Abstract
Drug induced liver injury (DILI) due to medications and herbal and dietary supplements (HDS) is a major cause of acute liver injury leading to liver transplantation (LT). This study used United Network for Organ Sharing LT data to analyze severe HDS induced acute liver injury in the United States.
By convention, patients with acute DILI are listed as “Acute Hepatic Necrosis” (AHN) under the subheading “AHN: Drug Other Specify”. All patients waitlisted from 1994 to 2020 were divided into 3 subgroups: “HDS DILI”, “Non-HDS DILI”, and “AHN: unknown drug. Analyses were performed to identify epidemiologic differences between HDS DILI and Non-HDS DILI patients. Sub-analysis was performed for transplanted patients, including longitudinal changes.
Of 1875 patients waitlisted for LT, 736 (39.2%) underwent LT. The proportion of Asian patients in the HDS DILI group was significantly higher compared to the non-HDS DILI group (17.4% v. 3.8%, p < 0.001). Excluding acetaminophen cases, the proportion of Black patients in the HDS DILI v. non-HDS group was significantly lower (8.7% v. 25.3%, p < 0.001). Waitlisted HDS DILI patients were significantly older (median age 38 y for HDS DILI vs 31 y for non-HDS DILI, p=0.03). Lastly, the number of patients requiring LT due to HDS DILI increased significantly over time with more than 70 % of cases occurring in the last 10 years (2010–2020) compared to the prior 15 years (1994–2009) (Ptrend =0.001).
Conclusion:
Ethnicity may help in identifying the cause of severe acute DILI, a growing problem as more patients experiment with HDS.
Keywords: Acute hepatic necrosis, Liver Transplantation, Drug Induced Liver Injury, Herbal and Dietary Supplement, Organ Procurement database
Background:
Drug induced liver injury (DILI) due to medications and herbal and dietary supplements (HDS) is the leading cause of severe acute liver injury in the United States and Western Europe (1,2). Amongst medications, acetaminophen is by far the most common cause of severe acute liver injury, whereas no single HDS product stands out in the same way. HDS include a large spectrum of agents used for a variety of medicinal and nutritional purposes including weight loss, bodybuilding, anxiety and depression, cardiovascular health, and pain management among others (3). There is no standard classification of HDS, which include vitamins, minerals, elements, herbal and botanical remedies, multi-ingredient compounds, and non-prescription anabolic steroids.
Americans are increasingly turning to complementary treatments such as HDS in addition to or instead of traditional medications. Data from the National Health Interview Surveys reveals that approximately 18% of American population used non-vitamin and non-mineral dietary supplements (4). Another study using data from the National Health and Nutrition Examination Survey reported that 52% of the US adults used supplements in 2011–2012 (5). HDS are readily available over the counter, do not require prescriptions from providers, and are not rigorously regulated by the Food and Drug Administration FDA (6). HDS use is not completely benign. Of HDS users, 4% reported experiencing an adverse event related to HDS consumption (7).
Increased use of HDS over time may lead to greater adverse hepatic events from mild to severe acute liver injury and possibly liver transplantation. Indeed, the US Drug-Induced Liver Injury Network (US-DILIN) reported the incidence of HDS DILI in their cohort increased significantly from 7% of collected non-acetaminophen DILI cases in 2004–2005 to 20% in 2013–2014, nearly a three-fold increase (8). Acute liver injury study groups across multiple Asian and European countries have reported that 20–40% of acute liver injury cases due to DILI are related to HDS use (9). Additionally, HDS related DILI in the US-DILIN cohort was significantly more likely to lead to death or liver transplantation (LT) compared to DILI from other medications (8). Also, in the US-DILIN cohort, Asian ethnicity was associated with an increased risk of death or liver transplantation (10). In the US Acute Liver Failure Study Group (ALFSG) cohort, both Blacks and Asians were overrepresented amongst those with severe liver injury related to DILI (11); no breakdown was provided with regard to DILI induction by HDS versus medications. These limited multi-site cohorts may not be representative of the national demographic and longitudinal patterns of severe liver injury due to DILI and specifically HDS consumption.
The United Network for Organ Sharing (UNOS) database provides information on all those waitlisted for liver transplantation in the United States. Earlier studies of acute liver failure cases in the UNOS database did not specifically examine HDS related cases nor ethnicity (12, 13). This study utilizes the UNOS database to provide a broad, updated evaluation of the demographic and longitudinal trends of severe acute liver injury due to DILI.
Methods:
Patients were identified by searching the UNOS database, which contains information regarding every organ donation and transplantation in the U.S. since October 1, 1987. There is no specific category for acute liver injury in the UNOS database. By convention, patients with drug induced acute liver injury are listed in the “Acute Hepatic Necrosis” (AHN) category under the subheading “AHN: Drug Other Specify”. We identified all patients who were waitlisted for LT for a diagnosis of “AHN” between January 1,1994 and December 31, 2020. We chose the calendar year 1994 as our starting point for data collection as there was no diagnosis code required for waitlisting prior to it. We excluded patients with AHN due to a non-DILI etiology or due to DILI but with no specific agent implicated. Patients with AHN due to DILI were categorized as HDS DILI or non-HDS DILI. In some analyses, the acetaminophen (APAP) cases were excluded from comparisons between HDS and non-HDS-non-APAP cases. We collected data on the characteristics of patients including their sex, age, ethnicity, and education level. MELD score at listing and the time of transplantation was available since 2002. Ethnicity was assigned based on Organ procurement and transplantation network (OPTN) database categories: Asian, Black, Hispanic, White, American Indian/Alaska Native, Pacific Islanders and Multiracial. Given the small number of patients in the latter three ethnicities, they were combined into “other ethnicities”, leaving 5 categories for comparison purposes. Ethnicity was mostly self-declared. Education is collected in OPTN database as follows: primary school, high school, college/some technical university, associate degree or post graduate degree. For simplicity, we divided the education categories into any education after high school vs no education beyond high school. One year transplant free survival of patients post transplantation was calculated and compared between groups.
Subgroup comparisons were also performed specifically on the waitlisted patients who ultimately underwent LT. Rurality was added to the comparisons for between transplanted patients until 2014 (supplementary file). Patients’ zip codes were used to determine whether they resided in rural or non-rural areas using U.S. Census Bureau official definitions. The U.S. Census Bureau defines rural as what is not urban—that is, after defining individual urban areas, rural is what is left. Urbanized areas are areas with 50,000 or more people. Urban clusters are areas with at least 2,500 but fewer than 50,000 people. We used US census data from 2010 and along with their zip codes to define urban and rural populations (14–16).
