Abstract
We assessed the genetic relatedness of sequential isolates of Candida parapsilosis during persistent or recurrent fungemia and the effect of central venous catheter (CVC) removal. Serial isolates of C. parapsilosis were obtained from 17 patients with persistent or recurrent fungemia over periods of up to 5 months. Forty-eight C. parapsilosis isolates from the blood of 17 patients were analyzed by electrophoretic karyotyping (EK) with pulsed-field gel electrophoresis (PFGE), revealing 25 different karyotypes. The strains sequentially isolated from each of seven patients whose fungemia resolved following CVC removal had the same karyotype. Two patients with fungemia that cleared without CVC removal each had two sequential isolates with different karyotypes. In six (75%) of the eight patients whose fungemia was recurrent even after CVC removal, the karyotypes of the pre- and post-CVC removal isolates were different, implying the emergence of a new strain. Overall, the sequential strains from each patient had identical karyotypes in 53% (9 of 17) of the patients and two different karyotypes in 47% (8 of 17). This study shows that EK with PFGE is useful for investigating persistent or recurrent fungemia due to C. parapsilosis and that recurrent fungemia due to C. parapsilosis is more likely caused by reinfection with a second strain.
Candida parapsilosis has become increasingly important as a cause of central venous catheter (CVC)-related candidemia (5, 6). Catheter-related C. parapsilosis fungemia has a higher clearance rate and a much lower rate of dissemination than infections due to other Candida species (7, 14). This can be explained in part by the powerful effect of catheter removal on clearance of the fungemia (7, 12) and by the relatively lower virulence of C. parapsilosis compared to other Candida species (14). However, we recently observed that some C. parapsilosis strains caused persistent or recurrent fungemia, sometimes despite CVC removal. Therefore, assessment of the genetic relationship of the sequential isolates of C. parapsilosis from blood cultures before and after CVC removal is helpful for monitoring of therapy.
There is a lack of substantial research on strain relationships among bloodstream isolates of C. parapsilosis obtained from sequential cultures of the same patient. Electrophoretic karyotyping (EK) with pulsed-field gel electrophoresis (PFGE) is a means of discriminating heterogeneous C. parapsilosis isolates using chromosome number or size (2, 10, 11). The goal of this study was to determine whether the same or different strains were responsible for persistent or recurrent fungemia due to C. parapsilosis and its relationship with CVC removal by performing EK on sequential C. parapsilosis isolates from the same patient.
MATERIALS AND METHODS
Isolates.
Forty-eight candidemic isolates were obtained from 17 patients, each of whom had one or more blood cultures positive for C. parapsilosis on 2 or more separate days. The patients were admitted to Chonnam National University Hospital between 1996 and 1999, and all had CVCs when the first positive cultures were obtained. No patients had neutropenia or evidence of invasive candidiasis. The fungemia was divided into the following categories: (i) fungemia that cleared after CVC removal, (ii) fungemia that cleared without CVC removal, and (iii) persistent or recurrent fungemia even after CVC replacement.
Microbiological studies.
All blood specimens were inoculated into BACTEC Plus Aerobic/F, BACTEC Plus Anaerobic/F, or BACTEC PEDS Plus/F medium bottles (Becton Dickinson, Towson, Md.). These were incubated in the BACTEC 9240 System (Becton Dickinson) at 35°C for 5 days or until the bottles were positive for colorimetric detection of CO2. Bottles proven to contain Candida species by Gram staining were selected and subcultured on blood agar plates or Sabouraud dextrose agar (SDA; Becton Dickinson). With CVC removal, the CVC tips (5 cm) were cultured by rolling the catheter across a blood agar plate four times. After a 48-h incubation at 35°C, the colonies were counted and the organisms were identified (8). C. parapsilosis was identified by assimilation tests, including conventional methods, using an API 20C and an ATB 32C (bioMerieux, Marcy l'Etoile, France) and by assessing the isolates on cornmeal agar (Difco Laboratories, Detroit, Mich.). The isolates were stored on potato dextrose agar at −70°C.
EK.
