of demographic and contextual variables that may predict outcome in people with BD as they age. Sex and education level are associated with mood symptom severity and outcome.15,16 For example, depressive symptoms may be more common and more severe in women with aging.17 Educational level is a strong predictor for cognitive reserve and thus socioeconomic status could be associated with outcome and mood symptom severity.18 Since lower education is a risk factor for late-life depression,19 older individuals with BD and less education may be particularly likely to have depressive symptoms.
This is a first-ever analysis of GAGE-BD. The first of two primary aims was to examine the association between BD symptoms and advancing age. The second aim was to examine the contribution of symptom severity and somatic burden to functioning across the age span. Findings are expected to inform future analyses within the GAGE-BD project as well as additional research focused on OABD.
Methods
Analytic overview
GAGE-BD comprises pooled data from multiple archival studies made possible by an international team of investigators who have an interest in BD and aging. The overarching approach and methods of GAGE-BD have been described elsewhere.5 This analysis used merged, baseline, cross-sectional data. For our first aim, investigating the association between BD symptoms and age, we hypothesized (H1a) that BD manic symptoms would attenuate with age, while depressive symptoms would be more prominent. We also expected (H1b) that sex and educational status would interact with age in relationship to symptoms, such that, particularly at older ages, women and those with lower education would have worse BD symptoms than men and those with higher education. For our second aim, investigating associations between age, somatic burden and functioning, we hypothesized (H2a) that older age, more severe manic and depressive symptoms, and greater somatic burden would be associated with poorer functioning. Additionally, we hypothesized (H2b) that age would moderate the associations of BD symptoms and somatic burden with functioning, specifically that older individuals would show a stronger relationship of mood symptoms and somatic burden with functioning.
Quality control, dataset intake process, data harmonization
The aggregate sample for this analysis, as of March 2020, was derived from 19 studies from 13 sites across the globe reporting data (total N = 1678) on adults with BD (N = 1377) as well as healthy volunteers (N = 301). This sample represents the first wave of datasets contributed to GAGE-BD, part of a larger vision to continue to accumulate datasets that can help researchers better understand aging and BD.
Studies/sites that contributed data are listed in Supplemental Tables 1 and 2. Approval to contribute data was obtained by local site institutional review boards or ethics committees at originating sites as appropriate and a data use agreement was executed between each contributing site and the GAGE-BD coordinating center (Case Western Reserve University School of Medicine, Cleveland, Ohio, USA). Investigators from each study participated in a centralized “intake” process wherein de-identified data was uploaded to a shared and secure on-line drive. Site investigators provided meta-data information (see Supplemental Tables 1 and 2) such as where the study was conducted, study inclusion and exclusion criteria, sample size, study design, and a data dictionary with variables listing and description. Investigators were also available to answer any coordinating site questions about the dataset and site principal investigators reviewed meta-data and descriptive data from their own sites to help ensure that findings were consistent with individual, previously reported results.
Measures
Selected data domains were collected across studies in identical format such as age in years, age of onset in years, and manic symptom severity using the Young Mania Rating Scale (YMRS)20 total score. These variables were used in the master dataset “as is.” It should be noted that while the method of recording age of onset was generally documented as chronological age in years, studies may have used different methods of defining onset (for example, first manic episode vs. first mood episode regardless of polarity).
Some variables required re-coding or harmonization based on meta-data or other variables, such as diagnostic group and subtype (e.g., BD Type I vs BD Type II), or whether individuals were currently employed. Generally, when data were re-coded or harmonized, some degree of granularity was lost. For example, depressive symptom severity from measures that used a continuous scale were re-grouped into ordinal categories by converting scores from either the Hamilton Depression Rating Scale (HAM-D)21 or Montgomery-Asberg Depression Rating Scale (MADRS)22 into severity bands following procedures established in preliminary work on dataset integration.23 Additionally, as might be expected in an archival dataset, not all measures were collected in each study.
Functional status was assessed using the Global Assessment of Functioning (GAF);24 however, GAF was only collected in approximately one-third of the total sample. Somatic comorbidity was assessed in individual studies using a variety of methods, including standardized measures such as the Cumulative Illness Rating Scale (CIRS)25 and the Charlson Comorbidity Index26 or clinical determination of selected comorbidity categories based on self-report, charts, or examination. Somatic comorbidity was harmonized into 8 binary variables, similar to a procedure used in a related project.27 Individual comorbidity variables were defined as whether the subject had positively endorsed having any diagnosis for a condition within the following domains: cardiovascular, respiratory, gastrointestinal, liver, renal, genitourinary, musculoskeletal, and endocrine. Summing across these 8 categories was used to construct a cumulative somatic comorbidity burden variable.
Data analysis
All variables were examined for distributional characteristics. YMRS was left-skewed (low manic symptom severity) and therefore log transformed to meet normality assumptions for analyses in which it was a predictor variable. The transformation resulted in a distribution that was nearly Gaussian, as indicated by more linear QQ plots (data not shown), and reductions in both skewness (2.0 → −0.12), and kurtosis (5.9 → −0.68). For the first aim, linear and ordered logistic mixed models were used with a random effect for study cohort for mania severity (log transformed YMRS) and depression symptom severity as the respective dependent variables. The independent variable of interest was age; sex, and education were included as covariates (H1a). We iteratively examined if the association between age on each outcome was moderated by sex or education through the inclusion of interaction terms (H1b), and followed up on significant interactions by comparing relationships within groups defined by a median split on education.
For the second aim, level of functioning as measured by GAF was the dependent variable and age an independent variable of interest with sex and education as covariates (H2a). In this linear mixed model with a random effect for study cohort, we iteratively included other independent variables of interest: mania severity, depression severity, and somatic comorbidity burden (H2a). We subsequently included interaction terms for age and mania severity, age and depression symptom severity, and age and comorbidity burden (H2b). To further characterize associations underlying any significant interaction terms, we performed age-stratified models (stratifying by median age of the total sample). For all analyses, a two-sided alpha of 0.05 was considered statistically significant.
We also conducted follow-up analyses removing the 4 studies that included symptom thresholds as part of study inclusion criteria, either by requiring euthymic status at baseline (CiBS-XR, COG-BD, UPMC studies) or a specific level of symptom severity (GERI SAD study). The total number of studies remaining was 15, comprising 1316 BD participants.
Results
Overall sample description
Only data from BD participants (N = 1,377) were analyzed; Table 1 shows descriptive variables for the sample. Most (N = 1,190, 86.4%) participants were aged over 50 years with a mean age of 60.8 years (SD = 12.19 years). There was a slight excess of women in the sample (55.1%), and among those with BD, 71.9 % had Type I disorder. Figure 1 shows the distribution of subject age in each data set. Supplemental Table 1 shows the studies that contributed data to this GAGE-BD analysis and the meta-data that describe selected relevant study characteristics. Most of the studies (N = 14, 74%) in this GAGE-BD analysis were observational. Of the 5 interventional studies, 80% (N = 4) were drug treatment studies. Supplemental Table 2 shows the inclusion and exclusion criteria for the individual studies. Note that some studies had inclusion/exclusion criteria that involved variables in the present analysis including mood symptom severity and medical comorbidities; a cohort variable was included in all models to account for this. Some studies were exclusively composed of OABD participants, while others had participants of mixed ages. Supplemental Table 3 shows descriptive data from individual studies.
