Abstract
PURPOSE:
To assess the repeatability and accuracy of corneal astigmatism measurement with a spectral-domain optical coherence tomography (OCT) system (Avanti, Optovue) and compare them with Scheimpflug imaging (Pentacam HR, Oculus) and swept-source optical biometry (IOLMaster 700, Carl Zeiss Mediatec AG).
SETTING:
Casey Eye Institute, Oregon Health & Science University, Portland, Oregon, USA
DESIGN:
Prospective cross-sectional observational study.
METHODS:
Sixty pseudophakic eyes with monofocal non-toric intraocular lens that previously had refractive surgery were analyzed. To assess accuracy, simulated keratometric (SimK) and net corneal astigmatism, obtained from each device were compared with subjective manifest refraction astigmatism. Repeatability for corneal astigmatism was assessed for OCT and Pentacam HR by the coefficient of repeatability from three repeated measures.
RESULTS:
Compared to manifest refraction, SimK readings produced with-the-rule (WTR) astigmatic bias which was reduced for net astigmatism for all the three devices. Except for OCT net astigmatism, all instruments significantly overestimated the magnitude of the astigmatism (linear mixed-effects model (LMM), P < .05). OCT net astigmatism showed the highest accuracy for manifest astigmatism prediction with the smaller 95% confidence ellipse for the mean difference vector. OCT net mean absolute difference was 0.57 D, significantly smaller than that of the other modalities (LMM, P <.05). Net corneal astigmatism measured with OCT showed the best repeatability (coefficient of repeatability = 0.29 D).
CONCLUSIONS:
OCT has the capability to measure net corneal astigmatism with higher precision and accuracy than Pentacam HR Scheimpflug imaging and IOLMaster 700 swept-source optical biometry in post refractive patients.
INTRODUCTION
For refractive surgery and cataract surgery, an accurate measurement of corneal shape is important to achieve a successful outcome. The measurement of corneal astigmatism is especially important for cataract surgery, as inaccurate pre-operative assessment could lead to an unsatisfying outcome. In patients going through cataract surgery, the pre-operative corneal astigmatism ranges between 0.25 D and 1.25 D in approximately 60% of eyes, and it is higher than 1.50 D in more than 20% of eyes.1 Toric intraocular lenses (IOL) have become a popular means to correct astigmatism at the time of cataract surgery. Pre-operative corneal astigmatism measurement is the largest contributor to residual astigmatism after toric IOL implantation.2–4 Thus a more accurate method to measure corneal astigmatism is critical in achieving better refractive and visual outcome.
Corneal astigmatism is usually estimated by simulated keratometry (SimK), which is based on the measurement of the anterior corneal surface. SimK uses the empiric keratometric index to extrapolate the power of the posterior corneal surface, assuming a uniform curvature of the cornea and a constant relation between anterior and posterior corneal surfaces. However, there can be significant deviations from these ideal assumptions even within the normal population. It is known that posterior corneal astigmatism has a significant influence in net astigmatism and, when neglected, is a major source of residual astigmatism after cataract surgery.5,6 This inaccuracy becomes more significant in eyes where the assumption of a constant relation between anterior and posterior cornea is no longer met, as it happens in patients with corneal ectatic diseases or after refractive surgery. Thus, SimK should not be the preferred technique for planning cataract surgery since it is not generally reliable for measuring the preoperative corneal astigmatism.7 The direct measurement of both corneal surfaces should provide a more reliable measurement of net corneal astigmatism.8 Scheimpflug imaging or swept-source optical biometry can directly measure posterior cornea and corneal thickness. Anterior segment optical coherence tomography (OCT) systems also permit the visualization and measurement of anterior and posterior corneal surfaces with higher image quality. High-speed OCT has shown its value in assessing net corneal power with precision and accuracy,7,9,10 and it has also been used to assess net corneal astigmatism.11 Compared to Scheimpflug and other slit imaging technologies, OCT has higher axial resolution and may provide better measurement of corneal surfaces, especially in eyes with corneal opacities and scars.12 Both anterior segment OCT systems and swept-source optical biometry utilize the OCT principle, but the former generally provide more detailed scanning of the cornea, at least in the current generation instruments.
The purpose of this study was to determine the repeatability and accuracy of corneal astigmatism measurements made with a spectral-domain OCT (SD-OCT) system and compare them with Scheimpflug imaging and swept-source optical biometry. For the assessment of accuracy, ground truth was approximated by manifest refraction in pseudophakic patients implanted with non-toric monofocal IOL. As refractive astigmatism is mainly determined by the cornea and the crystalline lens, for pseudophakic patients with non-toric IOL, corneal astigmatism becomes the only major source of refractive astigmatism. Therefore, the accuracy of corneal astigmatism measurements can be calculated as its deviation from manifest refraction astigmatism in these patients. We chose patients who had previous corneal refractive surgery, who were more likely to have significant deviations between simK and net corneal astigmatism.
METHODS
This prospective study was performed at Casey Eye Institute, Oregon Health and Science University (OHSU Portland, Oregon, USA) and approved by OHSU Institutional Review Board. The study followed the tenets of the Declaration of Helsinki and all participants signed an informed consent after the rationality and possible adverse effects of the study were explained. Cataract patients who had previous laser visual correction (laser in situ keratomileusis (LASIK), photorefractive keratectomy (PRK) or radial keratectomy (RK)) were enrolled in the study. Only patients with corrected distance visual acuity (CDVA) of 20/20 or better were included. Patients were also excluded from the study if they had any corneal surgery other than laser visual correction or if they had any corneal pathology. Manifest refraction, OCT scanning, Scheimpflug imaging and swept-source optical biometry were performed at least 1 month after the cataract surgery. Manifest refraction was obtained using a phoroptor and converted to the corneal plane.
A commercially available high-speed SD-OCT system (Avanti, Optovue Inc, CA) was used to acquire cross-sectional images of the cornea. Avanti OCT is FDA approved to perform retinal imaging, corneal imaging, and corneal mapping (pachymetry and epithelial thickness). In order to image the cornea an adaptor module was attached to the instrument. The speed of the system is 70,000 scans/second with an axial resolution of 5µm using a light source of 840 nm and a focal spot size of 15.5 µm ( diameter)13 when using the anterior segment adaptor. The scan pattern consisted of 5 mm line scans on 16 meridians centered on the pupil (Figure 1) which is repeated 10 times and completed in less than 1.5 seconds. Each meridional line has 640 axial scans. Subjects were instructed to place their head on the chinrest and fixate on the internal target of the machine.
