Abstract
Palmoplantar skin has several unique characteristics such as increased thickness, high resilience, hypopigmentation, and lack of hair follicles. The establishment of palmoplantar identity occurs through keratinocyte-fibroblast interactions, with keratin 9 expression and Wnt signaling playing key roles. Understanding how palmoplantar features develop may help efforts to reproduce them at both palmoplantar and non-palmoplantar body sites.
Keywords: Fibroblast, keratinocyte, volar, palm, sole, palmoplantar, keratin 9, Wnt, beta catenin, DKK1, DKK2, dsRNA, iRHOM2, EGF
Introduction
The skin includes an outer epidermis that protects against the external environment and consists mostly of keratinocytes. The underlying dermis contains fibroblasts and extracellular matrix, while the hypodermis contains adipose tissue. The skin varies by thickness, pigmentation, as well as presence of hair and sweat glands across body sites, and how the establishment of positional identity occurs has remained an intriguing question in skin biology research. More than 50 years ago, Billingham and Silvers (1967) showed through a series of heterotypic transplantation experiments in guinea pigs that epidermal identity is influenced by the underlying dermis. Specifically, transplantation of pure epidermal sheets to mismatched dermis between the ear, trunk, ands sole of guinea pigs often resulted in reprogramming of epidermal phenotype to match that of the dermal component (Billingham and Silvers, 1967). These findings highlight the importance of epithelial-mesenchymal interactions in determining epidermal identity.
From a functional perspective, palmoplantar skin (i.e., skin at the palms and soles) provides unique features that are essential for human daily activities. Increased epidermal thickness of palmoplantar skin allows resistance to abrasions, punctures, and lacerations during locomotion and manipulation of objects. Other defining characteristics of palmoplantar skin include presence of the stratum lucidum, relative hypopigmentation, and absence of hair follicles. Understanding factors responsible for the normal palmoplantar phenotype may support efforts to restore palmoplantar skin in cases of its dysfunction or loss.
The establishment of palmoplantar identity involves expression of keratin 9 and regulation of Wnt signaling
Keratin 9 (KRT9) is exclusively expressed in suprabasal keratinocytes of human palmoplantar epidermis, provides structural support for palmoplantar skin, and has been used as a marker for these body sites (Kim et al., 2016, Zhou et al., 2019). Although non-palmoplantar keratinocytes do not normally express KRT9, this may be induced by culturing non-palmoplantar keratinocytes with palmoplantar fibroblasts (Kim et al., 2016, Yamaguchi et al., 2008). Induction of KRT9 expression in keratinocytes likely involves the Wnt signaling pathway (Kim et al., 2016, Rinn et al., 2008, Xu et al., 2017, Yamaguchi et al., 2008), which has been implicated in various biological processes including cell growth and differentiation, limb development, hair cycling, and carcinogenesis (Jin and Yoon, 2012). The most well-studied canonical Wnt signaling pathway involves activation and translocation of β-catenin to the nucleus that affects the transcription of target genes (Jin and Yoon, 2012).
Thus far, multiple studies have identified canonical Wnt signaling as an important component of KRT9 expression, but their exact relationship remains uncertain. Yamaguchi et al. (2008) showed that Dickkopf-1 (DKK1), which disrupts the Wnt receptor LRP6 to inhibit canonical Wnt signaling, is expressed at higher levels in palmoplantar fibroblasts compared to non-palmoplantar fibroblasts. As Wnt signaling has a well-established role in hair follicle development, the inhibition of this pathway by increased levels of DKK1 may contribute to the characteristic lack of hair follicles at palmoplantar sites (Yamaguchi et al., 2008). Transfection studies also showed that DKK1 inhibits β-catenin-mediated suppression of microphthalmia-associated transcription factor (MITF), resulting in hypopigmentation of palmoplantar skin by decreasing melanocyte proliferation and differentiation (Yamaguchi et al., 2008). Treatment of cultured human keratinocytes with DKK1 resulted in modest upregulation of KRT9 mRNA expression, while treatment of reconstructed epidermal skin with DKK1 resulted in increased thickness and decreased pigmentation, with immunohistochemistry showing slight increase in KRT9-positive keratinocytes (Yamaguchi et al., 2008). Together, these results provide an attractive explanation for how DKK1-mediated inhibition of canonical Wnt signaling promotes characteristic palmoplantar features, including the expression of KRT9 (red arrow in Figure 1).