Table 1.
Descriptive statistics for GAGE-BD total sample (n=1377)
| Descriptive Variables | Number of Cohorts | Number of Participants | Mean (SD) or % |
|---|---|---|---|
|
| |||
| Age, years | 19 | 1374 | 60.79 (12.19) |
| Age range, years (n = 1374) | n/a | 18 – 95 | |
| Age >50 (n= 1374) | 1190 | 86.4% | |
| Sex (n=1375) | 19 | ||
| Male | 617 | 44.9% | |
| Female | 758 | 55.1% | |
| Diagnosis (n=1139) | 15 | ||
| Bipolar I | 819 | 71.9% | |
| Bipolar II | 264 | 23.2% | |
| Other bipolar | 56 | 4.9% | |
| Age of onset, years | 16 | 1105 | 31.78 (15.88) |
| Years of education | 14 | 842 | 11.99 (4.76) |
| Currently working (n=778) | 11 | ||
| Yes | 195 | 25.1% | |
| No | 583 | 74.9% | |
| GAF total | 8 | 524 | 62.03 (13.28) |
| Depression Band (n=811) | 12 | ||
| Absent | 397 | 49.0% | |
| Mild | 235 | 29.0% | |
| Moderate | 156 | 19.2% | |
| Severe | 23 | 2.8% | |
| YMRS total | 17 | 1148 | 4.27 (5.38) |
| BPRS total (18-item) | 3 | 228 | 28.64 (14.48) |
| Medical comorbidity | |||
| Cardiovascular comorbidity (n = 1278) | 17 | 560 | 43.8% |
| Respiratory comorbidity (n = 1139) | 15 | 409 | 35.9% |
| GI comorbidity (n = 953) | 13 | 235 | 24.7% |
| Hepatic/pancreatic comorbidity (n = 945) | 12 | 71 | 7.5% |
| Renal comorbidity (n = 899) | 11 | 57 | 6.3% |
| GU comorbidity (n = 715) | 10 | 149 | 20.8% |
| Musculoskeletal comorbidity (n = 993) | 14 | 402 | 40.5% |
| Endocrine comorbidity (n = 1280) | 17 | 430 | 33.6% |
Note: Of the 1377 BD participants represented in the total sample, there was some missing data for all measures, so sample size for each measure is given separately. SD = standard deviation,
GAF=Global Assessment of Functioning, score range 0–100 with higher scores indicating better functioning
YMRS = Young Mania Rating Scale, score range 0–60 with higher scores indicating more severe manic symptoms
BPRS= Brief Psychiatric Rating Scale, score range 18–126 with higher scores indicating more severe psychiatric symptoms
GI = gastro-intestinal, GU = genito-urinary
Figure 1.
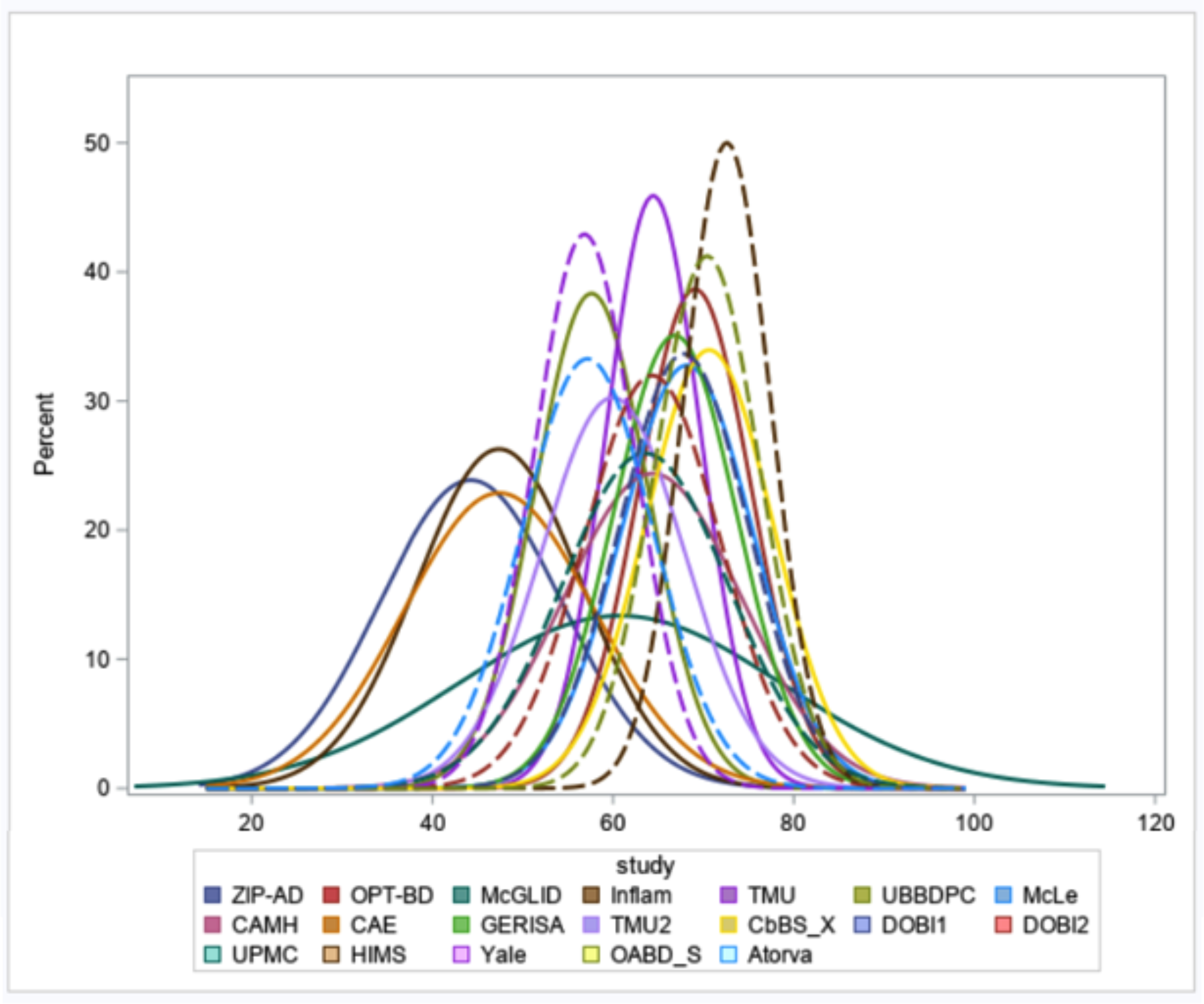
Age distributions for each cohort are shown. Abbreviations in the legend refer to study names as seen in Supplementary Table 1.