Figure 1.

OCT scan pattern used for corneal mapping.
Scheimpflug imaging was performed with Pentacam HR (Oculus Optikgerate GmbH, software version 1.22r09), which combines a slit illumination system with a Schiempflug camera to obtain tomographic images and provide a 3D reconstruction of the anterior chamber of the eye. Pentacam HR uses a 14 mm slit that is rotated 360 degrees along 25 meridians in one second and measures a total of up to 138,000 points. To obtain the net power of the cornea (i.e., takes into account anterior and posterior corneal surfaces), elevation data from anterior and posterior corneal surfaces is derived from the tomographic images and sagittal and tangential radii calculated from the elevation data. For this study, the Pentacam HR True Net Power (TNP) map was used. This map provides the net corneal power calculated using Gaussian optics formulae from anterior and posterior cornea sagittal radius and the refractive index of the different media (n = 1 for air, n = 1.376 for corneal tissue and n = 1.336 for the aqueous). Pentacam HR software allows calculating the True Net (TN) astigmatism for different circular area diameters and either centered at the pupil or at the corneal vertex. For this study, astigmatism was calculated in a 4-mm diameter circular area centered on the corneal vertex.
Swept-source optical biometry was performed with the IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany, software version 1.80.10.61129). In order to obtain the net power of the cornea, the anterior corneal power is obtained by central keratometry from 18 projected spots in the central 2.5 mm of the cornea while the corneal thickness and the posterior corneal power are obtained from the swept-source OCT scans using a light source of 1055-nm wavelength to obtain 2,000 A-scans per second. It measures six meridians, the scan width is 6 mm and it has an axial resolution of 22 µm in tissue.14 The scan pattern is repeated five times and the average calculated. Using thick lens formula, the IOLMaster total keratometry (TK) astigmatism is calculated using anterior and posterior corneal curvatures together with the corneal thickness.
Both Pentacam HR and IOL Master 700 software also provide SimK astigmatism using anterior corneal surface readings and the keratometric index (n = 1.3375, i.e., without measured information from the posterior cornea). SimK astigmatism is calculated as the difference between the steeper and flattest perpendicular meridians, whose angles are obtained from simulated keratometry.
OCT Derived Astigmatism Calculation
Raw OCT data were exported from Avanti and analyzed offline using a custom automated algorithm developed with Matlab (MathWorks Inc., Natick, MA) by Pavlatos et al.15 The OCT images were dewarped to correct for distortions due to refraction at tissue boundaries and differences in group indices of air, cornea, and aqueous media.16 The anterior and posterior corneal profiles were segmented and registered laterally and axially. Scans and sets with significant eye motion were identified and excluded from corneal surface reconstruction. For the remaining sets, an elevation map for each corneal surface was calculated by interpolating the elevation profiles along 16 equally spaced meridians. Finally, corneal surfaces from the repeated sets were registered at the vertex and averaged to obtain anterior and posterior corneal elevation maps.
Corneal elevation data were further fitted with eight orders of Zernike polynomials using an analytical zone of 4 mm of diameter centered either on the corneal vertex or the pupil as indicated in the results section. The second radial order Zernike polynomial coefficients measure the cardinal and oblique astigmatism components (X and Y, respectively).17,18 Net astigmatism for each vector component is given by the combination of the anterior and posterior components and the corneal thickness using formulas 1 and 2 below.
| (1) |
| (2) |
Where , , and are the Zernike coefficients corresponding to the oblique (subscript 3) and cardinal (subscript 5) astigmatism terms for anterior and posterior surfaces respectively, is the refractive index of the corneal tissue, is the refractive index of the aqueous medium, r is the radius of the analytic area, and CCT is the averaged 1 mm central corneal thickness.
Using a custom algorithm, the anterior corneal elevation maps were converted to axial power maps using the keratometric index of 1.3375. SimK was calculated as the difference between the orthogonal steepest and flattest meridians from the axial power map.
Data Analysis and Statistical Analysis
Both SimK astigmatism and net corneal astigmatism were analyzed for the three devices. Polar-coordinate astigmatism measurements (e.g. manifest refraction in spherocylindrical notation) were decomposed into rectangular coordinates (i.e., cardinal and oblique components) using the double angle formulas:
| (3) |
| (4) |
Where X is the cardinal component, Y is the oblique component, A is the magnitude in diopters and α is the axis of the astigmatism in degrees.
For OCT and Pentacam HR, three repeated measures were acquired to assess repeatability by means of the coefficient of repeatability calculated as where is the within subject standard deviation for the repeated measures.19 The coefficient of repeatability is reported separately for the cardinal and oblique components. Then the total vector repeatability is calculated as the square root of the sum of the squared orthogonal components.
To assess the accuracy of corneal net astigmatism by OCT, Pentacam HR and IOLMaster 700, post-cataract corneal astigmatism was compared to the manifest refraction astigmatism. Individual difference vectors between manifest and corneal astigmatism were calculated for each instrument and reported with the double angle astigmatism plots.20 To account for the correlation between left and right eyes, a linear mixed-effect model (LMM) was used to assess statistically significant differences between instruments (or methods) and manifest refraction astigmatism. For LMM a minimum of 41 eyes is required to have a power of 80% at the 5% significance level and an effect size of . The F test was used to compare the variance of corneal astigmatism measurements considering P < .05 as statistically significant. For the F test of equality of variance a minimum of 48 eyes is required to have a power of 80% at the 5% significance level assuming equal variances.