Figure 1. Signaling molecules and pathways likely involved in the development of palmoplantar characteristics.
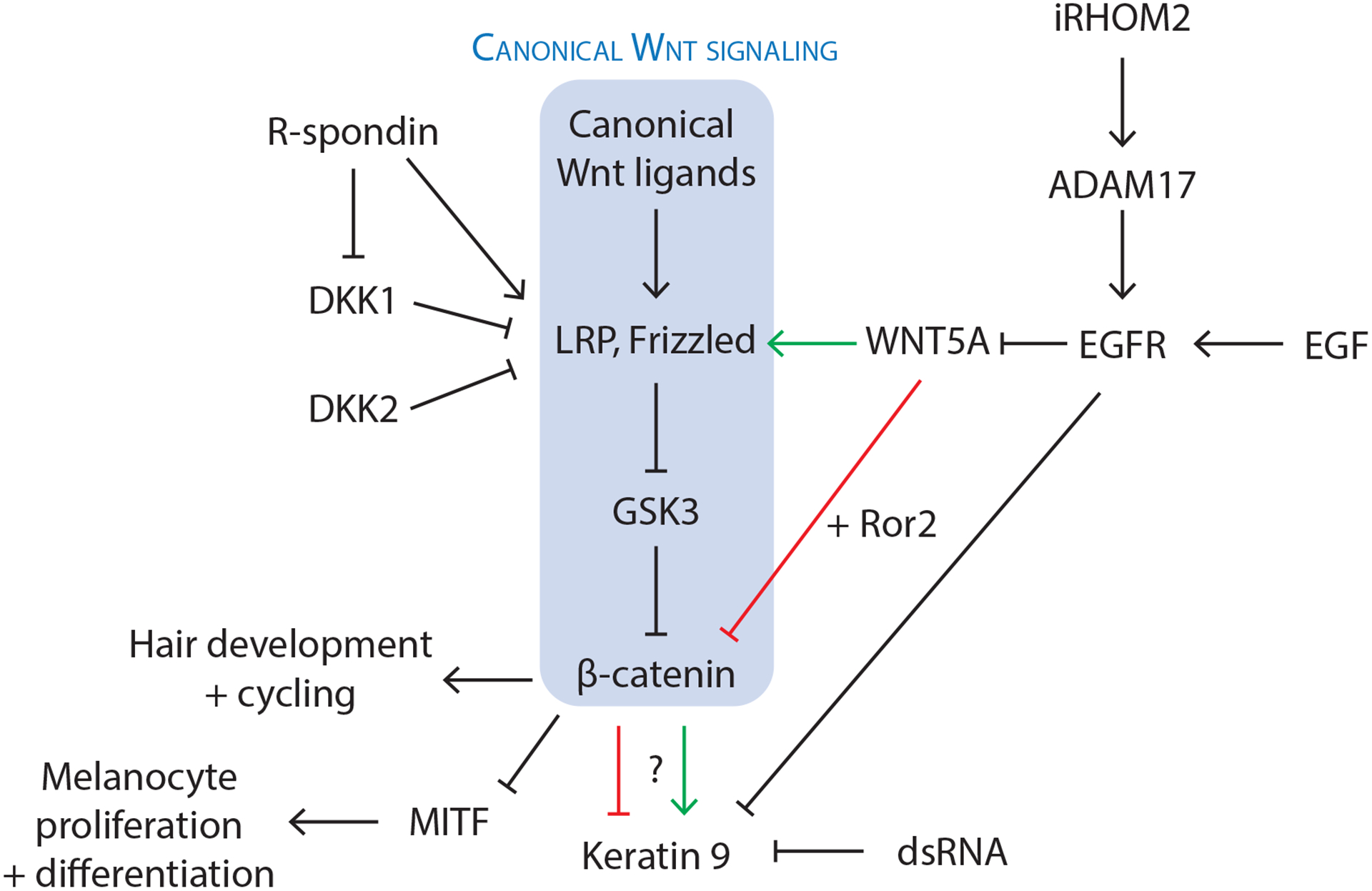
Expression of keratin 9 (KRT9) is a marker for palmoplantar keratinocyte differentiation, but there is uncertainty on whether KRT9 expression results from activation or inhibition of canonical Wnt signaling. Additional molecules including Dickkopf (DKK) proteins, R-spondin (RSPO) proteins, double-stranded RNA (dsRNA), inactive rhomboid protein 2 (iRHOM2), and epidermal growth factor (EGF) have been implicated in regulation of Wnt signaling or KRT9 expression, warranting further study on the interaction of different signaling pathways in the context of palmoplantar biology.