Forty-eight sequential C. parapsilosis bloodstream isolates from 17 patients were karyotyped by PFGE (GenePath system; Bio-Rad, Hercules, Calif.). EK with PFGE was performed as previously described (2, 10, 11). Briefly, one colony of each Candida isolate from the 48-h SDA cultures was incubated overnight at 37°C in 10 ml of YPD broth (glucose, 2%; yeast extract, 1%; Bacto Peptone [Difco], 2%). A 150-μl aliquot of cell suspension was evenly mixed with 30 U of lyticase (Sigma, St. Louis, Mo.) and 150 μl of 1.6% agarose (FMC BioProducts, Hercules, Calif.) that was previously melted and kept liquid at 50 to 55°C. Aliquots placed in plug molds were incubated for 20 min at room temperature. The agarose plugs were removed from the plug molds, placed in 500 μl of lyticase buffer containing 50 mM EDTA and lyticase (Sigma) at 100 U/ml for 2 h, and washed once in 2 ml of distilled water. The plugs were incubated in proteinase K solution (50 mM EDTA, 100 μg proteinase K [Gibco BRL, Life Technologies, Gaithersburg, Md.]) for 16 to 18 h at 50°C and washed five times in 50 mM sodium EDTA (pH 8.0). Candida chromosomal DNA was separated by PFGE using the GenePath system (Bio-Rad). Electrophoresis was performed for 48 h in 0.7% agarose gel (SeaKem GTG agarose; FMC BioProducts) in 0.5× TBE buffer (0.1 M Tris, 0.09 M boric acid, 0.01 M EDTA, pH 8.0) at 4 V/cm with initial and final switch times of 90 and 325 s, respectively. After electrophoresis, the gels were stained with 0.5 μg of ethidium bromide per ml, illuminated under UV light, and photographed. Isolates that differed by one or more bands were considered to have different karyotypes. All isolates were analyzed at least twice (mean, three times; range, two to five times) using a completely new procedure including subculturing of isolates from the original stock culture to SDA, preparation of DNA, and separation of the DNA by PFGE to ascertain pattern relatedness and to ensure reproducibility.
Antifungal susceptibility testing.
Antifungal susceptibility to amphotericin B, fluconazole, and itraconazole was tested using the standard methods of the National Committee for Clinical Laboratory Standards (9). Two reference strains, C. parapsilosis ATCC 22019 and Candida krusei ATCC 6258, were tested as quality control isolates in each antifungal susceptibility test. The final concentrations of the antifungal agents were 0.313 to 16 μg/ml for amphotericin B (Sigma), 0.125 to 64 μg/ml for fluconazole (Pfizer, Sandwich, United Kingdom), and 0.0313 to 16 μg/ml for itraconazole (Janssen Pharmaceutica, Beerse, Belgium). The amphotericin B MIC was defined as the lowest drug concentration at which there was no growth. The fluconazole and itraconazole MICs were defined as the lowest drug concentrations causing growth ≤20% of that of the control.
Clinical data and definitions.
The charts of the 17 patients with C. parapsilosis fungemia were reviewed retrospectively. Patient data (age, sex, and diagnosis), duration of each episode of fungemia, date of CVC removal, dates and dosage of antifungal drug administration, and fungemia outcome were recorded. Persistent candidemia was defined as two or more blood cultures positive for C. parapsilosis on 2 or more separate days. Recurrent fungemia was defined as positive blood cultures after an absence of fungemia for at least 3 days after CVC removal. Candidemia was defined as CVC related if other sources of infection were not found and if the semiquantitative catheter tip culture yielded more than 15 colonies of the same Candida species (8). Transient candidemia was defined as a single blood culture positive for a Candida strain that resolved without any specific intervention, such as catheter removal or antifungal therapy, or if the attending physician regarded the intervention as insignificant. Candidemia was defined as having cleared microbiologically if the blood culture became negative for the same C. parapsilosis strain, as proven by EK, at any time during a 5-month follow-up period.
RESULTS
Forty-eight C. parapsilosis isolates from the blood of 17 patients were analyzed by EK with PFGE. EK provided optimal resolution of the chromosome bands, particularly between molecular sizes of 1,600 and 900 kb. The high-molecular-weight chromosomal bands (>1,800 kb) were similar in size in all of the bloodstream C. parapsilosis isolates tested. Low-molecular-weight chromosomal bands (less than 900 kb) were absent. In the molecular size range of 1,600 to 900 kb, there was marked variation in the number and size of chromosomal bands. Twenty-five specific karyotypes were identified in 48 isolates from 17 patients, and identical karyotype patterns were not seen in the isolates from different patients. Sequential strains from each patient had identical karyotypes in 53% (9 of 17) of the patients and two different karyotypes in 47% (8 of 17).