RESULTS
The study analyzed sixty pseudophakic eyes of 39 patients aged between 49 and 78 years (mean ± standard deviation 67 ± 7 years). Among them, 17 patients were women (43.6 %) and 22 were men (56.4 %). Average anterior and posterior corneal power were 39.95 ± 3.74 (ranged from 32.06 to 48.16) diopter (D) and −5.88 ± 0.70 (ranged from −4.00 to −7.06) D, respectively. Thirty-five (58.3%) eyes had previous myopic LASIK, four (6.7%) had previous hyperopic LASIK, five (8.3%) had previous myopic PRK and sixteen (26.7%) previous RK. Average manifest refraction spherical equivalent before cataract surgery was −0.44 ± 2.45 D and average IOL power implanted was 21.52 ± 3.26 D. After cataract surgery, average manifest refraction was −0.49 ± 0.61 D and CDVA was 20/20 for 29 (48.3%) eyes and 20/15 for 31 (51.7%) eyes. For accuracy comparisons among the instruments, a sample size of 60 patients has a power of 95% at a 5% significance level assuming equal variances.
Table 1 provides population average corneal astigmatism by manifest refraction and the three different devices. Relative to manifest refraction, SimK astigmatism showed with-the-rule (WTR) astigmatic bias that was reduced for net corneal astigmatism with all three devices. Comparing with the manifest refraction, all measurements except the OCT net corneal astigmatism significantly overestimated the astigmatism vector magnitude.
Table 1.
Statistics of astigmatism measured by manifest refraction and corneal topography instruments
| Unit: diopters | Manifest refraction | OCT |
Pentacam HR |
IOLMaster 700 |
|||
|---|---|---|---|---|---|---|---|
| Net | SimK | TN | SimK | TK | SimK | ||
| Cardinal component | 0.24 ± 0.70 [−1.28 2.39] |
0.03 ± 0.51 [−0.76 1.68] |
−0.16 ± 0.91 [−1.82 3.03] |
0.10 ± 1.03 [−1.73 4.00] |
−0.10 ± 1.00 [−2.09 3.33] |
0.22 ± 1.03 [−1.81 3.23] |
0.06 ± 1.02 [−1.87 2.78] |
| Oblique component | 0.03 ± 0.43 [−1.28 1.18] |
0.00 ± 0.31 [−1.68 0.77] |
−0.02 ± 0.72 [−3.04 1.59] |
0.07 ± 0.59 [−1.61 1.34] |
0.17 ± 0.19 [0.01 1.37] |
0.20 ± 0.63 [−1.76 1.60] |
0.07 ± 0.63 [−2.27 1.60] |
| Magnitude | 0.63 ± 0.57 [0 2.42] |
0.49 ± 0.32 [0.03 2.68] |
0.96 ± 0.66 [0.04 3.19] |
0.96 ± 0.69 [0.06 4.00] |
0.81 ± 0.64 [0.07 3.35] |
0.96 ± 0.77 [0 3.23] |
0.97 ± 0.69 [0.08 2.8] |
| P value a | - | .10 | < .01* | < .01* | .02* | < .01* | < .01* |
Values reported as mean ± standard deviation [range]
Linear mixed effects model is used to compare the magnitude of manifest refraction astigmatism with corneal astigmatism measured with 3 different devices.
Statistically significant.
SimK = simulated keratometry; TK = Total Keratometry; TN = True Net.
Figure 2 illustrates the astigmatism difference vectors between manifest refraction and corneal astigmatism measurements using double angle plots. The 95% confidence ellipse for OCT net astigmatism is closer to the origin indicating better agreement compared to the other methods. The mean absolute difference (average of the absolute magnitude of difference vectors) between OCT net astigmatism and manifest refraction was 0.57 D, significantly (P < .01 by gamma mixed-effect model) lower than all of the other methods of measuring corneal astigmatism. For OCT, the mean absolute difference was significantly smaller for net astigmatism compared to SimK. For Pentacam HR and IOLMaster 700, there was no such improvement in mean absolute error for net corneal astigmatism over SimK. For Pentacam HR and IOLMaster 700, the mean absolute error was actually higher for net corneal astigmatism compared to SimK, but the difference was not statistically significant (gamma mixed-effect model, P > .05 for both cases). The centroids of the difference vectors (mean difference vector) showed that net astigmatism measured by all 3 methods have minimal bias (< 0.25 D) compared to manifest refraction. The SimK measurements all have a small WTR bias relative to manifest refraction. The pooled standard deviation from the centroid was 0.65 D for OCT net astigmatism, significantly (P<0.01 by F test) smaller than the other methods of measuring corneal astigmatism. This indicates that OCT net astigmatism had lower random measurement error. Table 2 shows astigmatism mean absolute difference and the mean difference vector (centroids and SD) between manifest refraction and corneal astigmatism calculated with OCT, Pentacam HR, and IOLMaster 700, for post-LASIK/PRK and post-RK eyes. Table 2 also provides the same statistics stratified by the type of astigmatism (with-the-rule [WTR], against-the-rule [ATR], or none) according to the manifest refraction. Only three eyes had oblique astigmatism therefore they were not included in the table. For SimK astigmatism, OCT, Pentacam HR, and IOLMaster 700 achieved very similar results in post-LASIK/PRK and post-RK eyes (P > .05 by gamma mixed-effect model). For net corneal astigmatism, the mean absolute difference with manifest refraction was significantly smaller for OCT measurements compared to that of Pentacam HR and IOLMaster 700 in post-RK patients and compared to that of Pentacam HR in post-LASIK/PRK patients (P < .05 by gamma mixed-effect model). There were no significant differences among eyes with different types of astigmatism (WTR, ATR, or none) except for OCT SimK where the agreement for ATR eyes was significantly worse than for WTR eyes (P < .05 by gamma mixed-effect model).
Figure 2.