Age and BD symptoms
Manic symptom severity in this sample was low, with a mean YMRS total score of 4.3 (SD = 5.4, range: 0–44). Depression severity was categorized among BD cases that included evaluable data for this domain (N = 811). We found no/minimal depression in 397 cases (49.0%), mild symptoms in 235 (29.0%), moderate symptoms in 156 (19.2%), and severe symptoms in 23 (2.8%). With respect to hypothesis H1a, Figure 2 shows a linear relationship between age and manic symptom severity on the YMRS total score, demonstrating a lower manic symptom severity among older participants, in the context of large variability of severity among OABD, ranging from absent to severe. This association was significant (p < 0.0001) even after adjusting for age of BD onset, sex, and education level (Table 2).
Figure 2.
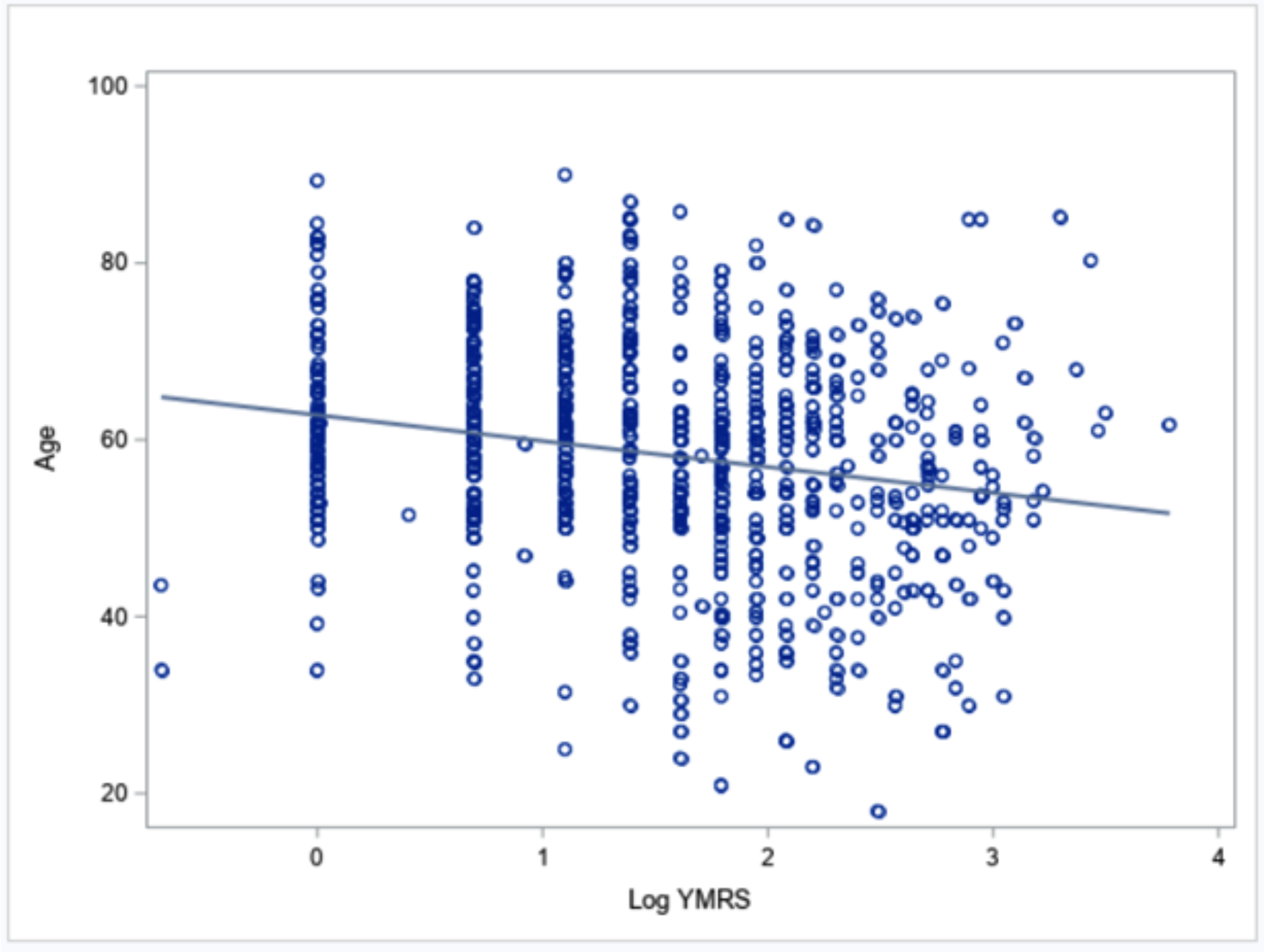
Scatter plot of the association between age and manic symptom severity, as measured by log YMRS, with best-fitting regression line.
Table 2.
Associations of age with BD symptoms
| Predictor | Log(YMRS)1 | Depression2 | ||
|---|---|---|---|---|
| β (95% CI) | p | OR (95% CI) | p | |
|
| ||||
| Age (unadjusted) | −0.015 (−0.020, −0.010) | <0.0001 | 1.04 (1.03, 1.06) | <0.0001 |
| Age (adjusted3) | −0.014 (−0.020, −0.008) | <0.0001 | 1.03 (1.021, 1.047) | <0.0001 |
| Age × Sex4 | −0.0003 (−0.012, 0.012) | 0.97 | 1.02 (0.998, 1.052) | 0.06 |
| Age × Education4 | 0.001 (−0.001, 0.003) | 0.20 | 0.99 (0.990, 0.998) | 0.002 |
Linear regression model with a random effect for study cohort
Ordered logistic model with a random effect for study cohort
Adjusted for sex and education
Models include main effects for age, sex, and education
Note: Log (YMRS) = Young Mania Rating Scale (log transformed)
OR=odds ratio, CI=confidence interval
Also with respect to hypothesis H1a, Figure 3 shows the linear relationship between age and total depressive symptom severity categorized along 4 bands of progressively more severe depressive symptoms. As with manic symptoms, there is lower depressive symptom severity among older individuals. This relationship was confirmed by results from the ordered logistic model (Table 2). For each 1-year increase in age, the odds of having less severe depression symptoms increases by 3% (OR = 1.03).
Figure 3.