Statistical analysis:
We compared the characteristics of patients waitlisted and transplanted due to AHN from HDS versus non-HDS DILI, both including and excluding patients with DILI due to acetaminophen. Comparisons between groups were performed using chi-square tests or Fisher exact tests for categorical variables and non-parametric Kruskal-Wallis rank-sum tests for continuous variables. Associations between ethnicity and having HDS as the cause of DILI-related AHN were calculated with univariate and multivariate logistic regression models. Multivariate models included age, sex, ethnicity, and calendar year. Trends by waitlist and transplantation year in the proportions of patients with HDS DILI among all DILI were calculated using univariate logistic regression models. All significance tests and resulting p values were two-sided with an alpha level of 0.05. Statistical analyses were performed using STATA SE 16 (Stata Corp LLC).
Results:
Categorization of AHN groups
Of 2446 patients waitlisted for LT within the UNOS “AHN” diagnostic category in the 26-year period studied, 1,875 had DILI with a specific agent implicated: 69 (3.7%) due to HDS and 1806 (96.3%) due to non-HDS DILI (Fig.1). The implicated HDS agents included herbal products, muscle building products, vitamins/minerals, and other dietary supplements (Fig. 2). Herbal products were the most common implicated agent in both those waitlisted and the transplanted subgroup. Use of body building supplements was marginally higher in those transplanted than in the waitlisted HDS group as a whole (32% v. 22%) (Fig. 2). The specific products that show up multiple times in the three main categories are: Oxyelite pro, Iron, and vitamin A. Acetaminophen (APAP) cases accounted for 1,371 (75.91%) of the non-HDS cases. The top 5 most common categories for non-apap non-HDS DILI includes: anti-tuberculosis drugs, antibiotics, anti-epileptics, anti-neoplastic agents and non-steroidal anti-inflammatory drugs. The top 5 implicated specific causes for non-APAP, non-HDS DILI includes: Isoniazid, Phenytoin, Propylthiouracil, Valproic acid and Trimethoprim/sulfamethoxazole (Table 1). All commonly known as causes of idiosyncratic liver injury (17). Of the HDS DILI cases, 59.4% underwent LT and only 38.5% of the non-HDS DILI cases had LT, though this increases to 61.8% if APAP cases are excluded.
Figure 1.
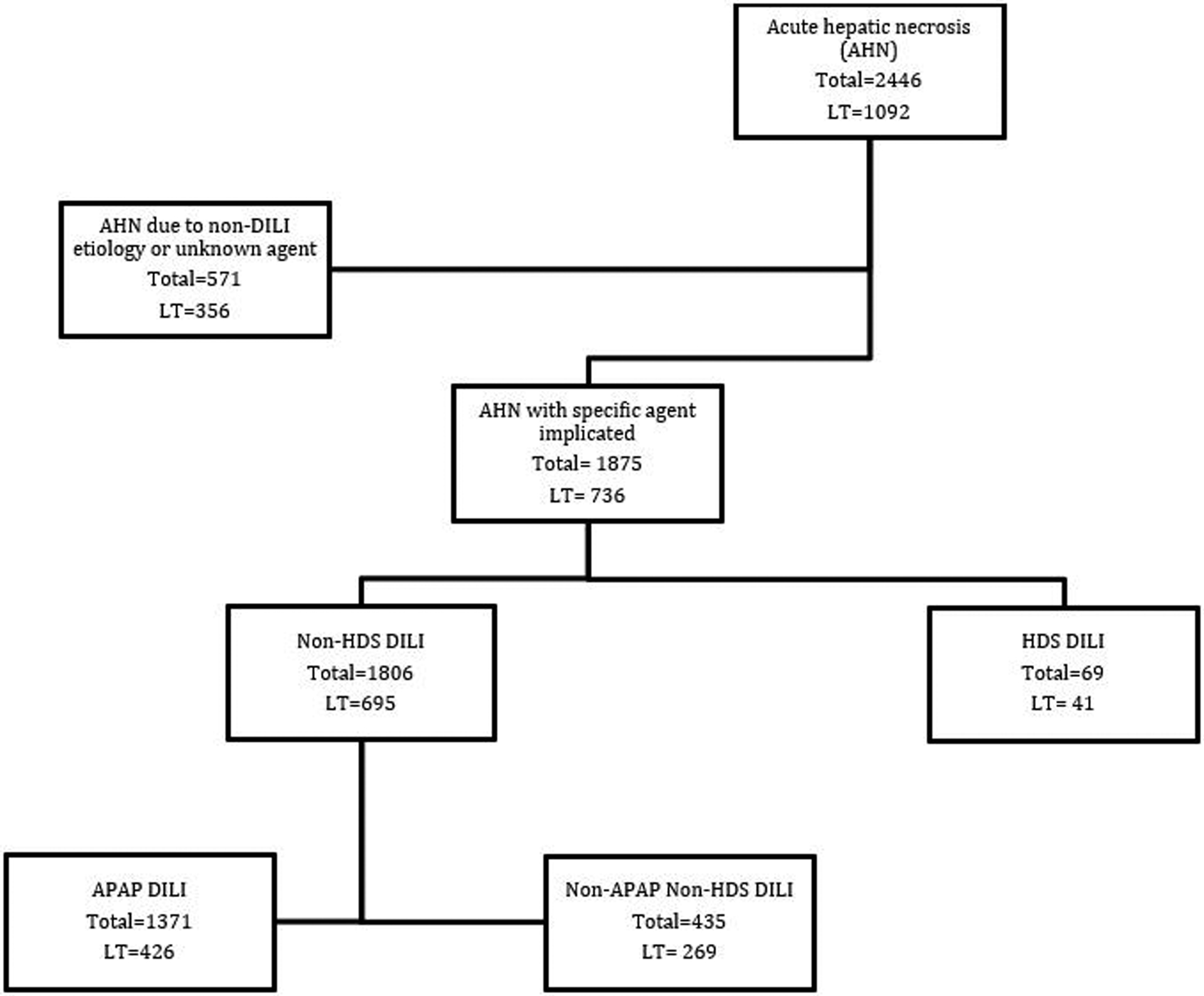
Flow chart of AHN patient subgroups with drug induced liver injury (DILI).
Both the total number of patients in each group and the number transplanted are shown.
AHN: acute hepatic necrosis, HDS: herbal and dietary supplements, APAP: acetaminophen, LT: liver transplantation), WL: Waitlist
Figure 2.