The EK results for sequential isolates from each patient were compared according to the outcome of fungemia after CVC removal. In the seven patients whose CVC-related fungemia cleared on CVC removal, the sequential strains from each patient had the same karyotype. In the two patients whose fungemia cleared without CVC removal, the two sequential isolates had different karyotypes. Six (75%) of the eight patients whose fungemia was recurrent even after CVC removal had isolates with different karyotypes pre- and post-CVC removal.
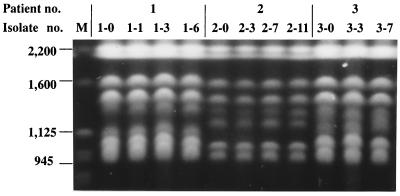
The clinical data for the patients whose fungemia cleared on CVC removal or without CVC removal are summarized in Table 1. Patients 1 to 7 were diagnosed as having CVC-related fungemia, and their blood cultures were positive for 2 to 11 days. In two of these patients (patients 3 and 5), the fungemia cleared after CVC removal without antifungal therapy, but the five other patients received fluconazole therapy after CVC removal. Patients 8 and 9 only had two positive blood cultures for C. parapsilosis, separated by 4 and 6 days, respectively. Both patients received antifungal therapy after the second positive blood culture was obtained, but their CVCs were not removed. Table 2 shows the EK patterns and antifungal susceptibilities of 23 bloodstream isolates from these nine patients. In patients 1 to 7, whose fungemia cleared on CVC removal, the sequential strains exhibited the same karyotype; the 19 isolates from these seven patients represented seven karyotypes. In patients 8 and 9, whose fungemia cleared without CVC removal, the two sequential isolates had different karyotypes (Table 2). Figure 1 shows the EK patterns for the sequential isolates from patients 1, 2, and 3.
TABLE 1.
Clinical summary for the patients whose fungemia cleared on CVC removal or without CVC removal
| Patient no. | Age/sexa | Admission cause | Fungemia durationb | CVC removal (dayb) | Antifungal drug, dose/day (therapy durationb) |
|---|---|---|---|---|---|
| 1 | 66 yr/F | Severe wound infection | 0–6 | Yes (6) | Fluconazole, 400 mg (9–21) |
| 2 | 1.5 mo/F | Low birth weight | 0–11 | Yes (10) | Fluconazole, 6 mg (10–25) |
| 3 | 2 mo/M | Fetal distress | 0–7 | Yes (3) | None |
| 4 | 60 yr/M | Chronic renal failure | 0–2 | Yes (2) | Fluconazole, 100 mg (2–17) |
| 5 | 64 yr/F | Gastric cancer | 0–2 | Yes (2) | None |
| 6 | 1 mo/M | Low birth weight | 0–3 | Yes (3) | Fluconazole, 6 mg (3–17) |
| 7 | 10 days/F | Low birth weight | 0–2 | Yes (3) | Fluconazole, 5 mg (3–19) |
| 8 | 40 yr/M | 3rd degree burn | 0, 4 | No | Fluconazole, 200 mg (4–8) |
| 9 | 61 yr/M | Liver cirrhosis | 0, 6 | No | Amphotericin B, 0.5 mg/kg (6–14) |
F, female; M, male.
Number of days after the first positive blood culture for each patient.
TABLE 2.