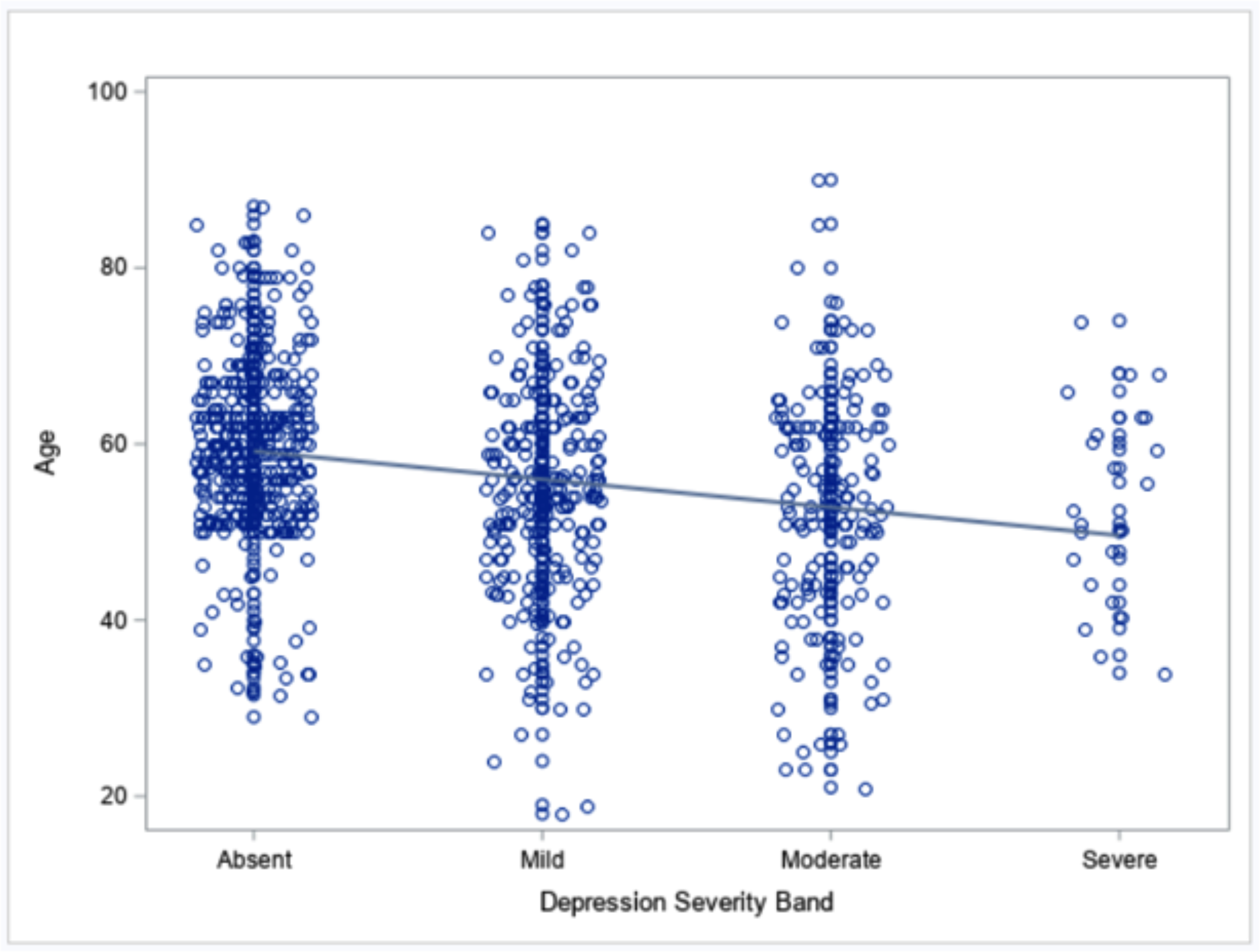
Scatter plot of association between age and depressive symptom severity, with best-fitting regression line.
With respect to moderator analyses (Hypothesis H1b), we found a significant interaction of age and education for the relationship to depression (Table 2). Specifically, the negative relationship of age and depression severity was more pronounced in those with fewer than 13 years of education (Supplemental Figure 1). Sex did not significantly interact with age in the relationship to depression severity, and neither education nor sex moderated age relationships with mania severity.
Age relationships with symptoms were unchanged when examining the subsample of studies that did not restrict the severity of mood symptoms in their inclusion criteria (data not shown).
Medical comorbidity and functioning
GAF scores were available in 8 contributing studies (N = 524) and the mean GAF was 62.0 (SD 13.3), which is consistent with individuals having some difficulty with social, occupational, and school functioning, but generally functioning relatively well. Supplemental Figure 2 shows the distribution of total GAF scores across studies.
Common somatic comorbidities in BD cases from datasets that included medical information were cardiovascular (43.8%, N = 560), musculoskeletal (40.5%, N = 402), endocrine (33.6%, N = 430), and respiratory conditions (35.9%, N = 409). Somatic burden overall was high (mean of 2.42 [SD = 1.97] out of 8). Older age was associated with greater somatic comorbidity, specifically for cardiovascular (p < 0.0001, 95% CI 0.010 – 0.017), gastrointestinal (p = 0.0007, 95% CI 0.003 – 0.011), musculoskeletal (p = 0.01, 95% CI 0.001 – 0.011) and endocrine (p = 0.08, 95% 0.0004 – 0.006) conditions.
With respect to hypothesis H2a, among BD cases, age (Models 1 to 4a), sex (Models 1 to 4a), and comorbidity burden (Model 4a) were not associated with functioning (Table 3). In a subset of studies that did not restrict mood symptoms in their study criteria (n=334), age was positively related to GAF in most models (Supplemental Table 4). Also, individual comorbidities were not associated with functioning (data not shown).
Table 3.
Associations from multivariate linear mixed models for Global Assessment of Functioning (GAF)
| Predictor | Model 11 | Model 2a1 | Model 3a1 | Model 4a1 | ||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
|
| ||||||||
| Age | 0.10 (−0.01, 0.20) | 0.07 | 0.04 (−0.06, 0.15) | 0.42 | 0.02 (−0.07, 0.11) | 0.62 | 0.11 (−0.01, 0.23) | 0.08 |
| Sex | −0.98 (−3.82, 1.87) | 0.50 | −1.13 (−3.92, 1.66) | 0.43 | −0.69 (−3.04, 1.67) | 0.57 | −0.91 (−3.78, 1.96) | 0.53 |
| Education | −0.39 (−0.74, −0.04) | 0.028 | −0.32 (−0.66, 0.03) | 0.07 | −0.03 (−0.32, 0.27) | 0.85 | −0.38 (−0.73, −0.03) | 0.034 |
| YMRS | −0.61 (−0.87, −0.36) | <0.0001 | ||||||
| Depression symptom severity | −9.14 (−10.44, −7.84) | <0.0001 | ||||||
| Comorbidity burden | −0.15 (−1.02, 0.72) | 0.73 | ||||||
Linear regression models with a random effect for study cohort
Note: CI=confidence interval, YMRS = Young Mania Rating Scale
There were strong relationships between BD symptoms and functioning, such that each 10 point increase in severity on the YMRS scale was associated with a 6.1 point decrease in functioning on the GAF scale (p = 0.0001; Model 2a and Figure 4). A similar relationship was evident for more severe depression symptoms, with an average decrease of 9 points on GAF for each transition to a higher category of depression symptom severity (p < 0.0001; Model 3a and Figure 5). Higher education appeared to be associated with poorer functioning (Models 1 and 4); however, this relationship did not persist when considering the effect of BD symptoms (Models 2a and 3a), nor was it seen in models of the subsample of studies without symptom restrictions. Thus, the initial observation was likely driven by the residual correlation between education and BD symptoms and not due to education level itself.
Figure 4.
