Abstract
Children exposed to prenatal maternal psychological distress are at elevated risk for a range of adverse outcomes; however, it remains poorly understood whether postnatal influences can ameliorate impairments related to prenatal distress. The current study evaluated if maternal care could mitigate child cognitive and emotional impairments associated with prenatal psychological distress. Prenatal maternal psychological distress was assessed (anxiety, depression, and perceived stress) for 136 mothers at 5 prenatal and 4 postpartum time points. Quality of maternal care (sensitivity to nondistress, positive regard and intrusiveness reverse-scored) were assessed during a mother-child play interaction at 6 and 12 months. Child cognitive function and negative emotionality were assessed at 2 years, using The Bayley Scales and the Early Childhood Behavior Questionnaire. Elevated prenatal distress was associated with poorer child cognitive function and elevated negative emotionality. Children exposed to elevated prenatal maternal distress did not, however, display these outcomes if they received high quality caregiving. Specifically, maternal care moderated the relation between prenatal psychological distress and child cognitive function and negative emotionality. This association remained after consideration of postnatal maternal psychological distress and relevant covariates. Sensitive maternal care was associated with altered offspring developmental trajectories, supporting child resilience following prenatal distress exposure.
Keywords: Fetal programming, maternal care, prenatal stress, resilience, cognitive function, depression, parenting
Fetal life is an exceptionally rapid period of neurological development, and is a time when the fetus is highly susceptible to both beneficial and harmful environmental influences (Barker, 1998). Prenatal maternal psychological distress is linked to profound and lasting consequences for developmental trajectories and increases risk for subsequent mental health problems (Davis et al., 2007; Davis & Sandman, 2010, 2012; Demers et al., 2020; Glynn et al., 2018; Van den Bergh et al., 2017). The evidence that prenatal maternal stress and mental health has long reaching implications for offspring psychopathology raises the need to investigate factors that can reduce or eliminate the consequences of prenatal adversity. High quality parental caregiving is a likely factor that may increase resiliency in the face of prenatal adversity (Davis et al., 2019; Kok et al., 2015; NICHD Early Childcare Research Network, 1999). The current study addresses an important knowledge gap by investigating whether high quality maternal caregiving during infancy ameliorates the consequences of prenatal maternal distress on child cognitive and emotional development, thereby shifting trajectories of risk towards more optimal mental health.
Fetal programming research illustrates that children exposed to maternal psychological distress (e.g. anxiety, depression, and stress) during pregnancy have poorer cognitive function and elevated negative emotionality during infancy, childhood, and adolescence (Blair et al., 2011; Glynn et al., 2018; Korja et al., 2017; Madigan et al., 2018; Mahrer et al., 2019; Sandman & Davis, 2010; Van den Bergh et al., 2005, 2017). Importantly, these prospective and longitudinal studies document the predictive importance of prenatal distress on child outcomes, even after covarying potential confounders, including postnatal maternal psychological distress. Further, an independent line of research focusing on postnatal experiences reports that sensitive and responsive maternal care is associated with benefits for child cognitive and emotional development, as well as adult psychological health (Davis et al., 2017; Deans, 2018; Fan et al., 2014; Farrell et al., 2019; Malmberg et al., 2016; Spinrad & Stifter, 2002). Maternal caregiving during the first year postpartum may be especially important, because this is a sensitive window for mother-infant relationship development (Ainsworth, 1979; Feldman, 2007).
Although the opportunity to assess the joint contributions of prenatal and postnatal experiences are rare in human research, and often are limited by a lack of objective assessment of maternal behavior (Sharp et al., 2012, 2015), experimental research with animals shows that high quality maternal care can compensate for exposure to prenatal maternal stress. Specifically, the adverse effects of prenatal maternal stress on offspring cognition, emotion stress regulation, and brain development can be prevented with experimental manipulations of maternal quality of care (Bogoch et al., 2007; Lemaire et al., 2006; Raineki et al., 2014; Wakshlak & Weinstock, 1990).
Several studies with clinical populations have tested the benefit of sensitive, responsive care on human infant development. Among mothers with an anxiety or a depression diagnosis during pregnancy, high quality of maternal care is associated with a reduction in the correlation between prenatal maternal mental health and offspring cortisol regulation at 4 months (Kaplan et al., 2008), on negative affect responses to still-face at 7 months (Grant et al., 2010a), and on Bayley cognitive development scores at 7 months (Grant et al., 2010b). In addition to the relatively small sample sizes of these studies (ranging from 47 to 84), limitations include reliance on assessment of a single diagnostic category (i.e., diagnoses of anxiety or depressive disorders; Grant et al., 2010a, 2010b; Kaplan et al., 2008). Reliance on a single timepoint assessment as well as diagnostic cut offs in a single disorder may not fully capture fetal exposure to maternal psychological distress for several reasons. First, there is compelling evidence that maternal distress across the spectrum and including in the non-clinical range is linked to child outcomes (Davis & Sandman, 2012; Glynn et al., 2018; Kingston et al., 2012; Krueger & Markon, 2006; Lee et al., 2007). Second, women frequently experience comorbidity of stress and internalizing symptoms from multiple disorders all of which may impact the fetus (Falah-Hassani et al., 2016; Glynn et al., 2018). For these reasons, it is important to assess prenatal exposure to maternal anxiety, depression and stress multiple times during gestation to create a reliable index of exposure to multiple types of maternal distress over the entire course of gestation and then evaluate the compensatory benefit of maternal care.
A recent study, that addressed these limitations, reported that high levels of maternal positive engagement at ages 2.5 to 5 years alleviated the cognitive delays associated with prenatal maternal distress and suggested that parental care may be an important target for intervention (Schechter et al., 2017). The present study extends the findings by Schechter et al. (2017) in two important ways. First, by assessing maternal care during the first postnatal year (6 and 12 months), a sensitive widow for attachment formation, this study assesses a potential target for early intervention. Second, the current study assesses both child cognitive function and negative emotionality, two important indices of early child development. Specifically, the current prospective research characterized maternal psychological distress (anxiety, depression, and perceived stress) across five gestational intervals and four postpartum time points. This approach was used to address the question: Does the quality of maternal care mitigate the link between prenatal exposure to maternal psychological distress and child cognitive function and negative emotionality?
Methods
Participants
Study participants included 136 mothers and their children (79 male, 57 female) participating in a longitudinal study evaluating the role of early experiences on development. Pregnant women were recruited from a large university medical center in Southern California. At recruitment, inclusion criteria were: (1) adult (≥ 18 years of age), (2) English-speaking, and (3) intrauterine, singleton pregnancy. Exclusion criteria at recruitment were: (1) presence of uterine or cervical abnormalities, (2) conditions such as endocrine, hepatic, or renal disorders, or use of corticosteroid medication, and (3) self-reported abuse of tobacco, alcohol, or recreational drugs in the pregnancy. An additional postnatal inclusion criterion for the current study was gestational age of 34 weeks or greater at birth. Descriptive information for the sample is provided in Table 1. All mothers provided informed, written consent for themselves and their child as approved by the Institutional Review Board for Protection of Human Subjects.
Table 1.
Sample Characteristics
| Maternal Characteristics (N = 136) | (M ± SD) (range) or (%)* |
|---|---|
| Age at Delivery | 30.28 ± 5.08 (19.06 – 44.63) |
| Cohabitating with Child’s Father | 91.0 |
| Primiparous | 43.4 |
| Ethnicity | |
| Non-Hispanic White | 50.7 |
| Latina | 29.4 |
| African American or Black | 2.9 |
| Asian | 8.1 |
| Multi-Ethnic | 8.8 |
| Household Income | |
| $0-$30,000 | 20.6 |
| $30,001-$60,000 | 25.7 |
| $60,001-$100,000 | 33.8 |
| Over $100,000 | 19.9 |
| Years of Education | 15.99 ± 2.35 (9 – 19) |
| Obstetric Complications Sum Score | 0.34 ± 0.56 (0 – 2) |
| Maternal Sensitivity Composite (6 months) | 9.98 ± 1.06 (6.5 – 12.0) |
| Maternal Sensitivity Composite (12 months) | 9.67 ± 1.67 (4.5 – 12.0) |
| Child Characteristics (N = 136) | |
| Sex (% Male) | 58.1, n=79 |
| Apgar Score (5 min) | 9.00 ± 0.27 (8 – 10) |
| Gestational Age at Birth (GAB) | 39.44 ± 1.26 (35.29 – 42.57) |
| Birth Weight Percentile** | 52.77 ± 27.90 (1 – 99) |
| Mental Developmental Index (MDI) | 97.71 ± 16.39 (54 – 140) |
| Negative Affectivity (ECBQ) | 2.80 ± 0.58 (1.38 – 5.08) |
Values presented as (means ± SD) or (%) where applicable
Birth weight percentile was calculated according to the infant’s sex and gestational age at birth.
Procedures
Measures of maternal psychological distress were collected at five time points prenatally and four time points postnatally. Maternal sensitivity was assessed at 6 and 12 months postpartum and child cognitive and emotional outcomes were evaluated at 2 years (see Figure 1 for study timeline). Gestational and child ages at each assessment are presented in the Appendix Table 1. Appendix Table 6 shows missing data for each measure.
Figure 1.
Study timeline
Measures
Maternal Psychological Distress
Maternal anxiety was assessed using the 10-item State Anxiety subscale of the State-Trait Personality Inventory (STAI; Spielberger, 1983). Maternal depressive symptoms were assessed using the 9-item short form of the Center for Epidemiologic Studies Depression Inventory (CES-D; Santor & Coyne, 1997). Generalized or non-specific perceptions of stress were assessed using the 12-item version of Cohen’s Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983). The internal consistencies of the three measures across time points were good (STAI α = .87 - .90, CES-D α = .85 - .88, PSS α = .87 - .91 in the current sample). Mean levels of maternal depression, stress, and anxiety and distress composites at prenatal and postnatal time points are presented in the Appendix Table 2. The correlation between the three distress indicators and the pattern of association for anxiety, stress, and depression across pregnancy are presented in Appendix Tables 3-5.
Mean depression, perceived stress, and anxiety scores were standardized and averaged to create a composite prenatal distress and a composite postnatal distress score. This distress composite score was used an index of prenatal exposure to multiple distress indicators, in order to provide a test of the ability of postnatal maternal sensitivity to compensate for prenatal maternal distress exposure. Prior research has indicated that the composite of anxiety, stress, and depression throughout pregnancy consistently predicts child outcomes (Glynn et al., 2018; Howland et al., 2020).
Quality of Maternal Care
Maternal care was characterized in the present investigation using a maternal sensitivity index developed by the NICHD early Child Care Study comprised of three indicators (a composite of positive regard, sensitivity to nondistress, and intrusiveness reverse-scored) that is a potent predictor of numerous child development outcomes (NICHD Early Child Care Research Network, 1997, 1999, 2001).
Mothers were video-recorded interacting with their infants in a semi-structured 10-minute play episode in the lab at 6 and 12 months of age. During this play interaction, mothers were given a standard set of age-appropriate toys and told to play with their infants as they would at home. Maternal behavior was scored from video using a coding system developed for the NICHD Study of Early Child Care and Youth Development (Glynn et al., 2016; NICHD Early Child Care Research Network, 1999). Based on standard procedures, a composite rating of maternal sensitivity was created by summing 4-point ratings of sensitivity to nondistress, positive regard, and intrusiveness (reverse-scored). A composite maternal sensitivity score was calculated by averaging 6- and 12-month scores. All coders were blind to other data gathered on study participants. Twenty percent of sessions were selected at random, without coder knowledge, and coded again by a second independent coder to obtain an index of inter-rater reliability. Reliability for each of the subscales were: sensitivity to nondistress (90%), intrusiveness reverse-scored (90%), and positive regard (93%).
Child Cognitive Function
The Bayley Scales of Infant Development, 2nd edition (BSID-II) Mental Developmental Index (MDI) was administered to assess child cognitive function at 2 years of age (Bayley, 1993). Examiners were directly supervised by a clinical psychologist. Interrater reliability, calculated on 15% of the assessments at each age, was 93%.
Child Negative Emotionality
Child negative emotionality at 2 years of age was assessed via maternal report on the Early Childhood Behavior Questionnaire (ECBQ; Putnam, Gartstein, & Rothbart, 2006). Prior research demonstrates that the ECBQ Negative Affectivity scale at 2 years has demonstrated inter-rater reliability and longitudinal stability (Putnam et al., 2006). Within the current sample, the Negative Affectivity scale had excellent internal consistency (α = .95).
Measurement of Covariates
Sociodemographic Characteristics
Maternal socioeconomic status, age, cohabitation with child’s father, and race and ethnicity were collected via maternal interview. Maternal socioeconomic status was calculated as the sum of standardized numbers of year of maternal education and standardized household income.
Pregnancy and Birth Outcomes
Maternal obstetric complications, parity, infant sex, birth weight, and 5-minute Apgar score were abstracted from the medical record. Estimated date of delivery was calculated utilizing both early ultrasound measures and date of last menstrual period based on ACOG guidelines, and used to assess gestational age at birth (Committee on Obstetric Practice, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine, 2017). An obstetric complications score was calculated, indicating the presence or absence of any pregnancy-related complications, including: prenatal infection, pregnancy-induced hypertension, gestational diabetes, oligohydramnios, polyhydramnios, preterm labor, anemia, vaginal bleeding, or placenta previa (Hobel, 1982).