EKs and antifungal susceptibilities of sequential C. parapsilosis isolates from patients whose fungemia cleared on CVC removal or without CVC removal
| Patient no. and Isolate no. | Date of isolation (mo/day/yr) | MICa (μg/ml)
|
Karyotype | ||
|---|---|---|---|---|---|
| AmB | Flu | Itra | |||
| 1 | |||||
| 1-0 | 11/16/98 | 0.5 | 1.0 | 0.06 | a |
| 1-1 | 11/17/98 | 0.5 | 1.0 | 0.06 | a |
| 1-3 | 11/19/98 | 0.5 | 1.0 | 0.06 | a |
| 1-6 | 11/22/98 | 0.5 | 1.0 | 0.06 | a |
| 2 | |||||
| 2-0 | 06/24/99 | 0.5 | 1.0 | 0.06 | b |
| 2-3 | 06/26/99 | 0.5 | 1.0 | 0.06 | b |
| 2-7 | 06/30/99 | 0.5 | 1.0 | 0.06 | b |
| 2-11 | 07/05/99 | 0.5 | 1.0 | 0.06 | b |
| 3 | |||||
| 3-0 | 07/18/99 | 0.5 | 0.25 | 0.03 | c |
| 3-3 | 07/21/99 | 0.5 | 0.25 | 0.03 | c |
| 3-7 | 07/25/99 | 0.5 | 0.25 | 0.03 | c |
| 4 | |||||
| 4-0 | 3/20/98 | 0.5 | 0.5 | 0.03 | d |
| 4-2 | 3/22/98 | 0.5 | 0.5 | 0.03 | d |
| 5 | |||||
| 5-0 | 05/11/98 | 1.0 | 1.0 | 0.25 | e |
| 5-2 | 05/13/98 | 1.0 | 1.0 | 0.25 | e |
| 6 | |||||
| 6-0 | 07/23/99 | 0.5 | 1.0 | 0.12 | f |
| 6-2 | 07/25/99 | 0.5 | 1.0 | 0.12 | f |
| 7 | |||||
| 7-0 | 09/10/99 | 0.5 | 0.5 | 0.03 | g |
| 7-2 | 09/12/99 | 0.5 | 0.5 | 0.03 | g |
| 8 | |||||
| 8-0 | 11/24/97 | 0.5 | 0.25 | 0.03 | h |
| 8-4 | 11/28/97 | 0.5 | 0.25 | 0.03 | i |
| 9 | |||||
| 9-0 | 10/31/97 | 0.5 | 0.25 | 0.03 | j |
| 9-6 | 11/06/97 | 0.5 | 0.25 | 0.03 | k |
AmB, amphotericin B; Flu, fluconazole; Itra, itraconazole.
FIG. 1.
EKs of sequential C. parapsilosis isolates from three patients (patients 1, 2, and 3) with fungemia that cleared on CVC removal. Sequential isolates from each patient showed the same karyotype. M, Saccharomyces cerevisiae chromosomal DNA size standards. Sizes are shown in in kilobases on the left.
Fungemia was recurrent even after CVC removal in eight patients (patients 10 to 17). The clinical summary for these patients is shown in Table 3. All eight patients were cared for in the intensive care unit (ICU) and had CVCs. The first episode of fungemia for all eight patients was diagnosed as CVC-related fungemia. Since all of the patients required continuous intravenous therapy and total parenteral nutrition via CVC, a new CVC was inserted after removal of the original. The time intervals between the first and recurrent episodes of fungemia ranged from 4 to 138 days (mean, 31 days). In patient 15, Candida albicans fungemia occurred between the first and recurrent episodes. Recurrent episodes of fungemia occurred during antifungal therapy in three patients (patients 13, 15, and 17).
TABLE 3.
Clinical summary for eight patients whose fungemia was recurrent even after CVC removal
| Patient no. | Age/sexa | Admission cause | Fungemia episode | Fungemia durationb | CVC removal (day[s]b) | Antifungal drug, dose/day (therapy durationb) |
|---|---|---|---|---|---|---|
| 10 | 25 days/M | Staphylococcus aureus sepsis | First | 0–3 | Yes (3) | None |
| Recurrent | 7–12 | No | Amphotericin, 0.5 mg/kg (9–18) | |||
| 11 | 62 yr/M | Myasthenia gravis | First | 0–7 | Yes (5) | Fluconazole, 200 mg (7–17) |
| Recurrent | 50–85 | Yes (85) | Fluconazole, 200 mg (86–100) | |||
| 12 | 52 yr/M | Bronchial asthma | First | 0–35 | Yes (3, 35) | Amphotericin, 0.5 mg/kg (11–35) |
| Recurrent | 48–67 | Yes (67) | Amphotericin, 0.5 mg/kg (66–78) | |||
| 13 | 40 yr/F | Tuberculous meningitis | First | 0–7 | Yes (8) | Amphotericin, 0.5 mg/kg (4–25) |
| Recurrent | 13–27,c 38 | Yes (28) | Amphotericin, 0.5 mg/kg (41–47) | |||
| 14 | 43 yr/M | Acute renal failure | First | 0–18 | Yes (18) | Amphoterin, 0.5 mg/kg (18–30) |
| Recurrent | 156–160 | Yes (160) | Amphotericin, 0.5 mg/kg (160–174) | |||
| 15 | 22 yr/M | Acute renal failure | First | 0 | Yes (3) | None; fluconazole, 200 mg (7–24)d |
| Recurrent | 19–27c | Yes (27) | Amphotericin, 0.5 mg/kg (25–34) | |||
| 16 | 76 yr/M | Intracranial hemorrhage | First | 0–3 | Yes (3) | Fluconazole, 400 mg (0–19) |
| Recurrent | 23 | No | None | |||
| 17 | 62 yr/M | Traffic accident | First | 0–2 | Yes (2) | Amphotericin, 0.5 mg/kg (4–11) |
| Recurrent | 11c | Yes (11) | Amphotericin, 0.5 mg/kg (11–26) |
M, male; F, female.