Kim et al. (2016) induced KRT9 expression in human foreskin and scalp keratinocytes by culturing them with plantar fibroblasts. Both gain-of-function and loss-of-function experiments, however, suggested that KRT9 expression results from activation, rather than inhibition of canonical Wnt signaling (Kim et al., 2016). Treatment of keratinocyte and plantar fibroblast co-cultures with increasing concentrations of a canonical Wnt signaling activator (SB216763, a glycogen synthase kinase-3 inhibitor) resulted in dose-dependent increase in KRT9 mRNA expression that was partially reversible through inhibition of canonical Wnt signaling with DKK1 (Kim et al., 2016). The authors similarly observed a dose-dependent decrease in KRT9 mRNA expression upon treating keratinocyte and plantar fibroblast co-cultures with increasing concentrations of DKK1, suggesting that canonical Wnt signaling promotes KRT9 expression in vitro (green arrow in Figure 1) (Kim et al., 2016). The contrasting roles of canonical Wnt signaling in KRT9 expression proposed in Yamaguchi et al. and Kim et al.’s studies may arise from different experimental conditions, such as cell types examined in culture (keratinocytes treated with DKK1 versus keratinocytes and plantar fibroblasts treated with SB216763 and/or DKK1, respectively).
Studies using mouse models have suggested that HOXA13 expression in fibroblasts induces KRT9 expression in palmoplantar keratinocytes and that this process is mediated through fibroblast-secreted WNT5A (Rinn et al., 2008). Consistent with this, Kim et al. (2016). found overlapping expression of epidermal WNT5A and KRT9 in normal human palmoplantar skin and in diseases with features of palmoplantar skin, including lichen simplex chronicus and psoriasis. WNT5A has been characterized as an atypical Wnt ligand that may activate or inhibit the canonical Wnt/β-catenin pathway depending on its receptor (Frizzled and Ror2, respectively) (Mikels and Nusse, 2006), and both mechanisms have been proposed to drive KRT9 expression in palmoplantar keratinocytes (Kim et al., 2016, Yamaguchi et al., 2008). While the expression of KRT9 often co-occurs with increased thickness of palmoplantar skin, caution should be taken in assuming a simple causal relationship between them, considering that dominant-negative mutations in KRT9 are associated with epidermolytic palmoplantar keratoderma (PPK), characterized by fragility with compensatory hyperkeratosis of human palmoplantar epidermis (OMIM # 144200).
Additional recent studies have showed that activation of canonical Wnt signaling is important for the maintenance of palmoplantar epidermis. Xu et al. (2017) found that deletion of WNT10A and β-catenin both resulted in reduction of KRT9 mRNA and protein levels in mouse footpad epidermis. Both the deletion of WNT10A and forced expression of the canonical Wnt signaling inhibitor DKK1 also independently resulted in regression of sweat ducts in mice (Xu et al., 2017). Consistent with findings in mice, loss of function mutation of WNT10A in human are associated with palmoplantar fissure and scaling similar to that seen in PPK, along with palmoplantar hypohidrosis, alopecia, and other tongue and dental abnormalities (Xu et al., 2017).