Maternal Intelligence
Maternal intelligence was assessed at a subsequent visit via the Perceptual Organization Index (POI) of the Wechsler Adult Intelligence Scale (WAIS-III) once postnatally. The POI was a proxy of general intelligence, because it is highly correlated with the general factor g of intelligence (r = .94) (Deary, 2001). The WAIS and its subscales are widely used and valid measures of intelligence. Previous studies demonstrate the validity of the WAIS, as well as the reliability of the POI score (r = .93) (Wechsler et al., 1997). The WAIS-III was administered at a postnatal visit (M = 5.34, SD = 1.99 years post-delivery).
Statistical Analyses
Sociodemographic and Obstetric Covariates
Potential covariates were selected based on the literature (Blair et al., 2011; Polanska et al., 2017) and included infant characteristics (biological sex at birth, gestational age at birth, birthweight percentile adjusted for sex, and 5-minute Apgar score), as well as obstetric (obstetric health related factors, parity) and maternal characteristics (socioeconomic status, age, cohabitation with infant’s father, race and ethnicity, and the WAIS, an index of intelligence). All regression models included covariates associated (p < .10) with the child outcome. Thus, the following covariates were included in cognitive function analyses: maternal age, socioeconomic status, parity, cohabitation with child’s father, maternal index of intelligence (POI), sum of obstetric risk, ethnicity (Non-Hispanic White or Latina), gestational age at birth (GAB), and child sex. Covariates included in negative emotionality analyses were maternal age, socioeconomic status, cohabitation with child’s father, maternal index of intelligence (POI), and ethnicity (Non-Hispanic White or Latina). The correlations between all potential covariates, prenatal and postnatal distress, maternal sensitivity, and child outcomes are presented in Appendix Table 7.
Psychological Distress, Maternal Care and Child Outcomes
Initial bivariate correlations were performed to test whether prenatal and postnatal maternal psychological distress were associated with child outcomes, cognitive function and negative emotionality. The relations between the maternal sensitivity composite and child outcomes were also evaluated via bivariate correlation.
Moderation of Prenatal Psychological Distress by Maternal Caregiving
Regression models tested the primary hypothesis that the maternal sensitivity composite moderates the relation between prenatal maternal psychological distress and child outcome. Continuous predictor variables were mean-centered in the regression model. Significant interactions were probed by calculating and plotting simple slopes. Finally, to test the potential contribution of postnatal maternal psychological distress, regression models included it as an additional covariate.
Secondary Analyses: Assessment of Sex Differences, Individual Distress Indicators, and Timing and Subscales of Maternal Care
Sex differences:
Secondary analyses were conducted in order to explore sex differences as follows 1) whether there are sex differences in the association between prenatal distress and child outcomes, 2) sex differences in the association between the maternal sensitivity composite and child outcomes and 3) whether the interaction between prenatal psychological distress and maternal sensitivity was moderated by child sex (three-way interaction).
Individual distress indicators:
To test whether the maternal sensitivity composite moderated the effect of each of the three distress indicators, regression models were conducted separately for prenatal anxiety, stress and depression (see Appendix Tables 7-8).
Timing and subscales of maternal care:
To evaluate whether care at 6 or 12 months was a more important moderator of prenatal distress (timing) interaction models were conducted with maternal sensitivity separately at 6 and 12 months (see Appendix Tables 9-10). Finally, to test the role of the individual subscales that comprise the sensitivity composite (positive regard, sensitivity to nondistress, and intrusiveness reverse-scored) in moderating the effect of prenatal maternal distress, moderation analyses were conducted separately with each of the three subscales (Appendix Tables 11-12).
Results
Child Developmental Outcomes
Descriptive information for the cognitive and emotional outcomes is shown in Table 1. Poorer child cognitive function was associated with higher child negative emotionality (r = −.32, p < .001).
Maternal Distress, Maternal Care and Child Cognitive Function
Elevated prenatal psychological distress composite was associated with poorer cognitive function (r = −.28, p = .001) and as shown in Appendix Table 5, the pattern of association was similar across the 5 gestational timepoints. Further, elevated postnatal psychological distress was associated with poorer child cognitive function at 2 years of age (r = −.26, p = .003). In contrast, a higher maternal sensitivity composite score was associated with enhanced child cognitive function (r = .47, p < .001).
Does Postnatal Care Moderate the Relation Between Prenatal Psychological Distress and Child Cognitive Function?
The maternal sensitivity composite moderated the association between prenatal distress and child cognitive function even with the inclusion of covariates (b = 2.80, t(119)= 2.25, p = .027; see Table 2). As shown in Figure 2, children exposed to elevated prenatal maternal distress and low maternal sensitivity exhibited the poorest cognitive performance, but children exposed to higher prenatal maternal distress who then received sensitive maternal care did not display deficits in cognitive function. This association remained when postnatal distress was additionally included as a covariate (b = 2.78, t(118)= 2.22, p = .028; see Appendix Table 13a).
Table 2.
Regression Model Examining the Association Between Prenatal Maternal Psychological Distress, Maternal Care and Child Cognitive Function
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Sensitivity Composite |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | B | B | SE B | β | B | SE B | β |
| Maternal Age | 0.038 | 0.334 | 0.011 | −0.062 | 0.333 | −0.019 | −0.042 | 0.327 | −0.013 |
| SES | 2.159† | 1.139 | 0.225 | 1.771 | 1.138 | 0.185 | 1.839 | 1.120 | 0.192 |
| Parity | −2.503 | 1.728 | −0.125 | −1.610 | 1.755 | −0.080 | −1.610 | 1.726 | −0.080 |
| Cohabitation with Child’s Father | 4.281 | 4.612 | 0.078 | 3.732 | 4.554 | 0.068 | 1.920 | 4.550 | 0.035 |
| Index of Intelligence (WAIS) | 0.166† | 0.097 | 0.174 | 0.144 | 0.096 | 0.151 | 0.130 | 0.094 | 0.137 |
| Obstetric Risk | −1.925 | 2.831 | −0.053 | −1.795 | 2.792 | −0.049 | −1.921 | 2.746 | −0.053 |
| Non-Hispanic White | −1.764 | 3.191 | −0.054 | −1.951 | 3.148 | −0.060 | −2.611 | 3.109 | −0.080 |
| Latina | −6.728† | 3.747 | −0.189 | −6.048 | 3.709 | −0.170 | −6.544† | 3.654 | −0.184 |
| Gestational Age at Birth (GAB) | 0.863 | 1.017 | 0.066 | 0.641 | 1.008 | 0.049 | 0.458 | 0.995 | 0.035 |
| Child Sex (male) | −6.473* | 2.464 | −0.195 | −6.244* | 2.432 | −0.188 | −6.703** | 2.400 | −0.202 |
| Prenatal Distress | −1.376 | 1.616 | −0.079 | −1.301 | 1.594 | −0.074 | −1.425 | 1.568 | −0.081 |
| Sensitivity Composite | 2.599* | 1.229 | 0.190 | 2.759* | 1.211 | 0.201 | |||
| Prenatal Distress* Sensitivity Composite | 2.813* | 1.245 | 0.161 | ||||||
| R2 | 0.379 | 0.401 | 0.426 | ||||||
| F | 6.715*** | 6.705*** | 6.794*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Figure 2.
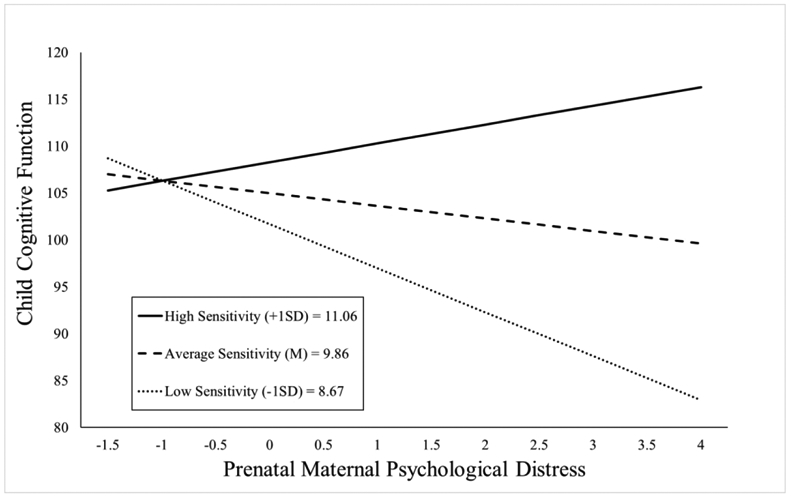
Maternal sensitivity composite was analyzed as a continuous variable using regression, but for illustrative purposes are depicted here as low (1 SD below the mean), average (at the mean), and high (1 SD above the mean) levels of maternal sensitivity. Prenatal maternal distress (on the x-axis) is the standardized composite of anxiety, depressive symptoms, and perceived stress scores. Children exposed to elevated prenatal maternal distress did not exhibit impaired cognitive function at age 2 if they received higher quality maternal caregiving.
Cognitive Function Secondary Analyses: Assessment of Sex Differences, Individual Distress Indicators, and Timing of Maternal Care
Sex differences:
Secondary analyses revealed that child sex did not moderate the effects of prenatal psychological distress (β = 0.070) or maternal sensitivity (β = 0.002) on child cognitive function; additionally, the three-way interaction of child sex, prenatal distress, and maternal sensitivity was nonsignificant (β = 0.072) (ps > .52).
Individual distress indicators:
The moderating effect of sensitivity on prenatal anxiety (β = 0.143), stress (β = 0.166), and depression (β = 0.114) examined separately yielded similar effect sizes (see Appendix Table 7).
Timing and subscales of maternal care:
The moderating role of maternal sensitivity on child cognitive function was similar when maternal sensitivity was examined separately at 6 months (β = 0.181) and 12 months (β = 0.158) (Appendix Table 9). Additionally, the moderating effect of the individual subscales that comprise the sensitivity composite, including positive regard (β = 0.154), sensitivity to nondistress (β = 0.151), and intrusiveness reverse-scored (β = 0.022 ), revealed similar effect sizes (Appendix Table 11).
Maternal Distress, Maternal Care, and Child Negative Emotionality
Elevated prenatal maternal psychological distress was associated with higher child negative emotionality at 2 years of age (r = .28, p = .002), and as shown in Appendix Table 5, the pattern of association was similar across the 5 gestational timepoints. Elevated postnatal maternal psychological distress (r = .25, p = .005) was associated with higher child negative emotionality at 2 years of age. A higher maternal sensitivity composite score was associated with lower child negative emotionality (r = −.24, p = .008).
Does Postnatal Care Moderate the Relation between Prenatal Psychological Distress and Child Negative Emotionality?
As shown in Figure 3, children exposed to elevated prenatal distress and who received high quality maternal caregiving exhibited low negative emotionality even after consideration of covariates (b = −0.11, t(109)= −2.01, p = .047; see Table 3). With the addition of postnatal maternal distress as a covariate this association remained statistically significant (b = −0.11, t(108)= −2.03, p = .045; see Appendix Table 13b).
Figure 3.
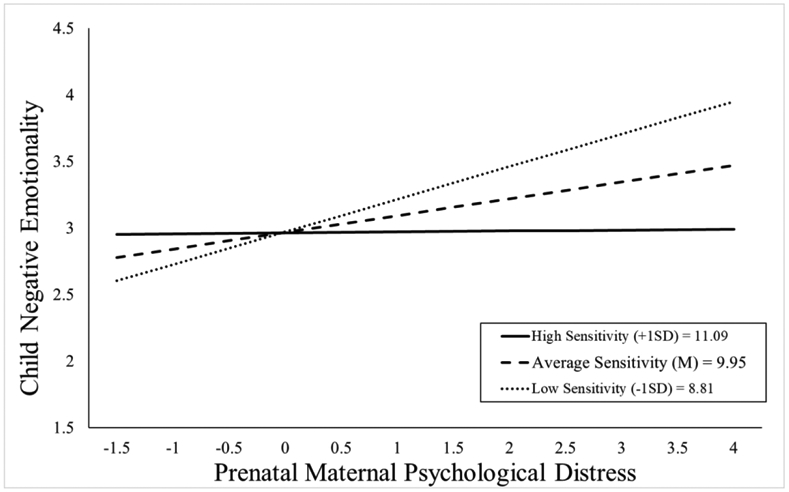
Maternal sensitivity composite was analyzed as a continuous variable using regression, but for illustrative purposes are depicted here as low (1 SD below the mean), average (at the mean), and high (1 SD above the mean) levels of maternal sensitivity. Prenatal maternal distress (on the x-axis) is the standardized composite of anxiety, depressive symptoms, and perceived stress scores. Children exposed to elevated prenatal maternal distress did not exhibit high negative emotionality at age 2 if they received higher quality maternal caregiving.
Table 3.