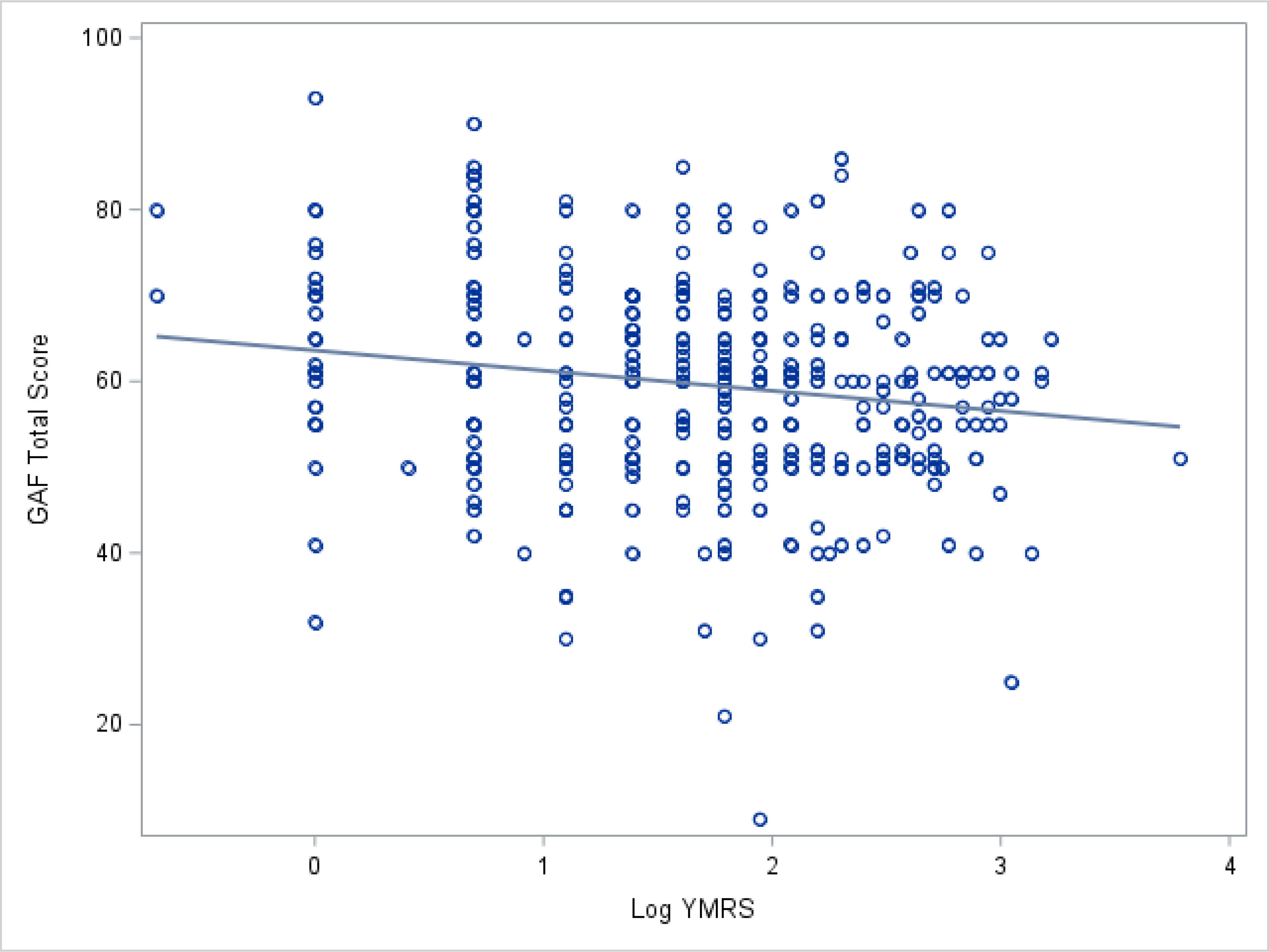
Scatter plot of the relationship of mania severity, as measured by log YMRS, to GAF total score.
Figure 5.
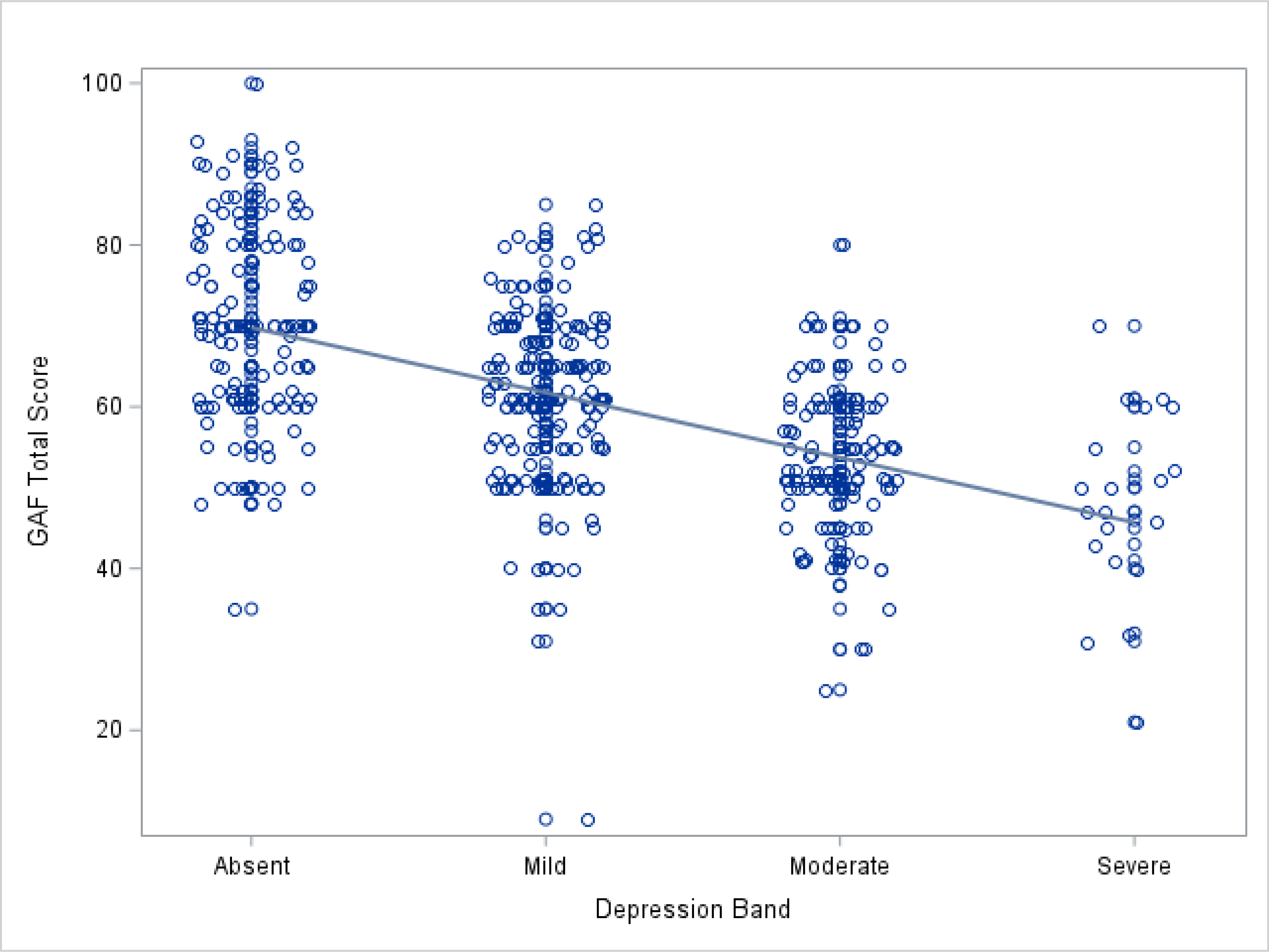
Scatter plot of the relationship of depression severity to GAF total score.