The newly characterized R-spondin (RSPO) family of secreted proteins are activators of Wnt/β-catenin signaling, with proposed mechanisms including activation of the cells surface receptors LRP6 and LGR4 responsible for propagation of Wnt signaling, as well as antagonism of Kremen/DKK1 or ZNRF3-mediated downregulation of the Wnt ligand receptors, LRP6 and Frizzled (Jin and Yoon, 2012). RSPO1 and RSPO4 are both secreted by fibroblasts, and loss of function mutations in human have been associated with palmoplantar hyperkeratosis and anonychia, respectively (Jin and Yoon, 2012). The fact that loss of function mutations in WNT10A and RSPO1 (which both lead to decreased canonical Wnt signaling) result in similar palmoplantar phenotypes as that seen with deletion of KRT9 support the model that canonical Wnt signaling promotes KRT9 expression in palmoplantar keratinocytes (green arrow in Figure 1).
Despite recent experimental support for activation of canonical Wnt signaling in the maintenance of palmoplantar skin integrity, inhibition of the same pathway has continued to be implicated in other palmoplantar features, including the absence of hair follicles. Song et al. (2018) found that high expression of DKK2 (an inhibitor of canonical Wnt signaling like DKK1) in the plantar dermis underlies the lack of hair development at plantar feet in mice, similar to the model involving DKK1 previously proposed by Yamaguchi et al. (2008). Wnt signaling in palmoplantar skin may be temporally and spatially coordinated to allow its activation and inhibition under different conditions, and it would be valuable in future studies to clarify how Wnt signaling-mediated regulation of palmoplantar characteristics (e.g., KRT9 expression, hair growth, pigmentation, sweat gland formation) may be time, cell type, and microenvironment-dependent in vivo.
Signaling pathways involving dsRNA, iRHOM2, and EGF may influence KRT9 expression and palmoplantar hyperkeratosis
Additional signaling molecules and pathways that may modulate palmoplantar features have been proposed. Zhou et al. (2019) observed autonomous expression of KRT9 by cultured palmoplantar keratinocytes in the absence of fibroblasts that decreased with keratinocyte passaging; this decrease in KRT9 expression by passaged palmoplantar keratinocytes occurred in parallel to an increase in the double-stranded RNA (dsRNA) receptor, DDX58, whose knockdown by siRNA was sufficient to rescue KRT9 expression (Zhou et al., 2019). Although dsRNA sensing is usually associated with the innate immune response and antiviral pathways, the inhibition of KRT9 expression in keratinocytes by exogenous synthetic dsRNA suggests that dsRNA may also inhibit keratinocyte differentiation (Zhou et al., 2019). Interestingly, this work also implied that KRT9 induction in volar keratinocytes is not solely from the influence of adjoining fibroblasts, but also a result of an intrinsic elevated capacity of isolated volar keratinocytes to express KRT9 (Zhou et al., 2019).
The inactive rhomboid protein 2 (iRHOM2), which may participate in antiviral responses, has also been implicated in the regulation of palmoplantar skin integrity, with hyperactivating mutations of its encoding gene RHBDF2 associated with the autosomal dominant condition, tylosis with esophageal cancer (Chao-Chu et al., 2021). iRHOM2 upregulates epidermal growth factor receptor (EGFR) signaling in keratinocytes through the sheddase enzyme ADAM17 (Chao-Chu et al., 2021), and its role in palmoplantar biology may be related to the prior finding that exogenous EGF inhibits KRT9 and Wnt5a expression in keratinocytes in vitro in a dose-dependent manner (Kim et al., 2016).
Improved understanding of epithelial-mesenchymal signaling at palmoplantar sites may provide insight for the treatment of conditions like PPK and support efforts to therapeutically induce palmoplantar features. Other potential areas of study include signaling pathways activated from different forms of mechanical stimulation. Additional unique qualities of palmoplantar skin such as its high concentration of eccrine glands and presence of friction ridges (e.g., fingerprints) also warrant further examination to clarify their biological connection with other palmoplantar features.
