Abstract
Background:
Mast cells are involved in many distinct pathological conditions, suggesting that they recognize and respond to various stimuli and thus require a rich repertoire of cell surface proteins. However, mast cell surface proteomes have not been comprehensively characterized.
Objective:
We aimed to further characterize the mast cell surface proteome to obtain a better understanding of how mast cells function in health and disease.
Methods:
We enriched for glycosylated surface proteins expressed in mouse bone marrow-derived cultured mast cells (BMCMCs) and identified them using mass spectrometry analysis. The presence of novel surface proteins in mast cells was validated by qPCR and flow cytometry analysis in BMCMCs and peritoneal mast cells (PMCs). We developed a clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (Cas9) gene editing approach to disrupt genes of interest in BMCMCs.
Results:
The glycoprotein enrichment approach resulted in the identification of 1270 proteins in BMCMCs, 378 of which were localized to the plasma membrane. The most common protein classes among plasma membrane proteins are small GTPases, receptors and transporters. One such cell surface protein is CD98 heavy chain (CD98hc) encoded by the Slc3a2 gene. Slc3a2 gene disruption resulted in a significant reduction in CD98hc expression, adhesion, and proliferation.
Conclusions:
Our study indicates that glycoprotein enrichment coupled with mass spectrometry can be used to identify novel surface molecules in mast cells. Moreover, we found that CD98hc plays an important role in mast cell function.
Keywords: Mast cells, glycoprotein enrichment, mass spectrometry, CRISPR/Cas9, surfaceome, CD98hc
Capsule summary
Glycoprotein enrichment and mass spectrometry enabled a comprehensive characterization of the mast cell surface proteome. CD98hc expression is required for optimal mast cell function.
INTRODUCTION
Mast cells are hematopoietic progenitor-derived, granule-containing immune cells that are widely distributed in tissues that interact with the external environment, such as the skin and mucosal tissues1. It is well-known that mast cells are significantly involved in IgE-mediated allergic reactions2. Moreover, recent clinical studies with therapies that can reduce mast cell numbers in severe asthma highlight the relevance of mast cells in human disease outcomes3. Recent studies using mouse modeling of mast cell or mast cell protease deficiency by our group and others indicate that mast cells and their granule proteases have pleiotropic regulatory roles in immunological responses and diseases, exemplified by bacterial and parasite infections, sepsis, auto-immune disease, and cancer4–6. Overall, these studies suggest that in addition to IgE-dependent mechanisms, mast cell function can be influenced by IgE-independent mechanisms involving cytokines, peptides, toll-like receptor ligands, or components of the complement system. The mast cell surface protein repertoire that transduces these stimuli is essential for the concerted biological function of mast cells in the complex signaling environments generated during homeostasis and disease. However, the mast cell surface proteome or surfaceome has yet not been comprehensively characterized.
Historically, antibody-based technologies have been utilized to characterize the cell surface proteomes of immune cells, including mast cells7, 8. However, antibody-based technologies such as flow or mass cytometry are limited in their multiplexing capabilities and by the availability of high-quality antibodies. For example, approximately 370 antigens are targeted by monoclonal antibodies to cluster of differentiation (CD) molecules9, representing only a fraction of the estimated 2,900 proteins in the surface exposed proteome10. Mass spectrometry-based technologies offer the advantage of multiplexed and unbiased detection of proteins independent of existing antibody availability. Since most cell surface proteins are glycosylated, the identification of most components of the cell surface proteome can be accomplished by the enrichment of glycoproteins exposed to the extracellular space. Glycoprotein enrichment in combination with mass spectrometry has been used to characterize the surfaceome of a relatively large number of tumor 11–13 and human primary cells including macrophages 14,15 and CD4+ T cells16. Therefore, we decided to use this approach to investigate the surface proteome profile of mast cells. Mass spectrometry analysis of BMCMC glycoprotein enriched samples resulted in the identification of 1,270 proteins. Plasma membrane proteins including GTPases, receptors and transporters were overrepresented in the glycoprotein enriched samples. Among the novel pool of surface proteins, we found that CD98hc was highly expressed in mast cells. Therefore, we investigated CD98hc’s contribution to mast cell function and observed that CD98hc deletion by a CRISPR/Cas9 approach significantly impaired mast cell ability to degranulate, proliferate and adhere to fibronectin.
METHODS
Mass spectrometry (MS) sample preparation
For glycoprotein enrichment, BMCMCs were treated with 10mM sodium periodate (NaIO4) (Bio-Rad, Hercules, CA) in PBS for 1h to oxidize hydroxyl groups on surface glycoproteins. Cells were lysed in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 0.002% sodium azide, 1% octyl-b-D-1-thioglucopyranoside) supplemented with a cocktail of serine and cysteine protease inhibitors (catalog number 11836170001, Sigma Aldrich, St. Louis, MO) and passed through a 27.5 gauge needle 15 times. Lysates were incubated overnight at room temperature with hydrazide beads (catalog number 153-6047, Bio-Rad) in coupling buffer (100 mM sodium acetate, 150 mM NaCl in H2O, pH 5.5). Bead-bound proteins were denatured with 8 M urea in 100 mM NH4HCO3. Immobilized denatured proteins were reduced with 50 mM dithiothreitol (DTT) for 1 h at 37°C, alkylated with 65 mM iodoacetamide (IAA) at room temperature in the dark for 30 min, and digested with trypsin (1:100, w/w) at 37 °C overnight. The digested tryptic peptides were recovered and stored at −80 °C until analysis. For whole cell proteomics, BMCMCs were treated with the same lysis, denaturation, reduction, alkylation, and digestion steps described for enriched glycoproteins.
Preparation of CRISPR/Cas9 reagents
CRISPR RNAs (crRNA) targeting Slc3a2 were identified using the CCTop design tool17 and the COSMID CRISPR design tool18 and commercially synthesized by Integrated DNA Technologies (IDT, Coralville, IA). The ribonucleoprotein (RNP) crRNA sequence targeting exon 2 in mouse cells was GGGTGCTCAAGTACTCCAGA.
Mock edited cells were generated with Alt-R® CRISPR-Cas9 Negative Control crRNA #1 (catalog 1072544, Integrated DNA Technologies, Inc., Coralville, IA).
Gene editing
Slc3a2 disruption was accomplished using the Neon transfection system (catalog number MPK1025, Thermo Fisher Scientific). crRNA targeting Slc3a2 and trans-activating RNA (IDT) were complexed at a 1:1 ratio, as per the manufacturer’s instructions. crRNA:trans-activating RNA complexes were mixed with Cas9 nuclease (IDT) at 1.2:1 M ratio (120 pmol RNA complex: 100 pmol Cas9 per reaction). Cells were washed with PBS and resuspended in Neon Buffer T at a final cell density of 4 x 107 cells/ml. Then, 100 pmol of complexed RNP per 4 x 106 cells was delivered to the cells by Neon electroporation (1450 V, 3 10 ms pulses). Cells were then transferred into pre-warmed cell culture medium with IL-3 (10 ng/ml) and 10% FBS. Cells were maintained in media after editing at a density of ~5 x 105 cells/ml. Seven days after gene editing, CD98hc− cells accounted for ≥85% of the total gene-edited BMCMCs.
Image analysis of gene edited cells
Cells were transferred onto glass slides using cytospin and stained with May-Grunwald Giemsa. Photographs were taken using a Leica DM4000 B microscope with a Leica DFC310 FX camera (Leica. Allendale, NJ). LAS V4.5 software was used for capturing the images (Leica). The images were analyzed using the ImageJ software (NIH, Bethesda, MD). The largest diameter of cells was quantified using the measure function. The metachromatic area of each cell was estimated as the integrated density of the photos converted into binary images.
Statistical analyses
Statistical tests were performed using GraphPad Prism version 8.4.3 (GraphPad Software, Inc., La Jolla, CA). Data were analyzed for statistical significance using the Mann Whitney U-test. P < 0.05 was considered statistically significant.
Detailed methods used in this study are included in the Methods section in this article’s Online Repository (available at www.jacionline.org), covering the following: C57BL/6 mice used, generation of BMCMCs, mass spectrometry analysis of cell surface proteins, single-cell sorting and qPCRs, flow cytometry, confocal microscopy, β-hexosaminidase release assay, PGD2 and cys-LT release assays, cytokine and chemokine release assays, cell counting and proliferation assay, adhesion assay, analysis of ERK phosphorylation, and colocalization immunofluorescence for cutaneous mastocytosis.