Regression Model Examining the Association Between Prenatal Maternal Psychological Distress, Maternal Care and Child Negative Emotionality
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Sensitivity Composite |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | −0.012 | 0.011 | −0.105 | −0.012 | 0.011 | −0.105 | −0.014 | 0.011 | −0.121 |
| SES | −0.023 | 0.041 | −0.065 | −0.021 | 0.042 | −0.061 | −0.023 | 0.041 | −0.065 |
| Cohabitation with Child’s Father | −0.172 | 0.193 | −0.079 | −0.173 | 0.194 | −0.080 | −0.122 | 0.193 | −0.056 |
| Index of Intelligence (WAIS) | −0.003 | 0.003 | −0.100 | −0.003 | 0.004 | −0.097 | −0.003 | 0.003 | −0.087 |
| Non-Hispanic White | −0.258* | 0.127 | −0.223 | −0.258* | 0.128 | −0.224 | −0.233† | 0.127 | −0.201 |
| Latina | 0.152 | 0.152 | 0.117 | 0.148 | 0.154 | 0.114 | 0.182 | 0.153 | 0.141 |
| Prenatal Distress | 0.125* | 0.060 | 0.201 | 0.124* | 0.061 | 0.199 | 0.126* | 0.060 | 0.204 |
| Sensitivity Composite | −0.008 | 0.048 | −0.016 | −0.003 | 0.047 | −0.006 | |||
| Prenatal Distress* Sensitivity Composite | −0.105* | 0.052 | −0.164 | ||||||
| R2 | 0.280 | 0.280 | 0.306 | ||||||
| F | 6.173*** | 5.358*** | 5.342*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Negative Emotionality Secondary Analyses: Assessment of Sex Differences, Individual Distress Indicators, and Timing of Maternal Care
Sex differences:
Secondary analyses revealed that child sex did not moderate the effects of prenatal psychological distress (β = 0.065) or maternal sensitivity (β = −0.133) on child negative emotionality; additionally, the three-way interaction of child sex, prenatal distress, and maternal sensitivity was nonsignificant (β = 0.104) (ps > .29).
Individual distress indicators:
The moderating effect of sensitivity on prenatal anxiety (β =−0.135), stress (β = −0.136), and depression (β =−0.165) examined separately yielded similar effect sizes (see Appendix Table 8).
Timing and subscales of maternal care:
The moderating role of maternal sensitivity on child negative emotionality was similar when maternal sensitivity was examined separately at 6 (β = −0.162) and 12 months (β = −0.176) (Appendix Table 10). Additionally, the moderating effect of the individual subscales that comprise the sensitivity composite, including positive regard (β = −0.083), sensitivity to nondistress (β = −0.151), and intrusiveness reverse-scored (β = −0.166), revealed similar effect sizes (Appendix Table 12).
Discussion
Consistent with the fetal programing literature, we show that prenatal exposure to maternal psychological distress (anxiety, depression and perceived stress) is associated with child cognitive and emotional vulnerabilities (Buss et al., 2010; Davis et al., 2007, 2019; Davis & Sandman, 2010; Glynn et al., 2018; Kingston et al., 2012; Van den Bergh et al., 2017). Further, our findings are consistent with decades of research that have established the importance of parental care during sensitive periods, such as the first postnatal year, for promoting optimal developmental outcomes (Ainsworth, 1979). The present study provides new evidence that high quality maternal care during the first postnatal year ameliorates the negative cognitive and emotional outcomes that follow exposure to prenatal maternal distress. These findings remained after considering potential confounding factors including SES, postnatal maternal distress, and maternal intelligence scores. These data indicate that consequences of fetal programming are malleable and that prenatal and postnatal experiences synergistically impact child development. Specifically, we find that high-quality caregiving can compensate for the impact of prenatal maternal distress by altering developmental trajectories and improving child mental health.
The present study addresses a key issue in the fetal programming literature by demonstrating that the consequences of prenatal exposures are modifiable by postnatal experiences. Our data are consistent with experimental rodent models that report manipulations of maternal care compensate for prenatal adversity (Bogoch et al., 2007; Lemaire et al., 2006; Raineki et al., 2014; Wakshlak & Weinstock, 1990) as well as studies showing that infants with a secure attachment relationship to their mother do not show behavioral problems following prenatal stress (Ali et al., 2020; Bergman et al., 2008). There are several important contributions of our project to the relatively small human literature evaluating whether maternal behavior towards her infant mitigates the consequences of prenatal stress (Grant et al., 2010a, 2010b; Kaplan et al., 2008; Schechter et al., 2017; Sharp et al., 2012, 2015). First, maternal distress was assessed repeatedly throughout gestation and we have shown previously that this composite measure of anxiety, stress, and depression symptoms predicts both cognitive and emotional outcomes through childhood and adolescence (Glynn et al., 2018; Howland et al., 2020). Second, we assessed a composite of maternal sensitivity twice during the first postnatal year, a sensitive window for attachment formation (Ainsworth, 1979). Thus, our design is a rigorous test of the hypothesis that high-quality maternal care can offset the impact of prenatal distress exposure after covarying for confounding factors including postnatal maternal distress. Third, we characterize both child cognitive and emotional function. Finally, these data provide evidence that infancy may be a sensitive window when maternal care can mitigate the consequences of prenatal maternal distress, thereby identifying a plausible target for early intervention following prenatal adversity.
Maternal care that is warm, sensitive and responsive to infant’s signals (Ainsworth et al., 1978), may play a particularly important role in child neurodevelopment and long-term developmental outcomes (Malmberg et al., 2016; Spinrad & Stifter, 2002; Wang et al., 2019). Thus, it is highly plausible that high quality maternal care during the first postnatal year may alter neurodevelopmental trajectories following prenatal exposures towards more optimal outcomes. We show that maternal care at 6 and 12 months similarly moderated the association between prenatal maternal distress and child outcomes, suggesting that care throughout the first postnatal year may be important for ameliorating the impact of prenatal distress (Appendix Tables 9-10). The maternal sensitivity composite in the current study measured positive regard, sensitivity to nondistress, and intrusiveness reverse-scored, key aspects of maternal behavior the influence development. Findings suggest that all of these components of maternal sensitivity captured by this measure contribute to positive child development following prenatal maternal distress (See Appendix Tables 11-12). Future research could consider other aspects of positive parenting and paternal parenting behaviors that may ameliorate the impact of prenatal maternal distress.
There are a number of potential mechanisms by which prenatal maternal psychological distress may have consequences for child cognitive and emotional development, including alterations to fetal neurodevelopment (Sandman, Class, et al., 2015; Sandman et al., 2016; Schuurmans & Kurrasch, 2013; Wu et al., 2020). Animal studies demonstrate changes in offspring brain structure following prenatal stress exposure, such as reduced hippocampal volume and neurogenesis (Bogoch et al., 2007; Charil et al., 2010). Children exposed to prenatal maternal psychological distress show reduced gray matter volume and thickness in frontal, temporal, and limbic areas, as well as reduced total gray matter density (Adamson et al., 2018; Buss et al., 2010; Davis et al., 2019; Demers et al., 2020; Sandman, Buss, et al., 2015). Cross-species studies provide mechanistic evidence that dendritic atrophy may be a pathway by which prenatal maternal distress disrupts offspring brain development (Curran et al., 2017; Sandman et al., 2018). These neural systems impacted by prenatal exposures may be modifiable by high-quality postnatal maternal care. The first year postpartum continues to be a sensitive window of heightened neuroplasticity and rapid neural growth (Gee, 2016; Gilmore et al., 2018; Knickmeyer et al., 2008) and maternal care may modify developmental trajectories. Consistent with this possibility, high quality maternal care is associated with enhanced child hippocampal volume growth; this growth trajectory is further associated with improved child emotion regulation (Luby et al., 2017) as well as with greater child gray matter volume at 8 years (Kok et al., 2015). Experimental animal and cross species research provide strong evidence that maternal care directly impacts neural circuits underlying cognitive and emotional vulnerabilities that are impacted by prenatal maternal distress (Gee, 2016; Granger et al., 2021; Liu et al., 2000; Rao et al., 2010).
The current study has several limitations. Although the use of a community sample highlights the importance of subclinical variation in maternal psychological symptoms of distress, the range of observed maternal psychological distress and maternal sensitivity is constrained. Because of this, the current study may underestimate the associations among prenatal distress, maternal sensitivity, and child outcomes. We also cannot completely disentangle the role of genetic influences. However, evidence from experimental animal research consistently reports the effects of maternal care on offspring outcomes are independent from effects of genetics, and provides experimental evidence that maternal care can compensate for prenatal exposures (Francis et al., 1999; Liu et al., 2000).
Decades of research have confirmed the prenatal period as a time of enhanced responsivity to environmental input, when maternal psychological distress can have a profound influence on child development (Bush et al., 2017; Davis et al., 2007, 2018; Davis & Sandman, 2010, 2012; Doyle et al., 2015; Van den Bergh et al., 2017; Vehmeijer et al., 2019). Few studies have directly assessed processes that promote resilience following prenatal adversity (Atzl et al., 2019; D’Anna-Hernandez & Rivera, 2014; Davis & Narayan, in press; Rosand et al., 2011). Our findings, coupled now with those from Schechter et al. (2017), provide strong support for postnatal prevention and intervention efforts to reduce the consequences of prenatal adversity. Efficacious interventions exist to support the transition to parenthood, and the development of positive parenting skills (Bick & Dozier, 2010; Eshel et al., 2006; Nillni et al., 2018). Thus, interventions to promote maternal psychological health and sensitive, responsive caregiving has enduring benefits that could thus mitigate the life-long cognitive and emotional consequences of prenatal psychological distress.
Acknowledgements:
We wish to thank the families who participated in this project. The assistance of Megan Faulkner, Natalie Hernandez, and Kendra Leak of the Women and Children’s Health and Well-Being Project, Department of Psychiatry & Human Behavior, University of California and the Department of Psychology, Chapman University is gratefully acknowledged. This research was supported by the National Institutes of Health [R01 HD065823; P50 MH096889; R03 MH86062; R01 HD51852; R01 NS041298].
Appendix
Appendix Table 1.
Mean Fetal/Child Age at Assessment
| Prenatal (Gestational Weeks) | Postnatal (Months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Fetal/Child Age | 15.63 (1.16) |
19.74 (1.06) |
25.74 (1.03) |
31.07 (0.89) |
36.83 (0.93) |
3.10 (0.50) |
6.06 (0.34) |
12.12 (0.32) |
24.17 (0.41) |
Values presented as Means (SD)
Appendix Table 2.
Mean Levels of Maternal Depression, Anxiety, Stress, and Distress Composites Across Prenatal and Postnatal Time points.
| Prenatal (Gestational Weeks) | Postnatal (Months) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 19 | 25 | 31 | 36 | Composite | 3 | 6 | 12 | 24 | Composite | |
| CES-D | 5.06 (4.44) |
4.44 (4.05) |
4.91 (4.64) |
5.53 (4.42) |
6.50 (5.17) |
5.21 (3.84) |
4.02 (3.95) |
4.05 (4.48) |
3.77 (4.49) |
4.29 (4.63) |
4.02 (3.57) |
| STAI | 17.07 (5.34) |
16.84 (5.25) |
16.46 (5.27) |
16.86 (5.39) |
17.35 (5.71) |
16.95 (4.56) |
16.81 (4.68) |
16.12 (4.98) |
16.65 (5.31) |
16.73 (5.51) |
16.60 (4.19) |
| PSS | 11.39 (6.43) |
10.45 (5.83) |
10.53 (6.97) |
10.31 (6.78) |
10.96 (6.43) |
10.72 (5.66) |
10.83 (6.56) |
9.93 (6.30) |
9.86 (5.94) |
10.81 (6.28) |
10.40 (5.20) |
Values presented as Means (SD)
Appendix Table 3.
Correlations between Prenatal Stress, Depression, and Anxiety Composites and Child Cognitive Function and Negative Emotionality
| Variable | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Depression Composite | — | ||||
| 2. Stress Composite | .792** | — | |||
| 3. Anxiety Composite | .842** | .792** | — | ||
| 4. Cognitive Function | −.242** | −.300** | −.245** | — | |
| 5. Negative Emotionality | .259** | .315** | .210* | −.323** | — |
p < .05.
p < .01.
Appendix Table 4a.
Correlations Between Depression (CESD) at Each Prenatal Timepoint, Prenatal Psychological Distress, Child Cognitive Function, and Child Negative Emotionality
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. CESD 15 wks | — | .746** | .661** | .625** | .533** | .792** | −.234** | .289** |
| 2. CESD 19 wks | .746** | — | .669** | .654** | .575** | .811** | −0.137 | .241** |
| 3. CESD 25 wks | .661** | .669** | — | .579** | .638** | .815** | −.252** | .274** |
| 4. CESD 31 wks | .625** | .654** | .579** | — | .665** | .774** | −.214* | 0.143 |
| 5. CESD 36 wks | .533** | .575** | .638** | .665** | — | .796** | −.207* | .227* |
| 6. Prenatal Psychological Distress | .792** | .811** | .815** | .774** | .796** | — | −.281** | .281** |
| 7. Cognitive Function 2 yrs | −.234** | −0.137 | −.252** | −.214* | −.207* | −.281** | — | −.323** |
| 8. Negative Emotionality 2yrs | .289** | .241** | .274** | 0.143 | .227* | .281** | −.323** | — |
p < .05.
p < .01.
Appendix Table 4b.