With respect to hypothesis H2b, age did not moderate the relationships of mania or comorbidity burden with functioning (pinteraction > 0.23; Table 4; Models 2b and 4b), but age did moderate the relationship of depression with functioning (pinteraction = 0.0028; Model 3b). To further characterize this interaction, we stratified by median age (Table 5). The magnitude of the association of higher depression to worse functioning was more pronounced in older BD patients (β = −10.9, p < 0.0001) than in those below the median age of 57 years (β = −7.4, p < 0.0001). In the follow-up analysis with the subset of studies that did not restrict symptom severity, depression showed a similar interaction result, and mania severity and comorbidity also interacted with age in predicting functioning (mania: pinteraction < .0001; comorbidity: pinteraction = 0.04; Supplemental Table 5). Older patients showed a particularly strong association between higher mania and worse functioning. For comorbidity, neither age group had a significant association with functioning, but the older group showed a numerically larger association of higher co-morbidities and better GAF scores (Supplemental Table 6).
Table 4.
Tests of age moderation in multivariate linear mixed models for Global Assessment of Functioning (GAF)
| Predictor | Model 2b1 | Model 3b1 | Model 4b1 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
|
| ||||||
| Age | 0.12 (−0.04,0.28) | 0.15 | 0.37 (0.13,0.62) | 0.0029 | 0.07 (−0.09,0.23) | 0.39 |
| Sex | −1.08 (−3.87,1.70) | 0.44 | −0.92 (−3.25,1.41) | 0.44 | −0.89 (−3.76,1.98) | 0.54 |
| Education | −0.30 (−0.65,0.04) | 0.08 | 0.06 (−0.23,0.35) | 0.70 | −0.39 (−0.74,−0.04) | 0.03 |
| YMRS | 0.07 (−1.07,1.20 ) | 0.91 | ||||
| YMRS * Age | −0.01 (−0.03,0.01) | 0.23 | ||||
| Depression symptom severity | −0.24 (−6.18,5.70) | 0.94 | ||||
| Depression symptom severity * Age | −0.16 (−0.27,−0.06) | 0.0028 | ||||
| Comorbidity burden | −1.49 (−5.50,2.52) | 0.47 | ||||
| Comorbidity burden * Age | 0.02 (−0.04,0.09) | 0.50 | ||||
Linear regression models with a random effect for study cohort
Note: CI=confidence interval, YMRS = Young Mania Rating Scale
Table 5.
Associations from multivariate linear mixed models for Global Assessment of Functioning (GAF) stratified by median age of the study population
| Predictor | Age ≤57 years1 | Age >57 years1 | ||
|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | |
|
| ||||
| Sex | 1.10 (−1.68, 3.88) | 0.44 | −2.83 (−6.77, 1.10) | 0.16 |
| Education | 0.19 (−0.34, 0.71) | 0.49 | −0.001 (−0.39, 0.39) | 0.99 |
| Depression symptom severity | −7.37 (−8.98, −5.76) | <0.0001 | −10.88 (−13.02, −8.74) | <0.0001 |
Linear regression models with a random effect for study cohort
Note: CI=confidence interval
Discussion
With the global population of older adults increasing more rapidly than any time in history, there is an urgent need for data that are specific to late-life among individuals with BD.28 This first-ever analysis of GAGE-BD suggests that global collaboration can yield a large, integrated dataset for powerfully testing hypotheses about OABD. In contrast to most studies on OABD to date, the large sample size allows for aggregate analyses that have the potential to help inform how aging, BD symptoms, somatic burden, and functioning inter-relate and facilitate investigations that would not be possible using only smaller and independent studies. Somewhat in contrast to our original hypotheses and after statistical control for sample effects, these initial results from GAGE-BD suggest that both BD manic symptoms and BD depressive symptom severity appear to be reduced in older age. Contrary to our predictions, this pattern of less severe BD symptoms in older age was broadly similar for men and women, and the negative age relationship was particularly pronounced among those with lower education, such that depression symptoms in late-life BD were less severe, not more severe, among less educated participants.
Our findings are generally in-line with some other reports.29 O’Rourke and colleagues similarly found an inverse association between duration of diagnosis and depressive symptoms in older people with BD29 and suggested that older adults with BD have acclimated to their diagnosis and symptoms, and have devised effective coping strategies. OABD who are referred to participate in research studies also may be those who have remained in treatment over time and are adherent to interventions. This may be particularly true of those with high levels of depressive symptoms.17,30 Another possibility is that the clinical rating scales for mania and depression may not be designed to recognize the way these symptoms manifest in later life. Relatedly, it is possible that older individuals with BD may, over time, find ways of managing illness that are positive and constructive, such as forming supportive relationships with other individuals, acceptance of BD diagnosis, mindfulness, education, identification of relapse triggers and signals, sleep and stress management, appropriate lifestyle changes, and a “stay-well” plan.31–33 Our finding that older BD patients with less education had less severe depressive symptoms was surprising given the idea that lower cognitive and brain reserve might predispose to late-life depression.19 In addition, some demographic variables, such as education, may be proxy markers of social advantage34 that further complicate how our findings might be interpreted. In some geographic and cultural contexts, higher educational attainment can reflect access to opportunities that are not available to individuals with less income and support.34 Longitudinal data and data that helps parse out social disadvantage factors, for example information on income and neighborhood stress or deprivation, are needed to determine whether lower education indeed promotes within-subject declines in depressive symptoms with age in BD.
Somatic comorbidity in the GAGE-BD sample is highly prevalent, particularly cardiovascular disease, seen in nearly half of individuals with OABD. This differs somewhat from a report by Kemp and colleagues derived from a mixed-age sample (mean age 39.2, SD ±12.4 years) which found the most frequently observed somatic conditions to be obesity, migraines, hypertension, hyperlipidemia, and asthma.35 Kemp and colleagues also reported an association between elevated somatic comorbidity burden and several clinical features of BD, including a higher rate of lifetime mood episodes.35 Our cohorts had widely varying inclusion/exclusion criteria, many of which involved the presence of somatic conditions, so our conclusions may not match those from epidemiologic samples.
Our original hypothesis that greater somatic burden would be associated with reduced functioning was not confirmed in the full sample of studies. This may be because our measure of functioning, the GAF, is not intended to reflect impairment due to physical or environmental limitations. It is also possible that the same acquired competence in mood symptom management that some people with OABD achieve might be helpful in optimizing functioning. Evidence from our follow-up analysis of only studies that did not restrict mood symptoms supports this idea, since an association of more comorbidity with poorer functioning was strongest in younger patients. Still, an important caveat is that these analyses were cross-sectional. Thus, interpretation of our findings must take into account the possibility that the overall impression of fewer BD symptoms and better functional status could be explained by a “healthy survivor” effect wherein individuals who die prematurely36 or who are too ill to participate in research studies could bias sample characteristics to favor older adults who are doing relatively well. Other authors37 have suggested that one approach to protect against overlooking survival-related biases has to do with study design and differentiating between an “inception cohort” and a “survivor cohort.” In an inception cohort, the unfolding of risk from an underlying event or condition is observed from the start of the condition; while in a survivor cohort, the risk is observed at a given point of time. Only the inception cohort can provide results free of the survivor-related biases. Future efforts to examine the trajectory of health in people with BD should include a diverse age range and include data that begins at time of BD diagnosis or even earlier.