RESULTS
Mast cell surface proteome profiling
To profile the cell surface proteome of mast cells, we used well-established approaches to enrich for glycoproteins15, 16, 19, which are disproportionately located on the plasma membrane20. Briefly, glycoproteins were oxidized prior to cell lysis and captured from the lysate by hydrazide beads. Following several washes in denaturing buffer, peptides digested from hydrazide beads were subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) for protein identification (Fig. E1A). Analysis of the protein expression profiles generated by this method demonstrated close agreement between replicates as well as readily discernable differences between BMCMC whole cell lysate and BMCMC glycoprotein enriched samples (Fig. E1B). Of the 1,750 proteins identified in BMCMC samples, 748 (~43%) were common between whole cell and enriched samples, 480 (~27%) were present in whole cell lysates only, and 522 (~30%) were present in glycoprotein enriched samples only. The glycoprotein enrichment process substantially increased the proportion of transmembrane domain-containing proteins in the samples relative to whole cell lysate samples from 11% to 26% (Fig. 1A). Additionally, analysis of annotated subcellular localization listed on UniProt and Genecards confirmed that plasma membrane proteins were over-represented in glycoprotein enriched samples relative to whole cell lysates from the same cells (Fig. 1A, 30% and 24%, respectively). This proportion of plasma membrane proteins is similar to that previously reported with the use of glycoprotein enrichment and mass spectrometry15, 19. All further analysis in the study utilized only the glycoprotein-enriched samples. The most highly represented plasma membrane-annotated proteins are listed in Table 1 (see full list in Table E1). Importantly, our approach detected surface molecules known to be expressed in mast cells in the enriched samples, validating its usefulness for identifying mast cell surface molecules. These expected surface molecules include the beta and gamma subunits of the high affinity receptor for IgE (FcεRIβ and FcεRIγ, respectively), the receptor for stem cell factor (c-Kit, also known as CD117), the type 1 receptor for transforming growth factor-beta (Tgfbr1), the receptor for IL-33 (Il1rl1, also known as St2) and the common beta subunit of the receptor for granulocyte-macrophage colony stimulating factor (GM-CSF), IL-3, and IL-5 (Csf2rb). We also observed numerous mast cell-associated leukocyte CD surface molecules among the plasma membrane proteins (Table E1). These CD molecules reported to be expressed during mast cell development including CD13 (Anpep), CD34, and CD117 (c-Kit)21 as well as those involved in mast cell activation including CD31 (Pecam)22, CD45 (Ptprc)23, CD4824, 25, CD63 (Lamp3)26, and CD107a (Lamp1)27.
Figure 1. Characterization of mast cell surface proteome.
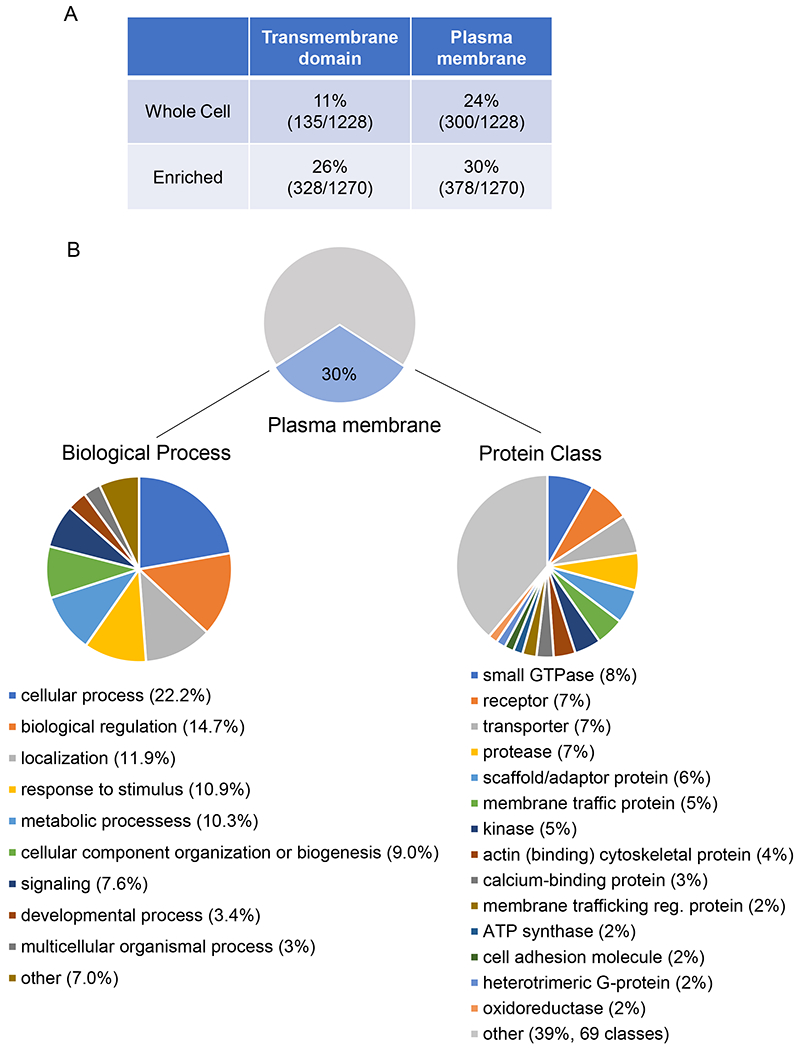
(A) Level of enrichment of number of transmembrane domain-containing proteins and cell surface proteins in glycoprotein-enriched samples compared to whole cell lysates. (B) Description of BMCMC cell surface proteome gene ontology (GO terms) by biological process (left) and PANTER protein classes (right).
Table 1. Top 30 represented mast cell plasma membrane annotated proteins.
Plasma membrane proteins in glycoprotein enriched BMCMC lysates (top 30 proteins identified sorted by normalized spectral abundance factor).
| Gene Name | UniProtKB Entry Name | Protein Name | Average number of peptides | Normalized peptide abundance |
|---|---|---|---|---|
| Atp5a1 | ATPA | ATP synthase subunit alpha, mitochondrial | 647.5 | 1.609362744 |
| Pdia3 | PDIA3 | Protein disulfide-isomerase A3 | 300 | 0.817938914 |
| Tmed10 | TMEDA | Transmembrane emp24 domain-containing protein 10 | 96.5 | 0.604409682 |
| Scin | ADSV | Adseverin | 300.5 | 0.578482641 |
| Arf3 | ARF3 | ADP-ribosylation factor 3 | 61 | 0.46384661 |
| Tagln2 | TAGL2 | Transgelin-2 | 61.5 | 0.426144919 |
| Rab7a | RAB7A | Ras-related protein Rab-7a | 63 | 0.419253803 |
| Rab14 | RAB14 | Ras-related protein Rab-14 | 58.5 | 0.375086815 |
| Arf5 | ARF5 | ADP-ribosylation factor 5 | 45 | 0.343771887 |
| P4hb | PDIA1 | Protein disulfide-isomerase (PDI) | 124.5 | 0.337299144 |
| Rab5c | RAB5C | Ras-related protein Rab-5C | 53 | 0.335955405 |
| Eef1a1 | EF1A1 | Elongation factor 1-alpha 1 | 111.5 | 0.332567166 |
| Phb | PHB | Prohibitin | 63.5 | 0.32199888 |
| Anpep | AMPN | Aminopeptidase N | 222.5 | 0.317256399 |
| Canx | CALX | Calnexin | 133.5 | 0.310751474 |
| Myh9 | MYH9 | Myosin-9 | 416 | 0.29230598 |
| Vdac1 | VDAC1 | Voltage-dependent anion-selective channel protein 1 | 61.5 | 0.285514099 |
| Hsp90ab1 | HS90B | Heat shock protein HSP 90-beta | 125.5 | 0.23793405 |
| Atp1b3 | AT1B3 | Sodium/potassium-transporting ATPase subunit beta-3 | 48 | 0.237285781 |
| Pdia6 | PDIA6 | Protein disulfide-isomerase A6 | 75 | 0.235160627 |
| Ifitm1 | IFM1 | Interferon-induced transmembrane protein 1 | 17.5 | 0.227438107 |
| Pdia4 | PDIA4 | Protein disulfide-isomerase A4 | 105 | 0.22702886 |
| Slc25a11 | M2OM | Mitochondrial 2-oxoglutarate/malate carrier protein | 51.5 | 0.226485846 |
| Gnai2 | GNAI2 | Guanine nucleotide-binding protein G(i) subunit alpha-2 | 58.5 | 0.226278247 |
| Stoml2 | STML2 | Stomatin-like protein 2, mitochondrial | 58 | 0.22612262 |
| Gng12 | GBG12 | Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-12 | 11 | 0.210576234 |
| Anxa5 | ANXA5 | Annexin A5 | 48.5 | 0.209973681 |
| Rhog | RHOG | Rho-related GTP-binding protein RhoG | 28.5 | 0.205855353 |
| Rac2 | RAC2 | Ras-related C3 botulinum toxin substrate 2 | 27.5 | 0.197982925 |
| Pgam1 | PGAM1 | Phosphoglycerate mutase 1 | 36 | 0.195256676 |
Gene ontology analysis of the plasma membrane-localized proteins by PANTHER28, 29 revealed that these proteins are involved in a wide variety of biological processes (Fig. 1B). The most annotated processes included cellular processes, biological regulation, response to stimulus, metabolic processes, cellular compartment organization, signaling, developmental process, and multicellular organismal process (Fig. 1B), reflecting the functional diversity of proteins at the mast cell plasma membrane. Additional PANTHER analysis of the protein classes represented in our dataset revealed that the most common protein classes in our dataset were small GTPases, receptors, and transporters (Fig. 1B, PANTHER protein class data) which are protein classes normally enriched at the plasma membrane in a variety of cell types. In these classes, we examined the most highly represented proteins in our dataset. The three most highly represented small GTPases were Rab proteins—Rab7a, Rab14, and Rab5c— which have all been reported to be involved in the endocytic pathway30 and to associate with macrophage phagosomes31, 32. In RBL-2H3 cells, a basophilic leukemia cell line, Rab7a and Rab14 are involved in exocytosis33, while Rab5c regulates secretory granule size through the fusion of secretory granules 34. Aside from the known mast cell receptors addressed above, the three most highly expressed receptors were Ly6a (Sca-1), plasminogen receptor (Plgrkt), and Spn (CD43). Ly6a is a murine bone marrow hematopoietic stem cell marker35 that has been found on some mast cell progenitor cells in mice as well as BMCMCs36 but not in granulocyte-myelocyte progenitors (GMPs), which are also thought to give rise to mast cells37. Plgrkt has not yet been characterized in mast cells but is reportedly involved in murine macrophage migration38. Spn is a sialoglycoprotein that is a negative regulator of mast cell adhesion likely involved in mast cell migration39. The highly expressed small GTPases and receptors reflect the potential relevance of these surface proteins in mast cell degranulation and ability to migrate; however, the most highly expressed transporters seen in our dataset do not currently have reported roles in mast cells despite their known contribution to cell function in other immune cells. These transporters include Atp1b3 (CD298), a sodium/potassium ATPase which has been reported to be involved in pain sensitivity in mice40 and human T cell activation41, 42; Clic1, a chloride ion channel that is important for macrophage function43, and CD98hc, the heavy chain of an amino acid transport heterodimer44 and integrin co-receptor45 implicated in B and T cell activation46, 47, 48. Overall, our data indicate that we were able to use glycoprotein enrichment and mass spectrometry to define the cell surface proteome of BMCMCs in a comprehensive manner that includes both known mast cell surface proteins as well as surface proteins of unknown mast cell function.