Correlations Between Anxiety (STAI) at Each Prenatal Timepoint, Prenatal Psychological Distress Composite, Child Cognitive Function, and Child Negative Emotionality
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. STAI 15 wks | — | .744** | .626** | .637** | .668** | .824** | −.244** | 0.165 |
| 2. STAI 19 wks | .744** | — | .620** | .620** | .598** | .788** | −0.113 | 0.148 |
| 3. STAI 25 wks | .626** | .620** | — | .603** | .679** | .796** | −0.167 | .189* |
| 4. STAI 31 wks | .637** | .620** | .603** | — | .719** | .792** | −.241** | .257** |
| 5. STAI 36 wks | .668** | .598** | .679** | .719** | — | .801** | −.266** | 0.143 |
| 6. Prenatal Psychological Distress | .824** | .788** | .796** | .792** | .801** | — | −.281** | .281** |
| 7. Cognitive Function 2 yrs | −.244** | −0.113 | −0.167 | −.241** | −.266** | −.281** | — | −.323** |
| 8. Negative Emotionality 2 yrs | 0.165 | 0.148 | .189* | .257** | 0.143 | .281** | −.323** | — |
p < .05.
p < .01.
Appendix Table 4c.
Correlations Between Perceived Stress (PSS) at Each Prenatal Timepoint, Prenatal Psychological Distress Composite, Child Cognitive Function, and Child Negative Emotionality
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. PSS 15 wks | — | .747** | .660** | .706** | .630** | .802** | −.289** | .323** |
| 2. PSS 19 wks | .747** | — | .696** | .707** | .625** | .796** | −.220* | .307** |
| 3. PSS 25 wks | .660** | .696** | — | .725** | .690** | .816** | −.266** | .287** |
| 4. PSS 31 wks | .706** | .707** | .725** | — | .739** | .820** | −.260** | .334** |
| 5. PSS 36 wks | .630** | .625** | .690** | .739** | — | .786** | −.236** | 0.134 |
| 6. Prenatal Psychological Distress | .802** | .796** | .816** | .820** | .786** | — | −.281** | .281** |
| 7. Cognitive Function 2 yrs | −.289** | −.220* | −.266** | −.260** | −.236** | −.281** | — | −.323** |
| 8. Negative Emotionality 2 yrs | .323** | .307** | .287** | .334** | 0.134 | .281** | −.323** | — |
p < .05.
p < .01.
Appendix Table 5.
Correlations for Psychological Distress at Each Timepoint
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Distress 15 wks | — | ||||||
| 2. Distress 19 wks | .804** | — | |||||
| 3. Distress 25 wks | .714** | .717** | — | ||||
| 4. Distress 31 wks | .742** | .724** | .718** | — | |||
| 5. Distress 36 wks | .701** | .666** | .722** | .776** | — | ||
| 6. Cognitive Function 2yrs | −.295** | −.173* | −.251** | −.267** | −.263** | — | |
| 7. Negative Emotionality 2yrs | .300** | .248** | .269** | .280** | 0.161 | −.323** | — |
p < .05.
p < .01.
Appendix Table 6.
Percent Missing Data for Each Timepoint
| Timepoint | N | % Missing Data |
||
|---|---|---|---|---|
| Depression (CESD) | Prenatal | 15 weeks | 125 | 11 |
| 19 weeks | 135 | 1 | ||
| 25 weeks | 135 | 1 | ||
| 31 weeks | 135 | 1 | ||
| 36 weeks | 98 | 38* | ||
| Postnatal | 3 months | 128 | 8 | |
| 6 months | 129 | 7 | ||
| 1 year | 128 | 8 | ||
| 2 years | 132 | 4 | ||
| Perceived Stress (PSS) | Prenatal | 15 weeks | 131 | 5 |
| 19 weeks | 134 | 2 | ||
| 25 weeks | 135 | 1 | ||
| 31 weeks | 135 | 1 | ||
| 36 weeks | 129 | 7 | ||
| Postnatal | 3 months | 129 | 7 | |
| 6 months | 131 | 5 | ||
| 1 year | 130 | 6 | ||
| 2 years | 135 | 1 | ||
| Anxiety (STAI) | Prenatal | 15 weeks | 124 | 12 |
| 19 weeks | 135 | 1 | ||
| 25 weeks | 134 | 2 | ||
| 31 weeks | 135 | 1 | ||
| 36 weeks | 127 | 9 | ||
| Postnatal | 3 months | 129 | 7 | |
| 6 months | 130 | 6 | ||
| 1 year | 130 | 6 | ||
| 2 years | 134 | 2 | ||
| Maternal Sensitivity Composite | 6 months | 126 | 10 | |
| 1 year | 125 | 11 |
Due to an administrative/data collection error, some participants were not administered CESD at 36 weeks
Appendix Table 7.
Correlations between Sociodemographic Covariates, Prenatal Distress, Maternal Care, and Child Outcomes
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gestational Age at Birth (GAB) | — | −0.082 | 0.156 | −.192* | −0.051 | 0.052 | −0.107 | −0.065 | −0.080 | 0.036 | 0.009 | 0.063 | −0.130 | −0.156 | .196* | 0.163 | −0.101 |
| 2. Maternal Age | −0.082 | — | .589** | .190* | .197* | .316** | −0.045 | .239** | −.279** | 0.145 | 0.160 | −0.108 | −.295** | −.206* | .287** | .238** | −.298** |
| 3. SES | 0.156 | .589** | — | −.203* | .353** | .556** | −0.075 | .250** | −.323** | −0.033 | 0.107 | −0.027 | −.450** | −.401** | .496** | .500** | −.367** |
| 4. Parity | −.192* | .190* | −.203* | — | 0.123 | −.297** | 0.061 | −.184* | .218* | 0.073 | 0.033 | 0.073 | −0.037 | −0.010 | −.352** | −.279** | 0.098 |
| 5. Cohabitation | −0.051 | .197* | .353** | 0.123 | — | 0.159 | −0.010 | −0.020 | 0.045 | −0.023 | .184* | −0.097 | −.422** | −.301** | 0.145 | .202* | −.197* |
| 6. Index of Intelligence (WAIS) | 0.052 | .316** | .556** | −.297** | 0.159 | — | −.305** | .364** | −.450** | −0.005 | 0.030 | 0.000 | −.317** | −.191* | .461** | .474** | −.352** |
| 7. Obstetric Risk | −0.107 | −0.045 | −0.075 | 0.061 | −0.010 | −.305** | — | −.235** | .221** | −0.040 | −0.060 | 0.011 | −0.001 | −0.094 | −0.167 | −0.165 | 0.105 |
| 8. Non-Hispanic White | −0.065 | .239** | .250** | −.184* | −0.020 | .364** | −.235** | — | −.655** | 0.027 | 0.054 | −0.046 | 0.031 | 0.086 | .253** | .220* | −.355** |
| 9. Latina | −0.080 | −.279** | −.323** | .218* | 0.045 | −.450** | .221** | −.655** | — | −0.106 | −0.060 | 0.105 | −0.069 | −0.093 | −.333** | −.329** | .331** |
| 10. Child Sex (male) | 0.036 | 0.145 | −0.033 | 0.073 | −0.023 | −0.005 | −0.040 | 0.027 | −0.106 | — | 0.110 | −0.075 | 0.069 | 0.100 | −0.015 | −.199* | 0.019 |
| 11. Apgar Score at 5 min | 0.009 | 0.160 | 0.107 | 0.033 | .184* | 0.030 | −0.060 | 0.054 | −0.060 | 0.110 | — | −0.007 | 0.009 | 0.009 | 0.017 | 0.030 | −0.016 |
| 12. Birthweight Percentile by Sex | 0.063 | −0.108 | −0.027 | 0.073 | −0.097 | 0.000 | 0.011 | −0.046 | 0.105 | −0.075 | −0.007 | — | −0.007 | −0.045 | −0.050 | −0.019 | −0.034 |
| 13. Prenatal Distress | −0.130 | −.295** | −.450** | −0.037 | −.422** | −.317** | −0.001 | 0.031 | −0.069 | 0.069 | 0.009 | −0.007 | — | .802** | −.239** | −.281** | .281** |
| 14. Postnatal Distress | −0.156 | −.206* | −.401** | −0.010 | −.301** | −.191* | −0.094 | 0.086 | −0.093 | 0.100 | 0.009 | −0.045 | .802** | — | −.212* | −.257** | .253** |
| 15. Maternal Sensitivity Composite | .196* | .287** | .496** | −.352** | 0.145 | .461** | −0.167 | .253** | −.333** | −0.015 | 0.017 | −0.050 | −.239** | −.212* | — | .468** | −.241** |
| 16. Cognitive Function | 0.163 | .238** | .500** | −.279** | .202* | .474** | −0.165 | .220* | −.329** | −.199* | 0.030 | −0.019 | −.281** | −.257** | .468** | — | −.323** |
| 17. Negative Emotionality | −0.101 | −.298** | −.367** | 0.098 | −.197* | −.352** | 0.105 | −.355** | .331** | 0.019 | −0.016 | −0.034 | .281** | .253** | −.241** | −.323** | — |
p < .05.
p < .01.
Appendix Tables 7-8: Analyses using Individual Distress Subscales (Depressive Symptoms, Anxiety Symptoms, & Perceived Stress)
Appendix Table 7a.
Regression Models Examining the Association Between Prenatal Maternal Depressive Symptoms and Child Cognitive Function, Including Postnatal Maternal Depressive Symptoms
| Model 1: Prenatal Depression | Model 2: Prenatal Depression and Sensitivity Composite |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | 0.084 | 0.336 | 0.025 | −0.020 | 0.335 | −0.006 | 0.003 | 0.333 | 0.001 |
| SES | 2.147† | 1.158 | 0.224 | 1.748 | 1.157 | 0.183 | 1.834 | 1.152 | 0.191 |
| Parity | −2.381 | 1.742 | −0.119 | −1.494 | 1.768 | −0.075 | −1.568 | 1.758 | −0.078 |
| Cohabitation with Child’s Father | 4.975 | 4.785 | 0.090 | 4.349 | 4.726 | 0.079 | 2.723 | 4.816 | 0.050 |
| Index of Intelligence (WAIS) | 0.185† | 0.096 | 0.194 | 0.161† | 0.096 | 0.169 | 0.145 | 0.096 | 0.152 |
| Obstetric Risk | −1.812 | 2.836 | −0.050 | −1.692 | 2.796 | −0.046 | −1.768 | 2.780 | −0.049 |
| Non-Hispanic White | −1.495 | 3.220 | −0.046 | −1.689 | 3.176 | −0.052 | −2.145 | 3.171 | −0.066 |
| Latina | −6.286† | 3.705 | −0.177 | −5.641 | 3.665 | −0.158 | −6.261† | 3.666 | −0.176 |
| Gestational Age at Birth (GAB) | 0.871 | 1.023 | 0.067 | 0.644 | 1.014 | 0.049 | 0.540 | 1.011 | 0.041 |
| Child Sex (male) | −6.490* | 2.485 | −0.196 | −6.253* | 2.452 | −0.189 | −6.360* | 2.439 | −0.192 |
| Postnatal Depression | −0.310 | 0.523 | −0.068 | −0.300 | 0.515 | −0.066 | −0.334 | 0.513 | −0.073 |
| Prenatal Depression | 0.042 | 0.551 | 0.010 | 0.035 | 0.543 | 0.008 | 0.033 | 0.540 | 0.008 |
| Sensitivity Composite | 2.615* | 1.235 | 0.191 | 2.817* | 1.235 | 0.205 | |||
| Prenatal Depression* | 0.494 | 0.320 | 0.114 | ||||||
| Sensitivity Composite | |||||||||
| R2 | 0.378 | 0.401 | 0.413 | ||||||
| F | 6.085*** | 6.125*** | 5.923*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 7b.
Regression Models Examining the Association Between Prenatal Maternal Anxiety Symptoms and Child Cognitive Function, including Postnatal Maternal Anxiety Symptoms
| Model 1: Prenatal Anxiety | Model 2: Prenatal Anxiety and Sensitivity Composite |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | 0.058 | 0.339 | 0.017 | −0.051 | 0.339 | −0.015 | −0.050 | 0.335 | −0.015 |
| SES | 2.205† | 1.146 | 0.230 | 1.835 | 1.144 | 0.192 | 1.879† | 1.130 | 0.196 |
| Parity | −2.432 | 1.732 | −0.121 | −1.532 | 1.762 | −0.076 | −1.410 | 1.741 | −0.070 |
| Cohabitation with Child’s Father | 4.967 | 4.587 | 0.090 | 4.291 | 4.535 | 0.078 | 3.578 | 4.493 | 0.065 |
| Index of Intelligence (WAIS) | 0.179† | 0.096 | 0.188 | 0.155 | 0.095 | 0.163 | 0.150 | 0.094 | 0.158 |
| Obstetric Risk | −1.812 | 2.844 | −0.050 | −1.700 | 2.805 | −0.047 | −1.731 | 2.771 | −0.048 |
| Non-Hispanic White | −1.532 | 3.254 | −0.047 | −1.820 | 3.212 | −0.056 | −2.132 | 3.176 | −0.065 |
| Latina | −6.353† | 3.716 | −0.178 | −5.688 | 3.679 | −0.160 | −5.428 | 3.635 | −0.152 |
| Gestational Age at Birth (GAB) | 0.846 | 1.041 | 0.065 | 0.627 | 1.032 | 0.048 | 0.354 | 1.028 | 0.027 |
| Child Sex (male) | −6.454* | 2.487 | −0.195 | −6.263* | 2.454 | −0.189 | −6.627** | 2.431 | −0.200 |
| Postnatal Anxiety | −0.195 | 0.489 | −0.050 | −0.107 | 0.484 | −0.027 | −0.119 | 0.478 | −0.031 |
| Prenatal Anxiety | −0.018 | 0.467 | −0.005 | −0.084 | 0.462 | −0.024 | −0.097 | 0.456 | −0.027 |
| Sensitivity Composite | 2.601* | 1.241 | 0.190 | 2.704* | 1.226 | 0.197 | |||
| Prenatal Anxiety* Sensitivity Composite | 0.470* | 0.235 | 0.143 | ||||||
| R2 | 0.377 | 0.400 | 0.419 | ||||||
| F | 6.063*** | 6.094*** | 6.086*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p < .05.
p < .01.
p < .001
Appendix Table 7c.