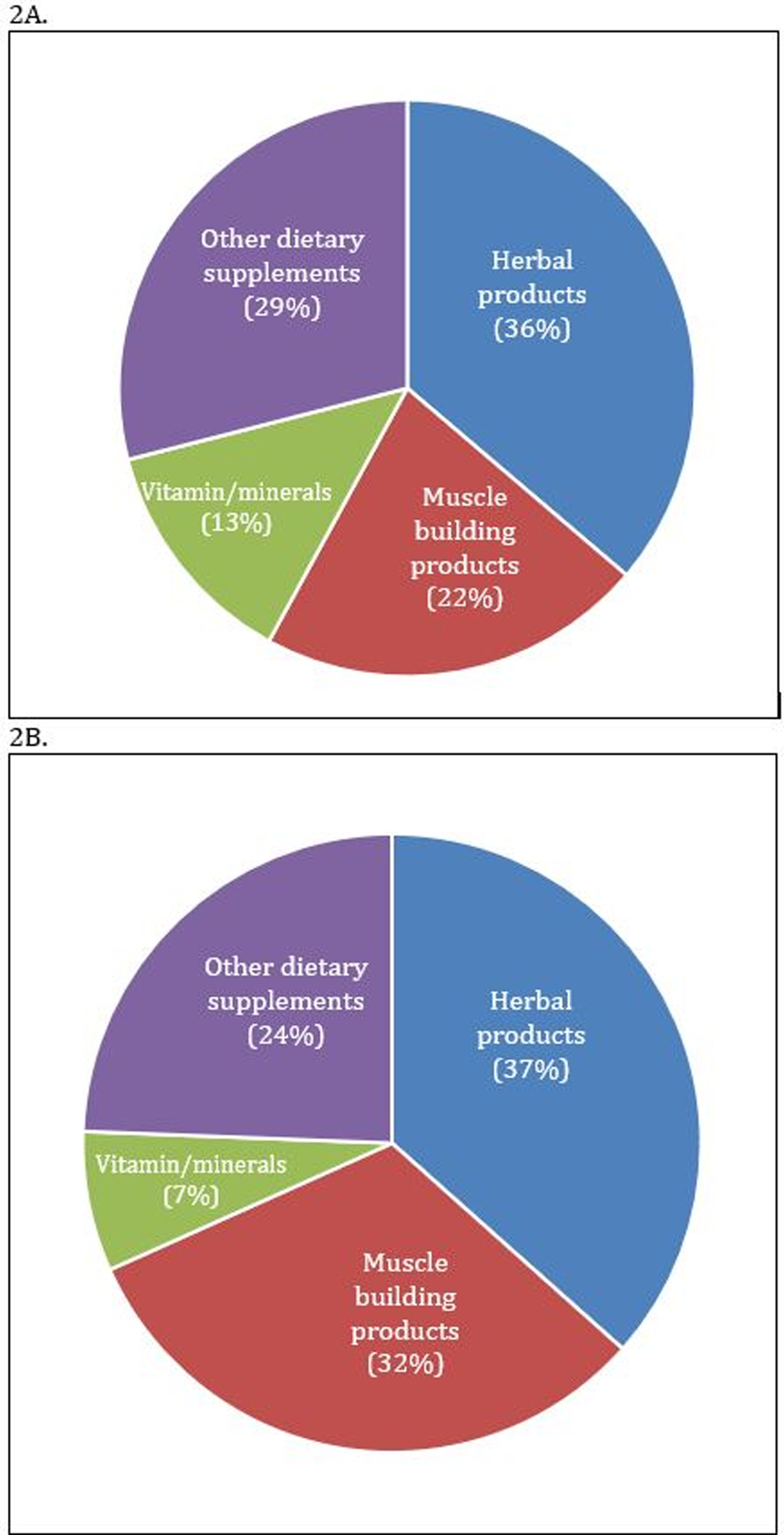
Breakdown of HDS products in waitlisted patients (A) and the subgroup of transplanted patients (B).
Use of muscle building HDS products were more frequent amongst the transplanted patient subgroup than the overall group of waitlisted patients.
HDS: Herbal and Dietary supplements
Table 1.
Proportions of patients waitlisted for AHN who were transplanted based on cause of DILI.
| Waitlisted (n = 1,875) | Transplanted (n = 736) | P | |
|---|---|---|---|
| Cause of DILI: | <0.001 | ||
| Acetaminophen | 1,371 | 426 (31.1%) | |
| Non-acetaminophen, non-HDS | 435 | 269 (61.8%) | |
| HDS | 69 | 41 (59.4%) | |
| Common categories of non-acetaminophen, non-HDS DILI: | 0.83 | ||
| Antituberculosis drugs1 | 88 | 52 (59.1%) | |
| Antibiotics2 | 75 | 50 (66.7%) | |
| Antiepileptic drugs3 | 66 | 39 (59.1%) | |
| Antineoplastic agents4 | 20 | 11 (55.0%) | |
| Nonsteroidal anti-inflammatory drugs (NSAIDs)5 | 20 | 12 (60.0%) | |
| Statins6 | 16 | 12 (75.0%) | |
| Antihypertensive drugs7 | 14 | 9 (64.3%) | |
| Antidepressants8 | 12 | 5 (41.7%) | |
| Antiretroviral therapy9 | 8 | 5 (62.5%) | |
| Azoles10 | 7 | 5 (71.4%) | |
| Common specific causes of non-acetaminophen, non-HDS DILI: | 0.17 | ||
| Isoniazid | 81 | 49 (60.5%) | |
| Phenytoin | 35 | 19 (54.3%) | |
| Propylthiouracil | 23 | 15 (65.2%) | |
| Valproic acid | 19 | 13 (68.4%) | |
| Trimethoprim/sulfamethoxazole | 16 | 10 (62.5%) | |
| Disulfiram | 14 | 13 (92.9%) | |
| Nitrofurantoin | 14 | 12 (85.7%) | |
| Amoxicillin/clavulanic acid | 12 | 7 (58.3%) | |
| Methotrexate | 10 | 5 (50.0%) |
Tests for significance with chi-squared tests or Fisher exact tests.
Includes: isoniazid (n = 81), rifampin (n = 3), and combination or unspecified antituberculosis drugs (n = 4).
Includes: trimethoprim/sulfamethoxazole (n = 16), nitrofurantoin (n = 14), amoxicillin/clavulanic acid (n = 12), azithromycin (n = 5), amoxicillin (n = 4), ciprofloxacin (n = 3), doxycycline (n = 3), levofloxacin (n = 3), minocycline (n = 2), clarithromycin (n = 1), dapsone (n = 1), metronidazole (n = 1), moxifloxacin (n = 1), telithromycin (n = 1), trovafloxacin (n = 1), vancomycin (n = 1), and combination or unspecified antibiotics (n = 6).