Identification and validation of novel surface proteins expressed in mast cells
The comprehensive nature of our glycoprotein enrichment technique allowed for the identification of mast cell surface proteins whose role in mast cell biology is not yet understood. The analysis of mast cell surface proteins by database searches (Pubmed and Google Scholar) revealed that approximately half of the mast cell plasma membrane proteins identified (223/378) have not previously been characterized in mast cells. The most highly represented novel mast cell surface proteins are listed in Table 2 (see full list in Table E2). All the novel mast cells surface genes were validated by independent verification of their expression in tissue mast cells using the Immunological Genome Project (ImmGen, http://www.immgen.org/databrowser/) database (Fig. E2). Gene ontology analysis of this set of surface proteins revealed that the uncharacterized surface proteins are involved in similar biological processes as the whole mast cell plasma membrane proteome (Fig. E3). Protein class analysis gave more dissimilar results as the most represented protein classes in this set of proteins are transporters, membrane traffic proteins, and scaffold/adaptor proteins (Fig. E3). Highly represented proteins in these classes include the transporters Atp1b3, Clic1, and CD98hc discussed above as well as Lman2 (VIP36), a lectin whose shedding stimulates macrophage phagocytosis49; and Lamtor1, a scaffold protein for the mechanistic target of rapamycin (mTOR) complex that is involved in regulating the innate immune response of macrophages50. To broadly verify the results of our proteomics analysis, a group of novel surface proteins was selected based on their wide range of estimated expression levels, and their involvement in a variety of biological processes reflecting the variety of our dataset (Table E3). Messenger RNA for all the selected novel surface proteins was detected in BMCMCs as well as PMCs, and both mouse mast cell types showed a similar pattern of expression among the genes measured (Fig. 2). Thus, our approach of the characterization of the mast cell’s surfaceome by glycoprotein enrichment and mass spectrometry led to the identification of a novel pool of surface proteins to investigate in the context of mast cell biology.
Table 2. Top 30 unique mast cell plasma membrane annotated proteins.
Plasma membrane proteins in glycoprotein enriched BMCMC lysates that have not been previously characterized in mast cells (top 30 proteins identified sorted by normalized spectral abundance factor).
| Gene Name | UniProt ID | Protein Name | Average number of peptides | Normalized peptide abundance | Expression in MC in Immgen | Reported in High Throughput Studies |
|---|---|---|---|---|---|---|
| Tmed10 | TMEDA | Transmembrane emp24 domain-containing protein 10 | 131.5 | 0.604 | M/H | Y |
| Scin | ADSV | Adseverin (Scinderin) | 300.5 | 0.578 | N-M* | Y |
| Tagln2 | TAGL2 | Transgelin-2 | 61.5 | 0.426 | H | N |
| Arf5 | ARF5 | ADP-ribosylation factor 5 | 45.0 | 0.344 | M | N |
| P4hb | PDIA1 | Protein disulfide-isomerase | 124.5 | 0.337 | X | Y |
| Canx | CALX | Calnexin | 133.5 | 0.311 | H | N |
| Myh9 | MYH9 | Myosin-9 | 416.0 | 0.292 | M | Y |
| Vdac1 | VDAC1 | Voltage-dependent anion-selective channel protein 1 | 61.5 | 0.286 | M | N |
| Atp1b3 | AT1B3 | Sodium/potassium-transporting ATPase subunit beta-3 | 48.0 | 0.237 | H | N |
| Pdia6 | PDIA6 | Protein disulfide-isomerase A6 | 75.0 | 0.235 | H | Y |
| Ifitm1 | IFM1 | Interferon-induced transmembrane protein 1 | 17.5 | 0.227 | VL-M* | Y |
| Pdia4 | PDIA4 | Protein disulfide-isomerase A4 | 105.0 | 0.227 | M-H | Y |
| Slc25a11 | M2OM | Mitochondrial 2-oxoglutarate/malate carrier protein | 51.5 | 0.226 | H | N |
| Stoml2 | STML2 | Stomatin-like protein 2 | 58.0 | 0.226 | M | N |
| Gng12 | GBG12 | Guanine nucleotide-binding protein | 11.0 | 0.211 | M | Y |
| Ppia | PPIA | Peptidyl-prolyl cis-trans isomerase A | 24.0 | 0.201 | M-H | Y |
| Pgam1 | PGAM1 | Phosphoglycerate mutase 1 | 36.0 | 0.195 | M | N |
| Itm2c | ITM2C | Integral membrane protein 2C | 37.0 | 0.189 | H-VH | N |
| Hsp90aa1 | HS90A | Heat shock protein HSP 90-alpha | 101.0 | 0.189 | M-H* | N |
| Clic1 | CLIC1 | Chloride intracellular channel protein 1 | 32.0 | 0.183 | H | N |
| Ddost | OST48 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit | 58.5 | 0.183 | H | N |
| Itm2b | ITM2B | Integral membrane protein 2B | 32.5 | 0.169 | VH | Y |
| Arf4 | ARF4 | ADP-ribosylation factor 4 | 21.0 | 0.161 | H | N |
| Plgrkt | PLRKT | Plasminogen receptor (KT) | 16.5 | 0.155 | H | N |
| Slc3a2 | 4F2 | 4F2 cell-surface antigen heavy chain (CD antigen CD98) | 58.0 | 0.152 | H | Y |
| Got2 | AATM | Aspartate aminotransferase, mitochondrial | 45.5 | 0.146 | M | N |
| Gnb2l1 | RACK1 | Receptor of activated protein C kinase 1 | 33.5 | 0.145 | H | N |
| Nme2 | NDKB | Nucleoside diphosphate kinase B | 15.5 | 0.142 | L | N |
| Mlec | MLEC | Malectin | 29.5 | 0.139 | M*-H | Y |
| Lman2 | LMAN2 | Vesicular integral-membrane protein VIP36 | 35.5 | 0.137 | H | N |
Figure 2. Validation of plasma membrane proteins identified but not previously characterized in mast cells.
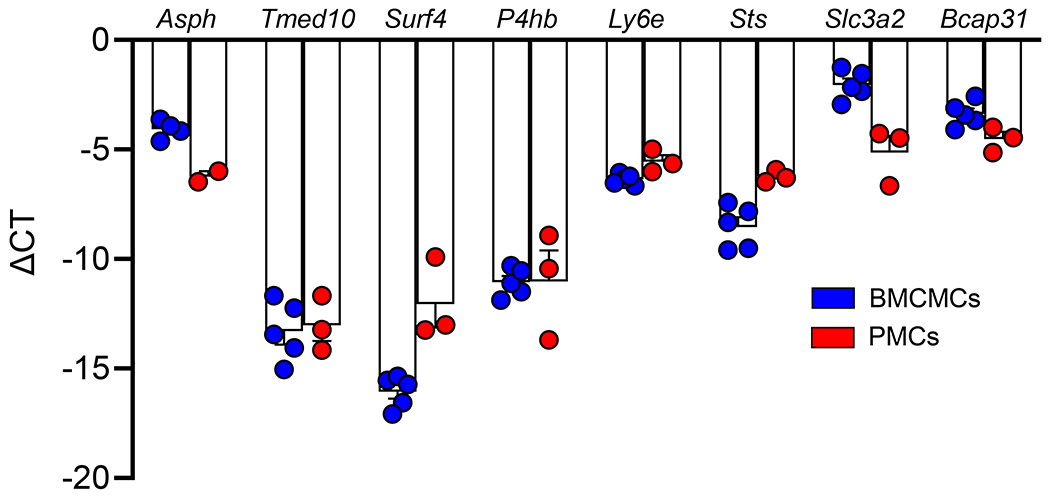
Expression of selected cell surface proteins in BMCMCs and PMCs by qPCR. Messenger RNA expression levels for the indicated transcripts are expressed as ΔCT values normalized against Gapdh as the reference transcript. Data are shown as mean + SEM of the average for duplicate specimens from 3-5 independent experiments, circles show values from individual experiments.
CD98hc is highly expressed in resting mast cells
The highest mRNA expression in the set of genes encoding for novel surface proteins was seen for Slc3a2 (Fig. 2). Slc3a2 encodes the heavy chain of CD98 (CD98hc), which is the only ubiquitously expressed heavy subunit of the heteromeric amino acid transporter CD98. CD98hc, carries the complex to the plasma membrane, whereas any of the six subunits of the L-type amino acid transporters (LATs) from the SLC7 family (LAT1, LAT2, xCT, y+LAT1, y+LAT2, and Asc-1) confers substrate specificity and constitutes the catalytic subunit of the transporter51.
In addition to CD98hc, mRNA for all the SLC7 subunits was detected in BMCMCs as well as PMCs (Fig. E4). Both mouse mast cell types showed a similar pattern of expression among the genes measured except for Asc-1 that is expressed at higher levels in BMCMCs than PMCs (Fig. E4).