Regression Models Examining the Association Between Prenatal Maternal Perceived Stress and Child Cognitive Function, Including Postnatal Maternal Perceived Stress
| Model 1: Prenatal Perceived Stress |
Model 2: Prenatal Perceived Stress and Sensitivity Composite |
Model 3 : Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | 0.066 | 0.335 | 0.020 | −0.038 | 0.336 | −0.011 | −0.012 | 0.330 | −0.004 |
| SES | 1.929† | 1.140 | 0.201 | 1.632 | 1.138 | 0.170 | 1.713 | 1.119 | 0.179 |
| Parity | −2.561 | 1.720 | −0.128 | −1.736 | 1.755 | −0.087 | −1.704 | 1.724 | −0.085 |
| Cohabitation with Child’s Father | 4.476 | 4.501 | 0.081 | 3.932 | 4.461 | 0.072 | 2.283 | 4.441 | 0.042 |
| Index of Intelligence (WAIS) | 0.144 | 0.099 | 0.151 | 0.125 | 0.098 | 0.132 | 0.126 | 0.097 | 0.133 |
| Obstetric Risk | −2.455 | 2.843 | −0.067 | −2.209 | 2.815 | −0.061 | −2.248 | 2.766 | −0.062 |
| Non-Hispanic White | −2.132 | 3.195 | −0.065 | −2.277 | 3.161 | −0.070 | −2.956 | 3.120 | −0.091 |
| Latina | −7.454† | 3.860 | −0.209 | −6.802† | 3.833 | −0.191 | −7.292† | 3.772 | −0.205 |
| Gestational Age at Birth (GAB) | 0.910 | 1.007 | 0.069 | 0.696 | 1.002 | 0.053 | 0.660 | 0.984 | 0.050 |
| Child Sex (male) | −6.443* | 2.452 | −0.194 | −6.257* | 2.427 | −0.189 | −6.943** | 2.404 | −0.209 |
| Postnatal Stress | −0.356 | 0.389 | −0.114 | −0.231 | 0.390 | −0.074 | −0.151 | 0.385 | −0.049 |
| Prenatal Stress | −0.094 | 0.395 | −0.033 | −0.155 | 0.392 | −0.054 | −0.152 | 0.386 | −0.053 |
| Sensitivity Composite | 2.393† | 1.248 | 0.174 | 2.496* | 1.227 | 0.182 | |||
| Prenatal Stress*Sensitivity Composite | 0.444* | 0.193 | 0.166 | ||||||
| R2 | 0.389 | 0.408 | 0.433 | ||||||
| F | 6.376*** | 6.299*** | 6.436*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 8a.
Regression Models Examining the Association Between Prenatal Maternal Depressive Symptoms and Child Negative Emotionality, Including Postnatal Maternal Depressive Symptoms
| Model 1: Prenatal Depression | Model 2: Prenatal Depression and Sensitivity Composite |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | B | B | SE B | β | B | SE B | B |
| Maternal Age | −0.016 | 0.011 | −0.134 | −0.016 | 0.011 | −0.134 | −0.018 | 0.011 | −0.155 |
| SES | −0.009 | 0.041 | −0.027 | −0.007 | 0.042 | −0.020 | −0.008 | 0.042 | −0.023 |
| Cohabitation with Child’s Father | −0.160 | 0.194 | −0.074 | −0.162 | 0.195 | −0.074 | −0.104 | 0.195 | −0.048 |
| Index of Intelligence (WAIS) | −0.005 | 0.003 | −0.136 | −0.004 | 0.004 | −0.132 | −0.004 | 0.003 | −0.112 |
| Non-Hispanic White | −0.292* | 0.127 | −0.253 | −0.293* | 0.128 | −0.253 | −0.277* | 0.126 | −0.239 |
| Latina | 0.128 | 0.149 | 0.099 | 0.124 | 0.151 | 0.096 | 0.169 | 0.150 | 0.131 |
| Postnatal Depression | 0.028 | 0.019 | 0.174 | 0.028 | 0.019 | 0.175 | 0.030 | 0.019 | 0.184 |
| Prenatal Depression | 0.015 | 0.021 | 0.091 | 0.014 | 0.021 | 0.088 | 0.015 | 0.021 | 0.090 |
| Sensitivity Composite | −0.011 | 0.047 | –0.023 | −0.013 | 0.047 | −0.025 | |||
| Prenatal Depression*Sensitivity Composite | −0.029* | 0.015 | −0.165 | ||||||
| R2 | 0.298 | 0.298 | 0.324 | ||||||
| F | 5.834*** | 5.148*** | 5.168*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 8b.
Regression Models Examining the Association Between Prenatal Maternal Anxiety Symptoms and Child Nesative Emotionality, Including Postnatal Maternal Anxiety Symptoms
| Model 1: Prenatal Anxiety | Model 2: Prenatal Anxiety and Sensitivity Composite |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | B | B | SE B | β | B | SE B | β |
| Maternal Age | −0.013 | 0.011 | −0.114 | −0.013 | 0.011 | −0.114 | −0.015 | 0.011 | −0.126 |
| SES | −0.023 | 0.041 | −0.067 | −0.021 | 0.042 | −0.060 | −0.021 | 0.042 | −0.061 |
| Cohabitation with Child–s Father | −0.258 | 0.192 | −0.119 | −0.259 | 0.193 | −0.119 | −0.250 | 0.191 | −0.115 |
| Index of Intelligence (WAIS) | −0.005 | 0.003 | −0.145 | −0.005 | 0.004 | −0.140 | −0.005 | 0.004 | −0.138 |
| Non-Hispanic White | −0.272* | 0.129 | −0.235 | −0.273* | 0.129 | −0.236 | −0.260* | 0.129 | −0.225 |
| Latina | 0.128 | 0.152 | 0.099 | 0.123 | 0.154 | 0.095 | 0.123 | 0.152 | 0.095 |
| Postnatal Anxiety | 0.028 | 0.018 | 0.201 | 0.028 | 0.018 | 0.200 | 0.031† | 0.018 | 0.217 |
| Prenatal Anxiety | −0.006 | 0.017 | –0.049 | −0.007 | 0.017 | −0.052 | −0.009 | 0.017 | −0.067 |
| Sensitivity Composite | −0.013 | 0.048 | −0.026 | −0.009 | 0.048 | −0.017 | |||
| Prenatal Anxiety*Sensitivity Composite | −0.016 | 0.010 | −0.135 | ||||||
| R2 | 0.276 | 0.276 | 0.294 | ||||||
| F | 5.240*** | 4.626*** | 4 499*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 8c.
Regression Models Examining the Association Between Prenatal Maternal Perceived Stress and Child Negative Emotionality, Including Postnatal Maternal Perceived Stress
| Model 1: Prenatal Perceived Stress |
Model 2: Prenatal Perceived Stress and Sensitivity Composite |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | −0.013 | 0.011 | −0.108 | −0.013 | 0.011 | −0.109 | −0.014 | 0.011 | −0.117 |
| SES | −0.017 | 0.040 | −0.050 | −0.017 | 0.041 | −0.049 | −0.020 | 0.041 | −0.056 |
| Cohabitation with Child’s Father | −0.184 | 0.189 | −0.085 | −0.184 | 0.190 | −0.085 | −0.136 | 0.191 | −0.062 |
| Index of Intelligence (WAIS) | −0.002 | 0.004 | −0.072 | −0.002 | 0.004 | −0.072 | −0.003 | 0.004 | −0.075 |
| Non-Hispanic White | −0.222† | 0.126 | −0.192 | −0.223† | 0.127 | −0.192 | −0.196 | 0.127 | −0.170 |
| Latina | 0.216 | 0.157 | 0.167 | 0.215 | 0.159 | 0.166 | 0.247 | 0.159 | 0.191 |
| Postnatal Stress | 0.013 | 0.016 | 0.112 | 0.013 | 0.016 | 0.112 | 0.012 | 0.016 | 0.102 |
| Prenatal Stress | 0.017 | 0.016 | 0.155 | 0.017 | 0.016 | 0.155 | 0.016 | 0.016 | 0.149 |
| Sensitivity Composite | −0.002 | 0.048 | −0.005 | 0.004 | 0.047 | 0.008 | |||
| Prenatal Stress*Sensitivity Composite | −0.014 | 0.009 | −0.136 | ||||||
| R2 | 0.297 | 0.297 | 0.314 | ||||||
| F | 5.810*** | 5.118*** | 4.946*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Tables 9-10: Analyses of Maternal Care at 6 and 12 months
Appendix Table 9a.
Regression Models Examining the Association Between Prenatal Maternal Psychological Distress and Child Cognitive Function, with Maternal Care at 6 months
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Sensitivity Composite at 6 months |
Model 3 : Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | 0.050 | 0.349 | 0.015 | 0.014 | 0.352 | 0.004 | 0.051 | 0.346 | 0.015 |
| SES | 2.102† | 1.160 | 0.221 | 1.973† | 1.172 | 0.207 | 2.024† | 1.150 | 0.212 |
| Parity | −2.224 | 1.807 | −0.110 | −2.208 | 1.810 | −0.110 | −2.589 | 1.784 | −0.128 |
| Cohabitation | 5.439 | 4.792 | 0.098 | 5.687 | 4.807 | 0.102 | 4.796 | 4.735 | 0.086 |
| Index of Intelligence (WAIS) | 0.175† | 0.099 | 0.186 | 0.158 | 0.101 | 0.167 | 0.138 | 0.100 | 0.146 |
| Obstetric Risk | −2.557 | 2.995 | −0.069 | −2.561 | 2.999 | −0.069 | −4.123 | 3.023 | 0.111 |
| Non-Hispanic White | −2.660 | 3.363 | −0.081 | −2.727 | 3.368 | −0.083 | −3.746 | 3.336 | −0.114 |
| Latina | −6.908† | 3.925 | −0.191 | −6.810† | 3.932 | −0.188 | −7.987* | 3.894 | −0.221 |
| Gestational Age at Birth (GAB) | 0.294 | 1.080 | 0.022 | 0.092 | 1.108 | 0.007 | −0.229 | 1.097 | −0.017 |
| Child Sex (male) | −5.719* | 2.586 | −0.171 | −5.376* | 2.621 | −0.161 | −6.271* | 2.602 | −0.188 |
| Prenatal Distress | −1.798 | 1.686 | −0.102 | −1.993 | 1.704 | −0.113 | −2.467 | 1.686 | −0.140 |
| Sensitivity Composite 6mo | 1.135 | 1.346 | 0.074 | 1.579 | 1.336 | 0.102 | |||
| Prenatal Distress* Sensitivity Composite 6mo | 3.886* | 1.710 | 0.181 | ||||||
| R2 | 0.383 | 0.387 | 0.414 | ||||||
| F | 6.252*** | 5.775*** | 5 930*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 9b.
Regression Models Examining the Association Between Prenatal Maternal Psychological Distress and Child Cognitive Function, with Maternal Care at 12 months
| Model 1: Prenatal Distress | Model 2: Prenatal Distress and Sensitivity Composite at 12 months |
Model 3 : Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | 0.159 | 0.385 | 0.047 | 0.152 | 0.377 | 0.045 | 0.249 | 0.375 | 0.074 |
| SES | 1.913 | 1.221 | 0.198 | 1.419 | 1.216 | 0.147 | 1.411 | 1.198 | 0.146 |
| Parity | −2.659 | 1.909 | −0.130 | −1.414 | 1.945 | −0.069 | −1.576 | 1.920 | −0.077 |
| Cohabitation | 4.206 | 4.870 | 0.073 | 2.621 | 4.822 | 0.045 | 0.560 | 4.860 | 0.010 |
| Index of Intelligence (WAIS) | 0.193† | 0.103 | 0.195 | 0.185† | 0.101 | 0.186 | 0.171† | 0.100 | 0.172 |
| Obstetric Risk | 0.021 | 3.157 | 0.001 | 0.520 | 3.102 | 0.014 | 1.108 | 3.071 | 0.029 |
| Non-Hispanic White | −2.254 | 3.396 | −0.068 | −2.449 | 3.330 | −0.074 | −2.528 | 3.283 | −0.076 |
| Latina | −7.350† | 3.901 | −0.204 | −6.623† | 3.837 | −0.184 | −6.660† | 3.783 | −0.185 |
| Gestational Age at Birth (GAB) | 0.703 | 1.140 | 0.049 | 0.671 | 1.117 | 0.047 | 0.531 | 1.104 | 0.037 |
| Child Sex (male) | −7.679** | 2.637 | −0.227 | −7.841** | 2.586 | −0.232 | −7.982** | 2.550 | −0.236 |
| Prenatal Distress | −1.551 | 1.757 | −0.082 | −0.913 | 1.744 | −0.048 | −0.738 | 1.721 | −0.039 |
| Sensitivity Composite 12mo | 2.141 | 0.915* | 0.215 | 1.950* | 0.907 | 0.196 | |||
| Prenatal Distress* Sensitivity Composite 12mo | 1.844* | 0.905 | 0.158 | ||||||
| R2 | 0.376 | 0.406 | 0.428 | ||||||
| F | 6.027*** | 6.207*** | 6.215*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 10a.