Includes: phenytoin (n = 35), valproic acid (n = 19), carbamazepine (n = 6), lamotrigine (n = 2), felbamate (n = 1), levetiracetam (n = 1), and combination or unspecified antiepileptic drugs (n = 2).
Includes: methotrexate (n = 10), azathioprine or 6-mercaptopurine (n = 5), asparaginase (n = 1), cyclophosphamide (n = 1), mitotane (n = 1), and combination or unspecified antineoplastic agents (n = 2).
Includes: diclofenac (n = 4), aspirin (n = 3), ibuprofen (n = 3), bromfenac (n = 2), indomethacin (n = 1), nabumetone (n = 1), naproxen (n = 1), salicylate (n = 1), and combination or unspecified NSAIDs (n = 4).
Includes: atorvastatin (n = 3), cerivastatin (n = 3), simvastatin (n = 3), ezetimibe/simvastatin (n = 2), fluvastatin (n = 1), pravastatin (n = 1), rosuvastatin (n = 1), or unspecified statin (n = 2).
Includes: methyldopa (n = 6), diltiazem (n = 2), hydralazine (n = 2), lisinopril (n = 2), valsartan (n = 1), and unspecified antihypertensive drug (n = 1).
Includes: nefazodone (n = 5), paroxetine (n = 2), amitriptyline (n = 1), bupropion (n = 1), duloxetine (n = 1), and unspecified tricyclic antidepressant (n = 2).
Includes: efavirenz/emtricitabine/tenofovir (n = 3), efavirenz (n = 1), nevirapine (n = 1), and combination or unspecified antiretroviral therapies for HIV (n = 3).
Includes: ketoconazole (n = 5), albendazole (n = 1), and itraconazole (n = 1).
AHN: acute hepatic necrosis, DILI: drug-induced liver injury, HDS: herbal and dietary supplements
Demographics of HDS DILI versus non-HDS DILI cases
The overall distribution of our study patients when categorized by race/ethnicity was 68.8% White, 13.6% Black, 10.5% Hispanic, 4.3% Asian, and 2.8% other, which is similar to the 2010 US Census data (72% White, 13% Black, 16% Hispanic, 5.6% Asian, 7% Other) (16). While those with Asian ethnicity made up only 5.6% of the total US population (16) they accounted for 17.1% of all cases of LT due to HDS DILI in our study. This large difference in proportions was not seen for the other ethnic or demographic groups studied.
Overall, waitlisted patients with DILI were predominantly female (74.5%) and White (68.7%), with a median age of 32 years (IQR 22–45). There were no significant differences in MELD score at listing or education level between patients waitlisted due to non-HDS versus HDS DILI (Table 2), but HDS DILI patients were more likely to be older (Median age 38 vs 31 y p=0.03) and male (40.6% vs 24.9% P=0.003). However, both above findings lost statistical significance when APAP was excluded due to the high prevalence of young woman in the APAP cases.
Table 2.
Characteristics of patients waitlisted for AHN due to HDS DILI vs non-HDS DILI.
| HDS DILI (n = 69) | Non-HDS DILI (n = 1,806) | P | Non-APAP, non-HDS DILI (n = 435) | P | |
|---|---|---|---|---|---|
| Sex: | |||||
| Female | 41 (59.4%) | 1,357 (75.1%) | 298 (68.5%) | ||
| Male | 28 (40.6%) | 449 (24.9%) | 0.003 | 137 (31.5%) | 0.14 |
| Age (years): | |||||
| Median [IQR] | 38 [23.5–53.5] | 31 [22–44] | 0.03 | 43 [27–55] | 0.17 |
| ≤19 | 10 (14.5%) | 340 (18.8%) | 58 (13.3%) | ||
| 20–29 | 14 (20.3%) | 467 (25.9%) | 68 (15.6%) | ||
| 30–39 | 12 (17.4%) | 390 (21.6%) | 63 (14.5%) | ||
| 40–49 | 13 (18.8%) | 323 (17.9%) | 88 (20.2%) | ||
| 50–59 | 13 (18.8%) | 184 (10.2%) | 97 (22.3%) | ||
| ≥60 | 7 (10.1%) | 102 (5.7%) | 0.12 | 61 (14.0%) | 0.82 |
| Ptrend 0.02 | Ptrend 0.24 | ||||
| Ethnicity: | |||||
| White | 39 (56.5%) | 1,250 (69.2%) | 217 (49.9%) | ||
| Black | 6 (8.7%) | 248 (13.7%) | 110 (25.3%) | ||
| Hispanic | 8 (11.6%) | 190 (10.5%) | 63 (14.5%) | ||
| Asian | 12 (17.4%) | 68 (3.8%) | 28 (6.4%) | ||
| Other | 4 (5.8%) | 50 (2.8%) | 17 (3.9%) | 0.001 | |
| Education beyond high school:1 | |||||
| No | 25 (47.2%) | 712 (59.1%) | 165 (56.7%) | ||
| Yes | 28 (52.8%) | 492 (40.9%) | 0.08 | 126 (43.3%) | 0.20 |
| Year listed: | |||||
| 1994–1999 | 18 (27.3%) | 410 (23.3%) | 133 (31.2%) | ||
| 2000–2004 | 1 (1.5%) | 371 (21.1%) | 90 (21.1%) | ||
| 2005–2009 | 10 (15.2%) | 419 (23.8%) | 83 (19.4%) | ||
| 2010–2014 | 18 (27.3%) | 311 (17.7%) | 64 (15.0%) | ||
| 2015–2020 | 22 (31.9%) | 295 (16.3%) | <0.001 | 65 (14.9%) | <0.001 |
| Ptrend 0.004 | Ptrend 0.001 | ||||
| MELD score at listing2, median [IQR] | 31 [26–37.5] | 35 [29–41] | 0.06 | 32 [27.5–39] | 0.66 |
Tests for significance with chi-squared tests or Fisher exact tests for categorical variables, Kruskal-Wallis rank tests for continuous variables, and univariate logistic regression for trends.
Missing for n = 618 patients (16 with HDS DILI and 602 with non-HDS DILI including 144 with non-APAP/non-HDS DILI).
Available for patients waitlisted in 2002 onward only and additionally missing for n = 17 patients with non-HDS DILI (including n = 2 with non-APAP/non-HDS DILI) from those years.