Our findings do suggest that worse BD symptom severity is associated with worse functioning in OABD, and the effect for depression on functioning is more severe for even older OABD patients. In the subsample of studies that did not restrict mood symptom severity, the association between mania and functioning also was most pronounced in OABD compared to younger patients. This might suggest the need for tailored care approaches that include targeting residual symptoms and providing these individuals with more social and other supports. Functioning in OABD is also likely affected by cognitive impairments, which, while not examined in the present analysis, will be the topic of future investigations with the GAGE-BD dataset.
This study had a number of additional limitations inherent in an analysis of archival data and use of an aggregate sample from diverse sources. Meta-data was heterogeneous, including variable inclusion criteria, aims and designs. Some studies in the sample had inclusion criteria that specifically included variables relevant to symptom severity, although our findings were broadly similar when these studies were excluded. Not surprisingly, samples differed with respect to demographic and clinical variables. Additionally, some of the domains of interest, such as depressive symptoms and somatic comorbidity, were assessed using different evaluation methods. Harmonization procedures to facilitate data analysis resulted in loss of information granularity as continuous measures were collapsed into broad or selective categories. The data also do not reflect the full spectrum of OABD, in particular, those who may have more severe manic symptoms.
In spite of these limitations, findings represent an approach to overcome some of the existing challenges to being able to study the important issue of aging and BD. An advantage of combining datasets is that it affords more power to detect smaller, yet still clinically meaningful, effects. Larger and combined datasets may also facilitate identification and characterization of sample sub-groups, such as Type I vs. Type II BD, sex-related differences and characteristics related to age of onset, that can be a target of clinical focus. The GAGE-BD study team continues to expand our sample and is in the process of adding a second wave of GAGE-BD contributing sites. The study team is also refining analytic approaches and planning new analyses, including longitudinal evaluations, that will explore population sub-groups and outcome domains with OABD that are not possible to examine in smaller, independent studies.
In conclusion, these first analyses from GAGE-BD suggest an attenuation of BD symptoms in OABD, with relatively good functional status despite extensive somatic burden. Findings also show that the relationship of depressive symptoms, and possibly mania symptoms, to poor functioning is particularly strong among older individuals. This international collaboration lays a path toward a better understanding of how aging may impact the evolution of BD and other chronic mental health disorders across the lifespan.
Supplementary Material
Supplemental Figure 2. Global Assessment of Functioning (GAF) total score distributions for each cohort are shown. Abbreviations in the legend refer to study names as seen in Supplementary Table 1.
Supplemental Figure 1. Interaction of age and education on depression severity is depicted with separate fit lines (and 95% confidence intervals) of the relationship of age and depression severity for education < 13 years (blue) and 13 or more years (red).
Acknowledgements:
The authors would like to thank the following people: Leon Flicker, Graeme J. Hankey, Bu B. Yeap, Jonathan Golledge, Melis Orhan, Nicole Korten, Tokie Kemp, Lejla Colic PhD, Luca Villa PhD, Susan Quatrano, Miranda Skurla, Patrick Monette, Beny Lafer, James Emanuel, Daniel M. Blumberger, and Tarek K. Rajji.
Funding:
This project was supported by the International Society for Bipolar Disorders (ISBD) Bowden Massey Strategic Research Initiative; US National Institute of Mental Health (R01MH070902, R01MH113230, R01MH084921); Pfizer; Glaxo Smith Kline; Merck; National Health and Medical Research Council of Australia; National Institute for Biomarker Research in Neuropsychiatry, INBION (FAPESP 14/50873–3; 2016/01302–9 and CNPq 465412/2014–9); Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS); Canadian Institutes for Health Research, grant 200017; Ministry of Science and Technology, Taiwan; Spanish Ministry of Science and Innovation (PI15/00283, PI18/00805) integrated into the Plan Nacional de I+D+I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER); the Instituto de Salud Carlos III; the CIBER of Mental Health (CIBERSAM); the Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2017 SGR 1365); the CERCA Programme; and the Departament de Salut de la Generalitat de Catalunya for the PERIS grant SLT006/17/00357.
Contributor Information
Martha Sajatovic, Case Western Reserve University School of Medicine, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Annemiek Dols, GGZ inGeest, Amsterdam UMC, VU Medical Center, Amsterdam Neuroscience, Amsterdam Public Health Research Institute, Amsterdam, the Netherlands.
Soham Rej, Lady Davis Institute, McGill University, Montreal, Canada.
Osvaldo P. Almeida, University of Western Australia, Perth, Australia.
Alexandra J.M. Beunders, GGZ inGeest, Amsterdam UMC, VU Medical Center, Amsterdam Neuroscience, Amsterdam Public Health Research Institute, Amsterdam, the Netherlands.
Hilary P. Blumberg, Department of Psychiatry, Yale School of Medicine, New Haven, Connecticut, USA.
Farren B.S. Briggs, Department of Population and Quantitative Health Sciences, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA
Brent P. Forester, Division of Geriatric Psychiatry, McLean Hospital, Belmont, Massachusetts, USA and Harvard Medical School, Boston, MA
Regan E. Patrick, Division of Geriatric Psychiatry, McLean Hospital, Belmont, MA, USA
Orestes V. Forlenza, Laboratory of Neuroscience (LIM-27), Department and Institute of Psychiatry, HCFMUSP, Faculdade de Medicina da Universidade de São Paulo, São Paulo, SP, Brazil
Ariel Gildengers, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Esther Jimenez, Bipolar and Depressive Disorders Unit, Hospital Clinic, University of Barcelona, IDIBAPS, CIBERSAM, Barcelona, Catalonia, Spain.
Eduard Vieta, Bipolar and Depressive Disorders Unit, Hospital Clinic, University of Barcelona, IDIBAPS, CIBERSAM, Barcelona, Catalonia, Spain.
Benoit Mulsant, Department of Psychiatry, University of Toronto, Center for Addiction & Mental Health, Toronto, Canada.
Sigfried Schouws, GGZ ingest, Amsterdam UMC, VU Medical Center, Amsterdam, the Netherlands.
Nadine Paans, GGZ inGeest, Amsterdam UMC, VU Medical Center, Amsterdam Public Health Research Institute, Amsterdam, the Netherlands.
Sergio Strejilevich, AREA, Assistance and Research in Affective Disorders, Buenos Aires, Argentina.
Ashley Sutherland, Department of Psychiatry, University of California San Diego, San Diego, CA, USA.
Shangying Tsai, Department of Psychiatry, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
Betsy Wilson, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA.