Regression Models Examining the Association Between Prenatal Maternal Psychological Distress and Child Negative Emotionality, with Maternal Care at 6 months
| Model 1: Prenatal Distress | Model 2: Prenatal Distress and Sensitivity Composite at 6 months |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | −0.019 | 0.012 | −0.156 | −0.018 | 0.012 | −0.150 | −0.017 | 0.012 | −0.142 |
| SES | −0.008 | 0.041 | −0.023 | −0.001 | 0.042 | −0.002 | −0.004 | 0.041 | −0.010 |
| Cohabitation | −0.225 | 0.202 | −0.099 | −0.259 | 0.205 | −0.115 | −0.270 | 0.202 | −0.119 |
| Index of Intelligence (WAIS) | −0.004 | 0.003 | −0.117 | −0.004 | 0.004 | −0.103 | −0.004 | 0.003 | −0.107 |
| Non-Hispanic White | −0.234† | 0.130 | −0.199 | −0.248† | 0.131 | −0.211 | −0.239† | 0.130 | −0.203 |
| Latina | 0.140 | 0.157 | 0.105 | 0.112 | 0.160 | 0.084 | 0.132 | 0.158 | 0.099 |
| Prenatal Distress | 0.159* | 0.062 | 0.249 | 0.158* | 0.062 | 0.247 | 0.156* | 0.061 | 0.244 |
| Sensitivity Composite 6mo | −0.052 | 0.053 | −0.090 | −0.076 | 0.054 | −0.132 | |||
| Prenatal Distress* Sensitivity Composite 6mo | −0.131† | 0.069 | −0.162 | ||||||
| R2 | 0.308 | 0.314 | 0.338 | ||||||
| F | 6.536*** | 5.838*** | 5.726*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 10b.
Regression Models Examining the Association Between Prenatal Maternal Psychological Distress and Child Negative Emotionality, with Maternal Care at 12 months
| Model 1: Prenatal Distress | Model 2: Prenatal Distress and Sensitivity Composite at 12 mo |
Model 3 : Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | −0.009 | 0.012 | −0.073 | −0.007 | 0.012 | −0.063 | −0.012 | 0.012 | −0.099 |
| SES | −0.026 | 0.042 | −0.074 | −0.032 | 0.044 | −0.089 | −0.030 | 0.043 | −0.085 |
| Cohabitation | −0.180 | 0.198 | −0.080 | −0.189 | 0.199 | −0.084 | −0.120 | 0.199 | −0.053 |
| Index of Intelligence (WAIS) | −0.004 | 0.004 | −0.103 | −0.004 | 0.004 | −0.109 | −0.003 | 0.004 | −0.093 |
| Non-Hispanic White | −0.265* | 0.131 | −0.226 | −0.268* | 0.132 | −0.228 | −0.25† | 0.130 | −0.214 |
| Latina | 0.203 | 0.156 | 0.155 | 0.208 | 0.157 | 0.159 | 0.242 | 0.156 | 0.185 |
| Prenatal Distress | 0.147* | 0.065 | 0.217 | 0.153* | 0.066 | 0.226 | 0.159* | 0.066 | 0.234 |
| Sensitivity Composite 12mo | 0.018 | 0.035 | 0.049 | 0.034 | 0.035 | 0.093 | |||
| Prenatal Distress* Sensitivity Composite 12mo | −0.076* | 0.038 | −0.176 | ||||||
| R2 | 0.305 | 0.307 | 0.334 | ||||||
| F | 6.399*** | 5.593*** | 5.564*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Tables 11-12: Maternal Sensitivity Subscale Analyses (Sensitivity to Nondistress, Intrusiveness Reverse-Scored, Positive Regard)
Appendix Table 11a.
Regression Model Examining Maternal Sensitivity to Nondistress as a Moderator of the Association Between Prenatal Distress and Child Cognitive Function
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Sensitivity to Nondistress |
Model 3 : Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | B | B | SE B | β | B | SE B | β |
| Maternal Age | 0.038 | 0.334 | 0.011 | −0.104 | 0.330 | −0.031 | −0.093 | 0.326 | −0.028 |
| SES | 2.159† | 1.139 | 0.225 | 1.627 | 1.129 | 0.170 | 1.635 | 1.113 | 0.171 |
| Parity | −2.503 | 1.728 | −0.125 | −1.459 | 1.730 | −0.073 | −1.481 | 1.706 | −0.074 |
| Cohabitation with Child’s Father | 4.281 | 4.612 | 0.078 | 3.678 | 4.505 | 0.067 | 1.888 | 4.522 | 0.034 |
| Index of Intelligence (WAIS) | 0.166† | 0.097 | 0.174 | 0.139 | 0.095 | 0.146 | 0.129 | 0.093 | 0.135 |
| Obstetric Risk | −1.925 | 2.831 | −0.053 | −1.435 | 2.768 | −0.039 | −1.242 | 2.731 | −0.034 |
| Non-Hispanic White | −1.764 | 3.191 | −0.054 | −1.435 | 3.116 | −0.044 | −1.921 | 3.081 | −0.059 |
| Latina | −6.728† | 3.747 | −0.189 | −5.478 | 3.686 | −0.154 | −5.920 | 3.640 | −0.166 |
| Gestational Age at Birth (GAB) | 0.863 | 1.017 | 0.066 | 0.632 | 0.996 | 0.048 | 0.464 | 0.985 | 0.035 |
| Child Sex (male) | −6.473* | 2.464 | −0.195 | −5.463* | 2.434 | −0.165 | −5.965* | 2.411 | −0.180 |
| Prenatal Distress | −1.376 | 1.616 | −0.079 | −1.324 | 1.577 | −0.076 | −1.403 | 1.555 | −0.080 |
| Sensitivity to Nondistress | 7.622** | 2.857 | 0.241 | 8.227** | 2.831 | 0.260 | |||
| Prenatal Distress* Sensitivity to Nondistress | 5.485* | 2.598 | 0.151 | ||||||
| R2 | 0.379 | 0.414 | 0.435 | ||||||
| F | 6.715*** | 7 | .060*** | 7.048*** | |||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 11b.
Regression Model Examining Maternal Intrusiveness Reverse-Scored as a Moderator of the Association Between Prenatal Distress and Child Cognitive Function
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Intrusiveness Reverse- Scored |
Model 3 : Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | B | B | SE B | β | B | SE B | β |
| Maternal Age | 0.038 | 0.334 | 0.011 | 0.008 | 0.333 | 0.002 | 0.002 | 0.335 | 0.001 |
| SES | 2.159† | 1.139 | 0.225 | 1.916† | 1.145 | 0.200 | 1.956† | 1.158 | 0.204 |
| Parity | −2.503 | 1.728 | −0.125 | −1.763 | 1.788 | −0.088 | −1.772 | 1.795 | −0.088 |
| Cohabitation with Child’s Father | 4.281 | 4.612 | 0.078 | 2.720 | 4.705 | 0.049 | 2.599 | 4.743 | 0.047 |
| Index of Intelligence (WAIS) | 0.166† | 0.097 | 0.174 | 0.148 | 0.097 | 0.156 | 0.149 | 0.097 | 0.156 |
| Obstetric Risk | −1.925 | 2.831 | −0.053 | −2.226 | 2.824 | −0.061 | −2.210 | 2.835 | −0.061 |
| Non-Hispanic White | −1.764 | 3.191 | −0.054 | −2.295 | 3.195 | −0.070 | −2.277 | 3.208 | −0.070 |
| Latina | −6.728† | 3.747 | −0.189 | −7.197† | 3.741 | −0.202 | −7.238† | 3.759 | −0.203 |
| Gestational Age at Birth (GAB) | 0.863 | 1.017 | 0.066 | 0.776 | 1.014 | 0.059 | 0.800 | 1.021 | 0.061 |
| Child Sex (male) | −6.473* | 2.464 | −0.195 | −6.717** | 2.457 | −0.203 | −6.808** | 2.488 | −0.205 |
| Prenatal Distress | −1.376 | 1.616 | −0.079 | −1.624 | 1.616 | −0.093 | −1.675 | 1.633 | −0.096 |
| Intrusiveness Reverse-Scored | 4.403 | 2.937 | 0.128 | 4.174 | 3.059 | 0.121 | |||
| Prenatal Distress* Intrusiveness Reverse-Scored | 0.977 | 3.497 | 0.022 | ||||||
| R2 | 0.379 | 0.390 | 0.391 | ||||||
| F | 6.715*** | 6.406*** | 5.874*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 11c.
Regression Model Examining Maternal Positive Regard as a Moderator of the Association Between Prenatal Distress and Child Cognitive Function
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Positive Regard |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | B | B | SE B | β | B | SE B | β |
| Maternal Age | 0.038 | 0.334 | 0.011 | 0.011 | 0.336 | 0.003 | 0.092 | 0.334 | 0.028 |
| SES | 2.159† | 1.139 | 0.225 | 2.110† | 1.143 | 0.220 | 2.099† | 1.128 | 0.219 |
| Parity | −2.503 | 1.728 | −0.125 | −2.425 | 1.734 | −0.121 | −2.627 | 1.713 | −0.131 |
| Cohabitation with Child’s Father | 4.281 | 4.612 | 0.078 | 4.728 | 4.659 | 0.086 | 3.989 | 4.609 | 0.073 |
| Index of Intelligence (WAIS) | 0.166† | 0.097 | 0.174 | 0.164† | 0.097 | 0.172 | 0.152 | 0.096 | 0.160 |
| Obstetric Risk | −1.925 | 2.831 | −0.053 | −1.816 | 2.840 | −0.050 | −2.281 | 2.810 | −0.063 |
| Non-Hispanic White | −1.764 | 3.191 | −0.054 | −1.753 | 3.197 | −0.054 | −2.491 | 3.174 | −0.076 |
| Latina | −6.728† | 3.747 | −0.189 | −6.319† | 3.794 | −0.177 | −6.571† | 3.745 | −0.185 |
| Gestational Age at Birth (GAB) | 0.863 | 1.017 | 0.066 | 0.792 | 1.023 | 0.060 | 0.503 | 1.019 | 0.038 |
| Child Sex (male) | −6.473* | 2.464 | −0.195 | −6.452* | 2.469 | −0.195 | −6.201* | 2.438 | −0.187 |
| Prenatal Distress | −1.376 | 1.616 | −0.079 | −1.219 | 1.633 | −0.070 | −1.059 | 1.613 | −0.060 |
| Positive Regard | 2.006 | 2.691 | 0.059 | 1.486 | 2.666 | 0.044 | |||
| Prenatal Distress* Positive Regard | 5.768* | 2.768 | 0.154 | ||||||
| R2 | 0.379 | 0.382 | 0.404 | ||||||
| F | 6.715*** | 6.179*** | 6.197*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 12a.
Regression Model Examining Maternal Sensitivity to Nondistress as a Moderator of the Association Between Prenatal Distress and Child Negative Emotionality
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Sensitivity to Nondistress |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | −0.012 | 0.011 | −0.105 | −0.012 | 0.011 | −0.105 | −0.014 | 0.011 | −0.120 |
| SES | −0.023 | 0.041 | −0.065 | −0.022 | 0.042 | −0.062 | −0.019 | 0.042 | −0.054 |
| Cohabitation with Child’s Father | −0.172 | 0.193 | −0.079 | −0.174 | 0.194 | −0.080 | −0.141 | 0.193 | −0.065 |
| Index of Intelligence (WAIS) | −0.003 | 0.003 | −0.100 | −0.003 | 0.004 | −0.097 | −0.003 | 0.004 | −0.090 |
| Non-Hispanic White | −0.258* | 0.127 | −0.223 | −0.259* | 0.128 | −0.224 | −0.235† | 0.127 | −0.203 |
| Latina | 0.152 | 0.152 | 0.117 | 0.149 | 0.154 | 0.115 | 0.187 | 0.154 | 0.145 |
| Prenatal Distress | 0.125* | 0.060 | 0.201 | 0.124* | 0.061 | 0.200 | 0.127* | 0.060 | 0.205 |
| Sensitivity to Nondistress | −0.013 | 0.109 | −0.011 | −0.026 | 0.108 | −0.022 | |||
| Prenatal Distress* Sensitivity to Nondistress | −0.214† | 0.116 | −0.151 | ||||||
| R2 | 0.280 | 0.280 | 0.302 | ||||||
| F | 6.173*** | 5.355*** | 5.246*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 12b.