AHN: acute hepatic necrosis, APAP: acetaminophen, DILI: drug-induced liver injury, HDS: herbal and dietary supplements, IQR: interquartile range, MELD = Model for End-stage Liver Disease
During the study period, 1092 patients were transplanted and categorized as “AHN” cases, of whom 736 had DILI with a specific agent implicated (Fig. 1). Of the 736 patients transplanted due to DILI, 41 (5.6%) had HDS DILI and 695 (94.4%) had non-HDS DILI including 426 (61.2%) due to APAP. APAP waitlisted cases were notably less likely to undergo LT (Table 1). Similar to the waitlisted DILI patients, overall, those transplanted due to DILI were predominantly female (71.7%) and White (66.3%), with a median age of 35 (IQR range: 25–47) years. There were no significant differences in age distribution between patients transplanted due to HDS versus non-HDS DILI, nor in sex, or educational level (Table 3). Interestingly, median MELD score at time of listing but not transplantation was lower in HDS as compared to non-HDS (31.5 vs 37 p= 0.02) cases. However, the finding was not statistically significant when APAP cases were excluded from the comparison. (Table 3)
Table 3.
Characteristics of patients transplanted for AHN due to HDS DILI vs non-HDS DILI.
| HDS DILI (n = 41) | Non-HDS DILI (n = 695) | P | Non-APAP, non-HDS DILI (n = 335) | P | |
|---|---|---|---|---|---|
| Sex: | |||||
| Female | 26 (63.4%) | 502 (72.2%) | 182 (67.7%) | ||
| Male | 15 (36.6%) | 193 (27.8%) | 0.22 | 87 (32.3%) | 0.59 |
| Age (years): | |||||
| Median [IQR] | 36 [24.5–52.5] | 35 [25–47] | 0.65 | 43 [27–54] | 0.18 |
| ≤19 | 3 (7.3%) | 82 (11.8%) | 33 (12.3%) | ||
| 20–29 | 12 (29.3%) | 179 (25.8%) | 44 (16.4%) | ||
| 30–39 | 9 (22.0%) | 152 (21.9%) | 41 (15.2%) | ||
| 40–49 | 6 (14.6%) | 145 (20.9%) | 56 (20.8%) | ||
| 50–59 | 8 (19.5%) | 81 (11.7%) | 55 (20.5%) | ||
| ≥60 | 3 (7.3%) | 56 (8.1%) | 0.64 | 40 (14.9%) | 0.24 |
| Ptrend 0.52 | Ptrend 0.34 | ||||
| Ethnicity: | |||||
| White | 22 (53.7%) | 466 (67.1%) | 140 (52.0%) | ||
| Black | 4 (9.8%) | 119 (17.1%) | 70 (26.0%) | ||
| Hispanic | 6 (14.6%) | 66 (9.5%) | 33 (12.3%) | ||
| Asian | 7 (17.1%) | 21 (3.0%) | 15 (5.6%) | ||
| Other | 2 (4.9%) | 23 (3.3%) | 0.001 | 11 (4.1%) | 0.03 |
| Education beyond high school:1 | |||||
| No | 14 (40.0%) | 269 (54.7%) | 100 (52.9%) | ||
| Yes | 21 (60.0%) | 223 (45.3%) | 0.09 | 89 (47.1%) | 0.16 |
| Year transplanted: | |||||
| 1994–1999 | 6 (15.4%) | 125 (18.6%) | 71 (27.0%) | ||
| 2000–2004 | 1 (2.6%) | 131 (19.4%) | 53 (20.2%) | ||
| 2005–2009 | 4 (10.3%) | 178 (26.4%) | 56 (21.3%) | ||
| 2010–2014 | 15 (38.5%) | 139 (20.6%) | 46 (17.5%) | ||
| 2015–2020 | 15 (36.6%) | 122 (17.6%) | <0.001 | 43 (16.0%) | <0.001 |
| Ptrend 0.001 | Ptrend <0.001 | ||||
| MELD score at listing2, median [IQR] | 31.5 [28–37] | 37 [30–42] | 0.02 | 33 [29–40] | 0.37 |
| MELD score at transplant3, median [IQR] | 35 [30–42.25] | 36 [29–41] | 0.70 | 34 [29–41] | 0.38 |
Tests for significance with chi-squared tests or Fisher exact tests for categorical variables, Kruskal-Wallis rank tests for continuous variables, and univariate logistic regression for trends.
Missing for n = 209 patients (6 with HDS DILI and 203 with non-HDS DILI including 80 with non-APAP/non-HDS DILI).
Available for patients waitlisted in 2002 onward only and additionally missing for n = 4 patients with non-HDS DILI due to APAP from those years.
Available for patients waitlisted in 2002 onward only and additionally missing for n = 4 patients with non-HDS DILI due to APAP from those years.
AHN: acute hepatic necrosis, APAP: acetaminophen, DILI: drug-induced liver injury, HDS: herbal and dietary supplements, IQR: interquartile range, MELD = Model for End-stage Liver Disease
However, the distributions of ethnicities differed significantly between non-HDS versus HDS patients for both waitlisted (Table 2) and transplanted (Table 3) patients.
Asians constituted 17.4% of patients waitlisted due to HDS DILI but only 3.8% (p<0.001) of those waitlisted due to non-HDS DILI (Table 2). Similarly, Asians constituted 17.1% of transplant recipients for HDS DILI but only 3.0 % (p<0.001) of transplant recipients due to non-HDS DILI (Table 3). Asian HDS use consisted predominantly of drugs categorized as supplements and not strictly herbal products. In multi-variate model for waitlisted patients, the risk of having HDS as the cause of DILI was almost 5 times higher for Asians versus Whites (OR 5.09, 95% CI: 2.5–10.3, p<0.001) (Table 4). Other significant associations identified were older age (p=0.06) and female sex (p=0.004). Blacks had a lower risk of have HDS as cause of DILI, however, it did not reach statistical significance (p=0.45) unless APAP cases were excluded. In multivariate models for transplantation adjusted for the risk of having HDS as the cause of DILI for transplant recipients was 5.8 times higher for Asian versus White patients (95% CI: 2.2–15.6, P <0.001) (Table 5). Similarly, as waitlist Blacks had a lower risk of have HDS as cause of DILI for transplantation, however, it did not reach statistical significance (p=0.55). Other predictors for were late transplantation years.
Table 4.