Number of days after the first positive blood culture in each patient.
Fungemia occurred during antifungal therapy in three patients.
Patient 15 underwent antifungal therapy for the treatment of C. albicans candidemia.
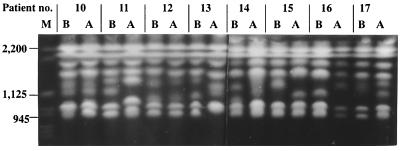
On EK, the 25 isolates from the first and recurrent episodes of fungemia in eight patients revealed 14 different types (Table 4). When the isolates from the separate episodes were compared, the index and recurrent episodes of fungemia were caused by different strains in six of the patients (patients 11 and 13 to 17) but by the same strain in two others (patients 10 and 12). Representative PFGE patterns are shown in Fig. 2.
TABLE 4.
EKs and antifungal susceptibilities of sequential C. parapsilosis isolates from patients whose fungemia was recurrent even after CVC removal
| Patient no. and isolate no. | Date of isolation (mo/day/yr) | MICa (μg/ml)
|
Karyotype | ||
|---|---|---|---|---|---|
| AmB | Flu | Itra | |||
| 10 | |||||
| 10-0 | 12/09/96 | 0.5 | 0.25 | 0.06 | l |
| 10-1 | 12/10/96 | 0.5 | 0.25 | 0.06 | l |
| 10-9 | 12/18/96 | 0.5 | 0.25 | 0.06 | l |
| 10-12 | 12/21/96 | 0.5 | 0.25 | 0.06 | l |
| 11 | |||||
| 11-0 | 06/09/99 | 0.5 | 1.0 | 0.06 | m |
| 11-52 | 07/31/99 | 0.5 | 0.5 | 0.06 | n |
| 11-68 | 08/16/99 | 0.5 | 0.5 | 0.06 | n |
| 11-78 | 08/26/99 | 0.5 | 0.5 | 0.06 | n |
| 12 | |||||
| 12-0 | 07/15/99 | 0.5 | 0.5 | 0.06 | o |
| 12-35 | 08/19/99 | 0.5 | 0.5 | 0.06 | o |
| 12-53 | 09/06/99 | 0.5 | 0.5 | 0.06 | o |
| 12-61 | 09/14/99 | 0.5 | 0.5 | 0.06 | o |
| 12-67 | 09/20/99 | 0.5 | 0.5 | 0.06 | o |
| 13 | |||||
| 13-0 | 09/13/99 | 0.5 | 0.25 | 0.03 | p |
| 13-14 | 09/27/99 | 1.0 | 0.5 | 0.06 | q |
| 13-18 | 10/01/99 | 1.0 | 0.5 | 0.06 | q |
| 13-38 | 10/21/99 | 1.0 | 0.5 | 0.06 | q |
| 14 | |||||
| 14-4 | 06/05/97 | 0.5 | 0.25 | 0.03 | r |
| 14-158 | 11/10/97 | 0.5 | 0.5 | 0.03 | s |
| 15 | |||||
| 15-0 | 09/26/97 | 0.5 | 0.5 | 0.03 | t |
| 15-27 | 10/23/97 | 0.5 | 0.25 | 0.03 | u |
| 16 | |||||
| 16-0 | 02/04/99 | 1.0 | 0.5 | 0.25 | v |
| 16-23 | 02/27/99 | 1.0 | 0.5 | 0.25 | w |
| 17 | |||||
| 17-0 | 09/06/99 | 1.0 | 1.0 | 0.25 | x |
| 17-11 | 09/17/99 | 0.5 | 0.5 | 0.06 | y |
AmB, amphotericin B; Flu, fluconazole; Itra, itraconazole.