Regression Model Examining Maternal Intrusiveness Reverse-Scored as a Moderator of the Association Between Prenatal Distress and Child Negative Emotionality
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Intrusiveness Reverse- Scored |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | −0.012 | 0.011 | −0.105 | −0.013 | 0.011 | −0.109 | −0.010 | 0.011 | −0.088 |
| SES | −0.023 | 0.041 | −0.065 | −0.017 | 0.042 | −0.050 | −0.030 | 0.042 | −0.087 |
| Cohabitation with Child’s Father | −0.172 | 0.193 | −0.079 | −0.154 | 0.196 | −0.071 | −0.076 | 0.198 | −0.035 |
| Index of Intelligence (WAIS) | −0.003 | 0.003 | −0.100 | −0.003 | 0.004 | −0.088 | −0.003 | 0.004 | −0.085 |
| Non-Hispanic White | −0.258* | 0.127 | −0.223 | −0.255* | 0.128 | −0.221 | −0.267* | 0.126 | −0.231 |
| Latina | 0.152 | 0.152 | 0.117 | 0.155 | 0.153 | 0.120 | 0.153 | 0.151 | 0.118 |
| Prenatal Distress | 0.125* | 0.060 | 0.201 | 0.126* | 0.060 | 0.203 | 0.142* | 0.060 | 0.228 |
| Intrusiveness Reverse-Scored | −0.073 | 0.128 | −0.052 | 0.020 | 0.136 | 0.014 | |||
| Prenatal Distress*Intrusiveness Reverse-Scored | −0.262† | 0.142 | −0.166 | ||||||
| R2 | 0.280 | 0.282 | 0.304 | ||||||
| F | 6.173*** | 5.409*** | 5.295*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 12c.
Regression Model Examining Maternal Positive Regard as a Moderator of the Association Between Prenatal Distress and Child Negative Emotionality
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Positive Regard |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | −0.012 | 0.011 | −0.105 | −0.012 | 0.011 | −0.105 | −0.014 | 0.012 | −0.124 |
| SES | −0.023 | 0.041 | −0.065 | −0.023 | 0.041 | −0.067 | −0.022 | 0.041 | −0.062 |
| Cohabitation with Child’s Father | −0.172 | 0.193 | −0.079 | −0.166 | 0.196 | −0.076 | −0.174 | 0.196 | −0.080 |
| Index of Intelligence (WAIS) | −0.003 | 0.003 | −0.100 | −0.003 | 0.003 | −0.100 | −0.003 | 0.003 | −0.099 |
| Non-Hispanic White | −0.258* | 0.127 | −0.223 | −0.258* | 0.128 | −0.223 | −0.238† | 0.129 | −0.206 |
| Latina | 0.152 | 0.152 | 0.117 | 0.158 | 0.155 | 0.122 | 0.171 | 0.156 | 0.132 |
| Prenatal Distress | 0.125* | 0.060 | 0.201 | 0.127* | 0.062 | 0.205 | 0.119† | 0.062 | 0.192 |
| Positive Regard | 0.022 | 0.108 | 0.018 | 0.022 | 0.108 | 0.018 | |||
| Prenatal Distress* Positive Regard | −0.127 | 0.128 | −0.083 | ||||||
| R2 | 0.280 | 0.280 | 0.287 | ||||||
| F | 6.173*** | 5.360*** | 4.873*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 13: Main Analyses Including Postnatal Maternal Distress
Appendix Table 13a.
Regression Models Examining the Association Between Prenatal Maternal Psychological Distress and Child Cognitive Function, Including Postnatal Maternal Distress
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Sensitivity Composite |
Model 3 : Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | B |
| Maternal Age | 0.072 | 0.338 | 0.022 | −0.035 | 0.338 | −0.011 | −0.018 | 0.332 | −0.005 |
| SES | 2.036† | 1.154 | 0.213 | 1.692 | 1.151 | 0.177 | 1.769 | 1.133 | 0.185 |
| Parity | −2.484 | 1.731 | −0.124 | −1.619 | 1.760 | −0.081 | −1.618 | 1.731 | −0.081 |
| Cohabitation | 4.675 | 4.652 | 0.085 | 4.029 | 4.603 | 0.073 | 2.193 | 4.601 | 0.040 |
| Index of Intelligence (WAIS) | 0.172† | 0.097 | 0.180 | 0.148 | 0.096 | 0.156 | 0.135 | 0.095 | 0.141 |
| Obstetric risk | −2.023 | 2.840 | −0.056 | −1.869 | 2.804 | −0.051 | −1.986 | 2.758 | −0.054 |
| Non-Hispanic White | −1.551 | 3.211 | −0.048 | −1.794 | 3.171 | −0.055 | −2.468 | 3.134 | −0.076 |
| Latina | −6.690† | 3.755 | −0.188 | −6.038 | 3.720 | −0.170 | −6.532† | 3.666 | −0.183 |
| Gestational Age at Birth (GAB) | 0.820 | 1.021 | 0.063 | 0.616 | 1.012 | 0.047 | 0.437 | 0.999 | 0.033 |
| Child sex (male) | −6.401* | 2.471 | −0.193 | −6.199* | 2.441 | −0.187 | −6.660** | 2.410 | −0.201 |
| Postnatal Distress | −1.701 | 2.334 | −0.095 | −1.224 | 2.316 | −0.068 | −1.080 | 2.279 | −0.060 |
| Prenatal Distress | 0.014 | 2.503 | 0.001 | −0.303 | 2.475 | −0.017 | −0.543 | 2.437 | −0.031 |
| Sensitivity Composite | 2.533* | 1.239 | 0.185 | 2.700* | 1.221 | 0.197 | |||
| Prenatal Distress* Sensitivity Composite | 2.796* | 1.249 | 0.161 | ||||||
| R2 | 0.382 | 0.403 | 0.427 | ||||||
| F | 6.176*** | 6.173*** | 6.283*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Appendix Table 13b.
Regression Models Examining the Association Between Prenatal Maternal Psychological Distress and Child Negative Emotionality, Including Postnatal Maternal Distress
| Model 1: Prenatal Distress |
Model 2: Prenatal Distress and Sensitivity Composite |
Model 3: Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| Maternal Age | −0.014 | 0.011 | −0.121 | −0.014 | 0.011 | −0.122 | −0.016 | 0.011 | −0.138 |
| SES | −0.013 | 0.041 | −0.037 | −0.012 | 0.042 | −0.033 | −0.013 | 0.042 | −0.037 |
| Cohabitation with Child’s Father | −0.195 | 0.192 | −0.090 | −0.196 | 0.193 | −0.090 | −0.145 | 0.192 | −0.067 |
| Index of Intelligence (WAIS) | −0.004 | 0.003 | −0.119 | −0.004 | 0.004 | −0.117 | −0.004 | 0.004 | −0.108 |
| Non-Hispanic White | −0.265* | 0.127 | −0.230 | −0.266* | 0.127 | −0.230 | −0.240† | 0.126 | −0.208 |
| Latina | 0.158 | 0.152 | 0.122 | 0.156 | 0.153 | 0.120 | 0.190 | 0.152 | 0.146 |
| Postnatal Distress | 0.122 | 0.084 | 0.194 | 0.122 | 0.084 | 0.194 | 0.123 | 0.083 | 0.196 |
| Prenatal Distress | 0.030 | 0.089 | 0.048 | 0.029 | 0.089 | 0.047 | 0.031 | 0.088 | 0.050 |
| Sensitivity Composite | −0.007 | 0.047 | −0.013 | −0.002 | 0.047 | −0.003 | |||
| Prenatal Distress* Sensitivity Composite | −0.105* | 0.052 | −0.165 | ||||||
| R2 | 0.294 | 0.294 | 0.320 | ||||||
| F | 5.718*** | 5.039*** | 5.076*** | ||||||
B = unstandardized coefficient. β= standardized coefficient.
p < .10
p< .05.
p < .01.
p< .001
Footnotes
Conflicts of Interest: None
References
- Adamson B, Letourneau N, & Lebel C (2018). Prenatal maternal anxiety and children’s brain structure and function: A systematic review of neuroimaging studies. Journal of Affective Disorders, 241, 117–126. 10.1016/j.jad.2018.08.029 [DOI] [PubMed] [Google Scholar]
- Ainsworth M, Blehar M, Waters E, & Wall S (1978). Patterns of attachment: Assessed in the strange situation and at home. [Google Scholar]
- Ainsworth MS (1979). Infant–mother attachment. American Psychologist, 34(10), 932–937. [DOI] [PubMed] [Google Scholar]
- Ali E, Letourneau N, Benzies K, Ntanda H, Dewey D, Campbell T, & Giesbrecht G (2020). Maternal prenatal anxiety and children’s externalizing and internalizing behavioral problems: The moderating roles of maternal-child attachment security and child sex. Canadian Journal of Nursing Research, 52(2), 88–99. 10.1177/0844562119894184 [DOI] [PubMed] [Google Scholar]
- Atzl VM, Grande LA, Davis EP, & Narayan AJ (2019). Perinatal promotive and protective factors for women with histories of childhood abuse and neglect. Child Abuse & Neglect, 91, 63–77. 10.1016/j.chiabu.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D (1998). In utero programming of chronic disease. Clinical Science, 95(2), 115–128. [PubMed] [Google Scholar]
- Bayley N (1993). Bayley Scales of Infant Development. Psychological Corporation. [Google Scholar]
- Bergman K, Sarkar P, Glover V, & O’Connor T. g. (2008). Quality of child–parent attachment moderates the impact of antenatal stress on child fearfulness. Journal of Child Psychology and Psychiatry, 49(10), 1089–1098. 10.1111/j.1469-7610.2008.01987.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, & Dozier M (2010). Mothers’ concentrations of oxytocin following close, physical interactions with biological and nonbiological children. Developmental Psychobiology, 52(1), 100–107. 10.1002/dev.20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, & Davis EP (2011). Prenatal maternal anxiety and early childhood temperament. Stress, 14(6), 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoch Y, Biala YN, Linial M, & Weinstock M (2007). Anxiety induced by prenatal stress is associated with suppression of hippocampal genes involved in synaptic function. Journal of Neurochemistry, 101(4), 1018–1030. [DOI] [PubMed] [Google Scholar]
- Bush NR, Jones-Mason K, Coccia M, Caron Z, Alkon A, Thomas M, Coleman-Phox K, Wadhwa PD, Laraia BA, Adler NE, & Epel ES (2017). Effects of pre- and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Development and Psychopathology, 29(5), 1553–1571. 10.1017/S0954579417001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, & Sandman CA (2010). High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology, 35(1), 141–153. 10.1016/j.psyneuen.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, & King S (2010). Prenatal stress and brain development. Brain Research Reviews, 65(1), 56–79. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. [PubMed] [Google Scholar]
- Committee on Obstetric Practice, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine. (2017). Committee Opinion No 700: Methods for estimating the due date. Obstetrics and Gynecology, 129(5), 150–154. [Google Scholar]
- Curran MM, Sandman CA, Davis EP, Glynn LM, & Baram TZ (2017). Abnormal dendritic maturation of developing cortical neurons exposed to corticotropin releasing hormone (CRH): Insights into effects of prenatal adversity? PloS One, 12(6), e0180311. 10.1371/journal.pone.0180311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna-Hernandez K, & Rivera KD (2014). Understanding and alleviating cultural stressors and health disparities in the perinatal outcomes of Mexican-American women. ZERO TO THREE, 34(4), 37–45. [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel CJ, Chicz-DeMet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child Adolescent Psychiatry, 46(6), 737–746. [DOI] [PubMed] [Google Scholar]
- Davis EP, Hankin BL, Glynn LM, Head K, Kim DJ, & Sandman CA (2019). Prenatal maternal stress, child cortical thickness, and adolescent depressive symptoms. Child Development. 10.1111/cdev.13252 [DOI] [PubMed] [Google Scholar]
- Davis EP, Hankin BL, Swales DA, & Hoffman MC (2018). An experimental test of the fetal programming hypothesis: Can we reduce child ontogenetic vulnerability to psychopathology by decreasing maternal depression? Development and Psychopathology, 30(3), 787–806. 10.1017/S0954579418000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, & Narayan AJ (in press). Pregnancy as a period of risk, adaptation, and resilience for mothers and infants. Developmental Psychopathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, & Sandman CA (2010). The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development, 81(1), 131–148. 10.1111/j.1467-8624.2009.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, & Sandman CA (2012). Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology, 37(8), 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Stout SA, Molet J, Vegetabile B, Glynn LM, Sandman CA, Heins K, Stern H, & Baram TZ (2017). Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proceedings of the National Academy of Sciences, 114(39), 10390–10395. 10.1073/pnas.1703444114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans CL (2018). Maternal sensitivity, its relationship with child outcomes, and interventions that address it: A systematic literature review. Early Child Development and Care, 1–24. 10.1080/03004430.2018.1465415 [DOI] [Google Scholar]
- Deary IJ (2001). Human intelligence differences: A recent history. Trends in Cognitive Sciences, 5(3), 127–130. 10.1016/S1364-6613(00)01621-1 [DOI] [PubMed] [Google Scholar]
- Demers CH, Aran O, Glynn LM, & Davis EP (2020). Prenatal programming of neurodevelopment: Imaging and structural changes. In Wazana A, Oberlander T, & Szekel E (Eds.), Prenatal Stress and Child Development. [Google Scholar]
- Doyle C, Werner E, Feng T, Lee S, Altemus M, Isler JR, & Monk C (2015). Pregnancy distress gets under fetal skin: Maternal ambulatory assessment & sex differences in prenatal development. Developmental Psychobiology, 57(5), 607–625. 10.1002/dev.21317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Daelmans B, Mello MCD, & Martines J (2006). Responsive parenting: Interventions and outcomes. Bulletin of the World Health Organization, 84(12), 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falah-Hassani K, Shiri R, & Dennis CL (2016). Prevalence and risk factors for comorbid postpartum depressive symptomatology and anxiety. Journal of Affective Disorders, 198, 142–147. 10.1016/j.jad.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Fan A, Buka S, Kosik RO, Chen YS, Wang SJ, & Eaton WW (2014). Association between maternal behavior in infancy and adult mental health: A 30-year prospective study. Comprehensive Psychiatry, 55(2). [DOI] [PubMed] [Google Scholar]
- Farrell AK, Waters TEA, Young ES, Englund MM, Carlson EE, Roisman GI, & Simpson JA (2019). Early maternal sensitivity, attachment security in young adulthood, and cardiometabolic risk at midlife. Attachment & Human Development, 21(1), 70–86. 10.1080/14616734.2018.1541517 [DOI] [PubMed] [Google Scholar]