Multivariate predictors of HDS as the cause of DILI-related AHN requiring waitlisting.
| Odds of HDS as cause of DILI-related AHN | Odds of HDS as cause of non-APAP DILI-related AHN | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (per year) | 1.02 | 0.99–1.03 | 0.06 | 0.98 | 0.96–0.99 | 0.02 |
| Year listed (per year, after 1994) | 1.05 | 1.01–1.08 | 0.009 | 1.07 | 1.03–1.11 | <0.001 |
| Sex: | 1.00 (ref) | 1.00 (ref) | ||||
| Female | 2.10 | 1.26–3.46 | 0.004 | 1.25 | 0.72–2.16 | 0.42 |
| Male | ||||||
| Ethnicity: | 1.00 (ref) | 1.00 (ref) | ||||
| White | 0.78 | 0.32–1.86 | 0.57 | 0.29 | 0.11–0.73 | 0.008 |
| Black | 1.36 | 0.61–2.97 | 0.45 | 0.65 | 0.28–1.50 | 0.31 |
| Hispanic | 5.09 | 2.50–10.33 | <0.001 | 2.09 | 0.95–4.56 | 0.07 |
| Asian | 2.59 | 0.87–7.62 | 0.09 | 1.38 | 0.42–4.49 | 0.59 |
| Other | ||||||
AHN: acute hepatic necrosis, APAP: acetaminophen, DILI: drug-induced liver injury, HDS: herbal and dietary supplements
Table 5.
Multivariate predictors of HDS as the cause of transplantation for DILI-related AHN.
| Odds of HDS as cause of DILI-related AHN transplant | Odds of HDS as cause of non-APAP DILI-related AHN transplant | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (per year) | 1.00 | 0.97–1.02 | 0.95 | 0.98 | 0.95–1.00 | 0.05 |
| Year listed (per year, after 1994) | 1.09 | 1.03–1.15 | 0.001 | 1.11 | 1.05–1.17 | <0.001 |
| Sex: | ||||||
| Female | 1.00 (ref) | 1.00 (ref) | ||||
| Male | 1.52 | 0.77–3.01 | 0.22 | 0.98 | 0.47–2.03 | 0.96 |
| Ethnicity: | ||||||
| White | 1.00 (ref) | 1.00 (ref) | ||||
| Black | 0.71 | 0.23–2.13 | 0.55 | 0.38 | 0.12–1.20 | 0.10 |
| Hispanic | 1.79 | 0.68–4.65 | 0.23 | 1.14 | 0.41–3.16 | 0.80 |
| Asian | 5.84 | 2.18–15.60 | <0.001 | 2.45 | 0.85–7.02 | 0.10 |
| Other | 1.79 | 0.38–8.32 | 0.46 | 1.31 | 0.24–6.95 | 0.75 |
AHN: acute hepatic necrosis, APAP: acetaminophen, DILI: drug-induced liver injury, HDS: herbal and dietary supplements
Temporal Trends in HDS DILI
Almost 60% of patients waitlisted for LT for HDS DILI were listed in 2010–2020, with a significant trend by listing year in the proportion due to HDS DILI (p for trend =0.004). Similarly, more than 70% of patients transplanted for HDS DILI were transplanted in 2010–2020, with a significant trend by year of transplant in the proportion due to HDS DILI (p for trend =0.001). Longitudinal trends in waitlisting and transplantation for HDS DILI persisted even if APAP cases were excluded (Tables 2 and 3).
One-year transplant-free survival of patients transplanted for AHN due to DILI.
HDS cases had good one year transplant-free survival as compared to APAP and non-APAP, non-HDS DILI cases after liver transplantation (89.7 % vs 74% vs 78.7 % P= 0.048). In multivariate modelling also the odds of one year transplant-free survival for transplantation due to non-APAP, non-HDS was 1.5 times the APAP post-transplant survival percentage (CI: 1.02 −2.32, P=0.04). Other predictors for one year transplant free survival were years transplanted (Table 6).
Table 6.
One-year transplant-free survival of patients transplanted for AHN due to DILI.
| Multivariate odds of 1-year transplant-free survival | ||||||
|---|---|---|---|---|---|---|
| n surviving / N | Survival (%) | Univariate P | OR | 95% CI | P | |
| Cause of DILI: | 0.048 | |||||
| Acetaminophen | 304 / 411 | 74.0% | 1.00 (ref) | |||
| Non-acetaminophen, non-HDS | 207 / 263 | 78.7% | 1.54 | 1.02–2.32 | 0.04 | |
| HDS | 35 / 39 | 89.7% | 2.07 | 0.81–5.28 | 0.13 | |
| Sex: | 0.18 | |||||
| Female | 386 / 513 | 75.2% | 1.00 (ref) | |||
| Male | 160 / 200 | 80.0% | 1.21 | 0.82–1.80 | 0.33 | |
| Age (years): | 0.22 | |||||
| ≤19 | 55 / 83 | 66.3% | 0.65 | 0.36–1.15 | 0.14 | |
| 20–29 | 143 / 183 | 78.1% | 1.00 (ref) | |||
| 30–39 | 120 / 158 | 76.0% | 0.99 | 0.60–1.64 | 0.98 | |
| 40–49 | 116 / 146 | 79.5% | 1.05 | 0.62–1.76 | 0.86 | |
| 50–59 | 69 / 85 | 81.2% | 0.88 | 0.46–1.66 | 0.69 | |
| ≥60 | 43 / 58 | 74.1% | 0.73 | 0.36–1.48 | 0.39 | |
| Ethnicity: | 0.61 | |||||
| White | 362 / 473 | 76.5% | 1.00 (ref) | |||
| Black | 86 / 119 | 72.3% | 0.69 | 0.43–1.09 | 0.11 | |
| Hispanic | 57 / 69 | 82.6% | 1.39 | 0.74–2.61 | 0.30 | |
| Asian | 21 / 27 | 77.8% | 0.69 | 0.26–1.77 | 0.44 | |
| Other | 20 / 25 | 80.0% | 1.18 | 0.42–3.30 | 0.75 | |
| Education beyond high school:1 | 0.997 | Excluded from model | ||||
| No | 211 / 271 | 77.9% | ||||
| Yes | 183 / 235 | 77.9% | ||||
| Year transplanted: | <0.001 | |||||
| 1994–1999 | 95 / 131 | 72.5% | 0.99 | 0.56–1.74 | 0.98 | |
| 2000–2004 | 84 / 132 | 63.6% | 0.72 | 0.42–1.23 | 0.23 | |
| 2005–2009 | 136 / 182 | 74.7% | 1.20 | 0.71–2.01 | 0.48 | |
| 2010–2014 | 134 / 154 | 87.0% | 2.72 | 1.47–5.03 | 0.001 | |
| 2015–2019 | 99 / 137 | 72.3% | 1.00 (ref) | |||
Excluded patients transplanted in 2020 due to <1 year follow-up available (n = 23).