Clinical applications of keratinocyte-fibroblast interactions in the context of palmoplantar biology
Despite remaining uncertainties in the mechanism by which palmoplantar fibroblasts induce positional identity of palmoplantar epidermis, clinical applications that take advantage of this quality are manifold. In the case of foot ulcers caused by diabetes mellitus or rheumatoid arthritis with the dermis still present, grafting non-palmoplantar epidermal sheets (which are easier to acquire) to the injury site and allowing induction of palmoplantar epidermal phenotype by fibroblasts in the underlying dermis has been proposed (Yamaguchi et al., 2008). In the context of full-body blistering skin diseases such as junctional epidermolysis bullosa, site-specific skin regeneration may also be beneficial for maintaining mechanical integrity at palmoplantar sites.
The inductive capacity of palmoplantar fibroblasts may be used to strengthen skin resilience and increase skin thickness at non-palmoplantar body sites, such as the sacral region and bony prominences in immobilized individuals who are prone to developing pressure ulcers. Amputees often develop stump dermatoses due to relative fragility of the stump site compared to normal palmoplantar skin; researchers have long considered whether full thickness grafting or autologous injection of palmoplantar fibroblasts to amputee stump sites may induce the ectopic development of palmoplantar features (Kim et al., 2016, Zhou et al., 2019) and a clinical trial investigating this is currently underway (ClinicalTrials.gov Identifier: NCT03947450). These exciting clinical applications may not only benefit diverse patient populations, but also further contribute to the discipline of regenerative medicine.
CLINICAL RELEVANCE.
Dysfunction in keratin 9 and Wnt signaling are associated with palmoplantar pathologies.
Regenerating palmoplantar-specific skin in foot ulcers may provide increased skin resilience.
Inducing palmoplantar features ectopically may prevent pressure ulcers and amputation stump dermatoses.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under R01AR074846 to LAG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to declare.
IRB approval status: Exempt from IRB review.
REFERENCES
- Billingham RE, Silvers WK. Studies on the Conservation of Epidermal Specificities of Skin and Certain Mucosas in Adult Mammals. Journal of Experimental Medicine 1967;125(3):429–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Chu J, Murtough S, Zaman N, Pennington DJ, Blaydon DC, Kelsell DP. iRHOM2: A Regulator of Palmoplantar Biology, Inflammation, and Viral Susceptibility. J Invest Dermatol 2021;141(4):722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YR, Yoon JK. The R-spondin family of proteins: emerging regulators of WNT signaling. Int J Biochem Cell Biol 2012;44(12):2278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Hossain MZ, Nieves A, Gu L, Ratliff TS, Mi Oh S, et al. To Control Site-Specific Skin Gene Expression, Autocrine Mimics Paracrine Canonical Wnt Signaling and Is Activated Ectopically in Skin Disease. Am J Pathol 2016;186(5):1140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a Protein Activates or Inhibits β-Catenin–TCF Signaling Depending on Receptor Context. PLoS Biology 2006;4(4):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Wang JK, Allen N, Brugmann SA, Mikels AJ, Liu H, et al. A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes & Development 2008;22(3):303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Boncompagni AC, Kim SS, Gochnauer HR, Zhang Y, Loots GG, et al. Regional Control of Hairless versus Hair-Bearing Skin by Dkk2. Cell Rep 2018;25(11):2981–91 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Horrell J, Snitow M, Cui J, Gochnauer H, Syrett CM, et al. WNT10A mutation causes ectodermal dysplasia by impairing progenitor cell proliferation and KLF4-mediated differentiation. Nature Communications 2017;8(1):15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Passeron T, Hoashi T, Watabe H, Rouzaud F, Yasumoto KI, et al. Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/β-catenin signaling in keratinocytes. The FASEB Journal 2008;22(4):1009–20. [DOI] [PubMed] [Google Scholar]
- Zhou R, Wang G, Kim D, Kim S, Islam N, Chen R, et al. dsRNA Sensing Induces Loss of Cell Identity. J Invest Dermatol 2019;139(1):91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