The increased proportion of transporters in the set of uncharacterized mast cell surface proteins (Fig. E3) suggests that transporters may play an important and understudied role in mast cells. Furthermore, examination of the RNAseq IMMGEN database52, revealed that Slc3a2 appears to be highly expressed in PMCs, heart mast cells and splenic basophils when compared to other immune cells (Fig. E2 and E5A). This observation was confirmed by flow cytometry on selected immune cells (Fig. E5B). We also confirmed that the CD98hc protein is strongly expressed in BMCMCs, though at lower levels than those observed in PMCs, by flow cytometry (Figs. 3A and 3B). Interestingly, confocal microscopy showed intracellular localization of CD98hc along with the cell surface staining observed by flow cytometry (Fig. 3C). It has been shown that CD98hc protein expression levels are upregulated in activated B and T cells46–48. On the contrary, Slc3a2 mRNA and CD98hc protein expression levels were not significantly increased in mast cells upon activation by IgE-dependent and IgE-independent stimuli (Figs. E6A and E6B).
Figure 3. Mast cells express CD98hc on the cell surface.
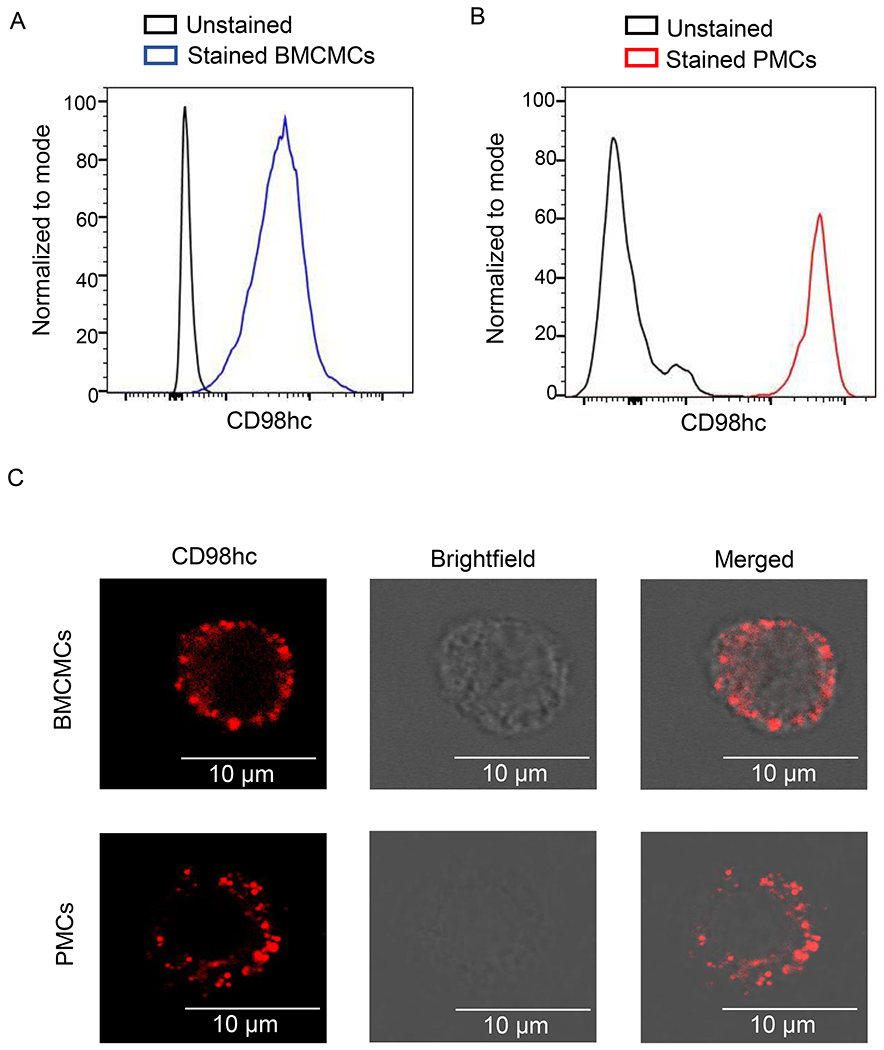
(A, B) Representative flow cytometry analysis of CD98hc expression in BMCMCs (A) and mast cell population in the peritoneal cavity (B). Cells in B were immunostained for CD98hc in addition to c-Kit and FcεRIα to identify mast cells in the peritoneal cavity. (C, D) Representative confocal images of BMCMCs (C) and PMCs (D) labeled first with rabbit anti-mouse CD98hc antibody and then with Alexa Fluor 488-conjugated donkey anti-rabbit antibody. Confocal microscopy images show staining for CD98hc (red in left panel), cell surface (brightfield microscopy in middle panel) and a merge of CD98hc staining and cell surface (right panel). Scale bar equals 10um. Flow cytometry data in A and B, and confocal images in C and D are representative of 3 independent experiments.
CD98hc deletion impairs the release of preformed mediators
Based on the findings that transporters are the most common type of uncharacterized mast cell surface proteins and the robust surface expression of CD98hc on resting mast cells as compared to other immune cells, we decided to examine CD98hc’s contribution to mast cell function.
Since CD98hc deficiency in mice is embryonically lethal53, we electroporated CRISPR-Cas9 ribonucleoprotein complexes into BMCMCs to delete CD98hc expression. Guide RNAs targeting exon 2 successfully disrupted the Slc3a2 gene as evidenced by T7 Endonuclease I assay (Fig. 4A) and confirmed using DNA sequencing and Inference of CRISPR Edits (ICE) analysis (~85% indel percentage). We generated a large population of BMCMCs that do not express CD98hc (85-95%) as measured by flow cytometry 7 days after gene editing (Fig. 4B). In contrast, CD98hc-deleted BMCMCs express similar levels of FcεRIα and c-Kit as mock-edited cells (Fig. E7). Moreover, BMCMC viability was not affected by CD98hc deletion at 7-8 days after gene editing (98.9% and 99.8% for mock edited and Slc3a2-disrupted BMCMCs, respectively). However, morphological characterization of CD98hc-deleted BMCMCs showed that these cells were smaller (Figs. 4C and 4D) and had a smaller metachromatic area per cell (Figs. 4C and 4E) than cells expressing CD98hc.
Figure 4. Slc3a2 disruption in BMCMCs partially abrogates mast cell degranulation.
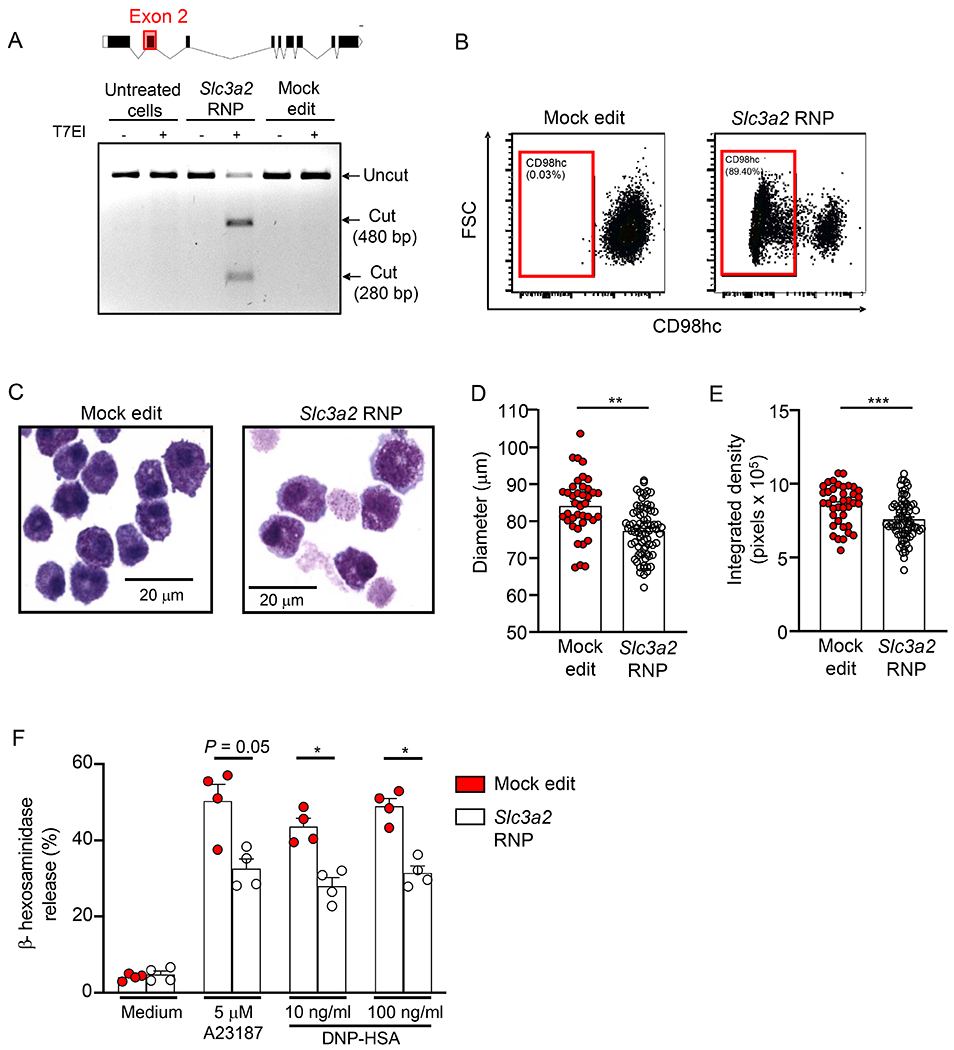
(A) Slc3a2 coding exons and representative T7 Endonuclease I (T7EI) assay showing ribonucleoprotein (RNP) cleavage. (B) Representative flow cytometry analysis of CD98hc expression and (C) cytospins stained with May-Grunwald-Giemsa of mock- and Slc3a2-edited BMCMCs. (D, E) Diameter (D) and metachromatic area (E) of mock- and Slc3a2-edited BMCMCs. (F) β-hexosaminidase release by mock- and Slc3a2-edited BMCMCs upon activation. In F mock- and Slc3a2-edited BMCMCs were sensitized with IgE mAb to DNP (2 μg/ml) overnight and then were challenged with either DNP-HSA (10 or 100 ng/ml) or calcium ionophore (A23187, 5 μM) for 1 h. Data in A-C are representative of 3 independent experiments. Data in D and E are shown as mean + SEM from 2 independent experiments, circles show values from one individual cell. Data in F are shown as mean + SEM of the average of duplicate specimens from 4 independent experiments, circles show values from individual experiments. *P < 0.05, ** P < 0.001, *** P < 0.0001.