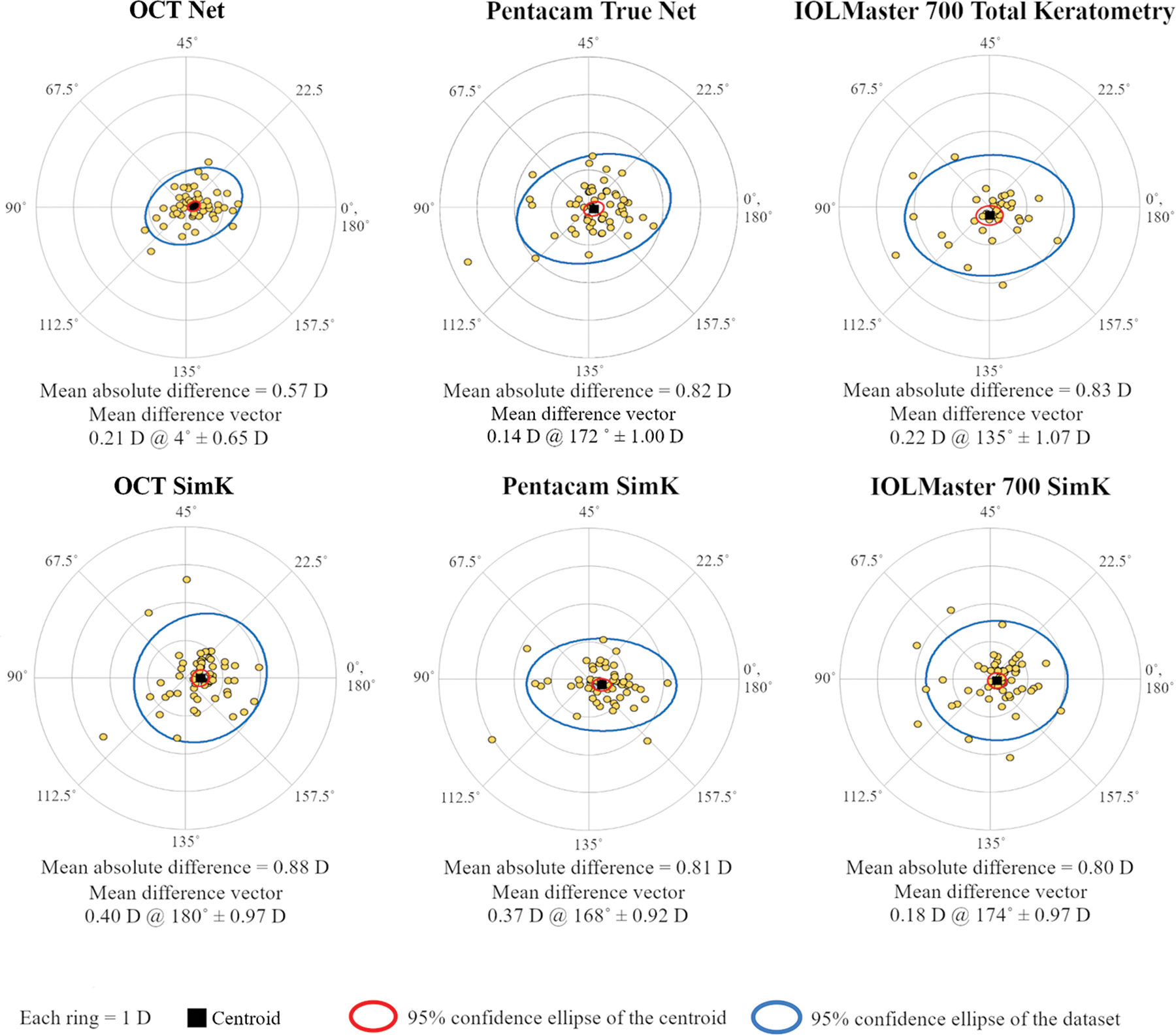
Double-angle plots showing the astigmatism difference vectors between manifest refraction and corneal astigmatism measurements by OCT, Pentacam HR, and IOLMaster 700. The OCT measurements shown here are centered on the corneal vertex. The mean absolute difference between corneal astigmatism and manifest refraction and the mean difference vector (centroid) are displayed under each double-angle plot.
Table 2.
Accuracy of Corneal Astigmatism Measurements Stratified by Previous Refractive Surgery and Type of Astigmatism
| Mean absolute difference |
Mean difference vector |
||||||
|---|---|---|---|---|---|---|---|
| OCT | Pentacam HR | IOLMaster 700 | OCT | Pentacam HR | IOL Master700 | ||
| Net astigmatism | LASIK/PRK (n = 44) | 0.49 D | 0.69 D* | 0.61 D | 0.20 D @ 6° ± 0.55 D | 0.31 D @ 174° ± 0.75 D | 0.30 D @ 164° ± 0.72 D |
| RK (n = 16) | 0.80 D | 1.18 D* | 1.28 D* | 0.23 D @ 3° ± 0.91 D | 0.32 D @ 87° ± 1.40 D | 0.68 D @ 105° ± 1.44 D | |
|
| |||||||
| WTR (n = 17) | 0.40 D | 0.59 D | 0.66 D | 0.21 D @ 95° ± 0.46 D | 0.06 D @ 142° ± 0.72 D | 0.29 D @ 120° ± 0.90 D | |
| ATR (n = 27) | 0.71 D | 0.90 D | 0.84 D | 0.47 D @ 4° ± 0.69 D | 0.14 D @ 175° ± 1.12 D | 0.22 D @ 119° ± 1.13 D | |
| Plano (n = 13) | 0.47 D | 0.89 D | 0.91 D | 0.23 @ 176° ± 0.49 D | 0.39 D @ 173° ± 1.00 D | 0.42 D @ 162° ± 1.05 D | |
|
| |||||||
| SimK astigmatism | LASIK/PRK (n = 44) | 0.79 D | 0.73 D | 0.67 D | 0.50 D @ 2° ± 0.73 D | 0.52 D @ 175° ± 0.67 D | 0.40 D @ 179° ± 0.68 D |
| RK (n = 16) | 1.14 D | 1.03 D | 1.15 D | 0.15 D @ 164° ± 1.42 D | 0.33 D @ 123° ± 1.29 D | 0.44 D @ 96° ± 1.35 D | |
|
| |||||||
| WTR (n = 17) | 0.60 D | 0.62 D | 0.60 D | 0.17 D @ 165° ± 0.64 D | 0.24 D @ 161° ± 0.70 D | 0.12 D @ 153° ± 0.75 D | |
| ATR (n = 27) | 1.00 Da | 0.87 D | 0.80 D | 0.45 D @ 4° ± 1.14 D | 0.43 D @ 165° ± 1.01 D | 0.10 D @ 6° ± 1.04 D | |
| None (n = 13) | 1.03 D | 0.85 D | 1.00 D | 0.64 D @ 179° ± 0.95 D | 0.59 D @ 173° ± 0.83 D | 0.55 D @ 173° ± 1.03 D | |
The accuracy of corneal astigmatism measurements were measured by the vector difference with post-cataract surgery manifest refraction. LASIK = laser in situ keratomileusis; PRK = photorefractive keratectomy; RK = radial keratectomy; WTR = with-the-rule astigmatism; ATR = against-the-rule astigmatism; D = diopter.