Tests for significance with chi-squared tests or Fisher exact tests for univariate comparisons and logistic regression for multivariate model.
Missing for n = 207 patients including 152 who were transplanted.
AHN: acute hepatic necrosis, DILI: drug-induced liver injury, HDS: herbal and dietary supplements
Discussion:
For patients presenting with acute liver injury, rapid identification of its etiology and severity is important to identify patients at risk for progression to liver failure and possibly death. Prior multi-site registration studies have characterized those with medication and HDS related acute liver injury to help providers more quickly assess similar cases. Utilizing the larger national UNOS database, we have further characterized severe cases of drug induced liver injury. Our study population of waitlisted patients likely included those with more severe liver injury than the prior US-DILIN studies and may be particularly useful in assessing hospitalized patients with drug induced liver injury.
The results of this study confirm several of the previously reported associations for acute liver injury due to medications and HDS with regards to female sex, older age, as well as its increasing incidence (9,11). Worse outcomes have been reported for Asians with DILI related acute liver injury (10) and those with HDS related acute liver injury (11). The finding that DILI related acute liver injury in waitlisted Asian patients was almost 5 times more likely to be due to HDS than for Whites may help explain the worse outcomes for Asians with DILI related acute liver injury. The opposite trend was noted for Blacks when excluding APAP cases.
In many cases of drug induced acute liver injury, patients are taking both HDS and non-HDS medications. For Asian patients with drug-induced liver injury, HDS is a more likely cause, while for Blacks, non-HDS, non-APAP medications is the more likely etiology.
Several factors may contribute to poorer outcomes from HDS related AHN. HDS are readily available over the counter, do not require prescriptions from providers, and are not rigorously regulated by the FDA. Clinical trials to assure their safety are not required prior to dissemination of most HDS. Additionally, the contents of HDS implicated in DILI are frequently mislabeled (18).
There is very limited data regarding the frequency and type of HDS use amongst different ethnic groups. In the 2002 Health and Diet Survey sponsored by the FDA, there was a significant difference in HDS use frequency by self-declared ethnic group with higher use reported in Whites and the “Other” group (4). Asian ethnicity was not studied individually and was included within “Other”, and details regarding the types of HDS being used were incomplete. In another survey of complementary and alternative medication, use rate of herbal and natural products was higher among those with Asian ethnicity, American Indians, or “multi-racial” individuals (19). Of note, in some Asian countries, HDS are a more common cause of DILI than acetaminophen or other conventional medications (20). Of course, HDS use may vary by country even amongst those of similar ethnic backgrounds.
Alternatively, as posed by US-DILIN researchers, genetic susceptibility, delayed presentation for care, or impaired liver regeneration in Asian patients would each conceivably contribute to worse outcomes from DILI related acute liver injury (10) even if HDS use is similar across ethnic groups. Indeed, HLA associations with genetic susceptibility to DILI from specific medications have been published suggesting an immune component to DILI (21). HLA alleles and haplotypes do vary significantly between ethnic groups. Some with Asian ethnicity may have increased susceptibility to DILI from specific HDS compared to other ethnic groups due to differences in HLA or non-HLA immune variant expression (22). Recently HLA-B*35:01 was identified as a high-risk allele for Polygonum multi-florum and green tea induced liver injury (23,24). Regardless of the mechanism for worse outcomes for HDS related DILI, clinicians should be particularly alert to HDS use amongst those with Asian ethnicity who develop severe acute liver injury.
The ALFSG network reviewed 927 patients who presented for management of ALF. These patients were 81.8% White, 12.8% Black, and 5.4% Asian, a similar population to our study (11). Within their cohort those classified as Asian or Black were significantly more likely to have ALF due to DILI than Whites. Our study did not include non-DILI causes of ALF so a direct comparison cannot be made. Their study did not report how many DILI cases were due to HDS use. They also found that those with Asian ethnicity and ALF were more likely to undergo LT with an odds ratio of 2.8, though this finding was attenuated and lost significance in the adjusted analysis. Another retrospective US cohort study utilizing the Drug-Induced Liver Injury Network (US DILIN) database, looked at 660 adults with definite, highly likely, or probable DILI with the aim of identifying demographic factors associated with early liver-related death or transplantation (11). In this group, self-declared Asian ethnicity was found to be independently associated with liver-related death or transplantation. Our data does not suggest increased risk of transplantation based on the ethnicity of those waitlisted for AHN due to DILI. Most US-DILIN patients were not waitlisted for liver transplantation as in our study population nor hospitalized as in the ALFSG cohort. Conceivably Whites with mild cases of DILI are more likely to enroll in US-DILIN than Asians.
Interestingly, in our study there were no significant differences in age distribution between patients transplanted due to HDS versus non-HDS DILI, however waitlisted patient due to HDS DILI were slightly older (Median age 38 vs 31 years for non-HDS DILI). There was also a difference in MELD score at listing with HDS DILI patient having a low MELD score at time for listing for transplant as compared to non-HDS DILI (31.5 vs 37). Likewise, with regard to sex, rurality and education level there was no significant differences between the patient groups. Some studies have seen an association between herbal medicine use and higher education levels as well with those between 25 and 49 years old (25,26). Our results did not indicate such a relationship, but our sample size was relatively small.
Independent of ethnicity this study showed that both the incidence and the proportion of cases of severe AHN due to HDS DILI in the US are increasing over time. As noted above, it is uncertain if this simply reflects increased use of HDS by the general population over time or a change in the types of HDS being consumed. These findings should encourage an increased awareness of the potential harm from HDS use amongst clinicians and purchasers of HDS.
There are several limitations to this study. As opposed to registry studies, one cannot verify data independently nor obtain additional clinical information as desired. The adjudication process utilized to diagnose DILI and to identify the implicated drug recorded in the UNOS database is likely quite variable and may not be highly rigorous. It would be beneficial to future studies if more specific data on the implicated HDS products and a more formal causality review process for non-APAP DILI listed cases were available. Despite utilization of a national database, the total sample size in our study remained small. For many cases within the database, information was missing regarding rurality and education. The conclusions regarding ethnicity are subject to error since data on ethnic differences in HDS use in the US are limited as mentioned above, the overall limited information regarding the total number of individuals using HDS is also a major barrier in assessing the overall safety of HDS use.