To assess whether CD98hc plays a role in regulating mast cell activation and/or degranulation, we measured β-hexosaminidase release upon IgE-dependent activation of mock edited and Slc3a2-disrupted BMCMCs. Stimulation of mock edited BMCMCs resulted in significant β-hexosaminidase release when compared with cells incubated in medium alone (Fig. 4F). In contrast, CD98hc-deleted BMCMCs (Slc3a2 RNP) showed a significant reduction in their ability to release β-hexosaminidase upon IgE-dependent activation. A similar reduction in β-hexosaminidase release was observed for CD98hc-deleted BMCMCs stimulated with calcium ionophore (A23187) (Fig. 4F). Based on this evidence, we hypothesized that CD98hc may influence the mast cell degranulation process, granule formation and/or storage of preformed mediators like β-hexosaminidase. Accordingly, we assessed whether CD98hc has an impact on the degranulation process itself by assessing the expression of the lysosomal-associated membrane protein 1 (LAMP1 or CD107a) which indicates granule mobilization towards the plasma membrane upon mast cell stimulation. As shown in Fig. E8, CD98hc-deleted BMCMCs exhibited a slight but not significant reduction in the percentage of cells that are positive for LAMP1 upon activation with either calcium ionophore (A23187) or IgE-dependent stimuli when compared with mock-edited cells. Overall, these data suggest that the reduced β-hexosaminidase release from CD98hc-deleted cells may be caused by defects in granule formation rather than a defect in the degranulation process itself.
Contrary to what was observed for β-hexosaminidase release, CD98hc deletion in BMCMCs did not impact the production and release of the arachidonic acid metabolites prostaglandin (PG)D2 and cys-leukotrienes (LTs) after IgE-dependent activation or stimulation with A23187 (Figs. E9A and E9B). Similarly, CD98hc deletion had no effect on the release of pro-inflammatory mediators such as tumor necrosis factor (TNF), IL-6 and monocyte chemoattractant protein-1 (MCP-1/CCL2) (Figs. E9C–E).
Taken together, this data indicates that CD98hc deletion impaired mast cell degranulation but did not affect the mast cell’s ability to produce and release arachidonic acid metabolites and newly formed pro-inflammatory mediators.
CD98hc contributes to mast cell adhesion to fibronectin and cell proliferation
CD98hc can also be expressed without LATs at the plasma membrane44, and it can have functions that are independent from its amino acid transporter role54. More specifically, CD98hc behaves as a co-receptor of β-integrins and amplifies their downstream signaling55, 56 promoting cell adhesion and proliferation46, 55. Therefore, we first examined whether CD98hc deletion influences the mast cell’s ability to adhere to fibronectin (FN), a ligand for alpha (5) and beta (1) integrin57, 58. For this purpose, we first ruled out that CD98hc deletion does not impact the expression of alpha 5 and beta 1 integrins (Fig. E10).
CD98hc-deleted BMCMCs exhibited a significant reduction in their ability to adhere to FN when compared with mock edited BMCMCs (Fig. 5A). Moreover, we observed that either SCF or IgE/antigen, stimuli known to promote mast cell adhesion to FN59, significantly increased the adhesion of mock edited cells and CD98hc-deleted cells to FN (Fig. 5A). However, CD98hc-deleted BMCMCs exhibited a significant reduction in their ability to adhere to FN after stimulation with either SCF or IgE/antigen when compared with mock edited cells (Fig. 5A).
Figure 5. Slc3a2 disruption in BMCMCs inhibits mast cell adhesion and proliferation.
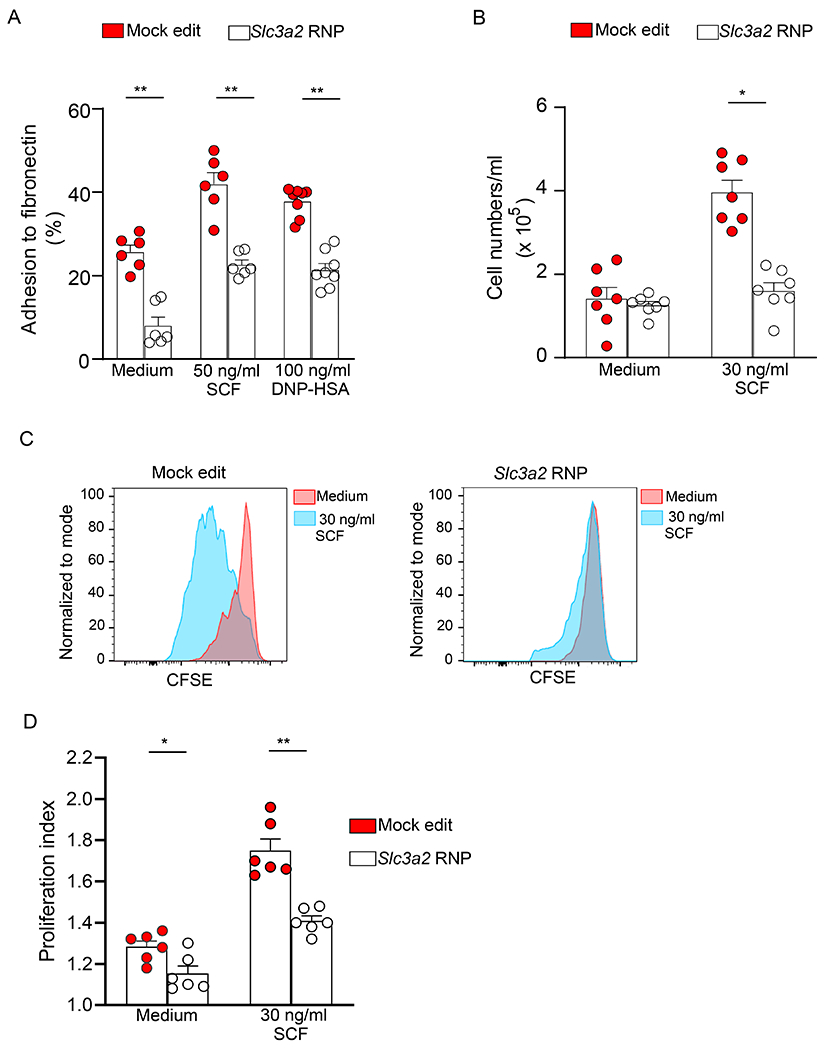
(A) Mock- and Slc3a2-edited BMCMC adhesion to 96-well plates coated with fibronectin (50 μg/ml) after the indicated treatment. Mock- and Slc3a2-edited BMCMCs were sensitized with IgE mAb to DNP (2 μg/ml) overnight, loaded with calcein-AM (3 μg/ml) and then challenged with DNP-HSA (100 ng/ml) or maintained in media supplemented with SCF (50 ng/ml). Percentage of fluorescent adherent cells relative to total number of adherent and non-adherent fluorescent cells was determined at 1 h after stimulation. (B-D) Numbers (B), representative flow cytometry analysis of CFSE dilution (C) and proliferation index D) at 5 d after incubation of mock- and Slc3a2-edited BMCMCs in media alone or in media supplemented with SCF (30 ng/ml). Data in A, B and D are shown as mean + SEM of the average of duplicate specimens from 4-6 independent experiments, circles show values from individual experiments. Flow cytometry data in C are representative of 3 independent experiments. *P < 0.05, ** P < 0.005.
Next, we examined the effects of CD98hc deletion on cell proliferation. Cell counting after plating equal numbers of cells and allowing them to grow for 5d showed that CD98hc-deleted cells were unable to significantly expand upon stimulation with SCF (Fig. 5B). To confirm whether the reduced cell expansion in CD98hc-deleted cells is due to impaired cell proliferation, we labeled the cells with carboxyfluorescein succinimidyl ester (CFSE) and analyzed the dilution of this fluorescent marker in subsequent generations of cells by flow cytometry. In BMCMC culture media, most cells stayed in the undiluted CFSE peak representing undivided cells (Fig. 5C) which is reflected in the proliferation index ranging from 1.08-1.30 (Fig. 5D). However, mock edited cells proliferated slightly more than CD98hc-deleted cells after 5d of culture (proliferation index of 1.18-1.36) (Figs. 5C and 5D). The addition of SCF to the culture media significantly stimulated proliferation in mock edited cells after 5d in culture as shown by a reduction in CFSE staining (Fig. 5C) and a significant increase in the proliferation index (1.63-1.96) when compared with cells incubated in media alone (Fig. 5D). In contrast, most of the CD98hc-deleted BMCMCs did not divide upon SCF stimulation as they remained in the undiluted CFSE peak and their proliferation index was significantly lower than that observed in mock edited cells (Fig. 5D, proliferation index 1.32-1.48). Overall, these data indicate that CD98hc plays a significant role in mast cell proliferation.