FIG. 2.
EKs of representative C. parapsilosis isolates before (B) and after (A) CVC removal from eight patients (patients 10 to 17) with recurrent fungemia. The isolates from patients 10 and 12 had the same karyotypes, and these patients were considered to have relapsing infections caused by the same strain, whereas the isolates from the other patients had different karyotypes, considered to represent different strains. M, S. cerevisiae chromosomal DNA size standards. Sizes are shown in kilobases on the left.
For two patients (patients 10 and 12), the index and subsequent isolates were the same strains, as indicated by the karyotype analysis. The respective time intervals between the two episodes were 4 and 13 days (Table 3). In patient 10, fungemia recurred following a 4-day clearance after CVC removal. The recurrent fungemia cleared after 9 days of amphotericin B therapy. In patient 12, C. parapsilosis was found in the blood continuously after removal of the first CVC (3 days after the first positive culture), probably because amphotericin B therapy was delayed. Although the patient's blood culture became negative after removal of a second CVC (35 days after the first positive culture), C. parapsilosis with the same karyotype reappeared in the blood after 13 days of negative cultures. The fungemia in patient 12 finally cleared on removal of the third CVC (67 days after the first positive culture), and amphotericin B therapy was restarted and continued for 12 days after a negative blood culture was obtained. The recurrent fungemia completely resolved in all eight patients. Two patients died (patients 16 and 17), but their deaths were not attributed to fungemia.
All of the C. parapsilosis isolates were susceptible to all three of the antifungal agents tested (Tables 2 and 4). For these isolates, the amphotericin B MICs ranged from 0.5 to 1.0 μg/ml, those of fluconazole were 0.25 to 1.0 μg/ml, and those of itraconazole were 0.03 to 0.25 μg/ml. A difference in the fluconazole MICs before and after CVC removal was observed in five patients (patients 11, 13, 14, 15, and 17), but they were within 1 tube dilution of each other. There was no significant increase in the MICs of amphotericin B, fluconazole, or itraconazole among the sequential isolates from each patient over the course of therapy.
DISCUSSION
C. parapsilosis is genetically heterogenous, and its karyotype has been used in the identification of strains or types in many different source groups (2–4). The EK patterns of C. parapsilosis isolates tend to converge rather than diverge and may ultimately not provide adequate discrimination. Of particular concern is the fact that most of the differences are due to fuzzy bands between 1,100 and 1,400 kb. However, to differentiate C. parapsilosis strains, karyotypes are more discriminatory than PFGE patterns obtained after digestion with low-frequency cleavage restriction endonuclease BssHII (2, 10) or SfiI (11).
Pfaller et al. (10) reported that restriction endonuclease analysis of genomic DNA (REAG) with BssHll, coupled with PFGE, added little additional discrimination among C. parapsilosis isolates to that achieved by EK analysis. They found that the majority of their isolates had the same REAG profile with BssHll (70% had the REAG 1 profile), whereas EK analysis identified 13 different types among the 42 isolates with the REAG 1 profile. They suggested that EK alone might be sufficient for epidemiologic typing of C. parapsilosis isolates. This study confirmed the effectiveness of EK for strain delineation of C. parapsilosis. Our EK typing method differentiated multiple isolates from 17 different patients and was able to differentiate multiple isolates from the same patient. Each EK pattern was stable when the organisms were retyped by using a completely new preparation of DNA and separating the DNA by PFGE. This study confirmed the chromosomal variability of C. parapsilosis blood isolates.
To date, the application of molecular typing in defining the relationship of sequential strains from C. parapsilosis fungemia from the same patient has been limited. Barton et al. (1) reported karyotype stability in serial isolates of C. albicans from neutropenic patients. We also found that the sequential bloodstream isolates of C. parapsilosis from most patients had the same karyotype. This suggests that for most patients, serial isolates of C. parapsilosis exhibit a stable karyotype. One episode of CVC-related fungemia caused by C. parapsilosis resulted from the proliferation of a single pathogenic strain.