In conclusion, severe DILI amongst Asians is more commonly due to HDS use than other ethnic groups, especially Blacks. Liver failure caused by HDS requiring LT should be expected to be a growing problem with increasing use of HDS.
Supplementary Material
Acknowledgement:
We would like to acknowledge Drs. Vivek Kesar and Ramit Singla for their valuable suggestions in preparation of manuscript.
Grant: NIDDK grant number. U01DK100928 (Icahn School of Medicine at Mount Sinai)
Abbreviations:
- ALFSG
Acute Liver Failure Study Group
- ALF
Acute Liver Failure
- APAP
Acetaminophen
- AHN
Acute Hepatic Necrosis
- DILI
Drug induced liver injury
- FDA
Food and Drug Administration
- HDS
Herbal and Dietary Supplements
- LT
Liver Transplantation
- OPTN
Organ Procurement and Transplantation Network
- ULN
Upper limit of normal
- UNOS
United Network for Organ Sharing
- WL
Wait list
Footnotes
Disclosures:
The authors have no conflict of interest to disclose.
References:
- 1.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007 [DOI] [PubMed] [Google Scholar]
- 2.Toma D, Lazar O, Bontas E. Acute Liver Failure. Liver Diseases. 2019;369–380. Published 2019 Jul 10. doi: 10.1007/978-3-030-24432-3_32 [DOI] [Google Scholar]
- 3.Rossi S, Navarro VJ, Herbs and Liver Injury: A Clinical Perspective. Clinical Gastroenterology and Hepatology 2014; 12:1069–1076 [DOI] [PubMed] [Google Scholar]
- 4.Clarke T, Black L et al. Trends in the Use of Complementary Health Approaches Among Adults: United States, 2002–2012. National health statistics reports; no 68. Hyattsville, MD: 2015 [PMC free article] [PubMed] [Google Scholar]
- 5.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA. 2016;316(14):1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Center for complementary and integrative health: https://www.nccih.nih.gov/health/using-dietary-supplements-wisely. Accessed January 2021.
- 7.Timbo BB, Ross MP, McCarthy PV, Lin C-T. Dietary Supplements in a National Survey: Prevalence of Use and Reports of Adverse Events. J Am Diet Assoc. 2006;106:1966–1974. [DOI] [PubMed] [Google Scholar]
- 8.Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from Herbals and Dietary Supplements in the US Drug Induced Liver Injury Network. Hepatology. 2014. October ; 60(4): 1399–1408. doi: 10.1002/hep.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grewal P, Ahmad J, Severe Liver Injury Due to Herbal and Dietary Supplements and the Role of Liver Transplantation World J Gastroenterol 2019. December 14; 25(46): 6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontana RJ, Hayashi PH, Gu J, et al. Idiosyncratic Drug-Induced Liver Injury Is Associated With Substantial Morbidity and Mortality Within 6 Months From Onset. Gastroenterology 2014;147:96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forde K et al. , Racial and ethnic differences in presentation, etiology and outcomes of acute liver failure in the United States, Clin Gastroenterol Hepatol 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mindikoglu AL, Magder LS, Regev A. Outcome of liver transplantation for drug-induced acute liver failure in the United States: Analysis of the united network for organ sharing database. Liver Transpl. 2009;15(7):719. [DOI] [PubMed] [Google Scholar]
- 13.Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10(8):1018–23. [DOI] [PubMed] [Google Scholar]
- 14.US Census bureau: https://www2.census.gov/geo/pdfs/reference/ua/Defining_Rural.pdf. Accessed January 2021.
- 15.US Census Bureau: https://mtgis-portal.geo.census.gov/arcgis/apps/MapSeries/index.html?appid=49cd4bc9c8eb444ab51218c1d5001ef6. Accessed December 2020.
- 16.Humes Karen R.; Jones Nicholas A.; Ramirez Roberto R. Overview of Race and Hispanic Origin: 2010” (PDF). United States Census Bureau. United States Department of Commerce; ) March 2011 [Google Scholar]
- 17.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148(7):1340–52.e7. doi: 10.1053/j.gastro.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro Victor, Avula Bharathi, Khan Ikhlas, Verma Manisha, Seeff Leonard, Serrano Jose, Stolz Andrew, Fontana Robert, and Ahmad Jawad. The Contents of Herbal and Dietary Supplements Implicated in Liver Injury in the United States Are Frequently Mislabeled. Hepatol Commun. 2019. Jun; 3(6): 792–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy J. Herb and supplement use in the US adult population. Clin Ther. 2005;27(11):1847–58. [DOI] [PubMed] [Google Scholar]
- 20.Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, et al. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology. 2019;156(8):2230–41. [DOI] [PubMed] [Google Scholar]
- 21.Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M, Barnhart HX, Bondon-Guitton E, Hayashi PH, Bessone F, Carvajal A, Cascorbi I, Cirulli ET, Chalasani N, Conforti A, Coulthard SA, Daly MJ, Day CP, Dillon JF, Fontana RJ, Grove JI, Hallberg P, Hernández N, Ibáñez L, Kullak-Ublick GA, Laitinen T, Larrey D, Lucena MI, Maitland-van der Zee AH, Martin JH, Molokhia M, Pirmohamed M, Powell EE, Qin S, Serrano J, Stephens C, Stolz A, Wadelius M, Watkins PB, Floratos A, Shen Y, Nelson MR, Urban TJ, Daly AK; International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of Liver Injury From Specific Drugs, or Groups of Drugs, With Polymorphisms in HLA and Other Genes in a Genome-Wide Association Study. Gastroenterology. 2017. Apr;152(5):1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad J, Odin JA, Epidemiology and Genetic Risk Factors of Drug Hepatotoxicity. Clinical Liver Disease 21 (2017) 55–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Rao T, Chen X, et al. HLA-B*35:01 Allele Is a Potential Biomarker for Predicting Polygonum multiflorum-Induced Liver Injury in Humans. Hepatology. 2019;70(1):346–357. doi: 10.1002/hep.30660 [DOI] [PubMed] [Google Scholar]
- 24.Hoofnagle JH, Bonkovsky HL, Phillips EJ, et al. HLA-B*35:01 and Green Tea-Induced Liver Injury [published online ahead of print, 2020 Sep 5]. Hepatology. 2020;10.1002/hep.31538. doi: 10.1002/hep.31538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional Medicine in the United States -- Prevalence, Costs, and Patterns of Use. N Engl J Med. 1993;328(4):246–52. [DOI] [PubMed] [Google Scholar]
- 26.Winslow LC, Kroll DJ. Herbs as Medicines. Arch Intern Med. 1998;158(20):2192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