CD98hc is highly expressed in mast cells from patients with cutaneous mastocytosis
It has been reported that SLC3A2 is differentially expressed by bone marrow mast cells (BMMCs) from patients with aggressive systemic mastocytosis versus both normal reactive BMMCs and BMMCs from patients with mild indolent systemic mastocytosis60. Therefore, we decided to examine CD98hc expression in skin biopsies obtained from patients with cutaneous mastocytosis. We found that mast cells from patients with cutaneous mastocytosis overexpress CD98hc when compared with mast cells from patients with urticaria as a disease control (Fig. 6).
Figure 6. CD98hc is highly expressed in mast cells from patients with cutaneous mastocytosis.
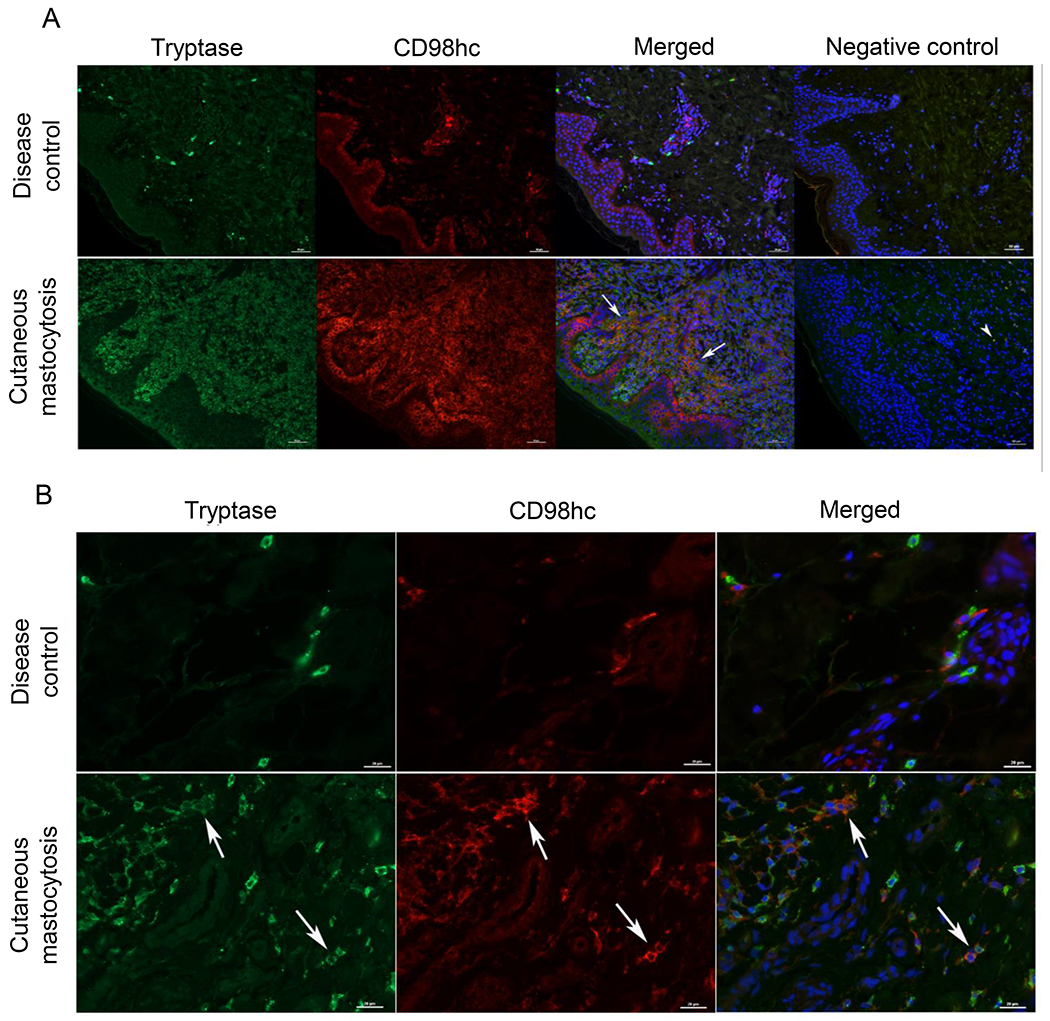
(A, B) Detection of CD98hc in mast cells located in skin biopsies from a disease control (chronic urticaria) and a newborn with congenital mastocytosis. Immunofluorescence was performed with anti-CD98hc (red fluorescence) and anti-tryptase mAb (green fluorescence) antibodies for mast cells located in skin. Nuclei were counterstained with DAPI (blue fluorescence). Mast cells expressing both CD98hc and tryptase are indicated with white arrows (yellow fluorescence). Negative controls were performed with secondary antibodies only (far right panel; arrowheads point to nonspecific signal). Images at 20x (A) and 40x (B) are representative of 4 separate disease controls and 3 separate patients with cutaneous mastocytosis.
DISCUSSION
The ability of mast cells to respond to various IgE-independent stimuli as well as their well-documented plasticity shaped by their microenvironment prompted us to hypothesize that a thorough characterization of the mast cell surface proteome could identify novel targets for the modulation of mast cell response in normal conditions and disease. Accordingly, our current study had two main goals: to develop a technique that provides a comprehensive characterization of the mast cell surface proteome, and to investigate the contribution of novel surface proteins to mast cell function by using CRISPR/Cas9 as a rapid and efficient method for the disruption of genes of interest.
The development of low input proteomics methods has allowed for the proteome profiling of resident tissue cells61. Proteome analysis of mast cells has been recently reported for primary human connective tissue mast cells from skin and fat62 and PMCs61, 62. These studies identified approximately 130-170 unique cell surface proteins in mast cells when mast cell proteome was compared with the proteome of macrophages58 and other immune cells61. These findings suggest that a relatively small number of surface proteins can be detected by mass spectrometry analysis of the whole mast cell proteome. Enrichment for glycoproteins, which are disproportionately located on the plasma membrane20, followed by mass spectrometry analysis has been successfully used as a comprehensive approach to characterize the cell surface proteome, or surfaceome, of immune cells including macrophages,15, 63 B cells, and T cells64. Based on these studies, we decided to use glycoprotein enrichment coupled with mass spectrometry to characterize the mast cell surface proteome. BMCMCs were used in our studies because these cells can be generated in the large numbers that are required to target plasma membrane proteins via glycoprotein enrichment. We recognize we should be careful about extrapolating findings from our study on BMCMCs to tissue mast cells. Comparative transcriptome analysis of BMCMCs and tissue mast cells revealed that these two populations show many similarities but also many differences65. Accordingly, we validated novel mast cell surface proteins in BMCMCs by independent verification of their expression in tissue mast cells using the Immunological Genome Project (ImmGen, http://www.immgen.org/databrowser/) database (Fig. E2). Moreover, we confirmed that PMCs express mRNA (Fig. 2) for a selected group of novel surface proteins with a wide range of estimated expression levels per our mass spectrometry data (Table E3).
Based on our analysis of annotated subcellular localization listed on UniProt and Genecards, we identified 378 plasma membrane proteins among the enriched glycoproteins, including GTPases, receptors, and transporters (Fig. 1B). The use of glycoprotein enrichment in combination with mass spectrometry typically leads to the identification of an average of 280 proteins, including 70 CD proteins, per cell type10. Based on this evidence we think that our approach has provided a comprehensive profiling of the mast cell surface proteome.
Despite the enrichment of plasma membrane proteins, we were not able to detect the presence of surface molecules known to be expressed by mast cells like the alpha subunit of the high affinity receptor for IgE (FcεRIα). Instead, we detected the FcεRIβ and FcεRIγ subunits (Supplemental Table E1). These observations are consistent with previous mass spectrometry analysis of mouse61, 62 and human mast cells66. We were also not able to detect surface innate immune sensors in BMCMCs such as toll-like receptor (TLR)2, TLR3, TLR4 and TLR7. Similar observations were recently reported in mass spectrometry studies of PMCs61, 62. Regardless of these observations, there is extensive literature supporting mast cell expression of these innate immune sensors that allow mast cells to respond to pathogens and contribute to host response to infection via secretion of cytokines, chemokines, and lipid mediators5, 67. It is well-known that plasma membrane proteins are under-represented in proteomic studies due to their low abundance and their hydrophobic nature68. Based on this evidence, we conclude that lack of expression of a surface protein in mast cells as determined by mass spectrometry is not definitive proof that the surface protein is not expressed in mast cells, and further assessment of its expression by alternative approaches is recommended.
Our analysis and subsequent validation of the plasma membrane proteins previously uncharacterized in mast cells revealed that CD98hc is highly expressed in both BMCMCs and PMCs (Figs. 2 and 3). Moreover, according to publicly available datasets (Fig. E3A) and our own flow cytometry data (Fig. E3B), mast cells express the highest levels of CD98hc amongst immune cells. Interestingly, resting mast cells express high CD98hc levels and mast cell activation does not increase CD98hc expression further (Figs. E6A and E6B). In contrast, other immune cells such as lymphocytes only express higher CD98hc levels upon activation, an event that seems to be required for clonal expansion of these cells46, 69. Overall, these observations suggest that CD98hc significantly contributes to mast cell function at steady state. To investigate the contribution of CD98hc to mast cell function, we developed a CRISPR/Cas9-based method to rapidly and efficiently delete CD98hc in BMCMCs (Figs. 4A and 4B). CD98hc-deleted BMCMCs were smaller in size (Figs. 4C and 4D), exhibited reduced metachromatic staining (Fig. 4C and 4E), and showed a significant reduction in β-hexosaminidase release when compared with mock edited cells (Fig. 4F). Moreover, we determined that CD98hc deletion does not impact the degranulation process itself as LAMP1 staining upon cell activation demonstrated that Slc3a2 disruption did not significantly affect mobilization of mast cell granules to the membrane (Fig. E8). These observations suggest that CD98hc may contribute to mast cell granule formation and hence to preformed mediator storage.