Branchini et al. (2) identified a considerable number of individual EK types of C. parapsilosis within a collection of clinical blood and catheter isolates from a single institution by EK analysis. Pfaller et al. (10) identified a total of 26 EKs among 60 clinical isolates of C. parapsilosis from five different medical centers. Both studies identified one or two major karyotypes among their isolates, which suggests a potential for cross-infection with C. parapsilosis among their patients. In our study, EK analysis of 48 isolates from 17 patients revealed 25 different karyotypes, each of which was unique to each patient. This showed that nearly every infection episode in a patient was caused by a distinct C. parapsilosis strain. Although an outbreak of C. parapsilosis fungemia related to long-term CVC use has been reported (6), we found no clustering of a predominant strain.
Recurrent fungemia caused by C. parapsilosis is barely mentioned in several reports on C. parapsilosis fungemia (5, 14). Here, we report eight cases of recurrent fungemia identified in a series of 50 patients with C. parapsilosis fungemia seen in our hospital over a 4-year period. Six of these eight patients were reinfected with new strains, as determined by PFGE. Thus, in certain clinical situations, recurrent fungemia caused by C. parapsilosis may represent reinfection (recurrence with a second strain) rather than relapse (recurrent disease with the index strain).
Among the cases of fungemia in which the karyotypes of the pre- and post-CVC removal isolates were different, the time intervals between the index and recurrent episodes exceeded 19 days in four of the patients but were 6 and 9 days, respectively, in two of the patients. On the other hand, the time intervals between the two episodes in the two cases of recurrent fungemia caused by the same karyotype strain were 4 and 13 days, respectively. This shows that reinfection or relapse cannot be differentiated by the time interval between the two episodes alone but requires EK analysis.
The pathogenesis of recurrent fungemia caused by C. parapsilosis may involve various host factors. Our eight patients with recurrent fungemia were all cared for in the ICU, and they required antibiotics and total parenteral nutrition via CVC for prolonged periods. Unlike other Candida species, C. parapsilosis causes nosocomial candidemia without prior colonization of other sites, suggesting that this yeast might gain direct access to the bloodstream via a vascular catheter or another environmental source (6, 14). It is theoretically possible that in severely ill patients, new strains of C. parapsilosis gain direct access to the bloodstream via a new CVC following the clearance of one episode of fungemia. In three patients (patients 13, 15, and 17) in our study, recurrent fungemia occurred during amphotericin B or fluconazole therapy. This confirms that catheter-related fungemia may develop despite antifungal therapy (12). Therefore, appropriate management to prevent further recurrence of C. parapsilosis fungemia should be considered for any severely debilitated patient with a CVC.
C. parapsilosis is the Candida species most frequently isolated from the hands of healthcare workers (HCWs) (6, 13). Levin et al. (6) reported the occurrence of an outbreak of C. parapsilosis fungemia related to long-term CVCs in which the hands of HCWs were implicated. They identified two karyotypes among isolates from five patients and three HCWs. The increasing frequency of C. parapsilosis fungemia in the ICU at our hospital led us to perform molecular typing of the isolates from ICU HCWs and to compare these with isolates from our patients. On 5 September 1999, surveillance cultures of the hands of ICU HCWs were performed using the broth wash technique (13). Five (20%) of 25 ICU HCWs were found to harbor C. parapsilosis on their hands. Of the 9 strains from these five HCWs, a total of eight different karyotypes were identified: one karyotype was shared by two isolates from two HCWs. Only one of the karyotypes of the HCW isolates was shared with patient isolates; an isolate with the same EK pattern as recurrent isolates of patient 11 was found on the hands of an HCW (data not shown). This demonstrated that although C. parapsilosis isolates have a high level of genetic diversity, nosocomial transmission might occur among patients and HCWs via the hands.
There has been a recent increase in the incidence of CVC-related fungemia in hospitalized patients (5, 6, 12). The proper management of patients with CVC-related candidemia is under debate. In our study, persistent fungemia cleared without CVC removal in two patients (patients 8 and 9) but the sequential isolates from each patient had different karyotypes. The first isolates from these two patients were recovered from blood just once and disappeared without specific management, such as CVC removal or antifungal therapy. This suggests that the isolates may cause transient fungemia or contaminate a blood culture. In this study, some fungemia cleared rapidly after CVC removal and did not require antifungal therapy. However, relapse with the same strain occurred after CVC removal in two patients (patients 10 and 12), reflecting the need for timely antifungal therapy. Antifungal susceptibility testing revealed no significant increase in the MIC of amphotericin B, fluconazole, or itraconazole among serial isolates of each patient over the course of therapy. Overall, all of the cases of persistent or recurrent C. parapsilosis fungemia in this study resolved successfully. This confirms the favorable prognosis of uncomplicated candidemia due to C. parapsilosis (5, 7).