- Feldman R (2007). Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48(3–4), 329–354. 10.1111/j.1469-7610.2006.01701.x [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, & Meaney MJ (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science; Washington, 286(5442), 1155–1158. [DOI] [PubMed] [Google Scholar]
- Gee DG (2016). Sensitive periods of emotion regulation: Influences of parental care on frontoamygdala circuitry and plasticity. In Rutherford HJV & Mayes LC (Eds.), Maternal brain plasticity: Preclinical and human research and implications for intervention. New Directions for Child and Adolescent Development; (Vol. 153, pp. 87–110). http://doi.wiley.com/10.1002/cad.20166 [DOI] [PubMed] [Google Scholar]
- Gilmore J, Knickmeyer R, & Gao W (2018). Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience, 19, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA, & Goldberg WA (2016). Gestational hormone profiles predict human maternal behavior at 1-year postpartum. Hormones and Behavior, 85, 19–25. 10.1016/j.yhbeh.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Howland M, Sandman CA, Davis EP, Phelan M, Baram TZ, & Stern H (2018). Prenatal maternal mood patterns predict child temperament and adolescent mental health. Journal of Affective Disorders, 228, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger SJ, Glynn LM, Sandman CA, Small SL, Obenaus A, Keator DB, Baram TZ, Stern H, Yassa MA, & Davis EP (2020). Aberrant maturation of the uncinate fasciculus follows exposure to unpredictable patterns of maternal signals. Journal of Neuroscience. 10.1523/JNEUROSCI.0374-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, McMahon C, Reilly N, & Austin MP (2010a). Maternal sensitivity moderates the impact of prenatal anxiety disorder on infant responses to the still-face procedure. Infant Behavior and Development, 33(4), 453–462. [DOI] [PubMed] [Google Scholar]
- Grant KA, McMahon C, Reilly N, & Austin MP (2010b). Maternal sensitivity moderates the impact of prenatal anxiety disorder on infant mental development. Early Human Development, 86(9), 551–556. 10.1016/j.earlhumdev.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Hobel CJ (1982). Identification of the patient at risk. In Perinatal medicine: Management of the high risk fetus and neonate. Williams & Wilkins. [Google Scholar]
- Howland MA, Sandman CA, Davis EP, Stern HS, Phelan M, Baram TZ, & Glynn LM (2020). Prenatal maternal mood entropy is associated with child neurodevelopment. Emotion. 10.1037/emo0000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan LA, Evans L, & Monk C (2008). Effects of mothers’ prenatal psychiatric status and postnatal caregiving on infant biobehavioral regulation: Can prenatal programming be modified? Early Human Development, 84(4), 249–256. 10.1016/j.earlhumdev.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston D, Tough S, & Whitfield H (2012). Prenatal and postpartum maternal psychological distress and infant development: A systematic review. Child Psychiatry and Human Development, 43(5), 683–714. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, & Gilmore J (2008). A structural MRI study of human brain development from birth to 2 years. Journal of Neuroscience, 28(47), 12176–12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok R, Thijssen S, Bakermans-Kranenburg MJ, Jaddoe VWV, Verhulst FC, White T, van IJzendoorn MH, & Tiemeier H (2015). Normal variation in early parental sensitivity predicts child structural brain development. Journal of the American Academy of Child & Adolescent Psychiatry, 54(10), 824–831. 10.1016/j.jaac.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Korja R, Nolvi S, Grant KA, & McMahon C (2017). The relations between maternal prenatal anxiety or stress and child’s early negative reactivity or self-regulation: A systematic review. Child Psychiatry & Human Development, 48(6), 851–869. 10.1007/s10578-017-0709-0 [DOI] [PubMed] [Google Scholar]
- Krueger RF, & Markon KE (2006). Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology, 2(1), 111–133. 10.1146/annurev.clinpsy.2.022305.095213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Lam SK, Sze Mun Lau S, Chong C, Chui H, & Fong D (2007). Prevalence, course, and risk factors for antenatal anxiety and depression. Obstetrics and Gynecology, 110(5), 1102–1112. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Le Moal M, Piazza P-V, & Abrous DN (2006). Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biological Psychiatry, 59(9), 786–792. 10.1016/j.biopsych.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Liu D, Diori J, Day JC, Francis DD, & Meaney MJ (2000). Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience, 3(8), 799. [DOI] [PubMed] [Google Scholar]
- Luby JL, Barch D, Whalen D, Tillman R, & Belden A (2017). Association between early life adversity and risk for poor emotional and physical health in adolescence: A putative mechanistic neurodevelopmental pathway. JAMA Pediatrics, 171(12), 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan S, Oatley H, Racine N, Fearon RMP, Schumacher L, Akbari E, Cooke JE, & Tarabulsy GM (2018). A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. Journal of the American Academy of Child & Adolescent Psychiatry, 57(9), 645–657.e8. 10.1016/j.jaac.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Mahrer N, Ramos I, Guardino C, Davis E, Ramey S, Shalowitz M, & Schetter C (2019). Pregnancy anxiety in expectant mothers predicts offspring negative affect: The moderating role of acculturation. Early Human Development, 141, 104932. 10.1016/j.earlhumdev.2019.104932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg LE, Lewis S, West A, Murray E, Sylva K, & Stein A (2016). The influence of mothers’ and fathers’ sensitivity in the first year of life on children’s cognitive outcomes at 18 and 36 months. Child: Care, Health and Development, 42(1), 1–7. 10.1111/cch.12294 [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. (1997). The effects of infant child care on infant-mother attachment security: Results of the NICHD study of early child care. Child Development, 68(5), 860–879. 10.2307/1132038 [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. (1999). Child care and mother-child interaction in the first three years of life. Developmental Psychology, 35(6), 1399–1413. [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. (2001). Nonmaternal care and family factors in early development: An overview of the NICHD Study of Early Child Care. Journal of Applied Developmental Psychology, 22(5), 457–492. 10.1016/S0193-3973(01)00092-2 [DOI] [Google Scholar]
- Nillni YI, Mehralizade A, Mayer L, & Milanovic S (2018). Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: A systematic review. Clinical Psychology Review, 66, 136–148. 10.1016/j.cpr.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska K, Krol A, Merecz-Kot D, Jurewicz J, Makowiec-Dabrowska T, Chiarotti F, Calamandrei G, & Hanke W (2017). Maternal stress during pregnancy and neurodevelopmental outcomes of children during the first 2 years of life. Journal of Paediatrics and Child Health, 53(3), 263–270. 10.1111/jpc.13422 [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, & Rothbart MK (2006). Measurement of fine-grained aspects of toddler temperament: The early childhood behavior questionnaire. Infant Behavior & Development, 29(3), 386–401. 10.1016/j.infbeh.2006.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Lucion A, & Weinberg J (2014). Neonatal handling: An overview of the positive and negative effects. Developmental Psychobiology, 56(8), 1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, Gee JC, Wang J, Hurt H, Detre JA, & Farah MJ (2010). Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. NeuroImage, 49(1), 1144–1150. 10.1016/j.neuroimage.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Røsand GB, Slinning K, Eberhard-Gran M, Røysamb E, & Tambs K (2011). Partner relationship satisfaction and maternal emotional distress in early pregnancy. BMC Public Health, 11(1), 161. 10.1186/1471-2458-11-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Head K, & Davis EP (2015). Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biological Psychiatry, 77(4), 324–334. 10.1016/j.biopsych.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Class QA, Glynn LM, & Davis EP (2015). Neurobehavioral disorders and developmental origins of health and disease. In Rosenfeld CS (Ed.), The Epigenome and Developmental Origins of Health and Disease (pp. 235–266). Academic Press/Elsevier. https://www.elsevier.com/books/the-epigenome-and-developmental-origins-of-health-and-disease/rosenfeld/978-0-12-801383-0 [Google Scholar]
- Sandman CA, Curran MM, Davis EP, Glynn LM, Head K, & Baram TZ (2018). Cortical thinning and neuropsychiatric outcomes in children exposed to prenatal adversity: A role for placental CRH? American Journal of Psychiatry, 175(5), 471–479. 10.1176/appi.ajp.2017.16121433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, & Davis EP (2010). Gestational stress influences cognition and behavior. Future Neurology, 5(5), 675–690. 10.2217/fnl.10.35 [DOI] [Google Scholar]
- Sandman CA, Glynn LM, Davis EP, Kisilevsky BS, & Reissland N (2016). Neurobehavioral consequences of fetal exposure to gestational stress. In Fetal Development: Research on Brain and Behavior, Environmental Influences and Emerging Technologies (pp. 229–265). Springer. https://link-springer-com.du.idm.oclc.org/chapter/10.1007/978-3-319-22023-9_13 [Google Scholar]
- Santor DA, & Coyne JC (1997). Shortening the CES–D to improve its ability to detect cases of depression. Psychological Assessment, 9(3), 233–243. [Google Scholar]
- Schechter JC, Brennan PA, Smith AK, Stowe ZN, Newport DJ, & Johnson KC (2017). Maternal prenatal psychological distress and preschool cognitive functioning: The protective role of positive parental engagement. Journal of Abnormal Child Psychology, 45(2), 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans C, & Kurrasch D (2013). Neurodevelopmental consequences of maternal distress: What do we really know? Clinical Genetics, 83(2), 108–117. 10.1111/cge.12049 [DOI] [PubMed] [Google Scholar]
- Sharp H, Hill J, Hellier J, & Pickles A (2015). Maternal antenatal anxiety, postnatal stroking and emotional problems in children: Outcomes predicted from pre- and postnatal programming hypotheses. Psychological Medicine, 45(2), 269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp H, Pickles A, Meaney M, Marshall K, Tibu F, & Hill J (2012). Frequency of infant stroking reported by mothers moderates the effect of prenatal depression on infant behavioural and physiological outcomes. PLOS ONE, 7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C (1983). Manual for the state-trait anxiety inventory. Consulting Psychologists Press, Inc. [Google Scholar]
- Spinrad TL, & Stifter CA (2002). Maternal sensitivity and infant emotional reactivity: Concurrent and longitudinal relations. Marriage & Family Review, 34(3–4), 243–263. 10.1300/J002v34n03_03 [DOI] [Google Scholar]
- Van den Bergh BRH, Mulder EJH, Mennes M, & Glover V (2005). Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neuroscience & Biobehavioral Reviews, 29(2), 237–258. 10.1016/j.neubiorev.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Van Den Heuvel M, Lahti M, Braeken M, Rooij S, Entringer S, Hoyer D, Roseboom T, Raikkonen K, King S, & Schwab M (2017). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience & Biobehavioral Reviews. https://www.ncbi.nlm.nih.gov/pubmed/28757456 [DOI] [PubMed] [Google Scholar]
- Vehmeijer FOL, Guxens M, Duijts L, & Marroun HE (2019). Maternal psychological distress during pregnancy and childhood health outcomes: A narrative review. Journal of Developmental Origins of Health and Disease, 10(3), 274–285. 10.1017/S2040174418000557 [DOI] [PubMed] [Google Scholar]
- Wakshlak A, & Weinstock W (1990). Neonatal handling reverses behavioral abnormalities induced in rats by prenatal stress. Physiology & Behavior, 48(2), 289–292. 10.1016/0031-9384(90)90315-U [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang H, Wee C-Y, Lee A, Poh JS, Chong Y-S, Tan KH, Gluckman PD, Yap F, Fortier MV, Rifkin-Graboi A, & Qiu A (2019). Maternal sensitivity predicts anterior hippocampal functional networks in early childhood. Brain Structure and Function, 224(5), 1885–1895. 10.1007/s00429-019-01882-0 [DOI] [PubMed] [Google Scholar]
- Wechsler D, Coalson DL, & Raiford SE (1997). WAIS-III: Wechsler adult intelligence scale. Psychological Corporation. [Google Scholar]
- Wu Y, Lu Y-C, Jacobs M, Pradhan S, Kapse K, Zhao L, Niforatos-Andescavage N, Vezina G, du Plessis AJ, & Limperopoulos C (2020). Association of prenatal maternal psychological distress with fetal brain growth, metabolism, and cortical maturation. JAMA Network Open, 3(1), e1919940–e1919940. 10.1001/jamanetworkopen.2019.19940 [DOI] [PMC free article] [PubMed] [Google Scholar]