Our findings seem to resemble those reported for other members of the solute carrier family which contribute to mast cell granule formation. For example, it has been shown that Slc15a4-deficient BMCMCs exhibit anomalous granule formation possibly due to the role of Slc15a4 in the transport of amino acids required for the synthesis of mast cell pre-formed mediators70.
Our studies were expanded to examine CD98hc’s contribution to cell functions that are not associated with its transport activity but with its capability to bind to cytoplasmic tails of integrin-β chains, thus mediating signals that control cell adhesion and growth71. Both adhesion and proliferation upon stimulation were significantly impacted by CD98hc deletion (Fig. 5). Overall, our studies point to a critical role for CD98hc in mast cell degranulation, adhesion, and proliferation.
It has been shown that CD98-deficiency selectively impairs sustained activation of Erk1/2 after BCR ligation, an event that promotes B cell proliferation46. Since Erk1/2 activation is also downstream of both FcεRI and c-Kit signalling72, we explored whether this pathway may integrate our findings on CD98hc contribution to mast cell degranulation, proliferation and adhesion. As shown in Fig. E11, analysis of Erk1/2 phosphorylation indicates that this signaling pathway is not altered in CD98hc-deleted BMCMCs. At this point, we think that multiple mechanisms are involved in how CD98hc influences mast cell function. This hypothesis is based on reports that showed that CD98hc facilitates amino acid transport using multiple partners with various amino acid specificities51, and that CD98hc has integrin-related activities that are independent from its function as an amino acid transporter46, 54–56. Studies are in progress in our laboratory to determine the mechanism (s) by which CD98hc is involved in mast cell biology.
Can CD98hc contribute to the outcome of mast cell-associated disorders in humans? To gain insight into this question, we found that CD98hc is highly expressed in mast cells from patients with cutaneous mastocytosis (Fig. 6). Mast cell CD98hc overexpression in mastocytosis is consistent with the increased demand of amino acids by rapidly proliferating cells73. This result supports exploring the possibility of targeting CD98hc to treat mastocytosis and other mast cell-associated disorders in a similar manner as an approach currently under investigation for the treatment of acute myeloid leukemia74, 75.
Conclusions
Overall, our studies indicate that glycoprotein enrichment in combination with mass spectrometry can be used to provide a comprehensive characterization of the mast cell surface proteome and hence improve our understanding of how mast cell function is influenced by signals generated by the environment in health and disease. Finally, we identified CD98hc as a surface protein that plays an important role in mast cell degranulation, adhesion and proliferation.
Supplementary Material
Clinical implications.
Characterization of the mast cell surface proteome can provide a better understanding of mast cell biology in the complex signaling environment that influences mast cell function in health and disease.
ACKNOWLEDGMENTS
We thank Warren Anderson and Ilana Meitlis for technical assistance.
Funding:
This work was supported by funding from the National Institutes of Health; Grant R21 AI144231 to A.M.P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used
- ATP
Adenosine triphosphate
- BMCMCs
bone marrow derived-cultured mast cells
- BMMCs
bone marrow mast cells
- Cas9
CRISPR associated protein 9
- CD
cluster of differentiation
- CD98hc
CD98 heavy chain
- CFSE
carboxyfluorescein succinimidyl ester
- CRISPR
clustered regularly interspaced short palindromic repeats
- Csf2rb
common beta subunit of the receptor for granulocyte-macrophage colony stimulating factor GM-CSF/IL-3/IL-5
- FcεRIβ
beta subunit of the high affinity receptor for IgE
- FcεRIγ
gamma subunit of the high affinity receptor for IgE
- FN
fibronectin
- GM-CSF
granulocyte-macrophage colony stimulating factor
- GTP
guanosine triphosphate
- Ig
immunoglobulin
- IL
interleukin
- ImmGen
Immunological Genome Project
- LAMP1
lysosomal-associated membrane protein 1
- LATs
L-type amino acid transporters
- LC-MS/MS
liquid chromatography-mass spectrometry-mass spectrometry
- LTs
leukotrienes
- MCP-1:
monocyte chemoattractant protein-1
- mTOR
mechanistic target of rapamycin
- Plgrkt
plasminogen receptor
- PMCs
peritoneal mast cells
- PG
prostaglandin
- RNP
ribonucleoprotein
- SCF
stem cell factor
- Tgfbr1
type 1 receptor for transforming growth factor-beta
- TLR
toll-like receptor
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflict of interest: The authors declare that they have no conflict of interests.
REFERENCES
- 1.Dudeck A, Koberle M, Goldmann O, Meyer N, Dudeck J, Lemmens S, et al. Mast cells as protectors of health. J Allergy Clin Immunol 2019; 144:S4–S18. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med 2012; 18:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill KN, Katz HR, Cui J, Lai J, Kazani S, Crosby-Thompson A, et al. KIT Inhibition by Imatinib in Patients with Severe Refractory Asthma. N Engl J Med 2017; 376:1911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivellese F, Nerviani A, Rossi FW, Marone G, Matucci-Cerinic M, de Paulis A, et al. Mast cells in rheumatoid arthritis: friends or foes? Autoimmun Rev 2017; 16:557–63. [DOI] [PubMed] [Google Scholar]
- 5.Piliponsky AM, Romani L. The contribution of mast cells to bacterial and fungal infection immunity. Immunol Rev 2018; 282:188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varricchi G, de Paulis A, Marone G, Galli SJ. Future Needs in Mast Cell Biology. Int J Mol Sci 2019; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz HR, LeBlanc PA, Russell SW. Two classes of mouse mast cells delineated by monoclonal antibodies. Proc Natl Acad Sci U S A 1983; 80:5916–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu YR, O’Koren EG, Hotten DF, Kan MJ, Kopin D, Nelson ER, et al. A Protocol for the Comprehensive Flow Cytometric Analysis of Immune Cells in Normal and Inflamed Murine Non-Lymphoid Tissues. PLoS One 2016; 11:e0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark G, Stockinger H, Balderas R, van Zelm MC, Zola H, Hart D, et al. Nomenclature of CD molecules from the Tenth Human Leucocyte Differentiation Antigen Workshop. Clin Transl Immunology 2016; 5:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bausch-Fluck D, Goldmann U, Muller S, van Oostrum M, Muller M, Schubert OT, et al. The in silico human surfaceome. Proc Natl Acad Sci U S A 2018; 115:E10988–E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bausch-Fluck D, Hofmann A, Bock T, Frei AP, Cerciello F, Jacobs A, et al. A mass spectrometric-derived cell surface protein atlas. PLoS One 2015; 10:e0121314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann A, Thiesler T, Gerrits B, Behnke S, Sobotzki N, Omasits U, et al. Surfaceome of classical Hodgkin and non-Hodgkin lymphoma. Proteomics Clin Appl 2015; 9:661–70. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca AL, da Silva VL, da Fonseca MM, Meira IT, da Silva TE, Kroll JE, et al. Bioinformatics Analysis of the Human Surfaceome Reveals New Targets for a Variety of Tumor Types. Int J Genomics 2016; 2016:8346198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raposo RA, Thomas B, Ridlova G, James W. Proteomic-based identification of CD4-interacting proteins in human primary macrophages. PLoS One 2011; 6:e18690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalxdorf M, Gade S, Eberl HC, Bantscheff M. Monitoring Cell-surface N-Glycoproteome Dynamics by Quantitative Proteomics Reveals Mechanistic Insights into Macrophage Differentiation. Mol Cell Proteomics 2017; 16:770–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graessel A, Hauck SM, von Toerne C, Kloppmann E, Goldberg T, Koppensteiner H, et al. A Combined Omics Approach to Generate the Surface Atlas of Human Naive CD4+ T Cells during Early T-Cell Receptor Activation. Mol Cell Proteomics 2015; 14:2085–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stemmer M, Thumberger T, Del Sol Keyer M, Wittbrodt J, Mateo JL. CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PLoS One 2015; 10:e0124633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cradick TJ, Qiu P, Lee CM, Fine EJ, Bao G. COSMID: A Web-based Tool for Identifying and Validating CRISPR/Cas Off-target Sites. Mol Ther Nucleic Acids 2014; 3:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song E, Zhu R, Hammoud ZT, Mechref Y. LC-MS/MS quantitation of esophagus disease blood serum glycoproteins by enrichment with hydrazide chemistry and lectin affinity chromatography. J Proteome Res 2014; 13:4808–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.In: nd, Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, et al. , editors. Essentials of Glycobiology. Cold Spring Harbor; (NY: ); 2009. [PubMed] [Google Scholar]
- 21.Jamur MC, Grodzki AC, Berenstein EH, Hamawy MM, Siraganian RP, Oliver C. Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood 2005; 105:4282–9. [DOI] [PubMed] [Google Scholar]
- 22.Wong MX, Roberts D, Bartley PA, Jackson DE. Absence of platelet endothelial cell adhesion molecule-1 (CD31) leads to increased severity of local and systemic IgE-mediated anaphylaxis and modulation of mast cell activation. J Immunol 2002; 168:6455–62. [DOI] [PubMed] [Google Scholar]
- 23.Grochowy G, Hermiston ML, Kuhny M, Weiss A, Huber M. Requirement for CD45 in fine-tuning mast cell responses mediated by different ligand-receptor systems. Cell Signal 2009; 21:1277–86. [DOI] [PubMed] [Google Scholar]