P < .05 Comparison with OCT using generalized linear mixed-effect model.
P < .05 Comparison between eyes with WTR, ATR, or no refractive astigmatism using generalized linear mixed-effect model.
The estimation of corneal astigmatism can be done in a circular area centered either in the corneal vertex or in the pupil. In order to ascertain whether there is any difference, we have further calculated OCT net astigmatism in a circular area of 4-mm diameter centered in the pupil and compared the results to those obtained with vertex centration. The mean ± standard deviation distance between corneal apex and pupil center was 0.33 mm ± 0.21 mm. Average shift of the pupil center respect to the corneal vertex was 0.10 mm nasally and 0.01 mm inferiorly. Figure 3 shows the double angle plot analyses of the difference vector between manifest refraction and OCT net astigmatism with pupil centration. The 95% confidence ellipse appear similar to that for vertex centration (see Figure 2). The mean absolute difference was 0.63 D, not significantly different from that for vertex centration.
Figure 3.
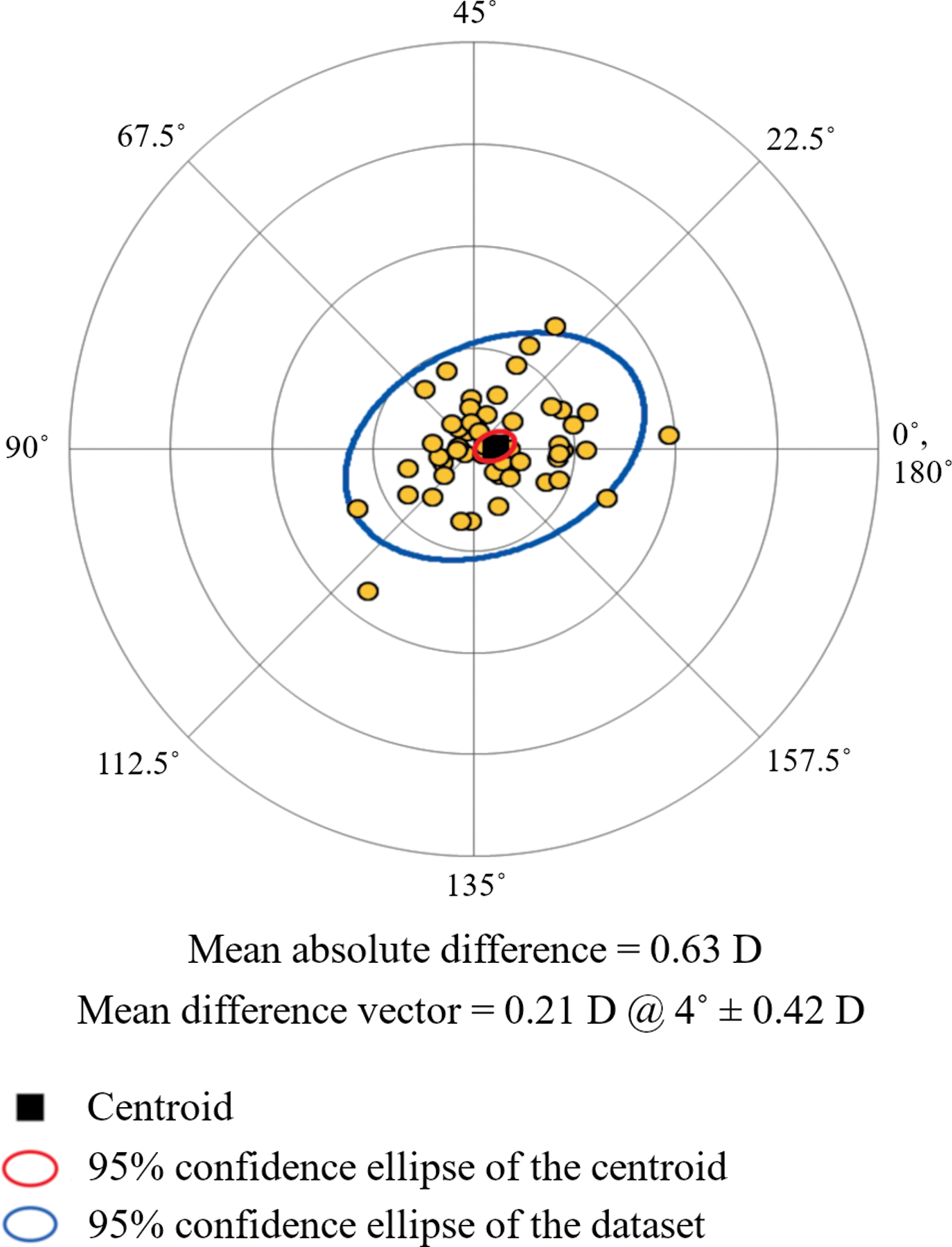
Double-angle plot of the astigmatism difference vectors between manifest refraction and net corneal astigmatism measured by OCT in a 4-mm diameter circular area centered on the pupil. The mean absolute difference between corneal astigmatism and manifest refraction and the mean difference vector (centroid) are displayed under the double-angle plot.
Table 3 shows the coefficient of repeatability for OCT and Pentacam HR for the astigmatism vector components and total vector. OCT achieved better repeatability compared to Pentacam HR for both net corneal astigmatism and SimK astigmatism. Compared to the repeatability of OCT net astigmatism, the coefficient of repeatability of OCT SimK, Pentacam HR TN and Pentacam HR SimK was significantly worse (F test, P < .01 in all cases).
Table 3.
Repeatability of corneal astigmatism measurements
| Coefficient of repeatability Unit: Diopters |
Cardinal component | Oblique component | Total vector |
|---|---|---|---|
| OCT Net | 0.22 | 0.19 | 0.29 |
| OCT SimK | 0.44* | 0.44* | 0.62* |
| Pentacam HR TN | 0.50* | 0.44* | 0.67* |
| Pentacam HR SimK | 0.47* | 0.42* | 0.63* |
Coefficient of repeatability =
P< .05 F test compared to OCT Net repeatability
SimK = simulated keratometry; TN = True Net
DISCUSSION
This study assessed the repeatability and accuracy of corneal astigmatism measured by OCT, compared to that obtained with Scheimpflug imaging and swept-source optical biometry. The OCT corneal astigmatism measurement we described in this study is investigational and not yet approved by the FDA.