In summary, this study showed that EK with PFGE is useful in the investigation of persistent or recurrent fungemia caused by C. parapsilosis. In addition, we observed that most recurrent fungemia due to C. parapsilosis was caused by reinfection with a second strain, as determined by EK.
REFERENCES
- 1.Barton R C, van Belkum A, Scherer S. Stability of karyotype in serial isolates of Candida albicans from neutropenic patients. J Clin Microbiol. 1995;33:794–796. doi: 10.1128/jcm.33.4.794-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branchini M L, Pfaller M A, Rhine-Charberg J, Frempong T, Isenberg H D. Genotypic variation and slime production among blood and catheter isolates of Candida parapsilosis. J Clin Microbiol. 1994;32:452–456. doi: 10.1128/jcm.32.2.452-456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carruba G, pontieri E, De Bernarids F, Martino P, Cassone A. DNA fingerprinting and electrophoretic karyotype of environmental and clinical isolates of Candida parapsilosis. J Clin Microbiol. 1991;29:916–922. doi: 10.1128/jcm.29.5.916-922.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassone A, De Bernardis F, Pontieri F, Carruba G, Girmenia C, Martino P, Fernandez-Rodriguez M, Quindos G, Ponton J. Biotypic diversity of Candida parapsilosis and its relationship to the clinical source and experimental pathogenicity. J Infect Dis. 1995;171:967–975. doi: 10.1093/infdis/171.4.967. [DOI] [PubMed] [Google Scholar]
- 5.Girmenia C, Martino P, De Bernardis F, Gentile G, Boccanera M, Monaco M, Antonucci G, Cassone A. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors, and differential pathogenicity of the causative strains. Clin Infect Dis. 1996;23:506–514. doi: 10.1093/clinids/23.3.506. [DOI] [PubMed] [Google Scholar]
- 6.Levin A S, Costa S F, Mussi N S, Basso M, Sinto S I, Machado C, Geiger D C, Villares M C B, Schreiber A Z, Barone A A, Branchini M L M. Candida parapsilosis fungemia associated with implantable and semi-implantable central venous catheters and the hands of healthcare workers. Diagn Microbiol Infect Dis. 1998;30:243–249. doi: 10.1016/s0732-8893(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 7.Levy I, Rubin L G, Vasishtha S, Tucci V, Sood S K. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin Infect Dis. 1998;26:1086–1088. doi: 10.1086/520277. [DOI] [PubMed] [Google Scholar]
- 8.Maki D G, Weise C E, Sarafin H W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296:1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Pfaller M A, Messer S A, Hollis R J. Variations in DNA subtype, antifungal susceptibility, and slime production among clinical isolates of Candida parapsilosis. Diagn Microbiol Infect Dis. 1995;21:9–14. doi: 10.1016/0732-8893(94)00114-c. [DOI] [PubMed] [Google Scholar]
- 11.Pontieri E, Gregori L, Gennarelli M, Ceddia T, Novelli G, Dallapiccola B, De Bernardis F, Carruba G. Correlation of SfiI macrorestriction endonuclease fingerprint analysis of Candida parapsilosis isolates with source of isolation. J Med Microbiol. 1996;45:173–178. doi: 10.1099/00222615-45-3-173. [DOI] [PubMed] [Google Scholar]
- 12.Rex J H. Editorial response: catheters and candidemia. Clin Infect Dis. 1996;22:467–470. doi: 10.1093/clinids/22.3.467. [DOI] [PubMed] [Google Scholar]
- 13.Strausbaugh L J, Sewell D L, Ward T T, Pfaller M A, Heitzman T, Tjoelker R. High frequency of yeast carriage on hands of hospital personnel. J Clin Microbiol. 1994;32:2299–2300. doi: 10.1128/jcm.32.9.2299-2300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weems J J., Jr Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin Infect Dis. 1992;14:756–766. doi: 10.1093/clinids/14.3.756. [DOI] [PubMed] [Google Scholar]