- 24.Malaviya R, Gao Z, Thankavel K, van der Merwe PA, Abraham SN. The mast cell tumor necrosis factor alpha response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc Natl Acad Sci U S A 1999; 96:8110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz S, Hernandez-Pando R, Abraham SN, Enciso JA. Mast cell activation by Mycobacterium tuberculosis: mediator release and role of CD48. J Immunol 2003; 170:5590–6. [DOI] [PubMed] [Google Scholar]
- 26.Kraft S, Jouvin MH, Kulkarni N, Kissing S, Morgan ES, Dvorak AM, et al. The tetraspanin CD63 is required for efficient IgE-mediated mast cell degranulation and anaphylaxis. J Immunol 2013; 191:2871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyons DO, Plewes MR, Pullen NA. Soluble transforming growth factor beta-1 enhances murine mast cell release of Interleukin 6 in IgE-independent and Interleukin 13 in IgE-dependent settings in vitro. PLoS One 2018; 13:e0207704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 2019; 47:D419–D26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mi H, Muruganujan A, Huang X, Ebert D, Mills C, Guo X, et al. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat Protoc 2019; 14:703–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 2014; 6:a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, et al. The phagosome proteome: insight into phagosome functions. J Cell Biol 2001; 152:165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Jagannath C, Rao PK, Singh CR, Lostumbo G. Analysis of phagosomal proteomes: from latex-bead to bacterial phagosomes. Proteomics 2010; 10:4098–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azouz NP, Matsui T, Fukuda M, Sagi-Eisenberg R. Decoding the regulation of mast cell exocytosis by networks of Rab GTPases. J Immunol 2012; 189:2169–80. [DOI] [PubMed] [Google Scholar]
- 34.Azouz NP, Zur N, Efergan A, Ohbayashi N, Fukuda M, Amihai D, et al. Rab5 is a novel regulator of mast cell secretory granules: impact on size, cargo, and exocytosis. J Immunol 2014; 192:4043–53. [DOI] [PubMed] [Google Scholar]
- 35.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science 1988; 241:58–62. [DOI] [PubMed] [Google Scholar]
- 36.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A 2005; 102:11408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A 2005; 102:18105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lighvani S, Baik N, Diggs JE, Khaldoyanidi S, Parmer RJ, Miles LA. Regulation of macrophage migration by a novel plasminogen receptor Plg-R KT. Blood 2011; 118:5622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drew E, Merzaban JS, Seo W, Ziltener HJ, McNagny KM. CD34 and CD43 inhibit mast cell adhesion and are required for optimal mast cell reconstitution. Immunity 2005; 22:43–57. [DOI] [PubMed] [Google Scholar]
- 40.LaCroix-Fralish ML, Mo G, Smith SB, Sotocinal SG, Ritchie J, Austin JS, et al. The beta3 subunit of the Na+,K+-ATPase mediates variable nociceptive sensitivity in the formalin test. Pain 2009; 144:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiampanichayakul S, Szekeres A, Khunkaewla P, Moonsom S, Leksa V, Drbal K, et al. Engagement of Na,K-ATPase beta3 subunit by a specific mAb suppresses T and B lymphocyte activation. Int Immunol 2002; 14:1407–14. [DOI] [PubMed] [Google Scholar]
- 42.Chruewkamlow N, Pata S, Mahasongkram K, Laopajon W, Kasinrerk W, Chiampanichayakul S. beta3 subunit of Na,K ATPase regulates T cell activation with no involvement of Na,K ATPase activity. Immunobiology 2015; 220:634–40. [DOI] [PubMed] [Google Scholar]
- 43.Jiang L, Salao K, Li H, Rybicka JM, Yates RM, Luo XW, et al. Intracellular chloride channel protein CLIC1 regulates macrophage function through modulation of phagosomal acidification. J Cell Sci 2012; 125:5479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, et al. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 1998; 395:288–91. [DOI] [PubMed] [Google Scholar]
- 45.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 1997; 390:81–5. [DOI] [PubMed] [Google Scholar]
- 46.Cantor J, Browne CD, Ruppert R, Feral CC, Fassler R, Rickert RC, et al. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat Immunol 2009; 10:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cantor J, Slepak M, Ege N, Chang JT, Ginsberg MH. Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity. J Immunol 2011; 187:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J Cell Sci 2012; 125:1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shirakabe K, Hattori S, Seiki M, Koyasu S, Okada Y. VIP36 protein is a target of ectodomain shedding and regulates phagocytosis in macrophage Raw 264.7 cells. J Biol Chem 2011; 286:43154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura T, Nada S, Takegahara N, Okuno T, Nojima S, Kang S, et al. Polarization of M2 macrophages requires Lamtor1 that integrates cytokine and amino-acid signals. Nat Commun 2016; 7:13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner CA, Lang F, Broer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol 2001; 281:C1077–93. [DOI] [PubMed] [Google Scholar]
- 52.Heng TS, Painter MW, Immunological Genome Project C. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 2008; 9:1091–4. [DOI] [PubMed] [Google Scholar]
- 53.Tsumura H, Suzuki N, Saito H, Kawano M, Otake S, Kozuka Y, et al. The targeted disruption of the CD98 gene results in embryonic lethality. Biochem Biophys Res Commun 2003; 308:847–51. [DOI] [PubMed] [Google Scholar]
- 54.Fenczik CA, Zent R, Dellos M, Calderwood DA, Satriano J, Kelly C, et al. Distinct domains of CD98hc regulate integrins and amino acid transport. J Biol Chem 2001; 276:8746–52. [DOI] [PubMed] [Google Scholar]
- 55.Feral CC, Nishiya N, Fenczik CA, Stuhlmann H, Slepak M, Ginsberg MH. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci U S A 2005; 102:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai S, Bulus N, Fonseca-Siesser PM, Chen D, Hanks SK, Pozzi A, et al. CD98 modulates integrin beta1 function in polarized epithelial cells. J Cell Sci 2005; 118:889–99. [DOI] [PubMed] [Google Scholar]
- 57.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell 1985; 40:191–8. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano RL, Ruoslahti E. The alpha v beta 1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol 1993; 122:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dastych J, Metcalfe DD. Stem cell factor induces mast cell adhesion to fibronectin. J Immunol 1994; 152:213–9. [PubMed] [Google Scholar]
- 60.Teodosio C, Garcia-Montero AC, Jara-Acevedo M, Sanchez-Munoz L, Pedreira CE, Alvarez-Twose I, et al. Gene expression profile of highly purified bone marrow mast cells in systemic mastocytosis. J Allergy Clin Immunol 2013; 131:1213–24, 24, e1–4. [DOI] [PubMed] [Google Scholar]
- 61.Myers SA, Rhoads A, Cocco AR, Peckner R, Haber AL, Schweitzer LD, et al. Streamlined Protocol for Deep Proteomic Profiling of FAC-sorted Cells and Its Application to Freshly Isolated Murine Immune Cells. Mol Cell Proteomics 2019; 18:995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plum T, Wang X, Rettel M, Krijgsveld J, Feyerabend TB, Rodewald HR. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 2020; 52:404–16 e5. [DOI] [PubMed] [Google Scholar]
- 63.Zarif JC, Yang W, Hernandez JR, Zhang H, Pienta KJ. The Identification of Macrophage-enriched Glycoproteins Using Glycoproteomics. Mol Cell Proteomics 2017; 16:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haverland NA, Waas M, Ntai I, Keppel T, Gundry RL, Kelleher NL. Cell Surface Proteomics of N-Linked Glycoproteins for Typing of Human Lymphocytes. Proteomics 2017; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akula S, Paivandy A, Fu Z, Thorpe M, Pejler G, Hellman L. How Relevant Are Bone Marrow-Derived Mast Cells (BMMCs) as Models for Tissue Mast Cells? A Comparative Transcriptome Analysis of BMMCs and Peritoneal Mast Cells. Cells 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gschwandtner M, Paulitschke V, Mildner M, Brunner PM, Hacker S, Eisenwort G, et al. Proteome analysis identifies L1CAM/CD171 and DPP4/CD26 as novel markers of human skin mast cells. Allergy 2017; 72:85–97. [DOI] [PubMed] [Google Scholar]
- 67.Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol 2012; 3:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elschenbroich S, Kim Y, Medin JA, Kislinger T. Isolation of cell surface proteins for mass spectrometry-based proteomics. Expert Rev Proteomics 2010; 7:141–54. [DOI] [PubMed] [Google Scholar]
- 69.Kurihara T, Arimochi H, Bhuyan ZA, Ishifune C, Tsumura H, Ito M, et al. CD98 Heavy Chain Is a Potent Positive Regulator of CD4+ T Cell Proliferation and Interferon-gamma Production In Vivo. PLoS One 2015; 10:e0139692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobayashi T, Tsutsui H, Shimabukuro-Demoto S, Yoshida-Sugitani R, Karyu H, Furuyama-Tanaka K, et al. Lysosome biogenesis regulated by the amino-acid transporter SLC15A4 is critical for functional integrity of mast cells. Int Immunol 2017; 29:551–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prager GW, Feral CC, Kim C, Flan J, Ginsberg MFI. CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J Biol Chem 2007; 282:24477–84. [DOI] [PubMed] [Google Scholar]
- 72.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev 2009; 228:149–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cano-Crespo S, Chillaron J, Junza A, Fernandez-Miranda G, Garcia J, Polte C, et al. CD98hc (SLC3A2) sustains amino acid and nucleotide availability for cell cycle progression. Sci Rep 2019; 9:14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glaser R, Zhang HY, Yao KT, Zhu HC, Wang FX, Li GY, et al. Two epithelial tumor cell lines (HNE-1 and HONE-1) latently infected with Epstein-Barr virus that were derived from nasopharyngeal carcinomas. Proc Natl Acad Sci U S A 1989; 86:9524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ablack JN, Ortiz J, Bajaj J, Trinh K, Lagarrigue F, Cantor JM, et al. MARCH Proteins Mediate Responses to Antitumor Antibodies. J Immunol 2020; 205:2883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