For the purpose of evaluating accuracy, the vector difference between corneal astigmatism and manifest refraction astigmatism was calculated. While other studies have compared the agreement between different corneal topography and tomography systems for corneal astigmatism measurement, they lacked a gold standard against which accuracy could be assessed.21–24 The benefit of assessing pseudophakic patients with nontoric monofocal IOL is that the cornea becomes the only significant contributor to refractive astigmatism. In this way, refractive astigmatism can be used as the ground truth to assess accuracy. Although the tilt of the IOL could introduce some astigmatism, it is relatively small – on the order of 0.11 D.25
Vector analysis showed that SimK tended to have a small WTR bias relative to manifest refraction. This bias was reduced with net corneal astigmatism, which also considers the posterior corneal surface. The WTR bias is most likely due to the fact that SimK neglects the posterior corneal astigmatism, which tend to be against-the-rule (ATR). Preussner et al.,26 also reported a reduction in WTR net astigmatism compared to SimK readings. The small systematic WTR bias only account for a small fraction of the difference between instruments since random deviations are greater. Overall, OCT net astigmatism was the most accurate since it had the smallest mean absolute difference relative to manifest refraction compared to the other instruments and to SimK. The accuracy of OCT, Pentacam HR, and IOLMaster 700 net corneal astigmatism measurements was comparable for both WTR and ATR eyes. OCT net corneal astigmatism measurement was more accurate than that of Pentacam HR in both post-LASIK/PRK eyes and post-RK eyes, showing the advantage of using OCT in both mildly and highly irregular corneas. OCT was also more accurate compared to IOLMaster 700 overall, though the difference was not significant in the post-LASIK/PRK subgroup.
OCT net astigmatism was also the only method that did not significantly overestimate the magnitude of astigmatism. Hoffman et al.27 used a SS-OCT (CASIA SS-1000, 1310 nm wavelength) with an axial resolution of 10 µm and a scanning speed of 30,000 A-scans per second using a radial scan pattern of 16 meridians and also found better prediction accuracy (mean absolute difference = 0.43 D) of post-cataract surgery refractive astigmatism than that of the Scheimpflug imaging (Pentacam HR, mean absolute difference = 0.70 D) and optical biometry (Lenstar LS 900, mean absolute difference = 0.56 D).
The repeatability for astigmatism measurement was assessed in this study for OCT and Pentacam HR Scheimpflug imaging. The repeatability of IOLMaster 700 was not assessed, as repeated measurements were not collected in our study. Astigmatism derived from OCT net readings was shown to have good test-retest repeatability with a coefficient of repeatability similar to that reported for manifest refraction,28 and significantly lower than that found for Pentacam HR. These results are in agreement with previous studies, where OCT astigmatism showed better repeatability than Scheimpflug imaging.29 Recently, Shajari et al.30 found that the coefficient of repeatability of astigmatism magnitude was 0.44 D for Pentacam HR TN and 0.39 D for Pentacam HR SimK, similar to previous results reported by Visser et al.31 who found a coefficient of repeatability of astigmatism magnitude of 0.42 D for Pentacam HR SimK. These literature values for Pentacam HR coefficient of repeatability were obtained from normal subjects, so it is not surprising that they were lower (better) than the Pentacam HR coefficient of repeatability for post-refractive surgery patients reported here. Post-LASIK eyes have non-uniform curvature in the anterior corneal surface, while post-RK eyes have non-uniform curvature in both anterior and posterior surfaces. Thus higher variability of the measurements could be expected from even small centration differences and this can be seen in the increased standard deviation of the mean difference vector between manifest refraction and corneal astigmatism for post-RK eyes (see Table 2). Overall, the OCT derived net corneal astigmatism measurement was very good, achieving a lower coefficient of repeatability in post-refractive surgery eyes than that obtained by Pentacam HR in both normal and post-refractive surgery eyes.
The better accuracy and repeatability of OCT obtained in our study could be due to several factors. The axial resolution of the Avanti SD-OCT system, at 5 μm, is 4 times finer than the 22 μm resolution of the IOLMaster 700’s swept-source OCT. From literature based on optical experiments, the axial resolution of Scheimpflug imaging is of the order of 50 μm, ten times lower than Avanti OCT.32 The finer resolution may enable more precise segmentation of anterior and posterior corneal surfaces. The IOLMaster 700 measures the anterior surface using 18 spot illumination (equivalent to 9 meridians) and maps the posterior surface using 6 meridional swept-source OCT scans. The smaller number of meridians could limit the precision of astigmatism measurement in comparison with the 16 meridians used by Avanti OCT. Although the Pentacam HR scans 25 meridians, this is accomplished in 1 second, which may introduce more motion error compared to the 0.15 seconds it takes Avanti OCT to scan 16 meridians. Pentacam HR also has the option to increase the number of measured meridians up to 50 with an acquisition time of two seconds. Both number of meridians and speed are important for the precision of the measurement; we have chosen to use the option of 25 meridians in one second as going from 25 to 50 meridians does not increase the precision, but the change from one to two seconds might increase the motion error.33 The custom topography software we used to process the Avanti OCT scan further reduces motion error by comparison between 10 sets of repeated scans.15 An additional small error might be added to Pentacam HR TN astigmatism calculation because it does not account for corneal thickness.7
Although both the Avanti and the IOLMaster 700 use OCT technology, the Avanti (70 kHz axial scans repetition rate) is 35 times faster than IOLMaster 700 (2 kHz). The higher speed reduces motion error and increases the accuracy of corneal surface measurements. This is consistent with previous results by Tang et al.7 that showed improved precision in corneal power measurement by increasing OCT speed from 2 kHz to 26 kHz.