Lisa T. Eyler, Department of Psychiatry, University of California San Diego, San Diego, CA, USA and Desert-Pacific Mental Illness Research Education and Clinical Center, VA San Diego Healthcare System, San Diego, CA, USA
Data Availability Statement:
Data is available as part of the GAGE-BD project and subject to the completion of appropriate data use agreements. Qualified scientists who wish to access the data should contact the study lead author.
References
- 1.Population Division. Department of Economic and Social Affairs; United Nations: World Population Ageing: 1950–2050. [Google Scholar]
- 2.Sajatovic M, Blow FC, Ignacio RV, Kales HC. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv 2004;55(9):1014–1021. [DOI] [PubMed] [Google Scholar]
- 3.Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord 2015;17(7):689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dols A, Kessing LV, Strejilevich SA, et al. Do current national and international guidelines have specific recommendations for older adults with bipolar disorder? A brief report. Int J Geriatr Psychiatry 2016;31(12):1295–1300. [DOI] [PubMed] [Google Scholar]
- 5.Sajatovic M, Eyler L, Rej S, et al. The global aging and geriatric experiments in bipolar disorder database (GAGE-BD) project: Understanding older age bipolar disorder by combining multiple datasets. Bipolar disorders 2019. May 13. [DOI] [PubMed] [Google Scholar]
- 6.Kessing LV. Diagnostic subtypes of bipolar disorder in older versus younger adults. Bipolar disorders 2006;8:56–64. [DOI] [PubMed] [Google Scholar]
- 7.Kessing LV, Hansen MG, Andersen PK. Course of illness in depressive and bipolar disorders. Naturalistic study, 1994–1999. Br J Psychiatry 2004;185:372–377. [DOI] [PubMed] [Google Scholar]
- 8.Jeon HJ, Baek JH, Ahn YM, et al. Review of cohort studies for mood disorders. Psychiatry Investig 2016. May;13(3):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry 2011. Jan;168(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Jurdi RK, Marangell LB, Petersen NJ, Martinez M, Gyulai L, Sajatovic M. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry 2008. Nov;16(11):922–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coryell W, Fiedorowicz J, Solomon D, Endicott J. Age transitions in the course of bipolar I disorder. Psychol Med 2009. 39(8):1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol 2012;25:20–25. [DOI] [PubMed] [Google Scholar]
- 13.Tsai SY, Kuo CJ, Chung KH, Huang YL, Lee HC, Chen CC. Cognitive dysfunction and medical morbidity in elderly outpatients with bipolar disorder. Am J Geriatr Psychiatry 2009;17:1004–1011. [DOI] [PubMed] [Google Scholar]
- 14.Gildengers AG, Whyte EM, Drayer RA, et al. Medical burden in late-life bipolar and major depressive disorders. Am J Geriatr Psychiatry 2008;16(3):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buoli M, Cesana BM, Dell’Osso B, et al. Gender-related differences in patients with bipolar disorder: a nationwide study. CNS Spectr 2019;24(6):589–596. [DOI] [PubMed] [Google Scholar]
- 16.Samame C, Martino DJ, Strejilevich SA. A quantitative review of neurocognition in euthymic late-life bipolar disorder. Bipolar Disorders 2013;15:633–644. [DOI] [PubMed] [Google Scholar]
- 17.Nivoli AM, Murru A, Pacchiarotti I, et al. Bipolar disorder in the elderly: a cohort study comparing older and younger patients. Acta Psychiatr Scand 2014. Nov;130(5):364–373. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenberg CM, Beekman AT, Deeg DJ, van Tilburg W. Sex differences in late-life depression. Acta Psychiatr Scand 2000. Apr;101(4):286–292. [PubMed] [Google Scholar]
- 19.Chang-Quan H, Zheng-Rong W, Yong-Hong L, Yi-Zhou X, Qing-Xiu L. Education and risk for late life depression: A meta-analysis of published literature. Intl J Psychiatry in Medicine. 2010;401(1):109–124. [DOI] [PubMed] [Google Scholar]
- 20.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 23.Eyler L, Aebi M, Daly R, Hansen K, Tatsuoka C, Young R, Sajatovic M. Understanding aging in bipolar disorder by integrating archival clinical research datasets. The American Journal of Geriatric Psychiatry 2019. April 18;(19):30336–303367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spitzer RL, Gibbon M, Williams JBW, et al. Global assessment of functioning (gaf) scale. In: Sederer LJ, Dickey B, eds. Outcome assessment in clinical practice. 1st ed. Williams & Wilkins, 1996. [Google Scholar]
- 25.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622–626. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care 2005;43(6):607–615. [DOI] [PubMed] [Google Scholar]
- 27.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the cumulative illness rating scale. Psychiatry Res 1992;41:237–248. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho AF, Firth J, Vieta E. Bipolar Disorder. N Engl J Med 2020. Jul 2;383(1):58–66. [DOI] [PubMed] [Google Scholar]
- 29.O’Rourke N, Heisel MJ, Canham SL, Sixsmith A, BADAS Study Team. Predictors of suicide ideation among older adults with bipolar disorder. PloS one 2017;12(11), e0187632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pallaskorpi S, Suominen K, Rosenström T, et al. Predominant polarity in bipolar I and II disorders: A five-year follow-up study. J Affect Disord 2019. Mar 1;246:806–813. [DOI] [PubMed] [Google Scholar]
- 31.Camp DL, Finlay WM, Lyons E. Is low self-esteem an inevitable consequence of stigma? An example from women with chronic mental health problems. Soc Sci Med 2002;55(5):823–834. [DOI] [PubMed] [Google Scholar]
- 32.Russell SJ, Browne JL. Staying well with bipolar disorder. Aust N Z J Psychiatry 2005;39(3):187–193. [DOI] [PubMed] [Google Scholar]
- 33.Sajatovic M, Jenkins JH, Safavi R, et al. Personal and societal construction of illness among individuals with rapid-cycling bipolar disorder: a life-trajectory perspective. Am J Geriatric Psychiatry 2008;16(9):718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shavers VL. Measurement of socioeconomic status in health disparities research. J National Medical Association 2007;99(9):1013–1023. [PMC free article] [PubMed] [Google Scholar]
- 35.Kemp DE, Sylvia LG, Calabrese JR, et al. General medical burden in bipolar disorder: findings from the LiTMUS comparative effectiveness trial. Acta Psychiatr Scand 2014;129(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crump C, Sundquist K, Winkeby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry 2013;70(9):931–939. [DOI] [PubMed] [Google Scholar]
- 37.Saracci R Survival-related biases survive well. International J Epidemiol 2007;36(1):244–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2. Global Assessment of Functioning (GAF) total score distributions for each cohort are shown. Abbreviations in the legend refer to study names as seen in Supplementary Table 1.
Supplemental Figure 1. Interaction of age and education on depression severity is depicted with separate fit lines (and 95% confidence intervals) of the relationship of age and depression severity for education < 13 years (blue) and 13 or more years (red).
Data Availability Statement
Data is available as part of the GAGE-BD project and subject to the completion of appropriate data use agreements. Qualified scientists who wish to access the data should contact the study lead author.