As for Pentacam HR, with OCT the analytical zone can be centered either at the corneal vertex or at the pupil center. We found these measurements to be essentially equivalent. This is in agreement with the Scheimpflug results of Dong et al.20 that also showed little differences between astigmatism magnitude measured using vertex versus pupil centration. In both normal and post-refractive surgery eyes, the vertex and pupil centers are close. So the equivalence of vertex and pupil centered astigmatism measurement may not be generalized to situations where the vertex could be further from the pupil, such as keratoconic eyes.
Another consideration for corneal astigmatism measurements should be the size of the analytical zone. In this study, we chose to use a 4-mm diameter analytic zone for both OCT and Pentacam HR. But the IOLMaster 700 uses a smaller 2.5 mm ring of projected spots for anterior measurement. The smaller measurement zone may be less precise for IOLMaster 700 measurements because our post-refractive surgery study population have less uniform corneal curvatures.
A limitation of the generalizability of this study is that the study population only included post-refractive surgery eyes. If we had studied normal eyes, all of the instruments would probably show better accuracy and repeatability, and there may be less difference between them. On the other hand, if we had studied more highly aberrated corneas (e.g. keratoconus), then the accuracy and repeatability would probably be worse, and there may be more difference between instruments.
Another limitation of this study is that we have not analyzed any Placido disk based instruments. Placido disk topographers have better spatial resolution than OCT and Scheimpflug imaging systems, but they only measure the anterior corneal surface. Therefore we do not expect to achieve better accuracy than instruments measuring both corneal surfaces.27
In the present study, the OCT corneal astigmatism was calculated using Gaussian optics. Therefore, OCT net astigmatism was compared to Pentacam true net astigmatism acquired using the Pentacam HR TNP map, which is also based on Gaussian optics. We are developing the assessment of OCT corneal astigmatism using the ray-tracing analysis. We will compare ray-tracing OCT corneal astigmatism Pentacam HR ray-tracing based corneal astigmatism (Corneal Refractive Power map) in a separate study. We also caution that the conclusion reached in this study is specific to the instrument models and may not be generalizable to other instruments based on the same type of technology. Dual Scheimpflug systems or other Scheimpflug systems that allow faster acquisitions may achieve higher precision by reducing motion error. Also, the combination of Placido disc based topography together with Scheimpflug imaging has been shown to be more precise that each technique on its own.27 Finally, the swept-source OCT technology used by the IOLMaster 700 is in principle capable of much higher speed and axial resolution and thus potentially higher performance.
In conclusion, this study shows an approach to measure net corneal astigmatism in post refractive surgery patients with OCT that can achieve higher precision and accuracy than other commonly used instruments. This high accuracy may potentially improve toric IOL selection. Further studies are needed to ascertain whether this approach may be advantageous in other types of patients.
What was known:
Scheimpflug imaging and swept-source optical biometry can estimate net corneal astigmatism by directly measuring anterior and posterior corneal surfaces with more accuracy compared to simulated keratometry.
Simulated keratometry has with-the-rule astigmatic bias compared relative to manifest refraction because neglecting the posterior corneal astigmatism.
What this paper adds:
Optical coherence tomography (OCT) provides the means to measure corneal astigmatism with significantly better accuracy than Scheimpflug imaging and swept-source optical biometry in patients post refractive surgery.
The repeatability of the measure of OCT net corneal astigmatism is significantly better to that from Pentacam HR True Net astigmatism
Acknowledgments
Financial Supports: Supported by the National Institutes of Health, Bethesda, MD (R01EY028755, R01EY029023, T32EY023211, P30EY010572); a research grant and equipment support from Optovue, Inc., Fremont, CA; unrestricted grants to Casey Eye Institute from Research to Prevent Blindness, Inc., New York, NY. The sponsors did not participate in the data collection, data management, or data analysis in the present study.
Footnotes
Financial Disclosures: Oregon Health and Science University (OHSU) and Drs. Li and Huang have a significant financial interest in Optovue, Inc., a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU.
References
- 1.Ferrer-Blasco T, Montés-Micó R, Peixoto-de-Matos SC, González-Méijome JM, Cerviño A. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg 2009;35(1):70–75. doi: 10.1016/j.jcrs.2008.09.027 [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann PC, Auel S, Hütz WW. Results of higher power toric intraocular lens implantation. J Cataract Refract Surg 2011;37(8):1411–1418. doi: 10.1016/j.jcrs.2011.02.028 [DOI] [PubMed] [Google Scholar]
- 3.Potvin R, Kramer BA, Hardten DR, Berdahl JP. Factors associated with residual astigmatism after toric intraocular lens implantation reported in an online toric intraocular lens back-calculator. J Refract Surg 2018;34(6):366–371. doi: 10.3928/1081597X-20180327-01 [DOI] [PubMed] [Google Scholar]
- 4.Visser N, Bauer NJC, Nuijts RMMA. Toric intraocular lenses: Historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg 2013;39(4):624–637. doi: 10.1016/j.jcrs.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 5.Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg 2012;38(12):2080–2087. doi: 10.1016/j.jcrs.2012.08.036 [DOI] [PubMed] [Google Scholar]
- 6.Savini G, Næser K. An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Investig Ophthalmol Vis Sci 2015;56(2):827–835. doi: 10.1167/iovs.14-15903 [DOI] [PubMed] [Google Scholar]
- 7.Tang M, Chen A, Li Y, Huang D. Corneal power measurement with Fourier-domain optical coherence tomography. J Cataract Refract Surg 2010;36(12):2115–2122. doi: 10.1016/j.jcrs.2010.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonn B, Klaproth OK, Kohnen T. Anterior surface–based keratometry compared with scheimpflug tomography–based total corneal astigmatism. Investig Ophthalmol Vis Sci 2014;56(1):291–298. doi: 10.1167/iovs.14-15659 [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Shekhar R, Huang D. Corneal Pachymetry Mapping with High-speed Optical Coherence Tomography. Ophthalmology 2006;113(5):792–799.e2. doi: 10.1016/j.ophtha.2006.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savini G, Schiano-Lomoriello D, Hoffer KJ. Repeatability of automatic measurements by a new anterior segment optical coherence tomographer combined with Placido topography and agreement with 2 Scheimpflug cameras. J Cataract Refract Surg 2018;44(4):471–478. doi: 10.1016/j.jcrs.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 11.McNabb RP, Farsiu S, Stinnett SS, Izatt JA, Kuo AN. Optical coherence tomography accurately measures corneal power change from laser refractive surgery. Ophthalmology 2015;122(4):677–686. doi: 10.1016/j.ophtha.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khurana RN, Li Y, Tang M, Lai MM, Huang D. High-speed Optical Coherence Tomography of Corneal Opacities. Ophthalmology 2007;114(7):1278–1285. doi: 10.1016/j.ophtha.2006.10.033 [DOI] [PubMed] [Google Scholar]
- 13.Nanji A, Redd T, Chamberlain W, et al. Application of Corneal Optical Coherence Tomography Angiography for Assessment of Vessel Depth in Corneal Neovascularization. Cornea 2020;39(5):598–604. doi: 10.1097/ICO.0000000000002232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullimore MA, Slade S, Yoo P, Otani T. An Evaluation of the IOLMaster 700. Eye Contact Lens 2019;45(2):117–123. doi: 10.1097/ICL.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 15.Pavlatos E, Huang D, Li Y. Eye motion correction algorithm for OCT-based corneal topography. Biomed Opt Express 2020;11(12):7343. doi: 10.1364/boe.412209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westphal V, Rollins A, Radhakrishnan S, Izatt J. Correction of geometric and refractive image distortions in optical coherence tomography applying Fermats principle. Opt Express 2002;10(9):397. doi: 10.1364/oe.10.000397 [DOI] [PubMed] [Google Scholar]
- 17.Schwiegerling J, Greivenkamp JE. Using corneal height maps and polynomial decomposition to determine cornea; aberration.pdf. Optom Vis Sci 1997;74(11):906–916. [DOI] [PubMed] [Google Scholar]
- 18.Robert Iskander D, Davis BA, Collins MJ, Franklin R. Objective refraction from monochromatic wavefront aberrations via Zernike power polynomials. Ophthalmic Physiol Opt 2007;27(3):245–255. doi: 10.1111/j.1475-1313.2007.00473.x [DOI] [PubMed] [Google Scholar]
- 19.Bland J, Altman D. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8(99):135–160. [DOI] [PubMed] [Google Scholar]
- 20.Dong J, Zhang Y, Zhou J, et al. Comparison of Corneal Power and Corneal Astigmatism of Different Diameter Zones Centered on the Pupil and Corneal Apex Using Scheimpflug Tomography. Cornea 2020;39(1):77–83. doi: 10.1097/ICO.0000000000002052 [DOI] [PubMed] [Google Scholar]
- 21.Özyol P, Özyol E. Agreement Between Swept-Source Optical Biometry and Scheimpflug-based Topography Measurements of Anterior Segment Parameters. Am J Ophthalmol 2016;169:73–78. doi: 10.1016/j.ajo.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 22.Molina-Martín A, Piñero DP, Caballero MT, de Fez D, Camps VJ. Comparative analysis of anterior corneal curvature and astigmatism measurements obtained with three different devices. Clin Exp Optom 2020;103(5):618–624. doi: 10.1111/cxo.13002 [DOI] [PubMed] [Google Scholar]
- 23.Tu R, Yu J, Savini G, et al. Agreement between two optical biometers based on large coherence length SS-OCT and scheimpflug imaging/Partial coherence interferometry. J Refract Surg 2020;36(7):459–465. doi: 10.3928/1081597X-20200420-02 [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Li Y, Savini G, et al. Comparison of anterior segment measurements obtained using a swept-source optical coherence tomography biometer and a Scheimpflug–Placido tomographer. J Cataract Refract Surg 2019;45(3):298–304. doi: 10.1016/j.jcrs.2018.10.033 [DOI] [PubMed] [Google Scholar]
- 25.Weikert MP, Golla A, Wang L. Astigmatism induced by intraocular lens tilt evaluated via ray tracing. J Cataract Refract Surg 2018;44(6):745–749. doi: 10.1016/j.jcrs.2018.04.035 [DOI] [PubMed] [Google Scholar]
- 26.Preussner PR, Hoffmann P, Wahl J. Impact of Posterior Corneal Surface on Toric Intraocular Lens (IOL) Calculation. Curr Eye Res 2015;40(8):809–814. doi: 10.3109/02713683.2014.959708 [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann PC, Abraham M, Hirnschall N, Findl O. Prediction of residual astigmatism after cataract surgery using swept source fourier domain optical coherence tomography. Curr Eye Res 2014;39(12):1178–1186. doi: 10.3109/02713683.2014.898376 [DOI] [PubMed] [Google Scholar]
- 28.Goss D, Grosvenor T. Reliability of refraction — a literature review. J Am Optom Assoc 1996;67(10):619–630. [PubMed] [Google Scholar]
- 29.Szalai E, Berta A, Hassan Z, Módis L. Reliability and repeatability of swept-source Fourier-domain optical coherence tomography and Scheimpflug imaging in keratoconus. J Cataract Refract Surg 2012;38(3):485–494. doi: 10.1016/j.jcrs.2011.10.027 [DOI] [PubMed] [Google Scholar]
- 30.Shajari M, Sonntag R, Ramsauer M, et al. Evaluation of total corneal power measurements with a new optical biometer. J Cataract Refract Surg 2020;46(5):675–681. doi: 10.1097/j.jcrs.0000000000000136 [DOI] [PubMed] [Google Scholar]
- 31.Visser N, Berendschot TTJM, Verbakel F, De Brabander J, Nuijts RMMA. Comparability and repeatability of corneal astigmatism measurements using different measurement technologies. J Cataract Refract Surg 2012;38(10):1764–1770. doi: 10.1016/j.jcrs.2012.05.036 [DOI] [PubMed] [Google Scholar]
- 32.Li X, Lawman S, Williams B, Shen Y, Zheng Y, ye sicong. Simultaneous Optical Coherence Tomography and Scheimpflug imaging using the same incident light. Opt Express 2020;28(26):39660–39676. doi: 10.1364/oe.405643 [DOI] [PubMed] [Google Scholar]
- 33.Reinstein DZ, Gobbe M, Archer TJ. Anterior segment biometry: A study and review of resolution and repeatability data. J Refract Surg 2012;28(7):509–520. doi: 10.3928/1081597X-20120620-02 [DOI] [PubMed] [Google Scholar]


